Abstract
Objective
To compare the periodontal bone height (PBH) of exclusive narghile smokers (ENS) with that of exclusive cigarette smokers (ECS).
Methods
Tunisian males aged 20–35 years who have been ENS for more than five narghile-years or ECS for more than five pack-years were recruited to participate in this comparative cross-sectional study. Information about oral health habits and tobacco consumption were gathered using a predetermined questionnaire. Plaque levels were recorded in four sites using the plaque index of Loe and Silness. The PBH was measured mesially and distally from digital panoramic radiographs of each tooth and expressed as a percentage of the root length. A PBH level ≤0.70 was applied as a cutoff reference value signifying bone loss. Student t-test and Chi2 test were used to compare quantitative and qualitative data of both groups.
Results
There were no significant differences between the ENS (n=60) and ECS (n=60) groups regarding age and the consumed quantities of tobacco (28±4 vs. 27±5 years, 7±3 narghile-years vs. 8±3 pack-years, respectively). Compared with the ECS group, the ENS group had a significantly higher plaque index (mean±SD values were 1.54±0.70 vs. 1.84±0.73, respectively). However, the two groups had similar means of PBH (0.85±0.03 vs. 0.86±0.04) and tooth brushing frequencies (1.1±0.8 vs. 0.9±0.6 a day, respectively) and had similar bone loss frequencies (15% vs. 12%, respectively).
Conclusions
Both ENS and ECS exhibited the same PBH reduction, which means that both types of tobacco smoking are associated with periodontal bone loss.
Keywords: hookah, shisha, tobacco, periodontal health, alveolar bone
Tobacco use is the major cause of mortality around the world (1). During the past decades, it has been responsible for over 5 million deaths per year (1). Therefore, tobacco use can be considered as a global threat. According to the World Health Organization, without efficient smoking control policies, the number of deaths will reach nearly 10 million by 2030 (2). Although the cigarette is the most popular form of tobacco, the use of narghile is increasing throughout the world (3). This practice is tradition in Eastern countries, and its prevalence is alarmingly high among Arab populations (3). Moreover, narghile use is often wrongly perceived as less harmful than cigarette smoking, and many studies on its damaging effects on health are controversial (4–7). Public health practitioners should tackle narghile use with extreme caution (8–11).
The chemical composition of narghile smoke includes many toxic and hazardous compounds (12). The adverse health effects of narghile use, especially on the cardiovascular and respiratory systems, have been reported in several studies (9, 12, 13). As inhalation of such toxic substances may affect the integrity of the oral cavity, and as dentists come across narghile smokers among their patients, it is fundamental to inform the patients of the significantly damaging impacts of narghile use on some components of the oral cavity, such as the periodontium (14). However, studies analyzing the adverse effects of narghile use on oral health (15–29) are scarce and present conflicting results (14).
Few studies have assessed the effects of narghile use on periodontal health (15–18, 20, 29), with only two focusing on periodontal bone height (PBH) (17, 29). The main conclusions of the above studies (15–18, 20, 29) were that compared with non-smokers, narghile smokers have a significantly higher mean gingival index (16), significantly higher frequencies of gingival bleeding (20, 29) and vertical defects (15), a significantly deeper probing pocket (18, 20, 29), and clinically significant attachment loss (20, 29). The two studies investigating the effect of narghile use on PBH and/or bone loss (17, 29) showed that they were significantly affected among exclusive narghile smokers (ENS) as compared with non-smokers, and that there was no statistically significant difference in the aforementioned parameters between ENS and exclusive cigarette smokers (ECS). Nevertheless, these two studies had some methodological limitations (14, 30), such as not being precise about the narghile tobacco type (tabamel and/or moassel and/or tombak) (17, 29), a heterogeneous population including subjects with large variations in the quantity of tobacco consumed (17) or daily smoking frequency (29), as well as elderly individuals (17). These limitations may affect the observations because diseases caused by narghile use are related to the tobacco type and/or quantity (8, 14), and the prevalence of periodontal disease increases with age (14, 31).
Taking into account the above methodological limitations (14, 17, 29, 30), the present study aimed at comparing some clinical and radiological data (i.e. plaque index, PBH, bone loss) determined in young adults who were exclusive tabamel smokers with those of ECS.
Population and methods
Study design
This was a comparative cross-sectional study carried out over 2 years (from October 2013 to September 2015). It was part of a project approved by the local ethical committee of Farhat HACHED University Hospital and conducted in accordance with the Declaration of Helsinki. The project aimed at evaluating the effect of the use of narghile on oral health on the basis of clinical, radiological and biological data. The clinical part of the study was conducted in the Department of Oral Medicine at the Basic Health Group, and radiological exams were performed in a private radiological center in Sousse, Tunisia.
In unpublished data, the adjusted prevalence rates of smoking among males aged 20 years and more living in Sousse were 12.7% for narghile and 40.1% for cigarettes. In Tunisia, the tobacco used during a narghile session weighs approximately 20 g (8).
Participants were individually informed about the purpose of the study and all of them signed an informed written consent form prior to the study. Subjects diagnosed with any oral pathology were given treatment or were scheduled for the right specialist.
