Abstract
The nature of the gastrointestinal microbiome determines the reservoir of lipopolysaccharide (LPS), which can migrate from the gut into the circulation, where it contributes to low-grade inflammation. Osteoarthritis (OA) is a low-grade inflammatory condition, and the elevation of levels of LPS in association with obesity and metabolic syndrome could contribute to OA. A bifactorial model of OA susceptibility and potentiation suggests that LPS primes the proinflammatory innate immune response via toll-like receptor 4 and that progression to a full-blown inflammatory response and structural damage of the joint results from coexisting complementary mechanisms, such as inflammasome activation or assembly by damage-associated molecular patterns in the form of fragmented cartilage-matrix molecules. LPS could be considered a major hidden risk factor that provides a unifying mechanism to explain the association between obesity, metabolic syndrome and OA.
Introduction
Osteoarthritis (OA), the most common form of joint disease and a major cause of pain and disability, affects ≥151 million individuals globally (data from 2002).1 For economic and ethical reasons, identification of the optimal treatment for individual patients is a pressing concern that is particularly challenging, owing to the heterogeneity of OA and the very large number of those affected. The goal of pairing patients with the most appropriate therapies will be achieved, in part, by the identification and characterization of subsets of disease.
Macrophage-associated inflammation has been shown in vivo to be a prevalent phenomenon in radiographic OA, although the exact pathogenesis is still unclear.2 Inflammation is thought to promote disease symptomatology and exacerbate disease progression in OA. In this Opinion article, we discuss the inflammatory processes involved in OA, along with findings related to a potential role of lipopolysaccharide (LPS) in the pathogenesis of chronic subacute inflammation in OA.
OA and low-grade inflammation
The concept of OA as a noninflammatory condition is slowly being supplanted by one in which low-grade inflammation (often subclinical) is not only prevalent in—but is also predictive of—articular chondropathy.3 The existence of this kind of low-grade inflammation in patients with OA is widespread, both locally and, when joint disease is generalized, systemically.2 The initiation and perpetuation of OA is intricately associated with activation of the innate immune system4 involving the macrophage-associated inflammatory response,5 activation of toll-like receptor (TLR) pathways,6 increases in levels of soluble CD14 (sCD14, a TLR co-receptor protein shed from activated proinflammatory macrophages)7 and soluble CD163 (a scavenger receptor for the haemoglobin–haptoglobin complex),7 complement activation8 and activation of the coagulation pathway (an intrinsic effector of innate immunity).9 TLRs are a group of membrane-associated pattern-recognition receptors that recognize fragments of the cartilage extracellular matrix (including fibronectin,10 hyaluronan,11,12 aggrecan13 and others14,15), bacterial lipopeptides and LPS. Relative to unaffected individuals, expression of TLRs is increased in OA, and this activation leads to elevated levels of nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) and sCD14.16 Elevated NF-κB can induce activated joint cells to produce catabolic cytokines and chemokines, such as TNF, IL-1β, IL-6, receptor activator of NF-κB ligand (RANKL) and IL-8, which in turn can increase the production of MMPs, decrease collagen and proteoglycan synthesis and further augment NF-κB activation.17
Responses to TLR2 and TLR4 ligands in fibroblast-like synoviocytes from patients with early OA are dependent on sCD14.16 The level of sCD14 in synovial fluid from patients with OA correlates with the abundance of activated macrophages in knees affected by OA, and with the severity and progression of knee OA.7 These results suggest that sCD14 shed from activated macrophages is a marker of activation of the innate immune system that has a pathogenic role in OA.
Chondrocytes contribute to the inflammatory process in OA through upregulation of the expression of TLR2 and TLR4, directly or indirectly activating matrix metalloproteinases (MMPs) such as MMP-1 and MMP-13,18,19 which contribute to cartilage degeneration.20 The alarmins S100A8 and S100A9 (Ca2+-binding proteins found in high amounts in the synovial fluids of patients with OA) have TLR4-dependent catabolic effects in osteoarthritic human chondrocytes.18,21,22 LPS can activate chondrocytes to induce production of complement C1r subcomponent, complement C3, complement factor B, mimecan (osteoglycin) and pentraxin 3, long (PTX3), leading to activation of the complement cascade and generation of active complement proteins,23 which can bind to receptors or deposit on cells of the synovium, leading to increased cytokine production. These results suggest that the innate inflammatory response induced by chondrocytes upon TLR4 activation not only exacerbates the progression of OA but also upregulates proinflammatory cytokines, exacerbating the existing low-grade inflammation and producing a recalcitrant, ‘chronic’ disease state.4
Inflammatory mechanisms of LPS
LPS is an outer-membrane component of gram-negative bacteria, released in constitutively produced outer-membrane vesicles that also contain proteins. Constituents of these vesicles are insensitive to proteases, suggesting they can transport cargo molecules over long distances from their sites of origin, via urine or blood, and deliver them in a concentrated manner.24 Vesicle-associated proteins can have proinflammatory or anti-inflammatory effects on host immune systems.24 The proinflammatory LPS is a glycolipid composed of a polysaccharide O-antigen, a core oligosaccharide and a highly conserved lipid A moiety. The toxicity of gram-negative bacteria and biological activities of LPS are mediated in large part by lipid A binding to the LPS-binding protein. LPS activates cells of the innate immune system, such as macrophages and neutrophils, which synthesize proinflammatory factors, such as IL-1β and TNF, MMPs and free radicals that lead to dramatic secondary inflammation in tissues.25 Recurrent exposure to subclinical LPS increases mortality and induces cardiac fibrosis in mice.26 Because of its pathophysiological properties, exogenous LPS has long been utilized in conjunction with collagen to induce arthritis in experimental animal models.25,27
The gram-negative flora of the terminal ileum and large intestine constitute a large reservoir of LPS.28 Absorption of LPS from the gut and transport to the portal vein is a normal physiological process.28 Low-grade inflammation can result from intestinal absorption of LPS.29–31 Although in humans LPS is normally associated with bacterial sepsis, shock and a high risk of mortality, low levels of LPS can be detected in the systemic circulation without acute illness,32 suggesting an intriguing aetiology for the low-grade inflammation associated with many chronic diseases including rheumatoid arthritis (RA),33 inflammatory bowel disease,34 type 1 diabetes mellitus,35 atopy36 and obesity.37 As little as a single injection of a very low or moderate dose of LPS (0.6–3 ng/kg) in a healthy individual can induce immediate adipose tissue inflammation and systemic insulin resistance.38 In RA, stimulation of the innate immune system, in part through the microbiome, has been postulated to be an early event in disease causation.39 Although a role for the microbiome has also been suggested for OA,40 LPS and the microbiome are currently unexplored factors in the pathogenesis of OA.
