Abstract
Feline herpesvirus type 1 (FHV-1) is a common and important cause of ocular surface disease, dermatitis, respiratory disease, and potentially intraocular disease in cats. However, many antiviral drugs developed for the treatment of humans infected with herpesviruses have been used to treat cats infected with FHV-1. Translational use of drugs in this manner ideally requires methodical investigation of their in vitro efficacy against FHV-1 followed by pharmacokinetic and safety trials in normal cats. Subsequently, placebo-controlled efficacy studies in experimentally-inoculated animals should be performed followed, finally, by carefully designed and monitored clinical trials in client-owned animals. This review is intended to provide a concise review of the available literature regarding the efficacy of antiviral drugs and other compounds with proven or putative activity against FHV-1, as well as a discussion of their safety in cats.
Keywords: Nucleoside analogues, antiviral therapy, feline herpesvirus, virology, lysine, interferon
Introduction
Feline herpesvirus type 1 (FHV-1) is a common and important cause of ocular surface disease, dermatitis, respiratory disease, and potentially intraocular disease in cats.[1] However, an increasing array of drugs with antiviral efficacy against FHV-1, and an improved understanding of their mechanisms of actions, indications, and limitations has led to critical improvements in veterinarians’ ability to control herpetic syndromes. In a 1995 report of 14 client-owned cats with herpetic ocular disease treated with topically applied trifluridine, idoxuridine, or vidarabine, 43% failed to improve or worsened.[2] More recently, a similar review described 59 client-owned cats with ocular disease attributed to FHV-1 and treated orally with famciclovir.[3] Clinical improvement was noted by the treating veterinarian in 85% of cats, and by their owners in 93% of cats. Clearly, antiviral therapy for FHV-1 has come a long way in 20 years. The present article is intended to provide a concise review of the available literature regarding the efficacy of antiviral drugs and other compounds with proven or putative activity against FHV-1, as well as a discussion of their safety in cats.
Antiviral Drugs
Conceivably, antiviral drugs could target any step in the viral replicative process from viral adsorption to release from the host cell. To date, however, most effective antiviral therapies target viral proteins responsible for DNA synthesis,[4, 5] and their safety depends, in large part, on how virus-specific that disruption of DNA is. Therefore, while most antiviral drugs have some efficacy against FHV-1, their safety in cats is not readily predicted from their behavior in other hosts, and their efficacy against FHV-1 is not predicted from their efficacy against other viruses – even the closely related human herpes simplex virus type 1 (HSV-1; Table 1). In addition, there are no drugs currently approved in the USA for treatment of herpetic disease in cats. These basic virologic concepts can be used to guide prescribing of antiviral drugs in general (Box 1).
Table 1.
Relative in vitro antiviral efficacy (expressed as IC50) against FHV-1 and HSV-1 for various antiviral drugs with references in brackets below the drug abbreviation.
| IC50 |
HPMP A ([31, 97]) |
IDU ([25, 58, 98]) |
GCV ([25, 59, 99]) |
PCV ([25, 29, 31, 43, 44, 100]) |
PMEDA
P ([59, 101]) |
BDVU ([31, 58, 102]) |
TFU
([31, 33, 58, 103]) |
CDV
([25, 29, 44, 59, 104, 105]) |
VDB ([58, 103]) |
ADV
([59, 106]) |
ACV
([25, 29, 31, 33, 44, 59, 102, 107]) |
PFA ([25, 59, 102]) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
FHV-
1 (μM) |
0.23 | 5.6 (4.3- 6.8) |
8.9 (5.2- 13) |
14 (1.2-130) |
14 | 18 (5.1- 30.1) |
19 (0.67- 1350) |
19 (7.9-168) |
21 | 73 | 150 (16-111000) |
187 (140- 230) |
|
HSV-
1 (μM) |
22 | 2.1 | 0.39 | 1.5 | 6.9 | 0.3 | 0.5 | 19 | 30 | 21 | 0.8 | 68 |
Median (range) reported in vitro efficacy of various antiviral agents against feline herpesvirus (FHV-1) and herpes simplex virus (HSV-1). Antiviral efficacy is expressed as the IC50 (concentration at which viral replication is inhibited by 50%), therefore a lower IC50 equates to greater efficacy.
Abbreviations: HPMPA: (S)-9-(3-hydroxy-2-phosphonylmethoxypropyl) adenine; IDU: Idoxuridine; GCV: Ganciclovir; PCV: Penciclovir; PMEDAP: 9-(2-phosphonylmethoxyethyl)-2, 6-diaminopurine; BDVU: Bromovinyldeoxyuridine; TFU: Trifluridine; CDV: Cidofovir; VDB: Vidarabine; ADV: Adefovir; ACV: Acyclovir; PFA: Foscarnet.
Box 1. Some important general concepts about antiviral agents that may be used to guide prescribing and expectations when using these drugs.
Because viruses are less capable than are bacteria of independent function and because FHV-1 is a DNA virus, drug targets for antiviral agents are less numerous and tend to be less specific than they are for antibacterial agents.
FHV-1 is an obligate intracellular organism which replicates within the host nucleus. Therefore, antiviral agents tend to be more toxic than antibacterial agents and often are sufficiently toxic that they may only be used topically and, even then, induce notable corneoconjunctival cytotoxicity.
All antiviral agents to date are virostatic, therefore they cannot target latent virus and must be administered frequently (systemically and topically).
No antiviral drug has proven antibacterial activity.
No antibacterial drug has proven antiviral activity.
Antiviral drugs safe in humans are not necessarily safe in cats.
Antiviral drugs effective against human herpesviruses are not necessarily effective against FHV-1.
Antiviral prodrugs metabolized to their active form by humans are not predictably metabolized by cats.
Whenever a drug developed for treatment of humans infected with a herpesvirus is used to treat a cat infected with FHV-1, 2 major assumptions must be made– that the drug is efficacious against FHV-1 and is safe in cats. For these reasons methodical investigation of in vitro efficacy against FHV-1, followed by pharmacokinetic and safety trials in normal cats, subsequent placebo-controlled efficacy studies in experimentally-inoculated animals, and, finally, carefully designed and monitored clinical trials in client-owned animals are critical. The remainder of this review summarizes data from such studies.
Nucleoside or Nucleotide Analogues for Topical Administration
Idoxuridine (5-iodo-2′-doexyuridine) is a thymidine analogue developed for treatment of humans infected with HSV-1.[6] It differs from thymidine by having a single iodide substitution at position 5 on the pyrimidine ring. Following intracellular phosphorylation, it competes with thymidine for incorporation into viral DNA thus rendering the resultant virus incapable of replication. However, as a nonspecific inhibitor of DNA synthesis, idoxuridine affects any process requiring thymidine, and host cells are similarly affected. Therefore, systemic therapy is not possible, and corneal toxicity can occur.[7] Where it is not commercially available, it can be obtained from a compounding pharmacy as an ophthalmic solution (0.1%) or ointment (0.5%). In a retrospective case series of cats with ocular disease attributed to FHV-1, 0.1% idoxuridine solution was used every 4-6 hours with improvement or resolution of clinical signs in 3 cats and no improvement or worsening in 4 cats.[2]
Vidarabine (adenine arabinoside; 9-β-D-arabinofuranasyladenine) is an adenosine analogue originally developed as a cancer chemotherapeutic[8] but subsequently found to be efficacious against varicella zoster virus[9] and HSV-1.[10] Following triphosphorylation, vidarabine disrupts DNA synthesis via effects on DNA polymerase. Like idoxuridine, vidarabine is non-selective in its effect and associated with notable host toxicity – especially if administered systemically.[11] Because it affects a viral replication step different from that targeted by idoxuridine, vidarabine may be effective in patients whose disease appears resistant to idoxuridine.[12] As a 3% ophthalmic ointment, vidarabine often appears to be better tolerated than many of the antiviral solutions including idoxuridine.[13] Where it is not available commercially, it can be obtained from a compounding pharmacist. In a retrospective case series of cats with ocular disease attributed to FHV-1, 3% vidarabine ointment was used every 4 to 6 hours with improvement noted in 1 cat and no improvement or worsening noted in 2 cats.[2]
Trifluridine (trifluorothymidine; 5-trifluoromethyl-2′-deoxyuridine; Viroptic®) is a fluorinated nucleoside analogue of thymidine. Its specific mechanism of action against HSV-1 is not completely understood and has not been reported in FHV-1. Following intracellular phosphorylation, it reduces DNA synthesis via inhibition of thymidilate synthetase. It is too toxic to be administered systemically but topically administered trifluridine is very effective at treating HSV-1 keratitis.[13] This is in part due to its superior corneal epithelial penetration in comparison to idoxuridine and vidarabine.[14] It is formulated as a 1% ophthalmic solution; however it frequently causes marked ocular irritation in cats. In a retrospective case series of cats with ocular disease attributed to FHV-1, 1% trifluridine solution was used every 4-8 hours with improvement in 1 cat and no improvement or worsening in 2 cats.[2]
Cidofovir (HPMPC; (S)-1-[3-hydroxy-2-(phosphonomethoxy)propyl]cytosine; Vistide®) is a cytosine analogue requiring 2 host-mediated but no virally-mediated phosphorylation steps.[15] Its safety arises from its relatively high affinity for viral DNA polymerase compared with human DNA polymerase.[16] Injectable cidofovir is administered intravenously or intravitreally to humans infected with herpesviruses; principally cytomegalovirus.[17, 18] Cidofovir applied as a 0.5% or 1.0% ophthalmic solution in rabbit models of human herpetic keratoconjunctivitis was equally effective when administered only twice daily as trifluridine administered 4-9 times daily,[19, 20] presumably due to the long tissue half-lives of cidofovir’s metabolites.[21] In a prospective, masked placebo-controlled study, a 0.5% ophthalmic solution of cidofovir compounded in methylcellulose and applied twice daily to cats experimentally infected with FHV-1 reduced viral shedding and clinical disease.[22] However, nasolacrimal stenosis has been reported in humans receiving cidofovir topically,[23, 24] and it is not commercially available as an ophthalmic agent. Therefore, although the in vitro[25] and short-term in vivo efficacy[22] of cidofovir against FHV-1 is proven, cats should be monitored for nasolacrimal cicatrization. Cidofovir 0.5% retained efficacy when compounded in normal saline and refrigerated (4 °C) or frozen (−20 or −80 °C) in plastic or glass for up to 6 months.[26] However, safety data including change in pH, tonicity, etc., and risk of contamination were not evaluated.
