Abstract
Objective:
Regarding the anticonvulsant effects of Satureja hortensis (S. hortensis) in Avicenna’s book: canon of medicine; the present study was undertaken to evaluate the anti- eplileptic effects of S. hortensis aqueous and ethanolic aerial part extracts. Furthermore, the mechanisms of their anticonvulsant activities were also evaluated.
Materials and Methods:
Seizure was induced by Pentylentetrazol (PTZ) and MES (maximal electroshock) models. Mice were randomly divided into 8 groups; negative control (normal saline, 10ml/Kg), positive control (diazepam, 2 mg/kg), S. hortensis aqueous and ethanolic extracts (200, 400 and 600 mg/kg). In PTZ test, latency to the first minimal clonic seizure (MCS), latency to the first generalized tonic–clonic seizures (GTCS), the total duration of seizures and protection against mortality were evaluated. In MES test, the stretching length of extremities and protection against mortality were recorded.
Results:
Aqueous and ethanolic extracts (400 and 600 mg/kg) significantly increased MCS and GTCS latencies in PTZ model. Three doses of the extracts decreased the total duration of seizure. These extracts did not show any protective effects on seizure induced by MES model. In PTZ model, flumazenil, an antagonist of benzodiazepine (BZD) site in the GABAA-BZD receptor complex and 7- nitroindazole (7- NI), a selective nNOS (neuronal nitric oxide synthase) inhibitor, reduced the prolongation of seizure latency.
Conclusion:
S. hortensis showed anticonvulsant activity in PTZ model and this effect may be mediated, at least partly, through interacting with nitric oxide and GABAA-BZD receptor complex.
Key Words: Satureja hortensis, Seizure, Pentylenetetrazol, Maximal electroshock, Flumazenil, 7-nitro- indazole
Introduction
Epilepsy is defined as one of the most common serious neurological illness which is characterized by recurrent seizures (Porter et al., 2001). The incidence of epilepsy is about 0.5-1%. It can occur at any age (Hosseinzadeh et al., 2013). Although several anti-epileptic drugs are used to treat convulsions, due to the incomplete medication of about 30% of patients, side effects of these drugs, and chronic nature of epileptic disease, herbal medicines are widely recommended (Gorgich et al., 2012). Herbal medicines are being used for the treatment of a variety of disorders including neurological diseases because of their safety, efficacy, cultural acceptability and fewer side effects (Semnani et al., 2007). According to the literature, some plants and their active constituents including Crocus sativus (Hosseinzadeh et al., 2007), Nigella sativa (Hosseinzadeh et al., 2004), Hypericum perforatum (Hosseinzadeh et al., 2004), Justicia extensa (Sowemimo et al., 2012), Annona senegalensis (Okoye et al., 2013), Zyzyphus jujube (Pahuja et al., 2012) , Harpagophytum procumbens (Mohamed et al., 2006), Sutherlandia frutescens (Ojewole et al., 2008), and Zingiber officinale (Hosseini et al., 2014), exhibit anticonvulsant activity.
Satureja hortensis L. (S. hortensis L.) is a plant belongs to Lamiaceae family (Labiatae). It is distributed in the Europe, Asia and northern Africa. This plant is also cultivated in Iran (Fathi et al., 2013. Kamkar et al., 2013). Besides its use in cookery, S. hortensis is utilized for treating many diseases including cardiovascular diseases, gastrointestinal disorders, muscle pains, cramps, and infectious diseases in traditional and modern medicines. Moreover, different properties including antibacterial, antifungal, antioxidant, analgesic and carminative effects have been attributed to this plant (Fathi et al., 2013; Dorman et al., 2004; Yazdanparast et al., 2012).
Since there are some reports in traditional medicine regarding the use of S. hortensis for the treatment of seizure (Asadi et al., 2012). The anticonvulsant activity of aqueous and ethanolic extracts of S. hortensis was evaluated in this study.
Materials and Methods
Animals
Male albino mice (weighing 25 ± 3 g) have been used in this study. Animals were housed in a ventilated room under a 12/12-hour light/dark cycle at 24 °C with free access to water and food. All animals in these experiments were carried out in accordance with Mashhad University of Medical Sciences, Ethical Committee Acts.
Plant
The aerial parts of S. hortensis were collected from Mashhad, and were identified by Mr. Joharchi and voucher samples were preserved for reference in the Herbarium of the Department of Pharmacognosy, School of Pharmacy, Mashhad (Voucher no. 1402).
Preparation of extract
S. hortensis aerial parts were cleaned, dried in shadow and powdered by a mechanical grinder. Then, the powder of leaves (100 g) was defatted with petroleum ether (40–60 °C) using the Soxhlet apparatus. For the ethanolic extract, the powder (100 g) was subsequently macerated in 1000 ml ethanol (70%, v/v) for 72 hours and the mixture was filtered and concentrated in vacuum at 40 °C. Then, to obtain dried powder, the residue was freeze dried. For the aqueous extract, 1000 ml distilled water was added to 100 g of aerial parts powder and was boiled for about 20 minutes. Then it was filtered. The extract was then concentrated in vacuum to the desired volume and then freeze dried.
