Abstract
The dopamine system plays an important role in the regulation of attention and motor behavior, subsequently, several dopamine-related genes have been associated with Attention Deficit/Hyperactivity Disorder (ADHD). Among them are the dopamine receptors D1 and D5 that mediate adenylyl cyclase activation through coupling with Gs-like proteins. We thus hypothesized that the Gs-like subunit Gαolf, expressed in D1-rich areas of the brain, contributes to the genetic susceptibility of ADHD. To evaluate the involvement of the Gαolf gene, GNAL, in ADHD, we examined the inheritance pattern of 12 GNAL polymorphisms in 258 nuclear families ascertained through a proband with ADHD (311 affected children) using the transmission/disequilibrium test (TDT). Categorical analysis of individual marker alleles demonstrated biased transmission of one polymorphism in GNAL intron 3 (rs2161961; P = 0.011). We also observed significant relationships between rs2161961 and dimensional symptoms of inattention and hyperactivity/impulsivity (P = 0.003 and P = 0.008). In addition, because of recent evidence of imprinting at the GNAL locus, secondary analyses were split into maternal and paternal transmissions to assess a contribution of parental effects. We found evidence of strong maternal effect, with preferential transmission of maternal alleles for rs2161961A (P = 0.005) and rs8098539A (P = 0.035). These preliminary findings suggest a possible contribution of GNAL in the susceptibility to ADHD, with possible involvement of parent-of-origin effects.
Keywords: Attention deficit/hyperactivity disorder, Genetics, G protein, Gαolf, Transmission/disequilibrium test, Dopamine receptor D1
1. Introduction
Attention Deficit/Hyperactivity Disorder (ADHD) is a common neurodevelopmental condition characterized by a pattern of inattention, hyperactivity and impulsivity. Current hypotheses on the biological basis of ADHD have centered on the dysregulation of fronto-striatal circuits and the neurotransmitters involved in these pathways. In particular, accumulating evidence implicate altered dopamine signalling in the disorder (Davids et al., 2003; Durston, 2003; Seeman and Madras, 1998; Viggiano et al., 2003) and genetic association of several genes engaged in dopamine signalling is supported by meta-analysis of pooled data (e.g. DRD4, DRD5, DAT1, and SNAP25) (Thapar et al., 2005).
We previously reported the association between the dopamine receptor D1 gene (DRD1) and ADHD (Misener et al., 2004), particularly between one haplotype and inattention symptoms (P = 0.008). Recently, we replicated the association between this haplotype and inattentive behaviors in children selected for reading difficulties (P = 0.004) (Luca et al., submitted for publication). Positive findings were also found for one DRD1 marker in an ADHD case-control sample (Bobb et al., 2005), although negative results were also obtained with smaller family-based samples for single markers (Bobb et al., 2005; Kirley et al., 2002). Our findings for DRD1 in ADHD symptoms are suggestive of a potential role of the D1/D5 signalling pathways in genetic susceptibility of this disorder. This is further supported by a large combined analysis of 14 independent samples of 1980 probands (P = 0.00005), odds ratio 1.24 (Lowe et al., 2004) for DRD5 (a D1-like receptor). In the same vein, we have recently reported evidence of association between ADHD and the calcyon gene, a D1-interacting protein (Laurin et al., 2005).
D1/D5 signalling mediates executive abilities including working memory (Goldman-Rakic et al., 2000), attention (Bayer et al., 2000; Granon et al., 2000), motor control (Dreher and Jackson, 1989; Meyer, 1993), and reward and reinforcement mechanisms (Beninger and Miller, 1998). Impairment of those functions is often observed in individuals with ADHD (Arnsten and Li, 2005b; Lijffijt et al., 2005; Luman et al., 2005; Martinussen et al., 2005; Willcutt et al., 2005). Moreover, a recent study in rodents suggested that D1 stimulation contributes to cognitive-enhancing effects of methylphenidate, a leading treatment for ADHD (Arnsten and Dudley, 2005a).
D1 signalling is mediated in the brain by the heterotrimeric G proteins Gs and Golf (Corvol et al., 2001; Zhuang et al., 2000), which cause activation of adenylyl cyclase, cAMP-dependant protein kinase, and DARPP32. D1 receptors also signal via phospholipase C-dependent mobilization of intracellular calcium (Undie and Friedman, 1990; Wang et al., 1995), likely involving calcyon (Lezcano et al., 2000). Lesion experiments and knockout studies have indicated that the coupling of D1 receptors to adenylyl cyclase is mostly provided by Gαolf in the striatal neurons, and that Gαolf is required for D1-mediated behaviour and biochemical effects in the striatum (Corvol et al., 2001; Herve et al., 1993; Zhuang et al., 2000). Gαolf appears to be highly regulated by receptor usage and availability of interacting/effector proteins (Corvol et al., 2004, 2001; Herve et al., 2001, 1993; Iwamoto et al., 2004; Schwindinger et al., 2003; Zhuang et al., 2000), suggesting that it represents a limiting factor in the coupling efficiency of D1 receptors.
Based on our previous finding for DRD1 in ADHD symptoms and the regulatory role played by Gαolf in D1 signalling, we believe that the Gαolf gene, GNAL, is a reasonable candidate for involvement in ADHD susceptibility. This is further supported by the locomotor behaviour of the mice deficient for Gαolf. When tested in open field exercises, the GNAL+/− mice exhibit a slight decrease in basal locomotor activity, while the −/− mice display locomotor hyperactivity (Belluscio et al., 1998; Schwindinger et al., 2003) similar to a D1 knockout (Xu et al., 1994a,b).
The GNAL gene is located on the short arm of chromosome 18 in a region that has been linked to bipolar disorder and schizophrenia (Berrettini, 2000; Schwab et al., 2000; Segurado et al., 2003), with some evidence of parent-of-origin effects (Gershon et al., 1996; Nothen et al., 1999; Stine et al., 1995). However, replication studies have led to conflicting results (Van Broeckhoven and Verheyen, 1998; Zill et al., 2003).
In the present study, we sought evidence for association between GNAL and ADHD in a sample of clinically ascertained nuclear families. We tested for the non-random transmission of alleles of 12 single nucleotide polymorphisms (SNPs) using the transmission/disequilibrium test (TDT) statistic (Spielman and Ewens, 1996). Given previous findings suggesting parent-of-origin effects at 18p and evidence of epigenetic modification of GNAL (Corradi et al., 2005), we also assessed transmissions from mothers and fathers separately. Finally, we performed quantitative analysis using ADHD inattentive and hyperactive/impulsive symptom counts.