Populations
Subjects were recruited by convenience sampling from acquaintances of people involved in the study and via flyers distributed in cafes in the city. Only male ENS or ECS aged between 20 and 35 years were included. The non-inclusion criteria were as follows: number of remaining teeth <20, tobacco use <5 pack-years for ECS or <5 narghile-years for ENS, jurak and/or tombac tobacco use, known systemic medical condition such as diabetes mellitus, previous head or neck radiation therapy, and consumption of drugs known to affect the periodontium such as antidepressants, anticonvulsants, cyclosporine A, or calcium antagonists. The number of analyzed teeth <20, because of the bad quality of the radiological exam, was applied as an exclusion criteria. Smokers were stratified into two groups: ENS and ECS.
Sample size
The sample size was estimated using the following formula (32): N=[(Z α/2)2×P×(1− P)×D]/E 2; P was the proportion of the main event of interest (i.e. bone loss), E was the margin of error, Z α/2 was the normal deviation for two-tailed alternative hypothesis at a level of significance, D was the design (=1 for simple random sampling). Previous literature gives an estimate of bone loss of 17% (p=0.17) in the surveyed population (17), and assuming 90% confidence interval (CI) (Z α/2=1.64) and 5% margin of error (E), the total sample size was 151 smokers.
Medical questionnaire
The subjects were interviewed using a non-standardized questionnaire written in Arabic. The questions were with closed answers and often dichotomous. Data were collected on sociodemographic variables, smoking habits (i.e. lifetime cigarette or narghile smoking, narghile smoking mixture), and oral hygiene (i.e. daily tooth brushing frequency) and yearly number of visits to the dentist.
Two schooling levels were arbitrarily defined: low (illiterate, primary education) and high (secondary and university education).
Two socioeconomic levels were defined according to professional status: low (e.g. unskilled workers, jobless) and high (e.g. skilled workers, farmers, managers).
The level of tobacco consumption was expressed in terms of narghile-years (‘narghile session per day’בnumber of years of consumption’) or pack-years (‘packs of cigarettes smoked per day’בnumber of years of consumption’) (8). According to their tobacco consumption levels, smokers were divided into two subgroups: 5–10 or 10–15 narghile-years or pack-years.
Applying ‘one daily tooth brushing frequency’ as a cutoff, smokers were classified arbitrarily into two subgroups: irregular (=0) and regular (≥1) daily tooth brushing.
Clinical examination
The examination was performed by an experienced dentist (MK in the authors list). The number of the remaining teeth was recorded. The Silness–Löe plaque index (33) was used to assess oral hygiene. It is a dental index that assesses the thickness of plaque at the cervical margin of the tooth (closest to the gum). Four scores are possible (0: no plaque; 1: a film of plaque adhering to the free gingival margin, adjacent to the tooth; 2: moderate accumulation of soft deposits within the gingival pocket or between the tooth and gingival margin; 3: abundance of soft matter within the gingival pocket and/or on the tooth and gingival margin). A plaque indicator was used to evaluate the plaque index. The presence of visible dental plaque was recorded on four sites (vestibular, lingual, mesial, and distal) of all existing teeth, except the third molars. As previously done by one author (20), three plaque index classes (0–1, 1–2, and 2–3) were arbitrarily defined.
Radiographic examination
Extraoral digital panoramic radiographs (RAYSCAN α, Ray, Hwaseong, South Korea) were performed to visualize and measure the marginal PBH.
To represent the mean PBH per individual, the mesial and distal bone heights were determined as a proportion of the root length from all measurable teeth (34). All images were analyzed by the same dentist (MK in the authors list) using an imaging software program (RAYSCAN's SMARTDent). The root length was measured from the cementoenamel junction to the root apex (17). The PBH was measured from the apex to a point where the lamina dura became continuous with the compact bone of the inter-dental septum (17). If any measure was not possible, the tooth was excluded. A bone height level ≤0.70 was used as a cutoff reference value signifying bone loss (17).
Statistical analysis
Distribution of variables was normal and results were expressed as mean±standard deviation (SD) (95% CI).
Student's t-test and Chi2 test were used to compare, respectively, the two groups’ quantitative and qualitative data.
All mathematical computations and statistical procedures were performed using Statistica software (Statistica Kernel version 6; Stat Software. France).
Significance was set at the 0.05 level.
Results
Among the 150 subjects, only 120 were retained (60 ENS and 60 ECS). Thirty smokers were excluded mainly because the number of teeth measured after the radiological analysis was <20.
Table 1 displays the smokers’ main characteristics. There were no significant differences between the two groups regarding age, quantities of consumed tobacco, and daily tooth brushing frequency. Compared with the ECS group, the ENS group had a significantly lower mean number of visits to the dentist and included significantly higher percentages of smokers having a low schooling level or having a high socioeconomic level.
Table 1.
