Abstract
Background
Toscana virus (TOSV) is an arbovirus belonging to the Bunyaviridae, a family of negative-stranded, enveloped RNA viruses. The virus can be transmitted to humans through the bite of an infected female sand fly of the genus Phlebotomus. Infections are usually asymptomatic but the virus is known to cause aseptic meningitis and/or meningo-encephalitis in the Mediterranean countries. Dogs are good sentinels for detection of viral circulation and are more easily accessible than wild animals.
Findings
In 2013 and 2014, we collected sera from 231 adult dogs living in 26 counties in two departments in Corsica, a French island in the Mediterranean. The virus microneutralization-based seroprevalence assay revealed a seropositivity of 3.9 % dogs on the eastern coast of Corsica.
Conclusions
Our study confirms the circulation of TOSV in Corsica. Accordingly, in geographical areas where dogs possess TOSV neutralizing antibodies, direct and indirect TOSV diagnosis should be implemented in patients presenting with febrile illnesses and central nervous system infections such as meningitis and encephalitis.
Keywords: Toscana virus, Dog, Corsica, France, Sand fly, Phlebotomus, Meningitis
Background
Toscana virus (TOSV) is an arbovirus belonging to the Bunyaviridae, a family of negative-stranded, enveloped RNA viruses. The virus can be transmitted to humans through the bite of an infected female sand fly of the genus Phlebotomus. The infection has previously been reported in countries located on the northern shores of the Mediterranean Sea (Italy, Croatia, France, Greece, Portugal and Spain), as well as in the east (Cyprus and Turkey) [1] and, recently, from North Africa (Morocco, Tunisia and Algeria) [2, 3]. Although it is believed that asymptomatic infections are frequent, TOSV is an important cause of aseptic meningitis and meningo-encephalitis, during the warm season (April to October) when sand flies are active [1]. In France, the first case of Toscana virus infection was reported in a German tourist returning from the region of Marseille, south-eastern France [4]. Since then, in France several autochthonous cases of TOSV infection have been described causing either meningitis [5, 6] or encephalitis [7]. Furthermore, myositis was reported as an additional clinical complication of TOSV virus [8].
In Corsica, the seroprevalence among human blood donors was 8.7 % in 2007 [9]. The presence of TOSV in P. perniciosus sand flies was revealed using PCR targeting the L-RNA segment [10]. Genetic analysis showed that Corsican TOSV belongs to lineage A [10]. Dogs and humans live in close contact and are both bitten by sand flies [11], the main transmission vectors of dog and human leishmaniasis. In Corsica, dogs are exposed to the bites of sand flies as evidenced by the rate of incidence of canine leishmaniasis [12]. Consequently, the aim of our study was to evaluate the TOSV seroprevalence in dogs in Corsica, a French island in the Mediterranean.
Methods
In 2013 and 2014, we collected sera from the radial veins of 231 adult dogs. The dogs were living in 26 communes in the two departments of Corsica (Fig. 1): in Haute-Corse [Aleria (n = 8), Biguglia (n = 4), Castirla (n = 7), Corte (n = 8), Erbajolo (n = 7), Favalello (n = 17), Ghisonaccia (n = 16), Mauracciole (n = 2), Riventosa (n = 4), Rogliano (n = 3), San’Andréa-di-Bozio (n = 15), Sermano (n = 4), Tavera (n = 12), Tomino (n = 4), Tralonca (n = 9), Ucciani (n = 15), Venaco (n = 11), Ventiseri (n = 20), Vico (n = 6) and Vivario (n = 9)] and in Corse-du-Sud [Afa (n = 7), Albertacce (n = 17), Altiani (n = 13), Brognano (n = 9), Casanova (n = 3) and Lecci (n = 1)]. Among the 231 dogs sampled, 147 were male (63.6 %), and 84 were female (36.4 %). The average age of dogs was four year-old (3–12 year-old) and most were wearing collars to reduce the likelihood of arthropod infestation. The dogs came from a range of sources including hunters’ dogs, shepherds’ dogs, military working dogs and some pet dogs. They all appeared to be in good health at the time of sampling and were examined with the assistance of their owners. Blood samples were centrifuged within 24 h of collection and the sera were subsequently frozen at -20 °C until being processed in the laboratory.
Fig. 1.