2. Materials and methods
2.1. Study sample and diagnostic assessment
The methods of assessment, characteristics of the subjects, and inclusion/exclusion criteria have been described previously, including the instruments used to collect information for the diagnosis of ADHD and co-morbid conditions (Barr et al., 1999; Laurin et al., 2005; Quist et al., 2000). Briefly, probands and their siblings between 7 and 16 years old were included if they met DSM-IV criteria for one of the three ADHD subtypes. The study sample was comprised of 258 nuclear families from the Toronto area, including 53 affected siblings. This gave a total of 311 affected children (251 boys and 60 girls) that were genotyped along with 209 fathers and 243 mothers. The sample consists of 194 parents-child trios and 64 families in which a single parent was genotyped. The distribution of the affected children among the DSM-IV ADHD subtypes was 14% of the predominantly hyperactive/impulsive subtype, 24% of the predominantly inattentive subtype and 62% of the combined subtype. All children were free of medication for 24 h before assessment. This protocol was approved by the Hospital for Sick Children’s Research Ethics Board and informed written consent or verbal assent (children) was obtained for all participants.
Information on ADHD symptoms was obtained using semi-structured interviews for parents (Parent Interview for Child Symptoms: PICS-IV; Ickowicz et al., 2006) and teachers (Teacher Telephone Interview: TTI-IV; Tannock et al., 2002). These instruments were used to determine symptom scores based on the nine DSM-IV criteria for both inattention and hyperactivity/impulsivity dimensions. In our study sample, parent-reported symptom scores range from 0 to 9 for both hyperactive/impulsive (mean = 5.54 ± 2.34) or inattentive (mean = 5.85 ± 1.99) behavior. The corresponding teacher-reported scores also range from 0 to 9 (mean = 4.17 ± 2.78 and 5.22 ± 2.21, respectively).
2.2. Genotyping
DNA was extracted from blood lymphocytes using a standard high salt extraction method (Miller et al., 1988). A total of 12 single nucleotide polymorphisms (SNPs) were genotyped using the ABI 7900-HT Sequence Detection System® (Applied Biosystems) and TaqMan 5′ nuclease assays for allelic discrimination (Livak, 1999). Primer and probe sequences are available on request. The PCR reactions (5 μl) contained 30 ng of genomic DNA, 10 μM of Taq-Man® Universal PCR Master Mix and 0.1 μl of allelic discrimination mix (Applied Biosystems). The thermal cycling conditions were 95 °C for 10 min and 40–60 cycles of 95 °C for 15 s and the annealing temperature (58–60 °C) for 1 min.
2.3. Statistical analysis
The TDTPHASE and PDTPHASE programs from the UNPHASED package v2.403 (Dudbridge, 2003) were used to test for biased transmission of individual marker alleles in relation to ADHD diagnosis (categorical analysis). TDTPHASE was used to test transmissions from mothers and fathers separately. Because 53 affected siblings were included in our study, we also provided the results of the TDT analysis using one randomly chosen sibling per family as well as examined the results using PDT, an extension of the TDT, which provides a valid test of association in the presence of linkage in families with multiple affected siblings and thus, is suitable for both family trios and sibling pair structures. Quantitative trait TDT analyses, examining the transmission of individual alleles in relation to inattentive and hyperactive/impulsive symptom scores were carried out using the FBAT program v1.5.5 (Horvath et al., 2001), with the additive model of inheritance. For the tests, we used population-based mean scores as an offset value to mean centre the trait. The coefficients of linkage disequilibrium (LD) between marker alleles, Δ2 and D′, were calculated using Haploview v2.03 (Barrett et al., 2005). Two-sided P-values were used for all results. Permutation testing was performed for TDTPHASE and PDTPHASE analyses. The corrected values for the most significant P values are reported together with the uncorrected P values. Our analysis of quantitative trait is not corrected for multiple tests as these are secondary analyses based on our findings from the categorical analyses.
3. Results
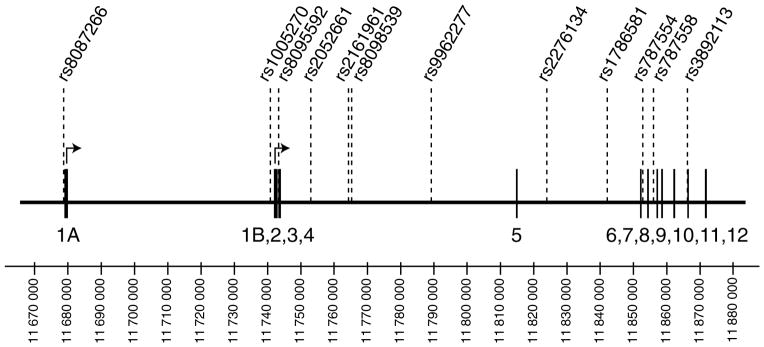
In this study, we selected a total of 12 non-coding SNPs across the GNAL gene to test for association between the GNAL gene and ADHD. Their location within the gene is presented in Fig. 1. Markers with high heterozygosity were initially chosen across the gene and additional markers were added to follow up positive findings. We included two SNPs described previously by Berrettini et al. (1998) in GNAL introns 3 (rs8095592) and 10 (rs3892113). Pairwise measures of linkage disequilibrium between adjacent SNPs are shown in Table 1. Parental allele frequencies are shown in Table 2. The allele frequencies of rs8095592 (G = 70%; A = 30%) and rs3892113 (T = 92%; G = 8%) are different from the ones reported by Berrettini et al. in a control sample drawn from an American population (rs8095592 G = 31%, A = 69%; and rs3892113 T = 84%, G = 16%), but were similar to the ones reported by Zill et al. (2002, 2003) in European controls (rs8095592 G = 69–72%, A = 28–31%; and rs3892113 T = 88–94%, G = 6–12%). We confirmed the allele identity of these two markers in our sample by sequencing. Genotypes demonstrated no significant departure from Hardy–Weinberg equilibrium.
Fig. 1.
Schematic representation of the GNAL gene and location of the genotyped polymorphisms within the gene. Exons are indicated by boxes and markers are shown above the gene. Two major alternative transcription start sites have been described for GNAL, giving rise to isoforms that differ in their first exons (1A or 1B), but share exons 2 to 12 (Corradi et al., 2005; Vuoristo et al., 2000).
