Abstract
Purpose
To explore the effects of personalized numeric prognostic information on physicians’ intentions to communicate prognosis to cancer patients at the end of life, and to identify factors that moderate these effects.
Methods
A factorial experiment was conducted in which 93 Family Medicine physicians were presented with a hypothetical case vignette depicting an end-stage gastric cancer patient seeking prognostic information. Physicians’ intentions to communicate prognosis were assessed before and after provision of personalized prognostic information, while emotional distress of the patient and ambiguity (manifest by imprecision) of the prognostic estimate were varied between subjects. General linear models were used to test the effects of personalized prognostic information, patient distress, and ambiguity on prognostic communication intentions, and explored potential moderating effects of: 1) perceived patient distress, 2) perceived credibility of prognostic models, 3) physician numeracy (objective and subjective), and 4) physician aversion to risk and ambiguity.
Results
Provision of personalized prognostic information increased prognostic communication intentions (p<.001, η2=.38), although experimentally-manipulated patient distress and prognostic ambiguity had no effects. Greater change in communication intentions was positively associated with higher perceived credibility of prognostic models (p=.007, η2=.10), higher objective numeracy (p=.01, η2=.09), female sex (p=.01, η2=.08), and lower perceived patient distress (p=.02, η2=.07). Intentions to communicate available personalized prognostic information were positively associated with higher perceived credibility of prognostic models (p=.02, η2=.09), higher subjective numeracy (p=.02, η2=.08), and lower ambiguity aversion (p=.06, η2=.04).
Conclusions
Provision of personalized prognostic information increases physicians’ prognostic communication intentions to a hypothetical end-stage cancer patient, and situational and physician characteristics moderate this effect. More research is needed to confirm these findings and to elucidate the determinants of prognostic communication at the end of life.
Introduction
Prognostic information is critically important in health care.1,2 For clinical decisions across the health care continuum—from disease prevention and screening to disease treatment and end-of-life (EOL) care—prognostic information allows physicians and patients to determine the appropriateness of medical interventions and to make decisions that are informed and consistent with patients’ values and preferences. This need is particularly great at the end of life, when patients must come to terms with impending death, and determine whether or when to pursue or forego curative or palliative treatment goals and interventions. These difficult tasks depend on prognostic information, which must thus be effectively communicated to patients. Empirical evidence suggests, furthermore, that terminally ill patients desire such information.1–3
Yet physicians have been shown to be reluctant to communicate prognostic information to patients.2,4–7 The reasons are multiple and incompletely understood, but include physicians’ lack of confidence in the accuracy of their own prognostic judgments.4,8–10 This lack of confidence is justifiable, given that physicians’ prognostic judgments have been shown to be inaccurate and optimistically biased, and evidence-based prognostic information has historically been lacking.9,10 The latter situation, however, has begun to change in recent years with the development of a growing number of clinical prediction models (CPMs)—multivariable statistical algorithms that utilize characteristics of patients, diseases, and treatments to produce personalized estimates of the probability of future health outcomes including mortality and survival.11,12 CPMs for estimating prognosis at the end of life have been increasingly disseminated—e.g., on public websites such as eprognosis.org—and implemented in clinical practice, and now provide physicians and patients with the prognostic information they need to make EOL care decisions that are informed, individualized, and based on patient values. A growing number of CPMs estimate short-term (6–12 month) survival, and may thus be useful for acute, critical EOL care decisions—in both hospitalized and non-hospitalized patients— including decisions to pursue palliative vs. curative treatment goals, or to refer patients for hospice care.13–17
Whether the availability of CPMs will increase the extent to which physicians communicate prognostic information to patients, however, remains unclear. The landmark SUPPORT study showed that the mere provision of evidence-based prognostic information does not necessarily enhance the clinical use and communication of such information by physicians.18 Numerous barriers may inhibit physicians’ capacity or willingness to communicate evidence-based, personalized prognostic information to patients.18 Structural factors, for example, are widely acknowledged and include lack of time and financial incentives for clinical discussions of prognosis.2,8,19,20
Situational barriers are another potentially important category and include clinical circumstances (e.g., disease acuity, severity, and trajectory) and patient characteristics as well as available clinical information.8 The emotional state of patients, for example, may be a particularly important barrier to prognostic disclosure by physicians. Patient emotional distress is common at the end of life and may be psychologically aversive to physicians, promoting avoidance of difficult conversations.21 Another potential barrier to prognostic disclosure is the irreducible uncertainty that arises from inherent limitations in the reliability, credibility, and adequacy of all prognostic estimates. This type of uncertainty, which decision theorists have termed “ambiguity,” is known to promote pessimistic appraisals of risk information and avoidance of decision making.22,23 This response may discourage physicians from communicating prognostic information to patients.
