Abstract
Background
The U.S. FDA has the authority to reduce cigarette nicotine content if found to benefit public health. Reduced nicotine content (RNC) cigarette use does not appear to increase harm exposure, but studies have not rigorously assessed smoking behavior or utilized a comprehensive panel of biomarkers. This study examined the effects of progressively decreasing RNC cigarettes on smoking behaviors, biomarkers of exposure, and subjective ratings.
Methods
158 daily, non-treatment-seeking smokers participated in a 35-day randomized, unblinded, parallel study. After a 5-day baseline period, participants were randomly assigned to an experimental group (n = 80) that smoked progressively decreasing RNC cigarettes during three 10-day periods, or control group (n =78) that smoked their own brand throughout the study.
Results
Daily cigarette consumption significantly increased for the intermediate RNCs (P’s < 0.001) but approached baseline rate for the lowest RNC (P = 0.686); in contrast, puffing behavior significantly decreased at intermediate levels and increased for the lowest RNC (P’s < 0.001). Cotinine and NNAL significantly decreased by RNC period (P’s ≤ 0.001–0.02), while CO boost initially increased (P’s = 0.001–0.005). 1-HOP did not change by period (P = 0.109).
Conclusions
Smoking behaviors changed by RNC period via CPD and puffing behavior. Biomarkers of exposure generally decreased with nicotine content.
Impact
Findings suggest that RNC use does not ubiquitously reduce smoking behaviors or biomarkers, yet the lowest RNC level tested may reduce harm exposure. This emphasizes the importance of utilizing multiple behavioral and biological measures to address the impact of RNC cigarette smoking.
Keywords: smoking behavior, nicotine, cotinine, topography, biomarkers
Introduction
Cigarette smoking is the leading cause of preventable death and a significant public health concern (1). Because nicotine is the primary addictive ingredient in cigarettes (2), decreasing its content in cigarettes has been proposed as a strategy for reducing tobacco-related morbidity and mortality (3). The United States Food and Drug Administration (FDA) has the authority to regulate and reduce cigarette nicotine content (4), if such regulation is shown to benefit public health. Rigorous empirical data on smoking behaviors and smoke exposure is needed to understand the impact a nicotine reduction agenda may have.
A concern with reducing cigarette nicotine content is that smokers may compensate, or increase their smoking frequency/intensity (e.g., increased daily consumption and/or puffing behavior) to maintain sufficient nicotine intake. Tobacco manufacturers previously manipulated cigarette design features (e.g., filter ventilation) to create “light” and “ultra-light” cigarettes (5), which had reduced machine-measured nicotine yield but contained comparable levels of nicotine and tar (6). Through more intensive puffing regimens, smokers could alter their smoking behaviors to achieve desired amounts of nicotine, while increasing exposure to other harmful non-nicotine cigarette constituents (7). Because reduced nicotine content (RNC) cigarettes contain a limited amount of nicotine, presumably these products would not sustain compensatory behaviors or increase harm exposure.
Accruing evidence (8–15) suggests that RNC cigarette use generally decreases toxicant exposure and nicotine dependence without causing long-term compensatory smoking (16), and further, may possibly facilitate cessation. Most recently, Donny and colleagues (15) conducted the first large-scale, double-blind, randomized controlled trial of RNC cigarettes, using a between-subjects design to assign smokers to use one of six types of RNC research cigarettes for six weeks. Those assigned to the lowest RNC cigarettes smoked fewer cigarettes/day and had lower total nicotine equivalents after six weeks compared to those assigned to own brand or moderate nicotine content cigarettes; however, participants’ daily cigarette consumption after six weeks of using very low nicotine content cigarettes did not decrease significantly from baseline when using participants’ own brand. While these findings, along with those of other, smaller RNC cigarette studies, are critically important in demonstrating that these products do not appear to increase harm, further research is required to more thoroughly assess the public health impact of RNC cigarettes. For example, brief use periods (10,14) and assessing behavior only through CPD (8,9), or with a single topography assessment (11), do not sufficiently capture the complexities and individual differences in smoking behavior. Further, because variations in smoking behavior can differentially affect exposure to carcinogenic cigarette constituents (17), a rigorous panel of biomarkers is needed to thoroughly characterize harm exposure (18).
The purpose of the present study was to investigate the effects of using progressively decreasing RNC cigarettes on smoking behavior and harm exposure measures. A secondary aim was to investigate these effects on subjective ratings, and to further explore associations of subjective ratings with use behaviors at each RNC cigarette level. This study aims to advance knowledge on RNC use and exposure and provide data addressing existing gaps through: 1) the use of multiple measures of smoking behavior including smoking topography, an objective measure of puffing behavior (19); 2) the use of a comprehensive panel of harm exposure measures; 3) a baseline period of own brand smoking to properly characterize individual differences; 4) the inclusion of a control group; and 5) utilizing a within-subject design to account for individual differences (e.g., nicotine dependence, sex, metabolism rate, brand preference) shown to affect both behavioral and biomarker outcomes.
Materials and Methods
Participants
Participants were recruited from October 2007 through February 2013 from the Philadelphia area through digital and print media by completing a telephone eligibility interview. Those eligible were ≥21 years of age, reported smoking ≥15 cigarettes per day (CPD) for ≥5 years, exclusively smoked filtered, non-menthol cigarettes, and were not currently trying/had no plans to quit in the next 2 months. Exclusion criteria were: consuming ≥25 alcohol-containing drinks per week, current use of nicotine replacement therapy or other non-cigarette nicotine-containing products, substance use disorders in the last 5 years, current or previous history of psychiatric disorders other than depression, past year myocardial infarction, current smoking of marijuana, pregnancy, and providing an initial carbon monoxide (CO) reading <10 ppm.
Procedure
The 35-day randomized, unblinded, single-site, parallel design laboratory study consisted of four periods: a 5-day period in which all participants smoked their own brand to establish baseline measures, followed by 3 successive 10-day periods in which the experimental group smoked 0.6 mg nicotine Quest® 1 brand cigarettes, then 0.3 mg nicotine Quest® 2 brand cigarettes, then 0.05 mg nicotine Quest® 3 brand cigarettes (Vector Tobacco Inc., Durham, NC). FTC-determined yields were 0.6, 0.3, and 0.05 mg, respectively; rod nicotine content varies based on assessment methods, but was estimated to be 8.9, 5.1–8.4, and 0.48–1.5 mg nicotine per cigarette, respectively (11,20–22). The control group smoked their own brand across all periods.