General characteristics of the study groups: exclusive narghile smokers (ENS, n=60) and exclusive cigarette smokers (ECS, n=60)
| ENS | ECS | p | ||
|---|---|---|---|---|
| Quantitative data: mean±SD [95% CI] | ||||
| Age (years) | 28.5±3.6 [27.5 to 29.4] | 27.3±5.0 [26.1 to 28.6] | 0.160 | |
| Quantity of tobacco used (NY or PY) | 7.1±2.9 [6.3 to 7.8] | 8.0±3.0 [7.2 to 8.8] | 0.090 | |
| Visit to the dentist | 0.0±0.0 | 0.1±0.3 [0.0 to 0.2] | 0.011* | |
| Daily tooth brushing frequency | 0.9±0.60 [0.8 to 1.1] | 1.1±0.85 [0.9 to 1.4] | 0.135 | |
| Qualitative data: number (percentage) | ||||
|
| ||||
| Schooling level | Low | 35 (58.3) | 23 (38.3) | 0.030† |
| High | 25 (41.7) | 37 (61.7) | ||
| Socioeconomic level | Low | 0 (0.0) | 8 (13.3) | 0.003† |
| High | 60 (100.0) | 52 (86.7) | ||
| Quantity of tobacco used (NY or PY) | 5–10 | 51 (85.0) | 42 (70.0) | 0.051 |
| 10–15 | 9 (15.0) | 18 (30.0) | ||
| Daily tooth brushing | Irregular brusher | 12 (20.0) | 16 (26.7) | 0.436 |
| Regular brusher | 48 (80.0) | 44 (73.3) | ||
NY: narghile-years; PY: pack-years.
p<0.05 (t-test): ENS versus ECS.
p<0.05 (Chi2): ENS versus ECS.
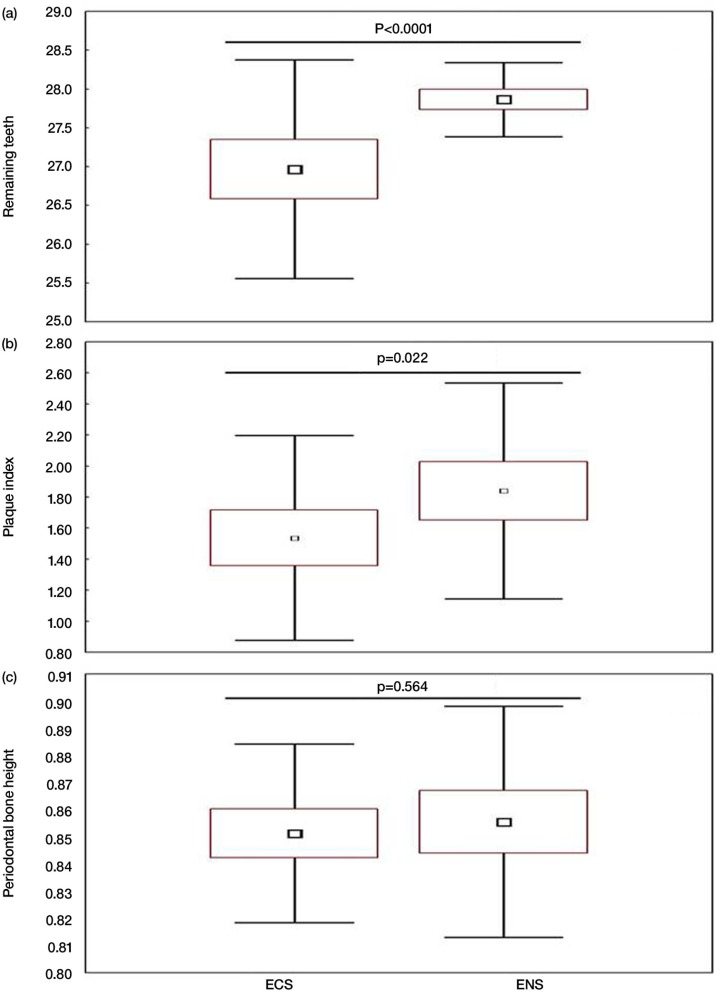
Figure 1 and Table 2 display, respectively, the clinical and radiological data and the plaque index intervals and bone loss of the two groups. Their main results were as follows:
The ENS group means±SD [95% CI] of remaining teeth (Fig. 1a) and plaque index (Fig. 1b) were significantly higher than those of the ECS group (respectively, 27.87±0.50 [27.74 to 28.00] vs. 26.97±1.48 [26.58 to 27.35] and 1.84±0.73 [1.65 to 2.03] vs. 1.54±0.70 [1.36 to 1.72]).
No significant difference was found between the two groups’ means±SD [95% CI] PBH (0.86±0.04 [0.84 to 0.87] vs. 0.85±0.03 [0.84 to 0.86], respectively) (Fig. 1c).
Fig. 1.
Remaining teeth, plaque index, and periodontal bone height of the study groups: exclusive narghile smokers (ENS, n=60) and exclusive cigarettes smokers (ECS, n=60). (a) Remaining teeth. (b) Plaque index. (c) Periodontal bone height. Data are shown as box-and-whisker plots. Small rectangle: mean; large rectangle: 95% CI; error bars: standard deviation. p (Student's t-test): ENS versus ECS.
Table 2.
Clinical and radiological data of the study groups: exclusive narghile smokers (ENS, n=60) and exclusive cigarette smokers (ECS, n=60)
| ENS | ECS | p | |||
|---|---|---|---|---|---|
| Plaque index classes | 0–1 | 4 (6.7) | 8 (13.3) | 0.386 | |
| 1–2 | 26 (43.3) | 37 (61.7) | |||
| 2–3 | 30 (50.0) | 15 (25.0) | |||
| Bone loss | Yes | 7 (11.7) | 9 (15.0) | 0.631 | |
| No | 53 (88.3) | 51 (85.0) | |||
Qualitative data: number (percentage).
p<0.05 (Chi2): ENS versus ECS.
