Abstract
Despite the enormous societal burden of alcohol and drug addiction and abundant research describing drug-induced maladaptive synaptic plasticity, there are few effective strategies for treating substance use disorders. Recent awareness that synaptic plasticity involves astroglia and the extracellular matrix is revealing new possibilities for understanding and treating addiction. We first review constitutive corticostriatal adaptations that are elicited by and shared between all abused drugs from the perspective of tetrapartite synapses, and integrate recent discoveries regarding cell-type specificity in striatal neurons. Next, we describe recent discoveries that drug-seeking is associated with transient synaptic plasticity that requires all four synaptic elements and is shared across drug classes. Finally, we prognosticate how considering tetrapartite synapses can provide new treatment strategies for addiction.
Keywords: drug addiction, synaptic plasticity, tetrapartite synapses, extracellular matrix
The Fourth Dimension of Drug Addiction
Iconic synaptic connections between neurons consist of specialized morphology and patterned expression of proteins in both pre- and post-synaptic elements. The traditional bipartite synapse provides mechanisms for regulating transmitter release probability and grading postsynaptic responses through ionotropic receptor-mediated ion fluxes and metabotropic receptor intracellular signaling cascades. Excitatory glutamatergic synapses constitute a majority of synaptic contacts in the brain, and extensive research over the last 50 years provides deep understanding not only of signaling produced by acute transmitter release, but how different stimulation patterns change synaptic efficacy by causing enduring changes in release probability and/or postsynaptic responses. Thus, long-term potentiation (LTP) and depression (LTD) are canonical changes in synaptic efficacy whereby the brain codes environmental experiences, and links these experiences to both adaptive behaviors and the behavioral changes characterizing drug addiction [1].
Glia account for over 50% of all cells in the brain, and traditionally function as structural and metabolic support for neurons [2]. In 1999, Araque et al. coined the term ‘tripartite’ synapse to account for the emerging role by astroglia in contributing to bipartite synaptic neurotransmission [3]. Astroglial processes tightly ensheath many excitatory synaptic contacts, and the patterned expression of glial excitatory amino acid transporters (EAAT1 and EAAT2) near the synaptic cleft is critical for eliminating synaptically released glutamate and maintaining the fidelity of synaptic transmission [4, 5]. More recent studies reveal that astroglia can release neuroactive molecules and thereby regulate synaptic transmission, termed gliotransmission (see Glossary). Changes in glial protein expression and morphology accompany enduring synaptic plasticity [6–9], and accumulating data demonstrate that astroglia have an important role in dynamically regulating drug-induced structural and functional plasticity [10].
The last 10–15 years has seen the gradual recognition that a fourth signaling domain plays a remarkable role in regulating synaptic transmission. Dityatev and colleagues proposed the term ‘tetrapartite’ synapse in 2010 to include the proteinacious extracellular matrix (ECM) as a key fourth element [11]. The ECM surrounds all components of the tripartite synapse (Figure 1A) and binds to glial and neuronal membrane receptors via cell adhesion molecules (CAMs). Transmembrane CAMs, such as integrins, ephrins, and cadherins play key roles in the development and maintenance of synapses and synaptic plasticity in the adult brain mediated via cell-cell and cell-ECM signaling cascades [12–15]. ECM proteins are secreted by both neurons and glia, which also release proteins that regulate ECM signaling via protein degradation [14, 16, 17], and, as first identified in 2002 by Kaczmarek and colleagues [18], catabolic activation of the ECM regulates dendritic spine morphology and synaptic plasticity in the mature CNS (for reviews, see [14, 17]). Key catabolic regulators of ECM signaling are the matrix metalloproteases (MMPs) that respond to synaptic activity and activate or inactivate proteins in the ECM [19]. Given the facts that the ECM thoroughly populates the extracellular space and that the extracellular space accounts for ~20% of human neuropil volume [19, 20], it is surprising that the ECM has been so slow to emerge as a critical component of drug-induced synaptic plasticity. MMP degradation of the ECM was first implicated in addiction by Wright and colleagues in 2003 ([21]; for reviews, see [17, 22–24]), and in this review we highlight the recent findings indicating that the deep focus on drug-induced synaptic plasticity in corticostriatal synapses needs to consider all four synaptic elements (Figure 2). Additionally, embedded throughout this review is a comparison of the synaptic plasticity induced by different chemical classes of addictive drugs that allows us to explore the hypothesis that the molecular underpinnings of synaptic plasticity that are shared between classes of addictive drug are most relevant for treating shared symptoms of drug addiction, such as the persistent vulnerability to relapse. We further suggest tetrapartite synaptic mechanisms of addiction may be a platform for integrating the relevant literatures generated for each drug class into a more unified understanding of the neurobiology of relapse to drug use.
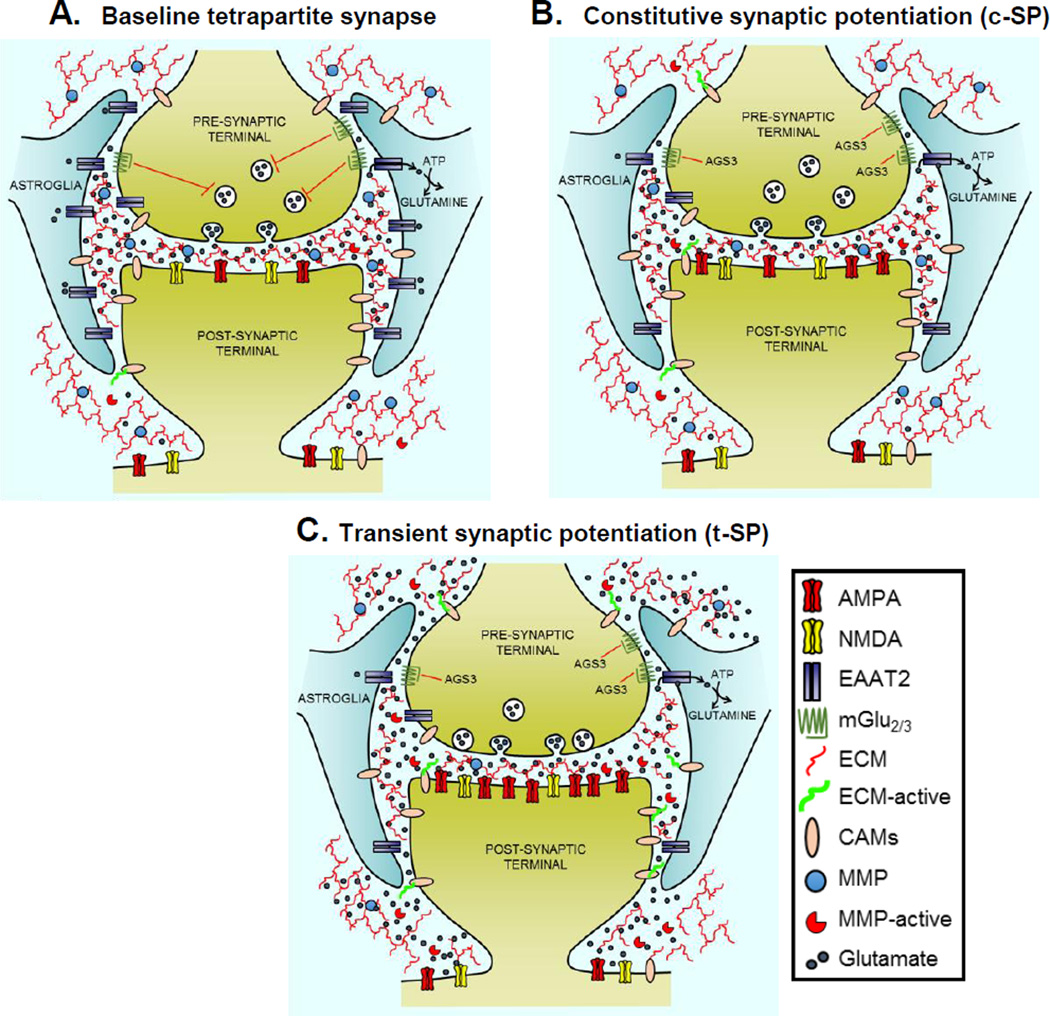
Figure 1.
Overview of the structural components of tetrapartite corticostriatal synapses (panel A), the constitutive adaptations (c-SP) seen after extended withdrawal from drug use (panel B), and the transient changes (t-SP) produced during cue- or drug-induced reinstatement of drug seeking (panel C). (A) Tetrapartite cortico-accumbens synapse consists of a pre- and postsynapse, astroglial fine processes surround the synaptic cleft, and the lattice-like structure of extracellular matrix (ECM) proteins. Signaling in the ECM is regulated by the catabolic activity of MMPs. Also shown are a subset of critical proteins that are affected by abused drugs, including AMPA and NMDA receptors, glial glutamate transporters (EAAT2), presynaptic mGlu2/3 signaling to provide inhibitory regulation of glutamate release probability, and cell adhesion molecules (CAMs). (B) Overview of the constitutive changes in protein function seen after extended withdrawal from addictive drugs. Constitutive changes shared by all drugs examined include down-regulated EAAT2 and signaling through presynaptic mGlu2/3 due to an increase in AGS3. Constitutive changes shared by all drugs tested except opioids include increases in AMPA receptors (including CP-receptors in the incubation paradigm), MMP2 activity to catabolically activate the ECM, and spine head diameter and/or spine number (not illustrated). None of these latter adaptations associated with postsynaptic c-SP are seen after withdrawal from heroin or morphine. (C) Illustration of the transient changes produced by presenting a drug-conditioned cue to reinstate lever pressing that are shared by all drugs tested to date (cocaine, nicotine and heroin). All transient changes illustrated occur in parallel with reinstated behavior and dissipate along with reinstated lever pressing by 120 min. Because of down-regulated EAAT2 and mGlu2/3, glutamate is more readily released and diffuses outside the synaptic cleft (seen for heroin, alcohol, nicotine, methamphetamine, cocaine). There is a marked increase in spine head diameter (not illustrated) and AMPA:NMDA ratio. Finally, there is an increase in MMP-9 activity and catabolic signaling through CAMs, possibly integrins.