Eligible participants scheduled an initial in-person laboratory visit at the University of Pennsylvania to provide written informed consent and confirm eligibility. Laboratory visits occurred every 5 days, and participants were randomized in a 1:1 ratio by an independent database assignment tracking program. During each visit participants smoked three cigarettes interspersed by 45 minutes; the first to standardize time since last cigarette, the second and third cigarettes smoked through a topography device to capture participants’ smoking behaviors. On Days 5, 15, and 25, prior to smoking the session’s last cigarette, the experimental group was switched to the next progressively lower RNC cigarette. Participants supplied CO samples before and after smoking, and provided subjective ratings following smoking each cigarette. Throughout the study, participants recorded CPD in a daily diary, and collected corresponding spent cigarette filters in a date-labeled resealable plastic bag to assist in tracking daily smoking rate. Research staff reconciled participants’ used filters and unused cigarettes at each visit with amount of product distributed, and with consumption during the previous laboratory visit/period; participants were asked to elaborate on discrepancies. At the end of each period, participants provided urine samples to be analyzed for biomarkers.
All sessions were scheduled between 8:00 AM and 12:00 PM. Each participant’s sessions commenced at the same time of day within 1 hour to minimize diurnal variation effects on primary outcomes. Study completers received $505 compensation. All procedures were approved by the university Institutional Review Board. This study was registered in accordance with the guidelines of the International Committee of Medical Journal Editors (ClinicalTrials.gov Identifier: NCT01202942).
Cigarettes
All cigarettes post-Day 5 were provided free of charge. Participants received 25% more cigarettes than their self-reported CPD to allow for compensatory smoking by increasing daily consumption and to ensure that product supply would not be depleted if the next session was delayed (maximum of 1 day). Research staff explained to participants that study participation indicated their agreement to smoke only study-supplied cigarettes. Participants were explicitly instructed that they were not required to consume all product, and that using non-study-supplied cigarettes would result in their removal. Staff assessed noncompliance at each visit by asking participants about non-study-supplied cigarette use and through counting returned spent filters and unused study-supplied cigarettes; those who reported using non-study-supplied cigarettes at any two visits post-Day 5 were removed (n = 3, see CONSORT Diagram in Figure 1). Participants received incentives based on returning used and unused cigarettes that were equal with amount distributed.
Figure 1.
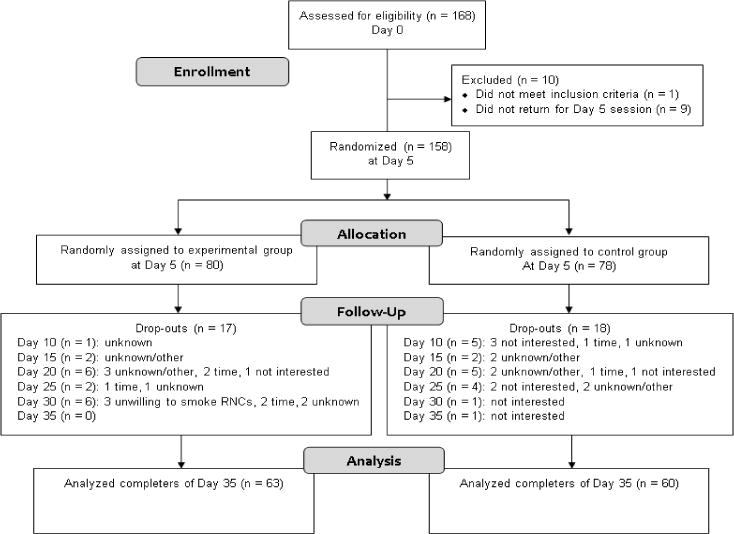
CONSORT flow diagram depicting study recruitment and retention.
Measures
Primary smoking behaviors were self-reported CPD and total puff volume; secondary behavioral measures were: mean puff volume, puff count, puff duration, interpuff interval, and peak velocity. In addition to recording CPD, participants collected all spent filters for each study day, increasing accuracy of daily consumption (23); r for self-reported CPD and filters = 0.98, P < 0.001, mean difference = 0.21 CPD, 95% CI = 0.15–0.27. Topography measures were obtained using the Clinical Research Support System (CReSS) smoking topography device (Borgwaldt KC, Richmond, VA); methodologies for data collection and processing were identical to those used in previous studies (23–26).
Biomarkers of exposure were urinary-derived measures previously used to assess tobacco and carcinogen exposure (18,27–29), including: nicotine and cotinine, the primary metabolite of nicotine; 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol (NNAL), a metabolite of the tobacco-specific lung carcinogen 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK); and 1-hydroxypyrene (1-HOP), a metabolite of pyrene and proxy for polycyclic aromatic hydrocarbon exposure. Urine samples were assayed at the University of Minnesota using standard procedures (29–31). Breath carbon monoxide (CO) was assessed at the onset of each session as a measure of daily smoke exposure, and before and after each cigarette smoked; the difference was defined as CO boost, a measure of smoke exposure from a single cigarette (24,25,32).
Subjective ratings were assessed using a 14-item cigarette characteristic rating scale (23–25,32). After smoking each cigarette, participants placed a vertical line along a 100 mm visual analog scale to indicate their rating for a certain feature (e.g., strength); anchors were item-specific (e.g., strength: 0=“Very weak”, 100=“Very strong”) with lower scores indicating more negative ratings.
Statistical Analysis
Baseline demographic and smoking variables were compared for study completers vs. non-completers, and for experimental vs. control groups. Categorical and continuous variables were analyzed using χ2 tests of independence and unpaired t-tests, respectively.
Several outcome measures were assessed repeatedly within each period to minimize the likelihood of data being compromised by an unexpected event or confound. Composite scores were the mean of all values obtained per period. To ensure that averaging outcomes within periods did not alter results (e.g., compensation occurring upon but abating after initial exposure to a specific nicotine content), we examined the effect of time on outcomes within each period; because there was no time effect for any outcome, these results are not presented.