Physician characteristics represent a final potential category of barriers to prognostic disclosure at the end of life. Beliefs and attitudes about the credibility or value of prognostic models and evidence, as well as differences in physicians’ numeracy—both the objective and subjective ability to understand and use numbers—may make physicians more or less able to use evidence-based prognostic information and comfortable disclosing such information to patients. Individual differences in physicians’ tolerance of ambiguity may also be influential. Ambiguity-tolerant physicians may be more accepting of the inherent, irreducible limitations in the accuracy and precision of prognostic estimates, and hence more comfortable sharing these estimates with patients. Similar effects might result from individual differences in physicians’ tolerance of uncertainty arising from other sources such as probability or risk (the fundamental indeterminacy of future outcomes).24
Although these and other factors may limit physician communication of prognosis at the end of life, empirical evidence on their effects is lacking and particularly with respect to situational and physician characteristics. The objective of the current study was to begin to address this problem. We conducted a factorial experiment, using a hypothetical vignette, aimed at exploring the potential effects of various situational and physician characteristics on physician interest in communicating evidence-based, personalized prognostic information: 1) patient affective state, 2) ambiguity in prognostic estimates, 3) physician perceptions of the credibility of prognostic models, 4) physician numeracy, and 5) physician tolerance of uncertainty (both risk and ambiguity). The ultimate aim of this experimental vignette study was to generate proof-of-principle evidence to guide more definitive future studies in clinical settings, and to inform the development of interventions to increase the extent and effectiveness of prognostic communication at the end of life.
Methods
Study population and recruitment
The study population consisted of a convenience sample of attending and resident Family Medicine physicians (N=312) belonging to a regional consortium of 5 Family Medicine residency programs in Maine and New Hampshire: Maine Medical Center; Maine-Dartmouth Family Medicine Residency; Eastern Maine Medical Center; Central Maine Medical Center; Dartmouth-Concord Hospital. From November 2013–January 2014, members of this consortium (N=158) participated in a separate study led by the research team, in which each physician completed a battery of measures assessing their tolerance of different types of uncertainty (e.g., risk, ambiguity). For the current study, members of this cohort were recruited individually by targeted emails and verbal communications at department meetings. An incentive of $50 per participating physician was provided to his or her residency program.
Study design
The study consisted of a 2×2 online factorial experiment employing a hypothetical case vignette with prognostic ambiguity and patient affect varied between subjects (Figure 1). The vignette was adapted from a case developed by Barnato et al. and used in a mixed-methods study of physician decision making in EOL care.25 The case depicted a patient with end-stage gastric carcinoma who is admitted to the hospital with respiratory distress, likely secondary to cancer progression. The vignette was modified to incorporate our factors of interest, as summarized in Figure 2. The vignette had two parts: Part 1 presented the case history, Part 2 presented prognostic information.
Figure 1.
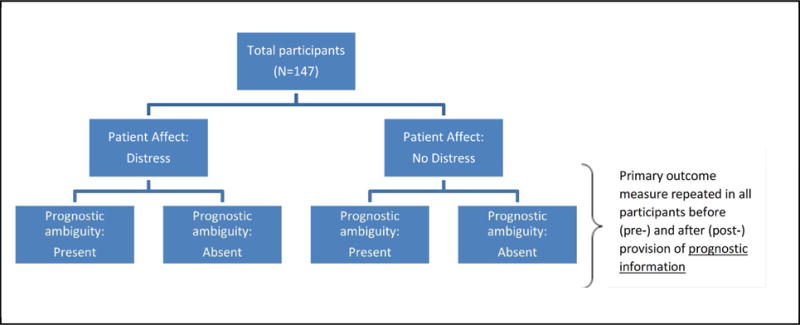
Experimental design and conditions.
Figure 2.

Clinical vignette (alternative experimental text bracketed in bold)
Prognostic information (absent, present) was operationalized in a pre/post manner, by presenting the physician participants with case information in Part 1 (without prognostic information) at which time they responded to measures assessing the outcomes of interest. Participants were then provided with an individualized mortality risk estimate calculated by a hypothetical CPM in Part 2, at which time they responded again to measures assessing the outcomes of interest.
The between-subjects prognostic ambiguity condition (absent, present) was operationalized by communicating a hypothetical individualized mortality risk estimate as either a point estimate (“12-month mortality risk estimate of 78%”) or a range (“12-month mortality risk estimate of 70–86%”). The between-subjects patient affect condition (emotional distress high, emotional distress low) was operationalized by the presence vs. absence of language describing a patient and family member as either distressed, tearful, and apprehensive. Participants were randomly assigned to experimental groups, and sent an email containing a URL to access the online study survey hosted through REDCap™, a secure, HIPAA compliant, web-based survey application.
Measures
Outcome measure
Prognostic communication intentions were measured using two items administered both before and after provision of prognostic estimates: 1) “How comfortable do you feel discussing prognosis with this patient and his family member?” and 2) “How likely are you to provide this patient and his family member with an estimate of prognosis?” Both questions utilized six-point Likert scales ranging from 0=“Not at all” to 5=“Extremely.” These questions were asked both before and after provision of prognostic estimates to each participant. Responses were averaged to form a single scale score (αbefore=.76 and αafter=.85).
Covariates and moderators
Physician characteristics included age, sex, years in practice since residency training, and current proportion of time spent in clinical practice.
Perceived credibility of prognostic models was measured using a 4-item scale (α=.76) developed for this study and ascertaining general perceptions of the accuracy, trustworthiness, and usefulness of prognostic models: 1–2) “In your opinion, how accurate [uncertain] are the risk estimates produced by clinical prediction models in general?”, 3) “Do you think that physicians should trust the risk estimates generated by clinical prediction models?” and 4) “Do you think that physicians should use clinical prediction models when determining prognosis?”. All questions utilized 6-point Likert scales ranging from “Not at all” to “Extremely.” Responses were averaged to form a single scale score.
