Abstract
Background
Although endometrial cancer is clearly influenced by hormonal factors, few epidemiologic studies have investigated the role of endogenous estrogens or especially estrogen metabolites.
Methods
We conducted a nested case-control study within the Women’s Health Initiative Observational Study (WHI-OS), a cohort of 93,676 postmenopausal women recruited between 1993–1998. Using baseline serum samples from women who were non-current hormone users with intact uteri, we measured 15 estrogens/estrogen metabolites via HPLC-MS/MS among 313 incident endometrial cancer cases (271 Type I, 42 Type II) and 354 matched controls, deriving adjusted odds ratios (OR) and 95% confidence intervals (CIs) for overall and subtype-specific endometrial cancer risk.
Results
Parent estrogens (estrone and estradiol) were positively related to endometrial cancer risk, with the highest risk observed for unconjugated estradiol (OR 5th vs. 1st quintile=6.19, 95% CI 2.95–13.03, ptrend=0.0001). Nearly all metabolites were significantly associated with elevated risks, with some attenuation after adjustment for unconjugated estradiol (residual risks of 2-3-fold). Body mass index (kg/m2, BMI) relations were somewhat reduced after adjustment for estrogen levels. The association with unconjugated estradiol was stronger for Type I than II tumors (phet=0.01).
Conclusions
Parent estrogens as well as individual metabolites appeared to exert generalized uterotropic activity, particularly for type I tumors. The effects of obesity on risk were only partially explained by estrogens.
Impact
These findings enhance our understanding of estrogen mechanisms involved in endometrial carcinogenesis but also highlight the need for studying additional markers that may underlie the effects on risk of certain risk factors, e.g., obesity.
Keywords: Endometrial cancer, estrogen metabolites, postmenopausal women
Introduction
Endometrial cancer is strongly influenced by hormonal factors, with established risk factors including obesity, menstrual and reproductive factors, and exogenous hormones (1). Despite acceptance of the hormonal etiology of endometrial cancer, relatively few studies have explored the role of endogenous estrogens (2). Although several epidemiologic investigations have shown strong associations with risk (3–6), results have generally been based on limited numbers of cases (e.g., 57–124 cases were involved with two of the studies). In addition, previous investigations have mainly relied on measurement of estrogens by radioimmunoassays, which have recognized limitations, particularly for measuring low concentrations of estrogens in postmenopausal women.
The use of mass spectrometry to measure endogenous hormones has opened up new research avenues (7). In addition to improving sensitivity, accuracy and reproducibililty, these assays have enabled concurrent measurement of estrogen metabolites, which purportedly have divergent effects on cancer development. The metabolism of estradiol or estrone with irreversible hydroxylation at the C-2, -4, or -16 positions of the steroid ring results in metabolites with varying mitogenic and genotoxic properties. Two major hypotheses about estrogen metabolites have emerged from experimental research, namely that 1) 16α-hydroxyestrone is carcinogenic because it can bind covalently to the estrogen receptor with strong mitogenic effects, and 2) the 2- and 4-hydroxylation catechol estrogen metabolites are carcinogenic because they can be oxidized into mutagenic quinones that form DNA adducts and lead to oxidative DNA damage (7).
Estrogen metabolites have been most extensively evaluated with respect to breast cancer, where initial attention focused on potential mitogenic effects, with the hypotheses that high levels of 16α-hydroxyestrone (16α-OHE1) would be directly associated with risk, whereas high ratios of 2-hydroxyestrone (2-OHE1) to 16α-OHE1 would be inversely related (8, 9). More recent studies have focused on genotoxicity (10), given that hydroxylation at the C-2 and C-4 positions produces catechol estrogens [notably 2-OHE1, 2-hydroxyestradiol (2-OHE2) and 4-hydroxyestrone (4-OHE1)], which, unless methylated, can be metabolized to mutagenic catechol quinones. Several studies have in fact found decreased risks of postmenopausal breast cancer associated with elevated ratios of 2-pathway metabolites relative to parent estrogens (11, 12), and one study found increased risks for higher levels of catechols to methylated catechols (12).
For endometrial cancer, a cancer known to be influenced by epithelial cell proliferation, (particularly in the absence of progesterone), metabolites that are mitogenic may be of particular interest. However, few investigations have explored the role of estrogen metabolites in endometrial carcinogenesis. One prospective study, involving 124 cases, that evaluated circulating 2-OHE1 and 16α-OHE1 levels using an enzyme immunoassay found some increases with high levels of both metabolites, but this relation did not persist after adjustment for estrone or estradiol levels (13). In another cohort study (66 cases), estradiol was significantly associated with risk, but there was no further discrimination of risk according to levels of metabolites or their ratios (14). Two endometrial cancer case-control studies have also evaluated estrogen metabolites, with suggestive relationships for certain metabolites (15, 16), although interpretation of the results was limited by small numbers and assessment of hormone levels after disease onset.
We assessed the relation of estrogen metabolites to endometrial cancer risk among participants in the Women’s Health Initiative Observational Study (WHI-OS). Strengths of this study included relatively large numbers of endometrial cancers as well as availability of detailed information on risk factors, enabling a thorough assessment of confounding and interactive effects. Given recent interest in the etiologic heterogeneity of endometrial cancers, we evaluated whether the effects of estrogen metabolites differed across distinct tumor subtypes, including Type I vs. II tumors, which are characterized by different hormonally-related risk factor profiles (17, 18).
Materials and Methods
Study population
The WHI-OS is a prospective cohort involving 93,676 postmenopausal women, ages 50–79 years, enrolled at U.S. centers between 1993–1998 (19, 20). Excluded were 148 women who had medical conditions with a predicted survival <3 years or adherence/retention issues, or who were participating in a clinical trial. The present study, conducted as a case-control study nested in the cohort, included incident invasive endometrial cancers diagnosed between study initiation and May 2012. Study subjects were excluded if they had a history of cancer at baseline other than non-melanoma skin cancer (n=11,674); were current users of exogenous hormones (38,419); had a hysterectomy at baseline or during follow-up (13,862); and did not have at least 1.1 mL of available pre-diagnosis baseline serum (720).
Cases were identified via self-reports and medical records and centrally adjudicated. The mean time from sample collection to diagnosis was 6.9 years (standard deviation=3.7 years; range=45 days–15.0 years). Controls were eligible WHI-OS cohort members selected from strata defined by age at blood draw (50–54, 55–59, 60–64, 65–69, 70–74, 75–79 years), year at blood draw (1993–1996, 1997–1998), race/ethnicity (white, black, Hispanic, other/unknown), and time since last menopausal hormone therapy (MHT) use (≤1, >1 year). Controls were alive and at risk of endometrial cancer (did not have hysterectomy) at the time of diagnosis of their matched case. Controls were shared with a nested case-control study of ovarian cancer (21) and drawn from strata containing endometrial cancer cases, with a ratio of at least 1 control per case, resulting in 313 endometrial cancer cases and 354 matched controls. Institutional review board clearance was received at the Fred Hutchinson Cancer Research Center (WHI Clinical Coordinating Center), as well as the clinical centers. All study participants provided written informed consent.
Laboratory assays
Stable isotope dilution HPLC-MS/MS was used to quantify 15 estrogens and estrogen metabolites (Figure 1), as previously detailed (22). Six labeled internal standards were used: deuterated 2-hydroxyestradiol, 2-methoxyestradiol and estriol (C/D/N Isotopes Inc, Pointe-Claire, QC, Canada); deuterated 16-epiestriol (Medical Isotopes Inc, Pelham, NH, USA); and 13C-labeled estrone and estradiol (Cambridge Isotope Laboratories, Andover, MA, USA).
Figure 1.
