Abstract
Background
Poly (ADP-ribose) polymerase (PARP) is essential for recognition and repair of DNA damage. In preclinical models, PARP inhibitors modulate topoisomerase I inhibitor-mediated DNA damage. This Phase I study determined the maximum tolerated dose (MTD), dose-limiting toxicities (DLTs), pharmacokinetics (PK) and pharmacodynamics (PD) of veliparib, an orally-bioavailable PARP 1/2 inhibitor, in combination with irinotecan.
Methods
Patients with advanced solid tumors were treated with 100 mg/m2 irinotecan on days 1 and 8 of a 21-day cycle. Twice-daily (BID) oral dosing of veliparib (10–50 mg) occurred days 3–14 (Cycle 1) and days −1–14 (subsequent cycles) followed by a 6-day rest. PK studies were conducted with both agents alone and in combination. Paired tumor biopsies were obtained after irinotecan alone and veliparib/irinotecan to evaluate PARP1/2 inhibition and explore DNA damage signals (nuclear γ-H2AX and pNBS1).
Results
Thirty-five patients were treated. DLTs included fatigue, diarrhea, febrile neutropenia, and neutropenia. The MTD was 100 mg/m2 irinotecan (days 1, 8) combined with veliparib 40 mg BID (days −1–14) on a 21-day cycle. Of 31 response-evaluable patients there were 6 (19%) partial responses. Veliparib exhibited linear PK, and there were no apparent PK interactions between veliparib and irinotecan. At all dose levels, veliparib reduced tumor poly(ADP-ribose) (PAR) content in the presence of irinotecan. Several samples showed increases in γ-H2AX and pNBS1 after veliparib/irinotecan compared to irinotecan alone.
Conclusions
Veliparib can be safely combined with irinotecan at doses that inhibit PARP catalytic activity. Preliminary antitumor activity justifies further evaluation of the combination.
INTRODUCTION
Poly (adenosine diphosphate-ribose) (PAR) polymerases 1 and 2 (PARP1 and PARP2) are members of an essential nuclear protein superfamily that play a role in recognition of DNA damage and facilitation of DNA repair. PARP inhibition has emerged as a promising strategy as monotherapy for cancers defective in homologous recombination (HR) repair, such as those arising in BReast CAncer susceptibility gene (BRCA) carriers (1–4), and as chemo-potentiation for a variety of DNA-damaging agents, including topoisomerase I poisons, alkylators, platinum-based agents and γ-irradiation (5–12). Recently, it has been shown that PARP inhibitors possess two activities: (1) inhibition of NAD+-competitive catalytic activity of PARP; and (2) ability to trap PARP-DNA complexes. Cytotoxicity of monotherapy in preclinical models has been correlated with the trapping of PARP-DNA complexes (13).
Several orally-bioavailable small molecule PARP inhibitors are under active clinical development, including veliparib (ABT-888) (14, 15). Importantly, PARP-DNA complex trapping is drug-specific, with talazoparib (BMN673) and olaparib demonstrating a greater ability than veliparib, while all of the compounds are potent catalytic PARP inhibitors (13, 16). These findings may explain why inhibition of the catalytic activity of PARP, assayed by tumor content of PAR, was demonstrated at a dose of 25 mg veliparib in a Phase 0 study (17), whereas doses of 300 mg and above were required for tumor responses in a monotherapy Phase 1 study of veliparib in BRCA-deficient cancers (18).
Preclinical synergism between PARP inhibition and topoisomerase I poisons has been firmly established (19–25). The precise mechanism of synergy remains under intense investigation. In vitro studies combining a PARP inhibitor with camptothecin or the camptothecin derivative irinotecan have demonstrated variable effects on the onset and magnitude of DNA damage, the persistence of DNA damage and the time required for cells to accomplish repair (20, 22). Additionally, whether inhibition of PARP catalytic activity is sufficient, or whether PARP-DNA trapping is required for potentiation of topoisomerase I inhibitor-mediated DNA damage remains controversial (26, 27). Nonetheless, in vivo, the addition of a PARP inhibitor has resulted in substantial potentiation of the antitumor efficacy of irinotecan and other topoisomerase I poisons against a variety of human tumor xenografts (19, 21, 22).
Based on these preclinical data, we conducted a clinical trial of veliparib combined with irinotecan in patients with advanced solid tumors. The primary objective was to determine the recommended phase II dose (RP2D) by evaluating the feasibility, safety, adverse events (AEs), dose limiting toxicities (DLTs), and the maximum tolerated dose (MTD). Secondary objectives were to characterize the pharmacokinetics (PK) of veliparib and irinotecan, alone and in combination, and to assess preliminary antitumor activity. Paired tumor biopsies obtained post-irinotecan and post-veliparib/irinotecan at timepoints established for quantifying veliparib-mediated reductions in PAR levels were used to confirm inhibition of PARP catalytic activity (17). Additionally, we studied modulation of irinotecan-induced DNA damage and repair by veliparib at these timepoints by measuring two key proteins of the DNA damage response machinery: nuclear phosphorylated histone 2AX (γ-H2AX) and phosphorylated Nijmegan breakage syndrome 1 (pNBS1) (28).
MATERIALS AND METHODS
Patient selection
Eligible patients had histologically or cytologically confirmed metastatic or unresectable malignancy for which standard curative or palliative measures were nonexistent or ineffective or for which irinotecan was a viable treatment regimen; Eastern Cooperative Oncology Group (ECOG) performance status ≤ 2; measurable disease (per RECIST) accessible for biopsy; and adequate organ and marrow function defined as absolute neutrophil count ≥ 1,500/µL, platelets ≥100,000/µL, aspartate aminotransferase and/or alanine aminotransferase ≤ 2.5 × upper limit of normal (ULN) or ≤ 5 × ULN if liver metastases present; bilirubin ≤ 1.5 × ULN; and creatinine ≤ 1.5 × ULN or calculated or measured creatinine clearance ≥ 60 mL/min/1.73 m2. Patients homozygous for the UGT1A1*28 allele (A(TA7)TAA) were ineligible due to the potential increased risk of toxicity associated with irinotecan (29, 30).
Prior chemotherapy, experimental therapy or radiotherapy to >5% of bone marrow must have been completed at least 4 weeks prior to treatment initiation. Patients who received prior radiation to ≥50% of their total marrow volume were excluded. CYP3A4 isoform-inducing drugs had to be discontinued at least 2 weeks prior to the first administration of irinotecan. Patients were also excluded if they had an uncontrolled intercurrent illness, prior history of seizures (based on the ability of veliparib to lower the seizure threshold), known active brain metastases, or a requirement for chronic maintenance of growth factor support.
Study treatment and design
Supplementary Figure S1 shows the study design and sample collection time points. Treatment cycles were 21 days. Irinotecan was administered intravenously at 100 mg/m2 over 90 minutes on Days 1 and 8, a dose and schedule that is tolerable in heavily pre-treated patients. Blood samples for single-agent irinotecan PK were collected on Cycle 1, Days 1–3 following administration of the first dose. Twice daily (BID) oral administration of veliparib (Abbvie, Inc.) began on Day 3 of Cycle 1 and continued until Day 14, followed by 6 days of no treatment (Days 15–20). Four dose levels of veliparib were tested: 10, 20, 40, and 50 mg BID. A single dose of veliparib was administered on Day 21 (Cycle 2, Day −1; i.e. one day prior to Cycle 2 irinotecan), for evaluation of single-agent veliparib PK. BID veliparib continued in Cycle 2 through Day 14, followed by a 6-day rest. Serial blood samples were obtained on Cycle 2, Days 8–10 for evaluation of veliparib and irinotecan PK when co-administered. For subsequent cycles, veliparib was administered BID from Day −1 through Day 14, followed by a 6-day rest (15 days on treatment/6 days off).