Risk aversion was measured using the 6-item Pearson Risk Attitude scale (α=.85) adapted from the 20-item risk-taking subscale of the Jackson Personality Index by Pearson et al. to study physicians’ aversion to risk in medical decision making.26 Among emergency room physicians, higher scores on the Risk Aversion scale have been associated with higher rates of admission for patients with acute chest pain.
Ambiguity aversion was measured using the 6-item Ambiguity Aversion in Medicine (AA-Med) scale (α=.65) developed by Han et al. to measure aversion to ambiguity—conceptualized specifically in behavioral decision theory terms, as uncertainty regarding the reliability, credibility, or adequacy of risk information.27 Greater ambiguity aversion has been shown to predict lower interest in a hypothetical ambiguous cancer screening test,27 pessimistic cognitive appraisals of screening tests,28 and lower breast cancer screening intentions.29
Objective numeracy was measured using a three-item scale modified from the Berlin Numeracy Test,30 which was made more relevant to physicians by altering one of the items to make it discuss the proportion of people in a small town who “have a genetic mutation” rather than who are “members of a choir.” A response-option typographical error made one item uninterpretable, leaving a two-item scale with potentially reduced measure reliability.
Subjective numeracy was measured using the STAT-confidence scale, designed to assess confidence in using medical statistics (α=.85).31
Manipulation check
A single item was used to assess the adequacy of the distress manipulation: “How distressed do you think the patient in this scenario was?” In addition, the perceived verisimilitude of the vignette was assessed by a single item, “How realistic is the scenario, based on your practice experience?” The items used a 6-item Likert response scale ranging from “Not at all” to “Extremely.”
Hypotheses
The following hypotheses were tested:
-
H1A series of experimentally-manipulated factors will influence prognostic communication intentions:
-
H1aProvision of prognostic information will increase communication intentions.
-
H1bPrognostic ambiguity will decrease communication intentions.
-
H1cPatient emotional distress will decrease communication intentions.
-
H1a
-
H2A series of factors will moderate the positive effect of providing prognostic information on prognostic communication intentions:
-
H2aRisk aversion: greater risk aversion will decrease the positive effect of prognostic information.
-
H2bAmbiguity aversion: greater ambiguity aversion will decrease the positive effect of prognostic information.
-
H2cPerceived credibility of prognostic models: greater perceived credibility will increase the positive effect of prognostic information.
-
H2dObjective numeracy: greater objective numeracy will increase the positive effect of prognostic information.
-
H2eSubjective numeracy: greater subjective numeracy will be associated with greater intentions to communicate numeric prognostic information.
-
H2a
Data analyses
A general linear modeling framework was used to assess the effect of the experimental manipulations and individual-difference and demographic measures on the primary outcome of communication intentions. Power was not determined a priori, given the study’s opportunistic nature (the sample consisted of an existing study cohort) and the absence of previous similar studies of the outcome of interest. The study was thus exploratory in nature, and aimed at generating initial effect size estimates. The analysis was completed in two stages since the experimental design was not fully crossed (the ambiguity manipulation only applied to Part 2 because no prognostic information was presented in Part 1). First, a linear model—with the change score for prognostic communication intentions as the dependent variable—was used to assess the effects of the provision of personalized prognostic information (i.e., from Part 1 to Part 2 of the vignette) on communication intentions, and to identify predictors of these effects. A second linear model was used to assess factors predicting intentions to communicate prognostic information once it was available (i.e., within Part 2 of the vignette). We used an automated model selection algorithm from the glmulti package in R to test all possible models and rank them by Bayesian Information Criterion (BIC).32 The model with the lowest BIC was retained as the best fitting model. Problems with traditional approaches to model selection include the reliance on arbitrary thresholds for p-values and that backward and forward step-wise methods will not always result in the same final model.33 However, as a sensitivity analysis, we also used a standard backward elimination procedure, which resulted in the same variable selection and pattern of associations. We then examined the final best fitting model, with and without the demographic control variables of gender, age, time since residency, and percent of time spent in clinical practice. All analyses were conducted with the R statistical software package (version 3.2.1).
Results
Of 158 participants in the original uncertainty tolerance study, 24 had either no valid contact information or were lost to follow-up. Of the remaining 134 individuals, 93 completed the experiment (response rate 69%). Participant characteristics are in Table 1.
Table 1.
Study population characteristics
| N | Percent | |
|---|---|---|
| Age | ||
| ≤30 | 23 | 25% |
| 31–40 | 28 | 30% |
| 41–50 | 23 | 25% |
| 51–60 | 14 | 15% |
| 61–70 | 5 | 5% |
| Sex | ||
| Male | 41 | 45% |
| Female | 51 | 55% |
| Program | ||
| Eastern Maine Med Ctr | 20 | 22% |
| Maine-Dartmouth | 18 | 19% |
| Maine Medical Center | 31 | 33% |
| Concord Hospital | 11 | 12% |
| Central Maine Med Ctr | 13 | 14% |
| Years since residency | ||
| 0 | 28 | 34% |
| 1–3 | 11 | 13% |
| 4–9 | 9 | 11% |
| 10–19 | 17 | 21% |
| 20–40 | 16 | 20% |
| Percent clinical time | ||
| 0–19% | 1 | 1% |
| 20–39% | 28 | 30% |
| 40–59% | 10 | 11% |
| 60–79% | 26 | 28% |
| 80–99% | 24 | 26% |
| 100% | 3 | 3% |
Manipulation checks
Participants perceived greater patient distress in the distress than in the non-distress condition (M=4.21 vs. 3.48, t=4.28, p<.001). Overall, 88% of participants rated the vignette as “fairly,” “very,” or “extremely” realistic, while 4% of participants rated it as “a little” or “not at all” realistic and were excluded from subsequent analyses (total N=89).
