Abstract
Metformin (Met) is an approved antidiabetic drug currently being explored for repurposing in cancer treatment based on recent evidence of its apparent chemopreventive properties. Met is weakly cationic and targets the mitochondria to induce cytotoxic effects in tumor cells, albeit not very effectively. We hypothesized that increasing its mitochondria-targeting potential by attaching a positively-charged lipophilic substituent would enhance the antitumor activity of Met. In pursuit of this question, we synthesized a set of mitochondria-targeted Met analogs (Mito-Mets) with varying alkyl chain lengths containing a triphenylphosphonium cation (TPP+). In particular, the analog Mito-Met10, synthesized by attaching TPP+ to Met via a 10-carbon aliphatic side chain, was nearly 1,000 times more efficacious than Met at inhibiting cell proliferation in pancreatic ductal adenocarcinoma (PDAC). Notably, in PDAC cells Mito-Met10 potently inhibited mitochondrial complex I, stimulating superoxide and AMPK activation, but had no effect in non-transformed control cells. Moreover, Mito-Met10 potently triggered G1 cell cycle phase arrest in PDAC cells, enhanced their radiosensitivity and more potently abrogated PDAC growth in preclinical mouse models, compared to Met. Collectively, our findings show how improving the mitochondrial targeting of Met enhances its anticancer activities, including in aggressive cancers like PDAC in great need of more effective therapeutic options.
Keywords: metformin, mitochondria-targeting, bioenergetics, metabolism, cell proliferation
Introduction
Metformin (Met) is a synthetic analog of a naturally-occurring biguanides. It is an anti-diabetic drug (1,2) that exerts anticancer effects in diabetic individuals with pancreatic cancer (3,4). Met exists as a hydrophilic cation (Fig. 1A) at physiological pH and weakly targets mitochondria (5). A prevailing view is that Met exerts antitumor effects by elevating cellular AMP/ATP ratio and activating the 5’-AMP-activated protein kinase (AMPK)/mTOR pathway (6,7) and/or by decreasing the circulating insulin and the blood glucose levels (8). Met also inhibits complex I in the mitochondrial electron transport chain, and tumor mitochondrial respiration (9). Met suppresses gluconeogenesis by inhibiting mitochondrial glycerophosphate dehydrogenase (10). The organic cation transporter is responsible for Met uptake into tumor cells, while more lipophilic Met analogs (e.g., phenformin) are taken up into cells via alternate mechanism(s) (11). Phenformin is more potent than Met in inhibiting pancreatic tumor cell proliferation (12). However, phenformin was taken off the market in the U.S. because of increased incidence of acidosis during anti-diabetic therapy (13). Additional clinical research repurposing phenformin as an antitumor drug was recently recommended (14).
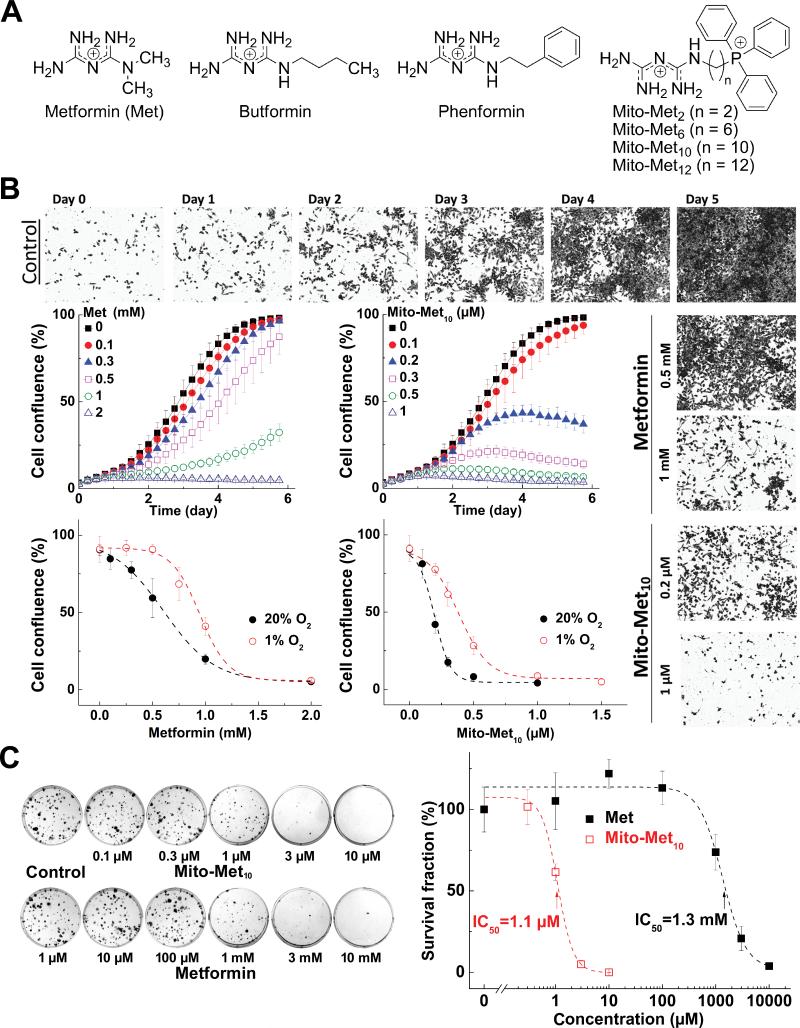
Figure 1. Effects of Met and Mito-Met10 on PDAC proliferation.
(A) Chemical structures. (B) Effects of Mito-Met10 and Met on PDAC proliferation. MiaPaCa-2 cells were treated with Mito-Met10 or Met and cell growth monitored over 6 days. Dose response of Met (left) or Mito-Met10 (right) on cell confluence is shown. Age-matched cells were cultured in three passages in either 20% or 1% oxygen immediately prior to treatment. (C) Effects of Mito-Met10 and Met on colony formation in MiaPaCa-2 cells. Cells were treated with Mito-Met10 or Met for 24 h. The survival fraction calculated is plotted against concentration. Dashed lines represent the fitting curves.
Previous reports suggest that mitochondria-targeted cationic agents induce antiproliferative and cytotoxic effects in tumor cells without markedly affecting normal cells (15,16). For example, conjugating a nitroxide, quinone, a chromanol moiety of α-tocopherol to the triphenylphosphonium (TPP+) group via an aliphatic linker increased their antiproliferative effect in tumor cells (15,16). Selective toxicity to tumor cells as compared to normal cells was attributed to enhanced uptake and retention of TPP+-containing compounds in tumor cell mitochondria (16). Met has been used in the clinic for over 50 years and has a very good safety profile (diabetic patients tolerate daily doses of 2-3 g) (1-4). Efforts to improve and enhance efficacy of Met involved modification of structure by attaching alkyl or aromatic groups (e.g., butformin, phenformin) (17) (Fig. 1A). We hypothesized that improved mitochondrial targeting of Met by attaching a positively-charged lipophilic substituent will result in a new class of mitochondria-targeted drugs with significantly increased antitumor potential. To this end, we synthesized and characterized several Met analogs (e.g., Mito-Met2, Mito-Met6, Mito-Met10, Mito-Met12, Fig. 1A) conjugated to an alkyl substituent containing a TPP+ moiety (Suppl. Fig. 1). The present results show that Mito-Met10 is nearly 1,000-fold more effective than Met in inhibiting pancreatic ductal adenoma cell (PDAC) proliferation in vitro and more effective than Met in abrogating PDAC tumor growth in vivo. Mito-Met10 inhibited mitochondrial complex I, stimulating superoxide generation and AMPK activation, more potently than Met in MiaPaCa-2 cells. Reports suggest that Met (1 mM) pretreatment followed by radiation resulted in enhanced cancer cell killing (17-19). In this study, we show that Mito-Met10 enhanced radiation sensitivity in PDAC to the same extent as did Met (1 mM) but at a 1,000-fold lower concentration (1 μM). The finding that relatively nontoxic mitochondria-targeted Met analogs alone or in combination with radiotherapy could inhibit pancreas cancer cell proliferation is highly relevant.