Table 1.
| 7266 | 5270 | 5592 | 2661 | 1961 | 8539 | 2277 | 6134 | 6581 | 7554 | 7558 | 2113 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 7266 | 0.045 | 0.204 | 0.203 | 0.253 | 0.376 | 0.130 | 0.109 | 0.679 | 0.116 | 0.190 | 0.061 | |
| 5270 | 0.001 | 0.912 | 0.767 | 0.743 | 0.724 | 0.745 | 0.776 | 0.330 | 0.319 | 0.306 | 0.019 | |
| 5592 | 0.041 | 0.384 | 0.887 | 0.862 | 0.872 | 0.801 | 0.812 | 0.557 | 0.317 | 0.391 | 0.067 | |
| 2661 | 0.041 | 0.276 | 0.787 | 0.895 | 0.908 | 0.844 | 0.848 | 0.652 | 0.350 | 0.427 | 0.281 | |
| 1961 | 0.050 | 0.330 | 0.580 | 0.639 | 0.986 | 0.886 | 0.880 | 0.967 | 0.234 | 0.341 | 0.294 | |
| 8539 | 0.108 | 0.184 | 0.574 | 0.630 | 0.583 | 0.973 | 0.889 | 0.933 | 0.338 | 0.402 | 0.028 | |
| 2277 | 0.010 | 0.417 | 0.390 | 0.439 | 0.621 | 0.437 | 0.894 | 0.916 | 0.237 | 0.238 | 0.050 | |
| 6134 | 0.005 | 0.530 | 0.270 | 0.298 | 0.406 | 0.247 | 0.519 | 1.000 | 0.415 | 0.414 | 0.620 | |
| 6581 | 0.038 | 0.004 | 0.026 | 0.035 | 0.061 | 0.094 | 0.042 | 0.034 | 0.553 | 0.609 | 0.033 | |
| 7554 | 0.009 | 0.072 | 0.068 | 0.084 | 0.048 | 0.058 | 0.051 | 0.105 | 0.017 | 0.960 | 0.587 | |
| 7558 | 0.031 | 0.050 | 0.133 | 0.160 | 0.105 | 0.107 | 0.039 | 0.080 | 0.027 | 0.705 | 0.611 | |
| 2113 | 0.000 | 0.000 | 0.001 | 0.003 | 0.003 | 0.000 | 0.000 | 0.006 | 0.000 | 0.009 | 0.012 |
D′ and Δ2 values are given in the top right and bottom left of the table, respectively.
SNP IDs are abbreviated to the final four digits.
Bold for D′ or Δ2 values >0.80, bolditalics for values between 0.80 and 0.60, italics for 0.59–0.40.
Table 2.
Transmission disequilibrium test for individual GNAL polymorphisms
| SNP | Allele | Freq. | Transmissions
|
TDT(all)
|
TDT(one sib)
|
PDT
|
||||
|---|---|---|---|---|---|---|---|---|---|---|
| T | NT | χ2 | P | χ2 | P | Z | P | |||
| rs8087266 G/A | G | 0.70 | 90 | 70 | 2.507 | 0.113 | 2.355 | 0.125 | 1.787 | 0.074 |
| rs1005270 T/C | T | 0.83 | 77 | 67 | 0.695 | 0.405 | 1.526 | 0.217 | 0.953 | 0.341 |
| rs8095592 G/A | G | 0.70 | 98 | 87 | 0.654 | 0.419 | 0.530 | 0.467 | 0.550 | 0.583 |
| rs2052661 G/C | G | 0.71 | 96 | 81 | 1.273 | 0.259 | 0.961 | 0.327 | 0.999 | 0.318 |
| rs2161961 A/G | A | 0.75 | 101 | 68 | 6.485 | 0.011* | 7.796 | 0.005* | 2.494 | 0.013* |
| rs8098539 A/G | A | 0.64 | 107 | 83 | 3.040 | 0.081 | 2.538 | 0.111 | 1.665 | 0.096 |
| rs9962277 C/T | C | 0.79 | 73 | 64 | 0.592 | 0.442 | 1.072 | 0.300 | 0.752 | 0.452 |
| rs2276134 T/C | T | 0.85 | 55 | 54 | 0.009 | 0.924 | 0.045 | 0.831 | −0.157 | 0.876 |
| rs1786581 C/T | C | 0.83 | 71 | 61 | 0.758 | 0.384 | 0.154 | 0.695 | 1.021 | 0.307 |
| rs787554 C/T | C | 0.78 | 72 | 88 | 1.603 | 0.206 | 0.954 | 0.329 | −1.265 | 0.206 |
| rs787558 T/C | T | 0.73 | 93 | 81 | 0.828 | 0.363 | 1.363 | 0.243 | 1.025 | 0.305 |
| rs3892113 T/G | T | 0.92 | 32 | 29 | 0.148 | 0.701 | 0.308 | 0.579 | 0.000 | 1.000 |
P value after 1000 permutations : PTDT(all) = 0.101; PTDT(one sib) = 0.071; PPDT = 0.101.
Categorical analysis revealed allelic association for rs2161961 (Table 2). Allele A was transmitted 101 times and untransmitted 68 times (PTDT(all)=0.011;PTDT(one sib)= 0.005; PPDT = 0.013). The other 11 markers failed to show a significant association (Table 1), although a weak trend was observed for rs8098539 (PTDT(all) = 0.081; PTDT(one sib) = 0.111; PPDT = 0.096), located 886 bp downstream and demonstrating strong, although not complete, LD with rs2161961 (D′ = 0.986, Δ2 = 0.583). When the results for rs2161961 were corrected for the total number of markers tested the results were no longer significant, although a trend was still evident (P = 0.071–0.101).
We also performed secondary analyses to examine the relationship of this gene to symptom counts of inattention or hyperactivity/impulsivity. Twin studies indicate that there are shared as well as independent genes contributing to the symptom dimensions of ADHD (Levy et al., 1997), thus we examine the genetic contribution to the dimensions separately. The marker rs2161961 showed significant association to both ADHD dimensions as rated by the parent (inattention: P = 0.003 and hyperactivity/impulsivity: P = 0.008) (Table 3).
Table 3.