Provision of personalized prognostic information: Effects and predictors
Consistent with predictions, there was a significant main effect of providing personalized prognostic information on prognostic communication intentions such that intentions were higher once prognostic information was present (M=3.52) as compared to when it was absent (M=2.87), F1,88=53.95, p<.001, η2=.38. Table 2 shows the parameter estimates and 95% CI’s for the best fitting model predicting change in communication intentions both with and without controls. Contrary to predictions, however, there were no significant effects of the experimental factors (patient distress or prognostic ambiguity) on prognostic communication intentions.
Table 2.
Best fitting linear model (with and without controls) for change in communication intention before and after prognostic information (N = 89).
| Predictor | Model 1 | Model 2 |
|---|---|---|
| Ambiguity Aversion | −0.04+ (−0.09, 0.003) |
−0.04 (−0.09, 0.02) |
| Risk Aversion | 0.02 (−0.02, 0.05) |
0.02 (−0.01, 0.06) |
| Credibility of prognostic models | 0.35* (0.04, 0.66) |
0.42** (0.12, 0.72) |
| Subjective numeracy | 0.007 (−0.10, 0.09) |
−0.005 (−0.11, 0.10) |
| Objective numeracy | 0.28* (0.03, 0.54) |
0.34* (0.08, 0.60) |
| Distress rating of patient | −0.27** (−0.47, −0.06) |
−0.25* (−0.47, −0.04) |
| Gender (1=male, 2=female) | – | 0.46** (0.10, 0.83) |
| Age | – | −0.02 (−0.33, 0.30) |
| Years since residency | – |
−0.01 (−0.05, 0.03) |
| Proportion of time spent in clinical | – |
−0.001 (−0.01, 0.005) |
Unstandardized regression coefficients (95% CI);
<.10,
p < .05,
p < .01.
However, several other variables moderated the influence of prognostic information on prognostic communication intentions in ways consistent with predictions. After accounting for demographic controls (Model 2), greater perceived credibility of prognostic models was associated with a larger increase in communication intention (p=.007, η2=.10) (Figure 3a), as was greater objective numeracy (p=.01, η2=.09) (Figure 3b). Furthermore, although patient emotional distress as an experimentally manipulated factor had no demonstrable moderating effect, greater perceived emotional distress was associated with a smaller increase in communication intention following provision of prognostic information when compared to less perceived emotional distress (p=.02, η2=.07) (Figure 3c). Being female was also associated with larger increases in communication intention (p=.01, η2=.08).
Figure 3a.
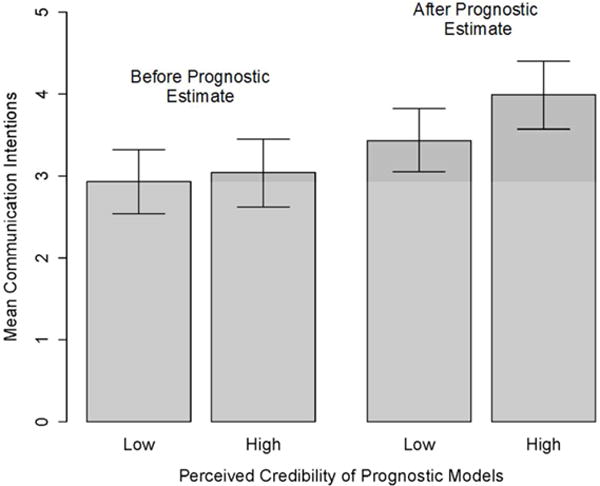
Moderators of the influence of prognostic information on prognostic communication intentions: Perceived credibility of prognostic models.
Figure 3b.
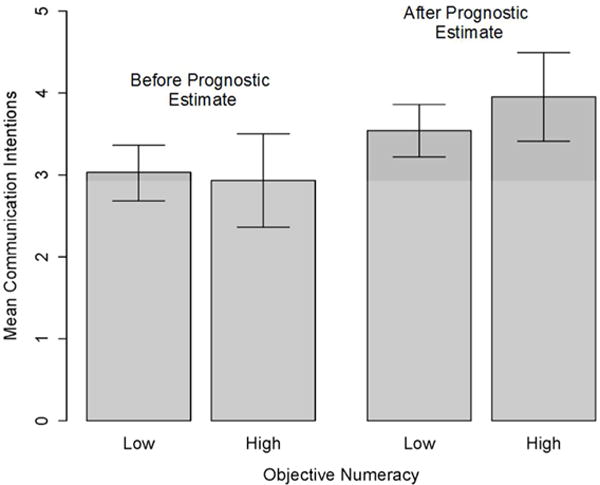
Moderators of the influence of prognostic information on prognostic communication intentions: Objective numeracy.