Purine Analogues and Their Oral Prodrugs
Acyclovir and Valacyclovir
Acyclovir (9-(2-hydroxyethoxymethy)guanine; Zovirax®, Avirax®) is the prototype of a group of antiviral drugs known as acyclic nucleoside analogues with all members requiring 3 phosphorylation steps for activation. The first step must be catalyzed by viral thymidine kinase,[27] thus increasing the safety of these drugs and permitting systemic administration to humans.[28] Unfortunately, FHV-1’s thymidine kinase phosphorylates acyclovir much less efficiently than does the HSV-1-encoded enzyme, likely explaining the relative lack of efficacy of acyclovir against FHV-1 (see Table 1).[29, 30] The second and third phosphorylation steps must be performed by host enzymes. To the authors’ knowledge, affinity of feline enzymes for acyclic nucleoside analogues has not been reported. In addition to relatively low antiviral potency against FHV-1,[25, 31] acyclovir has poor bioavailability and can cause bone marrow suppression when systemically administered to cats.[32] Oral administration of 50 mg/kg acyclovir to cats was associated with peak plasma concentrations of only 33 μM (approximately one third the IC50 for FHV-1).[32] Thus, systemic acyclovir administration is not recommended in cats. Application of acyclovir as 0.5% ophthalmic ointment 5 times daily in cats with ocular disease attributable to FHV-1 led to resolution of clinical signs after 10 days in an non-masked, non placebo-controlled study.[33] However, cats treated only 3 times daily took approximately twice as long to resolve and did so only once therapy was increased to 5 times daily. This suggests that at least 5 times daily topical application of acyclovir may produce corneal surface concentrations exceeding the IC50 for FHV-1 without causing clinically appreciable toxicity.
Valacyclovir (L-valine, 2-[(2-amino-1,6-dihydro-6-oxo-9H-purin-9-yl)methoxy]ethyl ester, monochloride; Valtrex®) is a prodrug developed to improve the bioavailability of acyclovir. In humans and cats, valacyclovir is more efficiently absorbed from the gastrointestinal tract than acyclovir is and, following absorption, is converted to acyclovir by a hepatic hydrolase.[34] Plasma concentrations of acyclovir exceeding the IC50 for FHV-1 can be achieved after oral administration of this drug to cats. However, in cats experimentally infected with FHV-1, valacyclovir induced potentially fatal hepatic and renal necrosis, along with bone marrow suppression, without reducing viral shedding or clinical disease severity. This likely resulted from the toxic plasma concentrations of acyclovir that were achieved.[35] Valacyclovir should never be administered to cats.
Ganciclovir and Valganciclovir
Ganciclovir (DHPG; 9-[[2-hydroxy-1-(hydroxymethyl)ethoxy]methyl]-guanine; Cytovene®, Zirgan®, Virgan®) is an acyclic nucleoside analogue with potent antiviral activity against HSV-1 and HSV-2.[36] It is approximately 10-fold more effective against FHV-1 in vitro than is acyclovir.[25] It is available for oral or intravenous administration in humans, where it is associated with more severe neurologic toxicity, neutropenia, and bacterial infections than is acyclovir.[37, 38] Additionally, an intravitreal sustained-release ganciclovir implant has been developed for treatment of cytomegalovirus retinitis in humans,[39] and a 0.15% ophthalmic gel is commercially available for treatment of acute human herpetic keratitis.[40]
Valganciclovir (L-Valine, 2-[(2-amino-1,6-dihydro-6-oxo-9H-purin-9-yl)methoxy]-3-hydroxypropyl ester; Valcyte®) is a prodrug of ganciclovir developed to address low oral bioavailability of ganciclovir,[41] and prescribed to treat cytomegalovirus retinitis in humans.[42] Although the in vitro efficacy of ganciclovir against FHV-1[25] and anecdotal reports of its topical administration to cats in Europe are very promising, to the authors’ knowledge, neither the safety nor pharmacokinetics of valganciclovir or ganciclovir in any form has been reported in cats.
Penciclovir and Famciclovir
Penciclovir (9-(4-hydroxy-3-hydroxymethylbut-1-yl)guanine; BRL39123; Denavir®, Vectavir®) is a nucleoside deoxyguanosine analogue with a similar mechanism of action to acyclovir and with potent antiviral activity against a number of human herpesviruses. Like acyclovir, it requires viral and cellular phosphorylation but is highly effective against FHV-1 in vitro[29, 43, 44] and in vivo.[45] In a rabbit model of human HSV-1 keratitis, a 3% penciclovir ointment administered once, twice or four times daily decreased epithelial keratitis severity. Thus, a topical ophthalmic penciclovir ointment may be effective in cats with FHV-1 keratitis and/or conjunctivitis, but, to the authors’ knowledge, there are no commercial or compounded preparations available for ophthalmic use.[19] Penciclovir is available as a 1% dermatologic cream for humans, but that should not be applied to the eye.
Famciclovir (2-(2-(2-amino-9H-Purin-9-yl)ethyl)-1,3-propanediol diacetate; Famvir®) is a highly bioavailable prodrug of penciclovir, which – once absorbed – is metabolized to penciclovir. In humans this metabolism is complex; requiring di-deacetylation to BRL42359, in the blood, liver, or small intestine, with subsequent oxidation to penciclovir by aldehyde oxidase in the liver (Fig. 1).[46-48] Neither famciclovir nor BRL42359 has any in vitro antiviral activity against FHV-1,[43] therefore complete metabolism to penciclovir is required. However, hepatic aldehyde oxidase activity in cats is about 2% of that seen in humans and lower than in any other species reported to date (Fig. 2).[49] Famciclovir pharmacokinetics in the cat are extremely complex and nonlinear (i.e., doubling of famciclovir dose does not lead to doubling of plasma penciclovir concentration) presumably due to saturation of the hepatic oxidase.[50] As a result very high plasma concentrations of BRL42359 accumulate in the cat.[50] Fortunately, this compound demonstrates very little cytotoxicity in vitro.[43] Table 2 summarizes the pharmacokinetic data available to date for penciclovir in tears and plasma of cats receiving one of numerous famciclovir dose regimens. Tissue concentration data are not yet available.
Figure 1.
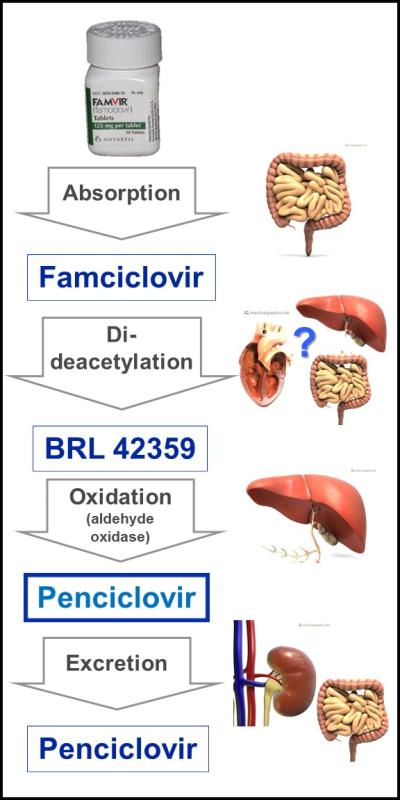
Absorption, metabolism, and excretion pathways of famciclovir in humans. Following oral administration, famciclovir is absorbed across the intestine and undergoes di-deacetylation. The exact site at which this step occurs is unclear but may be in the enterocytes, bloodstream, or liver. The inactive metabolite BRL42359 is then oxidized by a hepatic aldehyde oxidase to the active antiviral compound, penciclovir, which is ultimately excreted in feces and urine. Based upon famciclovir, BRL42359 and penciclovir concentrations in feline plasma following oral administration of famciclovir, similar steps likely occur in cats.[50, 52, 53] Anatomic images courtesy of www.MedicalGraphics.de (license # CC BY-ND 3.0 DE).