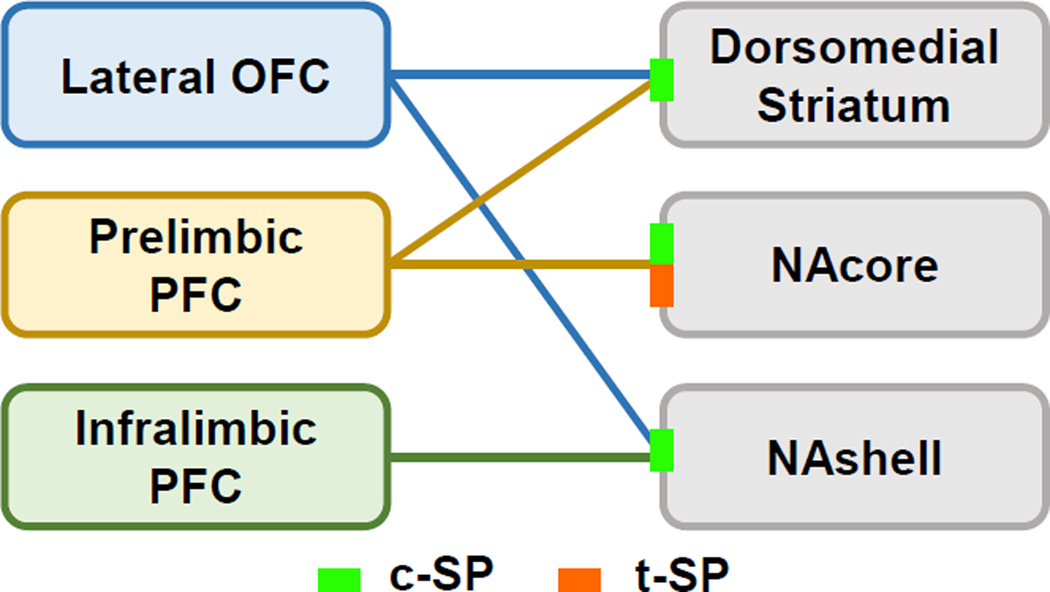
Figure 2.
Circuit diagram depicting glutamatergic corticostriatal projections that are affected by multiple classes of addictive drugs. Excitatory projections from PFC pyramidal neurons innervate dopamine D1R–and D2R–expressing medium spiny neurons in the dorsal and ventral subregions of the striatum. Pyramidal neurons in the lateral orbitofrontal cortex (OFC) project to neurons in the dorsomedial (DMS) and nucleus accumbens shell (NAshell). Excitatory projection neurons in the prelimbic and infralimbic prefrontal cortex (PFC) innervate the nucleus accumbens core (NAcore) and NAshell, respectively, and prelimbic pyramidal neurons also send excitatory projections to the DMS. Green line indicates that corticostriatal synapses in all three striatal subregions undergo one or another form of constitutive synaptic potentiation (c-SP), orange line indicates that only the PFC projections to the NAcore have to date been shown to undergo transient synaptic potentiation (t-SP) in response to a cue-, context-, or drug-induced reinstatement of drug-seeking.
Modeling Drug Addiction to Study Maladaptive Synaptic Plasticity
Research into the neurobiology of drug use generally focuses on two phases of the clinical syndrome. 1) Study of the transitory effects of acute drug administration and effects of early withdrawal from repeated drug use (see Box 1 for animal models used to provoke drug seeking). This strategy is most useful to understand the sequence of events that lead to the acquisition of drug dependence and compulsive drug-seeking behaviors. In general, research into the acquisition of drug seeking behaviors has triangulated on shared involvement of drug-facilitated dopamine release and signaling for developing drug-seeking behaviors across all chemical classes of addictive drug [25, 26]. 2) The second strategy is to study the long-lasting, constitutive changes in brain biology that underpin the enduring vulnerability to relapse. Akin to the ‘common mechanism’ status achieved by dopamine transmission as necessary for developing drug-seeking behaviors regardless of class of addictive drug, in this review we propose that shared corticostriatal glutamatergic plasticity is a ‘common mechanism’ for all addictive drugs. Accordingly, we focus discussion on the second phase of the clinical syndrome, the constitutive synaptic plasticity that endures after many weeks of withdrawal and may underpin the enduring vulnerability to relapse. In addition, we introduce recent experiments studying a third phase of the clinical syndrome, the synaptic plasticity accompanying the execution of drug seeking behavior that is initiated by a drug-associated contexts and cues.
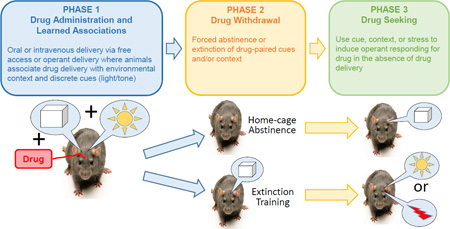
Box 1. Animal Models of Addiction Used to Study Corticostriatal Plasticity.
Animal models of addiction can be readily broken into two general protocols, noncontingent investigator administered drug and contingent self-administration of drug. Over the last 30 years there has been a migration away from noncontingent towards the more anthropomorphic self-administration models. Furthermore, the endeavor to anthropomorphize the self-administration model has inspired continuing evolution in duration of drug exposure and in characterizing an ‘addicted’ phenotype in a vulnerable subpopulation of animals. The evolution in model complexity is a trade-off in the experimental efficiency of noncontingent paradigms in favor of increasing face-validity, but it remains to be seen whether the anthropomorphic evolution of rodent models will benefit the treatment of human addiction. Nonetheless, when operationally defined in terms of rodent not human behavior, the distinct and overlapping neuroadaptations produced with different models identifies an array of molecular and circuit mechanisms that may be shared with humans.
To study addiction-associated adaptations in tetrapartite corticostriatal synapses, the various models employ the three stages shown below. Measurements are made at the end of Phase 2 to assess long-term, constitutive changes produced by drug administration that may create susceptibility to relapse. To assess the neurobiology of the drug seeking event itself, measurements are made during Phase 3. Almost all data discussed in this review are derived from Phase 2 and 3 using contingent drug self-administration in Phase 1, and the reader is referred to recent reviews outlining the noncontingent protocols [122, 123].
Contingent self-administration of drug in Phase 1 involves the intravenous or oral delivery of drug that is initiated by the animal. This can be an operant protocol where the animal lever presses or nose-pokes for drug, or it can be simply choosing between a bottle containing alcohol or water. Regardless, the animal learns to obtain drug and in the process develops learned associations with the drug-paired context and discrete environmental cues, such as a light or tone that signal drug delivery.
Drug seeking after forced abstinence [124]
When an animal is made abstinent without extinction training (Phase 2), return to the drug-paired context, with or without discrete cues previously associated with drug delivery triggers operant responding without drug delivery (Phase 3). Associated with this protocol is an ‘incubation’ process whereby the intensity of drug-seeking increases in proportion to the length of abstinence.
Reinstated drug seeking after extinction training [124]
Drug seeking can be triggered in an extinguished context by restoring a discrete cue previously associated with drug delivery or by providing a novel stress such as footshock. Thus, the animal is placed into the drug context without drug delivery until the operant response is extinguished (Phase 2), then the discrete cue (typically a light or tone) is restored to the operant response and the animal reinstates operant responding in the absence of drug delivery (Phase 3).
Protocols with increasing face validity [125]
Efforts to anthropomorphize self-administration and reinstatement behavior have involved two general modifications. First, the animal is allowed greater access to the drug (longer time per day and/or more days). In this case drug use generally escalates over time, which is proposed to model compulsive drug use. The second approach is to associate drug delivery with punishment, such as footshock, and set the punishment threshold such that a subpopulation of animals will continue to seek drug. These punishment ‘resistant’ animals are proposed to equate to the subpopulation of humans that develop addicted drug use.
Protocols unique to alcohol consumption
Like humans, rodents readily self-administer most addictive drugs, however, they typically do not drink alcohol in large enough amounts to model alcohol use disorder. Two strategies are employed to engender persistent heavy alcohol intake in rodents. The first is to give animals intermittent access to alcohol (24 h access with 24–48 h abstinence between drinking days), which causes the animals to escalate drinking over several weeks before reaching a stable baseline of heavy consumption and pharmacologically relevant blood alcohol levels (>0.08 g/dL). A second strategy for producing high drinking in rodents is to mix noncontingent with contingent drug administration. Most studies expose rodents to alcohol vapor in inhalation chambers in an intermittent fashion (16 h inhalation/8 h withdrawal) for 1–4 weeks, and contingent home-cage drinking or operant responding for oral alcohol self-administration is monitored before and after dependence is noncontingently induced in the inhalation chambers. This model is extensively used to study Phase 2 c-SP in corticostriatal circuitry, and may prove useful for studying the neurobiology of Phase 3 alcohol seeking.