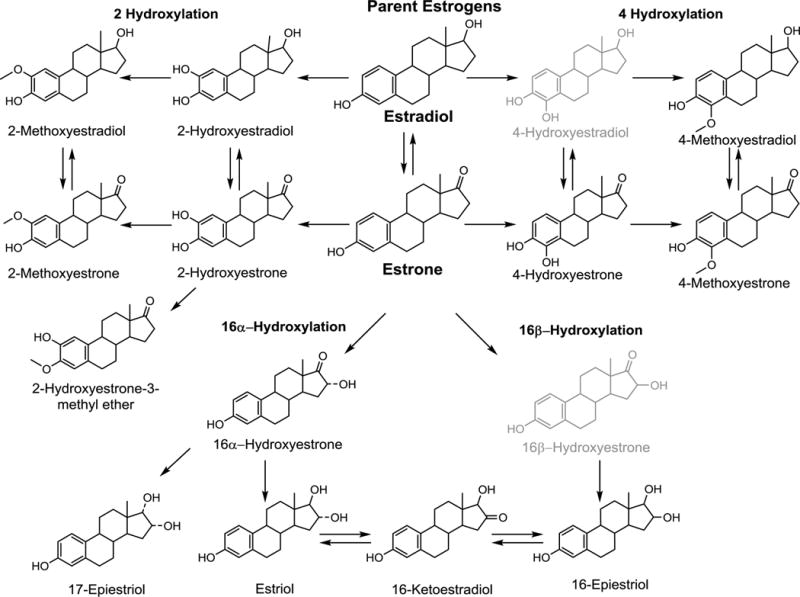
Formation of 2-, 4-, and 16-hydroxylation pathway estrogen metabolites from parent estrogens. The current serum estrogen metabolite assay measures 15 of the 17 metabolites pictured. 4-Hydroxyestradiol and 16β-Hydroxyestrone (in light gray) are not measured with the current assay due to very low abundance in circulation.
The serum method detects 15 estrogens and estrogen metabolites which circulate primarily as sulfated and/or glucuronidated conjugates. Five estrogen metabolites (estrone, estradiol, estriol, 2-methoxyestrone and 2-methoxyestradiol) were measured in unconjugated forms. The serum sample was split into two aliquots to measure the combined concentration of each of the 15 metabolites (sum of conjugated plus unconjugated forms) and the unconjugated forms. To measure the combined parent estrogen or estrogen metabolite level, an enzyme with sulfatase and glucuronidase activity was added to the samples to cleave any sulfate and glucoronide groups (22). To measure the unconjugated forms, the enzyme was not included in sample preparation. For metabolites with both combined and unconjugated measurements, the concentration of the conjugated form was calculated as the difference between the combined and unconjugated estrogen measurements. The limit of detection for each estrogen and estrogen metabolite measured was 10 fg on column (approximately 0.33–0.37 pmol/L) (22, 23). No samples had undetectable hormone levels. Laboratory coefficients of variation (CV) of masked technical replicates across batches were <6.0% for all hormones measured. Intraclass correlation coefficients (ICCs) ranged from 0.93–0.996 (median value 0.98).
Statistical analysis
We included self-reported never or former MHT pill/patch users and excluded individuals with unconjugated estradiol concentrations ≥184 pmol/L (~50 pg/mL; n=9), typically indicative of current exogenous hormone use. Individual estrogens and estrogen metabolites were categorized into quintiles based on control distributions.
Conditional logistic regression was used to estimate odds ratios (ORs) and 95% confidence intervals (CIs) of endometrial cancer risk while conditioning on matched case-control sets (determined by age and calendar year of blood draw, race/ethnicity, and time since last MHT use), and further adjusted for a priori potential confounding factors (education, body mass index (BMI), cigarette smoking status, years of oral contraception use, age at menarche, gravidity, age at menopause, never/former MHT use, and previous tubal ligation). Additional adjustment for other variables (e.g., parity) or finer adjustment for some factors (e.g., BMI) had minimal effects on derived associations. Tests for trend were based on the Wald statistic modeling the intra-category quintile median as a continuous parameter.
For analyses stratified by clinical characteristics of the cases, we used baseline category polytomous logistic regression models, with the controls as the reference group. Analyses stratified by BMI, age at blood draw, or duration of oral contraception use were evaluated using unconditional logistic regression models. The baseline category and unconditional logistic regression models were adjusted for matching factors and a priori selected potential confounding factors (listed above), with one exception: ever and time since last MHT use were combined (never, ≤1 year, >1 year). Likelihood ratio tests for the interaction across levels of BMI, age at blood draw, or duration of oral contraception use were computed based on cross-product terms with hormones categorized as the ordinal variable.
We evaluated associations stratified by histologic subtype [Type I (66 adenocarcinomas, 194 endometrioid carcinomas, 11 mucinous) vs. Type II (34 serous, 6 clear cell, 2 other tumors)], grade of the tumor among the endometrioid and adenocarcinomas [low-grade (grades 1–2) vs. high-grade (3–4)], and time between blood collection and diagnosis (<5 year, ≥ 5 years). Differences in risk estimates across subgroups were assessed using p-values (reported as p-heterogeneity) from unconditional logistic regression models that treated the largest subgroup as the reference category and excluded non-cases. We also conducted the following sensitivity analyses excluding: 1) individuals diagnosed within 2 years of blood draw (n=27), 2) potential outliers [greater than five standard deviations above the median; median excluded subjects per hormone n=10 (min-max: 7–17)], 3) diabetics (n=38), and 4) former MHT users (n=220).
Q-values which reflect false discovery rates (FDR) were calculated to account for multiple comparisons in the main analyses; all other analyses were considered exploratory and therefore not corrected for multiple comparisons. All p-values were two-sided; p-values <0.05 were considered statistically significant for uncorrected tests.
Results
The endometrial cancer cases and controls were comparably aged, being 64.5 and 64.0 years, respectively (Table 1). The majority of the subjects provided blood samples during 1993–1996 and were Caucasian. Approximately 4 percent of the subjects reported last MHT use within the year prior to study interview.
Table 1.
Demographic and health characteristics of endometrial cancer cases and matched controls.
| Endometrial cancer cases | Controls | |||
|---|---|---|---|---|
| n=313 | n=354 | |||
| Mean | SD | Mean | SD | |
| Age at baseline blood draw, years | 64.5 | 7.0 | 64.0 | 7.0 |
| Year of blood draw | n | % | n | % |
| 1993–1996 | 190 | 60.7 | 218 | 61.6 |
| 1997–1998 | 123 | 39.3 | 138 | 39.0 |
| Race/Ethnicity | ||||
| White | 284 | 90.7 | 319 | 90.1 |
| Black | 15 | 4.8 | 17 | 4.8 |
| Hispanic | 2 | 0.6 | 3 | 0.8 |
| Other | 12 | 3.8 | 15 | 4.2 |
| Time since last menopausal hormone therapy use, years | ||||
| >1 | 300 | 95.8 | 340 | 96.0 |
| ≤1 | 13 | 4.2 | 14 | 4.0 |
| Family Income | ||||
| <$35,000 | 110 | 35.1 | 133 | 37.6 |
| $35,000–$74,999 | 119 | 38.0 | 137 | 38.7 |
| ≥$75,000 | 60 | 19.2 | 57 | 16.1 |
| Education | ||||
| High school or less | 56 | 17.9 | 74 | 20.9 |
| Some post high school | 111 | 35.5 | 114 | 32.2 |
| College graduate | 144 | 46.0 | 163 | 46.0 |
| Alcohol use, drinks/week | ||||
| Never | 33 | 10.5 | 32 | 9.0 |
| Former | 52 | 16.6 | 59 | 16.7 |
| Current, <1 | 110 | 35.1 | 107 | 30.2 |
| Current, 1–<7 | 68 | 21.7 | 94 | 26.6 |
| Current, ≥7 | 48 | 15.3 | 62 | 17.5 |
| Smoking status | ||||
| Never | 163 | 52.1 | 177 | 50.0 |
| Former | 134 | 42.8 | 142 | 40.1 |
| Current | 13 | 4.2 | 32 | 9.0 |
| BMI, kg/m2 | ||||
| <25 | 74 | 23.6 | 161 | 45.5 |
| 25–29.9 | 90 | 28.8 | 104 | 29.4 |
| >30 | 148 | 47.3 | 88 | 24.9 |
| Ever pregnant | ||||
| No | 43 | 13.7 | 55 | 15.5 |
| Yes | 270 | 86.3 | 299 | 84.5 |
| Duration of oral contraceptive use, years | ||||
| Never | 221 | 70.6 | 214 | 60.5 |
| <5 | 50 | 16.0 | 72 | 20.3 |
| 5–<10 | 23 | 7.3 | 36 | 10.2 |
| ≥10 | 19 | 6.1 | 32 | 9.0 |
| Age at menopause, years | ||||
| <40 | 5 | 1.6 | 11 | 3.1 |
| 40–44 | 15 | 4.8 | 22 | 6.2 |
| 45–49 | 67 | 21.4 | 82 | 23.2 |
| 50–54 | 146 | 46.6 | 170 | 48.0 |
| ≥ 55 | 68 | 21.7 | 59 | 16.7 |
| Menopausal hormone therapy use | ||||
| Never | 237 | 75.7 | 210 | 59.3 |
| Former | 76 | 24.3 | 144 | 40.7 |
| Tubal Ligation | ||||
| No | 268 | 85.6 | 289 | 81.6 |
| Yes | 43 | 13.7 | 64 | 18.1 |
Expected associations were seen with respect to endometrial cancer risk factors, with cases generally having higher incomes, more education, higher BMIs, earlier ages at menarche and later ages at menopause, and less often reporting current smoking, long-term oral contraceptive usage and prior tubal ligation than controls.