At least three patients received study treatment on each cohort in standard 3+3 fashion. DLTs were based on toxicities observed during the first cycle. Toxicities were graded using the NCI Common Terminology Criteria for Adverse Events (CTCAE) version 3.0 until July 31, 2010, after which CTCAE version 4.0 was utilized. DLTs were defined as any grade ≥ 3 non-hematologic toxicity; grade ≥ 3 nausea, vomiting, or diarrhea uncontrolled by aggressive treatment; grade 4 granulocytopenia lasting ≥ 5 days without hematopoetic growth factor support; grade 4 thrombocytopenia or febrile neutropenia; inability to begin Cycle 2 of treatment (at full dose) within 2 weeks of the scheduled date of administration due to unresolved toxicity; Grade ≥ 2 non-hematological toxicity persisting beyond the first 42 days or the occurrence of Grade 2 toxicities that, in the judgment of the PI, were dose-limiting. For grade ≥ 3 electrolyte imbalance secondary to another toxicity, grading of the precipitating toxicity was used for DLT definition.
A new cycle of therapy did not begin until any toxicity recovered to ≤ grade 1, with no more than a 2-week delay permitted. Patients were also discontinued if there was a > 2 week delay in reinstitution of veliparib due to drug-related toxicity during a cycle. For a ≤ 2 week delay or a grade ≥ 3 toxicity related to veliparib, treatment proceeded with a one dose level reduction. Dose modifications for irinotecan followed those recommended in the package insert (recommended dose modifications for single-agent schedules) (31). Dose reductions could occur multiple times to the lowest protocol-specified dose level as long as there was clinical benefit. Dose re-escalation was not allowed.
Written informed consent was obtained from enrolled patients. Institutional Review Board approvals of the protocol and consent form were obtained at all sites. Protocol design and conduct complied with all applicable regulations, guidance, and local polices. The study was conducted in accordance with the Declaration of Helsinki. The trial (ClinicalTrials.gov identifier NCT00576654) was conducted under a National Cancer Institute (NCI)-sponsored Investigational New Drug (IND) application. Veliparib was supplied under a Collaborative Research and Development Agreement between Abbvie, Inc. and the Division of Cancer Treatment and Diagnosis, NCI. Irinotecan was obtained commercially.
PK evaluation
Plasma PK of veliparib and irinotecan were assessed for each agent alone and in combination. For single-agent irinotecan, blood samples were collected at pre-dose and at 0.5, 1.5 (immediately at the end of infusion), 2, 3.5, 5.5, 8.5, 28, and 48 hours (h) following the start of infusion on Cycle 1, Day 1. For single-agent veliparib, blood samples were collected at pre-dose and at 0.5, 1, 1.5, 3.5, 5.5, 8.5, 10, and 28 h after oral administration of a single dose on Cycle 2, Day −1. The PK of veliparib and irinotecan in combination were evaluated on Cycle 2, Day 8. Serial blood samples were collected at pre-dose and at 0.5, 1, 1.5, 2, 3.5, 5.5, 8.5, 10 (prior to the administration of the afternoon dose of veliparib), 28 and 48h following the administration of both drugs on day 8 (prior to veliparib dosing days 9 and 10). Following isolation of plasma by centrifugation, samples were stored at −80°C.
The plasma concentrations of veliparib and its main metabolite (A-925088) were determined using a validated liquid chromatography with tandem mass spectrometry (LC-MS/MS) method (32). The plasma concentrations of irinotecan and its major active metabolite SN-38 were determined using a validated high-performance liquid chromatographic method with fluorescence detection (33) with modifications described in the Supplementary Methods. The PK parameters of veliparib, irinotecan, and their metabolites, were estimated using non-compartmental analysis with WinNonlin software (Pharsight).
Analysis of Pharmacodynamic Biomarkers
Tumor sampling was designed to compare the levels of PAR as a PD biomarker of PARP 1/2 catalytic activity after irinotecan alone to those after the veliparib/irinotecan combination. Patients underwent tumor biopsies on Cycle 1 Day 2, ~28 hours after the start of irinotecan and again on Cycle 1 Day 9, ~28 hours after the second dose of irinotecan and ~4 hours after the morning dose of veliparib, a timepoint informed by preclinical studies and confirmed by the prior Phase 0 trial (17, 34).
Tumor PAR content was quantified using a validated, fit-for-purpose sandwich immunoassay (IA) of denatured tumor extracts (17, 34) and was performed according to NCI standard operating procedures (SOPs) detailed in the Supplementary Methods.
Whenever feasible, each biopsy procured a second pass specimen for surveying two nuclear PD biomarkers of DNA damage and repair (DDR): γ-H2AX and pNBS1. Nuclear γ-H2AX was quantified using a validated single-plex immunofluorescence microscopy assay (IFA) according to NCI-DCTD SOPs detailed in the Supplementary Methods. This assay employs ImagePro-based image analysis of all viable cell types in the specified number of objectively selected fields, excluding necrotic areas, and reports the percentage of their total nuclear area that is covered by the nuclear γ-H2AX staining pattern (%NAP, nuclear area positive). A preclinical fit-for-purpose study demonstrated the utility of this assay for quantifying the repair response to DNA damage caused by topoisomerase-1 inhibitors, including camptothecins (35).
The timing of the DDR response relative to reduction in tumor PAR levels in cancer patients is unknown; therefore, an exploratory multiplex IFA was developed to evaluate a second PD biomarker, pNBS1, in concert with γ-H2AX. After a modified antigen retrieval step replacing citrate with EDTA in the SOP for the validated γ-H2AX IFA (35), both γ-H2AX and pNBS1 biomarkers were evaluated in the same cut paraffin section after sequential staining on a Bond-max™ Autostainer with antibodies conjugated to different fluorochromes. Details of this exploratory IFA are in the Supplementary Methods. Following image capture, Tissue Studio software (Definiens, Carlsbad, CA) was used to quantify %NAP of γ-H2AX only in fields with a high content of viable tumor cells. Assessment of nuclear pNBS in paired biopsies was qualitative.
Statistical methods
Descriptive statistics were used to summarize the baseline patient characteristics, treatment-related adverse events and PK parameters. Confidence interval (CI) estimates of Grade ≥ 3 toxicity rates and response rates were calculated using Wilson’s method. The comparison of PK parameters of veliparib or irinotecan between single-agent and combination treatments was performed using a paired, two-sided Student’s t-Test. Associations between changes in PD markers, PK parameters and measures of clinical outcome were also assessed. We hypothesized a positive association between increasing veliparib dose and the percent reduction of PAR. This hypothetical gradient in PAR reduction was investigated with the exact version of the nonparametric Jonckheere-Terpstra test for ordered alternatives (1-sided), given the small sample sizes per dose level.
RESULTS
Patient Demographics
Thirty-five consented patients (Table 1) were enrolled between March 2008 and June 2011. Whenever possible, germline BRCA status documentation (performed by Myriad Genetics, Inc.) was obtained. All patients received at least one dose of study drug, with a median of 3 (range of 1–28) cycles of veliparib administered. Patients had ≥ 1 line of prior systemic therapy and had evidence of disease progression at enrollment. Four patients did not complete a full cycle of treatment and were not evaluable for response or MTD determination due to financial reasons (1), rapid clinical deterioration (1) or disease progression (2).
Table 1.