Drug Withdrawal and Constitutive Corticostriatal Synaptic Plasticity
Presynaptic and astroglial constitutive plasticity
Among the earliest observations regarding enduring shared adaptations in corticostriatal synapses is drug-induced down-regulation of signaling through mGlu2/3 presynaptic inhibitory autoreceptors and/or decreased expression and function of EAAT2 astroglial glutamate transporters [27–32]. For example, there is a deficit of mGlu2 expression in the prefrontal cortex (PFC) and nucleus accumbens of alcohol and cocaine treated rats and in human anterior cingulate cortex from alcoholics [31, 33–35]. Reduced mGlu2/3 signaling arises from down-regulated protein expression and/or up-regulated activator of G-protein signaling 3 (AGS3), which binds to and thereby inactivates Gia and promotes Gβγ signaling through mGlu2/3 [34, 36–38]. One or both of these constitutive adaptations are shared by alcohol, heroin, methamphetamine, and cocaine (Table 1). By increasing release probability and reducing glutamate elimination, these presynaptic and glial adaptations, respectively, facilitate elevated extracellular glutamate levels and glutamate spillover in the accumbens core (NAcore) during withdrawal and drug-, stress-, or cue-induced reinstatement of drug-seeking behavior. Synaptic glutamate spillover is derived from PFC afferents in NAcore [39, 40], and it occurs during reinstated drug-seeking for cocaine [39–43], methamphetamine [44], heroin [45], nicotine [28] and alcohol [46–48], but not for natural rewards such as food- or sucrose-seeking. In addition to driving elevated extracellular glutamate levels, these presynaptic and glial adaptations are critical for maintaining drug intake since stimulating mGlu2/3 agonists or inhibiting AGS3 binding to Gia prevent drug seeking in alcohol [31, 36, 47], heroin [38, 49], cocaine [37, 50, 51], methamphetamine [52], or nicotine [53] trained rodents. In support of the hypothesis that loss of mGlu2 autoreceptor control of glutamatergic signaling in the PFC is a key mechanism driving relapse, restoring mGlu2 expression in the infralimbic PFC prevents escalated drinking in alcohol dependent rats [34]. Similarly, restoring EAAT2 or chemogenetically stimulating astrocytes in NAcore inhibits reinstated drug-seeking across drug classes [8, 9, 43, 54–56], which is mediated by preventing synaptic glutamate spillover [32, 55, 57, 58]. Thus, mGlu2/3- and EAAT2-regulation of glutamatergic signaling are shared constitutive neuroadaptations across multiple drugs of abuse that may promote continued drug seeking behaviors.
Table 1.
Similar presynaptic and astroglial constitutive plasticity in cortico-accumbens glutamatergic synapses across drug classes that facilitate drug seeking behaviors.
Postsynaptic Constitutive Plasticity during Withdrawal
Recent research has identified a number of postsynaptic changes in corticostriatal circuitry that are best characterized in cocaine or alcohol treated animals, and include increases in AMPA receptor currents [30, 59–63], adaptations in thin dendritic spine density [63–68] and spine head size [30, 64, 65, 69–74], and altered capacity to induce synaptic plasticity [30, 61, 62, 66, 71, 75–80] (Table 2). These functional and morphological measures are consistent after protracted withdrawal from cocaine, nicotine and alcohol and constitute constitutive synaptic potentiation (c-SP) in corticostriatal circuits (Figure 1B), which occurs predominantly in dopamine D1R-, and to a lesser extent or not at all in D2R–expressing striatal medium spiny neurons (MSNs) [28, 63, 77, 81–83]. However, withdrawal from opioids does not produce any of these postsynaptic biomarkers of c-SP [84, 85]. This discrepancy indicates that postsynaptic c-SP may not be a candidate for the drug-induced plasticity critical for the shared behavioral phenotype of cue/context-reinstated drug-seeking. Nonetheless, although not shared across all drug classes, drug-seeking associated with drug-specific c-SP can be inhibited by reversing that specific adaptation. For example, withdrawal from cocaine is associated with the incorporation of calcium permeable AMPA receptors, and selectively blocking with Naspm or optogenetically reversing expression of these receptors selectively in D1 MSNs prevents augmented cocaine seeking after extended withdrawal (referred to as incubation, Box 1) [83, 86]. Also, the incubation of cocaine seeking is prevented by optogenetically depotentiating prelimbic cortex to NAcore synapses [87]. In addition, manipulating presynaptic (mGlu2/3) or astroglial (EAAT2) drug-induced adaptations in cocaine-withdrawn rats prevents postsynaptic c-SP and cue-induced reinstatement [57].
Table 2.
Similar postsynaptic and extracellular martix drug-induced constitutive synaptic plasticity in glutamatergic synapses in cortico-accumbens circuits across all drugs, except heroin.
| AMPA Receptor Function |
Spine Head Diameter |
Long-Term Potentiation/Long Term Depression |
Extracellular Matrix |
Refs | |
|---|---|---|---|---|---|
| Alcohol | ↑ | ↑ | ↓ LTD | ↑ PNNs ↑ CSPGs ↑ MMP-9 |
[60, 62, 63, 66, 75, 77, 91, 92, 95] |
| Cocaine | ↑ | ↑ | ↓ LTD/LTP | ↑ MMP-2 activity | [30, 69, 72, 76, 78, 79, 81–83, 99, 106, 118] |
| Nicotine | ↑ | ↑ | No data | ↑ MMP-2/9 activity | [28, 99] |
| Heroin | --* | -- | ↓ LTD/LTP | ↑ CSPGs -- MMP activity |
[58, 84, 98, 99] |
-- = no change
In contrast to postsynaptic potentiation produced by all addictive drugs except opioids, there is reduced capacity to electrically induce corticostriatal LTP and/or LTD in animals withdrawn from cocaine, heroin, or alcohol (Table 2) [66, 75–80]. The loss of LTD after extended cocaine exposure is associated with perseverative lever pressing in the presence of footshock stress [78]. For alcohol, the loss of LTD is present in D1R–expressing MSNs [75, 77]. Taken together, the literature indicates that the constitutive postsynaptic plasticity produced across classes of addictive drugs in accumbens neurons is a reduction in the ability to induce LTP and LTD, while changes in AMPA receptor insertion or spine morphology are drug specific, notably not produced by opioids such as morphine or heroin.
In addition to the accumbens, c-SP is produced by addictive drugs in deep-layer orbitofrontal cortex (OFC) and PFC pyramidal projection neurons [61, 71]. Chronic intermittent alcohol exposure markedly enhances spike timing-dependent LTP in layer V pyramidal neurons in the OFC and prelimbic PFC [61, 71]. This aberrant LTP is associated with deficits in behavioral flexibility, morphological adaptations in dendritic spines, and increases in synaptic glutamate receptor function [61, 65, 71]. Similarly, repeated cocaine produces robust LTP [88], enhances AMPA:NMDA current ratios [33], and reduces both endocannabinoid-and mGlu2/3-mediated LTD in prelimbic PFC [33]. Thus, while less studied than corticostriatal synapses, c-SP adaptations in PFC excitatory synapses also appear likely to promote relapse to drug-seeking across multiple classes of abused drug.
Constitutive Plasticity in the Extracellular Matrix
The brain parenchyma ECM is subdivided into two specialized compartments: perineuronal nets (PNNs) and interstitial neuropil ECM. PNNs are large stable aggregations primarily composed of hyaluronan, chrondroitin sulfate proteoglycans (CSPGs; e.g., aggrecan, brevican, neurocan, phosphocan, and versican), hyaluronan-CSPG link proteins, and Tenascin R [89]. In the case of parvalbumin-containing interneurons, PNNs support the high firing frequency of these fast-spiking cells by enveloping their soma and proximal dendrites [89]. To a lesser extent, PNNs also surround glutamatergic pyramidal neurons in cortex [63]. Neuropil ECM is ubiquitous in the extracellular space, and like PNNs, contains collagen, laminin, and fibronectin and associates with pre- and post-synaptic CAMs [90]. Through interactions with CAMs, perisynaptic ECM proteins function in mature brains to stabilize dendritic spine structure, and can be restructured through catabolism to regulate glutamatergic transmission and synaptic plasticity [13]. ECM degradation is accomplished largely by constitutive and inducible activity of MMPs (Box 2) [90]. Because study of the ECM is relatively new in the addiction field, in contrast to the rest of this review, below we describe constitutive ECM neuroadaptations produced in many brain areas by addictive drugs, then bring focus back to corticostriatal projections.
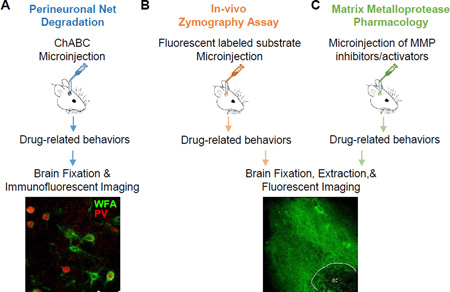
Box 2. Experimental approaches to measure and regulate the extracellular matrix.
Below is a brief description of common methods that can be used to study how ECM proteins influence drug-related behaviors. (A) The structure of perineuronal nets (PNNs) are enzymatically degraded by chondroitinase ABC (ChABC), an enzyme that degrades the glycosaminoglycan component of chondroitin sulfate proteoglycans that provide structural support and stabilize the extracellular matrix. ChABC is microinjected through guide cannula into discrete brain regions, and the degradation of the PNNs can persist for up to 4 weeks. Penicillinase, a β-lactamase, does not degrade PNNs and is typically used as a control. Drug-related behaviors can then be performed during this 4-week time window. At the end of the behavioral paradigm, multiplexed immunofluorescence that target PNN constituents can then determine extent of PNN degradation in certain cell types. Wisteria floribunda agglutinin (WFA) is a plant lectin that binds to terminal N-acetylgalactosamine residues predominantly expressed in PNNs. A representative image of WFA and paralbumin (PV) staining to label PNNs (green) and GABAergic parvalbumin-expressing interneurons (red) in mouse cortex is shown (image courtesy of Drs. Amy Lasek and Hu Chen). (B) Matrix metalloproteinases (MMPs) are proteolytic zinc-containing endopeptidases that degrade specific ECM proteins. MMP-2/9 are gelatinases, and an in-vivo zymography assay is used to directly measure gelatinase activity of MMP-2/9. A quenched, fluorescein-labeled gelatin is microinjected into a specific brain region, and a linear increase in fluorescence can be measured, the slope of which is a function of MMP-2/9 activity. Typically, microinjections are made at various times before or during drug seeking behaviors to assess the relationship between MMP-2/9 activity and behavior. Newer approaches for making in vivo estimates of fluorescence promise to markedly improve the capacity to dynamically evaluate MMP-2/9 activity over time during reinstated behavior (image courtesy of Dr. Michael Scofield, ac- anterior commissure). Also, while work in addiction has been based on a regional change in fluorescence, it is possible to quantify increases in MMP-2/9 activity at the level of neurons or synaptic puncta. (C) Microinjections of pharmacological inhibitors or MMP antibodies can be used to determine specific MMPs regulating drug-seeking behaviors.