The two groups included similar percentages of smokers having bone loss or divided according to the plaque index classes (Table 2).
Discussion
The main result of the present study was that the two groups made up of 60 ENS and 60 ECS have similar means of PBH and percentages of bone loss. Studies analyzing the effects of narghile use on oral health (15–29), especially the periodontium (15–18, 20, 29), are scarce. This was recently criticized in two letters to the editor (14, 30). To the best of the authors’ knowledge, only two comparative cross-sectional studies (17, 29), largely described in Table 3, investigated the effect of narghile use on PBH and/or bone loss.
Table 3.
Study designs, characteristics, and results of studies aiming to evaluate the effects of narghile use on periodontal bone height
| First author | Natto (17) | Javed (29) | Present study | ||
|---|---|---|---|---|---|
|
| |||||
| Study design | |||||
| Year of publication | 2005 | 2016 | |||
| Years of the study | NR | 2013–2014 | 2013–2015 | ||
| Town (country) | Jeddah (Saudi Arabia) | Riyadh (Saudi Arabia) | Sousse (Tunisia) | ||
| Study design | Cross-sectional Comparative | Cross-sectional Comparative | Cross-sectional Comparative | ||
| Recruitment method | Announcements/news paper | Visitors of a dental clinic for treatment | Flyers Acquaintances of people involved in the study |
||
| Name of the smoking device | Water pipe | Water pipe | Narghile | ||
| Inclusion criteria | >20 teeth | Healthy individuals Habitual ENS (>1 narghile/day for at least the past year) Habitual ECS (>1 cigarette/day for at least the past year) Non-S |
Male Age: 20–35 years Tabamel smokers |
||
| Non-inclusion (or exclusion) criteria | Pregnancy Unhealthy |
MS Systemic diseases Edentulous individuals Crowded teeth or occlusal trauma Alcohol consumers Tobacco chewers Lactating and/or pregnant females Medication use (antibiotics, non-steroidal anti-inflammatory drugs, steroids) ≤3 months Periodontal treatment ≤6 months |
<20 teeth Tobacco use <5 PY or <5 NY Jurak and/or Tombac smokers Diabetes mellitus, Previous head or neck radiation therapy Medication use: antidepressants. anticonvulsants, cyclosporine A, calcium antagonists |
||
| ENS | Yes | Yes | Yes | ||
| Calculated sample size | No | Yes (no reference cited) | Yes (32) | ||
| Number of ENS (M/F) | 117 (90/27) | 50 (50/0) | 60 (60/0) | ||
| Age of ENS (years) | 17–60a
M: 39 [37–41]b F: 38 [34–43]b |
485±62e years | 20–35a
284±36 [275–294]c |
||
| Starting narghile use age (years) | NR | NR | NR | ||
| Number of years of smoking | NR | 20.5±2.8e | NR | ||
| Type of tobacco | NR | NR | Tabamel | ||
| Method of narghile use quantification | RY | Times/daily Session duration |
NY | ||
| Quantity of narghile tobacco used | 57 [48–66]b RY 44d: <40 RY 56d: ≥40 RY |
Frequency of use: 4.7±1.1e times/daily Session duration: 50.2±6.7e min |
7.1±2.9 [6.3–7.8]e
85d: 5 ≤ NY<10 15d: ≥10 NY |
||
| Grams of tobacco/narghile session | NR | NR | 20 | ||
| Last narghile (h) | NR | NR | NR | ||
| Explorations | Clinical examination [4 sites (buccal, mesial, distal, lingual) for all teeth] Radiographic exam |
Clinical examination [6 sites (mesiobuccal, mid-buccal, distobuccal, distolingual/palatal, mid-lingual/palatal, and mesiolingual/palatal) for all teeth] Radiographic examination |
Clinical examination Radiographic examination |
||
| Questionnaires | Standardized without citing a reference | Non-standardized | Non-standardized | ||
| Used materials | Panoramic digital radiographs | Full mouth digital radiographs | Panoramic digital radiographs | ||
| Comparison with ECS |
n=72 (58 M/14 F) M: 36 [34–38]b years F: 38 [34–43]b years 230 [193–268]b CY 51d: <170 CY 49d: ≥170 CY |
n=50 (50 M/0 F) 50.1±3.5e years Duration of smoking: 22.3±6.5e years Frequency of use: 15.4±3.6e times/daily Session duration: 15.3±0.4e min |
n=60 (60 M/0 F) 27.3±5 [26.1–28.6]c years 70d: 5 ≤ PY <10 30d: ≥10 PY |
||
| Comparison with Non-S |
n=99 (56 M/43 F) M: 38 [35–41]b years F: 35 [32–39]b years |
n=100 (100 M/0 F) 46.5±4.2e years |
NA | ||
| Comparison with MS |
n=67 (51 M/16 F) M: 33 [31–35]b years F: 32 [28–37]b years 174 [141–207]b CY 24 [18–30]b RY |
NA | NA | ||
| Results | |||||
| PBH | BL (%) | BL (mm) | PBH | BL (%) | |
| ENS | 0.76 [0.75–0.78]b†‡ | 27d† | 5.1±0.8e† | 0.86±0.04 [0.84–0.87]c | 12d |
| ECS | 0.76 [0.74– 0.78]b | 24d | 5.6±1.2e | 0.85±0.03 [0.84–0.86]c | 15d |
| MS | 0.80 [0.79–0.82]b | 9d | NA | NA | NA |
| Non-S | 0.81 [0.79– 0.83]b | 6d | 2.2±0.9e | NA | NA |
| Other results | PBH decreases with age. Relative risk of BL (after adjustment for age) associated with narghile use and cigarette smoking compared with Non-S. |
The two groups had similar means of PBH and similar frequencies of BL. | |||
| Conclusions | Narghile use is associated with PBH reduction | The periodontal condition of ENS was equally as poor as ECS. | Both ENS and ECS exhibited the same PBH reduction suggesting that the two types of tobacco smoking are associated with BL. | ||
BL: bone loss. ECS: exclusive cigarette smoker. ENS: exclusive narghile smoker. F: female. M: male. Min: minutes. MS: mixed smoker. NA: not applied. Non-S: non-smoker. NR: not reported. NY: narghile-year. PY: pack-years. RY: run-years.