Creatinine-adjusted values for biomarkers were used to correct for urinary dilution (33,34). A natural log transformation was applied to values to meet normality and variance assumptions.
Primary outcomes were analyzed using linear mixed-effects regression models to account for correlated observations within individuals. Each model used an unstructured covariance structure and contained three fixed effect terms: period, group, and period × group interaction. Least square means and 95% confidence intervals (CI) were estimated for outcomes during each period. Reported P-values for all pair-wise comparisons conducted to follow-up significant interaction effects of group × period on each primary outcome were adjusted using a Bonferroni correction. A priori power analyses determined that sample of 140 participants (70 experimental, 70 control) would sufficiently detect differences at the P = 0.01 level with 80% power.
Pearson correlations were used to explore associations of subjective ratings with smoking behaviors during each study period among the experimental group.
Analyses were conducted using IBM-SPSS Statistics-v23 using two-tailed significance tests at the P < 0.05 level. Data are presented for the 123 participants who completed the entire study.
Results
Sample Characteristics
168 participants provided informed consent; 158 completed the 5-day baseline period and were randomized to the control (n = 78) or experimental group (n = 80). 123 participants (60 control, 63 experimental) completed the entire study (CONSORT Diagram; Figure 1).
On average, participants (65.0% male) were 40.68 years old (SD = 12.72; range = 21–64), smoked 20.81 CPD (SD = 5.57; 12*–40), smoked regularly for 24.01 years (SD = 12.84; 5–50), and were moderately nicotine dependent (M = 5.61; SD = 1.88; 1–9) as assessed by the Fagerström Test of Nicotine Dependence (35). The sample was predominately White (89.4%); ethnicity, irrespective of race, was non-Hispanic (96.7%).
Experimental and control groups did not differ on baseline demographic or smoking variables. Compared to study completers, non-completers were significantly younger (M ± SD = 31.83 ± 9.50; range = 21–56), t(72.35) = 4.49, P < 0.001, and smoked regularly for fewer years (16.43 ± 10.02; 5–41), t(69.02) = 3.70, P < 0.001.
Effects on Smoking Behaviors
There was a significant group × period interaction [F(3, 123.13) = 7.87, P < 0.001] effect on average CPD. Average cigarette consumption differed significantly by period among both the experimental [F(3, 122.79) = 24.31, P < 0.001] and control groups [F(3, 123.55) = 5.78, P = 0.001] (Figure 2A). Among the experimental group, relative to baseline, consumption significantly increased by 3.31 (95% CI = 1.97–4.66) and 4.85 (3.02–6.87) CPD during the 0.6 mg and 0.3 mg periods (P’s < 0.001), respectively, and was similar to baseline levels (mean increase = 1.22 CPD; 95% CI = −0.84–3.27) during the 0.05 mg period (P = 0.686). Full pairwise comparisons for the experimental group are indicated in Table 1. The control group smoked 1.69 (0.30–3.08, P = 0.009), 2.61 (0.73–4.50; P = 0.002), and 2.98 (0.86–5.11; P = 0.002) significantly more CPD than baseline during each subsequent period. The experimental group smoked significantly more CPD than controls during the 0.6 mg and 0.3 mg periods (P’s = 0.044 and 0.019, respectively).
Figure 2.
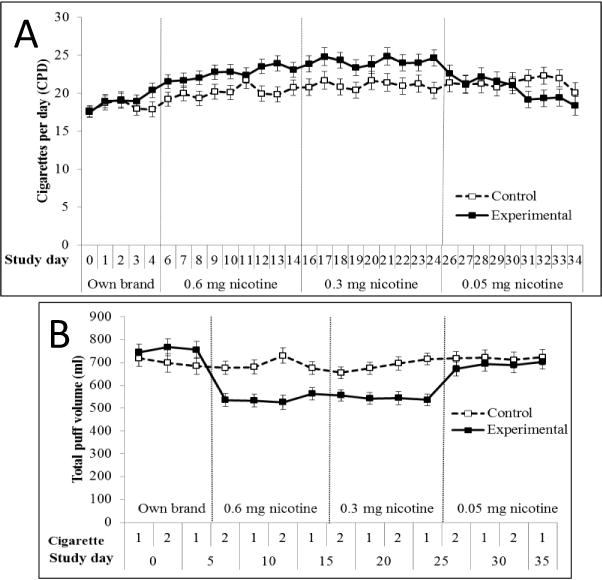
Average daily cigarette consumption (A) and total puff volume (B) throughout study in smokers using cigarettes of progressively reduced nicotine content (experimental group) vs. own brand (control group). Product switching days (i.e., Days 5, 15, and 25) are not depicted for 2A. Among the experimental group, daily cigarette consumption was significantly greater during the 0.6 and 0.3 mg nicotine cigarette periods compared to baseline and 0.05 mg nicotine cigarette periods. Total puff volume was significantly lower than baseline during all subsequent periods; total volume during the 0.05 mg nicotine cigarette period was significantly greater than during the 0.6 and 0.3 mg nicotine periods. Among the control group, daily cigarette consumption was significantly greater than baseline during all subsequent periods. There was no change in total puff volume across periods among controls. The experimental group smoked more cigarettes per day than controls during the 0.6 and 0.3 mg nicotine cigarette periods, and had lower total puff volume than controls during all periods following baseline.
Table 1.
Outcome measures during each study period among the experimental group.