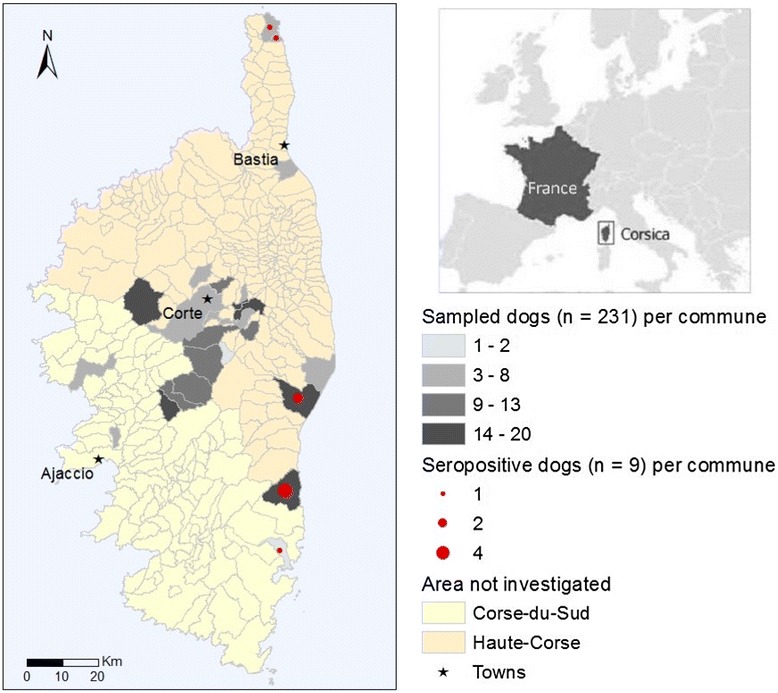
Map of Corsica showing the areas where dogs were sampled and where positive dogs were found
The seroprevalence study based on virus microneutralization (MN) was adapted from the previously described protocol [3]. The MN assay was performed in 96-well microtitre plates using Vero cells. In short, two-fold serial dilutions of 50 μl-serum aliquots were mixed with an equal volume of 1,000 TCID50 (Tissue Culture Infective Dose producing pathological change in 50 % of the cell culture inoculated) of TOSV (strain MRS2010-4319501) into 96-well plates, providing two-fold final dilutions between 1:20 and 1:160. Controls consisted of each serum (1:10) with Vero cells but without virus. After five days, the microplates were analysed under an inverted microscope, and the presence (neutralization titre at 10, 20, 40, 80 and 160) or absence (no neutralization) of cytopathic effect was noted. Cut-off titre for positivity was 20 [3, 13].
Results and discussion
The virus microneutralization-based assay is the most discriminative serological test adapted to differentiate the affinity of antibodies against different closely related viruses [14]. Of the 231 dogs, nine (eight males and one female) were seropositive (3.9 %) for TOSV antibodies. The seropositive dogs were all from five communes in the Haute-Corse [Tomino 1 positive/4 tested, Rogliano 1/3, Ghisonaccia 2/16, Ventiseri 4/20] and one from the Corse-du-Sud [Lecci 1/1] (Table 1). Seropositive dogs were all from the eastern coast of Corsica. This region has an average altitude of 37 m, while all the dogs from the central region were negative, corresponding to a region with a medium altitude of 576 m. The positive dogs were aged between three and 12 year-old (Table 1). The serological titres were low: 1/20 (n = 5) and 1/40 (n = 4), which was expected when a virus challenge dose of 1000TCID50 was used, thus rendering the assay more stringent compared with the commonly used 100TCID50; previous studies have shown a good correlation between both protocols [3]. The results from two TOSV neutralization-based seroprevalence studies reported in Tunisia were comparable with the results of the present study. In Kairouan, 5.6 % (11/147) of the seropositive dogs were living in an area with a high density of sand flies and where leishmaniasis is endemic [2]. In Bizerte, where leishmaniasis cases are uncommon, none of the 118 tested dogs was TOSV seropositive [2]. In Algeria (Kabylia), one serological survey showed 4.3 % positive sera among 93 dogs [15]. In contrast, the results reported from Turkey were much higher. The TOSV MN seroprevalence reported in southern Anatolia was 40.4 % (21/52); of these dogs, 15.5 % (24/155) were TOSV viraemic. In addition, two dogs were co-infected with Leishmania infantum [16]. In Granada (Spain), 48.3 % (138/286) of the dogs tested were positive for TOSV using indirect immunofluorescence (IFAT) [17]. However, this result should be considered with caution due to the possibility of cross-reactivity with other phleboviruses such as Granada virus, Massilia virus, Naples virus or Tehran virus [3]. This is the reason why we decided to use neutralization assay which is the most discriminative serological technique [18] and which is not hampered by cross-reactions due to antibodies elicited by sand fly-borne phleboviruses either from other antigenic groups (Sandfly fever Sicilian and Salehabad viruses) or from related viruses belonging to the Sandfly fever Naples species distinct from TOSV.