Figure 2.
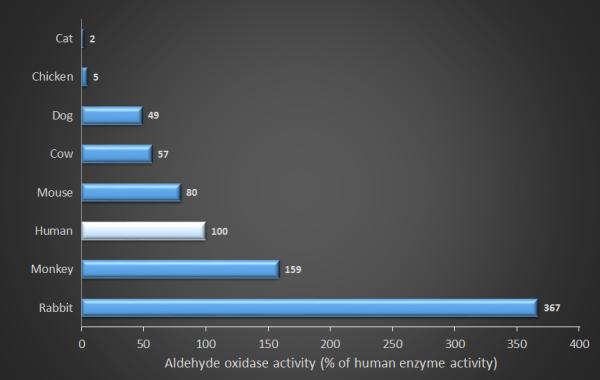
Relative activity of hepatic aldehyde oxidase in various species. All data are shown in percentage activity normalized against humans (100%). Original data from Dick et al.[49]
Table 2.
Maximum (Cmax) and minimum plasma and tear penciclovir concentrations and time to plasma and tear Cmax in cats administered a variety of famciclovir doses at various dose frequencies.
| Famciclovir dose |
Dose frequency |
Plasma [penciclovir] (ng/mL) |
Plasma penciclovir Tmax (h) |
Tear [penciclovir] (ng/mL) |
Tear penciclovir Tmax (h) |
||
|---|---|---|---|---|---|---|---|
| Cmax | Css(min) | Cmax | Css(min) | ||||
| 9-18 mg/kg[54] |
BID | 330 | 64 | 5.3 | ND | ND | ND |
| TID | 680 | 180 | 3.8 | ND | ND | ND | |
| 30 mg/kg[50] | BID | 2010 | 345 | 1.3 | 305 | 65 | 4.7 |
| TID | 1755 | 570 | 1.7 | 395 | 160 | 2.0 | |
| 40 mg/kg[50] | BID | 1945 | 445 | 2.3 | 375 | 55 | 3.3 |
| TID | 2210 | 790 | 2.5 | 750 | 150 | 2.6 | |
| 90 mg/kg[50] | BID | 2720 | 630 | 2.7 | 680 | 200 | 2.3 |
| TID | 2015 | 905 | 2.7 | 555 | 275 | 3.0 | |
Abbreviations: BID: twice daily; Cmax: Maximum observed drug concentration; Css(min): minimum observed drug concentration during the dosing interval at steady state; ND: Not done; TID; thrice daily; Tmax: Time to Cmax.
In addition to these pharmacokinetic data, recommendation of an appropriate famciclovir dose requires:
Knowledge of whether penciclovir concentrations in plasma, tears, or the infected tissues themselves are most relevant
Selection of an appropriate target penciclovir concentration based on in vitro IC50s which have reportedly ranged from 304 to 3500 ng/mL.[25, 31, 43, 44]
Knowledge of whether the targeted IC50 should be exceeded by the trough or the peak penciclovir concentrations, and for how long.
Together, these uncertainties have led to much controversy about the optimum famciclovir dose in cats, with reported doses ranging from 8 mg/kg once daily[51] to 140 mg/kg thrice daily.[3] The following data are provided to inform dose selection.
In the only masked, placebo-controlled efficacy trial to date, cats known to be infected with FHV-1 and given approximately 90 mg famciclovir/kg thrice daily per os achieved an approximate peak plasma penciclovir concentration of 2100 ng/mL.[45] Relative to control cats, treated cats had significantly reduced clinical signs, decreased serum globulin concentrations, reduced histologic evidence of conjunctivitis, decreased viral shedding and reduced serum FHV-1 titers, as well as increased goblet cell density.[45] A subsequent study showed that administration of a single dose of 40 mg/kg to uninfected healthy cats achieved nearly identical plasma penciclovir concentrations to those achieved with a single dose of 90 mg/kg.[52] A third study[53] revealed that cats receiving 40 mg/kg thrice daily had tear penciclovir concentrations likely to be effective against FHV-1 (using a target IC50 of 304 ng/mL)[29] for at least 3 hours after each dose (i.e., for ≥ 9 hours/day). In the most comprehensive pharmacokinetic study to date, healthy cats were administered famciclovir at 30, 40 or 90 mg/kg twice or thrice daily, and plasma and tear famciclovir, BRL42359, and penciclovir concentrations were measured.[50] This resulted in the recommendation that cats should receive 90 mg famciclovir/kg twice daily because this regimen achieved comparable plasma and tear penciclovir concentrations to those achieved with 90 mg/kg thrice daily, whereas the lower doses tested did not result in adequate tear penciclovir concentrations, even when administered thrice daily.
Perhaps most revealing so far, are data from a retrospective study comparing outcomes when famciclovir was administered thrice daily to cats with presumed herpetic disease at approximately 40 (n = 33 cats) or 90 mg/kg (n = 26 cats).[3] Median duration of therapy required for clinical improvement was significantly longer in cats administered 40 versus 90 mg/kg. Furthermore, cats in the 90 mg/kg group showed significantly greater and faster improvement than did cats in the 40 mg/kg group (Fig. 3). The reduction in treatment duration with the higher famciclovir dose was estimated to decrease overall client costs due to a reduction in total famciclovir administered (and potentially the number of recheck examinations required). These data, suggest that 90 mg/kg TID is clinically and cost effective. Meanwhile, pharmacokinetic data[50] suggest that tear and plasma penciclovir concentrations are similar whether cats receive 90 mg famciclovir /kg 2 or 3 times daily. Taken together, data from these 2 studies[3, 50] suggest that 90 mg famciclovir/kg twice daily is likely to be effective in treating cats with herpetic disease. Adverse events (most commonly gastrointestinal) potentially attributable to famciclovir were reported in 17% of cats receiving 40 or 90 mg famciclovir/kg thrice daily, but the prevalence was not different between the 2 dose groups.[3] Assessing all in vivo tolerance data for famciclovir, this drug appears to be markedly safer than acyclovir and valacyclovir - the only other systemic antiviral drugs to be orally administered to cats.[3, 32, 35, 45, 50, 52-54] However, patients administered famciclovir should be closely monitored, and assessment of a complete blood count, serum biochemistry panel and urinalysis should be considered in cats with known concurrent disease or cats expected to receive famciclovir for long periods. As in humans,[55] reduction in dose frequency should be considered in cats with renal insufficiency.[3, 50]
Figure 3.
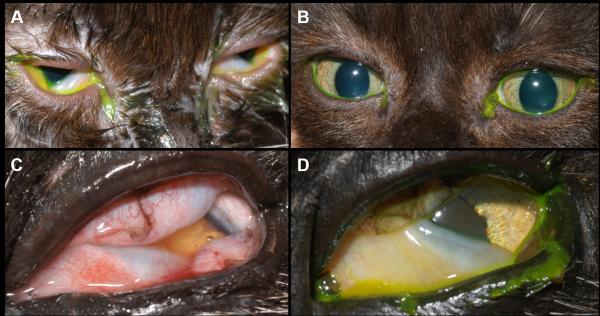
A 6-week-old, sexually intact male domestic short haired cat with blepharoconjunctivitis in both eyes and dendritic ulcerative keratitis in the right eye prior to (A) and following (B) 8 days of orally administered famciclovir given at 110 mg/kg thrice daily. Note the marked improvement in both eyes following treatment with famciclovir. Left eye of a 14-year-old, female spayed domestic medium haired cat with blepharokeratoconjunctivitis in both eyes prior to (C) and following (D) 15 days of orally administered famciclovir given at 85 mg/kg thrice daily. Note the improvement in the left eye following treatment with famciclovir.
Other Antiviral Drugs
Foscarnet (phosphonoformate; Foscavir®) mimics the anion pyrophosphate to selectively inhibit the pyrophosphate binding site on viral DNA polymerases at concentrations that do not affect human DNA polymerases.[56] Foscarnet is administered intravenously to treat cytomegalovirus retinitis or mucocutaneous acyclovir-resistant HSV infections in immunocompromised humans.[57] However, foscarnet has very low oral bioavailability (8%) in cats,[57] and markedly lower in vitro activity against FHV-1 in comparison to most other antiviral drugs reported;[25] its use in cats is not recommended.
Bromovinyldeoxyuridine,[31, 58] adefovir,[59] PMEDAP (9-(2-phosphonylmethoxyethyl)-2, 6-diaminopurine),[59] and HPMPA ((S)-9-(3-hydroxy-2-phosphonylmethoxypropyl) adenine)[31] have variable in vitro efficacy against FHV-1 (see Table 1). To the authors’ knowledge, their efficacy and safety when administered orally or topically to cats have not been reported.