Materials and Methods
Cell culture
MiaPaCa-2, PANC-1, HPNE, IEC-6 and MCF-10A cell lines were obtained from the American Type Culture Collection (Manassas, VA), where they were regularly authenticated. N27 cell line was a gift from Dr. Anumantha Kanthasamy (Iowa State University, Ames). FC-1242 cell line was a gift of Dr. David Tuveson and Dr. Dannielle Engle (Cold Spring Harbor Laboratory), and was derived from C57BL/6(B6) KPC transgenic mice that spontaneously developed pancreatic tumors. This cell line was engineered to express luciferase (FC-1242-luc) which enabled monitoring of in vivo tumor growth (20). All cells were obtained over the last five years, stored in liquid nitrogen and used within 20 passages after thawing.
Respiratory enzyme activity in intact and permeabilized cells
The mitochondrial function in intact and permeabilized cells was measured using a Seahorse XF96 Extracellular Flux Analyzer (Seahorse Bioscience, North Billerica, MA). Assays in intact cells were performed as previously described (21). Measurement of mitochondrial respiratory complexes in permeabilized cells was performed according to the manufacturer's instructions. Briefly, intact cells were permeabilized using 1 nM Plasma Membrane Permeabilizer (PMP, Seahorse Bioscience) immediately before oxygen consumption rate (OCR) measurement by XF96. The oxygen consumption derived from mitochondrial complex I or complex II activity was measured by providing different substrates to mitochondria, e.g., pyruvate/malate for complex I and succinate for complex II (22,23). Rotenone, malonate, and antimycin A were used as specific inhibitors of mitochondrial complex I, II, and III, respectively.
Clonogenic assay and cell proliferation assay
Cells were seeded as indicated in six-well plates and treated with Mito-Met10 or Met for 24 h. The plates were kept within the incubator and media changed every 3-4 days until the control cells formed sufficiently large clones. The cell survival fractions were calculated as before (21).
Cell proliferation was measured using a label-free, noninvasive cellular confluence assay by IncuCyte Live-Cell Imaging Systems (IncuCyte FLR, Essen Bioscience, Ann Arbor, MI), as described previously (21).
Three-dimensional spheroid cell culture
MiaPaCa-2 cells (5×103/well) were seeded in 96-well plates containing Matrigel (Corning). The culture medium (containing appropriate concentration of Met or Mito-Met10) was replaced every two days. At days 3, 7, and 14 the images were acquired using a Nikon Eclipse Ti inverted microscope (Nikon Inc., NY). Spheroid-forming cells were counted using the Nikon NIS Elements imaging software.
Cytotoxicity assay
To determine the cytotoxicity of Mito-Met analogs and other TPP+-conjugated compounds, we used the Sytox Green-based assay as described previously (16). MiaPaCa-2 cells were treated for 24 h, and dead cells were monitored in real time in the presence of 200 nM Sytox Green (Invitrogen) under an atmosphere of 5% CO2:95% air at 37°C. Data are represented as a percentage of dead cells after normalization to total cell number (measured with Sytox Green after a 3 h treatment with 0.065% Triton X-100) for each group.
Immunoblotting
Cells were serum-starved for 5 h (AMPK) or 24 h (FOXM1). Stimulations with Met or Mito-Met10 were performed for 30 min in serum-free medium (AMPK) or for 24 h in full growth media (FOXM1). After stimulation, cells were washed, lysed using a modified RIPA buffer and the proteins immunoblotted. Antibodies against FOXM1 (D12D5), Cyclin D1 (92G2), phospho-AMPK(40H9) or total AMPK (D5A2) anti-sera were from Cell Signaling Technology (Danvers, MA). Proteins were detected by chemiluminescence and quantified by densitometric analysis using the FluorChem HD2 software (Cell Biosciences, Santa Clara, CA).
Cell cycle analysis
PANC1 cells (5×105) were seeded into 35 mm dishes and immediately treated with 10 mM Met or 10 μM Mito-Met10 in complete media. At 48 h post-treatment, cells were fixed with 70% ethanol, permeabilized with 0.25% Triton X-100 in PBS, and stained using a propidium iodide/RNase solution (20 μg/ml and 10 μg/ml, respectively). Data were collected on the BD-LSR II flow cytometer (BD Sciences) and analyzed using Flow-Jo (Flow-Jo LLC, Ashland, OR).
Quantification of intracellular Mito-Met analogs by LC-MS/MS
Cells were grown on 10 cm dishes and incubated with the compounds for 24 h in full media. The protocol for extraction of Mito-Met analogs was the same as previously described, but without BHT (16). LC-MS/MS analyses were performed using a Kinetex Phenyl-Hexyl column (50 mm × 2.1 mm, 1.7 μm, Phenomenex) equilibrated with water:acetonitrile mixture (4:1) containing 0.1% formic acid. Compounds were eluted by increasing the content of acetonitrile from 20% to 100% over 4 min and detected using the MRM mode.
Radiation experiments
MiaPaCa-2, HPNE and MCF-10A cells (5×105/dish) were seeded in 6 cm dishes and treated with Mito-Met10 or Met for 24 h. The control cells were treated with the vehicle (0.1% DMSO), where appropriate. The cells were then exposed to X-radiation (0-6 Gy). After irradiation, cells were suspended and seeded at various densities (100-8,000 cells/well) in 6-well plates for clonogenic assay as described above. The plates were kept within the incubator and media changed every 4-6 days for 2 weeks. Wells with 10-50 sufficiently large clones were chosen for calculating the cell survival fractions.
In vivo studies and bioluminescence imaging
An orthotopic syngeneic engraftment model was used to assess metastatic homing and tumor progression following treatment with Met or Mito-Met10. Six to eight-week-old C57BL/6 mice were anesthetized with isoflurane and were injected with 1×106 luciferase-expressing FC-1242 (FC-1242-luc) cells (24). To generate FC-1242-luc cells, the firefly luciferase gene was cloned into the mammalian expression vector pcDNA3.1/hygro(−) cells transfected using the Lipofectamine 2000 transfection reagent (Life Technologies). A cell line stably expressing firefly luciferase was generated by culturing cells in growth medium supplemented with 0.5 mg/mL hygromycin and limited dilution cloning. A stable FC-1242-luc clone was expanded to generate a frozen stock. Cells were pretreated for 48 h with Met (1 mM) or Mito-Met10 (0.5 μM) and then orthotopically engrafted to the pancreas as described previously (24-26). Starting the day of implantation, mice were treated daily with 1 mg/kg Met or Mito-Met10 administered via an intraperitoneal injection in a 200 μL volume. Tumor growth and metastasis was monitored using bioluminescence imaging (Lumina IVIS 100, Perkin Elmer, Alameda, CA) on days 1, 7, and 13 (26,27). After 13 days, mice were euthanized, and the size of primary tumor determined using calipers. Metastasis to the liver, mesenteric lymph nodes, spleen and lung was assessed ex vivo using bioluminescence imaging.
Statistical Analysis
All statistical analyses were performed using GraphPad Prism 4 (San Diego, CA). Paired analyses were performed using a Student's t-test. Multiple comparisons were analyzed using a one-way ANOVA and Tukey post-hoc tests to identify pair-wise differences between distinct experimental groups. All values provided represent mean ± standard deviation.
Results
Syntheses and characterization of mitochondria-targeted Met analogs with varying side chain lengths
The Mito-Met analogs (Fig. 1A) were synthesized according to the reaction shown in Supplemental Figure 1E and characterized by NMR and mass spectrometry (Suppl. Fig. 1). Mito-Mets (n = 2, 6, 10, and 12) were obtained by reacting the corresponding aminoalkyltriphenylphosphonium substrate with dicyanamide and purified by preparative-HPLC. Experimental details are provided in Supplemental Figure 1.