Table 1.
Location, age and serological titres (microneutralization) of Toscana virus seropositive dogs
| Departement | Commune | Localisation | Age of dog | Titer |
|---|---|---|---|---|
| Haute-Corse | Tomino | 42°56'44"N, 9°26'28"E | 12 | 40 |
| Rogliano | 42°57'25"N, 9°25'08"E | 6 | 20 | |
| Ghisonaccia | 42°00'59''N, 9°24'18''E | 4 | 20 | |
| 9 | 40 | |||
| Ventiseri | 41°55'36''N, 9°24'19''E | 3 | 20 | |
| 9 | 20 | |||
| 9 | 40 | |||
| 9 | 20 | |||
| Corse du Sud | Lecci | 41°40'48"N, 9°19'05"E | 10 | 40 |
The seroprevalence of TOSV found in dogs in this study was fairly low. Historical records describe P. mascitii, P. perniciosus and Sergentomyia minuta on the whole island at altitudes lower than 800 m, with the latter two sand fly species being largely dominant. Phlebotomus mascitii has been described only in the north-eastern region. Phlebotomus papatasi, P. ariasi and P. perfiliewi were never reported [19]. Thus, the absence of seropositive dogs from the centre of Corsica is probably best explained by the fact that sand flies are rare in this mountainous region that seems to be colder than the flat coastal region. Furthermore, the use of insecticide collars for the prevention of leishmaniasis has probably reduced the number of infected dogs by decreasing the sand fly bites [20]. Our results suggest that the question of whether dogs serve as a reservoir for the Toscana virus remains unanswered and merits further environmental and experimental studies. Despite the lack of TOSV serological studies using neutralization tests in Corsican human populations, the results presented here, together with the direct evidence of TOSV in sand flies and high ELISA-based seroprevalence in blood donors [21], suggest that dogs are good sentinels for serosurvey studies of the type discussed. Our study contributes to the identification of Corsican areas where the virus currently circulates, and shows that the east coast is subject to substantial viral circulation. Accordingly, geographic areas where dogs possess TOSV neutralizing antibodies are good candidates for implementing direct and indirect TOSV diagnosis in patients presenting with febrile illnesses and central nervous system infections such as meningitis and encephalitis.
Conclusions
Our study indicates that dogs are promising sentinels for exposure to TOSV transmission by sand flies in the Mediterranean region. However, the potential of dogs as reservoirs of Toscana virus is yet unknown. Our data extend the knowledge of the distribution of this virus in Corsica, indicating higher prevalence of Toscana virus along the eastern coasts of Corsica. In geographic areas where dogs possess TOSV neutralizing antibodies, direct and indirect TOSV diagnosis should be implemented in patients presenting with febrile illnesses and central nervous system infections such as meningitis and encephalitis.
Abbreviations
ELISA, enzyme-linked immunosorbent assay; MN, microneutralization; TCID50, tissue culture infective dose producing pathological change in 50 % of the cell culture inoculated; TOSV, Toscana virus
Acknowledgements
This work was supported in part by the European Virus Archive goes Global (EVAg) project that has received funding from the European Union’s Horizon 2020 research and innovation programme under grant agreement No. 653316, the EDENext FP7 – No. 261504 EU project and this paper is catalogued by the EDENext Steering Committee as EDENext445 (http://www.edenext.eu). The work of RNC was done under the frame of the EurNegVec COST Action TD1303. This study was also supported by the AMIDEX project (No. ANR-11-IDEX-0001-02) funded by the “Investissements d’Avenir” French Government programme, managed by the French National Research Agency (ANR) and Foundation “Méditerranée Infection” (www.mediterranee-infection.com). The funders had no role in the study design, data collection and analyses, decision to publish, or preparation of the manuscript. We thank Thierry Segalen, Bernard Fabrizy and Sandrine Ferrandi for their help.
