Abstract
Navigation of the growth cone at the tip of the developing axon is crucial for the proper wiring of the nervous system. Mechanisms of actin-dependent growth cone steering, via signaling cascades, are well documented. Microtubules are also important in growth cone guidance, because their polarized invasion into the peripheral domain on one side of the growth cone is essential for it to turn in that direction. Classically, microtubules have been considered secondary players, invading the peripheral domain only where the actin cytoskeleton permits them to go. Presented here is evidence for an underappreciated mechanism by which signaling cascades can potentially affect growth cone turning, namely through regulatable forces imposed on the microtubules by molecular motor proteins.
Motor-Driven Forces Provide an Underappreciated Mechanism for Microtubule Participation in Growth Cone Turning
The growth cone is a fan-shaped structure at the tip of the growing axon that is receptive to environmental cues via signaling cascades that affect the cytoskeleton [1–3]. The fan shape comprises two domains called the central domain and the peripheral domain; the former is the microtubule-rich region contiguous with the shaft of the axon, and the latter is the actin-rich lamellar region that includes filopodia [4] (Figure 1). The motility of the growth cone is achieved through the coordinated behaviors of microtubules and the actin cytoskeleton. Axon extension, retraction, and turning in response to signaling cues require changes in the distribution of microtubules within the growth cone [1,3,5,6]. For the axon to turn, microtubules from the central domain must penetrate the transition zone to invade the peripheral domain, preferentially on the side of the growth cone in the direction of the turn [7]. Microtubules must be dynamic for growth cones to turn [8,9], and there are emerging roles in axon navigation of + TIPs, proteins that associate with the plus ends of elongating microtubules [10]. Even so, most research has focused on the actin cytoskeleton as the principal target of signaling events relevant to growth cone behaviors, with microtubule reconfigurations posited to passively follow changes in actin organization.
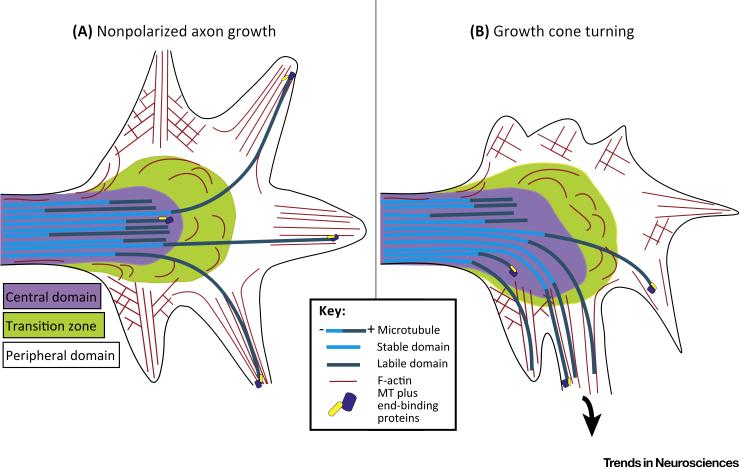
Figure 1. Growth Cone Cytoskeleton.
(A) The central domain of the growth cone is the microtubule (MT)-rich region contiguous with the axon shaft. The peripheral domain is the outer-most part of the growth cone. The peripheral domain comprises a broad flat lamellar region in which actin filaments are arranged as a meshwork, as well as elongated thin filopodia in which actin filaments are arranged as aligned bundles. The transition zone is the region between these two domains. Retrograde flow of the actin cytoskeleton in the peripheral domain pushes back most microtubules, compacting them in the central domain. Individual microtubules from the central domain are able to penetrate the transition zone to enter the peripheral domain during growth cone advance. (B) During growth cone turning, microtubules extend from the central domain through the transition zone preferentially into one side of the peripheral domain.
Dynamics (i.e., assembly, disassembly, and stabilization) are not the only behaviors that micro-tubules undergo within cells, as dramatically illustrated by the mitotic spindle, the most-studied microtubule array. Microtubule dynamics are essential for spindle formation and function, but so too are ATP-dependent forces on the microtubules generated by molecular motor proteins [11]. Cytoplasmic dynein and a variety of specialized kinesins generate forces on the microtubules that regulate their configuration and their interplay with one another, as well as with the actin cytoskeleton. The forces generated by mitotic microtubule-based motor proteins are crucial for the separation of the duplicated centrosomes in prophase, formation of the bipolar spindle, separation of the half-spindles at metaphase, and the final phases of cytokinesis during which daughter cells are pinched off from one another. These motor proteins are subjected to regulation by modifications (such as phosphorylation) and through interaction with partner proteins [12–19]. A decade and a half of work has revealed that terminally differentiated neurons repurpose much the same complement of motor proteins as used during cell division, as well as many of the same mechanisms that regulate them, to build the microtubule arrays of axons and dendrites [20–28]. In this Opinion, we focus on the role of motor-driven forces in reconfiguring microtubules in growth cones, which we posit to be an underappreciated but critical mechanistic aspect of axon navigation.