Other Compounds Investigated for Activity against FHV-1
Lysine is perhaps the best studied and yet maybe one of the more controversial of all of the other compounds with proven or putative antiviral efficacy against FHV-1 in cats. As with the antiviral drugs, initial interest arose from in vitro data and clinical trials in humans. Lysine’s antiviral effect is believed to arise because arginine is an essential amino acid for FHV-1[60] and HSV-1[61, 62] replication, and assumes that lysine antagonizes arginine availability to or utilization by these viruses during protein synthesis. This was hypothesized to affect protein synthesis of the virus more than the host because viral proteins had a higher arginine-to-lysine content than did human (and feline) proteins;[63] however recent analysis suggests that the difference in feline versus FHV-1 protein amino acid content is minimal.[64] Markedly elevated lysine concentrations in combination with notably low arginine concentrations suppress HSV-1[61, 62] and FHV-1[60] replication in vitro. However, this was not borne out with more physiologic amino acid concentrations.[65] In vivo data in cats are also contradictory. Oral administration of 500 mg L-lysine every 12 hours beginning 6 hours prior to inoculation with FHV-1 was associated with less severe conjunctivitis but similar viral shedding to cats receiving placebo.[66] In cats latently infected by experimental inoculation but without clinical signs, oral administration of 400 mg L-lysine once daily reduced viral shedding relative to placebo-treated cats.[67] Despite significant elevations in plasma lysine concentration, no change in plasma arginine concentration was observed in either study. Mild, reversible gastrointestinal disturbance potentially attributable to lysine administration was noted in some cats.[66] In the only study to assess bolus administration of lysine in naturally infected cats, 144 shelter-housed cats received 250 mg (kittens) or 500 mg (adult cats) lysine once daily for their entire shelter stay; outcomes were compared with an untreated control group. No significant treatment effect was detected for any parameter.[68]
Safety and efficacy of dietary lysine supplementation have also been assessed. No ill effects were seen in cats fed diets supplemented to up to 8.6% (dry matter) lysine.[69] In 2 subsequent efficacy trials, cats in environments where FHV-1 was enzootic were fed a diet supplemented to 5.1% lysine while control cats received a basal ration (approximately1% lysine).[70, 71] In both studies, disease was more severe and viral shedding was increased in cats fed the supplemented ration relative to those fed the basal diet. This may be partially explained by the observation that cats decreased their food (and therefore lysine) intake coincident with peak disease and viral presence.[70]
In summary, there is considerable variability among these studies, especially with respect to methodology, study population, and dose and method of lysine administration. However, taken together, data from these studies suggest that lysine is safe when orally administered to cats and, provided that it is administered as a bolus, may reduce viral shedding in latently infected cats and clinical signs in cats undergoing primary exposure to the virus. However, the stress of bolus administration in shelter situations may well negate its effects and data do not support dietary supplementation. Unfortunately, no clinical trials have been conducted on the group in which this drug is commonly used - client-owned cats with recurrent herpetic disease.
Interferons (IFNs) are cytokines with diverse immunological and antiviral functions and which may be divided into 4 groups (α, β, γ, and ω) and numerous subtypes. Viral infection stimulates cells to secrete IFNs into the extracellular space where they limit viral spread to adjacent cells without being virucidal. This knowledge should be used to set reasonable expectations of how therapeutically efficacious IFNs may be, and to decide in which patients and at what stages of disease they might be expected to be most effective.
Although IFNs likely play important physiological roles in the control of viral infections, in vitro data and clinical trials have produced conflicting and generally unsupportive results. In vitro tests using 1 × 105 - 5 × 105 IU/ml of recombinant human IFNα or feline IFNω reduced FHV-1 titer and/or cytopathic effect without observable cytotoxicity to the feline corneal cell line[72] or CRFK cells[73] on which the virus was grown. At higher concentrations, the effect of IFNω was greater than that of IFNα.[73] In another in vitro study, notable synergistic activity against FHV-1 was demonstrated when 10-62.5 μg/mL of acyclovir was combined with 10 or 100 IU/mL of human recombinant IFNα. The combination did not increase cytotoxicity but permitted a nearly eightfold reduction in acyclovir dose required to achieve maximal FHV-1 inhibition. Although synergy occurred when the IFNα was given before or after infection, pretreatment was more effective.[74] These data are supported by a study using a murine model of HSV-1 whereby concurrent oral acyclovir and intraperitoneal recurrent human IFNα and was more efficacious than either treatment alone.[75] In vivo investigation of nucleoside analogues in combination with IFN in cats are warranted before their use can be recommended.
To the authors’ knowledge, there have been only 2 experimental inoculation studies. In the first, 5 SPF cats were pretreated with 10,000 IU of recombinant feline IFNω OU q 12 hours and 2,000 IU administered PO q 24 hours for 2 days prior to viral inoculation; IFN therapy was not continued after inoculation.[76] No beneficial effects were shown. In the second study, twice daily subcutaneous administration of 108 IU/kg IFNα on two consecutive days prior to inoculation did lead to lower cumulative clinical scores for treated cats.[77] In clinical trials, there are reports of IFN administration to 37 client-owned[78] and 13 shelter-housed[79] cats testing negative for FeLV and FIV, 24 shelter housed cats testing negative for FeLV ± FIV,[80] and 16 shelter-housed cats testing positive for FeLV, FIV or both.[81] These cats were of widely ranging ages, and showed signs of acute,[78] unrecorded,[80, 81] or chronic unresponsive,[79] spontaneously-occurring upper respiratory disease. They were treated with recombinant human IFNα at 10,000 U/kg subcutaneously once daily for 14 days,[79] three 5-day cycles of once-daily subcutaneous injections of 1 million U/kg recombinant feline IFNω on Days 0, 14, and 60,[81] 1 drop of 1 million U/ml recombinant feline IFNω or human IFNα OU twice daily for 14 days,[80] or 2.5 million units of recombinant IFNω injected subcutaneously once on Day 0 followed by 0.5 million units applied every 8 hours for 21 days in each nostril and conjunctival sac (1 drop each) and the oral cavity (the remainder).[78] Only 2 of the studies were placebo-controlled; neither showed a significant treatment effect.[78, 80] Taken together, the data to date are not strongly supportive of interferon use in the management of herpetic disease in cats.
Lambda-carrageenan (λ-carrageenan) is a seaweed extract containing sulfated polysaccharides with in vitro activity against FHV-1 replication when used prior to but not following viral adsorption.[82] In vivo safety and efficacy of λ-carrageenan were examined in a placebo-controlled, masked study in vaccinated cats exposed for the first time to wild-type FHV-1.[82] Although well-tolerated, ophthalmic application of 1 drop of a 250 μg/mL λ-carrageenan solution before and after infection (n = 6 cats) or after infection only (n = 6 cats) did not reduce clinical signs. Reduction in virus isolation was noted only on Day 21 following inoculation. Other plant extracts with antiviral activity have undergone preliminary in vitro assessment but clinical safety or efficacy have not been reported.[83]
Leflunomide is an immunosuppressive agent with some antiviral efficacy against human herpesviruses.[84] In vitro efficacy studies with FHV-1 revealed significant and dose-dependent reduction in plaque number and - at higher concentrations only - viral load. However, at higher concentrations, some cytotoxicity was observed. Electron microscopy suggested a failure in viral tegument and external membrane assembly, which may indicate the mode of action.[85] Clinical studies are lacking.
Lactoferrin is a mammalian iron-binding glycoprotein that has antibacterial, antifungal, antiprotozoal, and antiviral properties. It is produced by mucosal epithelial cells and is present in tears and other body fluids. Lactoferrin has potent antiviral efficacy against FHV-1 replication in vitro, apparently via inhibition of adsorption or penetration of the virus into the cell.[86] Studies assessing the clinical relevance of these data are required.
Small interfering RNAs (siRNAs) are short (about 20-nucleotide), double-stranded sections of RNA designed to transfect a cell and knockdown expression of specific genes. To overcome the short-lived effect of transfection of native siRNAs, they can be incorporated into plasmids and thus extend their longevity, especially within rapidly dividing cells. Initial in vitro studies demonstrated antiviral activity of siRNAs targeting the FHV-1 glycoprotein D (gD) alone or the gD and DNA polymerase genes jointly, but not the DNA polymerase gene alone.[87, 88] However, intracellular delivery of these agents is essential but proving complex. Agents that facilitate siRNA delivery into corneal cells in vitro have been developed and they appear nontoxic in vitro and nonirritating when applied topically to normal cats’ eyes.[89] However, thus far, they have failed to deliver the siRNAs into corneal cells following topical application in vivo, perhaps due to rapid removal of the test substances from the ocular surface by tears.[89]
Probiotics were investigated in a prospective, placebo-controlled, pilot study[90] in which cats experimentally infected with FHV-1 for another study[22] were administered the probiotic Enterococcus faecium strain SF68. This clinical trial failed to reveal a significant treatment effect; however cats in both groups showed such minimal evidence of disease that a treatment effect may have been missed.
Summary
This review summarizes the current state of knowledge regarding antiviral drugs and other compounds in cats. It is not a “how to” manual for the treatment of the diverse range of clinical herpetic syndromes. However, some general comments are possible:
All antiviral drugs studied to date are virostatic and so cannot be used to cure infection, only to reduce replicating virus, and thereby the severity, duration, or both of clinical signs associated with infection.