Intriguingly, many of the mechanisms now identified as having roles in the pathogenesis of OA resemble the immune-activating functions of LPS (Figure 1).41 These mechanisms include activation of the innate immune response through TLR4 and its coreceptor, MD-2, which associates noncovalently with TLR4 on the cell surface and confers responsiveness to LPS,42,43 and also enhanced phagocytosis and cytotoxicity of macrophages2,5 activation of the complement cascade,44 activation of the coagulation cascade,44 activation of an innate-immune-system response in human adipose tissue in obesity and type 2 diabetes,29 and initiation of obesity.45 In mouse models, increased fat mass, body weight and low-grade inflammation in multiple tissues (liver, muscle and adipose tissue) can be induced by a high-fat diet or by subcutaneous LPS infusion.45 Moreover, mice lacking functional LPS receptors (Cd14-knock-out mice) are resistant to the induction of obesity and related metabolic syndrome by high-fat diet or subcutaneous LPS.45 These results are consistent with a known mechanism of activation of the innate immune system by LPS via its interaction with CD14–TLR4–MD-2 complexes.42,43 Broad-spectrum antibiotics (ampicillin with neomycin or norfloxacin) reduce endotoxaemia—LPS in the blood—along with inflammation, insulin resistance and fat-mass development in mice that are fed a high-fat diet or that are genetically obese (ob/ob), compared with similar, antibiotic-free mice.46,47 Germ-free mice with no intestinal microflora that are fed a high-fat diet do not develop inflammation.48 One controversial hypothesis suggests that saturated fatty acids promote low-grade inflammation through a TLR4-dependent pathway.49 LPS has been shown to interact with the TLR4–CD14 complex to initiate a signalling cascade, which in turn induces the expression of TLR2, further enhancing the innate immune response.50 These results are consistent with a pathogenic role of LPS originating in the gut in the mediation of the deleterious effects of high-fat feeding.
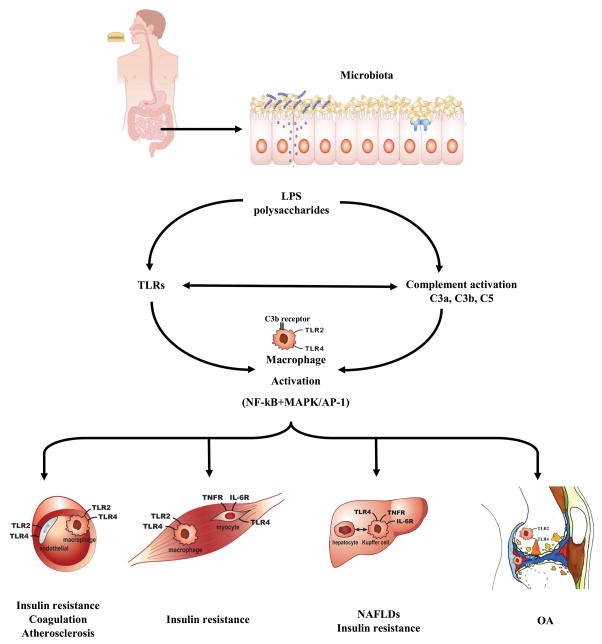
Figure 1.
The complement and TLR pathways in organ pathology. LPS can activate TLRs on macrophages in multiple organs including blood vessels, muscles, liver and joints. Activated complement components regulate intracellular signalling molecules such as MAPKs and transcription factors such as NF-κB, which can be enhanced by crosstalk from TLRs. The complement activation product C3b can directly activate macrophages. TLR activation can enhance complement protein biosynthesis and complement receptor expression. Thus, TLR and complement pathways can synergistically activate macrophages. This process can induce insulin resistance, coagulation and atherosclerosis in blood vessels, insulin resistance in muscles, and NAFLDs and insulin resistance in the liver, which in turn can reduce LPS clearance and increase levels of LPS in the systemic circulation. In joints, we propose that this process can exacerbate any underlying OA or pre-OA pathology, such as those initiated by excess mechanical load, or injury. Abbreviations: LPS, lipopolysaccharide; MAPK, mitogen-activated protein kinase; NAFLD, nonalcoholic fatty-liver disease; NF-κB, nuclear factor kappa-light-chain-enhancer of activated B cells; OA, osteoarthritis; TLR, toll-like receptor.
Systemic LPS concentrations
Systemic levels of LPS are elevated in obese individuals by several mechanisms (Figure 2).51,52 These mechanisms include impairment of clearance in the liver, and alterations in the gut microbiota, permeability, motility and enzyme levels, and serum levels of HDL cholesterol.
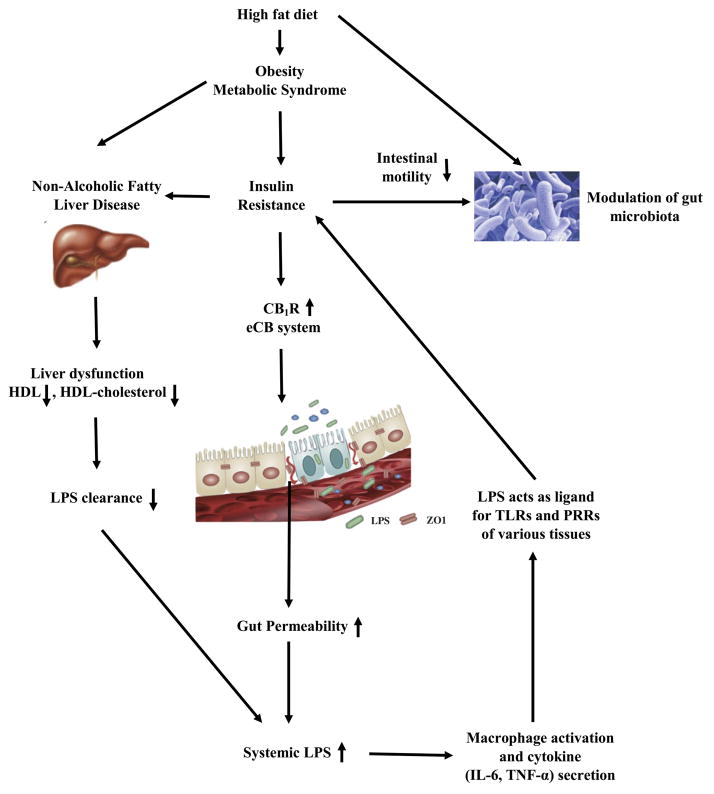
Figure 2.