Study design
The mice were randomly divided into 8 groups of six animals each: (1) negative control (normal saline, 10 ml/kg), (2) positive control (diazepam, 2 mg/kg), (3, 4, 5) ethanolic extract (200, 400, and 600 mg/kg, y) and (6, 7 and 8) aqueous extract (200,400 and 600 mg/kg). The selected doses of extracts were based on the calculated maximum tolerated dose in our pilot study.
Anticonvulsant activity
Pentylenetetrazol (PTZ) induced seizure test
Ethanolic extract, normal saline and diazepam were administrated intraperitoneally, 30 minutes prior to pentylenetetrazole (PTZ) (90 mg/Kg). The aqueous extract was administrated intraperitoneally, 60 minutes prior to PTZ (Hosseinzadeh et al., 2004). The animals were placed individually in plastic boxes and observed for 20 minutes.
In PTZ model, the latency to the first minimal clonic seizure (MCS), latency to the first generalized tonic–clonic seizures (GTCS), the total duration of seizures and protection against mortality were evaluated (Hosseinzadeh et al., 2000; Ramezani et al., 2004). In another experiment, flumazenil (10 mg/kg), an antagonist of benzodiazepine (BZD) site in the GABAA-BZD receptor complex (Hosseinzadeh et al., 2007) was administrated 30 minutes prior to the extracts. 7-NI (10 mg/kg), a selective nNOS inhibitor (Babbedge et al., 1993) was also administrated 60 minutes prior to S.hortensis L. extracts.
Maximal electroshock seizure (MES) test
Ethanolic extract, normal saline and diazepam were administrated intraperitoneally, 30 minutes prior to the MES test. The aqueous extract was administrated intraperitoneally, 60 minutes prior to the MES test (Hosseinzadeh et al., 2004). Then, the stimulus train was applied via ear-clip electrodes (sinusoidal pulses, 120 mA and 60 Hz, for 0.2 seconds) using a constant current stimulator (Digital Electroshock Model 150, EghbalTeb Co., Mashhad, Iran). A drop of 0.9% saline solution was applied on each ear of the animal prior to placing the electrode. The duration of hind limb tonic extension (HLTE), and the protection against mortality were recorded (Hosseinzadeh et al., 2000).