Mito-Met inhibits PDAC cell and PDAC spheroid growth more potently than Met
To compare the antiproliferative effects of Met and Mito-Met10, human MiaPaCa-2 cells were treated with Met (0.1 – 2 mM) or Mito-Met10 (0.1-1 μM). Cell proliferation curves, as measured by cell confluence kinetics and representative phase contrast images, are shown in Figure 1B. Under the conditions used, untreated cells reached 100% confluence in 5 to 6 days. Mito-Met10 treatment (0.2 and 1 μM) inhibited cell growth by 70% and > 90%, respectively. In contrast, Met inhibited cell growth by 80% at 1,000-fold higher concentrations (1 mM) (Fig. 1B). A similar trend was noticed at 20% and 1% oxygen (Fig. 1B). Mito-Met10 was also significantly more potent than Met in inhibiting proliferation of mouse pancreatic cells, FC-1242 (Suppl. Fig. 2).
We also monitored colony formation in MiaPaCa-2 cells after a 24-h treatment with different concentrations of Met or Mito-Met10 under similar conditions. Almost no colony formation was detected in cells treated with 3 μM of Mito-Met10 and 3 mM of Met (Fig. 1C left). The survival fraction analysis (Fig. 1C right) showed that the IC50 values determined for Met and Mito-Met10 were 1.3 mM and 1.1 μM, respectively. Thus, results from two independent cell growth assays revealed that Mito-Met10 is nearly 1,000-fold more potent than Met in inhibiting MiaPaCa-2 cell proliferation.
To compare the cytotoxic effects of Met, Mito-Met10 and selected other TPP+-linked compounds, MiaPaCa-2 cells were treated with Mito-Met10 (100 μM) or Met (30-100 mM) and other TPP+-linked compounds for 24 h and cell death was monitored in real time using the Sytox Green assay (Suppl. Fig. 3). Surprisingly, there was no detectable cell death induced by Mito-Met10 even at 100-fold higher concentrations (100 μM) than needed to completely block cell proliferation. In contrast, other mitochondria-targeted agents (e.g., Mito-CP, Mito-Q, Mito-CP-Ac, Mito-Tempol, and Mito-Chromanol) were considerably more cytotoxic to MiaPaCa-2 cells under these conditions. Intracellular ATP levels measured under these conditions for selected compounds are consistent with the results obtained using the Sytox Green assay.
We also tested the antiproliferative effects of Mito-Met10 and Met in the multicellular tumor spheroid model. As shown in Figure 2, Mito-Met10 (0.5 μM) more potently inhibited PDAC spheroid growth than Met (10 mM). Thus, both 2D and 3D cell growth assays indicate that Mito-Met10 is 1,000- to 10,000-fold more effective than Met in inhibiting PDAC proliferation.
Figure 2. Effects of Mito-Met10 and Met on PDAC spheroid growth.
(A) MiaPaCa-2 3D spheroids were treated with varying concentrations of Mito-Met10 or Met every other day with fresh medium and counted after 7 days of treatment. **, P < 0.01. (B) Representative images of MiaPaCa-2 spheroids. Scale bars: 100 μm.
Effects of Mito-Met analogs in normal and cancer cells: Fine tuning alkyl side chain length and potency
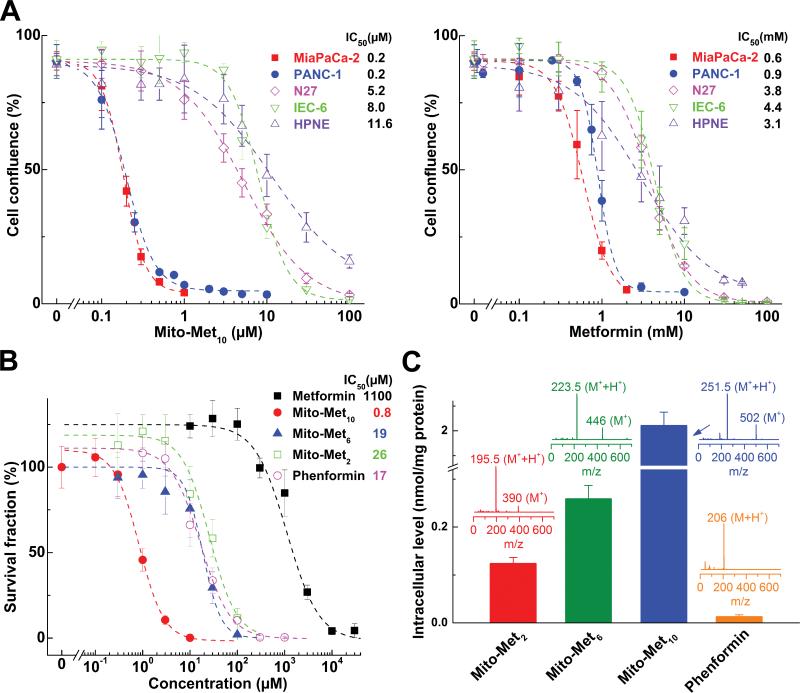
We compared the relative antiproliferative potencies of other Mito-Met analogs (Mito-Met2, Mito-Met6) with Mito-Met10, phenformin, and Met in normal and cancer cells. Figure 3A compares the antiproliferative effects of Mito-Met10 (left) and Met (right) in MiaPaCa-2 and PANC-1 and in nonmalignant control cells, HPNE, IEC-6, and N27 cells. Mito-Met10 and Met more potently inhibited proliferation of MiaPaCa-2 and PANC-1 cells as compared to normal cells (HPNE, IEC-6 and N27) (Fig. 3A). Furthermore, we performed detailed cell viability analyses in Mito-Met10 and Dec-TPP+(same length of the aliphatic linker as Mito-Met10)-treated MiaPaCa-2 and PANC-1 cells and in HPNE and IEC-6 control, nonmalignant cells. Over a wide range of concentrations and incubation times, Dec-TPP+ did not show any selectivity with regard to inhibition of cell viability in PDAC versus nonmalignant cells. However, Met conjugated to TPP+ (e.g., Mito-Met10) more potently inhibited pancreatic cancer cell viability as compared to normal cell viability, with significantly lower IC50values in MiaPaCa-2 and PANC-1 cells as compared to HPNE and IEC-6 cells (Suppl. Fig. 4). Figure 3B shows the relative potencies of Mito-Met analogs (Mito-Met2, Mito-Met6, and Mito-Met10, phenformin, and Met) in inhibiting MiaPaCa-2 colony formation. Mito-Met2 and Mito-Met6 were relatively less potent than Mito-Met10 in inhibiting MiaPaCa-2 cell proliferation (Fig. 3B and Suppl. Fig. 5A and B). Next we investigated the relative uptake of different Mito-Met analogs (Mito-Met2, Mito-Met6, and Mito-Met10) and phenformin in MiaPaCa-2 cells by LC-MS (Fig. 3C). Cells were treated with respective Met analog (1 μM) for 1 h. As shown in Figure 3C, there was a dramatic increase in Mito-Met cellular uptake as a function of increasing carbon-carbon side chain length and Mito-Met10 was taken up nearly 100-fold more than phenformin. Under these treatment conditions, Met uptake was considerably lower than phenformin.
Figure 3. Effects of Met and Mito-Met analogs on pancreatic cancer and normal cell proliferation.