Data are range (minimum and maximum).
Data are mean [95% CI].
Data are mean±SD [95% CI].
Data are percentages.
Data are mean±SD. Significant differences
ENS versus ECS;
ENS versus Non-S;
ENS versus MS.
Since PBH decreases with age (17, 31) and is positively related to sex (17), only males aged between 20 and 35 years were included. Inclusion of older smokers and/or of both males and females in the same group, as done by Natto et al. (17), could influence interpretation of the results. Contrary to the study by Natto et al. (17), and in reference to Javed et al. (29), some important non-inclusion criteria were applied (e.g. diabetes mellitus, head and/or neck radiation therapy, and use of some medications), which are known to affect periodontal health and are therefore considered as confounding factors (35, 36). Moreover, in the present study, only exclusive tabamel smokers were included. This important information (14), not mentioned in previous similar studies (17, 29), makes between-studies comparison complicated. In the case of the use of other types of narghile tobacco (such as tombak or jurak, frequently used in Saudi Arabia), and in comparison to tabamel, the pattern is different (8, 14, 37).
As done previously (17, 29), the present study applied a non-validated medical questionnaire. This could be considered as a methodological limitation, and it is imperative that a standardized epidemiological questionnaire be developed that could be applied in studies addressing the effects of narghile use on health (38).
In the present study, PBH was the main outcome used to evaluate periodontal health. Radiological exams evaluating PBH play a complementary role in assessing the periodontium in conjunction with periodontal probing (39). They have high sensitivity and reproducibility which yield fewer false-negative results compared with clinical records (39). However, it was preferable to collect clinical data such as gingival index, probing depth and tooth mobility. These data will be further investigated.
In the present study, the radiographic method used to evaluate bone loss was similar to that applied by Natto et al. (17) but was different from that used by Javed et al. (29). The latter study evaluated marginal bone loss defined as the vertical distance from 2 mm below the cementoenamel junction to the most crestal part of the marginal bone (29). Other researchers (15) aiming at detecting the vertical defect in the periodontal bone have performed a full set of intraoral radiographs including 16 periapical and 4 bitewing projections for smokers and non-smokers. Panoramic radiography is a suitable method for assessing marginal bone defects (34). However, it does not provide information on the height of the vestibular or lingual periodontal bone. Panoramic radiography is also a rapid method (34) that is not very expensive (30 Tunisian Dinars, the equivalent of 13 Euros). It has a lower radiation dose compared with a full mouth set of radiographs (40). As done by Natto et al. (17), the ratio of the PBH to the root length was preferred to the measurement in millimeters (as done by Javed et al. (29)) for evaluating the PBH. This method minimizes the effect of shortening or elongating the radiographic image (41). For more precision, one examiner measured the bone height measurements in all the radiographs. Although calculations may be influenced by a within-variability, measurement error is marginal and can be ignored when the radiographs are assessed by a single examiner (17, 29).
In addition to the methodological limitation concerning medical questionnaire described above, four other limitations (concerning blinding, convenience sampling, panoramic radiograph use especially for the anterior teeth, and the socioeconomic levels of smokers) were noted. The first limitation of the present study was the non-application of blinding. The last, applied in the studies by Natto et al. (17), during the radiological data analysis, and by Javed et al. (29), during the questionnaire and clinical examination, is an important methodological feature that can minimize bias and maximize the validity of the results (42). In future studies with similar aims as the present, researchers should strive to set up blinding not only for data analysts but also for radiological specialists and any other individuals involved in the study (42). In spite of the advantages of convenience sampling, such as the availability of data and the rapidity of data gathering, it has several disadvantages such as the risk of the sample not being representative of the population as a whole and volunteer bias. Although the panoramic radiographs used in the present study seem to be a more practical approach for a high number of participants and have less radiation, it would have been preferable to make a full mouth set of radiographs (40). The socioeconomic level has an impact on periodontal health (43). In the present study, 100% of ENS had a high socioeconomic level (Table 1), which could influence results. However, this higher percentage reflects the reality of narghile smokers in Tunisia, since a previous local study (44) showed that 67% of ENS had a high socioeconomic level.
