Abstract
Background
Trypanosoma cruzi is dispersed in nature through many transmission mechanisms among a high diversity of vectors and mammalian species, representing particular behaviors and habitats. Thus, each locality has a unique set of conditions underlying the maintenance of this parasite in the wild. The aim of the present study was to evaluate the life-cycle of T. cruzi in free-ranging coatis from the central region of the Brazilian Pantanal using a multi-factorial approach.
Methods
Three methodological blocks were used in the present study: (i) We evaluated T. cruzi infection using serological (ELISA) and parasitological (hemoculture) tests in free-ranging coatis captured from October 2010 to March 2012. In addition, we characterized T. cruzi isolates as DTUs (Discrete Typing Units); (ii) We evaluated Trypanosoma infection in species of Triatoma and Rhodnius inhabiting coati arboreal nests from May to September 2012 using parasitological and molecular assays; and (iii) We analyzed a set of longitudinal data (from 2005 to 2012) concerning the effects of T. cruzi infection on this coati population. Herein, we investigated whether seasonality, host sex, and host age influence T. cruzi prevalence and patterns of infection.
Results
The 2010–2012 period presented high seroprevalence on coatis (72.0 % ELISA) and a high percentage of individuals with infectivity competence (20.5 % positive hemoculture). All isolates presented TcI band patterns, occurring in single (n = 3) and mixed infections (1 TcI/T. rangeli; 4 with undefined characterization). Both male and female individuals presented the same transmission potential, expressed as positive hemoculture, which was only detected in the summer. However, overall, the data (2005–2012) highlighted the importance of females for T. cruzi maintenance in the winter. Moreover, twenty-three (67.6 %) bugs from five coati nests (71.4 %) were infected with flagellated forms. Seventeen samples were characterized as T. cruzi (TcI and TcIII genotypes).
Conclusion
In the Pantanal region, T. cruzi is transmitted in a complex, multifactorial, dynamic and non-linear transmission web. The coati nests might be inserted in this web, acting as important transmission foci at the arboreal stratum to different mammal species with arboreal or scansorial behavior.
Keywords: Trypanosoma cruzi, Nasua nasua, Rhodnius, Triatoma, Pantanal, Sylvatic cycle
Background
Most scientific knowledge is based on simplification analyses, including reductionist methodologies and linear and deterministic concepts. Both reductionism and determinism are closely related. The former relies on the analysis of a system through its individual components, while the latter supports the idea that every phenomenon in nature is determined by pre-existing causes, and each cause produces a unique effect (and vice versa), sustaining the concept of predictability [1]. However, many biological systems, including parasitism, are complex systems [2]. Complex systems exist on the edge of chaos: they might present regular and predictable behaviors, but can also show nonlinear conduct (unpredictability) in response to minor modifications [1]. Thus, in parasitology, one must consider the likelihood of unpredictable modifications.
Trypanosoma cruzi is a hemoflagellate parasite and the causative agent of Chagas disease in humans [3]. This species represents a heterogeneous group, partially explained by hybridization events [4, 5]. Currently, the international consensus defines six discrete typing units (DTUs) within this species: TcI-VI [6]. In nature, this protozoan is maintained through distinct and complex cycles via several transmission mechanisms among a wide range of vectors and mammalian host species across almost all American habitats [7, 8]. The vector bugs (Reduviidae: Triatominae) become infected through the ingestion of mammalian blood at the parasitemic phase of infection. Thus, infective competence of a mammalian host depends on high parasitemia. Under natural conditions, transmission to mammals occurs through (i) contact of the contaminated feces of the vector with mammalian mucosa or injured skin during the blood meal (contaminative route); (ii) predation of infected bugs and mammals or ingestion of contaminated foods and triatomine feces (oral route); and (iii) congenital route [8]. The ecology of T. cruzi is, therefore, immensely variable, and each locality has a unique set of conditions underlying the emergence and persistence of this parasite in wildlife. Therefore, many aspects of T. cruzi cycles are poorly understood. Furthermore, few studies have evaluated how host-vector interactions modulate the T. cruzi cycle at a given locality [9–12].
In the Nhecolândia region of the Brazilian Pantanal biome, T. cruzi infection in the brown-nosed coati, Nasua nasua (Procyonidae), has been extensively examined between 2005–2010. We previously observed the importance of these mammals as reservoirs of the main T. cruzi lineages, representing a transmission hub for T. cruzi dispersion. Both males and females and all age groups have been continuously exposed to the infection [13–15]. However, females were the principal potential dispersers of T. cruzi, presenting higher rates of parasitemia, evaluated using hemoculture, primarily during the winter season [14].
Coatis are gregarious, diurnal, scansorial, and generalist mammals [16], feeding mainly on arthropods and fruits [17]. They present a particular behavior of constructing arboreal nests for resting and birthing [18]. In the Nhecolândia region, these nests are constructed at different sites: open areas, along forest edges and within the forest [19]. Furthermore, coatis frequent different nests to rest on a daily basis. Additionally, these nests can be communal (JS de Lima, personal observation). Recent findings in this region have shown that a third of the sampled resting nests are infested with triatomine bugs belonging to the genera Rhodnius and Triatoma. Indeed, insects at different nymphal stages and adult specimens have been observed in these nests, indicating successful colonization. Precipitin tests revealed that these bugs fed on coati, bird, rodent and marsupial species [20]. Additionally, this habitat might act as a point of convergence and dispersion for triatomine bugs and mammal hosts infected with T. cruzi in the Pantanal region [21].
Herein, we continued the longitudinal study of T. cruzi transmission cycle in the Pantanal biome focusing on the DTU characterization of T. cruzi isolates from triatomine bugs inhabiting coati resting nests. We also describe T. cruzi infection in the same coati population, including an evaluation of previous data [13–15] and the results obtained in the present study, corresponding to a seven-year longitudinal period.