Median and inter-decile ranges of serum estrogen metabolites for cases vs. controls are shown in Table 2. The most abundant estrogen was conjugated estrone, followed by estriol. Several metabolites were detected at quite low levels, including metabolites in the 4-hydroxylation pathway, as well as the 2-methoxy metabolites. Cases showed higher levels of all parent estrogens and individual metabolites than controls.
Table 2.
Median and interdecile range (IDR) of estrogen metabolites among endometrial cancer cases and controls
| Cases | Controls | |||
|---|---|---|---|---|
| Estrogens and estrogen metabolites (pmol/L) | median | (IDR) | median | (IDR) |
| Parent Estrogens | ||||
| Estrone | 441.4 | (176.3–1239.8) | 293.9 | (137.5–880.9) |
| Unconjugated Estrone | 73.7 | (37.3–155.8) | 55.4 | (30.1–115.9) |
| Conjugated Estrone | 372.2 | (127.6–1096.8) | 238.8 | (98.1–792.1) |
| Estradiol | 68.2 | (29.4–172.6) | 49.3 | (21.9–154.7) |
| Unconjugated Estradiol | 20.0 | (6.3–55.0) | 11.6 | (4–38.8) |
| Conjugated Estradiol | 44.2 | (18.9–120) | 37.1 | (14.9–110.1) |
| 2-Hydroxylation Pathway | ||||
| 2-Hydroxyestrone | 80.9 | (43.5–178.2) | 64.9 | (32.8–164) |
| 2-Hydroxyestradiol | 19.0 | (11.0–43.0) | 15.9 | (8.3–40.6) |
| 2-Methoxyestrone | 50.8 | (28.2–104.5) | 41.7 | (24.8–89.5) |
| Unconjugated 2-Methoxyestrone | 12.8 | (6.3–26.4) | 10.4 | (4.4–23.3) |
| Conjugated 2-Methoxyestrone | 36.2 | (18.4–85.8) | 31.8 | (16.9–73.5) |
| 2-Methoxyestradiol | 15.9 | (8.0–36.8) | 12.4 | (6.6–32.9) |
| Unconjugated 2-Methoxyestradiol | 2.7 | (1.4–6.5) | 2.0 | (1.1–4.8) |
| Conjugated 2-Methoxyestradiol | 12.8 | (6.3–34.4) | 10.5 | (5.1–28.1) |
| 2-Hydroxyestrone-3-methyl ether | 9.1 | (5.1–19.0) | 7.6 | (4.2–17.1) |
| 4-Hydroxylation Pathway | ||||
| 4-Hydroxyestrone | 9.5 | (5.1–24.0) | 7.6 | (4.1–21.1) |
| 4-Methoxyestrone | 4.6 | (3.2–14.1) | 3.9 | (2.8–10.5) |
| 4-Methoxyestradiol | 2.1 | (1.2–5.6) | 1.7 | (0.9–4.1) |
| 16alpha-Hydroxylation Pathway | ||||
| 16alpha-Hydroxyestrone | 40.9 | (21.3–90.9) | 32.3 | (15.8–86.8) |
| Estriol | 181.3 | (87.8–421.9) | 140.4 | (63.5–391.5) |
| Unconjugated Estriol | 34.1 | (15.4–74.4) | 25.7 | (12.2–63.7) |
| Conjugated Estriol | 146.9 | (66.1–352.6) | 109.7 | (49.3–332) |
| 16-Ketoestradiol | 45.1 | (22.3–109.5) | 34.2 | (16.6–101.3) |
| 16-Epiestriol | 18.5 | (9.6–40.9) | 15.0 | (7.5–38.3) |
| 17-Epiestriol | 15.6 | (7.9–37.3) | 12.8 | (6.2–31.7) |
Adjusted ORs associated with the 5th vs. 1st quintile of the parent estrogens were 3.19 (95% CI 1.69–6.04) for estrone and 1.41 (0.75–2.67) for estradiol (Table 3). There was little difference in the association between unconjugated and conjugated estrone, but for estradiol the difference in risk was striking—being 6.19 (2.95–13.03) for unconjugated estradiol and 0.95 (0.51–1.77) for conjugated estradiol. There were significant trends across quintiles of estrone, regardless of conjugation, and for unconjugated estradiol (individual quintile ORs are shown in Supplemental Table 1).
Table 3.