The main result in this study was that ENS and ECS have similar means of PBH and percentages of bone loss. This result correlates with the two related studies (17, 29). Therefore, it appears that narghile use affects the PBH in the same way as cigarette smoking. Compared with the ENS and ECS PBH values and bone-loss percentages reported by Natto et al. (17), the present ones seem to be, respectively, higher and lower (Table 3). These differences may be explained by the inclusion in the present study, compared with the one by Natto et al. (17), of young subjects aged 20–35 years. On the one hand, the PBH decreases with age (17). On the other hand, in a subgroup of their total sample (ECS, ENS, mixed smokers) aged 17–30 years, Natto et al. (17) found PBH values similar to the present study (mean, 95% CI: 83.1%, 82.8–84.7%).
The biological mechanisms responsible for the effect of narghile use on PBH are still elusive (17). Two hypotheses could be speculated.
Due to the inhalation of hazardous and toxic compounds in narghile smoke (12), such as nicotine (17), compared with non-smokers, ENS have increased levels of nicotine and its principal saliva metabolite, that is, cotinine (45). However, compared with cigarette smoking, narghile use is associated with similar plasma nicotine levels (46). Wu et al. (47) suggested that nicotine upregulated interleukin-1β secretion, which may promote alveolar bone loss. These could explain the similarity of bone loss in ENS and ECS.
Implication of the matrix metalloproteinases in the degradation of periodontal tissues such as the alveolar bone (48).
To conclude, the present study suggests that narghile use is detrimental to periodontal health. In their daily practice, dentists are urged to encourage their patients to quit smoking both narghile and cigarettes. Besides, future research and gathering of clinical data, such as gingival index, periodontal probing depth, and tooth mobility, are encouraged.
Acknowledgements
Special thanks go to the American University of Beirut, Lebanon, for its financial support (Grant no. 201014).
The authors thank Dr. Nizar Brahem and Dr. Yassine Guermazi for their invaluable contribution to the radiographic examination.
Conflict of interest and funding
The authors declare that they have no conflicts of interest concerning this article. The American University of Beirut, Lebanon, supported a part of the present study (Grant no. 201014).
References
- 1.Mendez D, Alshanqeety O, Warner KE. The potential impact of smoking control policies on future global smoking trends. Tob Control. 2013;22:46–51. doi: 10.1136/tobaccocontrol-2011-050147. [DOI] [PubMed] [Google Scholar]
- 2.WHO report on the global tobacco epidemic (2011) Warning about the dangers of tobacco [downloaded 2016 May 30] Available from: http://apps.who.int/iris/bitstream/10665/70680/1/WHO_NMH_TFI_11.3_eng.pdf [cited 5 July 2011]
- 3.Akl EA, Gunukula SK, Aleem S, Obeid R, Jaoude PA, Honeine R, et al. The prevalence of waterpipe tobacco smoking among the general and specific populations: a systematic review. BMC Public Health. 2011;11:244. doi: 10.1186/1471-2458-11-244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Smith-Simone S, Maziak W, Ward KD, Eissenberg T. Waterpipe tobacco smoking: knowledge, attitudes, beliefs, and behavior in two U.S. samples. Nicotine Tob Res. 2008;10:393–8. doi: 10.1080/14622200701825023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chaouachi KT. The narghile (hookah, shisha, goza) epidemic and the need for clearing up confusion and solving problems related with model building of social situations. ScientificWorld Journal. 2007;7:1691–6. doi: 10.1100/tsw.2007.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chaouachi KT. Shisha confusion. Br Dent J. 2007;203:669–70. doi: 10.1038/bdj.2007.1124. [DOI] [PubMed] [Google Scholar]
- 7.Chaouachi K. Use & misuse of water-filtered tobacco smoking pipes in the world. Consequences for public health, research & research ethics. Open Med Chem J. 2015;9:1–12. doi: 10.2174/1874104501509010001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ben Saad H. The narghile and its effects on health. Part I: the narghile, general description and properties. Rev Pneumol Clin. 2009;65:369–75. doi: 10.1016/j.pneumo.2009.08.010. [DOI] [PubMed] [Google Scholar]
- 9.Ben Saad H. The narghile and its effects on health. Part II: the effects of the narghile on health. Rev Pneumol Clin. 2010;66:132–44. doi: 10.1016/j.pneumo.2009.08.011. [DOI] [PubMed] [Google Scholar]