Methods
Study area
Fieldwork was conducted at the Nhumirim ranch (56°39′W, 18°59′S), situated in the central region of the Pantanal, municipality of Corumbá, Brazilian state of Mato Grosso do Sul. This region is characterized by a mosaic of semideciduous forest, arboreal savannas, seasonally flooded fields covered by grasslands with dispersed shrubs and several temporary and permanent ponds [22]. The Pantanal is the largest Neotropical floodplain and is known for its biodiversity. Two well-defined seasons are recognized: a rainy summer (October to March) and a dry winter (April to September) [23]. Additionally, this area is subjected to multi-annual cycles of high flood and severe drought years [24]. Seasonal flood-drought cycles are the most striking ecological phenomena of the Pantanal, resulting in drastic changes in the landscape [25, 26].
Coati capture
Ten field expeditions were performed from October 2010 to March 2012 as a follow-up to the longitudinal studies conducted from March 2005 to February 2007 [13], May 2007 to February 2009 [14] and August 2009 to April 2010 [15]. Box traps, made of galvanized wire mesh, were baited with bacon. The captured animals were immobilized through intramuscular administration of tiletamine hydrochloride plus zolazepan hydrochloride (10 mg/kg) prior to ear-tag identification, blood sampling, weighing and sex identification. Based on dental conditions and body size measurements, coatis were classified as adult (> 2 year-old), subadult (between 6 month- and 2 year-old) or juvenile (< 6 month-old) [27]. Blood samples (5–10 ml) were obtained through the external saphenous vein puncture and stored in vacuum tubes (DB Vacutainer®, São Paulo, São Paulo, Brazil) containing EDTA for hemoculture and without anticoagulant for serum obtainment. The animals were released at the site of capture after full recovery from anesthesia. From October 2010 to March 2012, we obtained 40 samples from 35 individuals (22 males, 13 females; 30 adults, 1 subadult and 4 juveniles), resulting from 5 recaptures. The total capture effort included 1,188 trap nights.
Assessment of T. cruzi infection in coatis and interpretation of the results
To evaluate T. cruzi infection in coatis, we performed parasitological and serological assays. Hemoculture (HC) was accomplished under sterile conditions through the inoculation of 0.3 ml of each blood sample in NNN (Novy-McNeal-Nicolle) medium with a Liver Infusion Tryptose (LIT) overlay. The tubes were examined bi-weekly for 5 months. In case of trypanosomatid detection, the parasites were grown in LIT until log phase, cryopreserved, and deposited in the Trypanosoma Collection of Wild and Domestic Mammals and Vectors-COLTRYP (Fiocruz, Rio de Janeiro, Brazil) under accession numbers: 358, 360, 366–369, 476 and 477.
Additionally, we performed Enzyme-Linked Immunosorbent Assay (ELISA) using EIE-Chagas-Bio-Manguinhos kits (Bio-Manguinhos, Fiocruz, Rio de Janeiro, Rio de Janeiro, Brazil) kindly provided from the Laboratory of Diagnostic Technology/Fiocruz [28]. The cut-off value for the ELISA was defined as the mean optical absorbance of the negative controls plus 5 %. The anti-raccoon conjugate (Bethyl Laboratories, Inc., Montgomery, Texas, United States) was diluted 1:70,000, and each microtiter polystyrene plate contained 2 positive and 2 negative control samples.
Positive serological results indicate exposure to T. cruzi infection, while positive HC reveals significant T. cruzi parasitemia and thus a high potential for parasite transmission to triatomines. Seropositive individuals presenting negative results in the parasitological assay (HC) demonstrate sub-patent infection at the time of blood sampling.
Parasitological analysis of triatomine bugs
The capture and identification of triatomine bugs is described elsewhere [20]. Triatomine specimens were stored live in Falcon® tubes and submitted for Trypanosoma infection diagnosis to the Laboratory of Trypanosomatid Biology at Fiocruz, Rio de Janeiro, Brazil (transfer time: 24–48 h; mortality: 10.8 %).
We evaluated Trypanosoma spp. infection in 34 triatomine bugs from seven coati resting nests collected monthly from May to September 2012: 13 adults (11 Triatoma sordida and two Rhodnius stali) and 21 nymphs (five Triatoma sp., 13 Rhodnius sp. as well as three non-identified nymphs). Because of the lack of genital development, the nymphs were classified at the genus level.
Parasitological analysis was performed through fresh examination of the gut content macerated in 0.85 % saline solution supplemented with 10 % antimycotic/antibiotic solution (A5955, Sigma®, São Paulo, Brazil). The samples were examined under a light microscope for the presence of flagellated forms. The positive samples were further subjected to: (i) cultivation in NNN/LIT medium for posterior DNA extraction of the successfully isolated parasites (COLTRYP accession numbers: 483, 485, 491,492, 497–500, 507–512, 515 and 517); and (ii) direct DNA extraction using the Gentra® Puregene® kit (Qiagen®, Gaithersburg, Maryland, United States) according to the manufacturer’s instructions.
DNA extraction and molecular characterization
The isolates were washed in phosphate-buffered saline (0.15 M, pH = 7.2; centrifugation method: 4,000 rpm for 15 min at 4 °C). After the third procedure, the pellets were stored at -20 °C overnight. The pellets were resuspended in 50 μl of TE solution (10 mM Tris and 1 mM EDTA, pH 8.0) and incubated (56 °C for 2 h) with 10 μl of proteinase K (5 mg/ml) and 50 μl of 10 % sodium dodecyl sulfate. The genomic DNA was extracted three times with 500 μl of phenol-chloroform (1:1) and once with 500 μl of chloroform prior to precipitation with 45 μl of 3 M sodium acetate and 900 μl of ethanol [29]. The pellets obtained after centrifugation were suspended in 100 μl of distilled water. The DNA concentration was estimated after measuring the absorbance at 260 nm. The final template concentration (50 ng/μl) was achieved after dilution in distilled water.