Figure 3c.
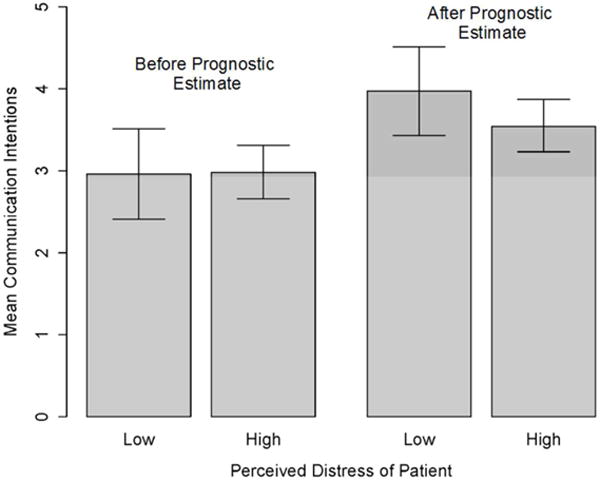
Moderators of the influence of prognostic information on prognostic communication intentions: Perceived patient distress.
Intentions to communicate available prognostic information: Predictors
The next set of models focused on intentions to communicate prognostic information once it was available (i.e., in Part 2 of the vignette), in a completely crossed between-subject comparison. Table 3 shows the parameter estimates and 95% CI’s for the best fitting model predicting communication intentions in part 2 both with and without controls. Contrary to predictions, there were no significant main effects of either experimentally manipulated factor (patient distress or prognostic ambiguity), or of risk aversion. Consistent with predictions, however, several other variables predicted prognostic communication intentions. After accounting for controls (model 2), greater ambiguity aversion was associated with lower prognostic communication intentions (p=.06, η2=.04), whereas greater perceived credibility of prognostic models (p=.02, η2=.09) and greater subjective numeracy (p=.02, η2=.08) were both associated with higher intentions.
Table 3.
Best fitting linear model for communication intentions after receiving prognostic information (N = 89).
| Predictor | Model 1 | Model 2 |
|---|---|---|
| Ambiguity Aversion |
−0.08+ (−0.15, −0.02) |
−0.06+ (−0.14, −0.01) |
| Credibility of prognostic models | 0.49** (0.07, 0.91) |
0.54* (0.97, 1.00) |
| Subjective numeracy | 0.19** (0.06, 0.32) |
0.18* (0.03, 0.34) |
| Objective numeracy | 0.03 (−0.31, 0.37) |
0.05 (−0.34, 0.45) |
| Gender (1=male, 2=female) | – | 0.29 (−0.27, 0.86) |
| Age | – |
−0.24 (−0.71, 0.23) |
| Years since residency | – | 0.02 (−0.04, 0.07) |
| Proportion of time spent in clinical | – |
−0.001 (−0.01, 0.01) |
Unstandardized regression coefficients (95% CI);
<.10,
p < .05,
p < .01.
Discussion
This study explored factors influencing family physicians’ intentions to communicate evidence-based, personalized prognostic information to a seriously ill cancer patient at the end of life. The study utilized a hypothetical vignette, focused on the responses of physicians and not patients, and examined communication intentions rather than actual behaviors; its findings are thus clearly preliminary. To our knowledge, however, the current study is the first to utilize experimental methods to quantify the potential effects of personalized prognostic information on physicians’ prognostic communication to dying patients, and to identify potential moderators of these effects. The study thus provides valuable proof-of-principle evidence to guide future research to understand situational and physician characteristics that influence physicians’ prognostic communication in EOL care.
The study’s primary finding was that the provision of personalized prognostic information increased physicians’ prognostic communication intentions. This finding is consistent with predictions, and endorses the value of efforts to develop and implement clinical prediction models in EOL care. If made available at the point of care, such models might finally provide physicians with the evidence and confidence they need to broach the issue of prognosis with dying patients.21
The equally important finding of our study, however, was that the effect of personalized prognostic information depended on several moderating factors. In our study, the positive effect of providing vs. not providing such information on prognostic communication intentions was associated with greater perceived credibility of prognostic models, greater objective numeracy, less perceived emotional distress of the patient, and physician gender. These findings are consistent with predictions, and add to growing evidence on the variety of ways in which objective numeracy—the ability to use numbers—influences people’s responses to and use of quantitative risk information.34 The negative effect of perceived patient distress on physicians’ prognostic communication intentions may manifest the influence of several factors. For example, people have been shown to neglect probabilistic information in emotionally rich situations,35 and may further avoid discussing prognosis with emotionally distressed patients due to fears of exacerbating emotional distress or undermining hope.36 More research is needed to test these and other alternative explanations.