Quantitative analysis of GNAL allele transmission in relation to ADHD symptom scores reported by parents (PICS-IV) and teacher (TTI-IV)
| Marker | Allele | Informative families | Inattention symptoms
|
Hyperactivity/impulsivity symptoms
|
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Parent (PICS-IV)
|
Teacher (TTI-IV)
|
Parent (PICS-IV)
|
Teacher (TTI-IV)
|
|||||||
| Z | P-value | Z | P-value | Z | P-value | Z | P-value | |||
| rs8087266 | G | 101 | 1.712 | 0.087 | 1.316 | 0.188 | 1.767 | 0.077 | 1.655 | 0.098 |
| A | −1.712 | 0.087 | −1.316 | 0.188 | −1.767 | 0.077 | −1.655 | 0.098 | ||
| rs1005270 | T | 85 | 1.141 | 0.254 | 0.116 | 0.908 | 0.133 | 0.895 | 0.024 | 0.981 |
| C | −1.141 | 0.254 | −0.116 | 0.908 | −0.133 | 0.895 | −0.024 | 0.981 | ||
| rs8095592 | G | 110 | 1.373 | 0.170 | 0.020 | 0.984 | 0.241 | 0.809 | −0.168 | 0.866 |
| A | −1.373 | 0.170 | −0.020 | 0.984 | −0.241 | 0.809 | 0.168 | 0.866 | ||
| rs2052661 | G | 109 | 1.345 | 0.179 | 0.083 | 0.934 | 0.863 | 0.388 | 0.442 | 0.659 |
| C | −1.345 | 0.179 | −0.083 | 0.934 | −0.863 | 0.388 | −0.442 | 0.659 | ||
| rs2161961 | A | 105 | 2.949 | 0.003 | 1.194 | 0.232 | 2.639 | 0.008 | 2.205 | 0.027 |
| G | −2.949 | 0.003 | −1.194 | 0.232 | −2.639 | 0.008 | −2.205 | 0.027 | ||
| rs8098539 | A | 119 | 1.771 | 0.077 | 1.105 | 0.269 | 1.302 | 0.193 | 1.315 | 0.189 |
| G | −1.771 | 0.077 | −1.105 | 0.269 | −1.302 | 0.193 | −1.315 | 0.189 | ||
| rs9962277 | C | 85 | 1.064 | 0.287 | 0.304 | 0.761 | 0.732 | 0.464 | 0.704 | 0.481 |
| T | −1.064 | 0.287 | −0.304 | 0.761 | −0.732 | 0.464 | −0.704 | 0.481 | ||
| rs2276134 | T | 74 | 0.494 | 0.622 | −0.006 | 0.995 | 0.173 | 0.863 | −0.067 | 0.946 |
| C | −0.494 | 0.622 | 0.006 | 0.995 | −0.173 | 0.863 | 0.067 | 0.946 | ||
| rs1786581 | C | 82 | −0.110 | 0.912 | 0.359 | 0.720 | −0.046 | 0.963 | 0.216 | 0.829 |
| T | 0.110 | 0.912 | −0.359 | 0.720 | 0.046 | 0.963 | −0.216 | 0.829 | ||
| rs787554 | C | 101 | −0.765 | 0.444 | −1.787 | 0.074 | −0.924 | 0.355 | −2.020 | 0.043 |
| T | 0.765 | 0.444 | 1.787 | 0.074 | 0.924 | 0.355 | 2.020 | 0.043 | ||
| rs787558 | T | 114 | 1.612 | 0.107 | 0.334 | 0.738 | 1.555 | 0.120 | −0.166 | 0.868 |
| C | −1.612 | 0.107 | −0.334 | 0.738 | −1.555 | 0.120 | 0.166 | 0.868 | ||
| rs3892113 | T | 41 | −0.141 | 0.888 | 0.257 | 0.797 | −0.219 | 0.827 | 0.140 | 0.889 |
| G | 0.141 | 0.888 | −0.257 | 0.797 | 0.219 | 0.827 | −0.140 | 0.889 | ||
As mentioned previously, genetic linkage studies for bipolar disorder and schizophrenia have suggested parent-of-origin effects for the chromosome 18p region encompassing GNAL. Furthermore, molecular studies assessing methylation status of the two promoters of the gene suggest that GNAL is subject to epigenetic regulation (Corradi et al., 2005). We thus examined the data for the presence of a parent-of-origin effect (Table 4). When assessing transmission separately for mothers and fathers, mothers appeared to transmit preferentially the rs2161961A allele (P = 0.005), as well as the rs8098539A alleles (P = 0.035). No bias was observed for paternal transmission of the same alleles (P = 0.271 and P = 0.502, respectively).
Table 4.
TDT analysis of GNAL polymorphisms by the sex of the transmitting parent
| SNP | Allele | Paternal transmissions
|
Maternal transmissions
|
||||||
|---|---|---|---|---|---|---|---|---|---|
| T | NT | χ2 (1 d.f.) | P | T | NT | χ2 (1 d.f.) | P | ||
| rs8087266 G/A | G | 36 | 25 | 1.995 | 0.158 | 34 | 25 | 1.378 | 0.240 |
| rs1005270 T/C | T | 31 | 26 | 0.439 | 0.508 | 34 | 29 | 0.397 | 0.529 |
| rs8095592 G/A | G | 37 | 32 | 0.363 | 0.547 | 42 | 36 | 0.462 | 0.497 |
| rs2052661 G/C | G | 37 | 36 | 0.014 | 0.907 | 42 | 28 | 2.819 | 0.093 |
| rs2161961 A/G | A | 38 | 29 | 1.213 | 0.271 | 49 | 25 | 7.926 | 0.005* |
| rs8098539 A/G | A | 43 | 37 | 0.450 | 0.502 | 46 | 28 | 4.423 | 0.035 |
| rs9962277 C/T | C | 27 | 28 | 0.018 | 0.893 | 33 | 23 | 1.795 | 0.180 |
| rs2276134 T/C | T | 23 | 24 | 0.021 | 0.884 | 24 | 22 | 0.087 | 0.768 |
| rs1786581 C/T | C | 28 | 29 | 0.018 | 0.895 | 34 | 23 | 2.136 | 0.144 |
| rs787554 C/T | C | 25 | 38 | 2.702 | 0.100 | 30 | 33 | 0.143 | 0.705 |
| rs787558 T/C | T | 31 | 33 | 0.063 | 0.803 | 44 | 30 | 2.665 | 0.103 |
| rs3892113 T/G | T | 16 | 10 | 1.397 | 0.237 | 15 | 18 | 0.273 | 0.601 |
P value after 1000 permutations: P = 0.054.