(A) Effects of Mito-Met10 (left) and Met (right) on cell proliferation (same conditions as in Fig. 1B). Cells were treated with Mito-Met10 or Met and cell growth monitored continuously. The cell confluence (as control groups reach 90% confluency) is plotted against concentration. Dashed lines represent the fitting curves used to determine the IC50 values. (B) MiaPaCa-2 cells were treated with metformin analogs for 24 h. The calculated survival fraction is plotted against concentration of the compounds. Dashed lines represent the fitting curves used for determination of the IC50 values. (C) Cellular uptake of phenformin and Mito-Met analogs was quantified using LC-MS/MS. Inserts show the mass spectra of the compounds. All Mito-Met analogs show up as monocations (M+) and dications (M++H+).
Effects of Met and Mito-Met analogs on mitochondrial bioenergetics in MiaPaCa-2 cells
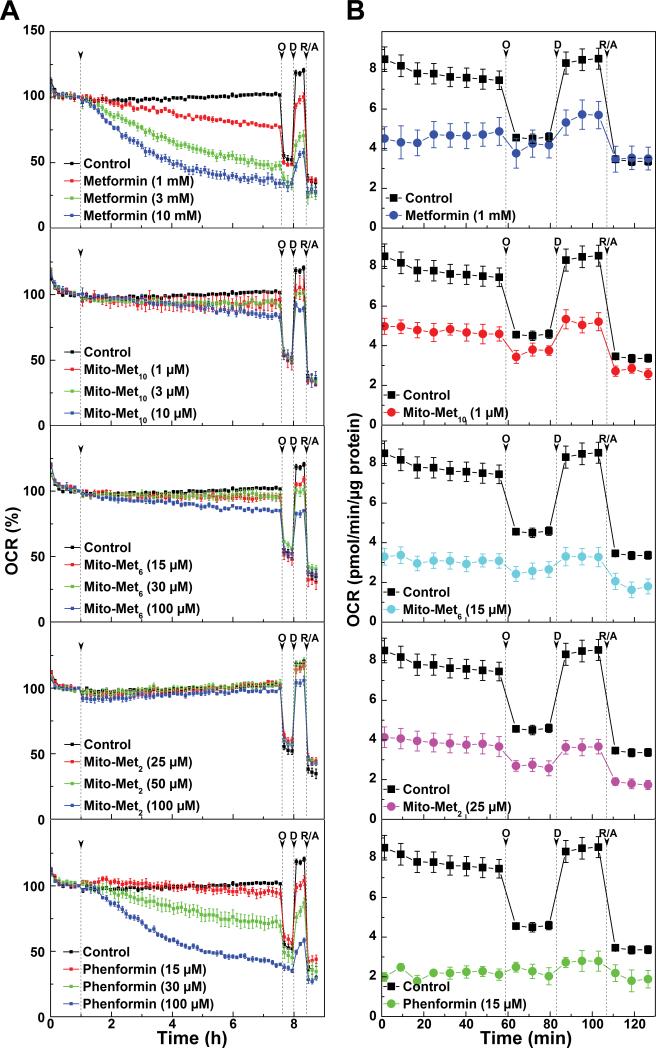
The oxygen consumption rates (OCR) were measured as a readout of mitochondrial function (28). We compared the immediate OCR changes in MiaPaCa-2 cells in response to different concentrations (e.g., at IC50 or higher values determined by clonogenic assay) of Met, Mito-Mets and phenformin (Fig. 4A). Met dose-dependently decreased the OCR at relatively higher concentrations (≈ 1-10 mM). In contrast, the more lipophilic phenformin inhibited OCR to the same extent (as did Met) at more than ten-fold lower concentration (Fig. 4A). Mito-Met10 inhibited proliferation most potently at sub-micromolar concentration, but did not inhibit OCR at 1 μM concentration. However, there was a slight inhibition at a higher concentration of Mito-Met10 (10 μM). Similar results were observed at higher concentrations of Mito-Met6 and no inhibition was noted with Mito-Met2 (Fig. 4A).
Figure 4. Effects of metformin analogs on mitochondrial respiration observed immediately and after 24 h pre-treatment in intact MiaPaCa-2 cells.
(A) Oxygen consumption rate (OCR) measured immediately before and after addition of Met analogs, and (B) OCR measured in intact cells after 24 h pre-treatment. The indices of mitochondrial function were determined by injecting oligomycin (O, 1 μg/ml) to inhibit ATP synthase, 2,4-dinitrophenol (D, 50 μM) to uncouple the mitochondria, and rotenone (R, 1 μM) and antimycin A (A, 10 μM) as inhibitors of complex I and complex III, respectively. Dashed vertical linesindicate the time of injection of the compounds.
Next we monitored the mitochondrial respiration rates in MiaPaCa-2 cells after longer treatment (24 h) with Met, Mito-Mets, and phenformin followed by a washout and replenishment with fresh assay media. A significant decrease in OCR was observed after a 24-h treatment with all compounds tested (Fig. 4B). These results demonstrate that the extent of OCR inhibition was dependent on the alkyl chain length with Mito-Met2, Mito-Met6 and Mito-Met10 showing similar effects at the concentrations of 25, 16 and 1 μM, respectively (Fig. 4B).
Effect of Mito-Met10 and Met on PDAC radiosensitivity, cell cycle, and AMPK activation
Both PDAC and control nonmalignant cells pretreated for 24 h with Met (1 mM) or Mito-Met (1 μM) were subjected to X-irradiation, followed by a clonogenic assay. As shown in Figure 5A, Met pretreatment decreased MiaPaCa-2 cell growth after irradiation, in agreement with previous reports indicating radiosensitizing effects of Met in PDAC (29,30). Radiation dose-response results show that 1 μM Mito-Met was as effective as 1 mM Met in radiosensitization (Fig. 5A), whereas no radiosensitization was observed in nonmalignant cells (MCF-10A or HPNE) pretreated with Mito-Met10 (Fig. 5A). Enhanced radiosensitivity in MiaPaCa-2 cells could be attributed to an increased uptake and retention of Mito-Met10 in these cells, as compared to normal (MCF-10A) cells (Suppl. Fig. 6). Detailed cell cycle analysis showed that treatment with Mito-Met10, but not Met, arrested PANC-1 cell growth in the G1 phase with a concomitant decrease in cyclin D1 levels (Fig. 5B).
Figure 5. Colony formation in cancer or normal cells with or without exposure to X-radiation, cell cycle analysis, and AMPK activation in Met- and Mito-Met10-treated PDAC cells.
(A) Cells were treated with Met (left) or Mito-Met10 (middle and right) 24 h before irradiation and clonogenic survival fraction was determined. *, P < 0.05 compared to radiation alone. (B) PANC-1 cells were treated with the compounds for 48 h and cell cycle distribution is shown as a histogram (left) and the percentage of cells in G1 phase (middle). Right panel shows the cyclin D1 protein level in treated cells.*, P < 0.05 and **, P < 0.01. (C, left) Immunoblot of FOXM1 in MiaPaCa-2 cells untreated (vehicle) or treated for 24 h with 1 and 10 μM Mito-Met10 or 1 and 10 mM Met. *, P < 0.05. (Middle, Right) Immunoblots of phosphorylated and total AMPK in MiaPaCa-2 (Middle) and FC-1242 (Right) cells treated with Mito-Met10 and Met. (D) The effect of AMPK inhibition on antiproliferative activity of Mito-Met10. (Left) MiaPaCa-2 cells were pretreated with 0.3 μM Compound C for 48 h, then cell proliferation was monitored in the presence or absence of 0.5 μM Mito-Met10. (Middle) MiaPaCa-2 cells were pretreated with 0.3 μM Compound C for 24 h, then treated in combination with 1 μM Mito-Met10 for another 24 h, and the colonies formed were counted after additional incubation in presence/absence of Compound C as indicated. (Right) The effect of Compound C on the survival fraction. *, P < 0.01 Mito-Met10 alone treatment vs. control. #, P < 0.01 Mito-Met10 alone vs. combination.