Statistical analysis
All results are expressed as mean±SEM. ANOVA followed by Tukey–Kramer test were performed to compare the means. p values less than 0.05 were considered as significant.
Results
Anticonvulsant activity
PTZ-induced seizure test
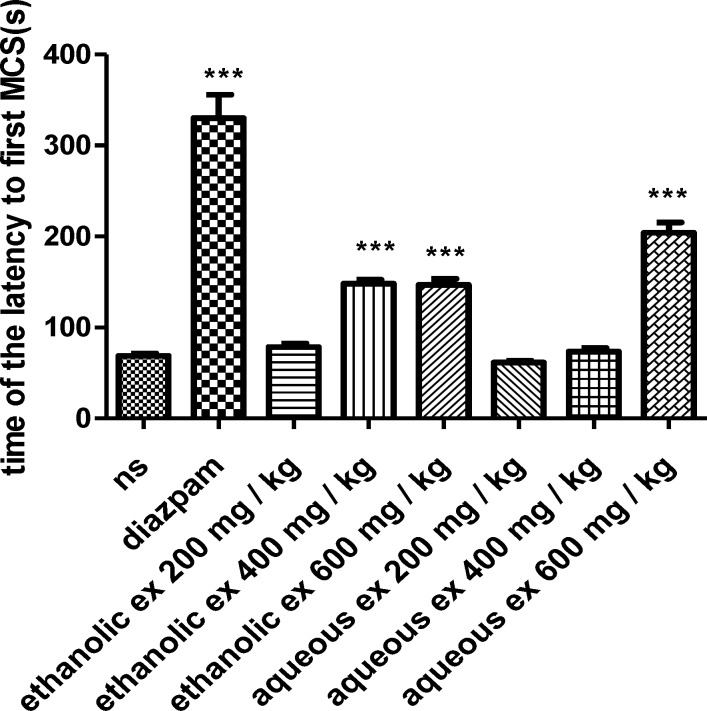
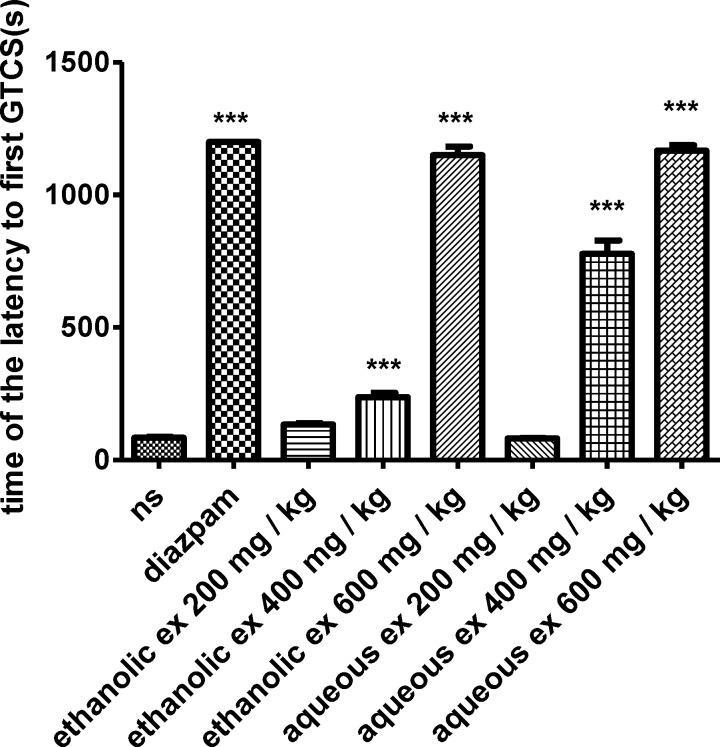
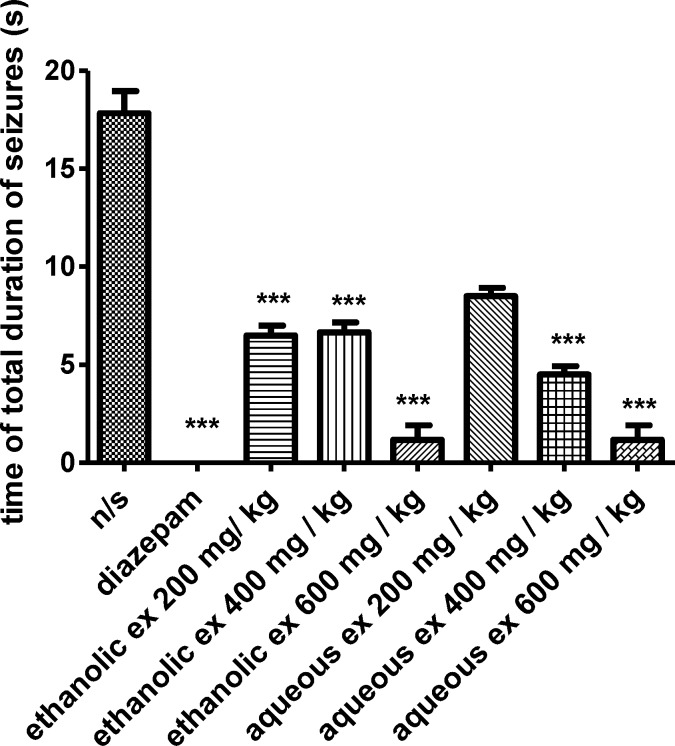
In the PTZ-induced seizure, administration of the ethanolic extract (400 and 600 mg/kg) (p< 0.001), increased the latency to the first minimal clonic seizure (MCS) (Figure 1) and latency to the first generalized tonic–clonic seizures (GTCS) (Figure 2), compared to the negative control group. The ethanolic extract (200, 400 and 600 mg/kg) (p<0.001) decreased the total duration of seizure (Figure 3). The aqueous extract (600 mg/kg) increased MCS (Figure 1) and GTCS (Figure 2) and decreased total duration of seizure (400 and 600 mg/kg) (Figure 3).
Figure 1.
Effect of ethanolic and aqueous extracts of S. hortensis on the latency to the first minimal clonic seizure (MCS) in PTZ-induced seizure in mice. Data are presented as mean ± SEM. Tukey Kramer, p<00.1 vs normal saline, n=6. (ns= Normal saline , ex= Extract
Figure 2.
Effect of ethanolic and aqueous extracts of S. hortensis on the latency to first generalized tonic-clonic seizure (GTCS) in PTZ-induced seizure in mice. Data presented as mean ± SEM. Tukey Kramer, p<00.1 vs normal saline, n=6. (ns= Normal saline , ex= Extract
Figure 3.
Effect of ethanolic and aqueous extracts of S. hortensis on total duration of seizures in PTZ-induced seizure in mice. Data presented as mean ± SEM. Tukey Kramer, p<00.1 vs normal saline, n=6. (ns= Normal saline , ex= Extract
Moreover, the aqueous extract (600 mg/kg) protected against mortality after 30 minutes and 24 hours (p<0.05) (Table 1).
Table 1.