Patient Characteristics
| Characteristic | N= 35 (%) |
|---|---|
| Gender | |
| Male | 7 (20) |
| Female | 28 (80) |
| Median (range) age in years | 54 (31–73) |
| Eastern Cooperative Oncology Group performance status | |
| 0 | 9 (26) |
| 1 | 24 (68) |
| 2 | 2 (6) |
| Tumor Type | |
| Breast | 15 (43) |
| Ovary | 9 (26) |
| Colon | 4 (11) |
| Esophagus | 4 (11) |
| Lung | 2 (6) |
| Anus | 1 (3) |
| Received prior systemic therapy | 35 (100) |
| Median no. prior therapies (range) | 4 (1–12) |
| Median no. veliparib cycles/patient (range) | 3 (1–28) |
Dose escalation and determination of MTD and RP2D
During dose escalation, 4 DLTs were observed. The first grade 3 toxicity, fatigue, occurred at the initial dose level (veliparib 10 mg BID). A second DLT of grade 4 neutropenia was observed at 20 mg BID. Two additional DLTs, one each of grade 3 diarrhea and febrile neutropenia occurred at 50 mg BID, resulting in expansion of the 40 mg veliparib BID cohort with eight additional patients. No DLTs were observed in the additional patients; therefore 40 mg veliparib BID administered on a 15 days on/6 days off schedule combined with 100 mg/m2 irinotecan on days 1 and 8 of a 21-day cycle was established as the MTD and RP2D.
Toxicity
The combination of veliparib and irinotecan was generally well tolerated (Table 2). The most common toxicities among the 35 patients treated across all dose levels included diarrhea (63%; 95% CI 46 – 77%), nausea (60%; 95% CI 44 – 74%), fatigue (60%; 95% CI 44 – 74%), neutropenia (49%; 95% CI 33 – 64%), and leukopenia (49%; 95% CI 33 – 64%). Beyond standard loperamide recommended during irinotecan treatment, 13 patients across dose levels required additional medications, including diphenoxylate and atropine, tincture of opium and in one case, octreotide injections surrounding irinotecan doses. In 4 patients who achieved a partial or mixed response, pegfilgrastim was used after the first cycle in order to maintain the initial dose of 20 or 40 mg veliparib BID with irinotecan. Thirteen patients required dose reductions during their treatment course, for diarrhea (6), dehydration (3), neutropenia (5), fatigue (2), nausea (3), or a combination of these toxicities. Eleven patients required dose interruption, most commonly for recovery of neutropenia. One ovarian cancer patient on the 20 mg BID veliparib dose experienced intolerable diarrhea, nausea and fatigue with irinotecan, even after two dose reductions within the first 6 cycles. She was permitted to remain on veliparib alone, and received an additional 11 cycles before progressive disease was documented.
Table 2.
Most Common (≥ 10%) treatment-related adverse events (maximum grade, all cycles)
| Adverse Event | Total # Patients With Event N=35(%) |
Veliparib Dose Level | |||||||
|---|---|---|---|---|---|---|---|---|---|
| 10 mg N=7 |
20 mg N=9 |
40 mg N=12 |
50 mg N=7 |
||||||
| All (%) |
Gr≥3 (%) |
All (%) |
Gr≥3 (%) |
All (%) |
Gr≥3 (%) |
All (%) |
Gr≥3 (%) |
||
| Diarrhea | 22 (63) | 5 (71) | 1 (14) | 5 (56) | 1 (11) | 8 (67) | - | 4 (57) | 1 (14) |
| Fatigue | 21 (60) | 5 (71) | 1 (14) | 5 (56) | 2 (22) | 6 (50) | - | 5 (71) | - |
| Nausea | 21 (60) | 4 (57) | - | 7 (78) | - | 6 (50) | - | 4 (57) | - |
| Leukopenia | 17 (49) | 4 (57) | 3 (43) | 4 (44) | 1 (11) | 4 (33) | 2 (17) | 5 (71) | 2 (29) |
| Neutropenia | 17 (49) | 4 (57) | 2 (29) | 4 (44) | 2 (22) | 5 (42) | 2 (17) | 4 (57) | 3 (43) |
| Anemia | 13 (37) | 4 (57) | 1 (14) | 1 (11) | - | 5 (42) | - | 3 (43) | - |
| Anorexia | 11 (31) | 3 (43) | - | 1 (11) | - | 4 (33) | - | 3 (43) | - |
| Vomiting | 9 (26) | 2 (29) | - | 3 (33) | - | 3 (25) | - | 1 (14) | - |
| Alopecia | 7 (20) | 1 (14) | - | 1 (11) | - | 4 (33) | - | 1 (14) | - |
| Lymphopenia | 6 (17) | 2 (29) | 2 (29) | 1 (11) | 1 (11) | 3 (25) | - | - | - |
| Dehydration | 5 (14) | 1 (14) | 1 (14) | 2 (22) | - | 1 (8) | - | 1 (14) | - |
| Hypoalbuminemia | 5 (14) | 1 (14) | - | 1 (11) | - | 3 (25) | - | - | - |
| Abdominal Pain | 4 (11) | 1 (14) | 1 (11) | 1 (8) | 1 (14) | ||||
Pharmacokinetics
PK samples for veliparib and irinotecan were obtained from 26 and 34 patients, respectively. The PK parameters estimates for veliparib following oral administration (Supplementary Table S1) were consistent with those reported previously (5, 6, 17). The systemic exposure (i.e., Cmax and AUClast) to veliparib or A-925088 increased with dose (Supplementary Figure S2). There was no appreciable difference in veliparib clearance (median CL/F: 18 versus 15 L/h, P > 0.05) and the AUC ratio of A-925088 to veliparib (median, 0.21 versus 0.14, P > 0.05) when veliparib was given alone or in combination with irinotecan. The PK parameters of irinotecan and SN-38 following intravenous infusion (Supplementary Table S2) were also consistent with those previously reported (36). There was no apparent difference in the PK parameters of irinotecan and SN-38 when irinotecan was given alone or in combination with veliparib (Supplementary Table S2). Collectively, these data suggest no PK interactions between veliparib and irinotecan.
Efficacy
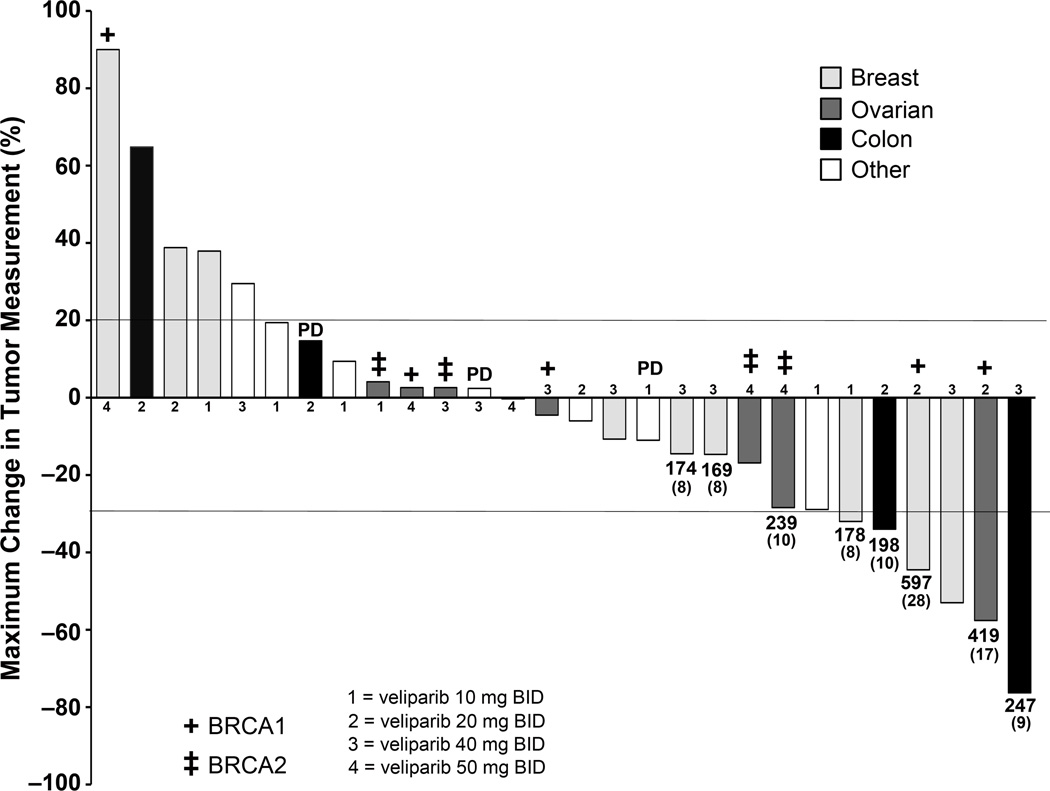
Thirty-one patients were evaluable for response. The maximum percent change in target lesions among the 28 patients with pre- and on-treatment radiographic assessments is shown in Figure 1. Six patients experienced a partial response (PR; mean 12.3 cycles; range 6–28 cycles) for a PR rate of 6/31 = 19%; 95% CI 9 – 36%. Three of these were advanced breast cancer patients, treated at the 10, 20 and 40 mg BID dose levels, for 8, 28 and 6 cycles, respectively; the patient initiated at the 20 mg BID dose level was a BRCA1 carrier who escalated to 40 mg BID after 13 cycles and remained on study for an additional 15 cycles. Partial responses also occurred in two colon cancer patients treated at the 20 and 40 mg BID dose levels for 10 and 9 cycles, respectively. The former patient had received prior irinotecan. The other two colorectal cancer patients enrolled, one of whom had disease with microsatellite instability, had both received prior irinotecan and had progressive disease after 2 cycles.