Once numeric prognostic information was made available to physicians (at Time 2), the likelihood to communicate it was influenced again by greater perceived credibility of prognostic models and also by greater subjective numeracy, and lower aversion to (greater tolerance of) ambiguity. Past research has indicated that physicians lower vs. higher in subjective numeracy are less likely to communicate numbers to explain screening test results to patients.37 The present results are consistent with these findings and add support to the notion that subjective numeracy drives motivational and emotional processes that exert influence—independent of objective numeracy—on the communication of prognostic information.34
These findings need to be confirmed, but are consistent with predictions and have several implications for future research and clinical practice. They suggest the need for further research to identify determinants of the perceived credibility of prognostic models, and to educate physicians about the accuracy, utility, and appropriate clinical use of these models. They also raise the need for more work to understand how ambiguity tolerance develops in physicians, and whether it can be “taught and nurtured.”38 Finally, our findings have practical import for efforts to use evidence-based prognostic models in EOL care. They suggest the importance of establishing and promoting the credibility of such models to physicians. They also suggest the need to enhance physicians’ capacity to not only understand and use quantitative information, but to effectively manage the emotional distress that both patients and physicians may experience during the dying process. More research is needed to determine how best to accomplish these challenging tasks.
The null findings of our study point to additional future research needs. Contrary to predictions, our manipulations of prognostic ambiguity and patient emotional distress did not influence physicians’ intentions to communicate prognostic information. One reason may have been that the study was underpowered, and our findings provide initial effect size information to power more definitive future studies. It is also possible that our experimental manipulations were insufficiently strong to elicit their intended effects. For example, the size of the statistical range used to operationalize ambiguity may have been too narrow to convey a degree of ambiguity that was psychologically or clinically significant. Similarly, although our manipulation check suggested otherwise, the manner in which emotional distress was conveyed in the vignettes may still have been insufficiently strong or vivid to elicit an emotional response; this may be a fundamental limitation of all paper-based vignettes. More research is needed to develop and test alternative experimental manipulations of these factors (e.g., using video stimuli or patient simulation). Finally, our study also found no influence of individual differences in aversion to risk—the other primary source of uncertainty in prognostic estimates39—on prognostic communication intentions. This finding supports the need to distinguish between specific types and sources of uncertainty (e.g., risk, ambiguity, complexity) when measuring uncertainty tolerance and assessing its effects.39
Our study had other limitations that further qualify its conclusions, aside from its hypothetical nature and focus on intentions rather than actual communication behaviors. The sample population was relatively small and limited to Family Medicine physicians in a single predominantly rural geographic region. Family Medicine physicians, furthermore, may have limited familiarity and experience not only estimating prognosis but providing acute hospital-based care for dying cancer patients. Although we believe that such physicians—particularly in rural practice settings—do routinely care for such patients, more work is needed to assess the generalizability of our findings to physicians in other specialties (e.g., oncology) and locations. Another fruitful direction for future work would be to assess the magnitude, accuracy, and associated uncertainty of physicians’ own prognostic estimates. We utilized only one clinical vignette focused on one cancer type, set of clinical circumstances and prognostic estimates, and hypothetical prognostic model. Confirming our findings will require the development and validation of additional vignettes depicting a wider range of clinical scenarios. Finally, our study focused only on the physician-side of the prognostic communication problem; future research should also examine patients’ responses to prognostic information.
Perhaps the most important limitation of our study was its singular focus on only the physician side of physician-patient prognostic communication. Clearly, patient perceptions and responses have equally critical effects on the extent, nature, and outcomes of the communication of personalized prognostic information between physicians and patients. Previous studies have shown, for example, that patients have negative perceptions of the competence of physicians who utilize computerized clinical decision support tools.40,41 Such perceptions may make both physicians and patients reluctant to use these tools. Our own work has also suggested that patients’ utilization of personalized prognostic information is limited by their lack of experience with using such information, a tendency to favor heuristic-over risk-based decision making strategies, and perceptions that model-derived risk information is less valuable than other types of evidence (e.g., emotions, recommendations of trusted physicians, personal narratives).42 More research is needed to examine how these and other patient-level factors influence both patients’ and physicians’ willingness to engage in communication about personalized prognostic information at the end of life.
Despite these limitations, our study provides important seminal evidence that the provision of evidence-based personalized prognostic information may increase physician-patient prognostic communication at the end of life, and that several potentially modifiable factors may enhance this effect. It remains for future research to confirm these findings and establish their generalizability, and to develop effective interventions to improve the use and communication of prognostic information in end-of-life care.
Acknowledgments
The study was funded by Contract HHSN261200800001E from the National Cancer Institute. We thank Deanna Williams for technical assistance in developing the web survey and study database, and to the Family Medicine Residency Programs at Maine Medical Center; Maine-Dartmouth Family Medicine Residency; Eastern Maine Medical Center; Central Maine Medical Center; Dartmouth-Concord Hospital for their participation in this study.