Effect of aqueous and ethanolic extracts of S. hortensis on mortality (%) in PTZ model after 30 minutes and 24 hours in mice. Fisher test. p<0.0 vs normal saline. n=6
| Agents | Protection against mortality (%) after 30 minutes | Protection against mortality (%) after 24 hours |
|---|---|---|
| Normal saline | 33 | 17 |
| Diazepam | 100* | 100* |
| Aqueous extract of S. hortensis (200mg/kg) | 50 | 33 |
| Aqueous extract of S. hortensis (400mg/kg) | 66 | 66 |
| Aqueous extract of S. hortensis (600mg/kg) | 83* | 83* |
| Ethanolic extract of S. hortensis (200mg/kg) | 50 | 50 |
| Ethanolic extract of S. hortensis (400mg/kg) | 66 | 66 |
| Ethanolic extract of S. hortensis (600mg/kg) | 83* | 83* |
MES test
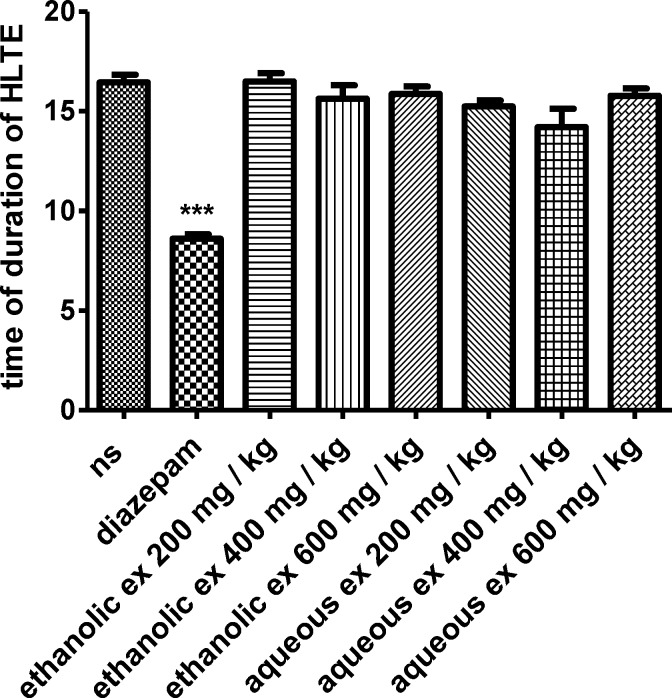
In the MES test, both extracts could not reduce the duration of hind limb tonic extension (HLTE) (Figure 4). Although the extract reduced the mortality (%) compared to the normal saline, this reduction was not statistically significant (Table 2).
Figure 4.
Effect of aqueous and ethanolic extracts of S. hortensis on duration of hind limbtonic extension (HLTE) in MES-induced seizure in mice. Data presented as mean ± SEM. Tukey Kramer, p<00.1 vs normal saline, n=6. (ns= Normal saline , ex= Extract
Table 2.
Effect of aqueous and ethanolic extracts of S. hortensis on mortality (%)in MES model in mice. Fisher test. p<0.0 vs normal saline. n=6
| Agents | Protection against mortality (%) |
|---|---|
| Normal saline | 16 |
| Diazepam | 100* |
| Aqueous extract of S. hortensis (200mg/kg) | 33 |
| Aqueous extract of S. hortensis (400mg/kg) | 33 |
| Aqueous extract of S. hortensis (600mg/kg) | 50 |
| Ethanolic extract of S. hortensis (200mg/kg) | 33 |
| Ethanolic extract of S. hortensis (400mg/kg) | 50 |