The Myosin II versus Dynein Competition
Myosin II is a well-studied bipolar actin-based motor protein that functions coordinately with actin dynamics to drive the retrograde flow of actin filaments within the growth cone. This retrograde actin flow provides traction for the growth cone to advance, while at the same time pushing microtubules backward from the peripheral domain into the central domain [29,30]. For microtubules to invade the peripheral domain from the central domain in a polarized fashion, the mechanism suggested has been that the actin cytoskeleton locally reconfigures or depolymerizes on the side of the growth cone in the direction of the turn, so that microtubules can then invade as a result of their dynamic properties. Indeed, local application of antiactin drugs can lead to microtubule invasion on the side of the growth cone exposed to the drug [31,32]. Without refuting the potential power of this mechanism to turn the growth cone, evidence also exists for a mechanism in which the microtubules are subjected to forces generated by microtubule-based motor proteins, with those forces in competition with the myosin II-driven retrograde flow of actin filaments. In this view, microtubules can utilize such forces to overcome the retrograde flow without the retrograde flow having to locally diminish to allow the microtubules to enter.
In the axon shaft, some of the microtubules are very short and undergo bouts of concerted and rapid transport, driven by cytoplasmic dynein [33]. Available data suggest that the cargo domain of cytoplasmic dynein binds to longer stationary microtubules or to the actin cytoskeleton, leaving the motor domain available to propel short microtubules down the axon with their plus-ends leading [34]. Long microtubules are not likely to individually move along other long microtubules of the same orientation because potential movements of microtubules by multiple dynein motors of random orientation would cancel one another out. However, these forces still exist, and can serve to integrate long microtubules with one another and with the actin cytoskeleton, similar to an isometric exercise. In this fashion, the dynein-driven forces enable the microtubule array to resist buckling and collapsing due to contractile actomyosin-based forces within the cortex of the axon [35,36]. Dynein-driven forces may also manifest as slow forward movement of the entire microtubule array during axon elongation [37].
In dynein-compromised neurons, the usual number of microtubules invading the peripheral domain of the growth cone is diminished [35,38]. Dynamic microtubules appear to stand still at the transition zone, despite being dynamic, because the retrograde flow of actin filaments pushes the microtubules back in the absence of an opposing dynein-based force. When challenged to turn in a substrate-based assay, the growth cones stall at the substrate interface, an effect that is correlated with the inability of microtubules to invade the peripheral domain [35]. These effects on microtubule distribution can be reversed if myosin II forces are experimentally inhibited, with microtubules then entering throughout the peripheral domain, regardless of whether cytoplasmic dynein is inhibited. Collectively, these observations reveal that microtubule invasion into the peripheral domain is enabled by dynein-driven forces that assist the micro-tubules in overcoming the retrograde actin flow. Consistent with this view, studies on Aplysia growth cones reveal modest forward movement of long microtubules in the peripheral domain of the growth cone [32], which is representative of the same dynein-driven forces that enable microtubules to resist the retrograde actin flow.
Kinesin-5 and Kinesin-12 Provide Polarity to Microtubule Invasion in Growth Cones
On the basis of the results discussed above, a model could be posited wherein environmental cues and signaling cascades regulate growth cone turning through a polarized increase or decrease in the forces generated by cytoplasmic dynein or myosin II. However, other studies indicate that at least two other motor proteins contribute to the balance of forces on growth cone microtubules, and suggest that these motor proteins are better candidates for providing the polarity of microtubule invasion underlying growth cone turning. These two motors, namely kinesin-5 (kif11, KSP, Eg5) and kinesin-12 (kif15), were once considered strictly mitotic, but have now been shown to have roles in regulating the distribution and transport of microtubules in both axons and dendrites. With regard to axon growth, regardless of whether kinesin-5 is experimentally inhibited by allosteric inhibitors or depleted by RNA interference, the result is faster-growing axons [28]. This is due in part to the fact that the frequency of transport of short microtubules is doubled as a result of kinesin-5 inhibition and/or depletion, and in part because the normal tendency of the axon to undergo bouts of partial retraction is suppressed when kinesin-5 is inhibited [28]. Mechanistically, the simplest interpretation is that kinesin-5 imposes a brake on the forces generated by cytoplasmic dynein, such that removing the brake increases the effects of dynein-driven forces. Indeed, the invasion of microtubules from the central domain of the growth cone into the peripheral domain dramatically increases when kinesin-5 is depleted or inhibited, and this occurs throughout the growth cone [39,40]. This effect is remarkably similar to that of myosin II inhibition [41], except that kinesin-5 inhibition does not diminish the retrograde flow of actin filaments. Just as with myosin II inhibition, this loss of polarity of microtubule invasion is accompanied by the growth cone no longer turning in response to either substrate cues or growth factors [39,40].
