Abstract
The murine Mls-1 antigen is the prototype of endogenous superantigens, molecules whose activities lead to deletion of T cells expressing certain T-cell receptor V beta genes from the mature repertoire. However, Mls-1 also stimulates T cells expressing these particular V beta genes (V beta 6, V beta 7, V beta 8.1, and V beta 9) in vitro, making it one of the strongest known T-cell activators. We have recently reported that the Mls-1 gene is closely linked to the endogenous mammary tumor virus Mtv-7. We now demonstrate that Mls-1 is encoded by the open reading frame in the U3 region of the long terminal repeat of Mtv-7. However, control of expression of this molecule seems complex, depending on the promoter used for the transfection experiments. The sequence of the Mtv-7 open reading frame differs from all other known mammary tumor virus open reading frame sequences in the 3' end, suggesting that the T-cell receptor V beta specificity is conferred by the C terminus of the molecule. The predicted structure of the protein encoded by the open reading frame is consistent with a type II transmembrane molecule where the C terminus is extracellular.
Full text
PDF
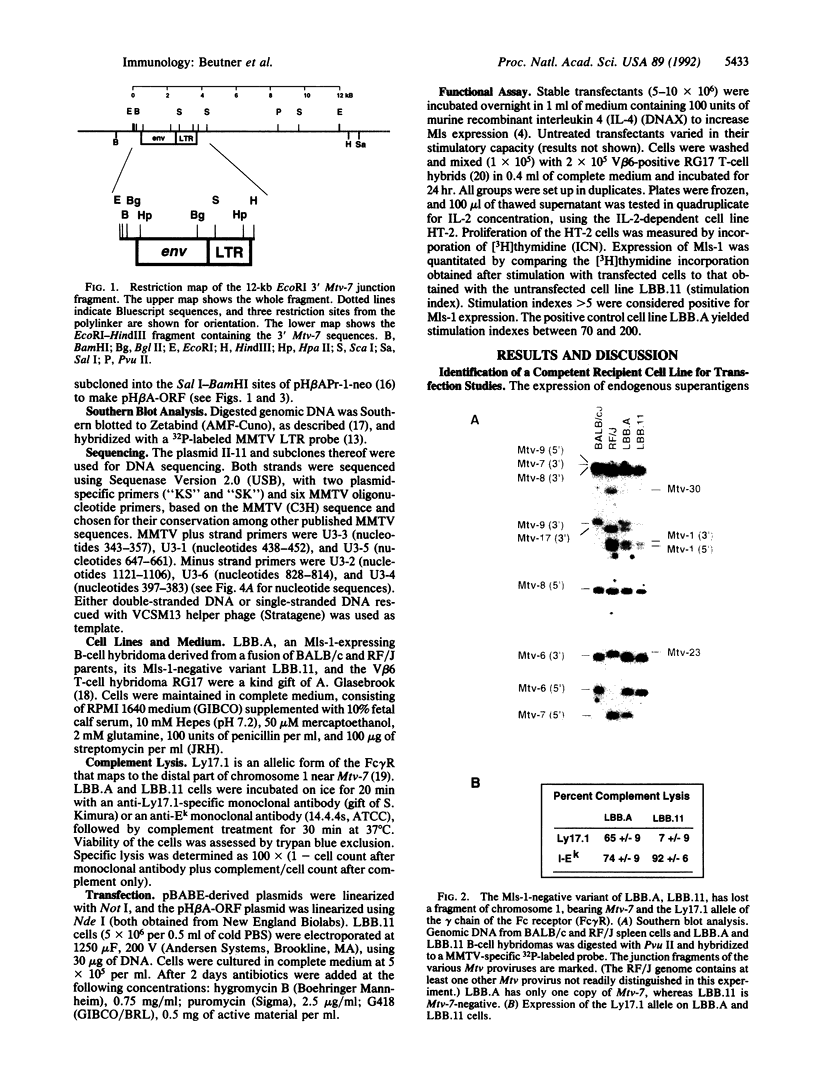

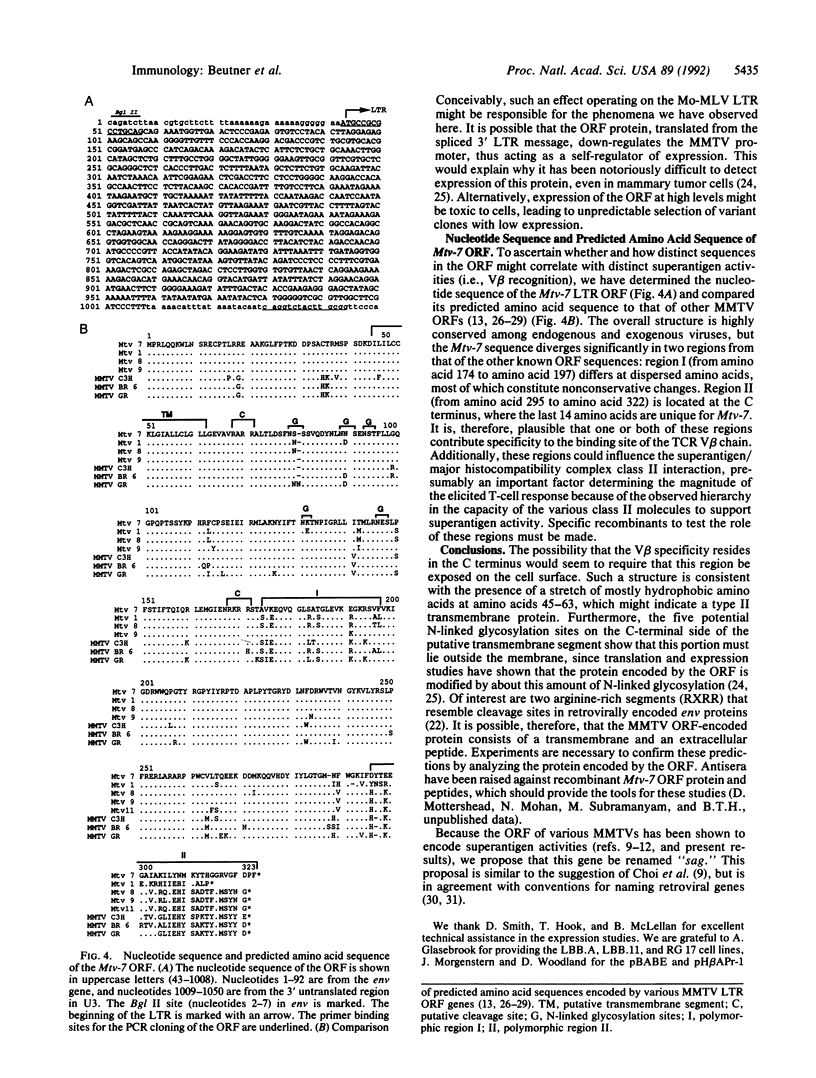

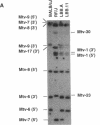
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Acha-Orbea H., Shakhov A. N., Scarpellino L., Kolb E., Müller V., Vessaz-Shaw A., Fuchs R., Blöchlinger K., Rollini P., Billotte J. Clonal deletion of V beta 14-bearing T cells in mice transgenic for mammary tumour virus. Nature. 1991 Mar 21;350(6315):207–211. doi: 10.1038/350207a0. [DOI] [PubMed] [Google Scholar]
- Brandt-Carlson C., Butel J. S. Detection and characterization of a glycoprotein encoded by the mouse mammary tumor virus long terminal repeat gene. J Virol. 1991 Nov;65(11):6051–6060. doi: 10.1128/jvi.65.11.6051-6060.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi Y., Kappler J. W., Marrack P. A superantigen encoded in the open reading frame of the 3' long terminal repeat of mouse mammary tumour virus. Nature. 1991 Mar 21;350(6315):203–207. doi: 10.1038/350203a0. [DOI] [PubMed] [Google Scholar]