| Ethanolic extract of S. hortensis (600mg/kg) | 66 |
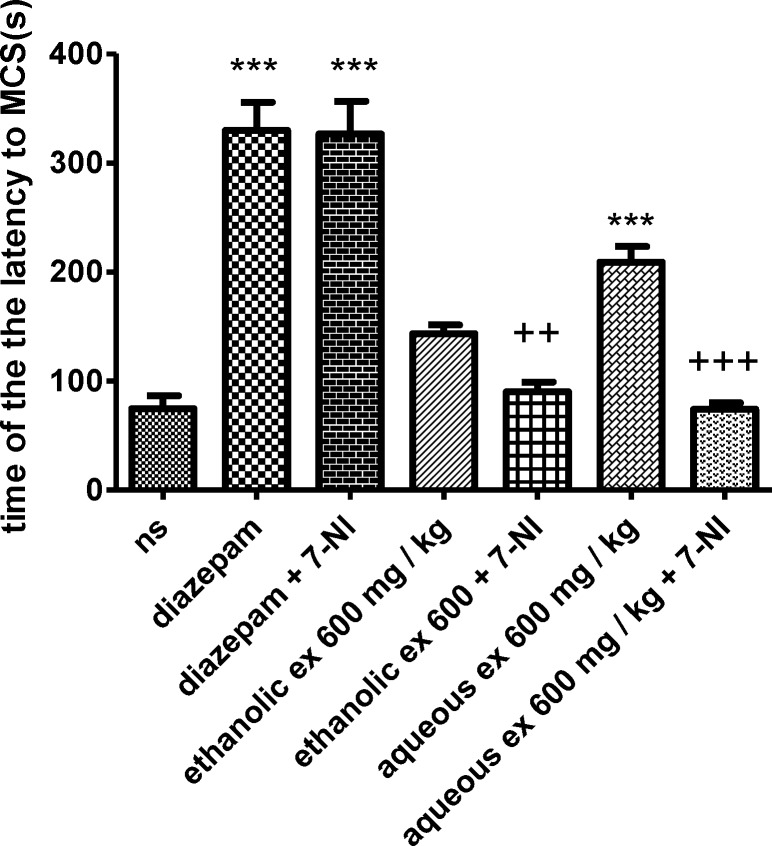
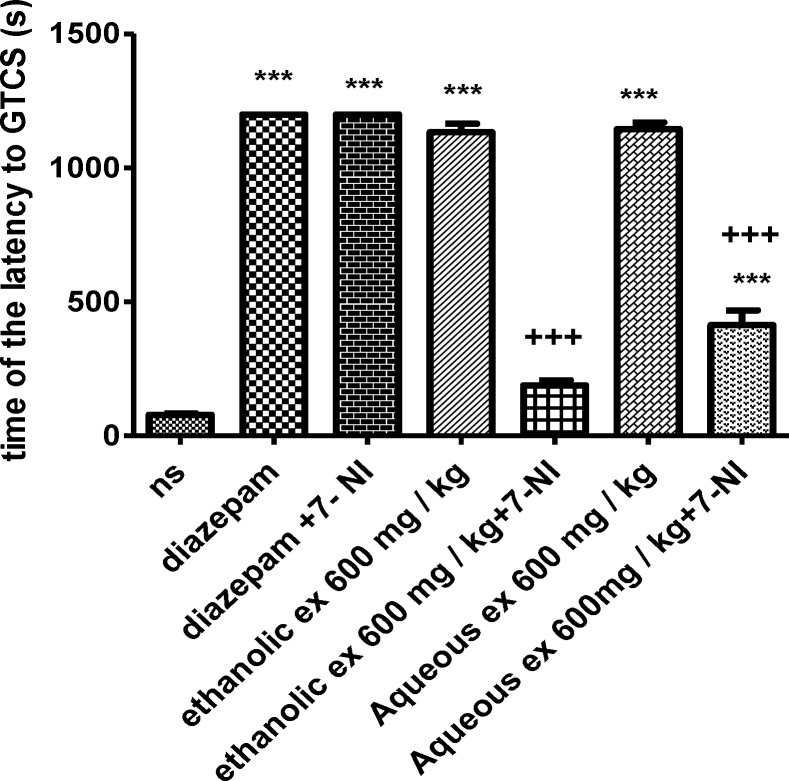
The effect of 7-NI on the anticonvulsant activity of S. hortensis extracts
The results showed that 7-NI significantly decreased the anticonvulsant effect of the aqueous and ethanolic extracts (600 mg/kg) against PTZ-induced seizures by reducing the latency to the first minimal clonic seizure (MCS) (p<0.001 and 0.01, respectively) (Figure 5) and the latency to the first generalized tonic–clonic seizures (GTCS) (p<0.001) (Figure 6).
Figure 5.
Effect of aqueous and ethanolic extracts of S. hortensis on the latency to first minimal clonic seizure (MCS) in PTZ-induced seizure in the presence and absence of 7-NI in mice. Data presented as mean ± SEM. Tukey Kramer, ***p<00.1 vs normal saline, +++ p<0.001 and ++ p<0.01 vs extracts received 7- nitroindazol. n=6. (ns= Normal saline , ex= Extract , 7-NI=7- nitroindazole
Figure 6.
Effect of aqueous and ethanolic extracts of S. hortensis on the latency to the first generalized tonic-clonic seizure (GTCS) in PTZ-induced seizure in the presence and absence of 7- NI in mice. Data presented as mean ± SEM. Tukey Kramer, ***p<00.1 vs normal saline, +++ p<0.001 vs extracts received 7- nitroindazol. n=6. (ns= Normal saline , ex= Extract , 7-NI=7-NI
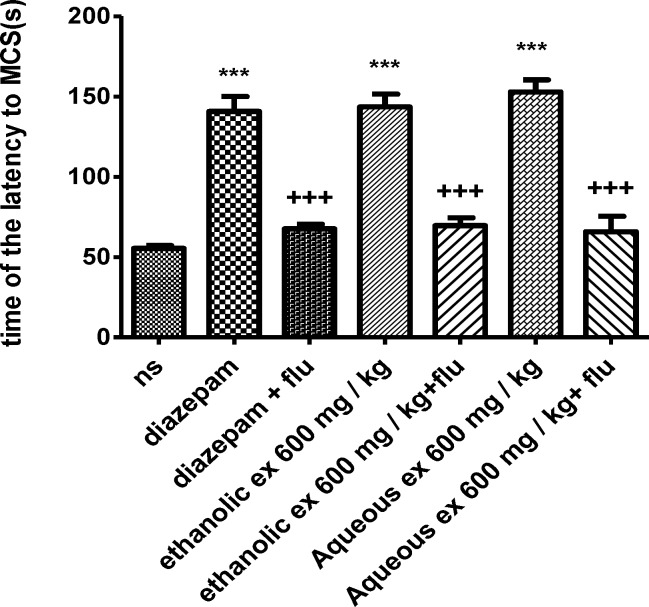
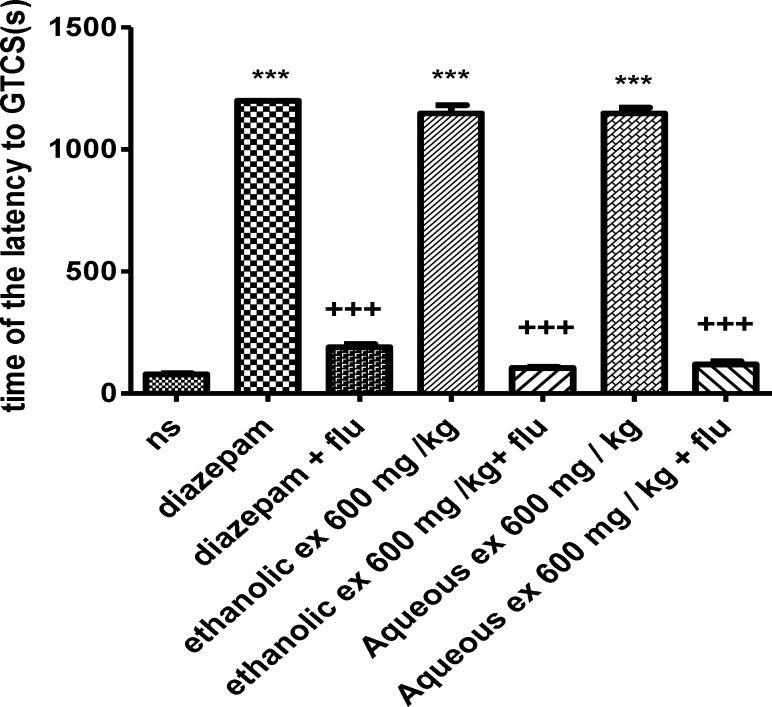
The effect of flumazenil on the anticonvulsant activity of S. hortensis extracts
Flumazenil reduced the anticonvulsant activity of the aqueous and ethanolic extracts (600 mg/kg) by decreasing the latency to the first minimal clonic seizure (MCS) (p<0.001) (Figure 7) and the latency to the first generalized tonic–clonic seizures (GTCS) (p<0.001) (Figure 8).