Availability of data and material
The data supporting the conclusions of this article are included within the article.
Authors’ contributions
RNC and BD designed the study. SA participated in serological testing. MD, BD, JLM and SGA collected samples and clinical data. MD, BD, SGA and RNC contributed to writing of the manuscript. All authors read and approved the final manuscript.
Competing interests
The authors declare that they have no competing interests.
Consent for publication
Not applicable.
Ethics approval and consent to participate
All dog-owners are consent for the sampling of their dogs, sampling was carried out by a veterinary doctor (Dr B. Davoust) with a broad experience in the epidemiology of infectious diseases in pet animals.
Contributor Information
Mustapha Dahmani, Email: mus.dahmani@gmail.com.
Sulaf Alwassouf, Email: sulaf_alwassouf@yahoo.com.
Sébastien Grech-Angelini, Email: sebastien.grech-angelini@corse.inra.fr.
Jean-Lou Marié, Email: jean-lou.marie@wanadoo.fr.
Bernard Davoust, Email: bernard.davoust@gmail.com.
Rémi N. Charrel, Email: remi.charrel@univ-amu.fr
References
- 1.Charrel RN, Bichaud L, de Lamballerie X. Emergence of Toscana virus in the mediterranean area. World J Virol. 2012;1(5):135–41. doi: 10.5501/wjv.v1.i5.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sakhria S, Alwassouf S, Fares W, Bichaud L, Dachraoui K, Alkan C, et al. Presence of sandfly-borne phleboviruses of two antigenic complexes (Sandfly fever Naples virus and Sandfly fever Sicilian virus) in two different bio-geographical regions of Tunisia demonstrated by a microneutralisation-based seroprevalence study in dogs. Parasit Vectors. 2014;7:476. doi: 10.1186/s13071-014-0476-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alkan C, Allal-Ikhlef A, Alwassouf S, Baklouti A, Piorkowski G, de Lamballerie X, et al. Virus isolation, genetic characterization, and seroprevalence of Toscana virus in Algeria. Clin Microbiol Infect. 2015;21(11):1040. doi: 10.1016/j.cmi.2015.07.012. [DOI] [PubMed] [Google Scholar]
- 4.Dobler G, Treibl J, Haass A, Frosner G, Woesner R, Schimrigk K. Toscana virus infection in German travellers returning from the Mediterranean. Infection. 1997;25:325. doi: 10.1007/BF01720413. [DOI] [PubMed] [Google Scholar]
- 5.Nougairede A, Bichaud L, Thiberville SD, Ninove L, Zandotti C, de Lamballerie X, et al. Isolation of Toscana virus from the cerebrospinal fluid of a man with meningitis in Marseille, France, 2010. Vector Borne Zoonotic Dis. 2013;13(9):685–8. doi: 10.1089/vbz.2013.1316. [DOI] [PubMed] [Google Scholar]
- 6.Hemmersbach-Miller M, Parola P, Charrel RN, Durand JP, Brouqui P. Sandfly fever due to Toscana virus: an emerging infection in southern France. Eur J Intern Med. 2004;15:316–7. doi: 10.1016/j.ejim.2004.05.006. [DOI] [PubMed] [Google Scholar]
- 7.Marlinge M, Crespy L, Zandotti C, Piorkowski G, Kaphan E, Charrel RN, et al. A febrile meningoencephalitis with transient central facial paralysis due to Toscana virus infection, southeastern France, 2014. Euro Surveill. 2014;19(48):20974. doi: 10.2807/1560-7917.ES2014.19.48.20974. [DOI] [PubMed] [Google Scholar]
- 8.Mosnier E, Charrel R, Vidal B, Ninove L, Schleinitz N, Harlé JR, et al. Toscana virus myositis and fasciitis. Med Mal Infect. 2013;43(5):208–10. doi: 10.1016/j.medmal.2013.04.002. [DOI] [PubMed] [Google Scholar]