Risk of endometrial cancer comparing the 5th to the 1st quintile for each estrogen metabolite, before and after adjustment for unconjugated estradiol
| OR1 | (95% CI) | p-trend2 | FDR | OR3 | (95% CI) | p-trend2 | |
|---|---|---|---|---|---|---|---|
| Parent Estrogens | |||||||
| Estrone | 3.19 | (1.69–6.04) | 0.0001 | 0.0004 | 2.70 | (1.34–5.45) | 0.003 |
| Unconjugated Estrone | 2.70 | (1.48–4.92) | 0.0002 | 0.0005 | 2.23 | (1.10–4.51) | 0.017 |
| Conjugated Estrone | 2.98 | (1.59–5.58) | 0.0002 | 0.0005 | 2.46 | (1.25–4.85) | 0.010 |
| Estradiol | 1.41 | (0.75–2.67) | 0.4531 | 0.4720 | |||
| Unconjugated Estradiol | 6.19 | (2.95–13.03) | 0.0001 | 0.0004 | |||
| Conjugated Estradiol | 0.95 | (0.51–1.77) | 0.6747 | 0.6747 | |||
| 2-Hydroxylation Pathway | |||||||
| 2-Hydroxyestrone | 3.00 | (1.56–5.78) | 0.0033 | 0.0055 | 2.41 | (1.21–4.82) | 0.048 |
| 2-Hydroxyestradiol | 2.31 | (1.22–4.37) | 0.0328 | 0.0373 | 1.82 | (0.93–3.55) | 0.237 |
| 2-Methoxyestrone | 2.12 | (1.16–3.88) | 0.0022 | 0.0042 | 1.77 | (0.94–3.33) | 0.025 |
| Unconjugated 2- Methoxyestrone |
2.69 | (1.46–4.96) | 0.0049 | 0.0074 | 2.00 | (1.03–3.89) | 0.099 |
| Conjugated 2-Methoxyestrone | 1.60 | (0.90–2.86) | 0.0246 | 0.0293 | 2.86 | (1.45–5.64) | 0.001 |
| 2-Methoxyestradiol | 3.35 | (1.76–6.37) | 0.0001 | 0.0004 | 2.45 | (1.23–4.85) | 0.018 |
| Unconjugated 2- Methoxyestradiol |
3.06 | (1.63–5.73) | 0.0006 | 0.0014 | 2.73 | (1.39–5.37) | 0.003 |
| Conjugated 2- Methoxyestradiol |
3.26 | (1.70–6.25) | 0.0002 | 0.0005 | 2.25 | (1.13–4.48) | 0.233 |
| 2-Hydroxyestrone-3-methyl ether |
2.83 | (1.45–5.51) | 0.0361 | 0.0392 | 2.45 | (1.24–4.84) | 0.025 |
| 4-Hydroxylation Pathway | |||||||
| 4-Hydroxyestrone | 3.04 | (1.59–5.80) | 0.0019 | 0.0040 | 2.46 | (1.31–4.60) | 0.001 |
| 4-Methoxyestrone | 2.89 | (1.57–5.31) | 0.0001 | 0.0004 | 3.01 | (1.51–6.03) | 0.001 |
| 4-Methoxyestradiol | 3.52 | (1.82–6.80) | 0.0001 | 0.0004 | 2.19 | (1.12–4.29) | 0.063 |
| 16alpha-Hydroxylation Pathway | |||||||
| 16alpha-Hydroxyestrone | 2.73 | (1.44–5.16) | 0.0050 | 0.0074 | 3.29 | (1.66–6.51) | 0.004 |
| Estriol | 4.00 | (2.09–7.67) | 0.0001 | 0.0004 | 1.37 | (0.76–2.48) | 0.101 |
| Unconjugated Estriol | 3.25 | (1.71–6.19) | 0.0002 | 0.0005 | 2.69 | (1.36–5.33) | 0.005 |
| Conjugated Estriol | 3.37 | (1.75–6.49) | 0.0032 | 0.0055 | 2.54 | (1.28–5.04) | 0.058 |
| 16-Ketoestradiol | 3.13 | (1.63–6.03) | 0.0053 | 0.0074 | 2.77 | (1.40–5.46) | 0.034 |
| 16-Epiestriol | 2.88 | (1.49–5.54) | 0.0060 | 0.0079 | 2.30 | (1.17–4.56) | 0.086 |
| 17-Epiestriol | 2.30 | (1.23–4.31) | 0.0146 | 0.0183 | 1.78 | (0.92–3.44) | 0.181 |
OR (quintile 5 vs. quintile 1) from model conditioned on matching factors [age at baseline, calendar year of blood draw, race/ethnicity, and recency of MHT use (≤1/>1 year)] and adjusted for education, BMI, cigarette smoking status, years of oral contraceptive use, age at menarche, gravidity, age at menopause, never/former MHT use, and previous tubal ligation.
p-values for trend across quintile (median value of category)
Same model as 1, also adjusted for unconjugated estradiol.
Nearly all of the individual metabolites also showed significant linear trends and associations with risk (the one exception being conjugated 2-methoxyestrone), with the magnitude of the risks ranging from 2.1 to 4.0. We cautiously (given high correlations of estrogens and metabolites—see Supplemental Table 2) also examined ratios of different estrogens and metabolites; however, we observed no convincing or statistically significant patterns of risk associated with these quantities (results not shown).
When we adjusted all other estrogens and metabolites for the strongest predictor of risk, namely unconjugated estradiol (as a continuous variable) (Table 3), we generally saw some attenuation in the observed risks, and several metabolite associations became non-significant (2-hydroxyestradiol, 2-methoxyestrone, estriol, 17-epiestriol). However, a number of the metabolites remained associated with risk, showing significant associations for the highest quintiles (magnitude of association 2.0–3.0) and significant linear trends.
Since endogenous estrogens are known to be correlated with BMI, we assessed relations of the various estrogens and metabolites across different BMI categories. This demonstrated that estrogens were generally associated with increased risks across all BMI categories (Parent estrogens shown in Table 4 and estrogen metabolites in Supplemental Table 3). Similar analyses were pursued according to different categories of age at diagnosis and years of use of oral contraceptives. While there was no evidence of interactive effects of estrogens with oral contraceptive usage, some of the estrogens showed stronger relations for older subjects, with interactions being statistically significant at p<0.05 for conjugated 2-methoxyestradiol, 4-methoxyestrone, and 17-epiestriol.
Table 4.
Risk of endometrial cancer comparing the 5th to the 1st quintile for parent estrogens across categories of BMI, age, and years of oral contraceptive use.
| BMI | OR1 | (95% CI) | p-trend | p-intx | Age | OR1 | (95% CI) | p-trend | p-intx | Years of OC Use | OR1 | (95% CI) | p-trend | p-intx | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Parent estrogens | |||||||||||||||
| Estrone | |||||||||||||||
| <25 | 3.62 | (1.00–13.11) | 0.02 | 0.41 | <60 | 2.44 | (0.63–9.44) | 0.25 | 0.36 | Never | 2.12 | (1.00–4.49) | 0.01 | 0.10 | |
| 25–29.9 | 1.08 | (0.33–3.54) | 0.29 | 60–69 | 2.60 | (1.03–6.54) | 0.003 | <5 yrs | 14.52 | (2.25–93.77) | 0.01 | ||||
| ≥30 | 8.06 | (1.82–35.77) | 0.004 | ≥70 | 10.60 | (2.80–40.10) | 0.001 | ≥5 yrs | 15.15 | (0.79–292.14) | 0.02 | ||||
| Unconjugated Estrone | |||||||||||||||
| <25 | 3.35 | (1.16–9.69) | 0.01 | 0.50 | <60 | 2.90 | (0.81–10.42) | 0.17 | 0.23 | Never | 1.71 | (0.85–3.44) | 0.04 | 0.12 | |
| 25–29.9 | 1.16 | (0.39–3.43) | 0.48 | 60–69 | 2.46 | (1.02–5.92) | 0.05 | <5 yrs | 29.90 | (4.08–219.16) | 0.003 | ||||
| ≥30 | 6.00 | (1.51–23.94) | 0.02 | ≥70 | 4.45 | (1.29–15.29) | 0.001 | ≥5 yrs | 7.12 | (0.50–100.97) | 0.008 | ||||
| Conjugated Estrone | |||||||||||||||
| <25 | 3.43 | (1.05–11.26) | 0.03 | 0.53 | <60 | 2.53 | (0.67–9.56) | 0.22 | 0.10 | Never | 2.01 | (0.95–4.26) | 0.04 | 0.15 | |
| 25–29.9 | 1.19 | (0.35–4.02) | 0.32 | 60–69 | 2.45 | (0.99–6.09) | 0.009 | <5 yrs | 10.60 | (2.12–53.14) | 0.005 | ||||
| ≥30 | 7.89 | (2.02–30.77) | 0.01 | ≥70 | 8.10 | (2.23–29.46) | 0.003 | ≥5 yrs | 7.43 | (0.39–142.68) | 0.06 | ||||
| Estradiol | |||||||||||||||
| <25 | 0.88 | (0.25–3.03) | 0.86 | 0.30 | <60 | 2.76 | (0.70–10.94) | 0.59 | 0.08 | Never | 1.22 | (0.57–2.60) | 0.33 | 0.36 | |
| 25–29.9 | 0.52 | (0.16–1.67) | 0.37 | 60–69 | 0.92 | (0.33–2.52) | 0.76 | <5 yrs | 3.75 | (0.54–26.15) | 0.69 | ||||
| ≥30 | 6.56 | (1.06–40.51) | 0.05 | ≥70 | 1.53 | (0.48–4.87) | 0.51 | ≥5 yrs | 6.23 | (0.45–85.47) | 0.52 | ||||
| Unconjugated Estradiol | |||||||||||||||
| <25 | 7.84 | (2.1–29.22) | 0.01 | 0.48 | <60 | 8.74 | (1.47–51.92) | 0.17 | 0.50 | Never | 4.19 | (1.84–9.56) | 0.01 | 0.25 | |
| 25–29.9 | 2.39 | (0.64–8.99) | 0.06 | 60–69 | 4.82 | (1.60–14.52) | 0.04 | <5 yrs | ** | 0.002 | |||||
| ≥30 | 6.60 | (1.02–42.75) | 0.05 | ≥70 | 12.61 | (3.17–50.13) | 0.001 | ≥5 yrs | 4.62 | (0.29–74.7) | 0.05 | ||||
| Conjugated Estradiol | |||||||||||||||
| <25 | 0.79 | (0.23–2.74) | 0.79 | 0.16 | <60 | 1.85 | (0.55–6.25) | 0.64 | 0.24 | Never | 0.78 | (0.37–1.66) | 0.66 | 0.10 | |
| 25–29.9 | 0.29 | (0.09–0.94) | 0.08 | 60–69 | 0.68 | (0.25–1.83) | 0.41 | <5 yrs | 1.26 | (0.22–7.16) | 0.99 | ||||
| ≥30 | 5.31 | (0.87–32.4) | 0.21 | ≥70 | 0.68 | (0.20–2.29) | 0.86 | ≥5 yrs | 7.41 | (0.54–100.96) | 0.19 | ||||
OR (quintile 5 vs. quintile 1) from model adjusted for matching factors [age at baseline, calendar year of blood draw, race/ethnicity, and recency of MHT use (never, ≤1/>1 year)] and education, cigarette smoking status, age at menarche, gravidity, age at menopause, and previous tubal ligation. Additional adjustment was made for BMI and years of oral contraceptive use, if relevant.