Kinesin-5 exists in cells as a homotetramer with four motor domains projected outward and, hence, is ideally suited to regulate microtubule–microtubule interactions. Kinesin-12 has some redundancy of function during mitosis with kinesin-5, and recent studies suggest that kinesin-12 behaves like a tetramer [42]. Reminiscent of kinesin-5 depletion or inhibition, depletion of kinesin-12 from cultured neurons and in vivo results in faster-growing axons with greater frequency of microtubule transport [26,43]. Neurons depleted of kinesin-12 also fail to turn properly in response to substrate cues. However, kinesin-12 has a myosin II-like domain that is not shared by kinesin-5, and this domain enables kinesin-12 to regulate microtubule–actin interactions in a manner that kinesin-5 cannot. Live cell imaging of growth cones reveals that microtubules entering filopodia display a ‘waggling’ effect, because they are assembling against the direction of actin retrograde flow. Depletion of kinesin-12 (but not of kinesin-5) obliterates the waggling effect, indicating an uncoupling of the microtubule from the actin bundle, reflecting a role for kinesin-12 in the normal interaction of these two cytoskeletal elements. These results suggest that kinesin-5 and kinesin-12 impose themselves on the force balance between cytoplasmic dynein and myosin II in such a way as to tip the balance one way or the other (Figure 2). In this manner, signaling cascades that locally regulate these motors can provide polarity to the force balance, and thereby underlie growth cone turning.
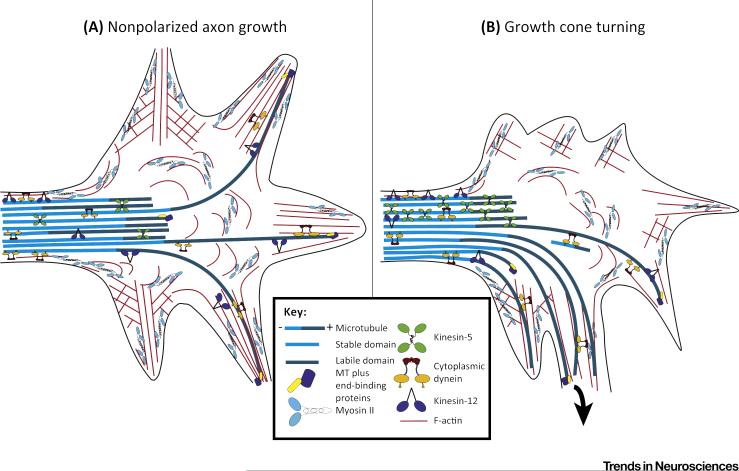
Figure 2. Motor-Driven Forces in the Growth Cone.
(A) During growth cone advance, cytoplasmic dynein generates forces on microtubules (MT) that enable some of them to resist the retrograde flow of the actin cytoskeleton in the peripheral domain of the growth cone. Kinesin-5 and kinesin-12 generate forces that oppose dynein-driven forces on the microtubules to resist their entry into the peripheral domain. Kinesin-5 acts more in the transition zone, while kinesin-12 acts more in the filopodia. (B) During growth cone turning, kinesin-5 and kinesin-12 forces become polarized to the side of the growth cone opposite to the direction of the turn. This enables microtubules to preferentially enter the side of the growth cone in the direction of the turn.