4. Discussion
In this study, we investigated the involvement of the G protein subunit αolf gene, GNAL, in ADHD. Our TDT analysis of 12 SNPs spanning GNAL in a clinically ascertained sample revealed the nominally significant association of rs2161961 alleles with ADHD diagnosis as well as with quantitative traits of inattention and hyperactivity/impulsivity as rated by parents. Moreover, we observed that these findings were most likely attributable to an excess of maternal transmissions.
Gαolf is a major regulatory target in D1 signalling and may modulate the intensity of the D1 response. The importance of the balance in D1 signalling in the brain has been highlighted by the demonstration that excessive as well as insufficient D1 receptor stimulation impairs prefrontal cognitive function (Arnsten and Li, 2005b; Williams and Castner, 2006). ADHD is a complex disorder that occurs across the developmental spectrum, with the course of the illness changing over time. Maturational changes in the dopamine system, particularly in D1 signalling, have been suggested to be involved in the emergence and course of ADHD (Andersen and Teicher, 2000; Diaz Heijtz et al., 2004). Interestingly, studies in rodents have shown that the levels of the Gαolf protein vary differentially across the developmental course in the striatum (Rius et al., 1994).
Pleiotropic effects of a susceptibility gene at 18p11.2 has been suggested by evidence of linkage in a proportion of families with bipolar disorder, recurrent major depression and/or schizophrenia (Balciuniene et al., 1998; Berrettini, 2000; Schwab et al., 2000; Segurado et al., 2003). Those findings are, however, not universal (Badner and Gershon, 2002; Lewis et al., 2003; Van Broeckhoven and Verheyen, 1999), and their interpretation is further complicated by the putative presence of a parent-of-origin effect in this region (Petronis, 2000). Stronger evidence of linkage has been reported in this region for bipolar families with paternal transmission of the phenotype (Gershon et al., 1996; Nothen et al., 1999; Stine et al., 1995), and with maternal pedigrees for schizophrenia (Schwab et al., 1998). The investigation of GNAL as a candidate gene in this region for both bipolar disorder (Tsiouris et al., 1996; Turecki et al., 1996; Zill et al., 2003) and major depression (Zill et al., 2002) yielded negative results. However, only one or two markers were assessed in each of these studies. To our knowledge, this is the first time that GNAL was investigated in relation to ADHD. Our findings of biased transmission of maternal, but not paternal, alleles of rs2161961 and rs8098539, are consistent with the presence of parental effect and with genomic imprinting of the GNAL gene region (Corradi et al., 2005).
Gαolf is a key effector molecule in signalling of the D1 receptor, for which evidence of association has been reported for ADHD (Bobb et al., 2005;Misener et al., 2004), particularly with the inattention symptoms in children with ADHD (Misener et al., 2004) or reading difficulties (Luca et al. submitted for publication). Most association studies have focused on neurotransmitter receptor or transporter level, however, G proteins are central in the regulation of different pathways. The findings of this study provide some support for the involvement of Gαolf in susceptibility to ADHD. We acknowledge, however, that these are preliminary results that require replication in independent samples, particularly in light of the number of markers tested for this gene as well as for other genes in this sample.
Acknowledgments
This work was supported by Postdoctoral Fellowships from the Hospital for Sick Children Research Training Centre (NL) and the Canadian Institutes of Health Research (NL) and by grants from The Hospital for Sick Children Psychiatric Endowment Fund (CLB), and the Canadian Institutes of Health Research MT14336 and MOP14336 (CLB).
References
- Andersen SL, Teicher MH. Sex differences in dopamine receptors and their relevance to ADHD. Neuroscience and Biobehavioral Reviews. 2000;24:137–41. doi: 10.1016/s0149-7634(99)00044-5. [DOI] [PubMed] [Google Scholar]
- Arnsten AF, Dudley AG. Methylphenidate improves prefrontal cortical cognitive function through alpha2 adrenoceptor and dopamine D1 receptor actions: relevance to therapeutic effects in attention deficit hyperactivity disorder. Behavioral and Brain Functions. 2005a;1:2. doi: 10.1186/1744-9081-1-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnsten AF, Li BM. Neurobiology of executive functions: catecholamine influences on prefrontal cortical functions. Biological Psychiatry. 2005b;57:1377–84. doi: 10.1016/j.biopsych.2004.08.019. [DOI] [PubMed] [Google Scholar]
- Badner JA, Gershon ES. Meta-analysis of whole-genome linkage scans of bipolar disorder and schizophrenia. Molecular Psychiatry. 2002;7:405–11. doi: 10.1038/sj.mp.4001012. [DOI] [PubMed] [Google Scholar]
- Balciuniene J, Yuan QP, Engstrom C, Lindblad K, Nylander PO, Sundvall M, et al. Linkage analysis of candidate loci in families with recurrent major depression. Molecular Psychiatry. 1998;3:162–8. doi: 10.1038/sj.mp.4000372. [DOI] [PubMed] [Google Scholar]
- Barr C, Wigg K, Sandor P. Catechol-O-methyltransferase and Gilles de la Tourette syndrome. Molecular Psychiatry. 1999;4:492–5. doi: 10.1038/sj.mp.4000549. [DOI] [PubMed] [Google Scholar]
- Barrett JC, Fry B, Maller J, Daly MJ. Haploview: analysis and visualization of LD and haplotype maps. Bioinformatics. 2005;21:263–5. doi: 10.1093/bioinformatics/bth457. [DOI] [PubMed] [Google Scholar]