Current small molecule inhibitors are useful, but proper experimental design is required to insure specificity. For example, when assessing MMP-2 versus MMP-9 activity, relative ligand specificity and the difficulty in controlling the actual concentration of drug diffusion through the neuropil after microinjection requires showing that both drugs have differential effects. Additionally, combining these pharmacological compounds with microinjections of fluorescein-labeled substrates can determine which MMPs are responsible for degradation of the ECM during drug-seeking behaviors (see micrograph comparison of fluorescence induced during reinstated in the presence or absence of an MMP-9 inhibitor) [99]. In vivo activation of MMPs is most readily achieved by microinjecting recombinant activated MMP. In addition to these approaches, transgenic and knockout rats and mice (e.g., MMP-9 KO mice and over-expressing transgenic rats, Thy-1.2-tissue plasminogen activator transgenic mice) and viral approaches can be used to study the ECM in models of drug addiction.
Binge-like ethanol drinking in mice increases PNNs and elevates expression of CSPGs aggrecan, brevican, and phosphacan in the insula, but not anterior cingulate or primary motor cortex [91]. In the OFC, PNNs and CSPGs brevican and neurocan are elevated in adult mice exposed to alcohol during adolescence [92]. Neurocan, a glial CSPG, is enhanced in stratum oriens of the CA1 and cultured astrocytes following short-term (24 hr) ethanol exposure [93]. Acute ethanol exposure also reduces MMP-9, but not MMP-2 levels in the hippocampus and PFC, and the acute ethanol-induced spatial memory deficits depend on disruption of MMP-9 activity [21]. Of translational relevance, MMP-9 activity is elevated in the dorsolateral PFC or hippocampus and serum of alcohol, cocaine and heroin addicts [94–97]. More specific for corticostriatal projections, a proteomic screen after extinction from heroin self-administration revealed a reduction in two PNN proteins, Tenascin R and brevican, in both prelimbic PFC and NAcore [98]. In cocaine extinguished rats MMP-2 activity is increased in the NAcore, and blocking MMP-2 inhibits cue-induced cocaine reinstatement [99]. In parallel with some forms of c-SP (Table 2), MMP-2 activity is also increased in the NAcore of nicotine, but not heroin extinguished rats [99]. Repeated methamphetamine treatment increases MMP-2/9 activity in the PFC and accumbens, and methamphetamine-induced behavioral sensitization and CPP is inhibited in MMP-deficient mice or by blocking MMPs [100, 101].
A number of studies point to enduring changes in CAM protein content produced by addictive drugs. For example, cocaine increases polysialylated neural CAM (NCAM) in the PFC [102], and NrCAM knock-out mice show reduced rewarding effects by alcohol, morphine, cocaine and amphetamine [103, 104]. Integrins are perhaps the most well characterized CAMs in terms of synaptic plasticity [105]. Extinction from cocaine self-administration is associated with an increase in the β3 integrin subunit in the NAcore [106], and treatment with the RGD peptide antagonist that prevents the binding of integrins to the ECM attenuates the increase in β3-integrin as well as the constitutive reduction in surface expression of GluR2 seen after withdrawal from cocaine [86, 107]. Integrin regulation of psychostimulant behavior is further supported by augmented cocaine-induced locomotor behavior in mice harboring homozygous deletion of the integrin β1 subunit or the β1 integrin signaling kinase Arg [108]. Finally, an elegant study linked Ras suppressor 1 (Rsu1) to a β integrin signaling pathway that destabilizes actin filaments and regulates alcohol intake, and polymorphisms in RSU1 are associated with alcohol dependence in adults and increases in NAc activation during reward processing and lifetime drinking in adolescents [109].
Pharmacological degradation of PNNs and perisynaptic ECM is used to demonstrate an important role of constitutive expression of the ECM in synaptic activity and plasticity (Box 2). For example, local chondroitinase-ABC (Ch-ABC) administration enhances the firing rate of hippocampal pyramidal neurons and increases the number of spontaneously active ventral tegmental area dopamine neurons [110]. Perhaps more dramatic, recombinant MMP-9 produces LTP in hippocampus without electrical stimulation [111]. Relevant to drug abuse, intra-hippocampal Ch-ABC administration enhances the locomotor response to acute amphetamine [110]. Depletion of PNNs in the amygdala prevents morphine- and cocaine-induced CPP when combined with extinction training. Also when combined with extinction training, intra-amygdala injections of Ch-ABC enhanced expression of plasticity-related proteins (i.e., BNDF and AMPA receptors) [112]. Degradation of PNNs in the prelimbic PFC reduces acquisition and reconsolidation without affecting the rate of extinction or spontaneous recovery of cocaine-induced CPP [113]. These authors also demonstrate that depletion of PNNs in the prelimbic PFC reduces inhibitory drive onto pyramidal neurons while enhancing intrinsic excitability [113].
General conclusions regarding withdrawal associated constitutive synaptic plasticity
Clearly the four elements of the tetrapartite corticostriatal synapse undergo enduring, constitutive adaptations following withdrawal from using addictive drugs. By filtering these adaptations through the hypothesis that those shared in common between different classes of addictive drug are likely most critical for shared symptoms of addiction, such as vulnerability to relapse, we can narrow the field of known adaptations to reduced elimination of synaptically released glutamate via down-regulated astroglial EAAT2 and to reduced presynaptic regulation of synaptic release probability via mGlu2/3. Also, addictive drugs share a diminished capacity for cortico-accumbens LTD and LTP, and in so far has been tested, this loss of postsynaptic plasticity depends on glutamate spillover since preventing spillover by restoring EAAT2 or tone onto mGlu2/3 receptors restores the capacity to induce LTP or LTD [79]. Unfortunately, studies with the ECM are too few to triangulate across classes of addictive drugs. None the less, widespread changes in PNNs, ECM proteins and MMPs occur in PFC and accumbens following withdrawal from addictive drugs, and future studies will determine which of these changes are shared across drug classes and can be reversed by restoring other shared tetrapartite adaptations, such as reduced EAAT2 and mGlu2/3 receptor signaling or reduced LTP/LTD.
Transient Corticostriatal Synaptic Plasticity
Postsynaptic Transient Plasticity during Reinstatement
In addition to studying corticostriatal c-SP produced by drug withdrawal, a research strategy has recently emerged to evaluate the synaptic plasticity associated with drug seeking induced by drug-associated cues or noncontingent drug injection. This has been termed transient synaptic potentiation (t-SP) [69], and is a transient LTP-like event that parallels the time course of drug-seeking behavior during reinstatement. Thus, 15 min after inducing drug-seeking by a cue, MSNs in the NAcore show increased electrophysiological (AMPA:NMDA ratio) and morphological (spine head diameter) measures of synaptic potentiation that are positively correlated with the number of active lever presses (drug-seeking) emitted during 15 min of cue-reinstated behavior [69] (Figure 1C). Both active lever pressing and t-SP return to levels measured in extinguished rats within 120 min after initiating drug seeking. Similar t-SP is present after context and cocaine-induced reinstatement [30, 72], and elevated AMPA receptor function appears to facilitate reinstatement of alcohol-seeking behavior [114, 115]. Also, 30 min after reinstating lever pressing with a cocaine injection there is an increase in Ser880 phosphorylation of GluR2, increased surface AMPA receptor expression, and enhanced prevalence of dendritic spines in NAshell [74, 116]. Cued reinstatement of nicotine seeking also produces t-SP in the NAcore that peaks in parallel with behavior after 15 min [28]. Noncontingent heroin administration induces t-SP with a similar time course to cocaine, with an increase in spine head diameter and density after 45 min of reinstatement [84]. Heroin administration enhances in-vivo field EPSPs in the NAcore of heroin-extinguished rats, beginning almost immediately after injection and enduring for nearly 3 hr before returning to baseline [84]. Interestingly, optogenetic inhibition of PFC afferents in the NAcore prevents drug-seeking behavior and morphological t-SP adaptations [117], suggesting that PFC inputs into the accumbens critically regulate t-SP.
Transient plasticity in the extracellular matrix during reinstatement
Evidence of the emerging role for the ECM in regulating synaptic plasticity, in particular the essential role of MMP-9 activity for inducing hippocampal LTP [19], led to studies examining whether the ECM participates in t-SP and reinstated lever pressing. Inhibiting ECM binding to integrins by microinjecting RGD into NAcore inhibits cocaine-induced reinstatement [107]. Similarly, inhibiting MMP activity suppresses the reinstatement of cocaine CPP, and the increases in MMP-9, but not MMP-2/3 activity in the PFC following cocaine-primed reinstatement of CPP [118]. Selectively inhibiting MMP-9 prevents cue- and cocaine-induced reinstatement of cocaine self-administration [99]. The role of MMP-9 in reinstated cocaine seeking is paralleled by a marked, transient increase in MMP-9 activity following cue-induced reinstatement, which also occurs at 15 min after cue-induced reinstatement of heroin and nicotine seeking. The induction of t-SP is linked to MMP-9 activation since inhibiting MMP-9 activity prevents both cue-induced increases in AMPA:NMDA current ratios and spine head diameter in NAcore MSNs [99]. In addition to MMP regulation of t-SP and reinstated drug seeking in the NAcore, treatment with a broad-spectrum MMP inhibitor (FN-439) into prelimbic PFC attenuates cue-induced heroin seeking [98] and escalated operant self-administration of alcohol [119].