The association of kinesin-5 with microtubules is regulated by its phosphorylation at Thr926, with the phosphorylated variant associating with microtubules [14,18,24]. In neurons, as the growth cone encounters a signal to turn, kinesin-5 becomes locally phosphorylated on the side of the growth cone opposite to the turn, thus enabling microtubules to better invade the side of the growth cone in the direction of the turn [39]. During mitosis of dividing cells, kinesin-5 is phosphorylated at Thr926 by CDK1 [18], but in neurons, CDK5 is the relevant kinase for phosphorylating kinesin-5 at Thr926 [24]. Expression of a Thr926-phosphomutant of kinesin-5 in neurons prohibits growth cone turning similarly to depleting kinesin-5 or pharmacologically inhibiting it with drugs, such as monastrol [40]. Kinesin-5 has a higher affinity for microtubules that are not extensively detyrosinated, which may explain why kinesin-5 preferentially accumulates in dendrites versus axons and in the growth cone relative to the axon shaft [24].
Less is known about kinesin-12. While the effects of inhibiting kinesin-12 are ostensibly similar to the effects of kinesin-5 inhibition on growth cone turning, the mechanism is likely to be different rather than redundant, because they do not compensate functionally for one another when the other is depleted or inhibited. Whereas kinesin-5 imposes its activity mainly in the transition zone of the growth cone [39,40], the forces of kinesin-12 are more relevant in the peripheral domain and filopodia, where the microtubules interact with actin filaments [26]. A potential link between kinesin-5 and kinesin-12 may be TPX2, which was originally discovered as a kinesin-12-interacting protein [44], but is now known to have a kinesin-5-binding site [17]. In neurons, TPX2 is multifunctional [16,19], with one of its functions to fortify the ability of kinesin-5 to behave as a brake. TPX2 does this by generating drag on the kinesin-5 motor via interactions with kinesin-5 as well as with the two microtubules with which kinesin-5 interacts [45]. In other cell types, TPX2 has been shown to have roles in the localization and recruitment of both kinesin-5 and kinesin-12 to microtubules [17,44]. A potential role for TPX2 in regulating kinesin-12 in neurons has not yet been explored.
Microtubule Severing in Growth Cones
Remodeling of cellular microtubule arrays can occur by severing of long microtubules into shorter ones, reorientation of the short microtubules (by molecular motor proteins), and subsequent elongation of the short microtubules into longer ones [46,47]. The coordinated activities of the microtubule-severing proteins that generate the short microtubules and the motor proteins that transport them have been referred to as the ‘cut and run’ mechanism [47]. Axons can pause or stall in their growth for prolonged periods of time and, when they do, the microtubules within the growth cone form a looped bundle that later splays apart for the axon to resume growth (Figure 3, Key Figure). When this happens, many of the microtubules undergo severing events that produce short highly motile microtubules that are propelled outward, throughout the peripheral domain [48] by a dynein-driven mechanism similar to what has been reported in non-neuronal migrating cells [49].
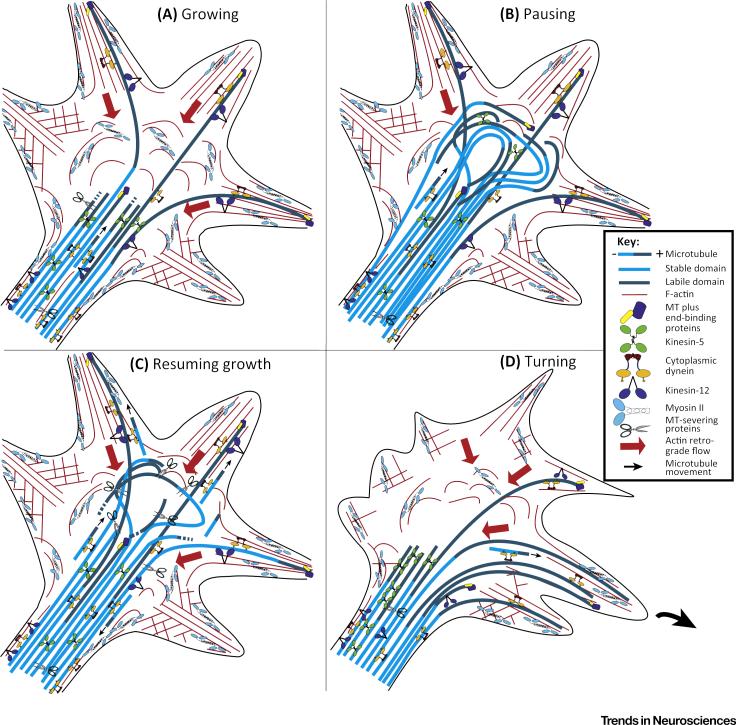
Figure 3. Motor-Driven Microtubule (MT) Behaviors during Different Phases of Growth Cone Activity.