- 9.de Lamballerie X, Tolou H, Durand JP, Charrel RN. Prevalence of Toscana virus antibodies in volunteer blood donors and patients with central nervous system infections in southeastern France. Vector Borne Zoonotic Dis. 2007;7(2):275–7. doi: 10.1089/vbz.2006.0637. [DOI] [PubMed] [Google Scholar]
- 10.Bichaud L, Izri A, de Lamballerie X, Moureau G, Charrel RN. First detection of Toscana virus in Corsica. France Clin Microbiol Infect. 2014;20(2):O101–4. doi: 10.1111/1469-0691.12347. [DOI] [PubMed] [Google Scholar]
- 11.Cotteaux-Lautard C, Leparc-Goffart I, Berenger JM, Plumet S, Pages F. Phenology and host preferences Phlebotomus perniciosus (Diptera: Phlebotominae) in a focus of Toscana virus (TOSV) in South of France. Acta Trop. 2016;153:64–9. doi: 10.1016/j.actatropica.2015.09.020. [DOI] [PubMed] [Google Scholar]
- 12.Aoun O, Mary C, Roqueplo C, Marié JL, Terrier O, Levieuge A, et al. Canine leishmaniasis in south-east of France: screening of Leishmania infantum antibodies (western blotting, ELISA) and parasitaemia levels by PCR quantification. Vet Parasitol. 2009;166(1-2):27–31. doi: 10.1016/j.vetpar.2009.08.006. [DOI] [PubMed] [Google Scholar]
- 13.Alkan C, Alwassouf S, Piorkowski G, Bichaud L, Tezcan S, Dincer E, et al. Isolation, genetic characterization and seroprevalence of Adana virus a novel phlebovirus belonging to the Salehabad virus complex in Turkey. J Virol. 2015;89(8):4080–91. doi: 10.1128/JVI.03027-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cusi MG, Savellini GG. Diagnostic tools for Toscana virus infection. Expert Rev Anti Infect Ther. 2011;9(7):799–805. doi: 10.1586/eri.11.54. [DOI] [PubMed] [Google Scholar]
- 15.Tahir D, Alwassouf S, Loudahi A, Davoust B, Charrel RN. Seroprevalence of Toscana virus in dogs from Kabylia (Algeria) Clin Microbiol Infect. 2016;22(3):e16–7. doi: 10.1016/j.cmi.2015.10.029. [DOI] [PubMed] [Google Scholar]
- 16.Dincer E, Gargari S, Ozkul A, Ergunay K. Potential animal reservoirs of Toscana virus and coinfections with Leishmania infantum in Turkey. Am J Trop Med Hyg. 2015;92(4):690–7. doi: 10.4269/ajtmh.14-0322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Navarro-Marí JM, Palop-Borrás B, Pérez-Ruiz M, Sanbonmatsu-Gámez S. Serosurvey study of Toscana virus in domestic animals, Granada, Spain. Vector Borne Zoonotic Dis. 2011;11(5):583–7. doi: 10.1089/vbz.2010.0065. [DOI] [PubMed] [Google Scholar]
- 18.Alkan C, Bichaud L, Lamballerie X, Alten B, Gould E, Charrel RN. Sandfly-borne phleboviruses of Eurasia and Africa: Epidemiology, genetic diversity, geographic range, control measures. Antiviral Res. 2013;100(1):54–74. doi: 10.1016/j.antiviral.2013.07.005. [DOI] [PubMed] [Google Scholar]
- 19.Rioux JA, Houin R, Leger N, Croset H, Deniau M, Poinsot S. Nouvelles stations Corses de Phlebotomus sergenti Parrot, 1917. Ann Parasitol Hum Comp. 1971;46:329–36. [PubMed] [Google Scholar]
- 20.Davoust B, Roqueplo C, Parzy D, Watier-Grillot S, Marié JL. A twenty-year follow-up of canine leishmaniosis in three military kennels in southeastern France. Parasit Vectors. 2013;6(1):323. doi: 10.1186/1756-3305-6-323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bosco-Lauth AM, Panella NA, Root JJ, Gidlewski T, Lash RR, Harmon JR, et al. Serological investigation of heartland virus (Bunyaviridae: Phlebovirus) exposure in wild and domestic animals adjacent to human case sites in Missouri 2012-2013. Am J Trop Med Hyg. 2015;92(6):1163–7. doi: 10.4269/ajtmh.14-0702. [DOI] [PMC free article] [PubMed] [Google Scholar]