Figure 1. Waterfall plot demonstrating the maximum percent change in target lesions among 28 patients with pre- and on-treatment radiographic assessments.
Diagnoses, dose levels and BRCA carrier status (for known subjects) are indicated. For subjects who remained on trial for approximately 6 months or longer, the number of days on study and number of cycles administered (parenthesized) are indicated beneath the bar. One of the colon cancer patients with progressive disease as best response had tumor with microsatellite instability.
Of the 9 patients with ovarian cancer enrolled to the study, all were BRCA1 or BRCA2 carriers. The sixth partial response occurred in a BRCA1 carrier with platinum-sensitive ovarian cancer, who had received 3 prior regimens and who was treated at the 20 mg BID dose level; she received combined irinotecan and veliparib for 6 cycles and veliparib alone for 11 cycles. A second patient with platinum-sensitive disease had been treated with 11 prior regimens, achieved stable disease (SD) as the best response and received 4 cycles of combination treatment. The remaining 7 ovarian cancer patients were considered to have platinum-resistant or refractory disease. Two of these patients experienced disease progression during the first cycle. The other 5 patients had been treated with 4–8 prior regimens, achieved SD as the best response, and received between 2–10 cycles of the combination.
One patient with advanced breast cancer on the 40 mg BID dose level had a mixed response (MR) and received 8 cycles. Thirteen patients met the criteria of SD after two cycles of treatment (median 2 cycles; range 2–10 cycles) for an SD rate of 13/31 = 42%; 95% CI 26 – 59%. There were 9 patients progression-free at 4 months (29%; 95% CI 16 – 47%), and 5 patients progression-free at 6 months after start of treatment (16%; 95% CI 7 – 33%).
Pharmacodynamics of catalytic PARP 1/2 inhibition
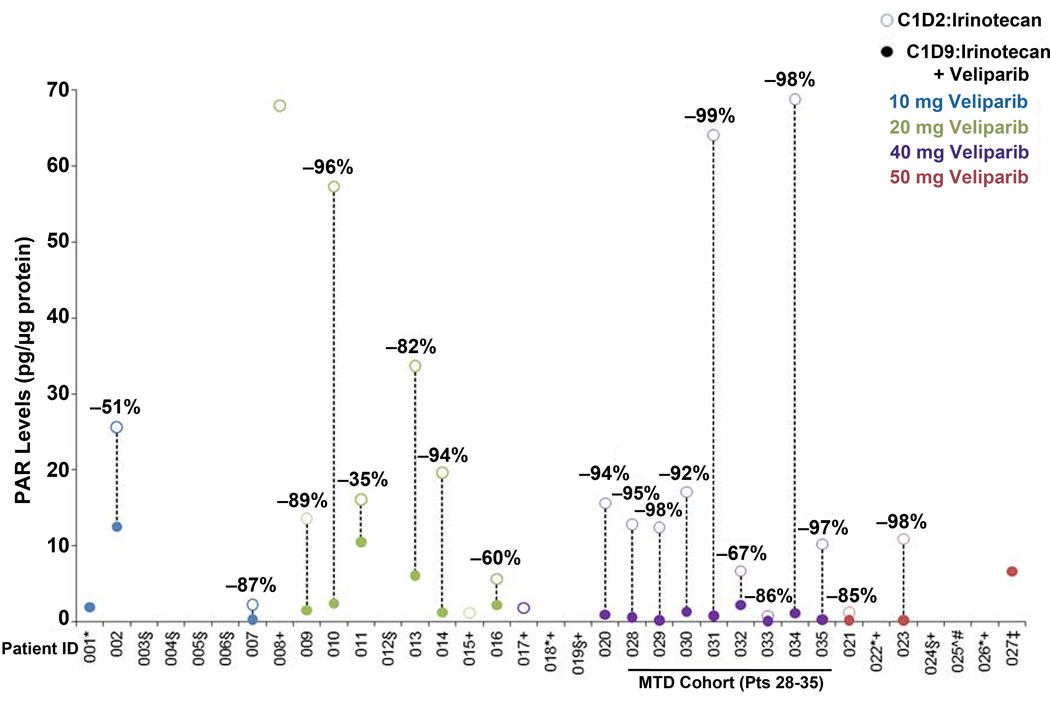
Paired biopsies were collected from 26 of the 35 patients; of these 26 pairs, 19 were fully evaluable for quantifying the primary PD endpoint of the PARP1/2 response to veliparib in the presence of irinotecan, including all eight patients enrolled in the MTD cohort at veliparib 40 mg BID (Figure 2 and Table 3). The reasons behind the attrition of seven biopsy pairs were (a) quality control failure in assay performance (5 pairs) with a root cause traced to a change in the supplier’s method for producing the commercial antibody that was addressed prior to patient 13 by modifying the assay SOP (see Supplementary Methods), (b) one pair with insufficient quality in the first biopsy, and (c) one pair in which both biopsies contained PAR levels below the assay Lower Limit of Quantitation (LLQ).
Figure 2. PAR content in paired tumor biopsies after irinotecan alone and after combined veliparib/irinotecan.
PAR levels in tumor obtained from biopsies performed on cycle 1 day 2, 28 hours after the first dose of irinotecan (open circles) and cycle 1, day 9, 28 hours after combined veliparib/irinotecan (closed circles), demonstrating substantial reductions in PAR after veliparib exposure across dose levels. Of 26 patients from whom paired biopsies were procured, 19 patients were evaluable for a quantitative change in PAR tumor content, including all 8 patients enrolled in the MTD cohort at 40-mg BID veliparib. *No Day 2 sample. ‡Day 2 sample, insufficient quality. ±Day 2 sample, insufficient protein. ∧Day 2 sample, < LLQ. +No Day 9 sample. #Day 9 sample, < LLQ. §Assay failed QC.
Table 3.