(A) During growth cone advance, dynein-driven forces permit a portion of the microtubules to overcome the retrograde flow of actin filaments so that these microtubules can invade the peripheral domain and filopodia. The invasion occurs in a nonpolarized fashion throughout the peripheral domain, so that the axon grows in a relatively straight trajectory. Severing in the labile portion of the microtubule (shown in dark blue) regulates the length of that portion of the microtubule, while severing in the stable portion of the microtubule (shown in light blue) creates short mobile microtubules. (B) When the growth cone pauses, the advance of the microtubule array results in a growth cone no longer compartmentalized into central and peripheral domains, dominated by a curved bundle of microtubules. (C) When the axon resumes growth, microtubules are rapidly unbundled, and microtubule severing is increased so that more short microtubules are propelled away from the bundle. (D) During turning, kinesin-5 and/or kinesin-12 forces become polarized to the side of the growth cone opposite to the direction of the turn, so that dynein-driven forces enable microtubule invasion only on the side of the growth cone in the direction of the turn. Small arrows indicate direction of movement of short microtubules. Large red arrows indicate retrograde actin flow.
Spastin, a microtubule-severing protein that concentrates in growth cones, has a preference for severing microtubules rich in post-translationally polyglutamylated tubulin [50,51], which is concentrated in the stable portion of the microtubule. Katanin, which is more evenly spread in the axon, has a preference for post-translationally acetylated tubulin [52], which is also concentrated in the stable portion of the microtubule. The preference of spastin and katanin for the stable portion of the microtubule is relevant to the production of short mobile microtubules because severing in the labile portion of the microtubule would cause it to disassemble. Katanin sensitivity is reduced by tau bound to the microtubule [53], which is especially interesting in light of new studies suggesting that tau is also a negative regulator of kinesin-5 [54]. Fidgetin is a microtubule-severing protein that severs in the labile portion of the microtubule, via a preference for unacetylated tubulin [55]. Within the growth cone, micro-tubules generally have their stable portions in the central domain, with the labile portions able to extend into the peripheral domain. Fidgetin is perhaps the most relevant of the severing proteins to axon navigation because severing in the labile portion of the microtubule affects the capacity of microtubules to assemble labile portions into the peripheral domain of the growth cone.
Concluding Remarks
How developing axons find their appropriate target tissues is one of the most crucial questions in developmental neurobiology. While the answer certainly includes the actin cytoskeleton, the importance of microtubules has languished by comparison in the contemporary literature. In our opinion, growth cone turning borrows a theme from mitosis, wherein a variety of molecular motors impose forces that are necessary for microtubules to undergo their needed behaviors. Evidence for such a scenario has been presented here, mainly focusing on three microtubule-based motors (cytoplasmic dynein, kinesin-5 and kinesin-12), and how they contribute to the polarized invasion of microtubules in the growth cone. Various other studies lend support to this motor-based perspective [49,56,57], with some suggesting that other motor proteins also participate [58]. The most exciting aspect of this developing model is regulation, because the activity of each motor is controlled by various factors, such as phosphorylation by particular kinases and/or interaction with co-factors (see Outstanding Questions). Relevant also are microtubule-severing proteins, because their activity affects how the motor-driven forces manifest on the microtubules. At present, there is little evidence that speaks to the signaling pathways by which environmental cues relevant to axon navigation might activate or deactivate particular motor proteins (and severing proteins) in functionally important regions of the growth cone to elicit polarized invasion of microtubules. The next frontier lies in elucidating these pathways to improve our understanding of the wiring of the nervous system during development. Progress on this frontier will hopefully provide new opportunities in the clinic for coaxing regenerating adult axons of the injured nervous system to renavigate to their appropriate targets.
Trends.
Polarized invasion of microtubules into one side only of the peripheral domain of the growth cone is required for the growth cone to turn.
Cytoplasmic dynein generates forces that enable some microtubules to overcome the myosin II-based retrograde flow of actin filaments in the growth cone, and thereby invade its peripheral domain.
Kinesin-5 and kinesin-12, traditionally considered mitotic motor proteins, act as polarizing motors that locally oppose cytoplasmic dynein-based forces in certain regions of the growth cone to enable microtubule invasion into other regions.
Signaling cascades can locally activate and deactivate the relevant motor proteins via mechanisms that include phosphorylation and interaction with partner proteins.
Outstanding Questions.
What are the means by which each relevant molecular motor protein is activated or deactivated in a manner that contributes to the turning of the growth cone in response to environmental cues?