Multiplex PCR amplification of the non-transcribed spacer of the mini-exon gene was performed [30]. This target classifies the T. cruzi population as TCI (corresponding to TcI DTU), TCII (TcII, TcV or TcVI DTUs) and zymodeme 3 (TcIII or TcIV DTUs) [31], and also detects Trypanosoma rangeli. Samples presenting TcII/TcV/TcVI or TcIII/TcIV band patterns were subjected to PCR amplification of the two targets: (i) the nuclear 1f8 gene, followed by restriction fragment length polymorphism (RFLP) analysis of DNA fragments digested with Alw21I enzyme [32], and/or (ii) histone 3 (H3), followed by RFLP analysis of the DNA fragments digested with Alul enzyme [33].
All reactions included sterile distilled water as a negative control and samples from T. cruzi strains of each genotype as positive controls. After electrophoresis, the amplified PCR products were visualized in ethidium bromide-stained agarose gels (2 %) under ultraviolet light.
Longitudinal data of T. cruzi infection in coatis from March 2005 to March 2012
We assembled all data associated with T. cruzi infection in coatis from previously published studies [13–15] and the present study, corresponding to a seven-year longitudinal period (March 2005 to March 2012). The data comprised 220 individuals. Sixty-one individuals were captured from two to six times, totaling 317 captures (Table 1).
Table 1.
Sampled coatis, captured from March 2005 to March 2012 at the Brazilian Pantanal
| Season | Male | Female | Total captured | ||
|---|---|---|---|---|---|
| S–SA–J | Total | S–SA–J | Total | ||
| Summer | 101–31–10 | 142 (67 %) | 61–8–5 | 74 (70 %) | 216 |
| Winter | 54–14–1 | 69 (33 %) | 26–3–3 | 32 (30 %) | 101 |
| Total | 155–45–11 | 211 | 87–11–8 | 106 | 317 |
Abbreviations: A, adult; SA, subadult, J, juvenile
We performed ELISA on all coati sera samples to compare the results with those of previous studies using an indirect immunofluorescence assay.
Statistical analysis
To analyze T. cruzi exposure in coatis from October 2010 to March 2012, we investigated differences between seroprevalence and positive HC rates for coati gender (Fisher’s exact test, α = 0.05). We did not evaluate the influence of age and seasonality, reflecting the small amount of records obtained during this period.
For the 2005–2012 period, we investigated differences in seroprevalence and positive HC rates between coati genders (Chi-square test, α = 0.05). Depending on load samples, both Fisher’s exact and Chi-square tests were used to access potential differences between positive HC rates for coati sex clustered according to age.
Results
Trypanosoma cruzi in coatis from October 2010 to March 2012
The results corroborate the maintenance of the essential role of the coati population in T. cruzi sylvatic cycle, as demonstrated by the high seroprevalence (72.0 % positive ELISA, n = 29) and high percentage of individuals with infectivity competence observed throughout the study period (20.5 % positive HC, n = 39) (Table 2). These rates did not differ among male and female individuals [Fisher’s exact test: ELISA (P > 0.99, n = 29); HC (P = 0.40, n = 39)]. Positive HC results were only detected in the summer (January - February-March). In addition, 21.7 % seropositive individuals exhibited significant parasitemia (i.e. positive HC; n = 23).
Table 2.
Seroprevalence (ELISA) and hemoculture (HC) of Trypanosoma cruzi in coatis from October 2010 to March 2012
| October 2010 - March 2012 | Positive ELISA | Positive HC | ||||
|---|---|---|---|---|---|---|
| Male | Female | Total | Male | Female | Total | |
| % (n) | % (n) | % (n) | % (n) | % (n) | % (n) | |
| Adult | 81.2 (16) | 77.8 (9) | 80 (25) | 17.4a (23) | 27.3b (11) | 20.6 (34) |
| Subadult | 0 (1) | – | 0 (1) | 0 (1) | – | 0 (1) |
| Juvenile | 50 (2) | 0 (1) | 33.3 (3) | 0 (2) | 50 (2) | 25 (4) |
| Total | 73.7 (19) | 70 (10) | 72 (29) | 15.4 (26) | 30.8 (13) | 20 (39) |
n = total number of samples analyzed
aFour samples from four recaptured male coatis
bOne sample from one recaptured female coati
Coatis were exposed to T. cruzi infection early in life, demonstrated as positive HC and ELISA results, respectively, observed in male and female individuals younger than six months.
Molecular characterization showed that all T. cruzi isolates (n = 8) presented TcI band patterns in single (samples 4, 6 and 7) and mixed infections with T. rangeli (sample 5). Furthermore, Trypanosoma rangeli occurred in three individuals (numbers 3, 5, and 8) from the 39 evaluated samples (7.7 %).
We could not define the genotype of samples 1, 2, 3 and 8, because of the lack of correspondence between the results of the mini-exon gene PCR (Fig. 1a) and the results of 1f8/Alw21I and H3/Alul assays (Fig. 1b, c). For example, the results of the mini-exon gene PCR of samples 1 and 2 displayed mixed infection with TcI + TCII (Fig. 1a), while 1f8/Alw21I and H3/Alul assays displayed only infection with TcI (Fig. 1b, c). Additionally, sample 3 presented a multiple band pattern in the multiplex PCR (Fig. 1).
Fig. 1.