- Coffin J. M., Varmus H. E., Bishop J. M., Essex M., Hardy W. D., Jr, Martin G. S., Rosenberg N. E., Scolnick E. M., Weinberg R. A., Vogt P. K. Proposal for naming host cell-derived inserts in retrovirus genomes. J Virol. 1981 Dec;40(3):953–957. doi: 10.1128/jvi.40.3.953-957.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crouse C. A., Pauley R. J. Molecular cloning and sequencing of the MTV-1 LTR: evidence for a LTR sequence alteration. Virus Res. 1989 Feb;12(2):123–137. doi: 10.1016/0168-1702(89)90059-2. [DOI] [PubMed] [Google Scholar]
- Donehower L. A., Fleurdelys B., Hager G. L. Further evidence for the protein coding potential of the mouse mammary tumor virus long terminal repeat: nucleotide sequence of an endogenous proviral long terminal repeat. J Virol. 1983 Mar;45(3):941–949. doi: 10.1128/jvi.45.3.941-949.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donehower L. A., Huang A. L., Hager G. L. Regulatory and coding potential of the mouse mammary tumor virus long terminal redundancy. J Virol. 1981 Jan;37(1):226–238. doi: 10.1128/jvi.37.1.226-238.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dyson P. J., Knight A. M., Fairchild S., Simpson E., Tomonari K. Genes encoding ligands for deletion of V beta 11 T cells cosegregate with mammary tumour virus genomes. Nature. 1991 Feb 7;349(6309):531–532. doi: 10.1038/349531a0. [DOI] [PubMed] [Google Scholar]
- Eicher E. M., Lee B. K. The NXSM recombinant inbred strains of mice: genetic profile for 58 loci including the Mtv proviral loci. Genetics. 1990 Jun;125(2):431–446. doi: 10.1093/genetics/125.2.431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Festenstein H. Immunogenetic and biological aspects of in vitro lymphocyte allotransformation (MLR) in the mouse. Transplant Rev. 1973;15:62–88. doi: 10.1111/j.1600-065x.1973.tb00111.x. [DOI] [PubMed] [Google Scholar]
- Frankel W. N., Rudy C., Coffin J. M., Huber B. T. Linkage of Mls genes to endogenous mammary tumour viruses of inbred mice. Nature. 1991 Feb 7;349(6309):526–528. doi: 10.1038/349526a0. [DOI] [PubMed] [Google Scholar]