Figure 7.
Effect of aqueous and ethanolic extracts of S. hortensis on the latency to first minimal clonic seizure (MCS) in PTZ-induced seizure in the presence and absence of flumazenil in mice. Data presented as mean ± SEM. Tukey Kramer, ***p<00.1 vs normal saline, +++ p<0.001 vs extracts received flumazenil. n=6. (ns= Normal saline , ex= Extract , flu= Flumazenil
Figure 8.
Effect of aqueous and ethanolic extracts of S. hortensis on the latency to first generalized tonic-clonic seizure (GTCS) in PTZ-induced seizure in the presence and absence of flumazenil in mice. Data presented as mean ± SEM. Tukey Kramer, ***p<00.1 vs normal saline, +++ p<0.001 vs extracts received flumazenil. n=6. (ns= Normal saline , ex= Extract , flu= Flumazenil
Discussion
This study indicated that ethanolic and aqueous extracts of S. hortensis exhibited anticonvulsant effects in the PTZ-induced seizure model but not in the MES induced seizure model. These extracts increased the latency to first minimal clonic seizure (MCS) and the latency to the first generalized tonic–clonic seizures (GTCS) and decreased the total duration of seizure compared to the negative control group especially at higher doses. Furthermore, pretreatment with flumazenil (10 mg/kg) or 7-NI (10 mg/kg), prior to extracts (600 mg/kg, i.p.), reduced the protective effect of S. hortensis against PTZ-induced seizure.
PTZ, is a chemical which blocks selectively the chloride channel coupled to the GABAA receptor complex (Seima et al., 1997). According to the documents, PTZ- induced seizure model could be used for the evaluation of absence epilepsy, so agents with anticonvulsant effects in the absence epilepsy are effective in PTZ-induced seizure model (Porter et al., 1984). Based on the data, S. hortensis extracts displayed anticonvulsant activity in the absence epilepsy.
Maximal electroshock (MES) induced seizure is a model to evaluate anticonvulsant properties of compounds that affect the tonic clonic epilepsy.[26] Neither ethanolic nor aqueous extracts of S. hortensis reduced the duration of hind limb tonic extension. Therefore, this plant was not effective in the tonic clonic epilepsy. It is also indicated that compounds which can inhibit voltage dependent sodium channels, show anticonvulsant properties in the MES induced seizure (Aquair et al., 2012). So, according to our data, this plant may not have any effect on the sodium channels.
It is found that changing in some neurotransmitter systems such as the glycine, glutamatergic, GABAergic and some molecules like nitric oxide could be considered as potential mechanisms involved in the induction of epilepsy (Engelborghs et al., 2000; Ure et al., 2000).
7-NI is considered as a selective inhibitor of neuronal NOS (nNOS) (Babbedge et al., 1993). It is established that 7-NI can abolish the anticonvulsant effects of agomelanine in PTZ- induced seizure model. Dastgheib et al (2014) concluded that agomelanine exerts its anti-epileptic effect partly due to the nNOS induction (Dastgheib et al., 2014). In our study, 7-NI was administrated 60 minutes prior to the extracts. Since 7-NI decreased the protective effect of S. hortensis against PTZ-induced seizure, its anticonvulsant effects may be attributed to the interaction with nitric oxide pathway.
Flumazenil is an antagonist of GABAA-benzodiazepine receptor (Hosseinzadeh et al., 2007). Administration of flumazenil 30 minutes prior to the extracts significantly decreased their anticonvulsant activity, so it might be concluded that this plant shows anticonvulsant effect through GABAA-benzodiazepine receptor complex.
It is documented that the effect of flumazenil (10 mg/kg) or 7-NI (10 mg/kg) alone on PTZ-induced seizure is similar to that of the control group (Borowicz et al., 2000). Based on these results and limitations of cost and time, the effect of these chemicals alone on PTZ-induced seizure was not evaluated in our study.
Our results also demonstrated that the ethanolic extract was likely as effective as the aqueous extract of S. hortensis in the absence epilepsy; suggesting that its effective constituents may be polar but some semi and non polar constituents may also be present.