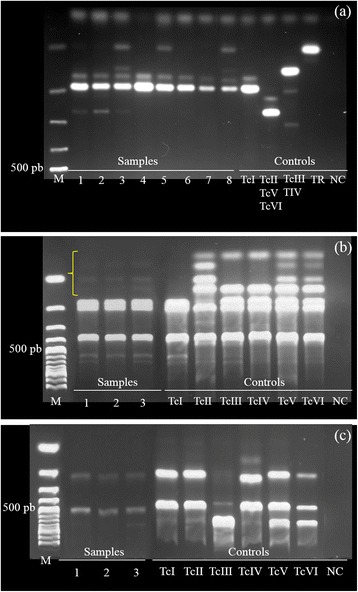
Trypanosoma cruzi genotyping from coatis at in the Pantanal region, Brazil. Agarose electrophoresis gels of (a) mini-exon multiplex PCR products; (b) 1f8 gene/Alw21I PCR-RFLP products; and (c) H3/Alul PCR-RFLP products. The brace in 1b shows non-characterizable weak bands. Abbreviations: M, Molecular weight markers (100 bp DNA ladder); TR, T. rangeli; NC, Negative control
Trypanosoma cruzi infection in coatis from March 2005 to March 2012
The sylvatic cycle of T. cruzi was highly stable, demonstrated as high annual proportions of seropositive coatis. Moreover, the role of the coati population as a T. cruzi reservoir was maintained during the entire period, as a relatively large portion of the population presented positive HC results each year (Fig. 2).
Fig. 2.
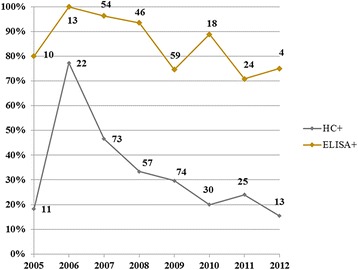
Annual fluctuations of positive HC and ELISA rates of T. cruzi infection in coatis from the Brazilian Pantanal. The numbers close to the dots correspond to the sample size
Although positive HC results were only detected in the summer during October 2010 to March 2012, all seven-year data showed that both male and female coatis presented higher HC rates in the winter. Moreover, this pattern was highly expressed in females (Fig. 3). The importance of females in T. cruzi transmission is summarized in Table 3.
Fig. 3.
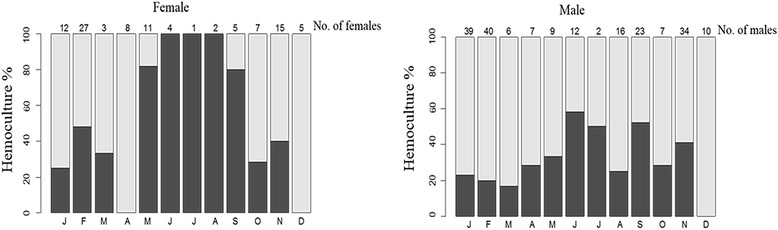
Proportion of female and male coatis with transmission potential (positive hemoculture) per month (accumulated from 2005 to 2012). The number of sampled coatis is indicated above the bars
Table 3.
Seroprevalence (ELISA) and hemoculture (HC) of Trypanosoma cruzi in coatis from March 2005 to March 2012
| 2005 - 2012 | Positive HC | Positive ELISA | ||||
|---|---|---|---|---|---|---|
| Male | Female | P-value | Male | Female | P-value | |
| % (n) | % (n) | % (n) | % (n) | |||
| Adult | 30.7 (150) | 42.7 (82) | P = 0.07; χ2 = 3.369 | 85.9 (92) | 93.8 (48) | P = 0.27; χ2 = 1.235 |
| Subadult | 32.5 (43) | 63.6 (11) | P = 0.08; Fisher’s exact test | 79.2 (24) | 85.7 (7) | P = 0.64; Fisher’s exact test |
| Juvenile | 27.3 (11) | 37.5 (8) | P > 0.99; Fisher’s exact test | 88.9 (9) | 83.3 (6) | P > 0.99; Fisher’s exact test |
| Total | 30.9 (204) | 44.5 (101) | P = 0.03a; χ2 = 5.521 | 84.8 (125) | 91.8 (61) | P = 0.27; χ2 = 1.220 with Yates correction |
n = total number of samples analyzed
aStatistical significance at 95 % confidence
Trypanosoma cruzi infection in triatomine bugs
Twenty-three (67.6 %; n = 34) triatomine bugs (Triatoma sp.: 56.2 %, n = 16; Rhodnius sp.: 80.0 %, n = 15) from five coati nests (71.4 %; n = 7) were infected with flagellated forms (positive fresh exam) [20]. Among the five coati nests harboring infected bugs, the infection rate in each nest was high, varying from 66.6 to 100 %.
Seventeen samples of four coati nests were initially characterized based on a mini-exon gene. All specimens were infected with T. cruzi: 13 single infections with TcI (76.4 %), a single infection with Z3 (5.8 %), and three co-infections with TcI and Z3 (17.6 %) (Fig. 4). PCR-RFLP analysis of the H3 target in two samples showed that Z3 corresponded to the TcIII DTU (Fig. 4). Two samples (TcI/Z3) could not be genotyped to DTUs. The TcI genotype was the most widely distributed, occurring in all sampled nests. On the other hand, Z3 was restricted to two nests and in Triatoma sp. bugs (Table 4).
Fig. 4.
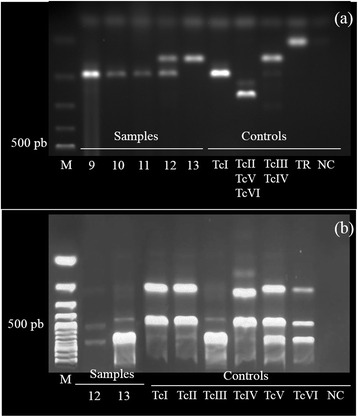
Trypanosoma cruzi genotyping from triatomine bugs in the Pantanal region, Brazil. Agarose electrophoresis gels of (a) mini-exon multiplex PCR products (representative samples); and (b) H3/Alul PCR-RFLP products. Abbreviations: M, Molecular weight markers (100 bp DNA ladder); TC, T. cruzi; TR, T. rangeli; NC, Negative control
Table 4.