Could not be estimated; too few cases in reference category
We also assessed the extent to which estrogens might explain the effects of obesity on endometrial cancer risk, comparing BMI estimates in models unadjusted for estrogens with estrogen-adjusted estimates. Without adjustment for estrogens and compared to a referent group of BMI<25, the ORs were 2.17 (95% CI 1.40–3.34) for BMI 25–29.9 and 3.97 (2.54–6.21) for BMI≥30. Additional adjustment for unconjugated estradiol resulted in an attenuation of these risks to 1.55 (0.97–2.48) and 2.25 (1.33–3.81), respectively.
Given interest in the etiologic heterogeneity of endometrial cancer, we also examined relations of the estrogens and metabolites according to several clinical characteristics (Table 5). A total of 271 (86.6%) cases were classified as Type I and 42 (13.4%) as Type II tumors. Significant heterogeneity in the associations of several estrogens were seen between the two tumor types, including unconjugated estradiol [OR for highest vs. lowest quintile for Type I tumors was 6.97 (95% CI 3.20–15.20) vs. 1.80 (0.43–7.49) for Type II tumors, phet=0.01]. Other significant differences were seen for unconjugated 2-methoxyestrone (3.47 vs. 0.95), 4-methoxyestrone (3.54 vs. 0.84) and unconjugated estriol (3.34 vs. 1.83). When we further limited the analyses to endometrioid tumors and adenocarcinomas and assessed whether there were differences in estrogen associations according to grade of the tumors, we observed stronger associations of unconjugated estradiol with risks of low-grade vs. high-grade tumors (OR 8.92 vs. 3.27), but the difference was not statistically significant (phet=0.14).
Table 5.
Risk of endometrial cancer comparing the 5th to the 1st quintile for each estrogen metabolite: Subtype analyses according to tumor type (I vs. II) and grade (low- vs. high-grade).
| Type I | Type II | Low grade | High Grade | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (n=271) | (n=42) | (n=189) | (n=65) | |||||||||||
| Parent Estrogens | OR2 | (95% CI) | p-trend3 | OR2 | (95% CI) | p-trend3 | phet | OR2 | (95% CI) | p-trend3 | OR2 | (95% CI) | p-trend3 | phet |
| Estrone | 3.41 | (1.79–6.50) | <0.001 | 3.39 | (0.90–12.73) | 0.14 | 0.48 | 3.38 | (1.65–6.96) | <0.001 | 3.16 | (1.09–9.13) | 0.01 | 0.63 |
| Unconjugated Estrone | 2.91 | (1.58–5.37) | <0.001 | 1.50 | (0.46–4.87) | 0.69 | 0.07 | 2.65 | (1.34–5.21) | <0.001 | 2.51 | (0.89–7.08) | 0.11 | 0.27 |
| Conjugated Estrone | 3.04 | (1.62–5.70) | <0.001 | 4.22 | (1.02–17.54) | 0.15 | 0.60 | 3.00 | (1.49–6.06) | <0.001 | 2.67 | (0.98–7.29) | 0.03 | 0.68 |
| Estradiol | 1.70 | (0.89–3.25) | 0.13 | 0.76 | (0.22–2.62) | 0.67 | 0.29 | 1.48 | (0.72–3.04) | 0.16 | 1.89 | (0.54–6.62) | 0.98 | 0.35 |
| Unconjugated Estradiol | 6.97 | (3.20–15.20) | <0.001 | 1.80 | (0.43–7.49) | 0.83 | 0.01 | 8.92 | (3.38–23.54) | <0.001 | 3.27 | (1.03–10.36) | 0.09 | 0.14 |
| Conjugated Estradiol | 1.16 | (0.62–2.19) | 0.64 | 0.48 | (0.13–1.77) | 0.28 | 0.26 | 1.05 | (0.52–2.13) | 0.50 | 1.26 | (0.39–4.08) | 0.57 | 0.45 |
| 2-Hydroxylation Pathway | ||||||||||||||
| 2-Hydroxyestrone | 2.99 | (1.55–5.78) | <0.001 | 3.38 | (0.58–19.67) | 0.78 | 0.16 | 3.56 | (1.65–7.69) | 0.001 | 2.30 | (0.81–6.48) | 0.06 | 0.62 |
| 2-Hydroxyestradiol | 2.43 | (1.28–4.63) | 0.008 | 2.56 | (0.56–11.72) | 0.71 | 0.40 | 3.16 | (1.47–6.82) | 0.006 | 1.63 | (0.62–4.29) | 0.16 | 0.62 |
| 2-Methoxyestrone | 2.51 | (1.36–4.65) | <0.001 | 0.90 | (0.28–2.90) | 0.81 | 0.16 | 2.27 | (1.16–4.45) | 0.001 | 3.94 | (1.30–11.92) | 0.01 | 0.93 |
| Unconjugated 2-Methoxyestrone | 3.47 | (1.82–6.62) | <0.001 | 0.95 | (0.30–3.05) | 0.74 | 0.02 | 3.40 | (1.63–7.11) | 0.003 | 3.45 | (1.16–10.23) | 0.03 | 0.72 |
| Conjugated 2-Methoxyestrone | 1.71 | (0.95–3.08) | 0.007 | 1.19 | (0.37–3.84) | 0.53 | 0.67 | 1.62 | (0.85–3.09) | 0.01 | 1.99 | (0.77–5.13) | 0.07 | 0.97 |
| 2-Methoxyestradiol | 4.19 | (2.18–8.07) | <0.001 | 1.48 | (0.43–5.08) | 0.66 | 0.08 | 3.50 | (1.68–7.28) | <0.001 | 5.14 | (1.76–14.99) | 0.00 | 0.42 |
| Unconjugated 2-Methoxyestradiol | 3.85 | (1.99–7.45) | <0.001 | 1.68 | (0.52–5.43) | 0.55 | 0.20 | 3.43 | (1.63–7.25) | <0.001 | 4.07 | (1.33–12.45) | 0.01 | 0.76 |
| Conjugated 2-Methoxyestradiol | 3.79 | (1.97–7.29) | <0.001 | 1.97 | (0.52–7.41) | 0.51 | 0.15 | 3.35 | (1.61–6.94) | <0.001 | 4.03 | (1.38–11.78) | 0.01 | 0.87 |
| 2-Hydroxyestrone-3-methyl ether | 3.58 | (1.80–7.11) | 0.004 | 1.08 | (0.25–4.60) | 0.81 | 0.17 | 3.93 | (1.80–8.58) | 0.003 | 2.33 | (0.73–7.43) | 0.43 | 0.18 |
| 4-Hydroxylation Pathway | ||||||||||||||
| 4-Hydroxyestrone | 3.19 | (1.67–6.11) | <0.001 | 2.73 | (0.59–12.63) | 0.82 | 0.12 | 4.17 | (1.93–9.03) | <0.001 | 1.91 | (0.72–5.08) | 0.07 | 0.55 |
| 4-Methoxyestrone | 3.54 | (1.91–6.59) | <0.001 | 0.84 | (0.23–3.06) | 0.96 | 0.03 | 3.20 | (1.61–6.37) | <0.001 | 4.13 | (1.6–10.66) | 0.00 | 0.30 |
| 4-Methoxyestradiol | 4.00 | (2.06–7.79) | <0.001 | 2.23 | (0.59–8.45) | 0.44 | 0.12 | 3.70 | (1.75–7.83) | <0.001 | 3.60 | (1.31–9.90) | 0.00 | 0.80 |
| 16alpha-Hydroxylation Pathway | ||||||||||||||
| 16alpha-Hydroxyestrone | 2.84 | (1.49–5.41) | 0.001 | 2.23 | (0.47–10.61) | 0.91 | 0.14 | 3.10 | (1.48–6.50) | 0.002 | 2.50 | (0.90–6.96) | 0.05 | 0.83 |
| Estriol | 4.14 | (2.14–8.00) | <0.001 | 3.38 | (0.75–15.20) | 0.34 | 0.21 | 4.85 | (2.20–10.66) | <0.001 | 3.53 | (1.32–9.46) | 0.00 | 0.40 |
| Unconjugated Estriol | 3.34 | (1.75–6.35) | <0.001 | 1.83 | (0.43–7.84) | 0.88 | 0.03 | 3.02 | (1.47–6.19) | <0.001 | 4.05 | (1.39–11.77) | 0.01 | 0.84 |
| Conjugated Estriol | 3.35 | (1.73–6.45) | <0.001 | 4.60 | (0.85–25.08) | 0.40 | 0.38 | 3.69 | (1.71–7.96) | 0.003 | 3.23 | (1.17–8.93) | 0.01 | 0.54 |
| 16-Ketoestradiol | 3.23 | (1.67–6.27) | 0.002 | 3.27 | (0.74–14.51) | 0.52 | 0.32 | 3.59 | (1.65–7.79) | 0.004 | 2.65 | (0.95–7.40) | 0.03 | 0.90 |
| 16-Epiestriol | 3.33 | (1.70–6.54) | 0.001 | 1.35 | (0.32–5.66) | 0.99 | 0.11 | 4.43 | (1.96–9.98) | <0.001 | 2.21 | (0.76–6.46) | 0.19 | 0.29 |
| 17-Epiestriol | 2.57 | (1.36–4.85) | 0.005 | 1.60 | (0.40–6.39) | 0.60 | 0.40 | 3.62 | (1.66–7.92) | 0.006 | 1.33 | (0.53–3.35) | 0.17 | 0.53 |