| Measures | Own brand | Quest® 1 (0.6 mg) | Quest® 2 (0.3 mg) | Quest® 3 (0.05 mg) |
|---|---|---|---|---|
| Smoking behaviors | ||||
| Cigarettes per daya,b,d,e,f | 19.33 (17.68–20.98) | 22.64 (20.93–24.36) | 24.18 (22.35–26.01) | 20.55 (18.53–22.58) |
| Total puff volume (ml)a,b,c,e,f | 756.01 (690.68–821.34) | 540.96 (489.42–592.51) | 545.10 (499.34–590.86) | 691.12 (632.34–749.91) |
| Mean puff volume (ml)a,b,c,e,f | 56.46 (52.45–60.47) | 52.21 (48.13–56.30) | 52.64 (48.91–56.37) | 61.13 (57.18–65.08) |
| Puff counta,b,c,e,f | 13.75 (12.63–14.88) | 10.84 (9.87–11.81) | 10.92 (9.92–11.91) | 11.75 (10.63–12.88) |
| Puff duration (sec)a,c,d,e,f | 1.76 (1.63–1.89) | 1.66 (1.53–1.79) | 1.75 (1.61–1.88) | 1.92 (1.79–2.05) |
| Interpuff interval (sec) | 24.37 (21.34–27.40) | 20.91 (18.36–23.46) | 19.05 (16.51–21.58) | 17.48 (15.04–19.91) |
| Maximum velocity (ml/sec)b,f | 49.47 (45.87–53.10) | 47.28 (43.47–51.08) | 45.63 (42.09–49.17) | 49.56 (45.57–53.55) |
|
| ||||
| Biomarkers | ||||
| Session onset CO (ppm)b,d,f | 27.33 (24.49–30.16) | 28.60 (25.78–31.43) | 31.94 (29.05–34.84) | 27.02 (24.11–29.94) |
| CO boost (ppm)b,c,d,e,f | 5.79 (5.12–6.46) | 6.10 (5.51–6.68) | 6.85 (6.23–7.46) | 4.88 (4.29–5.46) |
| Cotinine (ng/mg creatinine)b,c,d,e,f | 3229.23 (2670.44–3904.95) | 2807.36 (2344.90–3428.92) | 2059.05 (1635.98–2565.73) | 1130.03 (871.31–1465.57) |
| Nicotine (ng/mg creatinine)b,c,d,e,f | 1398.28 (1050.48–1861.24) | 1365.12 (1033.80–1802.63) | 814.85 (591.11–1124.39) | 475.33 (327.34–689.52) |
| NNAL (pmol/mg creatinine)a,b,c,e,f | 1.12 (0.92–1.36) | 0.84 (0.69–1.03) | 0.79 (0.64–0.97) | 0.63 (0.51–0.76) |
| 1-HOP (ng/mg creatinine) | 0.29 (0.25–0.34) | 0.34 (0.29–0.40) | 0.31 (0.26–0.37) | 0.28 (0.24–0.33) |
|
| ||||
| Subjective Ratings (0–100) | ||||
| Strengtha,b,c,d,e,f | 59.95 (56.03–63.88) | 46.43 (42.49–50.36) | 38.26 (34.35–42.17) | 28.79 (24.07–33.51) |
| Harshnessa,b,c,d,e | 48.94 (44.37–53.52) | 40.44 (36.00–44.89) | 35.12 (30.71–39.53) | 33.89 (28.90–38.88) |
| Heat | 34.74 (29.67–39.80) | 37.59 (32.50–42.68) | 38.86 (33.65–44.06) | 36.66 (30.66–42.65) |
| Draw | 32.98 (28.04–37.93) | 27.99 (23.73–32.26) | 25.90 (21.38–33.93) | 28.41 (22.89–33.93) |
| Taste (bad/good)b,c,d,e,f | 56.89 (52.42–61.37) | 51.64 (47.12–56.17) | 47.93 (43.39–52.47) | 38.31 (33.28–43.34) |
| Satisfaction from smokinga,b,c,d,e,f | 61.98 (57.13–66.83) | 53.73 (48.80–58.65) | 46.36 (41.01–51.71) | 31.78 (26.23–37.32) |
| Burn ratea,b,c,d | 56.81 (51.25–62.37) | 31.49 (26.30–36.68) | 27.06 (21.93–32.18) | 26.17 (20.55–31.78) |
| Taste (mild/not mild)a,b,c | 49.75 (44.82–54.68) | 38.25 (33.35–43.14) | 35.17 (30.06–40.28) | 35.52 (30.15–40.89) |
| Mildness (too mild/not too mild)a,b,c,d,e,f | 67.42 (62.68–72.16) | 53.63 (48.06–59.20) | 45.05 (39.41–50.69) | 37.73 (31.59–43.88) |
| Smoke harshness | 56.60 (51.35–61.85) | 59.18 (54.27–64.10) | 57.93 (52.61–63.25) | 52.65 (47.10–58.20) |
| Aftertastec,e,f | 45.80 (40.42–51.19) | 45.59 (40.59–50.59) | 44.50 (39.25–49.75) | 36.09 (30.61–41.57) |
| Stalenessc,e,f | 66.77 (60.81–72.73) | 63.95 (58.40–69.50) | 61.68 (55.83–67.53) | 52.01 (45.75–58.28) |
| Strength of smokea,b,c,d,e,f | 57.46 (53.51–61.40) | 45.76 (41.79–49.73) | 38.40 (34.30–42.49) | 32.25 (27.41–37.09) |
| Smoke smellc,e,f | 52.07 (46.65–57.49) | 51.75 (46.45–57.06) | 50.34 (44.57–56.11) | 42.29 (36.34–48.23) |
Data are presented as arithmetic mean (95% CI) for all outcomes except cotinine, nicotine, NNAL, and 1-HOP; these data are presented as geometric mean (95% CI). Superscript letters indicate significant Bonferroni-adjusted post-hoc comparisons at the P = 0.05 level;
= comparison between own brand and 0.6 mg periods;
= own brand and 0.3 mg;
= own brand and 0.05 mg;
= 0.6 and 0.3 mg;
= 0.6 and 0.05 mg;
= 0.3 and 0.05 mg.
Significant group × period interactions were found for total puff volume [F(3, 122) = 12.58, P < 0.001], mean puff volume [F(3, 122) = 13.22, P < 0.001], puff duration [F(3, 122) = 15.15, P < 0.001], puff number [F(3, 122) = 24.37, P < 0.001], and peak velocity [F(3, 122) = 3.91, P = 0.01], but not interpuff interval [F(3, 122) = 2.49, P = 0.063]. Topography measures differed by period within the experimental group (all P’s < 0.001; Table 1), but only puff number varied by period among controls (P = 0.004). Within the experimental group, total puff volume was significantly lower than baseline during all subsequent periods (P’s < 0.001–0.047; Figure 2B), although total volume during the 0.05 mg period was significantly greater than the 0.6 and 0.3 mg periods (P’s < 0.001). Fewer puffs were taken of all research cigarettes compared to baseline (P’s < 0.001); puff count was significantly greater during the 0.05 compared to 0.6 and 0.3 mg periods (P’s = 0.009 and 0.001, respectively). Compared to baseline, mean puff volume, duration, and peak velocity decreased during the 0.6 and 0.3 mg periods and increased during the 0.05 mg period; changes reaching statistical significance are indicated in Table 1. Among controls, puff count was greater during the final study period compared to baseline (P = 0.003). Compared to controls, the experimental group had lower total puff volume during the 0.6 and 0.3 mg periods (P’s < 0.001), lower puff count during all periods except baseline (P’s < 0.001–0.002), and greater mean puff volume and duration during the 0.05 mg period (P’s = 0.002 and 0.01).