Trypanosoma cruzi subpopulations in triatomine bugs per coati nest
| Nest | Species | Sampling method | |
|---|---|---|---|
| Gut content culture | Direct DNA extraction | ||
| A | Rhodnius stali | TcI | na |
| A | Rhodnius sp. | TcI | na |
| A | Rhodnius sp. | TcI | na |
| A | Rhodnius sp. | TcI | na |
| A | Rhodnius sp. | TcI | TcI |
| A | Rhodnius sp. | TcI | na |
| A | Rhodnius sp. | Nd | na |
| A | Triatoma sordida | TcI | TcI |
| A | Triatoma sordida | TcI | na |
| A | Triatoma sordida | TcI | na |
| A | Triatoma sordida | Nd | na |
| A | Triatoma sordida | TcI | TcI/Z3 |
| A | Triatoma sp. | TcIII | Z3 |
| A | Triatoma sp. | TcI | na |
| A | Unidentified | Nd | na |
| B | Rhodnius stali | TcI | nd |
| B | Rhodnius sp. | TcI | nd |
| C | Rhodnius sp. | TcI | na |
| C | Rhodnius sp. | Nd | na |
| D | Triatoma sordida | Nd | TcI/Z3 |
| D | Unidentified | TcI/TcIII | nd |
| E | Triatoma sordida | Nd | na |
Abbreviations: nd, not done; na, no amplification
One sample presented two different results concerning the T. cruzi subpopulation: the gut content culture showed single infection by TcI, whereas the direct DNA extraction of the gut content revealed mixed infection with TcI and Z3 (DTU characterization was not performed) (Table 4).
Discussion
The importance of the coati as a T. cruzi reservoir in the Nhecolândia region has been well recognized [13–15]. Herein, we confirmed the potential role of this population, particularly female individuals in the winter, in maintaining high and stable parasitemia, as observed in all data obtained during the entire seven-year period. Interestingly, the data obtained from October 2010 to March 2012 did not show this winter pattern, suggesting the unpredictability of the T. cruzi cycle and the role of a mammal host as a reservoir in a given area.
Indeed, in the two tours of Rocha FL at Nhumirim ranch from August 20 to August 31, 2013 (capture effort: 165 trap nights) and from August 18 to August 28, 2014 (capture effort: 180 trap nights), a distinct enzootic frame was revealed: low relative abundance of coatis (six and two captured coatis in 2013 and 2014, respectively) and undetected positive hemoculture (n = 8; unpublished results). Thus, the occurrence of a host population with a high transmission potential and the relative abundance and contact rate of the mammalian and vector populations are crucial factors for the epizootiology of T. cruzi in a given area [34]. These results suggest that the Pantanal climate might have changed coati abundance. The severe drought period in 2013 likely killed many animals and forced the emigration of resistant individuals. These findings show the complexity and unpredictability of T. cruzi transmission and highlight the influence of abiotic factors on host-parasite dynamics [35].
One feature of complex systems is the synergy of the components, thus, scientific methodology cannot reduce or deduce a system from the simplest parts [1]. Thus, the study of the T. cruzi cycle in a single species, the coati, represents a facet of the manifold host-parasite interactions occurring in the study region. Therefore, we further extended these studies to the role of T. cruzi vectors.
Triatomine bugs living in close association with free-ranging coatis have been observed in the Nhecolândia region [20, 21]. Herein, we observed that the majority of these nests sheltered triatomine bugs infected with T. cruzi, suggesting that coatis might be infected early in these nests through two mechanisms, the oral route and the contaminative route.
Furthermore, the coati nests might act as transmission foci in the arboreal stratum, where different T. cruzi populations are dispersed among vectors and different mammal species with arboreal or scansorial behaviors. Triatomine bugs can present habitat restriction and relatively low mobility when living in close association with a mammal species [36, 37], particularly in well-fed populations [38, 39]. However, a nest can be visited by different coati individuals and other mammalian species, including marsupials and rodents, as revealed through precipitin tests of triatomine feces and a camera trap record of a spiny rat (Thrichomys fosteri) visiting an abandoned coati nest [20]. The dynamics inside these shelters enhances the likelihood of T. cruzi dispersion.
We observed T. rangeli infection rate of 7.7 % in the coati population. Natural infections with T. rangeli in vectors have been reported in Brazil [40, 41], primarily in the Amazon region [42, 43]. However, this species was not detected in any of the 34 sampled bugs (Table 4), suggesting a niche for T. rangeli transmission other than the triatomine species inhabiting coati nests.
We observed the selective forces of axenic culture medium and highlighted the importance of DNA extraction directly from the gut content (Table 4). Parasite growth in vitro is an additional intermediate step that might have selected the TcI population to the detriment of Z3, resulting in a non-representative isolate [44]. This issue must be always considered when studying cultured isolates [45, 46]. Therefore, the detection of a particular subpopulation through growth in culture medium does not exclude mixed infections.
We could not define the genotype of T. cruzi isolates from four coatis, as the mini-exon band patterns were not consistent with the results of the RFLP analysis (Fig. 1). Additionally, we observed an isolate (sample 3) presenting multiple bands in the mini-exon target, as previously reported in four coati individuals [15]. These misinterpretations reflect heterogeneous populations with high genetic diversity isolated from free-ranging sylvatic hosts [47, 48], particularly with respect to coatis. This species displays many biological traits (eclectic feeding behavior, long-lived, long distance dispersal and both arboreal and terrestrial strata exploration in different habitats) that enhance the likelihood of exposure to various and heterogeneous T. cruzi subpopulations.
Conclusions
The essential role of coatis in T. cruzi sylvatic cycle was maintained during the 2010–2012 period. However, many biotic and abiotic factors might change the pattern of T. cruzi infection in a given population and the epizootiological profile. Therefore, in the Pantanal region, T. cruzi is transmitted in a complex, multifactorial, dynamic and non-linear transmission web. Coati nests might be inserted in this web, acting as important transmission foci at the arboreal stratum to different mammal species with arboreal or scansorial behaviors.
Ethics statement
This study was approved through the Ethical Commission for Experimentation with Animal Models (CEUA) of Fiocruz (registration number: P-292-06). The capture and sample collection were performed according to the Brazilian Government Institute for Wildlife and Natural Resources Care (IBAMA) regulations (license numbers 25078-2/2010, 28772-1/2011, 38787-1/2013, 38787-2/2014). Appropriate biosecurity techniques and individual protection equipment were used in all procedures for the collection and handling of the biological samples.