Type I (66 adenocarcinomas, 194 endometrioid carcinomas, 11 mucinous) vs. Type II (34 serous, 6 clear cell, 2 other); grade of tumor (low = grade 1,2, high = grade 3, 4) evaluated among endometrioid carcinomas and adenocarcinomas only.
OR (quintile 5 vs. quintile 1) from model adjusted for matching factors [age at baseline, calendar year of blood draw, race/ethnicity, and recency of MHT use (never, ≤1/>1 year)] and education, BMI, cigarette smoking status, years of oral contracteptive use, age at menarche, gravidity, age at menopause, and previous tubal ligation.
p-values for trend across quintile (median value of category)
Associations were not significantly different by time since blood draw (<5/≥ 5 years). In sensitivity analyses, ORs excluding cases diagnosed within 2 years of blood draw or diabetics remained unchanged. We also excluded individuals with outlier estrogen measurements, but given the small numbers involved this had minimal effects on derived associations. Finally, we restricted analyses to never users of MHT (excluding former users) and did not observe large alterations from previously derived risks.
Discussion
In this largest study to date addressing the relation of endogenous estrogens and estrogen metabolites to endometrial cancer risk, we found that endometrial cancer risk was directly associated with serum levels of endogenous estrogens, with the highest risks associated with levels of unconjugated estradiol. Using a highly sensitive, accurate and reproducible assay, we found that the association with unconjugated estradiol was stronger than generally has been seen with less robust assays, with subjects in the highest quintile showing a 6-fold increased risk compared to those in the lowest quintile. After adjustment for unconjugated estradiol, the associations with the other estrogens and metabolites were attenuated, but most continued to show risk elevations on the order to 2–3 fold for women in the highest vs. lowest quintiles.
Our findings provided little support for varying effects of different estrogen metabolites on endometrial cancer risk, in line with two prior small prospective studies (13, 14). Dissimilar to our hypothesis that endometrial cancer might be affected by metabolites that have unique mitogenic propereties, and in contrast to finding from several breast cancer studies (9, 11), we did not observe distinctive associations with high ratios of the 2- to 16-pathway estrogens. We also found no support for mutagenicity given that endometrial cancer risk was not related to increased levels of catechols to methylated catechols. Divergent findings for breast and endometrial cancer are further supported by experimental data, which fail to support unique antineoplastic activity of 2-hydroxy metabolites in endometrial tissue (24, 25) and instead are consistent with all three pathways (2, 4 and 16) having uterotropic activity that can result in endometrial proliferation (26) and endometrial tumor progression (27). Findings that unopposed estrogens are inversely associated with breast cancer risk (28), but show strong positive relations for endometrial cancer (28, 29), provide additional support for differing etiologic roles of estrogens on the two cancer sites.
Especially high risks of endometrial cancer have been related to obesity (particularly for Type I cancers), and it is widely accepted that this is partly due to higher levels of estrogens among obese postmenopausal women given peripheral conversion of androgens to estrogens in adipose tissue (30). Several investigations have explored the extent to which estrogens explain the effects of obesity on breast cancer risk (31, 32), with findings that the relations with obesity were attenuated and became non-significant after adjustment for estrogens—most notably estradiol. However, effects of estrogens on the obesity-endometrial cancer relation have been less well explored. We observed similar results as have been seen for breast cancer, namely an attenuation of risks associated with obesity after adjustment for unconjugated estradiol levels. However, for endometrial cancer, where obesity is more strongly related to risk than it is for breast cancer, we were unable to completely eliminate the obesity-associated risks, suggesting that there may be other mediators of the association. Thus, further attention seems warranted on such factors as insulin, insulin-like growth factors, androgens, and inflammatory factors, which are also related to endometrial cancer risk as well as BMI (3, 4).
Much recent interest has focused on the etiologic heterogeneity of endometrial cancer, specifically in whether there are differences between Type I and II tumors. It has long been speculated that the numerically predominant Type I tumors have hormonally-driven etiologies (excess estrogen exposure) that develop from endometrial hyperplasias; in contrast, Type II tumors are considered unrelated to typical endometrial cancer risk factors and endometrial hyperplasia (33). Risk factor differences, however, have only recently been robustly assessed in epidemiologic investigations. It has thus been of interest that these studies support risk factor differences, with accepted endometrial cancer risk factors (e.g., obesity, nulliparity, absence of cigarette smoking) being stronger for Type I than II tumors (17, 18, 34–36). As an extension, it would be expected that the effects of endogenous estrogens would also be differential; however, apart from one small investigation (37), these relations have not been explored.
Although estrogens appeared to influence both Type I and II tumors, our findings showed that the association of unconjugated estradiol was stronger for the Type I tumors, consistent with a central role for estrogens in influencing the progression of endometrial hyperplasias to these malignancies (38). Although we did not observe the difference to be statistically significant, we also observed unconjugated estradiol more strongly linked to low- than high-grade tumors. This finding supports recent epidemiologic (17) as well as clinical (39) data suggesting that the high-grade endometrioid tumors may be etiologically distinct from other Type I cancers and more resemble Type II cancers.