Next we investigated whether treatment with Mito-Met10 or Met would have an effect on the FOXM1 pathway. FOXM1 is a redox-responsive transcription factor that we have previously shown is down-regulated in malignant mesothelioma cells upon treatment with mitochondria-targeted nitroxides (31). Treatment of MiaPaCa-2 cells with Mito-Met10, but not Met, resulted in a significant decrease in FOXM1 levels (Fig 5C, left). Similar results were observed for PANC-1 cells (Suppl. Fig. 7). To better understand potential upstream signaling events, we investigated the AMPK-mTOR energy signaling pathway. The extent of AMPK activation, measured as a ratio of phosphorylated to total protein, showed a ~two-fold increase in active AMPK in human (Fig. 5C, middle) and a ~four-fold increase in pAMPK in murine FC-1242 PDAC cells treated with Mito-Met10 and Met (Fig. 5C, right). The increase in AMPK activation occurs at a 1,000-fold lower concentration of Mito-Met10 than Met. Treatment with dorsomorphin (Compound C), a potent, reversible AMPK inhibitor, counteracted the antiproliferative effects of Mito-Met10 (Fig. 5D), suggesting a role for AMPK signaling mechanism.
Effects of Mito-Met analogs and Met on complex I activity
To better understand the role of mitochondrial respiratory complex in Mito-Met and Met-mediated signaling pathways, we used permeabilized and intact cells. The use of permeabilized cells avoids differences in cellular uptake of compounds. Supplemental Figure 8 (left panel) shows OCR changes in control permeabilized cells and in rotenone- or malonate-treated cells. Rotenone (complex I inhibitor) greatly diminished OCR that was restored by added succinate. However, in the presence of malonate (complex II inhibitor) addition of succinate did not stimulate OCR. Antimycin A decreased both pyruvate- and succinate-induced OCR. These studies established the use of permeabilized cells in bioenergetics function assay (22,23). Next we used this model to probe the effects of Met and Mito-Met analogs. The IC50 values for Met and Mito-Met10 to inhibit complex I-mediated oxygen consumption upon injection to permeabilized cells were determined to be 0.8 mM and 2 μM, respectively (Suppl. Fig. 9). Next, we tested the effects of a 24 h pretreatment of intact cells with Mito-Met10 or Met on complex I activity. A significant inhibition of complex I-dependent OCR was observed (Fig. 6A and Suppl. Fig. 8). The IC50 values for complex I inhibition in MiaPaCa-2 cells determined for Met and Mito-Met10 were 1.1 mM and 0.4 μM, respectively. This increased potency of Mito-Met10 to inhibit complex I activity is consistent with the >1,000-fold enhanced antiproliferative effect of Mito-Met10 versus Met. In contrast to pancreatic cancer cells, higher concentrations of Mito-Met10 were required to inhibit complex I in HPNE and IEC6 cells (Fig. 6A and Suppl. Fig. 8). These results demonstrate the selectivity of Mito-Met10 in inhibiting mitochondrial respiration in PDACs and suggest that the enhanced antiproliferative effects of Mito-Met10 in PDAC cells are related to their enhanced ability to inhibit complex I activity.
Figure 6. Mito-Met10 and Met inhibit mitochondrial complex I activity and stimulate ROS in vitro and inhibit tumor growth and progression in vivo.
(A) Pancreatic cancer cells or normal nonmalignant cells were pretreated with Met or Mito-Met10 for 24 h. The mitochondrial complex I oxygen consumption (last OCR reading before succinate injection, as shown in Suppl. Fig. 8) is plotted against concentration of Met or Mito-Met10. Dashed lines represent the fitting curves used for determination of the IC50 values. (B) Characterization of intracellular oxidants induced by Met (1 mM), Mito-Met10 (1 μM) or rotenone (Rot, 1 μM) with HPLC-based analyses of the oxidation products derived from hydroethidine (HE) probe. (Left) Representative HPLC chromatograms for each treatment. (Middle) Quantitative analysis of the intracellular levels of the probe and its oxidation products. (Right) Schematic representation of the superoxide-dependent and - independent pathways of HE oxidation. Cells were pretreated with the indicated compounds for 24 h (Met and Mito-Met10) or for 1 h (Rot), followed by incubation with HE (10 μM) for 1 h. 2-Hydroxyethidium (2-OH-E+, marked in blue) is a superoxide-specific product, ethidium (E+, marked in red) is a non-specific oxidation product and diethidium (E+-E+, marked in green) is a product of one-electron oxidation of the probe. (C) Proposed signaling pathways activated by Mito-Mets. Mito-Met10 inhibits complex I, stimulates ROS production, and activates AMPK phosphorylation leading to antiproliferative effects. Changes due to the treatment with Mito-Met10 are shown by red block arrows. HE conversion to 2-OH-E+ is used for specific detection of superoxide. (D, top) Whole body bioluminescence images showing decreased tumor growth in mice treated with Mito-Met10 (1 mg/kg) and Met (1 mg/kg). (D, bottom, left) Total bioluminescence intensities (the light flux in photons/s) for individual FC-1242-luc autografts. Dotted colored lines represent individual mice treated with vehicle (black), Met (red) or Mito-Met10 (blue). As tumors become increasingly large and necrotic, the RLU values may decrease over time. Solid colored lines are linear regression curves for vehicle (black), Met (red) or Mito-Met10 (blue) treated animals. (D, bottom, right) Tumor volume measured using calipers of excised primary FC-1242 tumors. *, P < 0.05 and **, P < 0.01 vehicle vs. Mito-Met10.
Inhibition of complex I enhances formation of superoxide and other oxidants
One of the consequences of complex I inhibition is enhanced generation of cellular oxidizing species (32). We used the cell-permeable probe hydroethidine (HE) to detect reactive oxygen species. HPLC traces (Fig. 6B, left) and quantitative analyses of products are shown (Fig. 6B, middle). Results indicate that Mito-Met10 treatment of MiaPaCa-2 cells increased the formation of 2-hydroxyethidium (2-OH-E+), a diagnostic marker product of HE/superoxide reaction (Fig. 6B, right). In addition, a marked increase in diethidium (E+-E+) formation was observed (Fig. 6B, middle), indicating that Mito-Met10 also induced generation of one-electron oxidants. Under these conditions, Mito-Met10 did not stimulate O2•− formation in control HPNE cells (Fig. 6B, middle), consistent with its lack of inhibition of mitochondrial complex I at the concentration tested. However, at the concentrations equal or higher than IC50 (Fig. 3A) Mito-Met10 treatment increased the formation of 2-hydroxyethidium (2-OH-E+) in HPNE cells (Suppl. Fig. 10).
A proposed model for Mito-Met10-induced antiproliferative effect in PDAC via inhibition of mitochondrial complex I activity, enhanced O2•− generation, and AMPK activation is shown in Figure 6C.
Mito-Met inhibits tumor growth of KPC autografts in vivo
In vivo data show that Mito-Met suppresses tumor growth in a preclinical mouse model (Fig. 6D). Syngeneic KPC cells expressing firefly luciferase (FC-1242-luc) were orthotopically implanted in the pancreas of C57BL/6 mice and tumor growth monitored using bioluminescence imaging (Fig. 6D). Mice orthotopically autografted with FC-1242-luc cells (25) were treated daily with Met (1 mg/kg) or Mito-Met10 (1 mg/kg). Consistent with the cell culture data, Mito-Met10 was considerably more effective than Met in mitigating PDAC growth (Fig. 1B) and the treatment resulted in markedly smaller primary tumors at the completion of the experiment (Fig. 6D). Weekly tracking measurements of total radiance in FC-1242-luc engrafted mice (Fig. 6D, dotted lines) revealed a significant decrease in percent change of tumor burden throughout the course of the study. Over time, PDAC tumor bearing mice treated with Mito-Met10 had lower tumor burden when assessed at early, middle, and later time points as visualized by bioluminescent imaging, with smaller tumors at the completion of the study (Fig. 6D). Serum from these animals was collected, and hepatic and kidney toxicity tested using standard AST, ALT, AP, and BUN assays, respectively. As predicted from cell culture data (Fig. 3), neither Met nor Mito-Met10 elicited toxicity in vivo (Supplemental Table 1). Following administration of Mito-Met10 for two weeks in FC-1242-luc orthotopic mice, we detected an increased accumulation of this compound in liver, kidney, spleen, and tumor tissues (Suppl. Fig. 11). Collectively, results from the in vivo experiments indicate a potent antitumor activity of Mito-Met10, with negligible off-target toxicity.