General conclusions regarding transient synaptic potentiation during drug seeking
These initial studies demonstrate that reinstated drug seeking is associated with postsynaptic measures of t-SP that correlate with the intensity of drug seeking, and that postsynaptic t-SP occurs across multiple classes of addictive drug, including cocaine, nicotine and heroin. The co-occurrence of t-SP and some forms of c-SP across drug classes poses the question of whether t-SP in induced by spillover of synaptic glutamate arising during reinstated behavior that is also common across drugs. One strong indication that they are related is that inhibiting PFC inputs to the NAcore prevents both glutamate spillover and the t-SP induced during cocaine seeking [40, 69]. Although the mechanism of how c-SP influences t-SP is unknown, these studies identify MMPs as necessary for t-SP and the reinstatement of drug-seeking behaviors. MMP-9 is particularly promising target for regulation of shared plasticity across multiple abused drugs, and the development of highly selective MMP-9 inhibitors (e.g., GS-5747 [120]) that overcome the limitations of broad-spectrum MMP inhibitors [121] provide new therapeutic opportunities to treat addiction.
Therapeutic Implications and Concluding Remarks
The functional, biochemical, and morphological adaptations in tetrapartite synapses discussed in this review are emerging mechanisms driving drug-induced plasticity in corticostriatal circuits. Clearly experimental evidence involving glia and the ECM in drug-induced synaptic plasticity is nascent, and there are inconsistencies and gaps in the literature that leave open many questions (see Outstanding Questions). To parse this emerging literature, we have endeavored to highlight recent studies demonstrating similarities between multiple classes of abused drugs inducing c-SP, t-SP, and ECM adaptations. Generally, the findings summarized here suggest that withdrawal from alcohol, nicotine, psychostimulants and opioids inhibits the induction of LTD and LTP in accumbens. Also, with the notable exception of opioids, addictive drugs induce postsynaptic c-SP in accumbens MSNs quantified as increases in dendritic spines and AMPA currents, and the shared aspects of c-SP occur preferentially in dopamine D1R-, not D2R–containing MSNs.
Outstanding Questions.
Are shared mechanistic adaptations that are common between all abused drugs high priority pharmacotherapeutic targets for treating high rates of relapse in addicts?
Will a better understanding of drug-induced plasticity in the ECM and tetrapartite synapses in the corticostriatal circuitry provide effective targets to reduce drug-seeking behaviors?
How does synaptic glutamate spillover lead to the increase in MMP-9 and t-SP during cue-induced drug seeking?
Does restoring down-regulated EAAT2 or mGlu2/3 signaling reduce drug-seeking across all drug classes?
Which ECM proteins are critical substrates for the role played by MMPs in regulating drug seeking?
Which cell adhesion molecules mediate the transient synaptic potentiation produced by MMP-mediated catabolic activation of the ECM, and what is the subsequent intracellular signaling cascade underpinning the increase in AMPA receptors and dendritic spine head diameter?
Will inhibiting transient synaptic plasticity of the ECM induced by drug-associated cues or noncontingent drug injection reduce seeking behavior for other drugs like it has for cocaine?
What are the critical constitutive adaptations in tetrapartite synapses that contribute to transient synaptic plasticity that underpins the drive to seek cocaine?
If one considers only the shared aspects of synaptic plasticity, a number of potential pharmacotherapeutic targets emerge that may span across drug classes. For example, restoring down-regulated EAAT2 with drugs like N-acetylcysteine or ceftriaxone, or mGlu2/3 signaling with mGlu2/3 agonists or AGS3 peptide inhibitors reduces drug seeking across drug classes in animal models. Similarly, reversing the shared loss of electrically-induced LTD in NAc MSNs with either with N-acetylcysteine or through stimulating PFC inputs inhibits drug seeking (shown in alcohol, heroin and cocaine trained animals). Finally, the shared down-regulation of EAAT2 and the resulting transient glutamate spillover from PFC synapses in NAcore during reinstated drug-seeking, stimulates MMP-9 activity to initiate t-SP during cocaine-, heroin- and nicotine-seeking. This discovery opens additional therapeutic possibilities, including inhibitors of MMP-9 or the CAM effectors of ECM catabolism, such as integrins. While considerable work remains to fully integrate the ECM and tetrapartite synaptic physiology into the neurobiology of drug addiction, we propose that considering contributions by all four synaptic compartments in constitutive and drug-seeking induced transient synaptic potentiation is necessary for understanding synaptic plasticity that is shared across drug classes. Moreover, a more inclusive understanding of tetrapartite synaptic plasticity that is in common across drug classes is an unifying approach for moving forward our understanding of addiction and towards identifying pharmacotherapeutic targets for treating symptoms of drug addiction shared between all classes of addictive compounds.
Table 3.
Transient drug-induced synaptic plasticity mechanisms of cortico-accumbens glutamatergic synapses that are shared during the execution of drug seeking behaviors.
| AMPA Receptor Function |
Spine Head Diameter |
MMP-9 Activity | Refs | |
|---|---|---|---|---|
| Alcohol | ↑ | No data | No data | [114, 115] |
| Cocaine | ↑ | ↑ | ↑ | [30, 69, 72, 74, 99, 116] |
| Nicotine | ↑ | ↑ | ↑ | [28, 99] |
| Heroin | ↑ * | ↑ | ↑ | [84, 99] |
measured as field EPSPs
Trends Box.
Drug-induced plasticity involves all aspects of tetrapartite synapses.
Relapse involves common mechanisms that span multiple classes of abused drugs.
Tetrapartite synapses are novel targets for treating addiction.
Acknowledgments
This work was supported by the US National Institutes of Health AA020930, AA023288 (PJM), AA010761, AA019967, AA022701 (LJC), DA003906, DA012513 and DA015369 (PWK).
Glossary
- Cell adhesion molecules (CAMs)
are cell surface proteins that bind with extracellular matrix proteins and intracellular signaling systems that regulate synaptic function and morphology
- Chrondroitin sulfate proteoglycans (CSPGs)
are proteoglycans that provide structural support and stabilize the extracellular matrix
- Chondroitinase ABC (ChABC)
is an enzyme that degrades the glycosaminoglycan component of chondroitin sulfate proteoglycans
- Constitutive synaptic potentiation (c-SP)
are persistent neuroadaptations in tetrapartite synapses that occur after chronic drug exposure
- Excitatory amino acid transporter 2 (EAAT2)
is a glutamate and aspartate transporter that is highly expressed in astroglia and is essential for rapid removal of glutamate from the synaptic cleft
- Extracellular matrix
is a lattice-like support scaffold produced by neurons and glial cells that envelopes the soma, dendrites and synapses and functions in the mature brain to stabilize dendritic spine structure and regulate neurotransmission
- Gliotransmission
is bidirectional communication between astrocytes and neurons through the release of chemical transmitters, including taurine, ATP, D-serine, and glutamate
- In-vivo zymography
is a method to measure ECM remodeling using high-resolution imaging of the fluorescent degradation products of the introduced substrates
- Long-term potentiation (LTP)
is the persistent increase in the efficacy of synapses that is considered a critical cellular mechanism for learning and memory
- Long-term depression (LTD)
is a persistent weakening in synaptic strength that can influence storage of motor learning and memory
- Matrix metalloproteinases (MMPs)
are a large family of proteolytic zinc-containing endopeptidases that degrade specific ECM proteins. MMPs are either secreted into the extracellular milieu as proenzymes or are expressed in the plasma membrane
- Perineuronal nets (PNNs)
are ECM structures that surround the soma and proximal dendrites of some neurons (e.g., parvalbumin-containing fast-spiking interneurons in the cortex). Degradation of PNNs in the adult brain can influence synaptic plasticity
- Tetrapartite synapses
consist of pre- and post-synaptic compartments, astroglial fine membranous processes that surround the perisynaptic space, and the extracellular matrix
- Transient synaptic potentiation (t-SP)
is synaptic plasticity associated with drug seeking produced by drug-associated cues or noncontingent drug injection
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Schacher S, Hu JY. The less things change the more they are different: contributions of long-term synaptic plasticity and homeostasis to memory. Learn Mem. 2014;21(3):128–134. doi: 10.1101/lm.027326.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.De Pitta M, Brunel N, Volterra A. Astrocytes: Orchestrating synaptic plasticity? Neuroscience. 2015 doi: 10.1016/j.neuroscience.2015.04.001. [DOI] [PubMed] [Google Scholar]
- 3.Araque A, et al. Tripartite synapses: glia the unacknowledged partner. Trends Neurosci. 1999;22(5):208–215. doi: 10.1016/s0166-2236(98)01349-6. [DOI] [PubMed] [Google Scholar]
- 4.Allen NJ. Astrocyte regulation of synaptic behavior. Annu Rev Cell Dev Biol. 2014;30:439–463. doi: 10.1146/annurev-cellbio-100913-013053. [DOI] [PubMed] [Google Scholar]