Effects on Biomarkers of Exposure
Significant group × period interaction effects were found for CO boost [F(3, 123) = 15.43, P < 0.001] and session onset CO [F(3, 123) = 5.09, P = 0.002]. Both outcomes varied significantly by study period only among the experimental group (P’s < 0.001; Table 1). Compared to baseline, average CO boost among the experimental group did not change during the 0.6 mg period (change = 0.31 ppm; −0.45–1.06; P = 1.00), increased significantly by 1.05 ppm (0.32–1.79; P = 0.001) during the 0.3 mg period, and decreased significantly by 0.92 ppm (0.20–1.63; P = 0.005) during the 0.05 mg period (Figure 3A). Session onset CO increased during the 0.6 and 0.3 mg periods, and decreased during the 0.05 mg period; however, only the increase during the 0.3 mg period reached statistical significance (P < 0.001). The experimental group had significantly greater session onset CO and boost than the control group during the 0.3 mg period only (P’s ≤ 0.001).
Figure 3.
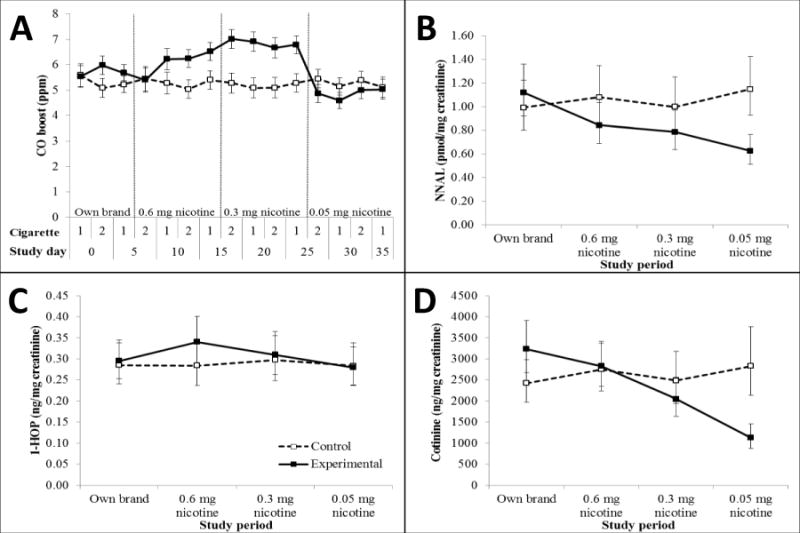
Carbon monoxide (CO) boost following each topography session (A), NNAL (B), 1-HOP (C), and cotinine (D) throughout study in smokers using cigarettes of progressively reduced nicotine content (experimental group) vs. own brand (control group). Data for urinary biomarkers are depicted as geometric means and 95% confidence intervals. Among the experimental group, CO boost was significantly greater during the 0.3 mg nicotine cigarette period compared to all other periods and to controls. CO boost was significantly lower during the 0.05 mg nicotine cigarette period relative to all previous periods. Cotinine and nicotine decreased progressively from baseline with each study period, although decreases from baseline were non-significant during the 0.6 mg nicotine period. NNAL levels were significantly lower than baseline during all subsequent periods, and during the 0.05 mg period compared to the 0.6 and 0.3 mg nicotine periods. Compared to controls, cotinine levels in the experimental group were significantly lower at baseline, and cotinine, nicotine, and NNAL levels were significantly lower during the 0.05 mg period. 1-HOP was similar across study periods and groups.
There was a significant interaction of group × period on cotinine [F(3, 104.82) = 18.75, P < 0.001), nicotine [F(3, 102.32 = 11.42, P < 0.001], and NNAL [F(3, 101.14) = 12.68, P < 0.001], but not 1-HOP [F(3, 105.22 = 0.97, P = 0.41] which was similar across study periods and groups (Figures 3B–D). Cotinine, nicotine, and NNAL were stable throughout for the control group (P’s > 0.2), and decreased from baseline with each study period for the experimental group (P’s ≤ 0.001); decreases were non-significant for 0.6 mg cotinine and nicotine (P’s > 0.3). NNAL levels were significantly lower than baseline during all subsequent periods (P’s < 0.001), and during the 0.05 mg period compared to the 0.6 and 0.3 mg periods (P’s = 0.004 and 0.02). Compared to controls, cotinine levels in the experimental group were significantly lower at baseline (P = 0.044), and cotinine, nicotine, and NNAL levels were significantly lower during the 0.05 mg period (P’s < 0.001).
Effects on Subjective Ratings
Significant interactions of group × period were found for all subjective rating items (P’s ≤ 0.001) except “heat”, “draw”, and “harshness of smoke.” Ratings differed by period for the experimental group only (P’s < 0.001; Table 1). Compared to baseline (own brand) ratings, the experimental group rated all research cigarettes more negatively on the “harshness”, “burn rate”, and “taste (mild/not mild)” items (P’s ≤ 0.001). Significant dose-response associations were observed for the “strength”, “satisfaction from smoking”, “strength of smoke”, and “too mild” items (P’s < 0.001–0.004), with the 0.05 mg and own brand cigarettes receiving the lowest and highest ratings, respectively.
Exploratory Associations of Subjective Ratings and Use Behaviors
Exploratory correlation analyses revealed few, modest associations between subjective rating items and smoking behaviors (r’s = 0.25–0.44, P’s ≤ 0.001–0.049). These associations varied based on nicotine content period, and thus did not produce a coherent trend that would help to better understand intensity of RNC product use. For example, “strength”, “harshness”, “heat”, and “taste (mildness)” ratings were negatively associated with interpuff interval (r’s = −0.39 to −0.28, P’s = 0.002–0.026) during the 0.6 mg and 0.3 mg nicotine periods, but were uncorrelated during the 0.05 mg nicotine period. No other subjective rating items were associated with use behaviors consistently across multiple nicotine periods.