Acknowledgments
We are grateful to Marcos Antônio Lima, Carlos Ardé, Maria Augusta Dario, Samanta das Chagas Xavier and Juliana Barros for technical support in the laboratory procedures. The authors would also like to thank Natalie Olifiers, Nilson Lino Xavier Filho, and Magyda Dahrough for assistance with the fieldwork and to Dr. Vera Bongertz for revising the English version.
Funding
This work was financially supported through grants from Fiocruz, Embrapa/Pantanal, and ChagasEpiNet 223034. Postgraduate grants were provided by the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) and the Fundação de Amparo à Pesquisa do Estado do Rio de Janeiro (FAPERJ) to F.M.A., CNPq to J.S.L., and Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (Capes) to F.L.R.
Availability of data and material
The datasets supporting the conclusions of this article are included within the article.
Authors’ contributions
FMA, AMJ, JSL, FLR, HMH and GMM drafted the manuscript, analyzed and interpreted the results. JSL and FLR collected data. FMA processed the samples. All authors have read and approved the final manuscript.
Competing interests
The authors declare that they have no competing interests.
Consent for publication
Not applicable.
Contributor Information
Fernanda Moreira Alves, Email: fernanda.alves@fiocruz.br.
Juliane Saab de Lima, Email: juliane.bioma@gmail.com.
Fabiana Lopes Rocha, Email: fabiana.rocha@triade.org.br.
Heitor Miraglia Herrera, Email: herrera@ucdb.br.
Guilherme de Miranda Mourão, Email: guilherme.mourao@embrapa.br.
Ana Maria Jansen, Email: jansen@ioc.fiocruz.br.
References
- 1.Mazzocchi F. Complexity in biology. Exceeding the limits of reductionism and determinism using complexity theory. EMBO Rep. 2008;9:10–14. doi: 10.1038/sj.embor.7401147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Horwitz P, Wilcox BA. Parasites, ecosystems and sustainability: an ecological and complex systems perspective. Int J Parasitol. 2005;35(7):725–732. doi: 10.1016/j.ijpara.2005.03.002. [DOI] [PubMed] [Google Scholar]
- 3.Chagas C. Nova tripanozomiaze humana. Estudos sobre a morfolojia e o ciclo evolutivo do Schizotrypanum cruzi n. gen, n. sp., ajente etiolojico de nova entidade morbida do homem. Mem Inst Oswaldo Cruz. 1909;1:11–80. doi: 10.1590/S0074-02761909000200008. [DOI] [Google Scholar]
- 4.Gaunt MW, Yeo M, Frame IA, Stothard JR, Carrasco HJ, Taylor MC, Mena SS, Veazey P, Miles GA, Acosta N, de Arias AR, Miles MA. Mechanism of genetic exchange in American trypanosomes. Nature. 2003;421(6926):936–939. doi: 10.1038/nature01438. [DOI] [PubMed] [Google Scholar]
- 5.Ocaña-Mayorga S, Llewellyn MS, Costales JA, Miles MA, Grijalva MJ. Sex, subdivision, and domestic dispersal of Trypanosoma cruzi lineage I in southern Ecuador. PLoS Negl Trop Dis. 2010;4(12) doi: 10.1371/journal.pntd.0000915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zingales B, Andrade SG, Briones MR, Campbell DA, Chiari E, Fernandes O, et al. A new consensus for Trypanosoma cruzi intraspecific nomenclature: second revision meeting recommends TcI to TcVI; Second Satellite Meeting. Mem Inst Oswaldo Cruz. 2009;104(7):1051–1054. doi: 10.1590/S0074-02762009000700021. [DOI] [PubMed] [Google Scholar]
- 7.Araújo A, Jansen AM, Reinhard K, Ferreira LF. Paleoparasitology of Chagas disease - a review. Mem Inst Oswaldo Cruz. 2009;104(1):9–16. doi: 10.1590/S0074-02762009000900004. [DOI] [PubMed] [Google Scholar]
- 8.Noireau F, Diosque P, Jansen AM. Trypanosoma cruzi: adaptation to its vectors and its hosts. Vet Res. 2009;40(2):26. doi: 10.1051/vetres/2009009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Canals M, Cruzat L, Molina MC, Ferreira A, Cattan PE. Blood host sources of Mepraia spinolai (Heteroptera: Reduviidae), wild vector of chagas disease in Chile. J Med Entomol. 2001;38(2):303–307. doi: 10.1603/0022-2585-38.2.303. [DOI] [PubMed] [Google Scholar]
- 10.Pineda V, Montalvo E, Alvarez D, Santamaría AM, Calzada JE, Saldaña A. Feeding sources and trypanosome infection index of Rhodnius pallescens in a Chagas disease endemic area of Amador County, Panama. Rev Inst Med Trop Sao Paulo. 2008;50(2):113–116. doi: 10.1590/S0036-46652008000200009. [DOI] [PubMed] [Google Scholar]
- 11.Ramsey JM, Gutiérrez-Cabrera AE, Salgado-Ramírez L, Peterson AT, Sánchez-Cordero V, Ibarra-Cerdeña CN. Ecological connectivity of Trypanosoma cruzi reservoirs and Triatoma pallidipennis hosts in an anthropogenic landscape with endemic Chagas disease. PLoS ONE. 2012;7(9) doi: 10.1371/journal.pone.0046013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bezerra CM, Cavalcanti LP, Souza RD, Barbosa SE, Xavier SC, Jansen AM, Ramalho RD, Diotaiut L. Domestic, peridomestic and wild hosts in the transmission of Trypanosoma cruzi in the Caatinga area colonised by Triatoma brasiliensis. Mem Inst Oswaldo Cruz. 2014;109(7):887–898. doi: 10.1590/0074-0276140048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Herrera HM, Lisboa CV, Pinho AP, Olifiers N, Bianchi RC, Rocha FL, et al. The coati (Nasua nasua, Carnivora, Procyonidae) as a reservoir host for the main lineages of Trypanosoma cruzi in the Pantanal region, Brazil. Trans R Soc Trop Med Hyg. 2008;102(11):1133–1139. doi: 10.1016/j.trstmh.2008.04.041. [DOI] [PubMed] [Google Scholar]