- 5.Takahashi K, Foster JB, Lin CL. Glutamate transporter EAAT2: regulation function and potential as a therapeutic target for neurological and psychiatric disease. Cell Mol Life Sci. 2015;72(18):3489–3506. doi: 10.1007/s00018-015-1937-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Divito CB, Underhill SM. Excitatory amino acid transporters: roles in glutamatergic neurotransmission. Neurochem Int. 2014;73:172–180. doi: 10.1016/j.neuint.2013.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ota Y, Zanetti AT, Hallock RM. The role of astrocytes in the regulation of synaptic plasticity and memory formation. Neural Plast. 2013;2013:185463. doi: 10.1155/2013/185463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Scofield MD, et al. Gq-DREADD Selectively Initiates Glial Glutamate Release and Inhibits Cue-induced Cocaine Seeking. Biol Psychiatry. 2015;78(7):441–451. doi: 10.1016/j.biopsych.2015.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bull C, et al. Rat nucleus accumbens core astrocytes modulate reward and the motivation to self-administer ethanol after abstinence. Neuropsychopharmacology. 2014;39(12):2835–2845. doi: 10.1038/npp.2014.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Scofield MD, Kalivas PW. Astrocytic dysfunction and addiction: consequences of impaired glutamate homeostasis. Neuroscientist. 2014;20(6):610–622. doi: 10.1177/1073858413520347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dityatev A, Seidenbecher CI, Schachner M. Compartmentalization from the outside: the extracellular matrix and functional microdomains in the brain. Trends Neurosci. 2010;33(11):503–512. doi: 10.1016/j.tins.2010.08.003. [DOI] [PubMed] [Google Scholar]
- 12.Dalva MB, McClelland AC, Kayser MS. Cell adhesion molecules: signalling functions at the synapse. Nat Rev Neurosci. 2007;8(3):206–220. doi: 10.1038/nrn2075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Levy AD, Omar MH, Koleske AJ. Extracellular matrix control of dendritic spine and synapse structure and plasticity in adulthood. Front Neuroanat. 2014;8:116. doi: 10.3389/fnana.2014.00116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wlodarczyk J, et al. Extracellular matrix molecules their receptors and secreted proteases in synaptic plasticity. Dev Neurobiol. 2011;71(11):1040–1053. doi: 10.1002/dneu.20958. [DOI] [PubMed] [Google Scholar]
- 15.Schubert D. The possible role of adhesion in synaptic modification. Trends Neurosci. 1991;14(4):127–130. doi: 10.1016/0166-2236(91)90078-9. [DOI] [PubMed] [Google Scholar]
- 16.Thalhammer A, Cingolani LA. Cell adhesion and homeostatic synaptic plasticity. Neuropharmacology. 2014;78:23–30. doi: 10.1016/j.neuropharm.2013.03.015. [DOI] [PubMed] [Google Scholar]
- 17.Tsilibary E, et al. Neural ECM proteases in learning and synaptic plasticity. Prog Brain Res. 2014;214:135–157. doi: 10.1016/B978-0-444-63486-3.00006-2. [DOI] [PubMed] [Google Scholar]
- 18.Szklarczyk A, et al. Matrix metalloproteinase-9 undergoes expression and activation during dendritic remodeling in adult hippocampus. J Neurosci. 2002;22(3):920–930. doi: 10.1523/JNEUROSCI.22-03-00920.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huntley GW. Synaptic circuit remodelling by matrix metalloproteinases in health and disease. Nat Rev Neurosci. 2012;13(11):743–757. doi: 10.1038/nrn3320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nicholson C, Sykova E. Extracellular space structure revealed by diffusion analysis. Trends Neurosci. 1998;21(5):207–215. doi: 10.1016/s0166-2236(98)01261-2. [DOI] [PubMed] [Google Scholar]
- 21.Wright JW, et al. Ethanol-induced impairment of spatial memory and brain matrix metalloproteinases. Brain Res. 2003;963(1–2):252–261. doi: 10.1016/s0006-8993(02)04036-2. [DOI] [PubMed] [Google Scholar]
- 22.Mizoguchi H, Yamada K, Nabeshima T. Matrix metalloproteinases contribute to neuronal dysfunction in animal models of drug dependence, Alzheimer’s disease and epilepsy. Biochem Res Int. 2011;2011:681385. doi: 10.1155/2011/681385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lubbers BR, et al. Neural ECM in addiction, schizophrenia and mood disorder. Prog Brain Res. 2014;214:263–284. doi: 10.1016/B978-0-444-63486-3.00012-8. [DOI] [PubMed] [Google Scholar]
- 24.Smith AC, Scofield MD, Kalivas PW. The tetrapartite synapse: Extracellular matrix remodeling contributes to corticoaccumbens plasticity underlying drug addiction. Brain Res. 2015;1628(Pt A):29–39. doi: 10.1016/j.brainres.2015.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Luscher C, Malenka RC. Drug-evoked synaptic plasticity in addiction: from molecular changes to circuit remodeling. Neuron. 2011;69(4):650–663. doi: 10.1016/j.neuron.2011.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pignatelli M, Bonci A. Role of Dopamine Neurons in Reward and Aversion: A Synaptic Plasticity Perspective. Neuron. 2015;86(5):1145–1157. doi: 10.1016/j.neuron.2015.04.015. [DOI] [PubMed] [Google Scholar]
- 27.Fischer-Smith KD, Houston AC, Rebec GV. Differential effects of cocaine access and withdrawal on glutamate type 1 transporter expression in rat nucleus accumbens core and shell. Neuroscience. 2012;210:333–339. doi: 10.1016/j.neuroscience.2012.02.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gipson CD, et al. Reinstatement of nicotine seeking is mediated by glutamatergic plasticity. Proc Natl Acad Sci U S A. 2013;110(22):9124–9129. doi: 10.1073/pnas.1220591110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Knackstedt LA, Melendez RI, Kalivas PW. Ceftriaxone restores glutamate homeostasis and prevents relapse to cocaine seeking. Biol Psychiatry. 2010;67(1):81–84. doi: 10.1016/j.biopsych.2009.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shen HW, et al. Prelimbic cortex and ventral tegmental area modulate synaptic plasticity differentially in nucleus accumbens during cocaine-reinstated drug seeking. Neuropsychopharmacology. 2014;39(5):1169–1177. doi: 10.1038/npp.2013.318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Barker JM, Lench DH, Chandler LJ. Reversal of alcohol dependence-induced deficits in cue-guided behavior via mGluR2/3 signaling in mice. Psychopharmacology (Berl) 2016;233(2):235–242. doi: 10.1007/s00213-015-4101-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Abulseoud OA, et al. Attenuation of ethanol withdrawal by ceftriaxone-induced upregulation of glutamate transporter EAAT2. Neuropsychopharmacology. 2014;39(7):1674–1684. doi: 10.1038/npp.2014.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kasanetz F, et al. Prefrontal synaptic markers of cocaine addiction-like behavior in rats. Mol Psychiatry. 2013;18(6):729–737. doi: 10.1038/mp.2012.59. [DOI] [PubMed] [Google Scholar]
- 34.Meinhardt MW, et al. Rescue of infralimbic mGluR2 deficit restores control over drug-seeking behavior in alcohol dependence. J Neurosci. 2013;33(7):2794–2806. doi: 10.1523/JNEUROSCI.4062-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Xie X, Steketee JD. Effects of repeated exposure to cocaine on group II metabotropic glutamate receptor function in the rat medial prefrontal cortex: behavioral and neurochemical studies. Psychopharmacology (Berl) 2009;203(3):501–510. doi: 10.1007/s00213-008-1392-4. [DOI] [PubMed] [Google Scholar]
- 36.Bowers MS, et al. Nucleus accumbens AGS3 expression drives ethanol seeking through G betagamma. Proc Natl Acad Sci U S A. 2008;105(34):12533–12538. doi: 10.1073/pnas.0706999105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bowers MS, et al. Activator of G protein signaling 3: a gatekeeper of cocaine sensitization and drug seeking. Neuron. 2004;42(2):269–281. doi: 10.1016/s0896-6273(04)00159-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yao L, et al. Activator of G protein signaling 3 regulates opiate activation of protein kinase A signaling and relapse of heroin-seeking behavior. Proc Natl Acad Sci U S A. 2005;102(24):8746–8751. doi: 10.1073/pnas.0503419102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.McFarland K, et al. Limbic and motor circuitry underlying footshock-induced reinstatement of cocaine-seeking behavior. J Neurosci. 2004;24(7):1551–1560. doi: 10.1523/JNEUROSCI.4177-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.McFarland K, Lapish CC, Kalivas PW. Prefrontal glutamate release into the core of the nucleus accumbens mediates cocaine-induced reinstatement of drug-seeking behavior. J Neurosci. 2003;23(8):3531–3537. doi: 10.1523/JNEUROSCI.23-08-03531.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Berglind WJ, et al. A single intra-PFC infusion of BDNF prevents cocaine-induced alterations in extracellular glutamate within the nucleus accumbens. J Neurosci. 2009;29(12):3715–3719. doi: 10.1523/JNEUROSCI.5457-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Li X, et al. Activation of mGluR7s inhibits cocaine-induced reinstatement of drug-seeking behavior by a nucleus accumbens glutamate-mGluR2/3 mechanism in rats. J Neurochem. 2010;114(5):1368–1380. doi: 10.1111/j.1471-4159.2010.06851.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Trantham-Davidson H, et al. Ceftriaxone normalizes nucleus accumbens synaptic transmission, glutamate transport and export following cocaine self-administration and extinction training. J Neurosci. 2012;32(36):12406–12410. doi: 10.1523/JNEUROSCI.1976-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Parsegian A, See RE. Dysregulation of dopamine and glutamate release in the prefrontal cortex and nucleus accumbens following methamphetamine self-administration and during reinstatement in rats. Neuropsychopharmacology. 2014;39(4):811–822. doi: 10.1038/npp.2013.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.LaLumiere RT, Kalivas PW. Glutamate release in the nucleus accumbens core is necessary for heroin seeking. J Neurosci. 2008;28(12):3170–3177. doi: 10.1523/JNEUROSCI.5129-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gass JT, et al. Alcohol-seeking behavior is associated with increased glutamate transmission in basolateral amygdala and nucleus accumbens as measured by glutamate-oxidase-coated biosensors. Addict Biol. 2011;16(2):215–228. doi: 10.1111/j.1369-1600.2010.00262.