Pathophysiological mechanisms underlying regulation of systemic LPS concentrations. A high-fat diet and insulin resistance associated with obesity can both adversely alter the gut flora (microbiome dysbiosis) leading to a constitutive increase in the delivery of LPS-containing bacterial outer-membrane vesicles to the liver. Insulin resistance also increases gut permeability for outer-membrane vesicles and LPS through alterations to the endocannabinoid system (upregulation of CB1) and downregulation of tight-junction proteins (ZO-1 and occludin) of the gut endothelium. Inadequate clearance of LPS by a fatty liver, owing to low HDL cholesterol production, can lead to the presence of LPS in the systemic circulation. These processes result in a vicious cycle because of the diabetes-promoting and proinflammatory actions of LPS throughout the body. For instance, LPS acts as a ligand for TLRs and PRRs in various tissues, inducing or exacerbating insulin resistance in the liver. Abbreviations: CB1, cannabinoid receptor 1; LPS, lipopolysaccharide; PRR, pattern-recognition receptor; TLR, toll-like receptor.
Impaired clearance
The principle clearance site for both septic and physiological levels of LPS is the liver. The Kupffer cells in the liver are the first line of defense against gut-derived toxins, preventing their entry into the systemic circulation. Impairment of this clearance and detoxification mechanism has been associated with liver diseases.28,31 One very common form of liver dysfunction associated with the presence of systemic LPS is nonalcoholic fatty-liver disease (NAFLD); the prevalence of NAFLD is astonishingly high in the general population (20–30%) and in obese individuals (75–100%).53 NAFLD impairs the ability of the liver to clear systemic LPS.28,31 Importantly, high fat intake also leads to acute postprandial exposure to circulating LPS,54 which can be attributed to the facilitation of the transport of bacterial LPS from the intestine to the circulation by chylomicrons that are enriched in high-fat diets. In addition, intestinal insults such as inflammatory bowel disease that result in increased intestinal permeability can contribute to elevation of circulating LPS.55
Altered gut microbiota
A high-fat diet changes the composition of the murine gut microbiota, resulting in reductions in the levels of some Gram-positive and Gram-negative species, but not in overall numbers of bacteria.57 Notably, compared with a normal diet, a high-fat diet significantly reduces numbers of the Gram-positive Bifidobacterium spp. that have been associated with reductions in intestinal LPS levels.86 Modification of the composition of the murine gut microbiota by the dietary addition of nondigestible carbohydrates, such as prebiotics, improves gut-barrier integrity, reduces metabolic endotoxaemia and lowers inflammation, compared with supplementation with nonprebiotics, or no carbohydrate addition.56–58 Introduction of prebiotic fibre to a high-fat diet specifically increases bifidobacterial numbers and reduces plasma LPS levels, compared with an unsupplemented high-fat diet.57
Obesity and gut permeability
Obesity has been linked to high intestinal permeability in both animal and human studies.30,46,51,52,59,60 The intestinal mucosa constitutes a selectively permeable barrier between the circulation and intestinal lumen. In a healthy state, the permeability should be well regulated to facilitate the necessary absorption without compromising barrier exclusion. The intercellular tight junctions formed by the interaction between internal membrane proteins and cytoskeletal components regulate intestinal permeability in response to physiological or pathological stimuli, including fatty acids and proinflammatory cytokines (especially IFN-γ and TNF) and are essential to maintain the integrity of the intestinal mucosa.61 A high-fat diet contributes to disruption of the expression and localization in the small intestine of the tight junction protein ZO-1 and occludin in mice.30,46,57,62
Endocannabinoids and gut permeability
Compelling evidence has shown that dysregulation of the tight control of endocannabinoid levels can result in elevation of LPS, leading to pathological conditions such as obesity and related metabolic syndrome or neurological disorders.63 LPS stimulates endocannabinoid synthesis,58,64 and the endocannabinoid system has an important role in the regulation of gut permeability. In lean wild-type mice, treatment with a cannabinoid receptor 1 (CB1) agonist increased both gut permeability and plasma LPS levels compared with untreated controls. Conversely, in genetically obese mice, treatment with a CB1 antagonist reduced plasma LPS levels compared with untreated controls.58 In an in vitro assay, cotreatment with LPS and a CB1 agonist had a greater effect than LPS alone on reduction of expression of the genes encoding occludin and ZO-1.58 High dietary intake of fatty acids can also increase endocannabinoid levels in different tissues, including the intestine,65 suggesting a mechanism whereby a high-fat diet can increase LPS, thereby triggering the development of obesity and metabolic syndrome.
Intestinal motility
Gut microbiota overgrowth and alteration are associated with reduced intestinal motility, an effect that is potentially mediated by insulin resistance.66 In a large cohort study,67 higher LPS concentrations were found in diabetic patients than in nondiabetic individuals, and serum levels of LPS-binding protein correlated with indicators of insulin resistance (levels of glycated haemoglobin and results of the homeostatic model assessment-1 algorithm).