Discussion
Human pancreatic ductal adenocarcinoma (PDAC) is the most severe and aggressive form of pancreatic cancer with limited chemo- and radiotherapeutic options to improve survival. Currently available standard-of-care chemotherapy offers limited survival benefit. There is critical unmet need for new therapeutic approaches to mitigate therapeutic resistance mechanisms and maximize multimodal treatment approaches in pancreatic cancer. Here we have developed new mitochondria-targeted metformin analogs that alone and in combination with radiotherapy markedly inhibited PDAC proliferation. The enhanced potency of Mito-Met10 is attributed to mitochondrial ROS-dependent activation of signal transduction pathway (involving AMPK activation) in PDAC cells. These mitochondria-targeted Met analogs may have significant clinical and translational potential in PDAC treatment.
Delocalized lipophilic cations inhibit tumor cell proliferation through selective accumulation into mitochondria and inhibition of mitochondrial respiration (15,16,32). The mitochondrial membrane potential is much higher (more negative inside) in tumor than in normal (nontransformed) cells (33). The cationic compounds tethered to an alkyl chain accumulate preferentially in tumor mitochondria depending upon the alkyl side chain length (15,16). TPP+-linked agents conjugated to an aromatic and heterocyclic groups (Mito-Chromanol, Mito-CP) also exerted selective cytostatic and cytotoxic effects in various tumor cells (15,16,34). Met exerts biological activity through alterations of cellular bioenergetics without itself undergoing any detectable metabolism (35). Selective targeting of cancer cell mitochondrial bioenergetics is an emerging chemotherapeutic strategy (36,37). We, therefore, surmised that enhancing its mitochondrial uptake would greatly increase Met's biological activity. Although several lipophilic variants of Met were synthesized and shown to exert increased antitumor potency, none of these modifications included mitochondrial targeting cationic function. In this study we showed and characterized, for the first time, that fine-tuning of Met structure by attaching a TPP+ group tethered to different alkyl chain lengths is synthetically feasible, and that these modified analogs increasingly target tumor mitochondria. Consistent with enhanced intracellular uptake, Mito-Met analogs were more potent than Met in their ability to inhibit PDAC proliferation. The antiproliferative potency of Mito-Met analogs increased with increasing length of the alkyl linker (Mito-Met10 > Mito-Met6 > Mito-Met2) (Fig. 3B and Suppl. Fig. 5). Elongation of the aliphatic linker to twelve carbon atoms (Mito-Met12) had no further effect on its antiproliferative efficacy, as compared to Mito-Met10 (not shown). A major reason for the selective antiproliferative effect of Mito-Met analogs in tumor cells is due to their preferential accumulation and inhibition of mitochondrial complex I activity in tumor cells as compared to normal, nonmalignant cells.
At present, the mechanism(s) responsible for the enhanced antiproliferative and radiosensitizing effects of Mito-Mets in cancer cells remain unknown. It is likely that Mito-Met10 exerts antiproliferative effects in PDACs via targeting the energy sensing bioenergetics pathway(s). Mito-Met10 activated AMPK in MiaPaCa-2 cells nearly 1,000-fold more potently than did Met (Fig. 5). AMPK, a master regulator of cellular energy homeostasis, is typically activated by enhanced intracellular AMP (38). Under conditions wherein intracellular ATP levels are decreased along with a concomitant increase in AMP (enhanced AMP/ATP ratio), AMPK is activated via phosphorylation of its threonine-172 residue (39). Previous research has shown that AMPK represses the FOXM1 transcription factor expression through inhibition of the AKT/FOXO3 signaling cascade, leading to regression of cervical cancer cell growth (31). Thus, it is conceivable that Mito-Met10 and related analogs act on the AKT/FOXO3/FOXM1 signaling pathway.
Recently, it was reported that ROS activate two proteins, AMP-activated kinase and hypoxia-inducible factor 1 (HIF-1α) in C. elegans and that balancing ROS at optimal levels was crucial for their health and longevity (40). Factors responsible for activation of AMPK still need to be ascertained, although mitochondrial ROS was implicated (40). We propose that Mito-Met10-mediated inhibition of complex I and superoxide generation, accompanied by activation of AMPK, play an important role in inhibiting PDAC proliferation (Fig. 6C).
PDAC radiosensitization: Met versus Mito-Met10
As reported previously (29), pretreatment with Met increased radiosensitivity of MiaPaCa-2 cells. The present data demonstrate that Mito-Met10 is significantly more effective than Met in PDAC radiosensitization (Fig. 5A). Prevailing views suggest that the antiproliferative effects of Met are mediated by activation of the AMPK pathway and/or improved tumor oxygenation (i.e., decreased hypoxia) due to inhibition of mitochondria, leading to decreased tumor cell respiration in irradiated tumors (29). The enhanced radiosensitivity upon treatment with Mito-Met analogs may be attributed to increased tumor oxygenation (i.e., decreased hypoxia). Tumor hypoxia (pO2 < 10 mmHg), an intrinsic property of numerous solid tumors including the pancreas, results from an imbalance between oxygen delivery and oxygen consumption (41). Studies suggest that decreasing oxygen consumption with pharmacologic drugs is an effective route for increasing tumor oxygenation and radiosensitivity (42-44). Met (1-10 mM) reportedly improves tumor oxygenation and enhances tumor radiosensitivity (29). The present results show that Mito-Met10 decreases mitochondrial respiration in MiaPaCa-2 cells after 24 h (Fig. 4B). Mito-Met10 inhibited mitochondrial complex I activity and tumor cell respiration at micromolar levels, whereas Met inhibited respiration to a similar extent at millimolar levels. It is likely that Mito-Met10 stimulates tumor oxygenation at concentrations 1,000-fold lower than that of Met. A plausible mechanism by which Mito-Met10 decreased mitochondrial respiration may be due to increased accumulation of Mito-Met10 in mitochondria, leading to enhanced inhibition of complex I in the mitochondrial electron transport chain (45). In the presence of radiation and Mito-Met10, it is possible that two or more mechanisms operate. AMPK-activating drugs increase tumor radiosensitivity (46). Radiation itself activates the AMPK energy sensor pathway (47). However, the degree to which AMPK induces tumor oxygenation and radiosensitivity remains poorly understood. Although Met inhibits growth of glioblastoma cells and mammalian target of rapamycin (mTOR) pathway, the effects were found to be independent of AMPK (48). The same study suggests that AMPK could potentially function as a tumor growth supporter. Met-induced mTOR inhibition and suppression of glioma proliferation were attributed to enhanced PRAS40's association with RAPTOR (48). Clearly, the antiproliferative mechanism of action of Met and Mito-Met may also be related to other mechanisms (i.e., activation of PRAS40/RAPTOR association).
Recent reports suggest that suppression of FOXM1 enhances the radiosensitivity of different human cancer cells (49). Mito-Met10, at concentrations 1,000-fold lower than Met, inhibited mitochondrial respiration, activated AMPK, and significantly decreased FOXM1 (Fig. 5C). Clearly, this is an exciting finding with significant potential to clinical translation and requires additional mechanistic studies. More recently, it was shown that at conventional antidiabetic doses of Met, there was no significant therapeutic effect in patients with advanced pancreatic cancer (50). The investigators suggested that more potent biguanides should be used in cancer treatment because of vastly reduced plasma concentrations typically detected in diabetic cancer patients treated with Met (50). It is conceivable that Mito-Met10 exhibiting a 1,000-fold higher potency than Met would achieve a therapeutically effective plasma concentration in humans.