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Griffin WC, 3rd, et al. Increased extracellular glutamate in the nucleus accumbens promotes excessive ethanol drinking in ethanol dependent mice. Neuropsychopharmacology. 2014;39(3):707–717. doi: 10.1038/npp.2013.256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Griffin WC, et al. Repeated cycles of chronic intermittent ethanol exposure increases basal glutamate in the nucleus accumbens of mice without affecting glutamate transport. Front Pharmacol. 2015;6:27. doi: 10.3389/fphar.2015.00027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bossert JM, et al. Activation of group II metabotropic glutamate receptors in the nucleus accumbens shell attenuates context-induced relapse to heroin seeking. Neuropsychopharmacology. 2006;31(10):2197–2209. doi: 10.1038/sj.npp.1300977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Baptista MA, Martin-Fardon R, Weiss F. Preferential effects of the metabotropic glutamate 2/3 receptor agonist LY379268 on conditioned reinstatement versus primary reinforcement: comparison between cocaine and a potent conventional reinforcer. J Neurosci. 2004;24(20):4723–4727. doi: 10.1523/JNEUROSCI.0176-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Peters J, Kalivas PW. The group II metabotropic glutamate receptor agonist, LY379268 inhibits both cocaine- and food-seeking behavior in rats. Psychopharmacology (Berl) 2006;186(2):143–149. doi: 10.1007/s00213-006-0372-9. [DOI] [PubMed] [Google Scholar]
- 52.Caprioli D, et al. Effect of the Novel Positive Allosteric Modulator of Metabotropic Glutamate Receptor 2 AZD8529 on Incubation of Methamphetamine Craving After Prolonged Voluntary Abstinence in a Rat Model. Biol Psychiatry. 2015;78(7):463–473. doi: 10.1016/j.biopsych.2015.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Justinova Z, et al. The Novel Metabotropic Glutamate Receptor 2 Positive Allosteric Modulator, AZD8529 Decreases Nicotine Self-Administration and Relapse in Squirrel Monkeys. Biol Psychiatry. 2015;78(7):452–462. doi: 10.1016/j.biopsych.2015.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Reissner KJ, et al. Glutamate transporter GLT-1 mediates N-acetylcysteine inhibition of cocaine reinstatement. Addict Biol. 2015;20(2):316–323. doi: 10.1111/adb.12127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sari Y, et al. Ceftriaxone treatment affects the levels of GLT1 and ENT1 as well as ethanol intake in alcohol-preferring rats. J Mol Neurosci. 2013;51(3):779–787. doi: 10.1007/s12031-013-0064-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Scofield MD, et al. Accumbens glutamate signaling underlies cued methamphetamine seeking. Addict Biol, Submitted [Google Scholar]
- 57.Moussawi K, et al. Reversing cocaine-induced synaptic potentiation provides enduring protection from relapse. Proc Natl Acad Sci U S A. 2011;108(1):385–390. doi: 10.1073/pnas.1011265108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Shen HW, et al. Synaptic glutamate spillover due to impaired glutamate uptake mediates heroin relapse. J Neurosci. 2014;34(16):5649–5657. doi: 10.1523/JNEUROSCI.4564-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.van Huijstee AN, Mansvelder HD. Glutamatergic synaptic plasticity in the mesocorticolimbic system in addiction. Front Cell Neurosci. 2015;8:466. doi: 10.3389/fncel.2014.00466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Marty VN, Spigelman I. Long-lasting alterations in membrane properties, k(+) currents and glutamatergic synaptic currents of nucleus accumbens medium spiny neurons in a rat model of alcohol dependence. Front Neurosci. 2012;6:86. doi: 10.3389/fnins.2012.00086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Nimitvilai S, et al. Chronic Intermittent Ethanol Exposure Enhances the Excitability and Synaptic Plasticity of Lateral Orbitofrontal Cortex Neurons and Induces a Tolerance to the Acute Inhibitory Actions of Ethanol. Neuropsychopharmacology. 20152015 doi: 10.1038/npp.2015.250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wang J, et al. Ethanol-mediated facilitation of AMPA receptor function in the dorsomedial striatum: implications for alcohol drinking behavior. J Neurosci. 2012;32(43):15124–15132. doi: 10.1523/JNEUROSCI.2783-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wang J, et al. Alcohol Elicits Functional and Structural Plasticity Selectively in Dopamine D1 Receptor-Expressing Neurons of the Dorsomedial Striatum. J Neurosci. 2015;35(33):11634–11643. doi: 10.1523/JNEUROSCI.0003-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Dumitriu D, et al. Subregional, dendritic compartment and spine subtype specificity in cocaine regulation of dendritic spines in the nucleus accumbens. J Neurosci. 2012;32(20):6957–6966. doi: 10.1523/JNEUROSCI.5718-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.McGuier NS, et al. Withdrawal from chronic intermittent alcohol exposure increases dendritic spine density in the lateral orbitofrontal cortex of mice. Alcohol. 2015;49(1):21–27. doi: 10.1016/j.alcohol.2014.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Spiga S, et al. Hampered long-term depression and thin spine loss in the nucleus accumbens of ethanol-dependent rats. Proc Natl Acad Sci U S A. 2014;111(35):E3745–E3754. doi: 10.1073/pnas.1406768111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Rasakham K, et al. Synapse density and dendritic complexity are reduced in the prefrontal cortex following seven days of forced abstinence from cocaine self-administration. PLoS One. 2014;9(7):e102524. doi: 10.1371/journal.pone.0102524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Radley JJ, et al. The Contingency of Cocaine Administration Accounts for Structural and Functional Medial Prefrontal Deficits and Increased Adrenocortical Activation. J Neurosci. 2015;35(34):11897–11910. doi: 10.1523/JNEUROSCI.4961-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Gipson CD, et al. Relapse induced by cues predicting cocaine depends on rapid transient synaptic potentiation. Neuron. 2013;77(5):867–872. doi: 10.1016/j.neuron.2013.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Gourley SL, et al. Arg kinase regulates prefrontal dendritic spine refinement and cocaine-induced plasticity. J Neurosci. 2012;32(7):2314–2323. doi: 10.1523/JNEUROSCI.2730-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kroener S, et al. Chronic alcohol exposure alters behavioral and synaptic plasticity of the rodent prefrontal cortex. PLoS One. 2012;7(5):e37541. doi: 10.1371/journal.pone.0037541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Stankeviciute NM, et al. Rapid transient potentiation of dendritic spines in context-induced relapse to cocaine seeking. Addict Biol. 2014;19(6):972–974. doi: 10.1111/adb.12064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Uys JD, et al. Chronic intermittent ethanol exposure and withdrawal leads to adaptations in nucleus accumbens core postsynaptic density proteome and dendritic spines. Addict Biol. 2015 doi: 10.1111/adb.12238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Gancarz AM, et al. Activin receptor signaling regulates cocaine-primed behavioral and morphological plasticity. Nat Neurosci. 2015;18(7):959–961. doi: 10.1038/nn.4036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Abrahao KP, et al. Locomotor sensitization to ethanol impairs NMDA receptor-dependent synaptic plasticity in the nucleus accumbens and increases ethanol self-administration. J Neurosci. 2013;33(11):4834–4842. doi: 10.1523/JNEUROSCI.5839-11.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Huang CC, et al. Cocaine withdrawal impairs metabotropic glutamate receptor-dependent long-term depression in the nucleus accumbens. J Neurosci. 2011;31(11):4194–4203. doi: 10.1523/JNEUROSCI.5239-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Jeanes ZM, Buske TR, Morrisett RA. Cell type-specific synaptic encoding of ethanol exposure in the nucleus accumbens shell. Neuroscience. 2014;277:184–195. doi: 10.1016/j.neuroscience.2014.06.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kasanetz F, et al. Transition to addiction is associated with a persistent impairment in synaptic plasticity. Science. 2010;328(5986):1709–1712. doi: 10.1126/science.1187801. [DOI] [PubMed] [Google Scholar]
- 79.Moussawi K, et al. N-Acetylcysteine reverses cocaine-induced metaplasticity. Nat Neurosci. 2009;12(2):182–189. doi: 10.1038/nn.2250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Shen H, Kalivas PW. Reduced LTP and LTD in prefrontal cortex synapses in the nucleus accumbens after heroin self-administration. Int J Neuropsychopharmacol. 2013;16(5):1165–1167. doi: 10.1017/S1461145712001071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Terrier J, Luscher C, Pascoli V. Cell-Type Specific Insertion of GluA2-Lacking AMPARs with Cocaine Exposure Leading to Sensitization, Cue-Induced Seeking, and Incubation of Craving. Neuropsychopharmacology. 2015 doi: 10.1038/npp.2015.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.MacAskill AF, Cassel JM, Carter AG. Cocaine exposure reorganizes cell type- and input-specific connectivity in the nucleus accumbens. Nat Neurosci. 2014;17(9):1198–1207. doi: 10.1038/nn.3783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Pascoli V, et al. Contrasting forms of cocaine-evoked plasticity control components of relapse. Nature. 2014;509(7501):459–464. doi: 10.1038/nature13257. [DOI] [PubMed] [Google Scholar]
- 84.Shen H, et al. Heroin relapse requires long-term potentiation-like plasticity mediated by NMDA2b–containing receptors. Proc Natl Acad Sci U S A. 2011;108(48):19407–19412. doi: 10.1073/pnas.1112052108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Robinson TE, Kolb B. Structural plasticity associated with exposure to drugs of abuse. Neuropharmacology. 2004;47(Suppl 1):33–46. doi: 10.1016/j.neuropharm.2004.06.025. [DOI] [PubMed] [Google Scholar]