Discussion
Study results illustrate the intricacies involved in understanding the effect of RNC cigarette use on smoking behavior and harm exposure. At moderate nicotine content, smokers increased daily cigarette consumption but puffed less intensely; at very low nicotine content, smokers’ CPD and total puff volume were similar to use of their own cigarette brand. Harm exposure findings were equally complex: NNAL and cotinine decreased with cigarette nicotine content, but RNC period had no effect on 1-HOP, consistent with other studies (8,9,11–13,36). Further, CO boost increased at moderate nicotine content, but decreased during very low nicotine content. Given the complex pattern of results, this study demonstrates the importance of utilizing rigorous behavioral and biological measures to fully elucidate the impact of RNC cigarette smoking, as using cigarettes with very low nicotine content does not ubiquitously lead to reductions in smoking behaviors or harm exposure.
Despite observing increased daily consumption during use of moderate nicotine RNC cigarettes, we observed declines in cotinine and NNAL. Although these findings could appear contradictory, they are consistent with previous studies (8,9). Findings suggest that reducing cigarette nicotine content is sufficient for decreasing exposure to certain toxic nicotine-derived combustion byproducts, even if participants increase their overall cigarette consumption. We also observed decreased puffing behavior, yet increased CO boost, during use of moderate RNC cigarettes. Although we would expect these behaviors to demonstrate the same directional relations with RNC use, previous studies have also found disparate associations (15,25). CO boost is a measure of one gaseous byproduct of cigarette use and does not represent all exposure. The Quest and other RNC cigarettes (e.g., Spectrums) have limited design and constituent information available, much like commercial cigarettes, so it remains unknown which constituents and additives may produce more carbon monoxide than participants’ own brand.
Study findings explicate previous work by demonstrating that very low nicotine content cigarettes generally do not increase measures of harm exposure or negative smoking behaviors, despite diverging from prior research (8,9,11) regarding behavioral results at moderate nicotine content. Such discrepancies may be due to study differences in assessment of cigarette consumption (e.g., using spent filters to complement self-report may have increased accuracy over daily dairy methods prone to recall bias), or length of exposure to each nicotine level (e.g., 7-day (11), 1-week (8), and 1-month (9) periods). Topography results also differed from a study by Hammond and O’Connor, which found no difference between RNC and own brand cigarettes on total or mean puff volume during the single topography assessment (11). Because the present study collected four topography assessments on three days throughout each 10-day nicotine content period, findings may better capture RNC effects on smoking behaviors; intraclass correlation (ICC) coefficients for the 0.6, 0.3, and 0.05 mg nicotine periods were 0.88, 0.87, and 0.90, respectively, indicating excellent agreement (ICC’s > 0.75). These differences are noteworthy, as many previous studies have shown that compensation via increased cigarette consumption does not occur with RNC cigarette use. The current study provides evidence that smoking behavior patterns may adjust and should be fully evaluated as nicotine content changes.
Subjective measure results were largely consistent with other blinded and open-label studies (8–10,14,25); Quest cigarettes generally received more negative subjective ratings than own brand. Where ratings differed among Quest cigarettes, the lowest RNC cigarettes were rated most negatively. These findings may indicate potential difficulties with consumer acceptance of RNCs should a nicotine reduction policy be implemented. However, additional research is needed to determine the extent to which subjective ratings predict consumer response to RNCs as exploratory analyses found few, modest associations between subjective ratings and use which varied by nicotine content.
Although findings provide important information regarding nicotine regulation, some caveats must be acknowledged. First, similar to Donny and colleagues (15), providing participants with free cigarettes is not representative of how smokers would obtain these products if commercially available, and may have increased daily cigarette consumption. However, similar methodology of providing free medication in clinical pharmacotherapy studies is standard practice, and providing cigarettes at no cost minimizes the influence of socioeconomic factors on purchasing premium cigarette brands. Moreover, the ability to contrast results in the experimental group against a control group mitigates much of these considerations. Second, the study sample consisted of heavy (i.e., ≥ 15 CPD), non-treatment-seeking smokers, selected intentionally because this group presumably may experience difficulty adjusting to a nicotine reduction policy. Because this group comprises only one segment of the smoking population, further restricted to include only healthy, non-menthol, non-psychiatric smokers recruited from a single large city, findings are not representative of the impact of RNC cigarettes among all smokers. Additionally, it is possible that increased RNC cigarette consumption resulted from participants’ sharing experimental cigarettes with other smokers. Also, we focused on RNC cigarette effects on puffing behavior simulating real world smoking conditions, and did not assess effects following tobacco abstinence. Further, although our collection of multiple topography assessments per study period represents a significant improvement over earlier studies, additional assessments may be necessary to capture behavioral adaptation to reduced nicotine content and explain biochemical findings. We also could not verify noncompliance with the RNC cigarettes beyond participants’ self-report and spent filter discrepancies, and thus cannot determine the impact of other cigarette use on study outcomes. Finally, Quest cigarettes may vary from participants’ own brand in unknown ways that cannot be fully accounted for without a full product and manufacturing disclosure; such variations may have independently affected study outcomes.
Results from this study are an important addition to understanding the impact nicotine regulation in cigarettes may have on public health. These findings provide support that at very low nicotine levels, negative smoking behaviors do not increase, and exposure to nicotine and many toxic cigarette constituents likely decrease. Donny and colleagues’ recent RCT of RNC cigarettes (15) demonstrated that smokers assigned to use very low nicotine cigarettes for six weeks had lower daily cigarette consumption and total nicotine equivalents compared to those assigned to own brand or moderate nicotine content cigarettes, although daily consumption of very low nicotine content cigarettes was not lower than daily consumption of own brand at baseline. Despite differences in design, sample sizes and composition, types of RNC cigarettes used and lengths of exposure, the present study also found that use of very low nicotine cigarettes decreased daily consumption, as well as nicotine and toxicant exposure, relative to use of moderate nicotine content cigarettes; further, cigarette consumption during use of the lowest nicotine content cigarettes was not statistically different from consumption during baseline use of own brand.