Appendix: Uncertainty Tolerance Measures
| A. |
Ambiguity Aversion in Medicine (Han et al, 2009)21
|
| B. |
Pearson Risk Attitude Scale (Pearson et al, 1995)20
|
All measures used a 6-point Likert response scale, ranging from “Strongly Disagree” to “Strongly Agree”
References
- 1.Clayton JM, Butow PN, Tattersall MH. The needs of terminally ill cancer patients versus those of caregivers for information regarding prognosis and end-of-life issues. Cancer. 2005;103(9):1957–1964. doi: 10.1002/cncr.21010. [DOI] [PubMed] [Google Scholar]
- 2.Hancock K, Clayton JM, Parker SM, et al. Truth-telling in discussing prognosis in advanced life-limiting illnesses: a systematic review. Palliat Med. 2007;21(6):507–517. doi: 10.1177/0269216307080823. [DOI] [PubMed] [Google Scholar]
- 3.Steinhauser KE, Christakis NA, Clipp EC, McNeilly M, McIntyre L, Tulsky JA. Factors considered important at the end of life by patients, family, physicians, and other care providers. JAMA. 2000;284(19):2476–2482. doi: 10.1001/jama.284.19.2476. [DOI] [PubMed] [Google Scholar]
- 4.Lamont EB, Christakis NA. Prognostic disclosure to patients with cancer near the end of life. Ann Intern Med. 2001;134(12):1096–1105. doi: 10.7326/0003-4819-134-12-200106190-00009. [DOI] [PubMed] [Google Scholar]
- 5.Daugherty CK, Hlubocky FJ. What are terminally ill cancer patients told about their expected deaths? A study of cancer physicians’ self-reports of prognosis disclosure. J Clin Oncol. 2008;26(36):5988–5993. doi: 10.1200/JCO.2008.17.2221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cartwright C, Onwuteaka-Philipsen BD, Williams G, et al. Physician discussions with terminally ill patients: a cross-national comparison. Palliat Med. 2007;21(4):295–303. doi: 10.1177/0269216307079063. [DOI] [PubMed] [Google Scholar]
- 7.Gattellari M, Voigt KJ, Butow PN, Tattersall MH. When the treatment goal is not cure: are cancer patients equipped to make informed decisions? J Clin Oncol. 2002;20(2):503–513. doi: 10.1200/JCO.2002.20.2.503. [DOI] [PubMed] [Google Scholar]
- 8.Christakis NA. Death Foretold: Prophecy and Prognosis in Medical Care. Chicago: University of Chicago; 1999. [Google Scholar]
- 9.Christakis NA, Lamont EB. Extent and determinants of error in physicians’ prognoses in terminally ill patients: prospective cohort study. West J Med. 2000;172(5):310–313. doi: 10.1136/ewjm.172.5.310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Glare P, Virik K, Jones M, et al. A systematic review of physicians’ survival predictions in terminally ill cancer patients. BMJ. 2003;327(7408):195–198. doi: 10.1136/bmj.327.7408.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Steyerberg EW. Clinical Prediction Models: a Practical Approach to Development, Validation, and Updating. New York: Springer; 2010. [Google Scholar]
- 12.Glare P, Christakis NA. Overview: advancing the clinical science of prognostication. In: Glare P, Christakis NA, editors. Prognosis in Advanced Cancer. New York: Oxford University Press; 2008. pp. 3–12. [Google Scholar]
- 13.Teno JM, Harrell FE, Jr, Knaus W, et al. Prediction of survival for older hospitalized patients: the HELP survival model. Hospitalized Elderly Longitudinal Project. J Am Geriatr Soc. 2000;48(5 Suppl):S16–24. doi: 10.1111/j.1532-5415.2000.tb03126.x. [DOI] [PubMed] [Google Scholar]
- 14.Fischer SM, Gozansky WS, Sauaia A, Min SJ, Kutner JS, Kramer A. A practical tool to identify patients who may benefit from a palliative approach: the CARING criteria. J Pain Symptom Manage. 2006;31(4):285–292. doi: 10.1016/j.jpainsymman.2005.08.012. [DOI] [PubMed] [Google Scholar]
- 15.Inouye SK, Bogardus ST, Jr, Vitagliano G, et al. Burden of illness score for elderly persons: risk adjustment incorporating the cumulative impact of diseases, physiologic abnormalities, and functional impairments. Med Care. 2003;41(1):70–83. doi: 10.1097/01.MLR.0000039829.60382.12. [DOI] [PubMed] [Google Scholar]
- 16.Walter LC, Brand RJ, Counsell SR, et al. Development and validation of a prognostic index for 1-year mortality in older adults after hospitalization. JAMA. 2001;285(23):2987–2994. doi: 10.1001/jama.285.23.2987. [DOI] [PubMed] [Google Scholar]
- 17.Han PK, Lee M, Reeve BB, et al. Development of a prognostic model for six-month mortality in older adults with declining health. Journal of pain and symptom management. 2012;43(3):527–539. doi: 10.1016/j.jpainsymman.2011.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Investigators TSP. A controlled trial to improve care for seriously ill hospitalized patients. The study to understand prognoses and preferences for outcomes and risks of treatments (SUPPORT) JAMA. 1995;274(20):1591–1598. [PubMed] [Google Scholar]
- 19.Clayton JM, Hancock KM, Butow PN, et al. Clinical practice guidelines for communicating prognosis and end-of-life issues with adults in the advanced stages of a life-limiting illness, and their caregivers. Med J Aust. 2007;186(12 Suppl):S77, S79, S83–108. doi: 10.5694/j.1326-5377.2007.tb01100.x. [DOI] [PubMed] [Google Scholar]
- 20.Lamont EB, Christakis NA. Physician factors in the timing of cancer patient referral to hospice palliative care. Cancer. 2002;94(10):2733–2737. doi: 10.1002/cncr.10530. [DOI] [PubMed] [Google Scholar]