Intestinal alkaline phosphatase
Intestinal alkaline phosphatase (IAP), which has an important role in LPS detoxification through dephosphorylation of the lipid portion of the endotoxin, is also implicated in the alteration of gut permeability in obese patients.68 In rats with an obesity-prone phenotype, a high-fat diet leads to altered gut microbiota, increased luminal LPS, decreased IAP activity and increased tight-junction permeability relative to a low-fat diet.69 Conversely, increased IAP activity is associated with the reduction of circulating levels of LPS.70
Exercise and HDL cholesterol
Systemic endotoxin levels negatively correlate with physical activity, being high in individuals with a sedentary lifestyle and low in those who are highly trained.71 Clinical studies72,73 have shown that aerobic exercise training can increase serum levels of HDL cholesterol from baseline, and HDL cholesterol is negatively correlated with LPS.67 Notably, clearance of HDL cholesterol by hepatocytes is mediated by control of expression of the scavenger receptor class B member 1 by leptin, and leptin deficiency results in high plasma HDL cholesterol.74 Leptin deficiency or absence of function in mice, which are associated with high levels of HDL cholesterol,75 prevent the development of OA even in the presence of a high-fat diet and obesity.76 This effect might be the result of elevation of HDL cholesterol in the absence of leptin protein or function leading to a high level of LPS clearance. Furthermore, the results of longitudinal studies have shown that physical exercise can reduce not only systemic LPS levels but also TLR4 activation, thereby further reducing the inflammatory response to LPS.77,78 The effects of diet and exercise on LPS might, at least in part, explain their synergistic benefits for OA.79
A potential role of LPS in OA
Increasingly, evidence suggests that endotoxaemia is intricately associated with several chronic diseases including type 2 diabetes, nonalcoholic steatohepatitis and cardiovascular disease (Figure 1). Given the compelling evidence for the association of obesity, metabolic syndrome, LPS, gut permeability, innate immunity and low-grade inflammation with OA, we hypothesize that LPS in the bloodstream has a role in a bifactorial model of OA pathogenesis and potentiation. One factor is the priming of the proinflammatory innate immune response by LPS, the second involves complementary mechanisms, such as joint injury and damage, which synergistically activate innate immunity (Figure 3). The rationale underlying this hypothesis is based on several lines of evidence.
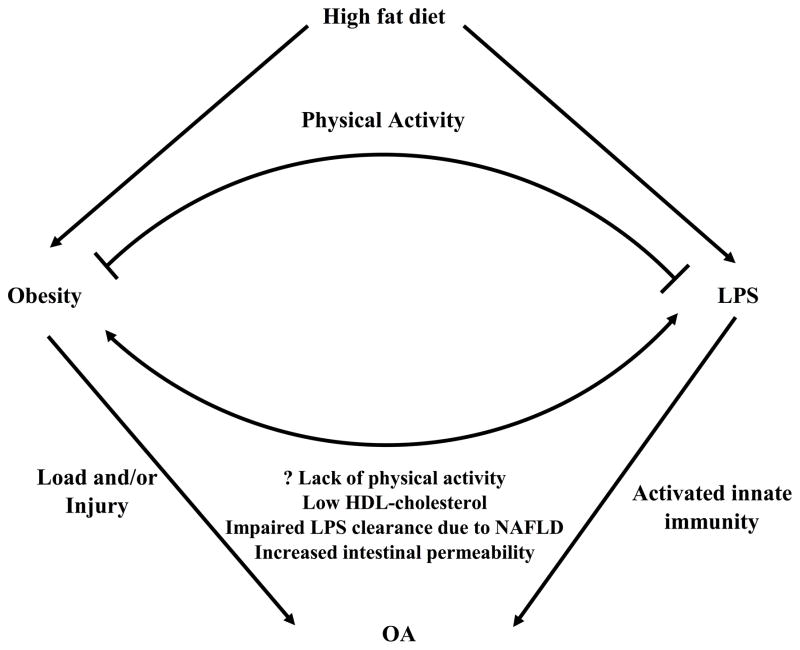
Figure 3.
Bifactorial model of OA pathogenesis involving LPS. The induction of obesity by a high-fat diet could lead to site-specific cartilage strains, which along with injury could be triggers for OA. Factors contributing to elevation of levels of systemic LPS include a high-fat diet with secondary insulin-resistance, gram-negative bacterial overload in the gastrointestinal tract, and a fatty liver with impairment of LPS clearance. High levels of systemic LPS can activate the innate immune system via macrophage stimulation, which can exacerbate the process of OA triggered by excess cartilage strains. A high level of physical activity can mitigate the effects of LPS, through enhancement of systemic levels of HDL cholesterol and LPS clearance, and through reduction of TLR4 activation to reduce the inflammatory response to LPS. Abbreviations: LPS, lipopolysaccharide; NAFLD, nonalcoholic fatty-liver disease; OA, osteoarthritis; TLR4, toll-like receptor 4.
Very small amounts of LPS, or fragments of hyaluronan (a damage-associated molecular pattern molecule that activates innate immune responses), cannot by themselves activate macrophages in vitro.80 However, the combination of these two factors synergistically activates macrophages to express high levels of OA-related cytokines.80 Also, obesity and metabolic syndrome are associated with insulin resistance, an altered gut microbiome and inflammation. Furthermore, insulin resistance and an altered gut microbiome could lead to impaired gut permeability, thereby increasing LPS absorption and resulting in the emergence of systemic metabolic endotoxaemia, and the induction of low-grade systemic inflammation. With the activation of the innate immune system, the whole process could lead to a pathological vicious cycle.
The functions of LPS can explain aspects of the association between obesity and OA81,82 particularly at non-weight-bearing joints—such as the twofold increased risk of hand OA in obese patients, which cannot be explained by biomechanical factors.83 Obesity is associated with compromised gut mucosa and elevated serum levels of LPS,51,52,59,60 providing a mechanism by which inflammation caused by obesity could result in OA. However, many factors potentially contribute to the low-grade inflammation seen in OA, including injuries and genetic factors, such as those regulating the level of the innate immune response, as reviewed previously.84 LPS could, therefore, be considered to be neither necessary nor sufficient, but rather an exacerbating factor in a bifactorial model of OA. Therapeutic strategies aimed at reducing systemic levels of LPS, such as modifications of the intestinal microbiome,85 could have benefits for the prevention and treatment of OA.
Conclusions
LPS causes both inflammation and obesity, and its clearance is altered through a variety of mechanisms in obesity. Altered clearance of LPS potentially explains how inflammation in obesity results in OA. LPS could, therefore, be considered a major hidden risk factor for OA. In addition to LPS, bacterial outer-membrane vesicles contain phospholipids, DNA, RNA and proteins,24 any one of which might also be found to exert subacute toxic or proinflammatory effects that could contribute to chronic disease. As profiling of the constituents of outer-membrane vesicles becomes more widespread, additional pathological mediators are likely to be uncovered. Understanding the relevance of LPS to human arthritides has profound implications for therapy. On the basis of current knowledge, LPS-lowering therapies could include a high-fibre diet, weight loss, exercise, antibiotics and, as a last resort, microbiome transplant.85 Surveillance of LPS levels could catalyze the development of new approaches to diagnosing and treating certain phenotypes of OA. In particular, circulating LPS concentrations could provide a new means of detecting individuals with a more-recalcitrant disease state, who might be at high risk for joint failure and who could be targeted with therapies aimed at lowering LPS levels.