Analysis of PAR Levels and Pharmacodynamic Biomarkers of DNA Damage Response in Paired Tumor Biopsy Specimens
| Patient Number |
Dose Level ABT-888 (mg) |
Best Response |
Validated PAR-IA | Validated Nuclear γH2Ax-qIFA1 (all cells in fields without necrosis) |
Exploratory Nuclear γH2Ax-qIFA1 (only fields with high content of viable tumor cells) |
Exploratory Nuclear pNBS1-qIFA2 (only fields with high content of viable tumor cells) |
|||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Day 2 Tumor PAR Content 28-hr of CPT-11 w/o ABT-888 (pg/µg protein) |
Day 9 Tumor PAR Content 28-hr of CPT-11 4-hr after a.m. ABT-888 dose (pg/µg protein) |
Decline in Tumor PAR from Day 2→9 (%) |
Day 2 Nuclear γH2Ax 28-hr of CPT-11 w/o ABT-888 (% NAP) |
Day 9 Nuclear γH2Ax 28-hr of CPT-11 4-hr after a.m. ABT-888 dose (% NAP) |
Day 2 Nuclear γH2Ax 28-hr of CPT-11 w/o ABT-888 (% NAP) |
Day 9 Nuclear γH2Ax 28-hr of CPT-11 4-hr after a.m. ABT-888 dose (% NAP) |
Day 2 Nuclear pNBS1 28-hr of CPT-11 w/o ABT-888 (% NAP) |
Day 9 Nuclear pNBS1 28-hr of CPT-11 4-hr after a.m. ABT-888 dose (% NAP) |
|||
| 002 | 10 | PD | 25.6 | 12.5 | −51 | < 5.0 | 9.5 | < 5.0 | < 5.0 | background | background |
| 010 | 20 | PD | 57.3 | 2.4 | −96 | 7.9 | 7.0 | < 5.0 | < 5.0 | background | background |
| 014 | 20 | PD | 19.6 | 1.2 | −94 | 9.8 | 7.1 | 8.4 | n/a | ++ | n/a |
| 016 | 20 | PD | 5.7 | 2.2 | −60 | < 5.0 | < 5.0 | n/a | n/a | ||
| 034 | 40 | PD | 68.8 | 1.2 | −98 | < 5.0 | 9.9 | 6.7 | < 5.0 | detectable | background |
| 006 | 10 | SD | n/a | n/a | n/a | < 5.0 | < 5.0 | < 5.0 | < 5.0 | background | background |
| 007 | 10 | SD | 2.3 | 0.3 | −87 | < 5.0 | < 5.0 | < 5.0 | < 5.0 | detectable | detectable |
| 028 | 40 | SD | 12.8 | 0.6 | −95 | < 5.0 | < 5.0 | < 5.0 | < 5.0 | detectable | + |
| 009 | 20 | PR | 13.6 | 1.6 | −89 | < 5.0 | < 5.0 | n/a | n/a | ||
| 012 | 20 | PR | n/a | n/a | n/a | 6.0 | < 5.0 | n/a | n/a | ||
| 013 | 20 | PR | 33.7 | 6.1 | −82 | < 5.0 | 5.7 | < 5.0 | < 5.0 | ++ | ++++ |
Paired biopsies for evaluation of PAR levels in first-pass core specimens also yielded second-pass core specimens in some patients that were sufficient for analysis of nuclear PD-biomarkers of DNA damage response (DDR). Note that nuclear pNBS1 was the only PD-biomarker of DDR that exhibited a signal at sampling times selected for evaluating PAR levels. Samples demonstrating evidence of increased DNA damage or persistent DNA repair after combined irinotecan/veliparib compared to irinotecan alone are bolded.
The validated single-plex IFA for nuclear γ-H2AX employs ImagePro-based image analysis and reports %NAP (nuclear area positivity) for all viable cells in non-necrotic fields. The exploratory multi-plex IFA for nuclear γ-H2AX employs a Definiens-based image analysis algorithm that reports %NAP only in fields predominantly composed of viable tumor cells. Background %NAP values of nuclear γ-H2AX in untreated tumor specimens are <5%, so %NAP values must exceed this threshold to demonstrate an increase in DDR.
The term “background” means <1% positive nuclei in all fields, “detectable” means a few positive nuclei in only some fields, and “++” and “++++” indicate progressively higher numbers of strongly positive nuclei in every field.
n/a-not assessable (insufficient cells for analysis); PD – progressive disease; PR – partial response; SD – stable disease
After thirteen BID doses, veliparib reduced PAR tumor levels in all PD-evaluable patients by 35–99%. The median PAR reduction across the veliparib dose levels of 10, 20, 40, and 50 mg was 69% (range 51–87%), 86% (range 35–96%), 95% (range 67–99%), and 92% (range 85–98%), respectively. This gradient in PAR reduction was statistically significant (p = 0.0184, 1-sided). However, there was no analogous positive association between the degree of PARP inhibition and best response; several patients with progressive disease as the best response exhibited > 90% reduction in tumor PAR content after veliparib (see Table 3). Nonetheless, the results demonstrate the effectiveness of veliparib at inhibiting PARP1/2 catalytic activity within 4 hours of its administration one day after irinotecan treatment.
DNA Damage and Repair Pharmacodynamics
Second pass core specimens from the paired biopsies of 11 patients yielded sufficient tissue for evaluating two nuclear PD biomarkers of DDR response: γ-H2AX and pNBS1. Modulation of nuclear γ-H2AX was initially evaluated using the validated single-plex IFA (41), and the response of this PD biomarker was low and highly variable (Table 3). In three sample pairs, the γ-H2AX NAP score (% nuclear area positive) increased above basal levels (i.e., >5% NAP) after veliparib/irinotecan compared with irinotecan alone (patients 2, 13 and 34). These patients had increases in NAP scores from < 5% after irinotecan alone to 9.5, 5.7 and 9.9% after veliparib/irinotecan, with PD, PR and PD as the best response, respectively. These %NAP values are considerably lower than those obtained in human tumor xenograft models that respond to topoisomerase 1 inhibitors, which reach 25% at 4 hours after dosing (35). Furthermore, these small changes in %NAP values were not confirmed in the exploratory multiplex assay, which uses an image analysis algorithm that reports %NAP only in fields predominately composed of viable tumor cells (Table 3 and Figure 3). In the remaining patients evaluable for DDR, the addition of veliparib to irinotecan treatment failed to increase nuclear γ-H2AX at the time of demonstrated decreases in tumor content of PAR (Table 3 and Supplementary Figure S3).