Are there other motor proteins beyond those studied thus far that have important functions in generating forces on microtubules in the growth cone that are relevant to its turning in response to environmental cues?
What are the different signaling cascades originating from the environmental cue that elicit changes in growth cone microtubule distribution via motor-driven forces?
Do some signaling cascades affect the actin cytoskeleton while others affect microtubules, or do the same signaling cascades affect them both in a coordinated way?
How are microtubule-severing proteins regulated during growth cone behaviors so that microtubules are severed when and where needed so that the motor forces manifest appropriately?
Can learning more about the role of motor-driven forces in axon navigation provide strategies for enabling regenerating axons to find their appropriate targets during recovery from nerve injury?
Acknowledgments
Related work by P.W.B. is funded from a grant from the National Institutes of Health. O.I.K. is the recipient of a predoctoral National Research Service Award from the National Institutes of Health.
References
- 1.Dent EW, Gertler FB. Cytoskeletal dynamics and transport in growth cone motility and axon guidance. Neuron. 2003;40:209–227. doi: 10.1016/s0896-6273(03)00633-0. [DOI] [PubMed] [Google Scholar]
- 2.Dent EW, et al. The growth cone cytoskeleton in axon outgrowth and guidance. Cold Spring Harb. Perspect. Biol. 2011;3:a001800. doi: 10.1101/cshperspect.a001800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lowery LA, Van Vactor D. The trip of the tip: understanding the growth cone machinery. Nat. Rev. Mol. Cell Biol. 2009;10:332–343. doi: 10.1038/nrm2679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Goldberg DJ, Burmeister DW. Stages in axon formation: observations of growth of Aplysia axons in culture using video-enhanced contrast-differential interference contrast microscopy. J. Cell Biol. 1986;103:1921–1931. doi: 10.1083/jcb.103.5.1921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Conde C, Caceres A. Microtubule assembly, organization and dynamics in axons and dendrites. Nat. Rev. Neurosci. 2009;10:319–332. doi: 10.1038/nrn2631. [DOI] [PubMed] [Google Scholar]
- 6.Geraldo S, Gordon-Weeks PR. Cytoskeletal dynamics in growth-cone steering. J. Cell Sci. 2009;122:3595–3604. doi: 10.1242/jcs.042309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tanaka E, Kirschner MW. The role of microtubules in growth cone turning at substrate boundaries. J. Cell Biol. 1995;128:127–137. doi: 10.1083/jcb.128.1.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Buck KB, Zheng JQ. Growth cone turning induced by direct local modification of microtubule dynamics. J. Neurosci. 2002;22:9358–9367. doi: 10.1523/JNEUROSCI.22-21-09358.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Suter DM, et al. Microtubule dynamics are necessary for SRC family kinase-dependent growth cone steering. Curr. Biol. 2004;14:1194–1199. doi: 10.1016/j.cub.2004.06.049. [DOI] [PubMed] [Google Scholar]
- 10.Bearce EA, et al. TIPsy tour guides: how microtubule plus-end tracking proteins (+TIPs) facilitate axon guidance. Front. Cell Neurosci. 2015;9:241. doi: 10.3389/fncel.2015.00241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cross RA, McAinsh A. Prime movers: the mechano-chemistry of mitotic kinesins. Nat. Rev. Mol. Cell Biol. 2014;15:257–271. doi: 10.1038/nrm3768. [DOI] [PubMed] [Google Scholar]
- 12.Kuijpers M. Dyneinregulator NDEL 1 controlspolarized cargo transport at the axon initial segment. Neuron. 2016;89:461–471. doi: 10.1016/j.neuron.2016.01.022. [DOI] [PubMed] [Google Scholar]
- 13.Tanaka T, et al. Lis1 and doublecortin function with dynein to mediate coupling of the nucleus to the centrosome in neuronal migration. J. Cell Biol. 2004;165:709–721. doi: 10.1083/jcb.200309025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Avunie-Masala R, et al. Phospho-regulation of kinesin-5 during anaphase spindle elongation. J. Cell Sci. 2011;124:873–878. doi: 10.1242/jcs.077396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Garcia K, et al. Tyrosines in the kinesin-5 head domain are necessary for phosphorylation by Wee1 and for mitotic spindle integrity. Curr. Biol. 2009;19:1670–1676. doi: 10.1016/j.cub.2009.