Acknowledgments
Z.H. wishes to acknowledge funding supportby the China Health Ministry Program (201302007). V.B.K. wishes to acknowledge funding supportby NIH/NIA OAIC P30-AG-028716.
Biographies
ZeYu Huang received his MD degree in 2010 from the West China Medical School, Sichuan University. He completed training as an orthopaedic surgeon in 2014 in the West China Hospital, ChengDu, People’s Republic of China. He is currently a PhD candidate supervised by Dr FuXing Pei in the Department of Orthopaedics, West China Hospital of Sichuan University, ChengDu, People’s Republic of China. Since August 2014, he has been a visiting scholar in the research laboratory of Dr Virginia Kraus at Duke University, NC, USA, focusing on metabolic syndrome, inflammation and osteoarthritis pathogenesis and biomarkers.
Virginia Byers Kraus, MD, PhD, is Professor of Medicine and adjunct Professor of Pathology and Orthopaedic Surgery at Duke University School of Medicine, NC, USA. She is a practicing Rheumatologist with over 20 years’ experience in musculoskeletal research focusing on osteoarthritis. She obtained a BSc from Brown University, RI, USA, and an MD and PhD from Duke University, and completed Residency (Internal Medicine) and Fellowship (Rheumatology) at Duke University. She served as the President of the Osteoarthritis Research Society International (2013–2015), is a national board member of the Arthritis Foundation, and recipient of the 2015 Kappa Delta award from the American Academy of Orthopaedic Surgeons and the Orthopaedic Research Society.
Footnotes
Competing interests
The authors declare no competing interests.
Author contributions
Both authors contributed equally to researching data for the article, substantial discussion of the content, writing the article and reviewing and revising the manuscript before submission.
References
- 1.Loeser RF, Goldring SR, Scanzello CR, Goldring MB. Osteoarthritis: a disease of the joint as an organ. Arthritis Rheum. 2012;64:1697–1707. doi: 10.1002/art.34453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kraus VB, et al. Direct in vivo evidence of activated macrophages in human osteoarthritis. Osteoarthritis Cartilage. 2013;21:S42. doi: 10.1016/j.joca.2016.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ayral X, Pickering EH, Woodworth TG, Mackillop N, Dougados M. Synovitis: a potential predictive factor of structural progression of medial tibiofemoral knee osteoarthritis -- results of a 1 year longitudinal arthroscopic study in 422 patients. Osteoarthritis Cartilage. 2005;13:361–367. doi: 10.1016/j.joca.2005.01.005. [DOI] [PubMed] [Google Scholar]
- 4.Scanzello CR, Plaas A, Crow MK. Innate immune system activation in osteoarthritis: is osteoarthritis a chronic wound? Curr Opin Rheumatol. 2008;20:565–572. doi: 10.1097/BOR.0b013e32830aba34. [DOI] [PubMed] [Google Scholar]
- 5.Bondeson J, et al. The role of synovial macrophages and macrophage-produced mediators in driving inflammatory and destructive responses in osteoarthritis. Arthritis Rheum. 2010;62:647–657. doi: 10.1002/art.27290. [DOI] [PubMed] [Google Scholar]
- 6.Scanzello CR, Plaas A, Crow MK. Innate immune system activation in osteoarthritis: is osteoarthritis a chronic wound? Curr Opin Rheumatol. 2008;20:565–572. doi: 10.1097/BOR.0b013e32830aba34. [DOI] [PubMed] [Google Scholar]
- 7.Daghestani HNPC, Kraus VB. Soluble macrophage biomarkers indicate inflammatory phenotypes in patients with knee osteoarthritis. Arthritis Rheumatol. 2015;67:956–965. doi: 10.1002/art.39006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang Q, et al. Identification of a central role for complement in osteoarthritis. Nat Med. 2011;17:1674–1679. doi: 10.1038/nm.2543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ritter SY, et al. Proteomic analysis of synovial fluid from the osteoarthritic knee: comparison with transcriptome analyses of joint tissues. Arthritis Rheum. 2013;65:981–992. doi: 10.1002/art.37823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Okamura Y, et al. The extra domain A of fibronectin activates Toll-like receptor 4. J Biol Chem. 2001;276:10229–10233. doi: 10.1074/jbc.M100099200. [DOI] [PubMed] [Google Scholar]
- 11.Scheibner KA, et al. Hyaluronan fragments act as an endogenous danger signal by engaging TLR2. J Immunol. 2006;177:1272–1281. doi: 10.4049/jimmunol.177.2.1272. [DOI] [PubMed] [Google Scholar]
- 12.Taylor KR, et al. Recognition of hyaluronan released in sterile injury involves a unique receptor complex dependent on Toll-like receptor 4, CD44, and MD-2. J Biol Chem. 2007;282:18265–18275. doi: 10.1074/jbc.M606352200. [DOI] [PubMed] [Google Scholar]
- 13.Lees S, et al. Bioactivity in an Aggrecan 32-mer Fragment Is Mediated via Toll-like Receptor 2. Arthritis Rheumatol. 2015;67:1240–1249. doi: 10.1002/art.39063. [DOI] [PubMed] [Google Scholar]
- 14.Yu L, Wang L, Chen S. Endogenous toll-like receptor ligands and their biological significance. J Cell Mol Med. 2010;14:2592–2603. doi: 10.1111/j.1582-4934.2010.01127.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gomez R, Villalvilla A, Largo R, Gualillo O, Herrero-Beaumont G. TLR4 signalling in osteoarthritis--finding targets for candidate DMOADs. Nat Rev Rheumatol. 2015;11:159–170. doi: 10.1038/nrrheum.2014.209. [DOI] [PubMed] [Google Scholar]