- Bayer LE, Brown A, Mactutus CF, Booze RM, Strupp BJ. Prenatal cocaine exposure increases sensitivity to the attentional effects of the dopamine D1 agonist SKF81297. Journal of Neuroscience. 2000;20:8902–8. doi: 10.1523/JNEUROSCI.20-23-08902.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belluscio L, Gold GH, Nemes A, Axel R. Mice deficient in G(olf) are anosmic. Neuron. 1998;20:69–81. doi: 10.1016/s0896-6273(00)80435-3. [DOI] [PubMed] [Google Scholar]
- Beninger RJ, Miller R. Dopamine D1-like receptors and reward-related incentive learning. Neuroscience and Biobehavioral Reviews. 1998;22:335–45. doi: 10.1016/s0149-7634(97)00019-5. [DOI] [PubMed] [Google Scholar]
- Berrettini WH. Susceptibility loci for bipolar disorder: overlap with inherited vulnerability to schizophrenia. Biological Psychiatry. 2000;47:245–51. doi: 10.1016/s0006-3223(99)00226-7. [DOI] [PubMed] [Google Scholar]
- Berrettini WH, Vuoristo J, Ferraro TN, Buono RJ, Wildenauer D, Ala-Kokko L. Human G(olf) gene polymorphisms and vulnerability to bipolar disorder. Psychiatric Genetics. 1998;8:235–8. doi: 10.1097/00041444-199808040-00006. [DOI] [PubMed] [Google Scholar]
- Bobb AJ, Addington AM, Sidransky E, Gornick MC, Lerch JP, Greenstein DK, et al. Support for association between ADHD and two candidate genes: NET1 and DRD1. American Journal of Medical Genetics Part B, Neuropsychiatric Genetics. 2005;134:67–72. doi: 10.1002/ajmg.b.30142. [DOI] [PubMed] [Google Scholar]
- Corradi JP, Ravyn V, Robbins AK, Hagan KW, Peters MF, Bostwick R, et al. Alternative transcripts and evidence of imprinting of GNAL on 18p11. 2. Molecular Psychiatry. 2005;10:1017–25. doi: 10.1038/sj.mp.4001713. [DOI] [PubMed] [Google Scholar]
- Corvol JC, Studler JM, Schonn JS, Girault JA, Herve D. Galpha(olf) is necessary for coupling D1 and A2a receptors to adenylyl cyclase in the striatum. Journal of Neurochemistry. 2001;76:1585–8. doi: 10.1046/j.1471-4159.2001.00201.x. [DOI] [PubMed] [Google Scholar]
- Corvol JC, Muriel MP, Valjent E, Feger J, Hanoun N, Girault JA, et al. Persistent increase in olfactory type G-protein alpha subunit levels may underlie D1 receptor functional hypersensitivity in Parkinson disease. Journal of Neuroscience. 2004;24:7007–14. doi: 10.1523/JNEUROSCI.0676-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davids E, Zhang K, Tarazi FI, Baldessarini RJ. Animal models of attention-deficit hyperactivity disorder. Brain Research Brain Research Reviews. 2003;42:1–21. doi: 10.1016/s0165-0173(02)00274-6. [DOI] [PubMed] [Google Scholar]
- Diaz Heijtz R, Scott L, Forssberg H. Alteration of dopamine D1 receptor-mediated motor inhibition and stimulation during development in rats is associated with distinct patterns of c-fos mRNA expression in the frontal-striatal circuitry. European Journal of Neuroscience. 2004;19:945–56. doi: 10.1111/j.0953-816x.2004.03154.x. [DOI] [PubMed] [Google Scholar]
- Dreher JK, Jackson DM. Role of D1 and D2 dopamine receptors in mediating locomotor activity elicited from the nucleus accumbens of rats. Brain Research. 1989;487:267–77. doi: 10.1016/0006-8993(89)90831-7. [DOI] [PubMed] [Google Scholar]
- Dudbridge F. Pedigree disequilibrium tests for multilocus haplotypes. Genetic Epidemiology. 2003;25:115–21. doi: 10.1002/gepi.10252. [DOI] [PubMed] [Google Scholar]
- Durston S. A review of the biological bases of ADHD: what have we learned from imaging studies? Mental Retardation and Developmental Disabilities Research Reviews. 2003;9:184–95. doi: 10.1002/mrdd.10079. [DOI] [PubMed] [Google Scholar]
- Gershon ES, Badner JA, Detera-Wadleigh SD, Ferraro TN, Berrettini WH. Maternal inheritance and chromosome 18 allele sharing in unilineal bipolar illness pedigrees. American Journal of Medical Genetics. 1996;67:202–7. doi: 10.1002/(SICI)1096-8628(19960409)67:2<202::AID-AJMG11>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- Goldman-Rakic PS, Muly EC, 3rd, Williams GV. D(1) receptors in prefrontal cells and circuits. Brain Research Brain Research Reviews. 2000;31:295–301. doi: 10.1016/s0165-0173(99)00045-4. [DOI] [PubMed] [Google Scholar]
- Granon S, Passetti F, Thomas KL, Dalley JW, Everitt BJ, Robbins TW. Enhanced and impaired attentional performance after infusion of D1 dopaminergic receptor agents into rat prefrontal cortex. Journal of Neuroscience. 2000;20:1208–15. doi: 10.1523/JNEUROSCI.20-03-01208.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herve D, Levi-Strauss M, Marey-Semper I, Verney C, Tassin JP, Glowinski J, et al. G(olf) and Gs in rat basal ganglia: possible involvement of G(olf) in the coupling of dopamine D1 receptor with adenylyl cyclase. Journal of Neuroscience. 1993;13:2237–48. doi: 10.1523/JNEUROSCI.13-05-02237.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herve D, Le Moine C, Corvol JC, Belluscio L, Ledent C, Fienberg AA, et al. Galpha(olf) levels are regulated by receptor usage and control dopamine and adenosine action in the striatum. Journal of Neuroscience. 2001;21:4390–9. doi: 10.1523/JNEUROSCI.21-12-04390.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horvath S, Xu X, Laird NM. The family based association test method: strategies for studying general genotype–phenotype associations. European Journal of Human Genetics. 2001;9:301–6. doi: 10.1038/sj.ejhg.5200625. [DOI] [PubMed] [Google Scholar]
- Ickowicz A, Schachar R, Sugarman R, Chen S, Millette C, Cook L. The parent interview for child symptoms (PICS): a situation-specific clinical-research interview for attention deficit hyperactivity and related disorders. Canadian Journal of Psychiatry. 2006;50:325–8. doi: 10.1177/070674370605100508. [DOI] [PubMed] [Google Scholar]