- 10.Ben Saad H, Chaouachi K. Comparison of cigarette and waterpipe smoking among pupils in the urban area of Sousse, Tunisia. Tunis Med. 2010;88:470–3. (Letter to editor), Pro Tunis Med. 2011; 89: 505–6. [PubMed] [Google Scholar]
- 11.Ben Saad H. Methodological problems in the article comparing lung function profiles and aerobic capacity of adult cigarette and hookah smokers after 12 weeks intermittent training. Libyan J Med. 2015;10 doi: 10.3402/ljm.v10.27760. 27760, doi: http://dx.doi.org/10.3402/ljm.v10.27760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bou Fakhreddine HM, Kanj AN, Kanj NA. The growing epidemic of water pipe smoking: health effects and future needs. Respir Med. 2014;108:1241–53. doi: 10.1016/j.rmed.2014.07.014. [DOI] [PubMed] [Google Scholar]
- 13.El-Zaatari ZM, Chami HA, Zaatari GS. Health effects associated with waterpipe smoking. Tob Control. 2015;24(Suppl 1):i31–43. doi: 10.1136/tobaccocontrol-2014-051908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Khemiss M, Rouatbi S, Berrezouga L, Ben Saad H. Critical analysis of the published literature about the effects of narghile use on oral health. Libyan J Med. 2015;10 doi: 10.3402/ljm.v10.30001. 30001, doi: http://dx.doi.org/10.3402/ljm.v10.30001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Baljoon M, Natto S, Abanmy A, Bergstrom J. Smoking and vertical bone defects in a Saudi Arabian population. Oral Health Prev Dent. 2005;3:173–82. [PubMed] [Google Scholar]
- 16.Natto S, Baljoon M, Abanmy A, Bergstrom J. Tobacco smoking and gingival health in a Saudi Arabian population. Oral Health Prev Dent. 2004;2:351–7. [PubMed] [Google Scholar]
- 17.Natto S, Baljoon M, Bergstrom J. Tobacco smoking and periodontal bone height in a Saudi Arabian population. J Clin Periodontol. 2005;32:1000–6. doi: 10.1111/j.1600-051X.2005.00813.x. [DOI] [PubMed] [Google Scholar]
- 18.Natto S, Baljoon M, Bergstrom J. Tobacco smoking and periodontal health in a Saudi Arabian population. J Periodontol. 2005;76:1919–26. doi: 10.1902/jop.2005.76.11.1919. [DOI] [PubMed] [Google Scholar]
- 19.Natto S, Baljoon M, Dahlen G, Bergstrom J. Tobacco smoking and periodontal microflora in a Saudi Arabian population. J Clin Periodontol. 2005;32:549–55. doi: 10.1111/j.1600-051X.2005.00710.x. [DOI] [PubMed] [Google Scholar]
- 20.Bibars AR, Obeidat SR, Khader Y, Mahasneh AM, Khabour OF. The effect of waterpipe smoking on periodontal health. Oral Health Prev Dent. 2015;13:253–9. doi: 10.3290/j.ohpd.a32671. [DOI] [PubMed] [Google Scholar]
- 21.El-Hakim IE, Uthman MAE. Squamous cell carcinoma and keratoacanthoma of the lower lip associated with ‘Goza’ and ‘Shisha’ smoking. Int J Dermatol. 1999;38:108–10. doi: 10.1046/j.1365-4362.1999.00448.x. [DOI] [PubMed] [Google Scholar]
- 22.Al-Belasy FA. The relationship of ‘shisha’ (water pipe) smoking to postextraction dry socket. J Oral Maxillofac Surg. 2004;62:10–14. doi: 10.1016/j.joms.2002.11.001. [DOI] [PubMed] [Google Scholar]
- 23.Ali AA. Histopathologic changes in oral mucosa of Yemenis addicted to water-pipe and cigarette smoking in addition to takhzeen al-qat. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2007;103:e55–9. doi: 10.1016/j.tripleo.2006.10.008. [DOI] [PubMed] [Google Scholar]
- 24.El-Setouhy M, Loffredo CA, Radwan G, Abdel Rahman R, Mahfouz E, Israel E, et al. Genotoxic effects of waterpipe smoking on the buccal mucosa cells. Mutat Res. 2008;655:36–40. doi: 10.1016/j.mrgentox.2008.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dangi J, Kinnunen TH, Zavras AI. Challenges in global improvement of oral cancer outcomes: findings from rural Northern India. Tob Induc Dis. 2012;10:5. doi: 10.1186/1617-9625-10-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Al-Attas SA, Ibrahim SS, Amer HA, Darwish ZE, Hassan MH. Prevalence of potentially malignant oral mucosal lesions among tobacco users in Jeddah, Saudi Arabia. Asian Pac J Cancer Prev. 2014;15:757–62. doi: 10.7314/apjcp.2014.15.2.757. [DOI] [PubMed] [Google Scholar]
- 27.Seifi S, Feizi F, Mehdizadeh M, Khafri S, Ahmadi B. Evaluation of cytological alterations of oral mucosa in smokers and waterpipe users. Cell J. 2014;15:302–9. [PMC free article] [PubMed] [Google Scholar]
- 28.Al-Amrah HJA, Aboznada OA, Alam MZ, ElAssouli MZM, Mujallid MI, ElAssouli SM. Genotoxicity of waterpipe smoke in buccal cells and peripheral blood leukocytes as determined by comet assay. Inhal Toxicol. 2014;26:891–6. doi: 10.3109/08958378.2014.970787. [DOI] [PubMed] [Google Scholar]
- 29.Javed F, Al-Kheraif AA, Rahman I, Millan-Luongo LT, Feng C, Yunker M, et al. Comparison of clinical and radiographic periodontal status between habitual water-pipe smokers and cigarette smokers. J Periodontol. 2016;87:142–7. doi: 10.1902/jop.2015.150235. [DOI] [PubMed] [Google Scholar]