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kahn OI, et al. TPX2 regulates neuronal morphology through kinesin-5 interaction. Cytoskeleton (Hoboken) 2015;72:340–348. doi: 10.1002/cm.21234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ma N, et al. TPX2 regulates the localization and activity of Eg5 in the mammalian mitotic spindle. J. Cell Biol. 2011;195:87–98. doi: 10.1083/jcb.201106149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cahu J, et al. Phosphorylation by Cdk1 increases the binding of Eg5 to microtubules in vitro and in Xenopus egg extract spindles. PLoS ONE. 2008;3:e3936. doi: 10.1371/journal.pone.0003936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mori D, et al. An essential role of the aPKC-Aurora ANDEL1 pathway in neurite elongation by modulation of microtubule dynamics. Nat. Cell Biol. 2009;11:1057–1068. doi: 10.1038/ncb1919. [DOI] [PubMed] [Google Scholar]
- 20.Baas PW. Microtubules and neuronal polarity: lessons from mitosis. Neuron. 1999;22:23–31. doi: 10.1016/s0896-6273(00)80675-3. [DOI] [PubMed] [Google Scholar]
- 21.Ahmad FJ, et al. Cytoplasmic dynein and dynactin are required for the transport of microtubules into the axon. J. Cell Biol. 1998;140:391–401. doi: 10.1083/jcb.140.2.391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ferhat L, et al. Expression of the mitotic motor protein Eg5 in postmitotic neurons: implications for neuronal development. J. Neurosci. 1998;18:7822–7835. doi: 10.1523/JNEUROSCI.18-19-07822.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sharp DJ, et al. Expression of a minus-end-directed motor protein induces Sf9 cells to form axon-like processes with uniform microtubule polarity orientation. J. Cell Sci. 1997;110:2373–2380. doi: 10.1242/jcs.110.19.2373. [DOI] [PubMed] [Google Scholar]
- 24.Kahn OI, et al. Effects of kinesin-5 inhibition on dendritic architecture and microtubule organization. Mol. Biol. Cell. 2015;26:66–77. doi: 10.1091/mbc.E14-08-1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lin S, et al. Mitotic motors coregulate microtubule patterns in axons and dendrites. J. Neurosci. 2012;32:14033–14049. doi: 10.1523/JNEUROSCI.3070-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu M, et al. Kinesin-12, a mitotic microtubule-associated motor protein, impacts axonal growth, navigation, and branching. J. Neurosci. 2010;30:14896–14906. doi: 10.1523/JNEUROSCI.3739-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Del Castillo U, et al. Interplay between kinesin-1 and cortical dynein during axonal outgrowth and microtubule organization in Drosophila neurons. Elife. 2015;4:e10140. doi: 10.7554/eLife.10140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Myers KA, Baas PW. Kinesin-5 regulates the growth of the axon by acting as a brake on its microtubule array. J. Cell Biol. 2007;178:1081–1091. doi: 10.1083/jcb.200702074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Suter DM, Forscher P. Substrate–cytoskeletal coupling as a mechanism for the regulation of growth cone motility and guidance. J. Neurobiol. 2000;44:97–113. [PubMed] [Google Scholar]
- 30.Bard L, et al. A molecular clutch between the actin flow and N-cadherin adhesions drives growth cone migration. J. Neurosci. 2008;28:5879–5890. doi: 10.1523/JNEUROSCI.5331-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Forscher P, Smith SJ. Actions of cytochalasins on the organization of actin filaments and microtubules in a neuronal growth cone. J. Cell Biol. 1988;107:1505–1516. doi: 10.1083/jcb.107.4.1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schaefer AW, et al. Coordination of actin filament and microtubule dynamics during neurite outgrowth. Dev. Cell. 2008;15:146–162. doi: 10.1016/j.devcel.2008.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.He Y, et al. Role of cytoplasmic dynein in the axonal transport of microtubules and neurofilaments. J. Cell Biol. 2005;168:697–703. doi: 10.1083/jcb.200407191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hasaka TP, et al. Role of actin filaments in the axonal transport of microtubules. J. Neurosci. 2004;24:11291–11301. doi: 10.1523/JNEUROSCI.3443-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Myers KA, et al. Antagonistic forces generated by cytoplasmic dynein and myosin-II during growth cone turning and axonal retraction. Traffic. 2006;7:1333–1351. doi: 10.1111/j.1600-0854.2006.00476.x. [DOI] [PubMed] [Google Scholar]
- 36.Ahmad FJ, et al. Motor proteins regulate force interactions between microtubules and microfilaments in the axon. Nat. Cell Biol. 2000;2:276–280. doi: 10.1038/35010544. [DOI] [PubMed] [Google Scholar]