- Frankel W. N., Stoye J. P., Taylor B. A., Coffin J. M. A linkage map of endogenous murine leukemia proviruses. Genetics. 1990 Feb;124(2):221–236. doi: 10.1093/genetics/124.2.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunning P., Leavitt J., Muscat G., Ng S. Y., Kedes L. A human beta-actin expression vector system directs high-level accumulation of antisense transcripts. Proc Natl Acad Sci U S A. 1987 Jul;84(14):4831–4835. doi: 10.1073/pnas.84.14.4831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes K. L., Palfree R. G., Hammerling U., Morse H. C., 3rd Alleles of the Ly-17 alloantigen define polymorphisms of the murine IgG Fc receptor. Proc Natl Acad Sci U S A. 1985 Nov;82(22):7706–7710. doi: 10.1073/pnas.82.22.7706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kappler J. W., Staerz U., White J., Marrack P. C. Self-tolerance eliminates T cells specific for Mls-modified products of the major histocompatibility complex. Nature. 1988 Mar 3;332(6159):35–40. doi: 10.1038/332035a0. [DOI] [PubMed] [Google Scholar]
- King L. B., Lund F. E., White D. A., Sharma S., Corley R. B. Molecular events in B lymphocyte differentiation. Inducible expression of the endogenous mouse mammary tumor proviral gene, Mtv-9. J Immunol. 1990 Apr 15;144(8):3218–3227. [PubMed] [Google Scholar]
- MacDonald H. R., Schneider R., Lees R. K., Howe R. C., Acha-Orbea H., Festenstein H., Zinkernagel R. M., Hengartner H. T-cell receptor V beta use predicts reactivity and tolerance to Mlsa-encoded antigens. Nature. 1988 Mar 3;332(6159):40–45. doi: 10.1038/332040a0. [DOI] [PubMed] [Google Scholar]
- Majors J. E., Varmus H. E. Nucleotide sequencing of an apparent proviral copy of env mRNA defines determinants of expression of the mouse mammary tumor virus env gene. J Virol. 1983 Sep;47(3):495–504. doi: 10.1128/jvi.47.3.495-504.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marrack P., Kushnir E., Kappler J. A maternally inherited superantigen encoded by a mammary tumour virus. Nature. 1991 Feb 7;349(6309):524–526. doi: 10.1038/349524a0. [DOI] [PubMed] [Google Scholar]
- Molina I. J., Cannon N. A., Hyman R., Huber B. T. Macrophages and T cells do not express Mlsa determinants. J Immunol. 1989 Jul 1;143(1):39–44. [PubMed] [Google Scholar]