- 30.Khemiss M, Ben Saad H. Clinical and radiographic periodontal status of exclusive narghile smokers: some sources of confusion. J Periodontol. 2016 in press. [Google Scholar]
- 31.Eke PI, Dye BA, Wei L, Thornton-Evans GO, Genco RJ. Cdc Periodontal Disease Surveillance workgroup: James Beck GDRP. Prevalence of periodontitis in adults in the United States: 2009 and 2010. J Dent Res. 2012;91:914–20. doi: 10.1177/0022034512457373. [DOI] [PubMed] [Google Scholar]
- 32.Kang M, Ragan BG, Park JH. Issues in outcomes research: an overview of randomization techniques for clinical trials. J Athl Train. 2008;43:215–21. doi: 10.4085/1062-6050-43.2.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Silness J, Loe H. Periodontal disease in pregnancy. II. Correlation between oral hygiene and periodontal condition. Acta Odontol Scand. 1964;22:121–35. doi: 10.3109/00016356408993968. [DOI] [PubMed] [Google Scholar]
- 34.Michalowicz BS, Aeppli DP, Kuba RK, Bereuter JE, Conry JP, Segal NL, et al. A twin study of genetic variation in proportional radiographic alveolar bone height. J Dent Res. 1991;70:1431–5. doi: 10.1177/00220345910700110701. [DOI] [PubMed] [Google Scholar]
- 35.Llambés F. Relationship between diabetes and periodontal infection. World J Diabetes. 2015;6:927. doi: 10.4239/wjd.v6.i7.927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ammajan RR, Joseph R, Rajeev R, Choudhary K, Vidhyadharan K. Assessment of periodontal changes in patients undergoing radiotherapy for head and neck malignancy: a hospital-based study. J Cancer Res Ther. 2013;9:630–7. doi: 10.4103/0973-1482.126461. [DOI] [PubMed] [Google Scholar]
- 37.Chaouachi K. Hookah (Shisha, Narghile) smoking and environmental tobacco smoke (ETS). A critical review of the relevant literature and the public health consequences. Int J Environ Res Public Health. 2009;6:798–843. doi: 10.3390/ijerph6020798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Akl EA, Gaddam S, Gunukula SK, Honeine R, Jaoude PA, Irani J. The effects of waterpipe tobacco smoking on health outcomes: a systematic review. Int J Epidemiol. 2010;39:834–57. doi: 10.1093/ije/dyq002. [DOI] [PubMed] [Google Scholar]
- 39.Hsiao CN, Ting CC, Shieh TY, Ko EC. Relationship between betel quid chewing and radiographic alveolar bone loss among Taiwanese aboriginals: a retrospective study. BMC Oral Health. 2014;14:133. doi: 10.1186/1472-6831-14-133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tugnait A, Clerehugh V, Hirschmann PN. The usefulness of radiographs in diagnosis and management of periodontal diseases: a review. J Dent. 2000;28:219–26. doi: 10.1016/s0300-5712(99)00062-7. [DOI] [PubMed] [Google Scholar]
- 41.Bergstrom J, Eliasson S. Cigarette smoking and alveolar bone height in subjects with a high standard of oral hygiene. J Clin Periodontol. 1987;14:466–9. doi: 10.1111/j.1600-051x.1987.tb02253.x. [DOI] [PubMed] [Google Scholar]
- 42.Karanicolas PJ, Farrokhyar F, Bhandari M. Practical tips for surgical research: blinding: who, what, when, why, how? Can J Surg. 2010;53:345–8. [PMC free article] [PubMed] [Google Scholar]
- 43.Borrell LN, Crawford ND. Socioeconomic position indicators and periodontitis: examining the evidence. Periodontol 2000. 2012;58:69–83. doi: 10.1111/j.1600-0757.2011.00416.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ben Saad H, Babba M, Boukamcha R, Ghannouchi I, Latiri I, Mezghenni S, et al. Investigation of exclusive narghile smokers: deficiency and incapacity measured by spirometry and 6-minute walk test. Respir Care. 2014;59:1696–709. doi: 10.4187/respcare.03058. [DOI] [PubMed] [Google Scholar]
- 45.Shafagoj YA, Mohammed FI, Hadidi KA. Hubble-bubble (water pipe) smoking: levels of nicotine and cotinine in plasma, saliva and urine. Int J Clin Pharmacol Ther. 2002;40:249–55. doi: 10.5414/cpp40249. [DOI] [PubMed] [Google Scholar]
- 46.Eissenberg T, Shihadeh A. Waterpipe tobacco and cigarette smoking: direct comparison of toxicant exposure. Am J Prev Med. 2009;37:518–23. doi: 10.1016/j.amepre.2009.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wu LZ, Duan DM, Liu YF, Ge X, Zhou ZF, Wang XJ. Nicotine favors osteoclastogenesis in human periodontal ligament cells co-cultured with CD4(+) T cells by upregulating IL-1β. Int J Mol Med. 2013;31:938–42. doi: 10.3892/ijmm.2013.1259. [DOI] [PubMed] [Google Scholar]
- 48.Silva N, Abusleme L, Bravo D, Dutzan N, Garcia-Sesnich J, Vernal R, et al. Host response mechanisms in periodontal diseases. J Appl Oral Sci. 2015;23:329–55. doi: 10.1590/1678-775720140259. [DOI] [PMC free article] [PubMed] [Google Scholar]