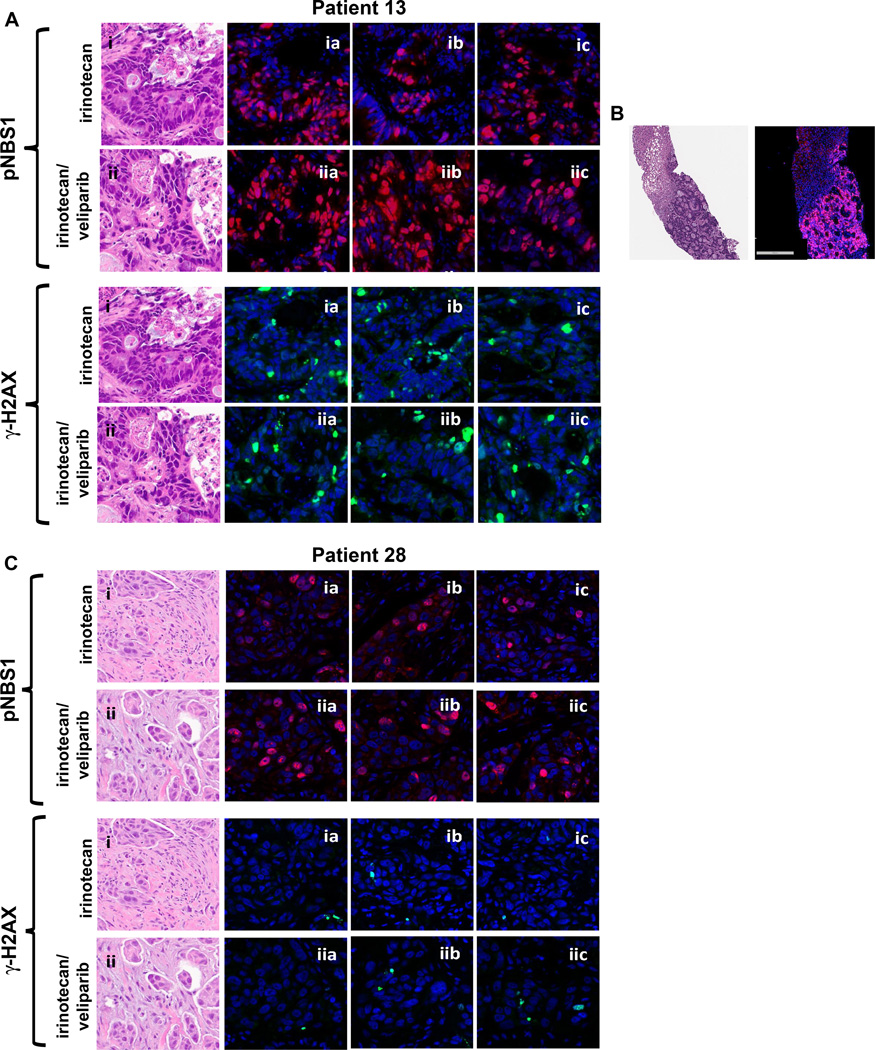
Figure 3. Assessment of γ-H2AX and pNBS in the exploratory multiplex immunofluorescence assay.
Multiplex immunofluorescence microscopy with Definiens image analysis was used to assess expression of γ-H2AX (green signal) and pNBS (red signal) after irinotecan alone and after combined veliparib/irinotecan. H&E panels and the “a” panels demonstrate cytology and pharmacodynamic response of adjacent sections. The “b” and “c” panels demonstrate typical staining patterns of other representative fields in the series of sections prepared from each biopsy specimen. (A) Sections from biopsies from patient 13 (colon cancer; best response PR). (B) Liver biopsy core post combination treatment from patient 13 demonstrating that pNBS staining is confined to tumor cells in the biopsy. Bar, 300 µm. (C) Sections from biopsies from patient 28 (TNBC; best response SD). Qualitatively all sections reveal a slight increase in nuclear pNBS staining post-combination treatment (Table 3).
Nuclear pNBS1 staining was assessed qualitatively in 7 sample pairs (Table 3). In 2 cases, nuclear pNBS1 staining was increased after veliparib/irinotecan compared with irinotecan alone (Table 3 and Figure 3). This included a robust biomarker response in a patient with colorectal cancer treated at the 20 mg dose level, who achieved a PR (patient 13). Importantly, pNBS1 staining was limited to tumor cells, as demonstrated by a liver biopsy in which both tumor and surrounding normal liver were analyzed (Figure 3 and Supplementary Figure S3). A second patient with triple-negative breast cancer treated at the 40 mg dose level, who was not a BRCA carrier and who achieved stable disease for 5.8 months, also had a slight increase in pNBS1 staining after combination treatment (patient 28). The other five paired biopsies did not demonstrate increased nuclear pNBS1 after the addition of veliparib (Table 3 and Supplementary Figure S4).
DISCUSSION
This trial defined the MTD of day 1, 8 irinotecan given in combination with 15 days of veliparib on a 21-day cycle. Although veliparib dosing was attenuated during the first cycle to accommodate PK and PD analyses, it is unlikely that defining DLT in the first cycle led to an underestimation of toxicities; of the 10 evaluable patients treated at 40mg veliparib BID, only 2 received dose reductions in the second and fourth cycles, respectively, indicating that the protocol-defined MTD was tolerable in the majority of patients.
Topoisomerase I poisons cause formation of a cleavable complex in which topoisomerase I is covalently attached to the DNA 3′ phosphate. PARP1 is activated by topoisomerase I-associated single-strand breaks, and via recruitment of X-ray repair cross-complementing protein 1 (XRCC1), promotes both removal of the cleavable complex by tyrosyl DNA phosphodiesterase-1 (TDP1) and subsequent completion of DNA repair (37–40). PARP inhibition therefore potentiates camptothecin-induced DNA damage by disabling the PARP1-XRCC1-TDP1 repair pathway (20, 37).
When PARP is inhibited, 3’ endonucleases may participate in the removal of the topoisomerase I cleavable complex. During S phase, camptothecin-stabilized Topo I-DNA complexes are converted to collapsed replication forks and double-strand breaks. Removal of the cleavable complex by the MRE11/RAD50/NBS1 (MRN) complex results in a lesion processed by HR (37). However, in the presence of a PARP inhibitor, there is inhibition of replication fork restart (41) and an increase in collapsed replication forks, so that PARP inhibition preferentially sensitizes S phase cells to topoisomerase I-mediated cytotoxicity (25). The cleavable complex may also be removed by XPF/ERCC1during the G2 phase (25, 42), so that cells with low ERCC1 levels are more sensitive to combined camptothecin/veliparib (42).
In this study, we assayed levels of nuclear γ-H2AX and pNBS1 in tumor after veliparib/irinotecan compared to irinotecan alone. γ-H2AX scores the presence of DNA double- strand breaks (28); phosphorylation of NBS1 (part of the MRN complex) indicates that DNA damage has been detected and suggests that the DNA repair process has been initiated. Increases in these biomarkers were sought to provide evidence either for (1) increased irinotecan-mediated DNA damage in the presence of veliparib; or (2) persistence of irinotecan-mediated DNA damage and repair in the presence of veliparib, resulting from a longer period of time required for DNA repair. The latter outcome is supported by one in vitro study, in which the increased detection of double-strand breaks after combination treatment was attributed to a significantly slowed repair process (20). Therefore, in samples demonstrating increased γ-H2AX or pNBS1 after veliparib/irinotecan compared to irinotecan alone, it is possible that DNA damage was nearly resolved at the 28- hour time point after irinotecan alone, but still ongoing at that time point after veliparib/irinotecan, consistent with veliparib-mediated retardation of repair. Among 4 biopsy pairs from patients who achieved SD or PR for which both of these markers were assessable, two demonstrated an increase in nuclear pNBS after combination treatment compared to irinotecan alone.
In the colorectal cancer case, adjacent normal liver was not similarly affected, likely because irinotecan-mediated cytotoxicity and its potentiation by PARP inhibition occurs primarily in cells with S phase DNA content (25), absent in non-cycling hepatocytes. A small amount of DNA damage during G1 may occur in response to irinotecan (25); if this occurred, the absence of pharmacodynamic effects in normal liver suggests proficient repair despite addition of veliparib. In either case, the lack of detectable DNA damage and repair markers in normal liver suggests a favorable therapeutic index for the irinotecan/veliparib combination.
However, increases in γ-H2AX foci after combination treatment measured using a validated single-plex assay did not correlate with response and were not confirmed with an exploratory multiplex assay. This discrepancy is likely related to the enhanced image analysis of the multiplex assay that is capable of excluding non-malignant cells from the biomarker evaluation, and points to the confounding influence of DDR responses by non-malignant yet cycling cells. In addition to technical issues, lack of corroboration of preclinical results may also be related to the timing of tumor sampling, designed in this trial to detect reduction of tumor PAR content after veliparib, and not optimized for detection of modulation of DDR in malignant cells. Finally, underlying biology not predicted by preclinical models may be contributing, including the possibility that γ-H2AX focus formation may be dispensable for initial recruitment of other DNA repair factors in some instances (43).