In summary, the present study findings complement those of Donny and colleagues (15) by providing further support of reduced smoking and exposure with very low nicotine content cigarettes, in the context of using a commercially available product. We chose the Quest cigarettes specifically for the present study to simulate how smokers would use commercially available – and marketed as – RNC cigarettes. Study cigarettes were marketed by the manufacturer using packaging and advertising strategies previously developed to increase consumer appeal, e.g., blue color, progressively numbered (ordinal and descriptive labeling) according to nicotine content. Thus, the results of the present study converge with those of Donny and colleagues, while also providing novel information on how smokers may use RNC cigarettes when commercially available and marketed to explicitly encourage reducing nicotine consumption. Future research is needed to better understand how marketing, labeling, and packaging of RNC cigarettes may alter perceptions and subsequent use patterns, as the tobacco industry will presumably manipulate these domains to promote cigarette use should nicotine content be regulated.
Acknowledgments
Financial support: This work was supported by R01 CA120594 to A. A. Strasser from the National Institutes of Health; M. Mercincavage’s salary was fully funded from a training core under P50 CA179546 from the National Institutes of Health and FDA Center for Tobacco Products (CTP). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH or the FDA.
Abbreviations
- 1-HOP
1-hydroxyprene
- CO
carbon monoxide
- CPD
cigarettes per day
- NNAL
4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol
- RNC
reduced nicotine content
Footnotes
Disclosure of potential conflicts of interest: None.
Reported range refers to daily consumption in the week preceding study enrollment rather than CPD reported during telephone eligibility interview. Five telephone-eligible participants reported smoking < 15 CPD at the in-person screening session. All smoked ≥ 15 CPD during the baseline period except two participants, who engaged in variable smoking behaviors. Overall results of primary analyses were unchanged when excluding these participants.
References
- 1.U.S. Department of Health and Human Services. The health consequences of smoking – 50 years of progress: a report of the Surgeon General. Atlanta, GA: Government Printing Office; 2014. [Google Scholar]
- 2.U.S. Department of Health and Human Services. The health consequences of smoking: nicotine addiction. A report of the Surgeon General. Washington, DC: Government Printing Office; 1988. [Google Scholar]
- 3.Benowitz NL, Henningfield JE. Establishing a nicotine threshold for addiction: the implications for tobacco regulation. N Engl J Med. 1994;331:123–5. doi: 10.1056/NEJM199407143310212. [DOI] [PubMed] [Google Scholar]
- 4.U.S. Congress. Family smoking prevention and tobacco control federal reform act. 111–31. 2009 [Google Scholar]
- 5.U.S. Department of Health and Human Services. Risks associated with smoking cigarettes with low machine-measured yields of tar and nicotine. Bethesda, MD: Public Health Service, National Institutes of Health, National Cancer Institute; 2001. Report No.: 13. [Google Scholar]
- 6.Kozlowski LT, O’Connor RJ. Cigarette filter ventilation is a defective design because of misleading taste, bigger puffs, and blocked vents. Tob Control. 2002;11:i40–50. doi: 10.1136/tc.11.suppl_1.i40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Benowitz NL. Risks associated with smoking cigarettes with low machine yields of tar and nicotine. National Cancer Institute; 2001. Compensatory smoking of low-yield cigarettes; pp. 39–63. [cited 2015 Jun 30] Available from: http://cancercontrol.cancer.gov/Brp/tcrb/monographs/13/m13_3.pdf. [Google Scholar]
- 8.Benowitz NL, Hall SM, Stewart S, Wilson M, Dempsey D, Jacob P. Nicotine and carcinogen exposure with smoking of progressively reduced nicotine content cigarette. Cancer Epidemiol Biomarkers Prev. 2007;16:2479–85. doi: 10.1158/1055-9965.EPI-07-0393. [DOI] [PubMed] [Google Scholar]
- 9.Benowitz NL, Dains KM, Hall SM, Stewart S, Wilson M, Dempsey D, et al. Smoking behavior and exposure to tobacco toxicants during 6 months of smoking progressively reduced nicotine content cigarettes. Cancer Epidemiol Biomarkers Prev. 2012;21:761–9. doi: 10.1158/1055-9965.EPI-11-0644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Benowitz NL, Jacob P, Herrera B. Nicotine intake and dose response when smoking reduced–nicotine content cigarettes. Clin Pharmacol Ther. 2006;80:703–14. doi: 10.1016/j.clpt.2006.09.007. [DOI] [PubMed] [Google Scholar]
- 11.Hammond D, O’Connor RJ. Reduced nicotine cigarettes: smoking behavior and biomarkers of exposure among smokers not intending to quit. Cancer Epidemiol Biomarkers Prev. 2014;23:2032–40. doi: 10.1158/1055-9965.EPI-13-0957. [DOI] [PubMed] [Google Scholar]
- 12.Hatsukami DK, Lemmonds C, Zhang Y, Murphy SE, Le C, Carmella SG, et al. Evaluation of carcinogen exposure in people who used “reduced exposure” tobacco products. JNCI J Natl Cancer Inst. 2004;96:844–52. doi: 10.1093/jnci/djh163. [DOI] [PubMed] [Google Scholar]