Supplementary Material
Acknowledgments
Financial support: This work was supported by grants from the NIH National Cancer Institute [U01 CA178960 (to M.B. Dwinell and B. Kalyanaraman) and R01 CA152810 (to B. Kalyanaraman)], the Medical College of Wisconsin Cancer Center (to M.B. Dwinell, B. Kalyanaraman and B. Johnson) and Aix-Marseille Université CNRS (to M. Hardy and O. Ouari).
Footnotes
Potential conflicts of interest: None
References
- 1.Dowling RJ, Goodwin PJ, Stambolic V. Understanding the benefit of metformin use in cancer treatment. BMC Med. 2011;9:33. doi: 10.1186/1741-7015-9-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sinnett-Smith J, Kisfalvi K, Kui R, Rozengurt E. Metformin inhibition of mTORC1 activation, DNA synthesis and proliferation in pancreatic cancer cells: dependence on glucose concentration and role of AMPK. Biochem Biophys Res Commun. 2013;430:352–7. doi: 10.1016/j.bbrc.2012.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sadeghi N, Abbruzzese JL, Yeung SC, Hassan M, Li D. Metformin use is associated with better survival of diabetic patients with pancreatic cancer. Clin Cancer Res. 2012;18:2905–12. doi: 10.1158/1078-0432.CCR-11-2994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Emami RA, Fisel P, Nies AT, Schaeffeler E, Schwab M. Metformin and cancer: from the old medicine cabinet to pharmacological pitfalls and prospects. Trends Pharmacol Sci. 2013;34:126–35. doi: 10.1016/j.tips.2012.11.005. [DOI] [PubMed] [Google Scholar]
- 5.Zhang X, Fryknas M, Hernlund E, Fayad W, De MA, Olofsson MH, et al. Induction of mitochondrial dysfunction as a strategy for targeting tumour cells in metabolically compromised microenvironments. Nat Commun. 2014;5:3295. doi: 10.1038/ncomms4295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pollak MN. Investigating metformin for cancer prevention and treatment: the end of the beginning. Cancer Discov. 2012;2:778–90. doi: 10.1158/2159-8290.CD-12-0263. [DOI] [PubMed] [Google Scholar]
- 7.Birsoy K, Sabatini DM, Possemato R. Untuning the tumor metabolic machine: Targeting cancer metabolism: a bedside lesson. Nat Med. 2012;18:1022–3. doi: 10.1038/nm.2870. [DOI] [PubMed] [Google Scholar]
- 8.Dowling RJ, Niraula S, Chang MC, Done SJ, Ennis M, McCready DR, et al. Changes in insulin receptor signaling underlie neoadjuvant metformin administration in breast cancer: a prospective window of opportunity neoadjuvant study. Breast Cancer Res. 2015;17:32. doi: 10.1186/s13058-015-0540-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bridges HR, Jones AJ, Pollak MN, Hirst J. Effects of metformin and other biguanides on oxidative phosphorylation in mitochondria. Biochem J. 2014;462:475–87. doi: 10.1042/BJ20140620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Madiraju AK, Erion DM, Rahimi Y, Zhang XM, Braddock DT, Albright RA, et al. Metformin suppresses gluconeogenesis by inhibiting mitochondrial glycerophosphate dehydrogenase. Nature. 2014;510:542–6. doi: 10.1038/nature13270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Segal ED, Yasmeen A, Beauchamp MC, Rosenblatt J, Pollak M, Gotlieb WH. Relevance of the OCT1 transporter to the antineoplastic effect of biguanides. Biochem Biophys Res Commun. 2011;414:694–9. doi: 10.1016/j.bbrc.2011.09.134. [DOI] [PubMed] [Google Scholar]
- 12.Miskimins WK, Ahn HJ, Kim JY, Ryu S, Jung YS, Choi JY. Synergistic anti-cancer effect of phenformin and oxamate. PLoS One. 2014;9:e85576. doi: 10.1371/journal.pone.0085576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kwong SC, Brubacher J. Phenformin and lactic acidosis: a case report and review. J Emerg Med. 1998;16:881–6. doi: 10.1016/s0736-4679(98)00103-6. [DOI] [PubMed] [Google Scholar]
- 14.Shackelford DB, Abt E, Gerken L, Vasquez DS, Seki A, Leblanc M, et al. LKB1 inactivation dictates therapeutic response of non-small cell lung cancer to the metabolism drug phenformin. Cancer Cell. 2013;23:143–58. doi: 10.1016/j.ccr.2012.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cheng G, Zielonka J, Dranka BP, McAllister D, Mackinnon AC, Jr, Joseph J, et al. Mitochondria targeted drugs synergize with 2-deoxyglucose to trigger breast cancer cell death. Cancer Res. 2012;72:2634–44. doi: 10.1158/0008-5472.CAN-11-3928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cheng G, Zielonka J, McAllister DM, Mackinnon AC, Jr, Joseph J, Dwinell MB, et al. Mitochondria-targeted vitamin E analogs inhibit breast cancer cell energy metabolism and promote cell death. BMC Cancer. 2013;13:285. doi: 10.1186/1471-2407-13-285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang J, Xia S, Zhu Z. Synergistic effect of phenformin in non-small cell lung cancer (NSCLC) ionizing radiation treatment. Cell Biochem Biophys. 2015;71(2):513–8. doi: 10.1007/s12013-014-0283-z. [DOI] [PubMed] [Google Scholar]
- 18.Fasih A, Elbaz HA, Hüttemann M, Konski AA, Zielske SP. Radiosensitization of pancreatic cancer cells by metformin through the AMPK pathway. Radiat Res. 2014;182(1):50–9. doi: 10.1667/RR13568.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sanli T, Rashid A, Liu C, Harding S, Bristow RG, Cutz JC, Singh G, Wright J, Tsakiridis T. Ionizing radiation activates AMP-activated kinase (AMPK): a target for radiosensitization of human cancer cells. Int J Radiat Oncol Biol Phys. 2010;78(1):221–9. doi: 10.1016/j.ijrobp.2010.03.005. [DOI] [PubMed] [Google Scholar]
- 20.Zimmerman NP, Roy I, Hauser AD, Wilson JM, Williams CL, Dwinell MB. Cyclic AMP regulates the migration and invasion potential of human pancreatic cancer cells. Mol Carcinog. 2015;54:203–15. doi: 10.1002/mc.22091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cheng G, Zielonka J, McAllister D, Tsai S, Dwinell MB, Kalyanaraman B. Profiling and targeting of cellular bioenergetics: inhibition of pancreatic cancer cell proliferation. Br J Cancer. 2014;111:85–93. doi: 10.1038/bjc.2014.272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Salabei JK, Gibb AA, Hill BG. Comprehensive measurement of respiratory activity in permeabilized cells using extracellular flux analysis. Nat Protoc. 2014;9:421–38. doi: 10.1038/nprot.2014.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Divakaruni AS, Wiley SE, Rogers GW, Andreyev AY, Petrosyan S, Loviscach M, et al. Thiazolidinediones are acute, specific inhibitors of the mitochondrial pyruvate carrier. Proc Natl Acad Sci U S A. 2013;110(14):5422–7. doi: 10.1073/pnas.1303360110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Roy I, McAllister DM, Gorse E, Dixon K, Piper CT, Zimmerman NP, et al. Pancreatic cancer cell migration and metastasis is regulated by chemokine-biased agonism and bioenergetic signaling. Cancer Res. 2015;75(17):3529–42. doi: 10.1158/0008-5472.CAN-14-2645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kim MP, Evans DB, Wang H, Abbruzzese JL, Fleming JB, Gallick GE. Generation of orthotopic and heterotopic human pancreatic cancer xenografts in immunodeficient mice. Nature Protocols. 2009;4:1670–80. doi: 10.1038/nprot.2009.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Roy I, Zimmerman NP, Mackinnon AC, Tsai S, Evans DB, Dwinell MB. CXCL12 chemokine expression suppresses human pancreatic cancer growth and metastasis. PLoS One. 2014;9(3):e90400. doi: 10.1371/journal.pone.0090400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wendt MK, Cooper AN, Dwinell MB. Epigenetic silencing of CXCL12 increases the metastatic potential of mammary carcinoma cells. Oncogene. 2008;27:1461–71. doi: 10.1038/sj.onc.1210751. [DOI] [PubMed] [Google Scholar]