FHV-1 causes long-term and marked reduction in goblet cell density[91] that famciclovir only partially mitigates.[45] As a result, a topical mucinomimetic agent such as hyaluronate is often required as an adjunct to antiviral therapy.[92]
Because antiviral drugs are virostatic, frequency of application of a topical agent and dose and frequency of a systemic agent are critical to therapeutic success.
In vitro selection of drug-resistant herpesviruses is performed by exposure to antiviral drug concentrations known to be ineffective.[93] Likewise, current guidelines for responsible antimicrobial stewardship reinforce the importance of appropriate dosing.[94] Under-dosing of antiviral agents, therefore, is likely to induce resistant viruses.
There are no clear guidelines regarding duration of therapy or, more specifically, when antiviral agents should be initiated and stopped. However, it appears reasonable that antiviral agents should be considered when signs are severe, persistent, or recurrent, particularly when there is corneal involvement, and especially ulceration. Because epithelial replication, latency and reactivation, and persistence are such interdependent and sequential phases of herpetic disease, interruption of any one of them is expected to limit the virus’ abilities to cause subsequent disease. Therefore, aggressive treatment of herpetic disease may limit disease progression and minimize frequency and severity of recurrences. Likewise, prudent antimicrobial practice would suggest that therapy should be continued for a period after clinical signs are absent. The length of this period should be tailored to the individual based, in part, upon duration and severity of the signs being treated. Tapering of topically applied antiviral agents must be done with appropriate consideration for their lowest effective frequency as virostatic agents. For example, the recommended reduction in trifluridine dose as human herpetic keratitis improves is from 9 to 5 times daily, but not lower.[95] By comparison, tapering of orally administered antiviral drugs is never advised in acute herpetic syndromes, but is practiced in some herpes prophylaxis regimens, but only to a dose proven to be effective.[95] Even in human patients with renal impairment and in whom metabolism of systemically administered antiviral agents is expected to be reduced, dose magnitude reduction is not recommended; rather, dose frequency is preferred.[95] Indeed, there is good evidence that reduction or tapering of antiviral dose leads to a resurgence in the herpesviral fraction of the microbiome.[96]
Acknowledgments
Supported in part by a grant from the National Institute of Health K08 EY021142.
References
- 1.Maggs DJ. Update on pathogenesis, diagnosis, and treatment of feline herpesvirus type 1. Clin Tech Small Anim Pract. 2005;20:94–101. doi: 10.1053/j.ctsap.2004.12.013. [DOI] [PubMed] [Google Scholar]
- 2.Stiles J. Treatment of cats with ocular disease attributable to herpesvirus infection: 17 cases (1983-1993) J Am Vet Med Assoc. 1995;207:599–603. [PubMed] [Google Scholar]
- 3.Thomasy SM, Shull O, Outerbridge CAL, C. C, Freeman KS, Strom AR, Kass PH, Maggs DJ. Oral administration of famciclovir for treatment of spontaneous ocular, respiratory or dermatologic disease attributed to feline herpesvirus type-1: A retrospective review in 59 client-owned cats. J Am Vet Med Assoc. 2016 doi: 10.2460/javma.249.5.526. in press. [DOI] [PubMed] [Google Scholar]
- 4.De Clercq E. Antivirals and antiviral strategies. Nat Rev Microbiol. 2004;2:704–720. doi: 10.1038/nrmicro975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.James SH, Prichard MN. Current and future therapies for herpes simplex virus infections: mechanism of action and drug resistance. Curr Opin Virol. 2014;8:54–61. doi: 10.1016/j.coviro.2014.06.003. [DOI] [PubMed] [Google Scholar]
- 6.Luntz MH, MacCallum FO. Treatment of herpes simplex keratitis with 5-iodo-2′-deoxyuridine. Br J Ophthalmol. 1963;47:449–456. doi: 10.1136/bjo.47.8.449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kaufman HE. Antimetabolite drug therapy in herpes simplex. Ophthalmol. 1980;87:135–139. doi: 10.1016/s0161-6420(80)35273-1. [DOI] [PubMed] [Google Scholar]
- 8.LePage GA, Khaliq A, Gottlieb JA. Studies of 9-beta-D-arabinofuranosyladenine in man. Drug Metab Dispos. 1973;1:756–759. [PubMed] [Google Scholar]
- 9.Johnson MT, Buchanan RA, Luby JP, Mikulec D. Treatment of varicella-zoster virus infections with adenine arabinoside. J Infect Dis. 1975;131:225–229. doi: 10.1093/infdis/131.3.225. [DOI] [PubMed] [Google Scholar]
- 10.Brightbill FS, Kaufman HE. Adenine arabinoside therapy in corneal stromal disease and iritis due to herpes simplex. Ann Ophthalmol. 1974;6:25–32. [PubMed] [Google Scholar]
- 11.Lauter CB, Bailey EJ, Lerner AM. Microbiologic assays and neurological toxicity during use of adenine arabinoside in humans. J Infect Dis. 1976;134:75–79. doi: 10.1093/infdis/134.1.75. [DOI] [PubMed] [Google Scholar]
- 12.Nesburn AB, Robinson C, Dickinson R. Adenine arabinoside effect on experimental idoxuridine-resistant herpes simplex infection. Invest Ophthalmol. 1974;13:302–304. [PubMed] [Google Scholar]
- 13.Chin GN. Treatment of herpes simplex keratitis with idoxuridine and vidarabine: a double-blind study. Ann Ophthalmol. 1978;10:1171–1174. [PubMed] [Google Scholar]
- 14.O'Brien WJ, Edelhauser HF. The corneal penetration of trifluorothymidine, adenine arabinoside, and idoxuridine: a comparative study. Invest Ophthalmol Vis Sci. 1977;16:1093–1103. [PubMed] [Google Scholar]
- 15.Cihlar T, Chen MS. Identification of enzymes catalyzing two-step phosphorylation of cidofovir and the effect of cytomegalovirus infection on their activities in host cells. Mol Pharmacol. 1996;50:1502–1510. [PubMed] [Google Scholar]
- 16.Neyts J, Snoeck R, Schols D, Balzarini J, De Clercq E. Selective inhibition of human cytomegalovirus DNA synthesis by (S)-1-(3-hydroxy-2-phosphonylmethoxypropyl)cytosine [(S)-HPMPC] and 9-(1,3-dihydroxy-2-propoxymethyl)guanine (DHPG) Virology. 1990;179:41–50. doi: 10.1016/0042-6822(90)90271-r. [DOI] [PubMed] [Google Scholar]
- 17.Bronson JJ, Ferrara LM, Hitchcock MJ, Ho HT, Woods KL, Ghazzouli I, Kern ER, Soike KF, Martin JC. (S)-1-(3-hydroxy-2-(phosphonylmethoxy)propyl)cytosine (HPMPC): a potent antiherpesvirus agent. Adv Exp Med Biol. 1990;278:277–283. doi: 10.1007/978-1-4684-5853-4_28. [DOI] [PubMed] [Google Scholar]
- 18.Snoeck R, Sakuma T, De Clercq E, Rosenberg I, Holy A. (S)-1-(3-hydroxy-2-phosphonylmethoxypropyl)cytosine, a potent and selective inhibitor of human cytomegalovirus replication. Antimicrob Agents Chemother. 1988;32:1839–1844. doi: 10.1128/aac.32.12.1839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kaufman HE, Varnell ED, Thompson HW. Trifluridine, cidofovir, and penciclovir in the treatment of experimental herpetic keratitis. Arch Ophthalmol. 1998;116:777–780. doi: 10.1001/archopht.116.6.777. [DOI] [PubMed] [Google Scholar]
- 20.Romanowski EG, Bartels SP, Gordon YJ. Comparative antiviral efficacies of cidofovir, trifluridine, and acyclovir in the HSV-1 rabbit keratitis model. Invest Ophthalmol Vis Sci. 1999;40:378–384. [PubMed] [Google Scholar]
- 21.Neyts J, Snoeck R, Balzarini J, De Clercq E. Particular characteristics of the anti-human cytomegalovirus activity of (S)-1-(3-hydroxy-2-phosphonylmethoxypropyl)cytosine (HPMPC) in vitro. Antiviral Res. 1991;16:41–52. doi: 10.1016/0166-3542(91)90057-x. [DOI] [PubMed] [Google Scholar]