There have been reports that monoterpenoid phenols such as carvacrol show anticonvulasant activity in PTZ and MES induced seizure models through interacting with GABA A receptor (Lucindo et al., 2010).
Carvacrol is abundantly found in the essential oils of the lamiaceae family (Lucindo et al., 2010). So it may be concluded that the protective effect of this plant on PTZ-induced seizures is at least partly due to the presence of these constituents, but further investigations to explore the main constituents required for the anticonvulsant activity of this plant are recommended.
This study indicated that S. hortensis could exert anticonvulsant activity in the PTZ model and this effect may be mediated, at least partly, through interaction with nitric oxide and GABAA-BZD receptor complex.
Acknowledgment
The authors are thankful to the Vice Chancellor of Research, Mashhad University of Medical Sciences for financial supports. The results described in this paper are a part of a Pharm. D. thesis.
Conflict of interest
The authors declare that there is no conflict of interest.
References
- 1.Aguiar C, Almeida A, Araujo P, Vasconcelos GS, Chaves EMC, Do vale OC. Anticonvulsant effects of agomelatine in mice. Epilepsy Behav. 2012;24:324–328. doi: 10.1016/j.yebeh.2012.04.134. [DOI] [PubMed] [Google Scholar]
- 2.Asadi-Pooya A, Nikseresht A, Yaghoubi E. Old Remedies for Epilepsy: Avicenna’s Medicine. Iran Red Crescent Med J. 2012;14:174–177. [PMC free article] [PubMed] [Google Scholar]
- 3.Babbedge R, Bland-Ward P, Hart S, Moore PK. Inhibition of rat cerebellar nitric oxide synthase by 7-nitro indazole and related substituted indazoles. Br J Pharmacol. 1993;110:225–228. doi: 10.1111/j.1476-5381.1993.tb13796.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Borowicz KK, Luszczki J, Kleinrok Z, Czuczwar SJ. 7-Nitroindazole, a nitric oxide synthase inhibitor, enhances the anticonvulsive action of ethosuximide and clonazepamagainst pentylenetetrazol-induced convulsions. J Neural Transm. 2000;101:1117–1126. doi: 10.1007/s007020070025. [DOI] [PubMed] [Google Scholar]
- 5.Castel-Branco M, Alves G, Figueiredo I, Falcao A, Caramona MM. The maximal electroshock seizure (MES) model in the preclinical assessment of potential new antiepileptic drugs. Methods Find Exp Clin Pharmacol. 2009;31:101–106. doi: 10.1358/mf.2009.31.2.1338414. [DOI] [PubMed] [Google Scholar]
- 6.Dastgheib M, Moezi L. Acute and chronic effects of agomelatine on intravenous penthylenetetrazol-induced seizure in mice and the probable role of nitric oxide. Eur J Pharmacol. 2014;736:10–15. doi: 10.1016/j.ejphar.2014.04.039. [DOI] [PubMed] [Google Scholar]
- 7.Dorman H, Hiltunen R. Fe (III) reductive and free radical-scavenging properties of summer savory (Satureja hortensis L) extract and sub-fractions. Food chem. 2004 ;88:193–199. [Google Scholar]
- 8.Engelborghs S, D’Hooge R, De Deyn P. Pathophysiology of epilepsy. Acta Neurol Belg. 2000 ;100:201–213. [PubMed] [Google Scholar]
- 9.Fathi A, Sahari M, Barzegar M. Antioxidant Activity of Satureja hortensis L Essential Oil and its Application in Safflower Oil. J Med Plants. 2013;12:51–67. [Google Scholar]
- 10.Gorgich E, Komeili G, Zahra Z, Ebrahimi S. Comparing anticonvulsive effect of melissa officinalis` hydro-alcoholic extract and phenytoin in rat. J Health Scope. 2012;1:44–48. [Google Scholar]
- 11.Hosseini A, Mirazi N. Acute administration of ginger (Zingiber nofficinale rhizomes) extract on timedintravenous pentylenetetrazol infusionseizure model in mice. Epilepsy Res. 2014;108:411–419. doi: 10.1016/j.eplepsyres.2014.01.008. [DOI] [PubMed] [Google Scholar]
- 12.Hosseinzadeh H, Karimi G R, Rakhshanizadeh M. Anticonvulsant effects of aqueous and ethanolic extracts of Hypericum perforatum L in mice. J Med Plants. 2004 ;3:23–30. [Google Scholar]
- 13.Hosseinzadeh H, Khosravan V. Anticonvulsant effects of aqueus and ethanolic extracts of Crocus sativus L stigmas in mice. Arch Irn Med. 2007;5:44–47. [Google Scholar]
- 14.Hosseinzadeh H, Madanifard M. Anticonvulsant effects of Coriandrum sativum L seeds extracts in mice. Arch Iranian Med. 2000;3:182–184. [Google Scholar]