- 16.Nair A, et al. Synovial fluid from patients with early osteoarthritis modulates fibroblast-like synoviocyte responses to toll-like receptor 4 and toll-like receptor 2 ligands via soluble CD14. Arthritis Rheum. 2012;64:2268–2277. doi: 10.1002/art.34495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kapoor M, Martel-Pelletier J, Lajeunesse D, Pelletier JP, Fahmi H. Role of proinflammatory cytokines in the pathophysiology of osteoarthritis. Nat Rev Rheumatol. 2011;7:33–42. doi: 10.1038/nrrheum.2010.196. [DOI] [PubMed] [Google Scholar]
- 18.Schelbergen RF, et al. Alarmins S100A8 and S100A9 elicit a catabolic effect in human osteoarthritic chondrocytes that is dependent on Toll-like receptor 4. Arthritis Rheum. 2012;64:1477–1487. doi: 10.1002/art.33495. [DOI] [PubMed] [Google Scholar]
- 19.Xie DL, Hui F, Meyers R, Homandberg GA. Cartilage chondrolysis by fibronectin fragments is associated with release of several proteinases: stromelysin plays a major role in chondrolysis. Arch Biochem Biophys. 1994;311:205–212. doi: 10.1006/abbi.1994.1228. [DOI] [PubMed] [Google Scholar]
- 20.Zhang Q, et al. Differential Toll-like receptor-dependent collagenase expression in chondrocytes. Ann Rheum Dis. 2008;67:1633–1641. doi: 10.1136/ard.2007.079574. [DOI] [PubMed] [Google Scholar]
- 21.van Lent PL, et al. Active involvement of alarmins S100A8 and S100A9 in the regulation of synovial activation and joint destruction during mouse and human osteoarthritis. Arthritis Rheum. 2012;64:1466–1476. doi: 10.1002/art.34315. [DOI] [PubMed] [Google Scholar]
- 22.Schelbergen RF, et al. Treatment efficacy of adipose-derived stem cells in experimental osteoarthritis is driven by high synovial activation and reflected by S100A8/A9 serum levels. Osteoarthritis Cartilage. 2014;22:1158–1166. doi: 10.1016/j.joca.2014.05.022. [DOI] [PubMed] [Google Scholar]
- 23.Haglund L, Bernier SM, Onnerfjord P, Recklies AD. Proteomic analysis of the LPS-induced stress response in rat chondrocytes reveals induction of innate immune response components in articular cartilage. Matrix Biol. 2008;27:107–118. doi: 10.1016/j.matbio.2007.09.009. [DOI] [PubMed] [Google Scholar]
- 24.Bonnington KE, Kuehn MJ. Protein selection and export via outer membrane vesicles. Biochim Biophys Acta. 2014;1843:1612–1619. doi: 10.1016/j.bbamcr.2013.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lorenz W, Buhrmann C, Mobasheri A, Lueders C, Shakibaei M. Bacterial lipopolysaccharides form procollagen-endotoxin complexes that trigger cartilage inflammation and degeneration: implications for the development of rheumatoid arthritis. Arthritis Res Ther. 2013;15:R111. doi: 10.1186/ar4291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lew WY, et al. Recurrent exposure to subclinical lipopolysaccharide increases mortality and induces cardiac fibrosis in mice. PLoS One. 2013;8:e61057. doi: 10.1371/journal.pone.0061057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yoshino S, Sasatomi E, Ohsawa M. Bacterial lipopolysaccharide acts as an adjuvant to induce autoimmune arthritis in mice. Immunology. 2000;99:607–614. doi: 10.1046/j.1365-2567.2000.00015.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fox ES, Thomas P, Broitman SA. Hepatic mechanisms for clearance and detoxification of bacterial endotoxins. J Nutr Biochem. 1990;1:620–628. doi: 10.1016/0955-2863(90)90020-l. [DOI] [PubMed] [Google Scholar]
- 29.Creely SJ, et al. Lipopolysaccharide activates an innate immune system response in human adipose tissue in obesity and type 2 diabetes. Am J Physiol Endocrinol Metab. 2007;292:E740–747. doi: 10.1152/ajpendo.00302.2006. [DOI] [PubMed] [Google Scholar]
- 30.Brun P, et al. Increased intestinal permeability in obese mice: new evidence in the pathogenesis of nonalcoholic steatohepatitis. Am J Physiol Gastrointest Liver Physiol. 2007;292:G518–525. doi: 10.1152/ajpgi.00024.2006. [DOI] [PubMed] [Google Scholar]
- 31.Harte AL, et al. Elevated endotoxin levels in non-alcoholic fatty liver disease. J Inflamm (Lond) 2010;7:15. doi: 10.1186/1476-9255-7-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Miller MA, et al. Ethnic and sex differences in circulating endotoxin levels: A novel marker of atherosclerotic and cardiovascular risk in a British multi-ethnic population. Atherosclerosis. 2009;203:494–502. doi: 10.1016/j.atherosclerosis.2008.06.018. [DOI] [PubMed] [Google Scholar]
- 33.Scher JU, et al. Expansion of intestinal Prevotella copri correlates with enhanced susceptibility to arthritis. Elife. 2013;2:e01202. doi: 10.7554/eLife.01202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Garrett WS, et al. Enterobacteriaceae act in concert with the gut microbiota to induce spontaneous and maternally transmitted colitis. Cell Host Microbe. 2010;8:292–300. doi: 10.1016/j.chom.2010.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Brugman S, et al. Antibiotic treatment partially protects against type 1 diabetes in the Bio-Breeding diabetes-prone rat. Is the gut flora involved in the development of type 1 diabetes? Diabetologia. 2006;49:2105–2108. doi: 10.1007/s00125-006-0334-0. [DOI] [PubMed] [Google Scholar]
- 36.van Nimwegen FA, et al. Mode and place of delivery, gastrointestinal microbiota, and their influence on asthma and atopy. J Allergy Clin Immunol. 2011;128:948–55. e1–3. doi: 10.1016/j.jaci.2011.07.027. [DOI] [PubMed] [Google Scholar]
- 37.Turnbaugh PJ, et al. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature. 2006;444:1027–1031. doi: 10.1038/nature05414. [DOI] [PubMed] [Google Scholar]
- 38.Mehta NN, et al. Experimental endotoxemia induces adipose inflammation and insulin resistance in humans. Diabetes. 2010;59:172–181. doi: 10.2337/db09-0367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Arend WP, Firestein GS. Pre-rheumatoid arthritis: predisposition and transition to clinical synovitis. Nat Rev Rheumatol. 2012;8:573–586. doi: 10.1038/nrrheum.2012.134. [DOI] [PubMed] [Google Scholar]