- 21.Hallen SA, Hootsmans NA, Blaisdell L, Gutheil CM, Han PK. Physicians’ perceptions of the value of prognostic models: the benefits and risks of prognostic confidence. Health Expect. 2014 doi: 10.1111/hex.12196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ellsberg D. Risk, ambiguity, and the Savage axioms. Quart J Econ. 1961;75:643–669. [Google Scholar]
- 23.Camerer C, Weber M. Recent developments in modeling preferences: uncertainty and ambiguity. Journal of Risk and Uncertainty. 1992;5:325–370. [Google Scholar]
- 24.Han PK. Conceptual, methodological, and ethical problems in communicating uncertainty in clinical evidence. Med Care Res Rev. 2013;70(1 Suppl):14S–36S. doi: 10.1177/1077558712459361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Barnato AE, Hsu HE, Bryce CL, et al. Using simulation to isolate physician variation in intensive care unit admission decision making for critically ill elders with end-stage cancer: a pilot feasibility study. Crit Care Med. 2008;36(12):3156–3163. doi: 10.1097/CCM.0b013e31818f40d2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pearson SD, Goldman L, Orav EJ, et al. Triage decisions for emergency department patients with chest pain: do physicians’ risk attitudes make the difference? J Gen Intern Med. 1995;10(10):557–564. doi: 10.1007/BF02640365. [DOI] [PubMed] [Google Scholar]
- 27.Han PK, Reeve BB, Moser RP, Klein WM. Aversion to ambiguity regarding medical tests and treatments: measurement, prevalence, and relationship to sociodemographic factors. J Health Commun. 2009;14(6):556–572. doi: 10.1080/10810730903089630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Han PK, Williams AE, Haskins A, et al. Individual differences in aversion to ambiguity regarding medical tests and treatments: association with cancer screening cognitions. Cancer Epidemiol Biomarkers Prev. 2014;23(12):2916–2923. doi: 10.1158/1055-9965.EPI-14-0872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yeh VM, Schnur JB, Margolies L, Montgomery GH. Dense Breast Tissue Notification: Impact on Women’s Perceived Risk, Anxiety, and Intentions for Future Breast Cancer Screening. Journal of the American College of Radiology: JACR. 2015;12(3):261–266. doi: 10.1016/j.jacr.2014.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cokely ET, Galesic M, Schulz E, Ghazal S, Garcia-Retamero R. Measuring risk literacy: The Berlin numeracy test. Judgment and Decision Making. 2012;7(1):25–47. [Google Scholar]
- 31.Woloshin S, Schwartz LM, Welch HG. Patients and medical statistics. Interest, confidence, and ability. J Gen Intern Med. 2005;20(11):996–1000. doi: 10.1111/j.1525-1497.2005.00179.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Calcagno V, de Mazancourt M. glmulti: An R Package for Easy Automated Model Selection with (Generalized) Linear Models. Journal of Statistical Software. 2010;34(12):1–29. [Google Scholar]
- 33.Venables WN, Ripley BD. Modern applied statistics with S-PLUS. 3rd. New York, NY: Springer-Verlag; 1997. [Google Scholar]
- 34.Peters E, Bjalkebring P. Multiple numeric competencies: When a number is not just a number. J Pers Soc Psychol. 2015;108:802–822. doi: 10.1037/pspp0000019. [DOI] [PubMed] [Google Scholar]
- 35.Hsee CK, Rottenstreich Y. Music, Pandas, and Muggers: On the Affective Psychology of Value. J Exper Psych: Gen. 2004;133:23–30. doi: 10.1037/0096-3445.133.1.23. [DOI] [PubMed] [Google Scholar]
- 36.Clayton JM, Hancock K, Parker S, et al. Sustaining hope when communicating with terminally ill patients and their families: a systematic review. Psychooncology. 2008;17(7):641–659. doi: 10.1002/pon.1288. [DOI] [PubMed] [Google Scholar]
- 37.Anderson BL, Obrecht NA, Chapman GB, Driscoll DA, Schulkin J. Physicians’ communication of Down syndrome screening test results: the influence of physician numeracy. Genetics in medicine: official journal of the American College of Medical Genetics. 2011;13(8):744–749. doi: 10.1097/GIM.0b013e31821a370f. [DOI] [PubMed] [Google Scholar]
- 38.Geller G. Tolerance for ambiguity: an ethics-based criterion for medical student selection. Acad Med. 2013;88(5):581–584. doi: 10.1097/ACM.0b013e31828a4b8e. [DOI] [PubMed] [Google Scholar]
- 39.Han PK, Klein WM, Arora NK. Varieties of uncertainty in health care: a conceptual taxonomy. Med Decis Making. 2011;31(6):828–838. doi: 10.1177/0272989X11393976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Arkes HR, Shaffer VA, Medow MA. Patients derogate physicians who use a computer-assisted diagnostic aid. Med Decis Making. 2007;27(2):189–202. doi: 10.1177/0272989X06297391. [DOI] [PubMed] [Google Scholar]
- 41.Shaffer VA, Probst CA, Merkle EC, Arkes HR, Medow MA. Why do patients derogate physicians who use a computer-based diagnostic support system? Med Decis Making. 2013;33(1):108–118. doi: 10.1177/0272989X12453501. [DOI] [PubMed] [Google Scholar]
- 42.Han PK, Hootsmans N, Neilson M, et al. The value of personalised risk information: a qualitative study of the perceptions of patients with prostate cancer. BMJ open. 2013;3(9):e003226. doi: 10.1136/bmjopen-2013-003226. [DOI] [PMC free article] [PubMed] [Google Scholar]