- 15.Hosseinzadeh H, Parvardeh S. Anticonvulsant effects of thymoquinone, the major constituent of Nigella sativa seeds, in mice. Phytomedicine. 2004;11:56–64. doi: 10.1078/0944-7113-00376. [DOI] [PubMed] [Google Scholar]
- 16.Hosseinzadeh H, Ramezani M, Shafaei H, Taghiabadi E. Anticonvulsant effect of berberis integerrima L root extracts in mice. J Acupunct Meridian Stud. 2013 ;6:12–17. doi: 10.1016/j.jams.2012.07.018. [DOI] [PubMed] [Google Scholar]
- 17.Hosseinzadeh H, Sadeghnia H. Protective effect of safranal on pentylenetetrazol-induced seizures in the rat: Involvement of GABAergic and opioids systems. Phytomedicine. 2007;14:256–262. doi: 10.1016/j.phymed.2006.03.007. [DOI] [PubMed] [Google Scholar]
- 18.Kamkar A, Tooryan F, Akhondzadeh Basti A, Misaghi A, Shariatifar N. Chemical composition of summer savory (Satureja hortensis L) essential oil and comparison of antioxidant activity with aqueous and alcoholic extracts. J Vet Res. 2013;68:183–190. [Google Scholar]
- 19.Lucindo J, Quintans-Junior A G, Guimaraes B E S. Carvacrol, borneol and citral reduce convulsant activity in rodents. African J Biotechnol. 2010;9:6566–6572. [Google Scholar]
- 20.Mahomed I M, Ojewole J A O. Anticonvulsant activity of Harpagophytum procumbens DC [Pedaliaceae] secondary root aqueous extract in mice. Brain Res Bull. 2006;69:57–62. doi: 10.1016/j.brainresbull.2005.10.010. [DOI] [PubMed] [Google Scholar]
- 21.Mihajilov-krstev T, Radnovia D, Kitic D, Stojanovic-Radic Z, Zlatkovic B. Antimicrobial activity of saturega hortensis L essential oil against pathogenic microbial srtains. Arch Biol Sci. 2010;62:159–166. [Google Scholar]
- 22.Ojewole J Anticonvulsant property of Sutherlandia frutescens R BR. (variety Incana E MEY)[Fabaceae] shoot aqueous extract. Brain Res Bull. 2008;75:126–132. doi: 10.1016/j.brainresbull.2007.08.002. [DOI] [PubMed] [Google Scholar]
- 23.Okoye T, Akah P A, Omeje EO, Okoye FBC, Nworu CS. Anticonvulsant effect of kaurenoic acid isolated from the root bark of Annona senegalensis. Pharmacol Biochem Behav. 2013 ;109:38–43. doi: 10.1016/j.pbb.2013.05.001. [DOI] [PubMed] [Google Scholar]
- 24.Pahuja M, Mehla J, Kumar Gupta Y. Anticonvulsant and antioxidative activity of hydroalcoholic extract of tuber of Orchis mascula in pentylenetetrazole and maximal electroshock induced seizures in rats. Ethnopharmacol J. 2012;142:23–27. doi: 10.1016/j.jep.2012.04.006. [DOI] [PubMed] [Google Scholar]
- 25.Porter R J, Cereghino J J, Gladding G D, Hessie BJ, Kupferberg HJ, Scovile B. Antiepileptic drug development program. Cleveland Clin. 1984 ;51:293–305. doi: 10.3949/ccjm.51.2.293. [DOI] [PubMed] [Google Scholar]
- 26.Porter R, Meldrum B, Katzung B. Basic and clinical pharmacology. New York: Mc Graw Hill; 2001. Antiseizure Drugs; 395 pp. [Google Scholar]
- 27.Ramezani M, Hosseinzadeh H, Samizadeh S. Antinociceptive effects of Zataria multiflora Boiss fractions in mice. J Ethnopharmacol. 2004;91:167–170. doi: 10.1016/j.jep.2003.12.016. [DOI] [PubMed] [Google Scholar]
- 28.Sejima H, Ito M, Kishi K, Tsuda H, Shiraishi H. Regional excitatory and inhibitory amino acid concentration in pentylenetetrazole kindling and kindled rat brain. Brain Develop. 1997;19:171–175. doi: 10.1016/s0387-7604(96)00492-5. [DOI] [PubMed] [Google Scholar]
- 29.Semnani M, Saeedi M, Mahdavi M. Study and comparison of the antimicrobial activity of methanolic extracts of several species of Stachys and Phlomis. J Mazandaran Univ Med Sci. 2007;57:57–66. [Google Scholar]
- 30.Sowemimo A, Adio O, Fageyinbo S. Anticonvulsant activity of the methanolic extract of Justicia extensa T Anders. Ethnopharmacol J. 2012;138:679–699. doi: 10.1016/j.jep.2011.09.043. [DOI] [PubMed] [Google Scholar]
- 31.Ure J A, Perassolo M. Update on the pathophysiology of the epilepsies. J Neurol Sci. 2000;177:1–17. doi: 10.1016/s0022-510x(00)00356-7. [DOI] [PubMed] [Google Scholar]
- 32.Yazdanparast R, Shahriyary L. Comparative effects of Artemisia dracunculus, Satureja hortensis and Origanum majorana on inhibition of blood platelet adhesion, aggregation and secretion. Vasc pharmacol. 2008;48:32–7. doi: 10.1016/j.vph.2007.11.003. [DOI] [PubMed] [Google Scholar]