- 86.Conrad KL, et al. Formation of accumbens GluR2-lacking AMPA receptors mediates incubation of cocaine craving. Nature. 2008;454(7200):118–121. doi: 10.1038/nature06995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ma YY, et al. Bidirectional modulation of incubation of cocaine craving by silent synapse-based remodeling of prefrontal cortex to accumbens projections. Neuron. 2014;83(6):1453–1467. doi: 10.1016/j.neuron.2014.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Lu H, et al. Elevated BDNF after cocaine withdrawal facilitates LTP in medial prefrontal cortex by suppressing GABA inhibition. Neuron. 2010;67(5):821–833. doi: 10.1016/j.neuron.2010.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Wang D, Fawcett J. The perineuronal net and the control of CNS plasticity. Cell Tissue Res. 2012;349(1):147–160. doi: 10.1007/s00441-012-1375-y. [DOI] [PubMed] [Google Scholar]
- 90.Shinoe T, Goda Y. Tuning synapses by proteolytic remodeling of the adhesive surface. Curr Opin Neurobiol. 2015;35:148–155. doi: 10.1016/j.conb.2015.08.005. [DOI] [PubMed] [Google Scholar]
- 91.Chen H, He D, Lasek AW. Repeated Binge Drinking Increases Perineuronal Nets in the Insular Cortex. Alcohol Clin Exp Res. 2015;39(10):1930–1938. doi: 10.1111/acer.12847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Coleman LG, Jr, et al. Adolescent binge ethanol treatment alters adult brain regional volumes cortical extracellular matrix protein and behavioral flexibility. Pharmacol Biochem Behav. 2014;116:142–151. doi: 10.1016/j.pbb.2013.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Zhang X, et al. Arylsulfatase B modulates neurite outgrowth via astrocyte chondroitin-4-sulfate: dysregulation by ethanol. Glia. 2014;62(2):259–271. doi: 10.1002/glia.22604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Sillanaukee P, et al. Matrix metalloproteinase-9 is elevated in serum of alcohol abusers. Eur J Clin Invest. 2002;32(4):225–229. doi: 10.1046/j.1365-2362.2002.00975.x. [DOI] [PubMed] [Google Scholar]
- 95.Rubio-Araiz A, et al. Disruption of blood-brain barrier integrity in postmortem alcoholic brain: preclinical evidence of TLR4 involvement from a binge-like drinking model. Addict Biol. 2016 doi: 10.1111/adb.12376. [DOI] [PubMed] [Google Scholar]
- 96.Kovatsi L, et al. Alterations in serum MMP and TIMP concentrations following chronic heroin abuse. Toxicol Mech Methods. 2013;23(5):377–381. doi: 10.3109/15376516.2012.758681. [DOI] [PubMed] [Google Scholar]
- 97.Mash DC, et al. Gene expression in human hippocampus from cocaine abusers identifies genes which regulate extracellular matrix remodeling. PLoS One. 2007;2(11):e1187. doi: 10.1371/journal.pone.0001187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Van den Oever MC, et al. Extracellular matrix plasticity and GABAergic inhibition of prefrontal cortex pyramidal cells facilitates relapse to heroin seeking. Neuropsychopharmacology. 2010;35(10):2120–2133. doi: 10.1038/npp.2010.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Smith AC, et al. Synaptic plasticity mediating cocaine relapse requires matrix metalloproteinases. Nat Neurosci. 2014;17(12):1655–1657. doi: 10.1038/nn.3846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Mizoguchi H, et al. Reduction of methamphetamine-induced sensitization and reward in matrix metalloproteinase-2 and −9-deficient mice. J Neurochem. 2007;100(6):1579–1588. doi: 10.1111/j.1471-4159.2006.04288.x. [DOI] [PubMed] [Google Scholar]
- 101.Mizoguchi H, et al. Role of matrix metalloproteinase and tissue inhibitor of MMP in methamphetamine-induced behavioral sensitization and reward: implications for dopamine receptor down-regulation and dopamine release. J Neurochem. 2007;102(5):1548–1560. doi: 10.1111/j.1471-4159.2007.04623.x. [DOI] [PubMed] [Google Scholar]
- 102.Mackowiak M, et al. Cocaine enhances ST8SiaII mRNA expression and neural cell adhesion molecule polysialylation in the rat medial prefrontal cortex. Neuroscience. 2011;186:21–31. doi: 10.1016/j.neuroscience.2011.04.030. [DOI] [PubMed] [Google Scholar]
- 103.Ishiguro H, et al. NrCAM-regulating neural systems and addiction-related behaviors. Addict Biol. 2014;19(3):343–353. doi: 10.1111/j.1369-1600.2012.00469.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Ishiguro H, et al. NrCAM in addiction vulnerability: positional cloning drug-regulation haplotype-specific expression and altered drug reward in knockout mice. Neuropsychopharmacology. 2006;31(3):572–584. doi: 10.1038/sj.npp.1300855. [DOI] [PubMed] [Google Scholar]
- 105.Wehrle-Haller B, Bastmeyer M. Intracellular signaling and perception of neuronal scaffold through integrins and their adapter proteins. Prog Brain Res. 2014;214:443–460. doi: 10.1016/B978-0-444-63486-3.00018-9. [DOI] [PubMed] [Google Scholar]
- 106.Wiggins AT, Pacchioni AM, Kalivas PW. Integrin expression is altered after acute and chronic cocaine. Neurosci Lett. 2009;450(3):321–323. doi: 10.1016/j.neulet.2008.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Wiggins A, et al. Integrins modulate relapse to cocaine-seeking. J Neurosci. 2011;31(45):16177–16184. doi: 10.1523/JNEUROSCI.3816-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Warren MS, et al. Integrin beta1 signals through Arg to regulate postnatal dendritic arborization, synapse density and behavior. J Neurosci. 2012;32(8):2824–2834. doi: 10.1523/JNEUROSCI.3942-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Ojelade SA, et al. Rsu1 regulates ethanol consumption in Drosophila and humans. Proc Natl Acad Sci U S A. 2015;112(30):E4085–E4093. doi: 10.1073/pnas.1417222112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Shah A, Lodge DJ. A loss of hippocampal perineuronal nets produces deficits in dopamine system function: relevance to the positive symptoms of schizophrenia. Transl Psychiatry. 2013;3:e215. doi: 10.1038/tp.2012.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Nagy V, et al. Matrix metalloproteinase-9 is required for hippocampal late-phase long-term potentiation and memory. J Neurosci. 2006;26(7):1923–1934. doi: 10.1523/JNEUROSCI.4359-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Xue YX, et al. Depletion of perineuronal nets in the amygdala to enhance the erasure of drug memories. J Neurosci. 2014;34(19):6647–6658. doi: 10.1523/JNEUROSCI.5390-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Slaker M, et al. Removal of perineuronal nets in the medial prefrontal cortex impairs the acquisition and reconsolidation of a cocaine-induced conditioned place preference memory. J Neurosci. 2015;35(10):4190–4202. doi: 10.1523/JNEUROSCI.3592-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Sanchis-Segura C, et al. Involvement of the AMPA receptor GluR-C subunit in alcohol-seeking behavior and relapse. J Neurosci. 2006;26(4):1231–1238. doi: 10.1523/JNEUROSCI.4237-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Cannady R, et al. Enhanced AMPA receptor activity increases operant alcohol self-administration and cue-induced reinstatement. Addict Biol. 2013;18(1):54–65. doi: 10.1111/adb.12000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Famous KR, et al. Phosphorylation-dependent trafficking of GluR2-containing AMPA receptors in the nucleus accumbens plays a critical role in the reinstatement of cocaine seeking. J Neurosci. 2008;28(43):11061–11070. doi: 10.1523/JNEUROSCI.1221-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Stefanik MT, Kupchik YM, Kalivas PW. Optogenetic inhibition of cortical afferents in the nucleus accumbens simultaneously prevents cue-induced transient synaptic potentiation and cocaine-seeking behavior. Brain Struct Funct. 2015 doi: 10.1007/s00429-015-0997-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Brown TE, et al. Increase in matrix metalloproteinase-9 levels in the rat medial prefrontal cortex after cocaine reinstatement of conditioned place preference. Synapse. 2008;62(12):886–889. doi: 10.1002/syn.20562. [DOI] [PubMed] [Google Scholar]
- 119.Smith AW, et al. Plasticity associated with escalated operant ethanol self-administration during acute withdrawal in ethanol-dependent rats requires intact matrix metalloproteinase systems. Neurobiol Learn Mem. 2011;96(2):199–206. doi: 10.1016/j.nlm.2011.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Marshall DC, et al. Selective Allosteric Inhibition of MMP9 Is Efficacious in Preclinical Models of Ulcerative Colitis and Colorectal Cancer. PLoS One. 2015;10(5):e0127063. doi: 10.1371/journal.pone.0127063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Vandenbroucke RE, Libert C. Is there new hope for therapeutic matrix metalloproteinase inhibition? Nat Rev Drug Discov. 2014;13(12):904–927. doi: 10.1038/nrd4390. [DOI] [PubMed] [Google Scholar]
- 122.Steketee JD, Kalivas PW. Drug wanting: behavioral sensitization and relapse to drug-seeking behavior. Pharmacol Rev. 2011;63(2):348–365. doi: 10.1124/pr.109.001933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Bardo MT, Bevins RA. Conditioned place preference: what does it add to our preclinical understanding of drug reward? Psychopharmacology (Berl) 2000;153(1):31–43. doi: 10.1007/s002130000569. [DOI] [PubMed] [Google Scholar]
- 124.Venniro M, Caprioli D, Shaham Y. Animal models of drug relapse and craving: From drug priming-induced reinstatement to incubation of craving after voluntary abstinence. Prog Brain Res. 2016;224:25–52. doi: 10.1016/bs.pbr.2015.08.004. [DOI] [PubMed] [Google Scholar]
- 125.Piazza PV, Deroche-Gamonet V. A multistep general theory of transition to addiction. Psychopharmacology (Berl) 2013;229(3):387–413. doi: 10.1007/s00213-013-3224-4. [DOI] [PMC free article] [PubMed] [Google Scholar]