- 13.Hatsukami DK, Kotlyar M, Hertsgaard LA, Zhang Y, Carmella SG, Jensen JA, et al. Reduced nicotine content cigarettes: effects on toxicant exposure, dependence and cessation. Addiction. 2010;105:343–55. doi: 10.1111/j.1360-0443.2009.02780.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hatsukami DK, Heishman SJ, Vogel RI, Denlinger RL, Roper-Batker AN, Mackowick KM, et al. Dose-response effects of Spectrum research cigarettes. Nicotine Tob Res. 2012;15:1113–21. doi: 10.1093/ntr/nts247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Donny EC, Denlinger RL, Tidey JW, Koopmeiners JS, Benowitz NL, Vandrey RG, et al. Randomized trial of reduced-nicotine standards for cigarettes. N Engl J Med. 2015;373:1340–9. doi: 10.1056/NEJMsa1502403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hatsukami DK, Donny EC, Koopmeiners JS, Benowitz NL. Compensatory smoking from gradual and immediate reduction in cigarette nicotine content. Cancer Epidemiol Biomarkers Prev. 2015;24:472–6. doi: 10.1158/1055-9965.EPI-14-0739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Harris J. Incomplete compensation does not imply reduced harm: Yields of 40 smoke toxicants per milligram nicotine in regular filter versus low-tar cigarettes in the 1999 Massachusetts Benchmark Study. Nicotine Tob Res. 2004;6:797–808. doi: 10.1080/1462220042000274266. [DOI] [PubMed] [Google Scholar]
- 18.Yuan J-M, Butler LM, Stepanov I, Hecht SS. Urinary tobacco smoke-constituent biomarkers for assessing risk of lung cancer. Cancer Res. 2014;74:401–11. doi: 10.1158/0008-5472.CAN-13-3178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee E, Malson J, Waters A, Moolchan E, Pickworth W. Smoking topography: Reliability and validity in dependent smokers. Nicotine Tob Res. 2003;5:673–9. doi: 10.1080/1462220031000158645. [DOI] [PubMed] [Google Scholar]
- 20.Becker K, Rose J, Albino A. A randomized trial of nicotine replacement therapy in combination with reduced-nicotine cigarettes for smoking cessation. Nicotine Tob Res. 2008;10:1139–48. doi: 10.1080/14622200802123294. [DOI] [PubMed] [Google Scholar]
- 21.Walker N, Howe C, Bullen C, Grigg M, Glover M, McRobbie H, et al. The combined effect of very low nicotine content cigarettes, used as an adjunct to usual Quitline care (nicotine replacement therapy and behavioural support), on smoking cessation: a randomized controlled trial: Low nicotine content cigarettes for quitting. Addiction. 2012;107:1857–67. doi: 10.1111/j.1360-0443.2012.03906.x. [DOI] [PubMed] [Google Scholar]
- 22.MacQueen DA, Heckman BW, Blank MD, Janse Van Rensburg K, Evans DE, Drobes DJ. Transient compensatory smoking in response to placebo cigarettes. Psychopharmacology. 2012;223:47–54. doi: 10.1007/s00213-012-2685-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Strasser AA, Ashare RL, Kaufman M, Tang KZ, Mesaros AC, Blair IA. The effect of menthol on cigarette smoking behaviors, biomarkers and subjective responses. Cancer Epidemiol Biomarkers Prev. 2013;22:382–9. doi: 10.1158/1055-9965.EPI-12-1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Strasser AA, Tang KZ, Sanborn PM, Zhou JY, Kozlowski LT. Behavioral filter vent blocking on the first cigarette of the day predicts which smokers of light cigarettes will increase smoke exposure from blocked vents. Exp Clin Psychopharmacol. 2009;17:405–12. doi: 10.1037/a0017649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Strasser AA, Lerman C, Sanborn PM, Pickworth WB, Feldman EA. New lower nicotine cigarettes can produce compensatory smoking and increased carbon monoxide exposure. Drug Alcohol Depend. 2007;86:294–300. doi: 10.1016/j.drugalcdep.2006.06.017. [DOI] [PubMed] [Google Scholar]
- 26.Strasser AA, Benowitz NL, Pinto A, Tang KZ, Hecht SS, Carmella SG, et al. Nicotine metabolite ratio predicts smoking topography and carcinogen biomarker level. Cancer Epidemiol Biomarkers Prev. 2011;20:234–38. doi: 10.1158/1055-9965.EPI-10-0674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hecht SS. Human urinary carcinogen metabolites: biomarkers for investigating tobacco and cancer. Carcinogenesis. 2002 Jun 1;23:907–22. doi: 10.1093/carcin/23.6.907. [DOI] [PubMed] [Google Scholar]
- 28.Hecht SS. Lung carcinogenesis by tobacco smoke. Int J Cancer. 2012;131:2724–32. doi: 10.1002/ijc.27816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hecht SS, Carmella SG, Chen M, Dor Koch JF, Miller AT, Murphy SE, et al. Quantitation of urinary metabolites of a tobacco-specific lung carcinogen after smoking cessation. Cancer Res. 1999;59:590–6. [PubMed] [Google Scholar]
- 30.Carmella SG, Han S, Fristad A, Yang Y, Hecht SS. Analysis of total 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol (NNAL) in human urine. Cancer Epidemiol Biomarkers Prev. 2003;12:1257–61. [PubMed] [Google Scholar]
- 31.Hochalter JB, Zhong Y, Han S, Carmella SG, Hecht SS. Quantitation of a Minor Enantiomer of Phenanthrene Tetraol in Human Urine: Correlations with Levels of Overall Phenanthrene Tetraol, Benzo[ a ]pyrene Tetraol, and 1-Hydroxypyrene. Chem Res Toxicol. 2011;24:262–8. doi: 10.1021/tx100391z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Strasser AA, Pickworth WB, Patterson F, Lerman C. Smoking topography predicts abstinence following treatment with nicotine replacement therapy. Cancer Epidemiol Biomarkers Prev. 2004;13:1800–4. [PubMed] [Google Scholar]
- 33.Benowitz NL, Dains KM, Dempsey D, Herrera B, Yu L, Jacob P. Urine nicotine metabolite concentrations in relation to plasma cotinine during low-level nicotine exposure. Nicotine Tob Res. 2009;11:954–60. doi: 10.1093/ntr/ntp092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Muscat JE, Liu A, Richie JP., Jr A comparison of creatinine vs. specific gravity to correct for urinary dilution of cotinine. Biomarkers. 2011;16:206–11. doi: 10.3109/1354750X.2010.538084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Heatherton TF, Kozlowski LT, Frecker RC, Fagerström KO. The Fagerström Test for Nicotine Dependence: a revision of the Fagerström Tolerance Questionnaire. Br J Addict. 1991;86:1119–27. doi: 10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- 36.Benowitz NL, Nardone N, Dains KM, Hall SM, Stewart S, Dempsey D, et al. Effect of reducing the nicotine content of cigarettes on cigarette smoking behavior and tobacco smoke toxicant exposure: 2-year follow up: Reduced nicotine content cigarettes. Addiction. 2015;110:1667–75. doi: 10.1111/add.12978. [DOI] [PMC free article] [PubMed] [Google Scholar]