This study had some notable strengths, as well as a few limitations. Strengths included the relatively large number of cases identified through a well-defined population for whom prediagnostic estrogen levels could be measured and adjusted for other important endometrial cancer risk factors. The assay used to measure estrogens and their metabolites has extremely high sensitivity and specificity. Nonetheless, many of the metabolites were highly correlated with each other, presenting problems for disentangling effects. Additionally, despite the relatively large number of endometrial cases, few were diagnosed with Type II tumors, limiting our ability to explore etiologic heterogeneity across tumor subtypes. We also included only postmenopausal women, conducted assays on a single serum sample collected at baseline, and did not measure sex hormone blinding globulin levels. Furthermore, estrogens were circulating measures, whereas more biologically meaningful assessments may derive from tissue assays, which are methodologically challenging (40).
In summary, our results support a central role for estrogens in endometrial cancer. Although the strongest effect was for unconjugated estradiol, other estrogens and estrogen metabolites may also contribute to risk. The association with unconjugated estradiol was restricted to Type I cancers, consistent with an important role for estrogenic influences in the progression of endometrial hyperplasia to cancer. Additional attention focused on estrogen metabolism in relation to other endometrial cancer biomarkers and as measured in tissue may further our understanding of endometrial carcinogenesis.
Supplementary Material
Acknowledgments
The authors would like to also acknowledge the following short list of WHI investigators
Program Office: (National Heart, Lung, and Blood Institute, Bethesda, Maryland) Jacques Rossouw, Shari Ludlam, Dale Burwen, Joan McGowan, Leslie Ford, and Nancy Geller
Clinical Coordinating Center: Fred Hutchinson Cancer Research Center, Seattle, WA (Garnet Anderson, Ross Prentice, Andrea LaCroix, and Charles Kooperberg)
Investigators and Academic Centers: Brigham and Women’s Hospital, Harvard Medical School, Boston, MA (JoAnn E. Manson); MedStar Health Research Institute/Howard University, Washington, DC (Barbara V. Howard); Stanford Prevention Research Center, Stanford, CA (Marcia L. Stefanick); The Ohio State University, Columbus, OH (Rebecca Jackson); University of Arizona, Tucson/Phoenix, AZ (Cynthia A. Thomson); University at Buffalo, Buffalo, NY (Jean Wactawski-Wende); University of Florida, Gainesville/Jacksonville, FL (Marian Limacher); University of Iowa, Iowa City/Davenport, IA (Robert Wallace); University of Pittsburgh, Pittsburgh, PA (Lewis Kuller); Wake Forest University School of Medicine, Winston-Salem, NC (Sally Shumaker)
Women’s Health Initiative Memory Study: (Wake Forest University School of Medicine, Winston-Salem, NC) Sally Shumaker
For a list of all the investigators who have contributed to WHI science, please visit: https://www.whi.org/researchers/Documents%20%20Write%20a%20Paper/WHI%20Investigator%20Long%20List.pdf)
Financial Support: This work was supported in part by the Intramural Research Program of the National Cancer Institute. The WHI program is funded by the National Heart, Lung, and Blood Institute, National Institutes of Health, U.S. Department of Health and Human Services through contracts HHSN268201100046C, HHSN268201100001C, HHSN268201100002C, HHSN268201100003C, HHSN268201100004C, and HHSN271201100004C.
Footnotes
Conflicts of Interest: There are no conflicts of interest to disclose.
References
- 1.Kaaks R, Lukanova A, Kurzer MS. Obesity, endogenous hormones, and endometrial cancer risk: a synthetic review. Cancer epidemiology, biomarkers & prevention: a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2002;11:1531–43. [PubMed] [Google Scholar]
- 2.Brown SB, Hankinson SE. Endogenous estrogens and the risk of breast, endometrial, and ovarian cancers. Steroids. 2015;99:8–10. doi: 10.1016/j.steroids.2014.12.013. [DOI] [PubMed] [Google Scholar]
- 3.Allen NE, Key TJ, Dossus L, Rinaldi S, Cust A, Lukanova A, et al. Endogenous sex hormones and endometrial cancer risk in women in the European Prospective Investigation into Cancer and Nutrition (EPIC) Endocrine-related cancer. 2008;15:485–97. doi: 10.1677/ERC-07-0064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gunter MJ, Hoover DR, Yu H, Wassertheil-Smoller S, Manson JE, Li J, et al. A prospective evaluation of insulin and insulin-like growth factor-I as risk factors for endometrial cancer. Cancer epidemiology, biomarkers & prevention: a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2008;17:921–9. doi: 10.1158/1055-9965.EPI-07-2686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lukanova A, Lundin E, Micheli A, Arslan A, Ferrari P, Rinaldi S, et al. Circulating levels of sex steroid hormones and risk of endometrial cancer in postmenopausal women. International journal of cancer Journal international du cancer. 2004;108:425–32. doi: 10.1002/ijc.11529. [DOI] [PubMed] [Google Scholar]
- 6.Zeleniuch-Jacquotte A, Akhmedkhanov A, Kato I, Koenig KL, Shore RE, Kim MY, et al. Postmenopausal endogenous oestrogens and risk of endometrial cancer: results of a prospective study. British journal of cancer. 2001;84:975–81. doi: 10.1054/bjoc.2001.1704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ziegler RG, Fuhrman BJ, Moore SC, Matthews CE. Epidemiologic studies of estrogen metabolism and breast cancer. Steroids. 2015;99:67–75. doi: 10.1016/j.steroids.2015.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bradlow HL, Sepkovic DW, Klug T, Osborne MP. Application of an improved ELISA assay to the analysis of urinary estrogen metabolites. Steroids. 1998;63:406–13. doi: 10.1016/s0039-128x(98)00041-5. [DOI] [PubMed] [Google Scholar]
- 9.Fishman J, Schneider J, Hershcope RJ, Bradlow HL. Increased estrogen-16 alpha-hydroxylase activity in women with breast and endometrial cancer. Journal of steroid biochemistry. 1984;20:1077–81. doi: 10.1016/0022-4731(84)90021-9. [DOI] [PubMed] [Google Scholar]
- 10.Cavalieri E, Chakravarti D, Guttenplan J, Hart E, Ingle J, Jankowiak R, et al. Catechol estrogen quinones as initiators of breast and other human cancers: implications for biomarkers of susceptibility and cancer prevention. Biochimica et biophysica acta. 2006;1766:63–78. doi: 10.1016/j.bbcan.2006.03.001. [DOI] [PubMed] [Google Scholar]
- 11.Dallal CM, Tice JA, Buist DS, Bauer DC, Lacey JV, Jr, Cauley JA, et al. Estrogen metabolism and breast cancer risk among postmenopausal women: a case-cohort study within B~FIT. Carcinogenesis. 2014;35:346–55. doi: 10.1093/carcin/bgt367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fuhrman BJ, Schairer C, Gail MH, Boyd-Morin J, Xu X, Sue LY, et al. Estrogen metabolism and risk of breast cancer in postmenopausal women. Journal of the National Cancer Institute. 2012;104:326–39. doi: 10.1093/jnci/djr531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zeleniuch-Jacquotte A, Shore RE, Afanasyeva Y, Lukanova A, Sieri S, Koenig KL, et al. Postmenopausal circulating levels of 2- and 16alpha-hydroxyestrone and risk of endometrial cancer. British journal of cancer. 2011;105:1458–64. doi: 10.1038/bjc.2011.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dallal CM, Lacey JV, Jr, Pfeiffer RM, Bauer DC, Falk RT, Buist DS, et al. Estrogen Metabolism and Risk of Postmenopausal Endometrial and Ovarian Cancer: the B approximately FIT Cohort. Horm Cancer. 2016;7:49–64. doi: 10.1007/s12672-015-0237-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Audet-Walsh E, Lepine J, Gregoire J, Plante M, Caron P, Tetu B, et al. Profiling of endogenous estrogens, their precursors, and metabolites in endometrial cancer patients: association with risk and relationship to clinical characteristics. The Journal of clinical endocrinology and metabolism. 2011;96:E330–9. doi: 10.1210/jc.2010-2050. [DOI] [PubMed] [Google Scholar]