- 40.Metcalfe D, et al. Does endotoxaemia contribute to osteoarthritis in obese patients? Clin Sci (Lond) 2012;123:627–634. doi: 10.1042/CS20120073. [DOI] [PubMed] [Google Scholar]
- 41.Todar K. Online Textbook of Bacteriology. 2013 [Google Scholar]
- 42.Ohto U, Fukase K, Miyake K, Shimizu T. Structural basis of species-specific endotoxin sensing by innate immune receptor TLR4/MD-2. Proc Natl Acad Sci U S A. 2012;109:7421–7426. doi: 10.1073/pnas.1201193109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shimazu R, et al. MD-2, a molecule that confers lipopolysaccharide responsiveness on Toll-like receptor 4. J Exp Med. 1999;189:1777–1782. doi: 10.1084/jem.189.11.1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fernandez-Puente P, et al. Identification of a panel of novel serum osteoarthritis biomarkers. J Proteome Res. 2011;10:5095–5101. doi: 10.1021/pr200695p. [DOI] [PubMed] [Google Scholar]
- 45.Cani PD, et al. Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes. 2007;56:1761–1772. doi: 10.2337/db06-1491. [DOI] [PubMed] [Google Scholar]
- 46.Cani PD, et al. Changes in gut microbiota control metabolic endotoxemia-induced inflammation in high-fat diet-induced obesity and diabetes in mice. Diabetes. 2008;57:1470–1481. doi: 10.2337/db07-1403. [DOI] [PubMed] [Google Scholar]
- 47.Membrez M, et al. Gut microbiota modulation with norfloxacin and ampicillin enhances glucose tolerance in mice. Faseb j. 2008;22:2416–2426. doi: 10.1096/fj.07-102723. [DOI] [PubMed] [Google Scholar]
- 48.Rabot S, et al. Germ-free C57BL/6J mice are resistant to high-fat-diet-induced insulin resistance and have altered cholesterol metabolism. Faseb j. 2010;24:4948–4959. doi: 10.1096/fj.10-164921. [DOI] [PubMed] [Google Scholar]
- 49.Shi H, et al. TLR4 links innate immunity and fatty acid-induced insulin resistance. J Clin Invest. 2006;116:3015–3025. doi: 10.1172/JCI28898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ojaniemi M, et al. TLR-2 is upregulated and mobilized to the hepatocyte plasma membrane in the space of Disse and to the Kupffer cells TLR-4 dependently during acute endotoxemia in mice. Immunol Lett. 2006;102:158–168. doi: 10.1016/j.imlet.2005.08.009. [DOI] [PubMed] [Google Scholar]
- 51.Shen J, Obin MS, Zhao L. The gut microbiota, obesity and insulin resistance. Mol Aspects Med. 2013;34:39–58. doi: 10.1016/j.mam.2012.11.001. [DOI] [PubMed] [Google Scholar]
- 52.Jialal I, Huet BA, Kaur H, Chien A, Devaraj S. Increased toll-like receptor activity in patients with metabolic syndrome. Diabetes Care. 2012;35:900–904. doi: 10.2337/dc11-2375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Henao-Mejia J, et al. Inflammasome-mediated dysbiosis regulates progression of NAFLD and obesity. Nature. 2012;482:179–185. doi: 10.1038/nature10809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Harte AL, et al. High fat intake leads to acute postprandial exposure to circulating endotoxin in type 2 diabetic subjects. Diabetes Care. 2012;35:375–382. doi: 10.2337/dc11-1593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gardiner KR, et al. Significance of systemic endotoxaemia in inflammatory bowel disease. Gut. 1995;36:897–901. doi: 10.1136/gut.36.6.897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cani PD, et al. Changes in gut microbiota control inflammation in obese mice through a mechanism involving GLP-2-driven improvement of gut permeability. Gut. 2009;58:1091–1103. doi: 10.1136/gut.2008.165886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cani PD, et al. Selective increases of bifidobacteria in gut microflora improve high-fat-diet-induced diabetes in mice through a mechanism associated with endotoxaemia. Diabetologia. 2007;50:2374–2383. doi: 10.1007/s00125-007-0791-0. [DOI] [PubMed] [Google Scholar]
- 58.Muccioli GG, et al. The endocannabinoid system links gut microbiota to adipogenesis. Mol Syst Biol. 2010;6:392. doi: 10.1038/msb.2010.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chen X, Devaraj S. Monocytes from metabolic syndrome subjects exhibit a proinflammatory m1 phenotype. Metab Syndr Relat Disord. 2014;12:362–366. doi: 10.1089/met.2014.0017. [DOI] [PubMed] [Google Scholar]
- 60.Jialal I, Kaur H, Devaraj S. Toll-like receptor status in obesity and metabolic syndrome: a translational perspective. J Clin Endocrinol Metab. 2014;99:39–48. doi: 10.1210/jc.2013-3092. [DOI] [PubMed] [Google Scholar]
- 61.Griffin TM, Huebner JL, Kraus VB, Yan Z, Guilak F. Induction of osteoarthritis and metabolic inflammation by a very high-fat diet in mice: effects of short-term exercise. Arthritis Rheum. 2012;64:443–453. doi: 10.1002/art.33332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Jayashree B, et al. Increased circulatory levels of lipopolysaccharide (LPS) and zonulin signify novel biomarkers of proinflammation in patients with type 2 diabetes. Mol Cell Biochem. 2014;388:203–210. doi: 10.1007/s11010-013-1911-4. [DOI] [PubMed] [Google Scholar]
- 63.Lambert DM, Muccioli GG. Endocannabinoids and related N-acylethanolamines in the control of appetite and energy metabolism: emergence of new molecular players. Curr Opin Clin Nutr Metab Care. 2007;10:735–744. doi: 10.1097/MCO.0b013e3282f00061. [DOI] [PubMed] [Google Scholar]