- Iwamoto T, Iwatsubo K, Okumura S, Hashimoto Y, Tsunematsu T, Toya Y, et al. Disruption of type 5 adenylyl cyclase negates the developmental increase in Galphaolf expression in the striatum. FEBS Letters. 2004;564:153–6. doi: 10.1016/S0014-5793(04)00333-3. [DOI] [PubMed] [Google Scholar]
- Kirley A, Hawi Z, Daly G, McCarron M, Mullins C, Millar N, et al. Dopaminergic system genes in ADHD: toward a biological hypothesis. Neuropsychopharmacology. 2002;27:607–19. doi: 10.1016/S0893-133X(02)00315-9. [DOI] [PubMed] [Google Scholar]
- Laurin N, Misener VL, Crosbie J, Ickowicz A, Pathare T, Roberts W, et al. Association of the Calcyon Gene (DRD1IP) with attention deficit/hyperactive disorder. Molecular Psychiatry. 2005;10:1117–25. doi: 10.1038/sj.mp.4001737. [DOI] [PubMed] [Google Scholar]
- Levy F, Hay DA, McStephen M, Wood C, Waldman I. Attention-deficit hyperactivity disorder: a category or a continuum? Genetic analysis of a large-scale twin study. Journal of the American Academy of Child and Adolescent Psychiatry. 1997;36:737–44. doi: 10.1097/00004583-199706000-00009. [DOI] [PubMed] [Google Scholar]
- Lewis CM, Levinson DF, Wise LH, DeLisi LE, Straub RE, Hovatta I, et al. Genome scan meta-analysis of schizophrenia and bipolar disorder, part II: Schizophrenia. American Journal of Human Genetics. 2003;73:34–48. doi: 10.1086/376549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lezcano N, Mrzljak L, Eubanks S, Levenson R, Goldman-Rakic P, Bergson C. Dual signaling regulated by calcyon, a D1 dopamine receptor interacting protein. Science. 2000;287:1660–4. doi: 10.1126/science.287.5458.1660. [DOI] [PubMed] [Google Scholar]
- Lijffijt M, Kenemans JL, Verbaten MN, van Engeland H. A meta-analytic review of stopping performance in attention-deficit/hyperactivity disorder: deficient inhibitory motor control? Journal of Abnormal Psychology. 2005;114:216–22. doi: 10.1037/0021-843X.114.2.216. [DOI] [PubMed] [Google Scholar]
- Livak KJ. Allelic discrimination using fluorogenic probes and the 5′ nuclease assay. Genetic Analysis. 1999;14:143–9. doi: 10.1016/s1050-3862(98)00019-9. [DOI] [PubMed] [Google Scholar]
- Lowe N, Kirley A, Hawi Z, Sham P, Wickham H, Kratochvil CJ, et al. Joint analysis of the DRD5 marker concludes association with attention-deficit/hyperactivity disorder confined to the predominantly inattentive and combined subtypes. American Journal of Human Genetics. 2004;74:348–56. doi: 10.1086/381561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luman M, Oosterlaan J, Sergeant JA. The impact of reinforcement contingencies on AD/HD: a review and theoretical appraisal. Clinical Psychology Review. 2005;25:183–213. doi: 10.1016/j.cpr.2004.11.001. [DOI] [PubMed] [Google Scholar]
- Martinussen R, Hayden J, Hogg-Johnson S, Tannock R. A meta-analysis of working memory impairments in children with attention-deficit/hyperactivity disorder. Journal of the American Academy of Child and Adolescent Psychiatry. 2005;44:377–84. doi: 10.1097/01.chi.0000153228.72591.73. [DOI] [PubMed] [Google Scholar]
- Meyer ME. Effects of intraaccumbens dopamine agonist SK&F38393 and antagonist SCH23390 on locomotor activities in rats. Pharmacology Biochemistry and Behavior. 1993;45:843–7. doi: 10.1016/0091-3057(93)90130-l. [DOI] [PubMed] [Google Scholar]
- Miller SA, Dykes DD, Polesky HF. A simple salting out procedure for extracting DNA from human nucleated cells. Nucleic Acids Research. 1988;16:1215. doi: 10.1093/nar/16.3.1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misener V, Luca P, Azeke O, Crosbie J, Waldman I, Tannock R, et al. Linkage of the dopamine receptor D1 gene to attention-deficit/hyperactivity disorder. Molecular Psychiatry. 2004;9:500–9. doi: 10.1038/sj.mp.4001440. [DOI] [PubMed] [Google Scholar]
- Nothen MM, Cichon S, Rohleder H, Hemmer S, Franzek E, Fritze J, et al. Evaluation of linkage of bipolar affective disorder to chromosome 18 in a sample of 57 German families. Molecular Psychiatry. 1999;4:76–84. doi: 10.1038/sj.mp.4000454. [DOI] [PubMed] [Google Scholar]
- Petronis A. The genes for major psychosis: aberrant sequence or regulation? Neuropsychopharmacology. 2000;23:1–12. doi: 10.1016/S0893-133X(00)00127-5. [DOI] [PubMed] [Google Scholar]
- Quist JF, Barr CL, Schachar R, Roberts W, Malone M, Tannock R, et al. Evidence for the serotonin HTR2A receptor gene as a susceptibility factor in attention deficit hyperactivity disorder (ADHD) Molecular Psychiatry. 2000;5:537–41. doi: 10.1038/sj.mp.4000779. [DOI] [PubMed] [Google Scholar]
- Rius RA, Mollner S, Pfeuffer T, Loh YP. Developmental changes in Gs and G(olf) proteins and adenylyl cyclases in mouse brain membranes. Brain Research. 1994;643:50–8. doi: 10.1016/0006-8993(94)90007-8. [DOI] [PubMed] [Google Scholar]
- Schwab SG, Hallmayer J, Lerer B, Albus M, Borrmann M, Honig S, et al. Support for a chromosome 18p locus conferring susceptibility to functional psychoses in families with schizophrenia, by association and linkage analysis. American Journal of Human Genetics. 1998;63:1139–52. doi: 10.1086/302046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwab SG, Hallmayer J, Albus M, Lerer B, Eckstein GN, Borrmann M, et al. A genome-wide autosomal screen for schizophrenia susceptibility loci in 71 families with affected siblings: support for loci on chromosome 10p and 6. Molecular Psychiatry. 2000;5:638–49. doi: 10.1038/sj.mp.4000791. [DOI] [PubMed] [Google Scholar]