- Morgenstern J. P., Land H. Advanced mammalian gene transfer: high titre retroviral vectors with multiple drug selection markers and a complementary helper-free packaging cell line. Nucleic Acids Res. 1990 Jun 25;18(12):3587–3596. doi: 10.1093/nar/18.12.3587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicolas J. F., Wegmann D., Lebrun P., Kaiserlian D., Tovey J., Glasebrook A. L. Relationship of B cell Fc receptors to T cell recognition of Mls antigen. Eur J Immunol. 1987 Nov;17(11):1561–1565. doi: 10.1002/eji.1830171106. [DOI] [PubMed] [Google Scholar]
- Pullen A. M., Choi Y., Kushnir E., Kappler J., Marrack P. The open reading frames in the 3' long terminal repeats of several mouse mammary tumor virus integrants encode V beta 3-specific superantigens. J Exp Med. 1992 Jan 1;175(1):41–47. doi: 10.1084/jem.175.1.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Racevskis J., Prakash O. Proteins encoded by the long terminal repeat region of mouse mammary tumor virus: identification by hybrid-selected translation. J Virol. 1984 Sep;51(3):604–610. doi: 10.1128/jvi.51.3.604-610.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salmons B., Erfle V., Brem G., Günzburg W. H. naf, a trans-regulating negative-acting factor encoded within the mouse mammary tumor virus open reading frame region. J Virol. 1990 Dec;64(12):6355–6359. doi: 10.1128/jvi.64.12.6355-6359.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodland D. L., Happ M. P., Gollob K. J., Palmer E. An endogenous retrovirus mediating deletion of alpha beta T cells? Nature. 1991 Feb 7;349(6309):529–530. doi: 10.1038/349529a0. [DOI] [PubMed] [Google Scholar]
- Woodland D. L., Lund F. E., Happ M. P., Blackman M. A., Palmer E., Corley R. B. Endogenous superantigen expression is controlled by mouse mammary tumor proviral loci. J Exp Med. 1991 Nov 1;174(5):1255–1258. doi: 10.1084/jem.174.5.1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodland D., Happ M. P., Bill J., Palmer E. Requirement for cotolerogenic gene products in the clonal deletion of I-E reactive T cells. Science. 1990 Feb 23;247(4945):964–967. doi: 10.1126/science.1968289. [DOI] [PubMed] [Google Scholar]
- von Boehmer H., Sprent J. Expression of M locus differences by B cells but not T cells. Nature. 1974 May 24;249(455):363–365. doi: 10.1038/249363a0. [DOI] [PubMed] [Google Scholar]