- 14.Alves FM, Olifiers N, Bianchi R de C, Duarte AC, Cotias PM, D'Andrea PS, et al. Modulating variables of Trypanosoma cruzi and Trypanosoma evansi transmission in free-ranging Coati (Nasua nasua) from the Brazilian Pantanal region. Vector Borne Zoonotic Dis. 2011;11(7):835–41. [DOI] [PubMed]
- 15.Rocha FL, Roque AL, de Lima JS, Cheida CC, Lemos FG, de Azevedo FC, Arrais RC, Bilac D, Herrera HM, Mourão G, Jansen AM. Trypanosoma cruzi infection in neotropical wild carnivores (Mammalia: Carnivora): at the top of the T. cruzi transmission chain. PLoS ONE. 2013;8(7) doi: 10.1371/journal.pone.0067463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gompper ME, Decker DM. Nasua nasua. Mamm Species. 1998;580:1–9. doi: 10.2307/3504444. [DOI] [Google Scholar]
- 17.Bianchi RC, Campos RC, Xavier-Filho NL, Olifiers N, Gompper ME, Mourão GM. Intraspecific, interspecific, and seasonal differences in the diet of three mid-sized carnivores in a large neotropical wetland. Acta Theriol. 2014;59:13–23. doi: 10.1007/s13364-013-0137-x. [DOI] [Google Scholar]
- 18.Emmons L, Feer F. Neotropical rainforest mammals: a field guide. 2. Chicago: The University of Chicago Press; 1997. [Google Scholar]
- 19.Olifiers N, Bianchi RC, Mourão GM, Gompper ME. Construction of arboreal nests by brown-nosed coatis, Nasua nasua (Carnivora: Procyonidae) in the Brazilian Pantanal. Zooologia. 2009;26(3):571–574. doi: 10.1590/S1984-46702009000300023. [DOI] [Google Scholar]
- 20.de Lima JS, Rocha FL, Alves FM, Lorosa ES, Jansen AM, Mourão GM. Infestation of arboreal nests of coatis by triatomine species, vectors of Trypanosoma cruzi, in a large Neotropical wetland. J Vector Ecology. 2015;40:2. doi: 10.1111/jvec.12177. [DOI] [PubMed] [Google Scholar]
- 21.Santos FM, Jansen AM, Mourão GM, Jurberg J, Nunes AP, Herrera HM. Triatominae (Hemiptera, Reduviidae) in the Pantanal region: association with Trypanosoma cruzi and different habitats and vertebrate hosts. Rev Soc Bras Med Trop. 2015;48:5. doi: 10.1590/0037-8682-0184-2015. [DOI] [PubMed] [Google Scholar]
- 22.Rodela LG. Unidades de vegetação e pastagens nativas do Pantanal da Nhecolândia, Mato Grosso do Sul [dissertation] São Paulo: Universidade de São Paulo; 2006. p. 2006. [Google Scholar]
- 23.Soriano BMA. Simposósio sobre recursos naturais e sócio-econômicos do Pantanal, 2, 1996. Corumbá: Embrapa Pantanal: Corumbá, MS. Manejo e conservação: anais; 1999. Caracterização climática da sub-região da Nhecolândia, Pantanal-MS; pp. 151–158. [Google Scholar]
- 24.Alho CJR, Silva JSV. Effects of severe floods and droughts on wildlife of the Pantanal Wetland (Brazil) - A review. Animals. 2012;2:591–610. doi: 10.3390/ani2040591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Alho CJR. Biodiversity of the Pantanal: response to seasonal flooding regime and to environmental degradation. Braz J Bio. 2008;68(4):957–966. doi: 10.1590/S1519-69842008000500005. [DOI] [PubMed] [Google Scholar]
- 26.Mourão G, Calheiros DF, Oliveira MD, Padovani C, Fischer E, Tomas W, Campos Z. Respostas ecológicas de longo prazo a variações plurianuais das enchentes no Pantanal. In: Tabarelli M, Rocha CFD, Romanowski HP, Rocha O, Lacerda LD, editors. PELD-CNPq: Dez anos do programa de pesquisas ecológicas de longa duração no Brasil: achados, lições e perspectivas. Recife: Ed. Universitária da UFPE; 2013. pp. 89–116. [Google Scholar]
- 27.Olifiers N, Bianchi RC, D’Andrea PS, Mourão GM, Gompper ME. Estimating age of carnivores from the Pantanal region of Brazil. Wildlife Biol. 2010;16:389–399. doi: 10.2981/09-104. [DOI] [Google Scholar]
- 28.Alves FM. A complexidade, multifatoriedade e não linearidade da rede de transmissão do Trypanosoma cruzi (Trypanosomatida: Trypanosomatidae) em quatis (Carnivora: Procyonidae: Nasua nasua) de vida livre do Pantanal/MS, um estudo longitudinal [dissertation] Rio de Janeiro: Oswaldo Cruz Foundation; 2013. [Google Scholar]
- 29.Sambrook J, Fritsch EF, Maniatis T. Molecular Cloning: A Laboratory Manual. New York: Cold Spring Harbor Press; 1989. [Google Scholar]
- 30.Fernandes O, Santos SS, Cupolillo E, Mendonça B, Derre R, Junqueira ACV, Santos LC, Sturm NR, Naiff RD, Barrett TB, Campbell D, Coura JR. A mini-exon multiplex polymerase chain reaction to distinguish the major groups of Trypanosoma cruzi and T. rangeli in the Brazilian Amazon. Trans R Soc Trop Med Hyg. 2001;95(1):97–99. doi: 10.1016/S0035-9203(01)90350-5. [DOI] [PubMed] [Google Scholar]