- 37.Roossien DH, et al. Cytoplasmic dynein pushes the cyto-skeletal meshwork forward during axonal elongation. J. Cell Sci. 2014;127:3593–3602. doi: 10.1242/jcs.152611. [DOI] [PubMed] [Google Scholar]
- 38.Grabham PW, et al. Cytoplasmic dynein and LIS1 are required for microtubule advance during growth cone remodeling and fast axonal outgrowth. J. Neurosci. 2007;27:5823–5834. doi: 10.1523/JNEUROSCI.1135-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nadar VC, et al. Kinesin-5 is essential for growth-cone turning. Curr. Biol. 2008;18:1972–1977. doi: 10.1016/j.cub.2008.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nadar VC, et al. Microtubule redistribution in growth cones elicited by focal inactivation of kinesin-5. J. Neurosci. 2012;32:5783–5794. doi: 10.1523/JNEUROSCI.0144-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Turney SG, Bridgman PC. Laminin stimulates and guides axonal outgrowth via growth cone myosin II activity. Nat. Neurosci. 2005;8:717–719. doi: 10.1038/nn1466. [DOI] [PubMed] [Google Scholar]
- 42.Drechsler H, et al. The Kinesin-12 Kif15 is a processive track-switching tetramer. Elife. 2014;3:e01724. doi: 10.7554/eLife.01724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Xu M, et al. Kinesin-12 influences axonal growth during zebrafish neural development. Cytoskeleton (Hoboken) 2014;71:555. doi: 10.1002/cm.21193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wittmann T, et al. TPX2, a novel Xenopus MAP involved in spindle pole organization. J. Cell Biol. 2000;149:1405–1418. doi: 10.1083/jcb.149.7.1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Balchand SK, et al. TPX2 Inhibits Eg5 by interactions with both motor and microtubule. J. Biol. Chem. 2015;290:17367–17379. doi: 10.1074/jbc.M114.612903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yu W, et al. The microtubule-severing proteins spastin and katanin participate differently in the formation of axonal branches. Mol. Biol. Cell. 2008;19:1485–1498. doi: 10.1091/mbc.E07-09-0878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Baas PW, et al. Microtubules cut and run. Trends Cell Biol. 2005;10:518–524. doi: 10.1016/j.tcb.2005.08.004. [DOI] [PubMed] [Google Scholar]
- 48.Dent EW, et al. Reorganization and movement of micro-tubules in axonal growth cones and developing interstitial branches. J. Neurosci. 1999;19:8894–8908. doi: 10.1523/JNEUROSCI.19-20-08894.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Abal M, et al. Microtubule release from the centrosome in migrating cells. J. Cell Biol. 2002;159:731–737. doi: 10.1083/jcb.200207076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lacroix B, et al. Tubulin polyglutamylation stimulates spastin-mediated microtubule severing. J. Cell Biol. 2010;189:945–954. doi: 10.1083/jcb.201001024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Valenstein ML, Roll-Mecak A. Graded control of microtubule severing by tubulin glutamylation. Cell. 2016;164:911–921. doi: 10.1016/j.cell.2016.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sudo H, Baas PW. Acetylation of microtubules influences their sensitivity to severing by katanin in neurons and fibroblasts. J. Neurosci. 2010;30:7215–7226. doi: 10.1523/JNEUROSCI.0048-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Qiang L, et al. Tau protects microtubules in the axon from severing by katanin. J. Neurosci. 2006;26:3120–3129. doi: 10.1523/JNEUROSCI.5392-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bouge A-L, Parmentier M-L. Tau excess impairs mitosis and kinesin-5 function, leading to aneuploidy and cell death. Dis. Model. Mech. 2016;9:307–319. doi: 10.1242/dmm.022558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Leo L, et al. Vertebrate fidgetin restrains axonal growth by severing labile domains of microtubules. Cell Rep. 2015;12:1723–1730. doi: 10.1016/j.celrep.2015.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Suter DM, Miller KE. The emerging role of forces in axonal elongation. Prog. Neurobiol. 2011;94:91–101. doi: 10.1016/j.pneurobio.2011.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.O'Toole M, et al. Measurement of subcellular force generation in neurons. Biophys. J. 2015;108:1027–1037. doi: 10.1016/j.bpj.2015.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sapir T, et al. Shootin1 acts in concert with KIF20B to promote polarization of migrating neurons. J. Neurosci. 2013;33:11932–11948. doi: 10.1523/JNEUROSCI.5425-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]