- 28.Dranka BP, Zielonka J, Kanthansamy AG, Kalyanaraman B. Alterations in bioenergetics function induced by Parkinson's disease mimetic compounds: Lack of correlation with superoxide generation. J Neurochem. 2012;122:941–51. doi: 10.1111/j.1471-4159.2012.07836.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Song CW, Lee H, Dings RP, Williams B, Powers J, Santos TD, et al. Metformin kills and radiosensitizes cancer cells and preferentially kills cancer stem cells. Sci Rep. 2012;2:362. doi: 10.1038/srep00362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zannella VE, Dal PA, Muaddi H, McKee TD, Stapleton S, Sykes J, et al. Reprogramming metabolism with metformin improves tumor oxygenation and radiotherapy response. Clin Cancer Res. 2013;19:6741–50. doi: 10.1158/1078-0432.CCR-13-1787. [DOI] [PubMed] [Google Scholar]
- 31.Cunniff B, Benson K, Stumpff J, Newick K, Held P, Taatjes D, et al. Mitochondrial-targeted nitroxides disrupt mitochondrial architecture and inhibit expression of peroxiredoxin 3 and FOXM1 in malignant mesothelioma cells. J Cell Physiol. 2013;228:835–45. doi: 10.1002/jcp.24232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Modica-Napolitano JS, Aprille JR. Delocalized lipophilic cations selectively target the mitochondria of carcinoma cells. Adv Drug Deliv Rev. 2001;49:63–70. doi: 10.1016/s0169-409x(01)00125-9. [DOI] [PubMed] [Google Scholar]
- 33.Beckham TH, Lu P, Jones EE, Marrison T, Lewis CS, Cheng JC, et al. LCL124, a cationic analog of ceramide, selectively induces pancreatic cancer cell death by accumulating in mitochondria. J Pharmacol Exp Ther. 2013;344:167–78. doi: 10.1124/jpet.112.199216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Weinberg F, Hamanaka R, Wheaton WW, Weinberg S, Joseph J, Lopez M, et al. Mitochondrial metabolism and ROS generation are essential for Kras-mediated tumorigenicity. Proc Natl Acad Sci U S A. 2010;2010;107(19):8788–93. doi: 10.1073/pnas.1003428107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dowling RJ, Goodwin PJ, Stambolic V. Understanding the benefit of metformin use in cancer treatment. BMC Med. 2011;9:33. doi: 10.1186/1741-7015-9-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhang X, Fryknas M, Hernlund E, Fayad W, De MA, Olofsson MH, et al. Induction of mitochondrial dysfunction as a strategy for targeting tumour cells in metabolically compromised microenvironments. Nat Commun. 2014;5:3295. doi: 10.1038/ncomms4295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cheng G, Zielonka J, McAllister D, Hardy M, Ouari O, Joseph J, et al. Antiproliferative effects of mitochondria-targeted cationic antioxidants and analogs: role of mitochondrial bioenergetics and energy-sensing mechanism. Cancer Lett. 2015;365:96–106. doi: 10.1016/j.canlet.2015.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Inoki K, Zhu T, Guan KL. TSC2 mediates cellular energy response to control cell growth and survival. Cell. 2003;115:577–90. doi: 10.1016/s0092-8674(03)00929-2. [DOI] [PubMed] [Google Scholar]
- 39.Hardie DG, Ashford ML. AMPK: regulating energy balance at the cellular and whole body levels. Physiology (Bethesda) 2014;29:99–107. doi: 10.1152/physiol.00050.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hwang AB, Ryu EA, Artan M, Chang HW, Kabir MH, Nam HJ, et al. Feedback regulation via AMPK and HIF-1 mediates ROS-dependent longevity in Caenorhabditis elegans. Proc Natl Acad Sci U S A. 2014;111(42):E4458–67. doi: 10.1073/pnas.1411199111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Secomb TW, Hsu R, Ong ET, Gross JF, Dewhirst MW. Analysis of the effects of oxygen supply and demand on hypoxic fraction in tumors. Acta Oncol. 1995;34:313–6. doi: 10.3109/02841869509093981. [DOI] [PubMed] [Google Scholar]
- 42.Durand RE, Biaglow JE. Radiosensitization of hypoxic cells of an in vitro tumor model by respiratory inhibitors. Radiat Res. 1977;69:359–66. [PubMed] [Google Scholar]
- 43.Bol V, Bol A, Bouzin C, Labar D, Lee JA, Janssens G, et al. Reprogramming of tumor metabolism by targeting mitochondria improves tumor response to irradiation. Acta Oncol. 2015;54(2):266–74. doi: 10.3109/0284186X.2014.932006. [DOI] [PubMed] [Google Scholar]
- 44.Crokart N, Radermacher K, Jordan BF, Baudelet C, Cron GO, Grégoire V, et al. Tumor radiosensitization by antiinflammatory drugs: evidence for a new mechanism involving the oxygen effect. Cancer Res. 2005;65(17):7911–6. doi: 10.1158/0008-5472.CAN-05-1288. [DOI] [PubMed] [Google Scholar]
- 45.Millard M, Pathania D, Shabaik Y, Taheri L, Deng J, Neamati N. Preclinical evaluation of novel triphenylphosphonium salts with broad-spectrum activity. PLoS One. 2010;5:e13131. doi: 10.1371/journal.pone.0013131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sanli T, Storozhuk Y, Linher-Melville K, Bristow RG, Laderout K, Viollet B, et al. Ionizing radiation regulates the expression of AMP-activated protein kinase (AMPK) in epithelial cancer cells: modulation of cellular signals regulating cell cycle and survival. Radiother Oncol. 2012;102:459–65. doi: 10.1016/j.radonc.2011.11.014. [DOI] [PubMed] [Google Scholar]
- 47.Isebaert SF, Swinnen JV, McBride WH, Begg AC, Haustermans KM. 5-aminoimidazole-4-carboxamide riboside enhances effect of ionizing radiation in PC3 prostate cancer cells. Int J Radiat Oncol Biol Phys. 2011;81:1515–23. doi: 10.1016/j.ijrobp.2011.06.1964. [DOI] [PubMed] [Google Scholar]
- 48.Liu X, Chhipa RR, Pooya S, Wortman M, Yachyshin S, Chow LM, et al. Discrete mechanisms of mTOR and cell cycle regulation by AMPK agonists independent of AMPK. Proc Natl Acad Sci U S A. 2014;111(4):E435–44. doi: 10.1073/pnas.1311121111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Halasi M, Gartel AL. Suppression of FOXM1 sensitizes human cancer cells to cell death induced by DNA-damage. PLoS One. 2012;7(2):e31761. doi: 10.1371/journal.pone.0031761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kordes S, Pollak MN, Zwinderman AH, Mathôt RA, Weterman MJ, Beeker A, et al. Metformin in patients with advanced pancreatic cancer: a double-blind, randomised, placebo-controlled phase 2 trial. Lancet Oncol. 2015;16(7):839–47. doi: 10.1016/S1470-2045(15)00027-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.