- 31.Aliaga C, Brenière SF, Barnabé C. Further interest of miniexon multiplex PCR for a rapid typing of Trypanosoma cruzi DTU groups. Infect Genet Evol. 2011;11(5):1155–1158. doi: 10.1016/j.meegid.2010.11.013. [DOI] [PubMed] [Google Scholar]
- 32.Rozas M, De Doncker S, Adaui V, Coronado X, Barnabé C, Tibyarenc M, Solari A, Dujardin JC. Multilocus polymerase chain reaction restriction fragment-length polymorphism genotyping of Trypanosoma cruzi (Chagas disease): taxonomic and clinical applications. J Infect Dis. 2007;195:1381–1388. doi: 10.1086/513440. [DOI] [PubMed] [Google Scholar]
- 33.Westenberger SJ, Barnabé C, Campbell DA, Sturm NR. Two hybridization events define the population structure of Trypanosoma cruzi. Genetics. 2005;171(2):527–543. doi: 10.1534/genetics.104.038745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kribs-Zaleta C. Estimating contact process saturation in sylvatic transmission of Trypanosoma cruzi in the United States. PLoS Negl Trop Dis. 2010;4(4) doi: 10.1371/journal.pntd.0000656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vale PF, Wilson AJ, Best A, Boots M, Little TJ. Epidemiological, evolutionary, and coevolutionary implications of context-dependent parasitism. Am Nat. 2011;177(4):510–521. doi: 10.1086/659002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Costa J, Lorenzo M. Biology, diversity and strategies for the monitoring and control of triatomines - Chagas disease vectors. Mem Inst Oswaldo Cruz. 2009;104:46–51. doi: 10.1590/s0074-02762009000900008. [DOI] [PubMed] [Google Scholar]
- 37.Noireau F, Dujardin JP. Biology of Triatominae. In: Telleira J, Tibayrenc M, editors. American Trypanosomiasis. Chagas Disease One Hundred Years of Research. New York: Elsevier; 2010. pp. 149–168. [Google Scholar]
- 38.Patterson J. The effect of larval nutrition on egg production in Rhodnius prolixus. J Insect Physiol. 1979;25:311–314. doi: 10.1016/0022-1910(79)90018-0. [DOI] [Google Scholar]
- 39.Schofield CJ. Triatominae: biology and control. West Sussex: Eurocommunica Publications; 1994. [Google Scholar]
- 40.Lucena DT, Vergetti JG. Infecção natural de Panstrongylus megistus (Burmeister, 1835) por Trypanosoma rangeli (Tejera, 1920) no interior do estado de Alagoas. Rev Inst Med Trop Sao Paulo. 1973;15(4):171–178. [PubMed] [Google Scholar]
- 41.Steindel M, Dias Neto E, Pinto CJ, Grisard EC, Menezes CL, Murta SM, et al. Randomly amplified polymorphic DNA (RAPD) and isoenzyme analysis of Trypanosoma rangeli strains. J Eukaryot Microbiol. 1994;41(3):261–267. doi: 10.1111/j.1550-7408.1994.tb01506.x. [DOI] [PubMed] [Google Scholar]
- 42.Valente SA, Da Costa Valente V, Das Neves Pinto AY, De Jesus Barbosa César M, Dos Santos MP, Miranda CO, et al. Analysis of an acute Chagas disease outbreak in the Brazilian Amazon: human cases, triatomines, reservoir mammals and parasites. Trans R Soc Trop Med Hyg. 2008;103(3):291–297. doi: 10.1016/j.trstmh.2008.10.047. [DOI] [PubMed] [Google Scholar]
- 43.Dias FB, Quartier M, Diotaiuti L, Mejía G, Harry M, Lima AC, et al. Ecology of Rhodniusrobustus Larrousse, 1927 (Hemiptera, Reduviidae, Triatominae) in Attalea palm trees of the Tapajós River Region (Pará State, Brazilian Amazon) Parasit Vectors. 2014;7:154. doi: 10.1186/1756-3305-7-154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Deane MP, Jansen AM, Mangia RHR, Gonçalves AM, Morel CM. Are our laboratory “strains” representative samples of Trypanosoma cruzi populations that circulate in nature? Mem Inst Oswaldo Cruz. 1984;79:19–24. doi: 10.1590/S0074-02761984000500006. [DOI] [Google Scholar]
- 45.Bosseno MF, Yacsik N, Vargas F, Brenière SF. Selection of Trypanosoma cruzi clonal genotypes (clonet 20 and 39) isolated from Bolivian triatomines following subculture in liquid medium. Mem Inst Oswaldo Cruz. 2000;95(5):601–607. doi: 10.1590/S0074-02762000000500002. [DOI] [PubMed] [Google Scholar]
- 46.Ortiz S, Zulantay I, Apt W, Saavedra M, Solari A. Transferability of Trypanosoma cruzi from mixed human host infection to Triatoma infestans and from insects to axenic culture. Parasitol Int. 2014;64(1):33–36. doi: 10.1016/j.parint.2014.09.005. [DOI] [PubMed] [Google Scholar]
- 47.Llewellyn MS, Rivett-Carnac JB, Fitzpatrick S, Lewis MD, Yeo M, Gaunt MW, et al. Extraordinary Trypanosoma cruzi diversity within single mammalian reservoir hosts implies a mechanism of diversifying selection. Int J Parasitol. 2011;41(6):609–614. doi: 10.1016/j.ijpara.2010.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lima VS, Jansen AM, Messenger LA, Miles MA, Llewellyn MS. Wild Trypanosoma cruzi I genetic diversity in Brazil suggests admixture and disturbance in parasite populations from the Atlantic Forest region. Parasit Vectors. 2014;7:263. doi: 10.1186/1756-3305-7-263. [DOI] [PMC free article] [PubMed] [Google Scholar]