- 22.Fontenelle JP, Powell CC, Veir JK, Radecki SV, Lappin MR. Effect of topical ophthalmic application of cidofovir on experimentally induced primary ocular feline herpesvirus-1 infection in cats. Am J Vet Res. 2008;69:289–293. doi: 10.2460/ajvr.69.2.289. [DOI] [PubMed] [Google Scholar]
- 23.Sherman MD, Feldman KA, Farahmand SM, Margolis TP. Treatment of conjunctival squamous cell carcinoma with topical cidofovir. Am J Ophthalmol. 2002;134:432–433. doi: 10.1016/s0002-9394(02)01569-6. [DOI] [PubMed] [Google Scholar]
- 24.Gordon YJ, Romanowski EG, Araullo-Cruz T. Topical HPMPC inhibits adenovirus type 5 in the New Zealand rabbit ocular replication model. Invest Ophthalmol Vis Sci. 1994;35:4135–4143. [PubMed] [Google Scholar]
- 25.Maggs DJ, Clarke HE. In vitro efficacy of ganciclovir, cidofovir, penciclovir, foscarnet, idoxuridine, and acyclovir against feline herpesvirus type-1. Am J Vet Res. 2004;65:399–403. doi: 10.2460/ajvr.2004.65.399. [DOI] [PubMed] [Google Scholar]
- 26.Stiles J, Gwin W, Pogranichniy R. Stability of 0.5% cidofovir stored under various conditions for up to 6 months. Vet Ophthalmol. 2010;13:275–277. doi: 10.1111/j.1463-5224.2010.00784.x. [DOI] [PubMed] [Google Scholar]
- 27.Darby G, Larder BA, Bastow KF, Field HJ. Sensitivity of viruses to phosphorylated 9-(2-hydroxyethoxymethyl)guanine revealed in TK-transformed cells. J Gen Virol. 1980;48:451–454. doi: 10.1099/0022-1317-48-2-451. [DOI] [PubMed] [Google Scholar]
- 28.Whitley RJ. The past as prelude to the future: history, status, and future of antiviral drugs. Ann Pharmacother. 1996;30:967–971. doi: 10.1177/106002809603000911. [DOI] [PubMed] [Google Scholar]
- 29.Hussein IT, Menashy RV, Field HJ. Penciclovir is a potent inhibitor of feline herpesvirus-1 with susceptibility determined at the level of virus-encoded thymidine kinase. Antiviral Res. 2008;78:268–274. doi: 10.1016/j.antiviral.2007.10.015. [DOI] [PubMed] [Google Scholar]
- 30.Hussein IT, Miguel RN, Tiley LS, Field HJ. Substrate specificity and molecular modelling of the feline herpesvirus-1 thymidine kinase. Arch Virol. 2008;153:495–505. doi: 10.1007/s00705-007-0021-6. [DOI] [PubMed] [Google Scholar]
- 31.Williams DL, Fitzmaurice T, Lay L, Forster K, Hefford J, Budge C, Blackmore K, Robinson JC, Field HF. Efficacy of antiviral agents in feline herpetic keratitis: results of an in vitro study. Curr Eye Res. 2004;29:215–218. doi: 10.1080/02713680490504849. [DOI] [PubMed] [Google Scholar]
- 32.Owens JG, Nasisse MP, Tadepalli SM, Dorman DC. Pharmacokinetics of acyclovir in the cat. J Vet Pharmacol Ther. 1996;19:488–490. doi: 10.1111/j.1365-2885.1996.tb00087.x. [DOI] [PubMed] [Google Scholar]
- 33.Williams DL, Robinson JC, Lay E, Field H. Efficacy of topical aciclovir for the treatment of feline herpetic keratitis: results of a prospective clinical trial and data from in vitro investigations. Vet Rec. 2005;157:254–257. doi: 10.1136/vr.157.9.254. [DOI] [PubMed] [Google Scholar]
- 34.de Miranda P, Burnette TC. Metabolic fate and pharmacokinetics of the acyclovir prodrug valaciclovir in cynomolgus monkeys. Drug Metab Dispos. 1994;22:55–59. [PubMed] [Google Scholar]
- 35.Nasisse MP, Dorman DC, Jamison KC, Weigler BJ, Hawkins EC, Stevens JB. Effects of valacyclovir in cats infected with feline herpesvirus 1. Am J Vet Res. 1997;58:1141–1144. [PubMed] [Google Scholar]
- 36.Smith KO, Galloway KS, Kennell WL, Ogilvie KK, Radatus BK. A new nucleoside analog, 9-[[2-hydroxy-1-(hydroxymethyl)ethoxyl]methyl]guanine, highly active in vitro against herpes simplex virus types 1 and 2. Antimicrob Agents Chemother. 1982;22:55–61. doi: 10.1128/aac.22.1.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ernst ME, Franey RJ. Acyclovir- and ganciclovir-induced neurotoxicity. Ann Pharmacother. 1998;32:111–113. doi: 10.1345/aph.17135. [DOI] [PubMed] [Google Scholar]
- 38.Burns LJ, Miller W, Kandaswamy C, DeFor TE, MacMillan ML, Van Burik JA, Weisdorf DJ. Randomized clinical trial of ganciclovir vs acyclovir for prevention of cytomegalovirus antigenemia after allogeneic transplantation. Bone Marrow Transplant. 2002;30:945–951. doi: 10.1038/sj.bmt.1703770. [DOI] [PubMed] [Google Scholar]
- 39.Anand R, Nightingale SD, Fish RH, Smith TJ, Ashton P. Control of cytomegalovirus retinitis using sustained release of intraocular ganciclovir. Arch Ophthalmol. 1993;111:223–227. doi: 10.1001/archopht.1993.01090020077027. [DOI] [PubMed] [Google Scholar]
- 40.Chou TY, Hong BY. Ganciclovir ophthalmic gel 0.15% for the treatment of acute herpetic keratitis: background, effectiveness, tolerability, safety, and future applications. Ther Clin Risk Manag. 2014;10:665–681. doi: 10.2147/TCRM.S58242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jung D, Dorr A. Single-dose pharmacokinetics of valganciclovir in HIV- and CMV-seropositive subjects. J Clin Pharmacol. 1999;39:800–804. doi: 10.1177/00912709922008452. [DOI] [PubMed] [Google Scholar]
- 42.Segarra-Newnham M, Salazar MI. Valganciclovir: A new oral alternative for cytomegalovirus retinitis in human immunodeficiency virus-seropositive individuals. Pharmacotherapy. 2002;22:1124–1128. doi: 10.1592/phco.22.13.1124.33527. [DOI] [PubMed] [Google Scholar]
- 43.Groth AD, Contreras MT, Kado-Fong HK, Nguyen KQ, Thomasy SM, Maggs DJ. In vitro cytotoxicity and antiviral efficacy against feline herpesvirus type 1 of famciclovir and its metabolites. Vet Ophthalmol. 2014;17:268–274. doi: 10.1111/vop.12094. [DOI] [PubMed] [Google Scholar]
- 44.Hussein IT, Field HJ. Development of a quantitative real-time TaqMan PCR assay for testing the susceptibility of feline herpesvirus-1 to antiviral compounds. J Virol Methods. 2008;152:85–90. doi: 10.1016/j.jviromet.2008.05.018. [DOI] [PubMed] [Google Scholar]
- 45.Thomasy SM, Lim CC, Reilly CM, Kass PH, Lappin MR, Maggs DJ. Evaluation of orally administered famciclovir in cats experimentally infected with feline herpesvirus type-1. Am J Vet Res. 2011;72:85–95. doi: 10.2460/ajvr.72.1.85. [DOI] [PubMed] [Google Scholar]
- 46.Clarke SE, Harrell AW, Chenery RJ. Role of aldehyde oxidase in the in vitro conversion of famciclovir to penciclovir in human liver. Drug Metab Dispos. 1995;23:251–254. [PubMed] [Google Scholar]
- 47.Vere Hodge RA, Sutton D, Boyd MR, Harnden MR, Jarvest RL. Selection of an oral prodrug (BRL 42810; famciclovir) for the antiherpesvirus agent BRL 39123 [9-(4-hydroxy-3-hydroxymethylbut-l-yl)guanine; penciclovir] Antimicrob Agents Chemother. 1989;33:1765–1773. doi: 10.1128/aac.33.10.1765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Filer CW, Allen GD, Brown TA, Fowles SE, Hollis FJ, Mort EE, Prince WT, Ramji JV. Metabolic and pharmacokinetic studies following oral administration of 14C-famciclovir to healthy subjects. Xenobiotica. 1994;24:357–368. doi: 10.3109/00498259409045899. [DOI] [PubMed] [Google Scholar]
- 49.Dick RA, Kanne DB, Casida JE. Identification of aldehyde oxidase as the neonicotinoid nitroreductase. Chem Res Toxicol. 2005;18:317–323. doi: 10.1021/tx049737i. [DOI] [PubMed] [Google Scholar]
- 50.Sebbag L, Thomasy SM, Woodward AP, Knych HK, Maggs DJ. Pharmacokinetic modeling of penciclovir and BRL42359 in plasma and tears so as to optimize oral famciclovir dosing recommendation in cats. Am J Vet Res. 2016 doi: 10.2460/ajvr.77.8.833. in press. [DOI] [PubMed] [Google Scholar]