- 64.Liu J, et al. Lipopolysaccharide induces anandamide synthesis in macrophages via CD14/MAPK/phosphoinositide 3-kinase/NF-kappaB independently of platelet-activating factor. J Biol Chem. 2003;278:45034–45039. doi: 10.1074/jbc.M306062200. [DOI] [PubMed] [Google Scholar]
- 65.Artmann A, et al. Influence of dietary fatty acids on endocannabinoid and N-acylethanolamine levels in rat brain, liver and small intestine. Biochim Biophys Acta. 2008;1781:200–212. doi: 10.1016/j.bbalip.2008.01.006. [DOI] [PubMed] [Google Scholar]
- 66.Cuoco L, et al. Eradication of small intestinal bacterial overgrowth and oro-cecal transit in diabetics. Hepatogastroenterology. 2002;49:1582–1586. [PubMed] [Google Scholar]
- 67.Pussinen PJ, Havulinna AS, Lehto M, Sundvall J, Salomaa V. Endotoxemia is associated with an increased risk of incident diabetes. Diabetes Care. 2011;34:392–397. doi: 10.2337/dc10-1676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bates JM, Akerlund J, Mittge E, Guillemin K. Intestinal alkaline phosphatase detoxifies lipopolysaccharide and prevents inflammation in zebrafish in response to the gut microbiota. Cell Host Microbe. 2007;2:371–382. doi: 10.1016/j.chom.2007.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.de La Serre CB, et al. Propensity to high-fat diet-induced obesity in rats is associated with changes in the gut microbiota and gut inflammation. Am J Physiol Gastrointest Liver Physiol. 2010;299:G440–448. doi: 10.1152/ajpgi.00098.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Everard A, Geurts L, Van Roye M, Delzenne NM, Cani PD. Tetrahydro iso-alpha acids from hops improve glucose homeostasis and reduce body weight gain and metabolic endotoxemia in high-fat diet-fed mice. PLoS One. 2012;7:e33858. doi: 10.1371/journal.pone.0033858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lira FS, et al. Endotoxin levels correlate positively with a sedentary lifestyle and negatively with highly trained subjects. Lipids Health Dis. 2010;9:82. doi: 10.1186/1476-511X-9-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kodama S, et al. Effect of aerobic exercise training on serum levels of high-density lipoprotein cholesterol: a meta-analysis. Arch Intern Med. 2007;167:999–1008. doi: 10.1001/archinte.167.10.999. [DOI] [PubMed] [Google Scholar]
- 73.Blazek A, Rutsky J, Osei K, Maiseyeu A, Rajagopalan S. Exercise-mediated changes in high-density lipoprotein: impact on form and function. Am Heart J. 2013;166:392–400. doi: 10.1016/j.ahj.2013.05.021. [DOI] [PubMed] [Google Scholar]
- 74.Neofytou E, O’Brien CG, Couture LA, Wu JC. Hurdles to clinical translation of human induced pluripotent stem cells. J Clin Invest. 2015;125:2551–2557. doi: 10.1172/JCI80575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Silver DL, Jiang XC, Tall AR. Increased high density lipoprotein (HDL), defective hepatic catabolism of ApoA-I and ApoA-II, and decreased ApoA-I mRNA in ob/ob mice. Possible role of leptin in stimulation of HDL turnover. J Biol Chem. 1999;274:4140–4146. doi: 10.1074/jbc.274.7.4140. [DOI] [PubMed] [Google Scholar]
- 76.Griffin TM, Huebner JL, Kraus VB, Guilak F. Extreme obesity due to impaired leptin signaling in mice does not cause knee osteoarthritis. Arthritis Rheum. 2009;60:2935–2944. doi: 10.1002/art.24854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Oliveira AG, et al. Physical exercise reduces circulating lipopolysaccharide and TLR4 activation and improves insulin signaling in tissues of DIO rats. Diabetes. 2011;60:784–796. doi: 10.2337/db09-1907. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 78.Olesen J, et al. Impact of training status on LPS-induced acute inflammation in humans. J Appl Physiol (1985) 2015;118:818–829. doi: 10.1152/japplphysiol.00725.2014. [DOI] [PubMed] [Google Scholar]
- 79.Messier SP, et al. Exercise and weight loss in obese older adults with knee osteoarthritis: a preliminary study. J Am Geriatr Soc. 2000;48:1062–1072. doi: 10.1111/j.1532-5415.2000.tb04781.x. [DOI] [PubMed] [Google Scholar]
- 80.Stabler T, Montell E, Verges J, Kraus V. Attentuation of hyaluronan fragment induced inflammatory response in macrophages by chondroitin sulphate. Osteoarthritis and Cartilage. 2015;23:A263–A264. [Google Scholar]
- 81.Anderson JJ, Felson DT. Factors associated with osteoarthritis of the knee in the first national Health and Nutrition Examination Survey (HANES I). Evidence for an association with overweight, race, and physical demands of work. Am J Epidemiol. 1988;128:179–189. doi: 10.1093/oxfordjournals.aje.a114939. [DOI] [PubMed] [Google Scholar]
- 82.Felson DT, Anderson JJ, Naimark A, Walker AM, Meenan RF. Obesity and knee osteoarthritis. The Framingham Study. Ann Intern Med. 1988;109:18–24. doi: 10.7326/0003-4819-109-1-18. [DOI] [PubMed] [Google Scholar]
- 83.Yusuf E, et al. Association between weight or body mass index and hand osteoarthritis: a systematic review. Ann Rheum Dis. 2010;69:761–765. doi: 10.1136/ard.2008.106930. [DOI] [PubMed] [Google Scholar]
- 84.Kraus VB. In: Rheumatology. Hochberg M, Silman A, Smolen J, Weinblatt M, Weisman M, editors. Elsevier; 2014. pp. 1498–1547. [Google Scholar]
- 85.West CE, et al. The gut microbiota and inflammatory noncommunicable diseases: associations and potentials for gut microbiota therapies. J Allergy Clin Immunol. 2015;135:3–13. doi: 10.1016/j.jaci.2014.11.012. quiz 14. [DOI] [PubMed] [Google Scholar]
- 86.Griffiths EA, et al. In vivo effects of bifidobacteria and lactoferrin on gut endotoxin concentration and mucosal immunity in Balb/c mice. Dig Dis Sci. 2004;49:579–589. doi: 10.1023/b:ddas.0000026302.92898.ae. [DOI] [PubMed] [Google Scholar]