- 16.Zhao H, Jiang Y, Liu Y, Yun C, Li L. Endogenous estrogen metabolites as biomarkers for endometrial cancer via a novel method of liquid chromatography-mass spectrometry with hollow fiber liquid-phase microextraction. Hormone and metabolic research = Hormon- und Stoffwechselforschung = Hormones et metabolisme. 2015;47:158–64. doi: 10.1055/s-0034-1371865. [DOI] [PubMed] [Google Scholar]
- 17.Brinton LA, Felix AS, McMeekin DS, Creasman WT, Sherman ME, Mutch D, et al. Etiologic heterogeneity in endometrial cancer: evidence from a Gynecologic Oncology Group trial. Gynecologic oncology. 2013;129:277–84. doi: 10.1016/j.ygyno.2013.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Setiawan VW, Yang HP, Pike MC, McCann SE, Yu H, Xiang YB, et al. Type I and II endometrial cancers: have they different risk factors? Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2013;31:2607–18. doi: 10.1200/JCO.2012.48.2596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Design of the Women’s Health Initiative clinical trial and observational study. The Women’s Health Initiative Study Group. Controlled clinical trials. 1998;19:61–109. doi: 10.1016/s0197-2456(97)00078-0. [DOI] [PubMed] [Google Scholar]
- 20.Langer RD, White E, Lewis CE, Kotchen JM, Hendrix SL, Trevisan M. The Women’s Health Initiative Observational Study: baseline characteristics of participants and reliability of baseline measures. Annals of epidemiology. 2003;13:S107–21. doi: 10.1016/s1047-2797(03)00047-4. [DOI] [PubMed] [Google Scholar]
- 21.Trabert B, Brinton LA, Anderson GL, Pfeiffer RM, Falk RT, Strickler HD, et al. Circulating estrogens and postmenopausal ovarian cancer risk in the Women’s Health Initiative Observational Study. Cancer epidemiology, biomarkers & prevention: a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2016 doi: 10.1158/1055-9965.EPI-15-1272-T. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xu X, Roman JM, Issaq HJ, Keefer LK, Veenstra TD, Ziegler RG. Quantitative measurement of endogenous estrogens and estrogen metabolites in human serum by liquid chromatography-tandem mass spectrometry. Analytical chemistry. 2007;79:7813–21. doi: 10.1021/ac070494j. [DOI] [PubMed] [Google Scholar]
- 23.Loud JT. Circulating estrogens and estrogens within the breast among postmenopausal BRCA1/2 mutation carriers. Breast cancer research and treatment. 2014;148:691–2. doi: 10.1007/s10549-014-3186-1. [DOI] [PubMed] [Google Scholar]
- 24.Newbold RR, Liehr JG. Induction of uterine adenocarcinoma in CD-1 mice by catechol estrogens. Cancer research. 2000;60:235–7. [PubMed] [Google Scholar]
- 25.Reddy VV, Hanjani P, Rajan R. Synthesis of catechol estrogens by human uterus and leiomyoma. Steroids. 1981;37:195–203. doi: 10.1016/s0039-128x(81)80017-7. [DOI] [PubMed] [Google Scholar]
- 26.Zhu BT, Conney AH. Functional role of estrogen metabolism in target cells: review and perspectives. Carcinogenesis. 1998;19:1–27. doi: 10.1093/carcin/19.1.1. [DOI] [PubMed] [Google Scholar]
- 27.Takahashi M, Shimomoto T, Miyajima K, Yoshida M, Katashima S, Uematsu F, et al. Effects of estrogens and metabolites on endometrial carcinogenesis in young adult mice initiated with N-ethyl-N′-nitro-N-nitrosoguanidine. Cancer letters. 2004;211:1–9. doi: 10.1016/j.canlet.2004.01.029. [DOI] [PubMed] [Google Scholar]
- 28.Anderson GL, Chlebowski RT, Aragaki AK, Kuller LH, Manson JE, Gass M, et al. Conjugated equine oestrogen and breast cancer incidence and mortality in postmenopausal women with hysterectomy: extended follow-up of the Women’s Health Initiative randomised placebo-controlled trial. The Lancet Oncology. 2012;13:476–86. doi: 10.1016/S1470-2045(12)70075-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Trabert B, Wentzensen N, Yang HP, Sherman ME, Hollenbeck AR, Park Y, et al. Is estrogen plus progestin menopausal hormone therapy safe with respect to endometrial cancer risk? International journal of cancer Journal international du cancer. 2013;132:417–26. doi: 10.1002/ijc.27623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Siiteri PK. Adipose tissue as a source of hormones. The American journal of clinical nutrition. 1987;45:277–82. doi: 10.1093/ajcn/45.1.277. [DOI] [PubMed] [Google Scholar]
- 31.Key TJ, Appleby PN, Reeves GK, Roddam A, Dorgan JF, Longcope C, et al. Body mass index, serum sex hormones, and breast cancer risk in postmenopausal women. Journal of the National Cancer Institute. 2003;95:1218–26. doi: 10.1093/jnci/djg022. [DOI] [PubMed] [Google Scholar]
- 32.Rinaldi S, Key TJ, Peeters PH, Lahmann PH, Lukanova A, Dossus L, et al. Anthropometric measures, endogenous sex steroids and breast cancer risk in postmenopausal women: a study within the EPIC cohort. International journal of cancer Journal international du cancer. 2006;118:2832–9. doi: 10.1002/ijc.21730. [DOI] [PubMed] [Google Scholar]
- 33.Bokhman JV. Two pathogenetic types of endometrial carcinoma. Gynecologic oncology. 1983;15:10–7. doi: 10.1016/0090-8258(83)90111-7. [DOI] [PubMed] [Google Scholar]
- 34.Felix AS, Weissfeld JL, Stone RA, Bowser R, Chivukula M, Edwards RP, et al. Factors associated with Type I and Type II endometrial cancer. Cancer causes & control: CCC. 2010;21:1851–6. doi: 10.1007/s10552-010-9612-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McCullough ML, Patel AV, Patel R, Rodriguez C, Feigelson HS, Bandera EV, et al. Body mass and endometrial cancer risk by hormone replacement therapy and cancer subtype. Cancer epidemiology, biomarkers & prevention: a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2008;17:73–9. doi: 10.1158/1055-9965.EPI-07-2567. [DOI] [PubMed] [Google Scholar]
- 36.Yang HP, Wentzensen N, Trabert B, Gierach GL, Felix AS, Gunter MJ, et al. Endometrial cancer risk factors by 2 main histologic subtypes: the NIH-AARP Diet and Health Study. American journal of epidemiology. 2013;177:142–51. doi: 10.1093/aje/kws200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sherman ME, Sturgeon S, Brinton LA, Potischman N, Kurman RJ, Berman ML, et al. Risk factors and hormone levels in patients with serous and endometrioid uterine carcinomas. Modern pathology: an official journal of the United States and Canadian Academy of Pathology, Inc. 1997;10:963–8. [PubMed] [Google Scholar]
- 38.Sherman ME. Theories of endometrial carcinogenesis: a multidisciplinary approach. Modern pathology: an official journal of the United States and Canadian Academy of Pathology, Inc. 2000;13:295–308. doi: 10.1038/modpathol.3880051. [DOI] [PubMed] [Google Scholar]
- 39.Zannoni GF, Vellone VG, Arena V, Prisco MG, Scambia G, Carbone A, et al. Does high-grade endometrioid carcinoma (grade 3 FIGO) belong to type I or type II endometrial cancer? A clinical-pathological and immunohistochemical study. Virchows Archiv: an international journal of pathology. 2010;457:27–34. doi: 10.1007/s00428-010-0939-z. [DOI] [PubMed] [Google Scholar]
- 40.Stanczyk FZ, Mathews BW, Sherman ME. Relationships of sex steroid hormone levels in benign and cancerous breast tissue and blood: A critical appraisal of current science. Steroids. 2015;99:91–102. doi: 10.1016/j.steroids.2014.12.011. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