- 51.Malik R, Lessels NS, Webb S, Meek M, Graham PG, Vitale C, Norris JM, Power H. Treatment of feline herpesvirus-1 associated disease in cats with famciclovir and related drugs. J Feline Med Surg. 2009;11:40–48. doi: 10.1016/j.jfms.2008.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Thomasy SM, Whittem T, Bales JL, Ferrone M, Stanley SD, Maggs DJ. Pharmacokinetics of penciclovir in healthy cats following oral administration of famciclovir or intravenous infusion of penciclovir. Am J Vet Res. 2012;73:1092–1099. doi: 10.2460/ajvr.73.7.1092. [DOI] [PubMed] [Google Scholar]
- 53.Thomasy SM, Covert JC, Stanley SD, Maggs DJ. Pharmacokinetics of famciclovir and penciclovir in tears following oral administration of famciclovir to cats: a pilot study. Vet Ophthalmol. 2012;15:299–306. doi: 10.1111/j.1463-5224.2011.00984.x. [DOI] [PubMed] [Google Scholar]
- 54.Thomasy SM, Maggs DJ, Moulin NK, Stanley SD. Pharmacokinetics and safety of penciclovir following oral administration of famciclovir to cats. Am J Vet Res. 2007;68:1252–1258. doi: 10.2460/ajvr.68.11.1252. [DOI] [PubMed] [Google Scholar]
- 55.Boike SC, Pue MA, Freed MI, Audet PR, Fairless A, Ilson BE, Zariffa N, Jorkasky DK. Pharmacokinetics of famciclovir in subjects with varying degrees of renal impairment. Clin Pharmacol Ther. 1994;55:418–426. doi: 10.1038/clpt.1994.51. [DOI] [PubMed] [Google Scholar]
- 56.Hess G, Arnold W, Meyer zum Buschenfelde KH. Inhibition of hepatitis-B-virus DNA polymerase by phosphonoformate: studies on its mode of action. J Med Virol. 1980;5:309–316. doi: 10.1002/1096-9071(1980)5:4<309::aid-jmv1890050407>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 57.De Clercq E. Selective anti-herpesvirus agents. Antivir Chem Chemother. 2013;23:93–101. doi: 10.3851/IMP2533. [DOI] [PubMed] [Google Scholar]
- 58.Nasisse MP, Guy JS, Davidson MG, Sussman W, De Clercq E. In vitro susceptibility of feline herpesvirus-1 to vidarabine, idoxuridine, trifluridine, acyclovir, or bromovinyldeoxyuridine. Am J Vet Res. 1989;50:158–160. [PubMed] [Google Scholar]
- 59.van der Meulen K, Garre B, Croubels S, Nauwynck H. In vitro comparison of antiviral drugs against feline herpesvirus 1. BMC Vet Res. 2006;2:13. doi: 10.1186/1746-6148-2-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Maggs DJ, Collins BK, Thorne JG, Nasisse MP. Effects of L-lysine and L-arginine on in vitro replication of feline herpesvirus type-1. Am J Vet Res. 2000;61:1474–1478. doi: 10.2460/ajvr.2000.61.1474. [DOI] [PubMed] [Google Scholar]
- 61.Tankersley RW., Jr. Amino Acid Requirements of Herpes Simplex Virus in Human Cells. J Bacteriol. 1964;87:609–613. doi: 10.1128/jb.87.3.609-613.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Griffith RS, DeLong DC, Nelson JD. Relation of arginine-lysine antagonism to herpes simplex growth in tissue culture. Chemotherapy. 1981;27:209–213. doi: 10.1159/000237979. [DOI] [PubMed] [Google Scholar]
- 63.Kaplan AS, Shimono H, Ben-Porat T. Synthesis of proteins in cells infected with herpesvirus. 3. Relative amino acid content of various proteins formed after infection. Virology. 1970;40:90–101. doi: 10.1016/0042-6822(70)90382-x. [DOI] [PubMed] [Google Scholar]
- 64.Bol S, Bunnik EM. Lysine supplementation is not effective for the prevention or treatment of feline herpesvirus 1 infection in cats: a systematic review. BMC Vet Res. 2015;11:284. doi: 10.1186/s12917-015-0594-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Cave NJ, Dennis K, Gopakumar G, Dunowska M. Effects of physiologic concentrations of l-lysine on in vitro replication of feline herpesvirus 1. Am J Vet Res. 2014;75:572–580. doi: 10.2460/ajvr.75.6.572. [DOI] [PubMed] [Google Scholar]
- 66.Stiles J, Townsend WM, Rogers QR, Krohne SG. Effect of oral administration of L-lysine on conjunctivitis caused by feline herpesvirus in cats. Am J Vet Res. 2002;63:99–103. doi: 10.2460/ajvr.2002.63.99. [DOI] [PubMed] [Google Scholar]
- 67.Maggs DJ, Nasisse MP, Kass PH. Efficacy of oral supplementation with L-lysine in cats latently infected with feline herpesvirus. Am J Vet Res. 2003;64:37–42. doi: 10.2460/ajvr.2003.64.37. [DOI] [PubMed] [Google Scholar]
- 68.Rees TM, Lubinski JL. Oral supplementation with L-lysine did not prevent upper respiratory infection in a shelter population of cats. J Feline Med Surg. 2008;10:510–513. doi: 10.1016/j.jfms.2008.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Fascetti AJ, Maggs DJ, Kanchuk ML, Clarke HE, Rogers QR. Excess dietary lysine does not cause lysine-arginine antagonism in adult cats. J Nutr. 2004;134:2042S–2045S. doi: 10.1093/jn/134.8.2042S. [DOI] [PubMed] [Google Scholar]
- 70.Maggs DJ, Sykes JE, Clarke HE, Yoo SH, Kass PH, Lappin MR, Rogers QR, Waldron MK, Fascetti AJ. Effects of dietary lysine supplementation in cats with enzootic upper respiratory disease. J Feline Med Surg. 2007;9:97–108. doi: 10.1016/j.jfms.2006.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Drazenovich TL, Fascetti AJ, Westermeyer HD, Sykes JE, Bannasch MJ, Kass PH, Hurley KF, Maggs DJ. Effects of dietary lysine supplementation on upper respiratory and ocular disease and detection of infectious organisms in cats within an animal shelter. Am J Vet Res. 2009;70:1391–1400. doi: 10.2460/ajvr.70.11.1391. [DOI] [PubMed] [Google Scholar]
- 72.Sandmeyer LS, Keller CB, Bienzle D. Effects of interferon-alpha on cytopathic changes and titers for feline herpesvirus-1 in primary cultures of feline corneal epithelial cells. Am J Vet Res. 2005;66:210–216. doi: 10.2460/ajvr.2005.66.210. [DOI] [PubMed] [Google Scholar]
- 73.Siebeck N, Hurley DJ, Garcia M, Greene CE, Kostlin RG, Moore PA, Dietrich UM. Effects of human recombinant alpha-2b interferon and feline recombinant omega interferon on in vitro replication of feline herpesvirus-1. Am J Vet Res. 2006;67:1406–1411. doi: 10.2460/ajvr.67.8.1406. [DOI] [PubMed] [Google Scholar]
- 74.Weiss RC. Synergistic antiviral activities of acyclovir and recombinant human leukocyte (alpha) interferon on feline herpesvirus replication. Am J Vet Res. 1989;50:1672–1677. [PubMed] [Google Scholar]
- 75.Cerruti RL, Connell EV, Trown PW, Sim IS. Synergistic interaction between interferon-alpha and acyclovir in the treatment of herpes simplex virus type 1 infection in mice. Antiviral Res. 1985;(Suppl 1):217–223. doi: 10.1016/s0166-3542(85)80031-0. [DOI] [PubMed] [Google Scholar]
- 76.Haid C, Kaps S, Gonczi E, Hassig M, Metzler A, Spiess BM, Richter M. Pretreatment with feline interferon omega and the course of subsequent infection with feline herpesvirus in cats. Vet Ophthalmol. 2007;10:278–284. doi: 10.1111/j.1463-5224.2007.00550.x. [DOI] [PubMed] [Google Scholar]
- 77.Cocker FM, Howard PE, Harbour DA. Effect of human alpha-hybrid interferon on the course of feline viral rhinotracheitis. Vet Rec. 1987;120:391–393. doi: 10.1136/vr.120.16.391. [DOI] [PubMed] [Google Scholar]
- 78.Ballin AC, Schulz B, Helps C, Sauter-Louis C, Mueller RS, Hartmann K. Limited efficacy of topical recombinant feline interferon-omega for treatment of cats with acute upper respiratory viral disease. Vet J. 2014;202:466–470. doi: 10.1016/j.tvjl.2014.09.030. [DOI] [PubMed] [Google Scholar]
- 79.Fenimore A, Carter K, Fankhauser J, Hawley JR, Lappin MR. Evaluation of intranasal vaccine administration and high-dose interferon- alpha 2b therapy for treatment of chronic upper respiratory tract infections in shelter cats. J Feline Med Surg. 2015 doi: 10.1177/1098612X15596199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Slack JM, Stiles J, Leutenegger CM, Moore GE, Pogranichniy RM. Effects of topical ocular administration of high doses of human recombinant interferon alpha-2b and feline recombinant interferon omega on naturally occurring viral keratoconjunctivitis in cats. Am J Vet Res. 2013;74:281–289. doi: 10.2460/ajvr.74.2.281. [DOI] [PubMed] [Google Scholar]