Since a pNBS1 signal was detectable in samples negative for γ-H2AX response, pNBS1 may be useful for assessing DDR endpoints in future trials. A larger sample size will be required to determine if increased pNBS1 post-combination treatment correlates with clinical outcome. Use of pNBS will also require determination of the optimal sampling window after topoisomerase 1 inhibition to measure this biomarker. In fact, a later time point might have demonstrated completion of repair (absent pNBS) after irinotecan alone that was still ongoing (persistent pNBS) after veliparib/irinotecan in a larger number of samples. It is also possible that NBS1 is involved in the removal of topoisomerase I cleavable complexes to a greater extent in some tumor cells than others. Assessment of XRCC1 or TDP1 recruitment into nuclear foci might have been a more direct measure of the action of veliparib in the presence of irinotecan, but quantitative IFAs for XRCC1 and TDP1 are not yet developed.
The MTD of veliparib when combined with irinotecan of 40 mg BID is comparable to doses used in the Phase 0 study (17). Consistent with the inhibition of PARP catalytic activity, our PD data demonstrated reduction of tumor PAR content during veliparib exposure. Although the timing of biopsies has not allowed us to document persistent reduction in PAR over the 12-hour dosing interval, in the Phase 0 trial, a single dose of 50 mg veliparib did reduce PAR levels measured at 24 hours by 76% and 97% in two patients, respectively (17).
In contrast to the documented effects on PARP catalytic activity, it is unlikely that PARP-DNA trapping has been achieved because monotherapy cytotoxicity, linked to PARP-DNA trapping, requires veliparib doses > 300 mg BID, with 400 mg BID as the monotherapy MTD (18). The compromise in PARP inhibitor dose compared to the monotherapy MTD is similar to the doses of veliparib given in combination with other DNA damaging agents (5, 44); the need for dose reduction has also been observed when other PARP inhibitors are combined with chemotherapy (10, 45, 46).
The requirement for PARP-DNA trapping in the potentiation of topoisomerase I poisoning by PARP inhibition is unclear. PARP1−/− cells are hypersensitive to camptothecin, and the addition of a PARP inhibitor does not further sensitize these cells, suggesting that the absence of PARP catalytic activity is sufficient for sensitization (26). However, in other studies, parental and PARP1−/− mouse embryonic fibroblasts have indistinguishable camptothecin sensitivities; transfection with catalytically inactive PARP1 or its isolated DNA binding domain sensitizes to camptothecin, consistent with a model in which small molecule inhibitors convert PARP1 into a protein that potentiates topoisomerase I poisons by binding to damaged DNA and preventing repair (27). If trapping of PARP-DNA complexes is a component of topoisomerase I inhibitor-mediated sensitization, alterative schedules using higher veliparib doses may be required.
In this study, PRs were seen in BRCA carriers and WT patients. HR is required to faithfully process double-strand breaks arising during S phase following combined topoisomerase I and PARP inhibition, so that HR-deficient tumors may be overall more vulnerable. For this reason, the irinotecan/veliparib combination is being studied in two triple-negative breast cancer populations, including those with and without BRCA germline mutations.
In summary, we have demonstrated the ability to safely inhibit PARP1/2 catalytic activity in combination with topoisomerase I inhibition. Further studies are necessary to reproducibly demonstrate modulation of the DDR in the context of loss of PARP1/2 function. Ultimately, randomized trials will be required to definitively demonstrate increased efficacy afforded by PARP inhibition in concert with DNA damage.
Supplementary Material
TRANSLATIONAL RELEVANCE.
In preclinical models, poly(ADP-ribose) [PAR] polymerase (PARP)1/2 inhibitors enhance the activity of topoisomerase I inhibitors. This Phase 1 trial established the tolerability of the PARP inhibitor veliparib in combination with irinotecan. The MTD of veliparib was lower than that required for monotherapy cytotoxicity, but consistent with catalytic inhibition of PARP, evidenced by paired tumor biopsies obtained after irinotecan alone and after veliparib/irinotecan demonstrating veliparib-mediated reduction in PAR content. In several samples, expression of γ-H2AX and pNBS1 was increased following veliparib/irinotecan compared to irinotecan, with the greatest increase in pNBS noted in one patient who achieved PR. In other patients, similarly increased γ-H2AX and pNBS1 levels were not observed. Administration schedules accommodating higher veliparib doses or alternative tumor sampling time points may be required to reproducibly demonstrate modulation by veliparib of the extent and repair of irinotecan-mediated DNA damage. Nonetheless, the observed clinical responses suggest that the combination merits further evaluation.
Acknowledgments
The authors appreciate the technical support of Lan Tran, Cameron Marlow, Candace Robinson, Will Yutzy, and Scott Lawrence and wish to thank Dr. Deborah Wilsker for expert assistance compiling the antibody qualification data and Dr. Yvonne Evrard for expert data reduction and presentation of the clinical PD results.
FUNDING SUPPORT
This project was funded with federal funds from the National Cancer Institute (NCI), NIH, under contract or grant numbers NCI U01-CA062487, U01-CA062490, NCI R21-CA135572, and CA-22453.
This project has been funded in part with federal funds from the National Cancer Institute, National Institutes of Health, under Contract No. HHSN261200800001E. The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. Government.