- Schwindinger WF, Betz KS, Giger KE, Sabol A, Bronson SK, Robishaw JD. Loss of G protein gamma 7 alters behavior and reduces striatal alpha(olf) level and cAMP production. Journal of Biological Chemistry. 2003;278:6575–9. doi: 10.1074/jbc.M211132200. [DOI] [PubMed] [Google Scholar]
- Seeman P, Madras BK. Anti-hyperactivity medication: methylphenidate and amphetamine. Molecular Psychiatry. 1998;3:386–96. doi: 10.1038/sj.mp.4000421. [DOI] [PubMed] [Google Scholar]
- Segurado R, Detera-Wadleigh SD, Levinson DF, Lewis CM, Gill M, Nurnberger JI, Jr, et al. Genome scan meta-analysis of schizophrenia and bipolar disorder, part III: bipolar disorder. American Journal of Human Genetics. 2003;73:49–62. doi: 10.1086/376547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spielman RS, Ewens WJ. The TDT and other family-based tests for linkage disequilibrium and association. American Journal of Human Genetics. 1996;59:983–9. [PMC free article] [PubMed] [Google Scholar]
- Stine OC, Xu J, Koskela R, McMahon FJ, Gschwend M, Friddle C, et al. Evidence for linkage of bipolar disorder to chromosome 18 with a parent-of-origin effect. American Journal of Human Genetics. 1995;57:1384–94. [PMC free article] [PubMed] [Google Scholar]
- Tannock R, Hum M, Masellis M, Humphries T, Schachar R. Teacher telephone interview for children’s academic performance, attention, behavior and learning: DSM-IV Version (TTI-IV) The Hospital for Sick Children; Toronto, Canada: 2002. Unpublished Document. [Google Scholar]
- Thapar A, O’Donovan M, Owen MJ. The genetics of attention deficit hyperactivity disorder. Human Molecular Genetics. 2005;14(Spec No. 2):R275–82. doi: 10.1093/hmg/ddi263. [DOI] [PubMed] [Google Scholar]
- Tsiouris SJ, Breschel TS, Xu J, McInnis MG, McMahon FJ. Linkage disequilibrium analysis of G-olf alpha (GNAL) in bipolar affective disorder. American Journal of Medical Genetics. 1996;67:491–4. doi: 10.1002/(SICI)1096-8628(19960920)67:5<491::AID-AJMG11>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- Turecki G, Alda M, Grof P, Martin R, Cavazzoni PA, Duffy A, et al. No association between chromosome-18 markers and lithium-responsive affective disorders. Psychiatry Research. 1996;63:17–23. doi: 10.1016/0165-1781(96)02864-8. [DOI] [PubMed] [Google Scholar]
- Undie AS, Friedman E. Stimulation of a dopamine D1 receptor enhances inositol phosphates formation in rat brain. Journal of Pharmacology and Experimental Therapeutics. 1990;253:987–92. [PubMed] [Google Scholar]
- Van Broeckhoven C, Verheyen G. Chromosome 18 workshop. Psychiatric Genetics. 1998;8:97–108. doi: 10.1097/00041444-199800820-00014. [DOI] [PubMed] [Google Scholar]
- Van Broeckhoven C, Verheyen G. Report of the chromosome 18 workshop. Amercian Journal of Medical Genetics. 1999;88:263–70. [PubMed] [Google Scholar]
- Viggiano D, Ruocco LA, Sadile AG. Dopamine phenotype and behaviour in animal models: in relation to attention deficit hyperactivity disorder. Neuroscience and Biobehavioral Reviews. 2003;27:623–37. doi: 10.1016/j.neubiorev.2003.08.006. [DOI] [PubMed] [Google Scholar]
- Vuoristo JT, Berrettini WH, Overhauser J, Prockop DJ, Ferraro TN, Ala-Kokko L. Sequence and genomic organization of the human G-protein Golfalpha gene (GNAL) on chromosome 18p11, a susceptibility region for bipolar disorder and schizophrenia. Molecular Psychiatry. 2000;5:495–501. doi: 10.1038/sj.mp.4000758. [DOI] [PubMed] [Google Scholar]
- Wang HY, Undie AS, Friedman E. Evidence for the coupling of Gq protein to D1-like dopamine sites in rat striatum: possible role in dopamine-mediated inositol phosphate formation. Molecular Pharmacology. 2003;48:988–94. [PubMed] [Google Scholar]
- Willcutt EG, Pennington BF, Olson RK, Chhabildas N, Hulslander J. Neuropsychological analyses of comorbidity between reading disability and attention deficit hyperactivity disorder: in search of the common deficit. Developmental Neuropsychology. 2005;27:35–78. doi: 10.1207/s15326942dn2701_3. [DOI] [PubMed] [Google Scholar]
- Williams GV, Castner SA. Under the curve: critical issues for elucidating D1 receptor function in working memory. Neuroscience. 2006;139:263–76. doi: 10.1016/j.neuroscience.2005.09.028. [DOI] [PubMed] [Google Scholar]
- Xu M, Hu XT, Cooper DC, Moratalla R, Graybiel AM, White FJ, et al. Elimination of cocaine-induced hyperactivity and dopamine-mediated neurophysiological effects in dopamine D1 receptor mutant mice. Cell. 1994a;79:945–55. doi: 10.1016/0092-8674(94)90026-4. [DOI] [PubMed] [Google Scholar]
- Xu M, Moratalla R, Gold LH, Hiroi N, Koob GF, Graybiel AM, et al. Dopamine D1 receptor mutant mice are deficient in striatal expression of dynorphin and in dopamine-mediated behavioral responses. Cell. 1994b;79:729–42. doi: 10.1016/0092-8674(94)90557-6. [DOI] [PubMed] [Google Scholar]
- Zhuang X, Belluscio L, Hen R. G(olf)alpha mediates dopamine D1 receptor signaling. Journal of Neuroscience. 2000;20:RC91. doi: 10.1523/JNEUROSCI.20-16-j0001.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zill P, Engel R, Baghai TC, Zwanzger P, Schule C, Minov C, et al. Analysis of polymorphisms in the olfactory G-protein Golf in major depression. Psychiatric Genetics. 2002;12:17–22. doi: 10.1097/00041444-200203000-00002. [DOI] [PubMed] [Google Scholar]
- Zill P, Malitas PN, Bondy B, Engel R, Boufidou F, Behrens S, et al. Analysis of polymorphisms in the alpha-subunit of the olfactory Gprotein Golf in lithium-treated bipolar patients. Psychiatric Genetics. 2003;13:65–9. doi: 10.1097/01.ypg.0000057881.80011.45. [DOI] [PubMed] [Google Scholar]