- 81.Gil S, Leal RO, Duarte A, McGahie D, Sepulveda N, Siborro I, Cravo J, Cartaxeiro C, Tavares LM. Relevance of feline interferon omega for clinical improvement and reduction of concurrent viral excretion in retrovirus infected cats from a rescue shelter. Res Vet Sci. 2013;94:753–763. doi: 10.1016/j.rvsc.2012.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Stiles J, Guptill-Yoran L, Moore GE, Pogranichniy RM. Effects of lambda-carrageenan on in vitro replication of feline herpesvirus and on experimentally induced herpetic conjunctivitis in cats. Invest Ophthalmol Vis Sci. 2008;49:1496–1501. doi: 10.1167/iovs.07-1245. [DOI] [PubMed] [Google Scholar]
- 83.Bagla VP, McGaw LJ, Eloff JN. The antiviral activity of six South African plants traditionally used against infections in ethnoveterinary medicine. Vet Microbiol. 2012;155:198–206. doi: 10.1016/j.vetmic.2011.09.015. [DOI] [PubMed] [Google Scholar]
- 84.Knight DA, Hejmanowski AQ, Dierksheide JE, Williams JW, Chong AS, Waldman WJ. Inhibition of herpes simplex virus type 1 by the experimental immunosuppressive agent leflunomide. Transplantation. 2001;71:170–174. doi: 10.1097/00007890-200101150-00031. [DOI] [PubMed] [Google Scholar]
- 85.Williams CR, Sykes JE, Mehl M, MacLeod JS, Lindsay LL, Poland AM, Chen YJ, Kyles AE, Waldman WJ, Gregory CR. In vitro effects of the active metabolite of leflunomide, A77 1726, on feline herpesvirus-1. Am J Vet Res. 2007;68:1010–1015. doi: 10.2460/ajvr.68.9.1010. [DOI] [PubMed] [Google Scholar]
- 86.Beaumont SL, Maggs DJ, Clarke HE. Effects of bovine lactoferrin on in vitro replication of feline herpesvirus. Vet Ophthalmol. 2003;6:245–250. doi: 10.1046/j.1463-5224.2003.00301.x. [DOI] [PubMed] [Google Scholar]
- 87.Wilkes RP, Kania SA. Use of interfering RNAs targeted against feline herpesvirus 1 glycoprotein D for inhibition of feline herpesvirus 1 infection of feline kidney cells. Am J Vet Res. 2009;70:1018–1025. doi: 10.2460/ajvr.70.8.1018. [DOI] [PubMed] [Google Scholar]
- 88.Wilkes RP, Kania SA. Evaluation of the effects of small interfering RNAs on in vitro replication of feline herpesvirus-1. Am J Vet Res. 2010;71:655–663. doi: 10.2460/ajvr.71.6.655. [DOI] [PubMed] [Google Scholar]
- 89.Wilkes RP, Ward DA, Newkirk KM, Adams JK, Kania SA. Evaluation of delivery agents used for introduction of small interfering RNAs into feline corneal cells. Am J Vet Res. 2013;74:243–247. doi: 10.2460/ajvr.74.2.243. [DOI] [PubMed] [Google Scholar]
- 90.Lappin MR, Veir JK, Satyaraj E, Czarnecki-Maulden G. Pilot study to evaluate the effect of oral supplementation of Enterococcus faecium SF68 on cats with latent feline herpesvirus 1. J Feline Med Surg. 2009;11:650–654. doi: 10.1016/j.jfms.2008.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Lim CC, Reilly CM, Thomasy SM, Kass PH, Maggs DJ. Effects of feline herpesvirus type 1 on tear film break-up time, Schirmer tear test results, and conjunctival goblet cell density in experimentally infected cats. Am J Vet Res. 2009;70:394–403. doi: 10.2460/ajvr.70.3.394. [DOI] [PubMed] [Google Scholar]
- 92.Cullen CL, Njaa BL, Grahn BH. Ulcerative keratitis associated with qualitative tear film abnormalities in cats. Vet Ophthalmol. 1999;2:197–204. doi: 10.1046/j.1463-5224.1999.00082.x. [DOI] [PubMed] [Google Scholar]
- 93.Sarisky RT, Quail MR, Clark PE, Nguyen TT, Halsey WS, Wittrock RJ, O'Leary Bartus J, Van Horn MM, Sathe GM, Van Horn S, Kelly MD, Bacon TH, Leary JJ. Characterization of herpes simplex viruses selected in culture for resistance to penciclovir or acyclovir. J Virol. 2001;75:1761–1769. doi: 10.1128/JVI.75.4.1761-1769.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Guardabassi L, Prescott JF. Antimicrobial stewardship in small animal veterinary practice: from theory to practice. Vet Clin North Am Small Anim Pract. 2015;45:361–376. doi: 10.1016/j.cvsm.2014.11.005. [DOI] [PubMed] [Google Scholar]
- 95.White ML, Chodosh J. Herpes Simplex Virus Keratitis: A Treatment Guideline - 2014. In: Ophthalmology AAo, editor. Clinical Guidelines. American Academy of Ophthalmology Clinical Guideline; Boston: 2014. [Google Scholar]
- 96.De Vlaminck I, Khush KK, Strehl C, Kohli B, Luikart H, Neff NF, Okamoto J, Snyder TM, Cornfield DN, Nicolls MR, Weill D, Bernstein D, Valantine HA, Quake SR. Temporal response of the human virome to immunosuppression and antiviral therapy. Cell. 2013;155:1178–1187. doi: 10.1016/j.cell.2013.10.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Andrei G, Snoeck R, Goubau P, Desmyter J, De Clercq E. Comparative activity of various compounds against clinical strains of herpes simplex virus. Eur J Clin Microbiol Infect Dis. 1992;11:143–151. doi: 10.1007/BF01967066. [DOI] [PubMed] [Google Scholar]
- 98.Babiuk LA, Meldrum B, Gupta VS, Rouse BT. Comparison of the antiviral effects of 5-methoxymethyldeoxyuridine with 5-iododeoxyuridine, cytosine arabinoside, and adenine arabinoside. Antimicrob Agents Chemother. 1975;8:643–650. doi: 10.1128/aac.8.6.643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Chen ZJ, Liu WG, Song JZ. [Experimental studies of 9-(1, 3-dihydroxy-2-propoxymethyl)-guanine(DHPG) against herpes simplex virus] Yao Xue Xue Bao. 1989;24:331–334. [PubMed] [Google Scholar]
- 100.Boyd MR, Bacon TH, Sutton D, Cole M. Antiherpesvirus activity of 9-(4-hydroxy-3-hydroxy-methylbut-1-yl)guanine (BRL 39123) in cell culture. Antimicrob Agents Chemother. 1987;31:1238–1242. doi: 10.1128/aac.31.8.1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.De Clercq E, Sakuma T, Baba M, Pauwels R, Balzarini J, Rosenberg I, Holy A. Antiviral activity of phosphonylmethoxyalkyl derivatives of purine and pyrimidines. Antiviral Res. 1987;8:261–272. doi: 10.1016/s0166-3542(87)80004-9. [DOI] [PubMed] [Google Scholar]
- 102.Gadler H, Larsson A, Solver E. Nucleic acid hybridization, a method to determine effects of antiviral compounds on herpes simplex virus type 1 DNA synthesis. Antiviral Res. 1984;4:63–70. doi: 10.1016/0166-3542(84)90026-3. [DOI] [PubMed] [Google Scholar]
- 103.Ayisi NK, Gupta SV, Babiuk LA. Combination chemotherapy: interaction of 5-methoxymethyldeoxyuridine with trifluorothymidine, phosphonoformate and acycloguanosine against herpes simplex viruses. Antiviral Res. 1985;5:13–27. doi: 10.1016/0166-3542(85)90011-7. [DOI] [PubMed] [Google Scholar]
- 104.Sandmeyer LS, Keller CB, Bienzle D. Effects of cidofovir on cell death and replication of feline herpesvirus-1 in cultured feline corneal epithelial cells. Am J Vet Res. 2005;66:217–222. doi: 10.2460/ajvr.2005.66.217. [DOI] [PubMed] [Google Scholar]
- 105.Bronson JJ, Ghazzouli I, Hitchcock MJ, Webb RR, 2nd, Martin JC. Synthesis and antiviral activity of the nucleotide analogue (S)-1-[3-hydroxy-2-(phosphonylmethoxy)propyl]cytosine. J Med Chem. 1989;32:1457–1463. doi: 10.1021/jm00127a010. [DOI] [PubMed] [Google Scholar]
- 106.Bestman-Smith J, Boivin G. Herpes simplex virus isolates with reduced adefovir susceptibility selected in vivo by foscarnet therapy. J Med Virol. 2002;67:88–91. doi: 10.1002/jmv.2195. [DOI] [PubMed] [Google Scholar]
- 107.Collins P. The spectrum of antiviral activities of acyclovir in vitro and in vivo. J Antimicrob Chemother. 1983;12(Suppl B):19–27. doi: 10.1093/jac/12.suppl_b.19. [DOI] [PubMed] [Google Scholar]