Footnotes
Presented in part at the 2011 annual meeting of the American Society of Clinical Oncology, Chicago, IL
REFERENCES
- 1.Fong PC, Boss DS, Yap TA, Tutt A, Wu P, Mergui-Roelvink M, et al. Inhibition of poly(ADP-ribose) polymerase in tumors from BRCA mutation carriers. N Engl J Med. 2009;361:123–134. doi: 10.1056/NEJMoa0900212. [DOI] [PubMed] [Google Scholar]
- 2.Kaufman B, Shapira-Frommer R, Schmutzler RK, Audeh MW, Friedlander M, Balmana J, et al. Olaparib Monotherapy in Patients With Advanced Cancer and a Germline BRCA1/2 Mutation. J Clin Oncol. 2014 doi: 10.1200/JCO.2014.56.2728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gartner EM, Burger AM, Lorusso PM. Poly(adp-ribose) polymerase inhibitors: a novel drug class with a promising future. Cancer J. 2010;16:83–90. doi: 10.1097/PPO.0b013e3181d78223. [DOI] [PubMed] [Google Scholar]
- 4.Kummar S, Chen A, Parchment RE, Kinders RJ, Ji J, Tomaszewski JE, et al. Advances in using PARP inhibitors to treat cancer. BMC Med. 2012;10:25. doi: 10.1186/1741-7015-10-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kummar S, Chen A, Ji J, Zhang Y, Reid JM, Ames M, et al. Phase I study of PARP inhibitor ABT-888 in combination with topotecan in adults with refractory solid tumors and lymphomas. Cancer Res. 2011;71:5626–5634. doi: 10.1158/0008-5472.CAN-11-1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kummar S, Ji J, Morgan R, Lenz HJ, Puhalla SL, Belani CP, et al. A phase I study of veliparib in combination with metronomic cyclophosphamide in adults with refractory solid tumors and lymphomas. Clin Cancer Res. 2012;18:1726–1734. doi: 10.1158/1078-0432.CCR-11-2821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tan A, Toppmeyer D, Stein M, Moss R, Gounder M, Lindquist D, et al. Phase I trial of veliparib (ABT-888), a poly(ADP-ribose) polymerase (PARP) inhibitor, in combination with doxorubicin and cyclophosphosphamide in breast cancer and other solid tumors. J Clin Oncol. 2011;29(suppl):A3041. [abstract] [Google Scholar]
- 8.Viswanathan S, Wesolowski R, Layman R, ALejandra G, Miller B, Chalmers J, et al. A phase I dose-escalation study of ABT-888 (veliparib) in combinatino with carboplatin in HER2-negative metastatic breast cancer (MBC) J Clin Oncol. 2011;29(suppl):TPS106. [abstract] [Google Scholar]
- 9.Somlo G, Frankel PH, Luu TH, Ma C, Arun B, Garcia AB, et al. Phase II trial of single agent PARP inhibitor ABT-888 (veliparib) followed by post-progression therapy of veliparib with carboplatin in aptiens with BRCA-associated metastatic breast cancer: California Cancer Consortium trial PHII-96. J Clin Oncol. 2014;32(suppl):A1021. [abstract] [Google Scholar]
- 10.Balmana J, Tung NM, Isakoff SJ, Grana B, Ryan PD, Saura C, et al. Phase I trial of olaparib in combination with cisplatin for the treatment of patients with advanced breast, ovarian and other solid tumors. Ann Oncol. 2014;25:1656–1663. doi: 10.1093/annonc/mdu187. [DOI] [PubMed] [Google Scholar]
- 11.Reiss K, Herman J, ZAhurak M, Brade A, Dawson L, Scardina A, et al. A phase I study of veliparib (ABT-888) in combinatino with low-dose fractionated whole abdominal radiation therapy (LDFWAR) in patients with advanced solid malignancies and peritoneal carcinomatosis. J Clin Oncol. 2014;32(suppl):A4139. doi: 10.1158/1078-0432.CCR-14-1552. [abstract] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Czito B, Mulcahy M, WR S, Vaghefi H, Jameson G, Deluca A, et al. The safety and tolerability of veliparib plus capecitabine and radiation in subjects with locally advanced rectal cancer: results of a phase 1b study. J Clin Oncol. 2014;32(5s suppl):A3634. [abstract] [Google Scholar]
- 13.Murai J, Huang SY, Das BB, Renaud A, Zhang Y, Doroshow JH, et al. Trapping of PARP1 and PARP2 by Clinical PARP Inhibitors. Cancer Res. 2012;72:5588–5599. doi: 10.1158/0008-5472.CAN-12-2753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Penning TD, Zhu GD, Gandhi VB, Gong J, Liu X, Shi Y, et al. Discovery of the Poly(ADP-ribose) polymerase (PARP) inhibitor 2-[(R)-2-methylpyrrolidin-2-yl]-1H–benzimidazole-4-carboxamide (ABT-888) for the treatment of cancer. J Med Chem. 2009;52:514–523. doi: 10.1021/jm801171j. [DOI] [PubMed] [Google Scholar]
- 15.Donawho CK, Luo Y, Luo Y, Penning TD, Bauch JL, Bouska JJ, et al. ABT-888, an orally active poly(ADP-ribose) polymerase inhibitor that potentiates DNA-damaging agents in preclinical tumor models. Clin Cancer Res. 2007;13:2728–2737. doi: 10.1158/1078-0432.CCR-06-3039. [DOI] [PubMed] [Google Scholar]
- 16.Murai J, Huang SY, Renaud A, Zhang Y, Ji J, Takeda S, et al. Stereospecific PARP Trapping by BMN 673 and Comparison with Olaparib and Rucaparib. Mol Cancer Ther. 2014;13:433–443. doi: 10.1158/1535-7163.MCT-13-0803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kummar S, Kinders R, Gutierrez ME, Rubinstein L, Parchment RE, Phillips LR, et al. Phase 0 clinical trial of the poly (ADP-ribose) polymerase inhibitor ABT-888 in patients with advanced malignancies. J Clin Oncol. 2009;27:2705–2711. doi: 10.1200/JCO.2008.19.7681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Puhalla S, Beumer JH, Pahuja S, Appleman LJ, Tawbi HA, Stoller RG, et al. Final results of a phase 1 study of the single-agent veliparib in patients with either BRCA1/2-mutated cancer, platinum-refractory ovarian, or basal-like breast cancer. J Clin Oncol. 2014;32(suppl):A2570. [abstract] [Google Scholar]
- 19.Calabrese CR, Almassy R, Barton S, Batey MA, Calvert AH, Canan-Koch S, et al. Anticancer chemosensitization and radiosensitization by the novel poly(ADP-ribose) polymerase-1 inhibitor AG14361. J Natl Cancer Inst. 2004;96:56–67. doi: 10.1093/jnci/djh005. [DOI] [PubMed] [Google Scholar]
- 20.Smith LM, Willmore E, Austin CA, Curtin NJ. The novel poly(ADP-Ribose) polymerase inhibitor, AG14361, sensitizes cells to topoisomerase I poisons by increasing the persistence of DNA strand breaks. Clin Cancer Res. 2005;11:8449–8457. doi: 10.1158/1078-0432.CCR-05-1224. [DOI] [PubMed] [Google Scholar]
- 21.Miknyoczki S, Chang H, Grobelny J, Pritchard S, Worrell C, McGann N, et al. The selective poly(ADP-ribose) polymerase-1(2) inhibitor, CEP-8983, increases the sensitivity of chemoresistant tumor cells to temozolomide and irinotecan but does not potentiate myelotoxicity. Mol Cancer Ther. 2007;6:2290–2302. doi: 10.1158/1535-7163.MCT-07-0062. [DOI] [PubMed] [Google Scholar]
- 22.Miknyoczki SJ, Jones-Bolin S, Pritchard S, Hunter K, Zhao H, Wan W, et al. Chemopotentiation of temozolomide, irinotecan, and cisplatin activity by CEP-6800, a poly(ADP-ribose) polymerase inhibitor. Mol Cancer Ther. 2003;2:371–382. [PubMed] [Google Scholar]
- 23.Davidson D, Wang Y, Aloyz R, Panasci L. The PARP inhibitor ABT-888 synergizes irinotecan treatment of colon cancer cell lines. Invest New Drugs. 2013;31:461–468. doi: 10.1007/s10637-012-9886-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tentori L, Leonetti C, Scarsella M, Muzi A, Mazzon E, Vergati M, et al. Inhibition of poly(ADP-ribose) polymerase prevents irinotecan-induced intestinal damage and enhances irinotecan/temozolomide efficacy against colon carcinoma. Faseb J. 2006;20:1709–1711. doi: 10.1096/fj.06-5916fje. [DOI] [PubMed] [Google Scholar]
- 25.Znojek P, Willmore E, Curtin NJ. Preferential potentiation of topoisomerase I poison cytotoxicity by PARP inhibition in S phase. Br J Cancer. 2014;111:1319–1326. doi: 10.1038/bjc.2014.378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Murai J, Zhang Y, Morris J, Ji J, Takeda S, Doroshow JH, et al. Rationale for poly(ADP-ribose) polymerase (PARP) inhibitors in combination therapy with camptothecins or temozolomide based on PARP trapping versus catalytic inhibition. J Pharmacol Exp Ther. 2014;349:408–416. doi: 10.1124/jpet.113.210146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Patel AG, Flatten KS, Schneider PA, Dai NT, McDonald JS, Poirier GG, et al. Enhanced killing of cancer cells by poly(ADP-ribose) polymerase inhibitors and topoisomerase I inhibitors reflects poisoning of both enzymes. J Biol Chem. 2012;287:4198–4210. doi: 10.1074/jbc.M111.296475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Redon CE, Nakamura AJ, Zhang YW, Ji JJ, Bonner WM, Kinders RJ, et al. Histone gammaH2AX and poly(ADP-ribose) as clinical pharmacodynamic biomarkers. Clin Cancer Res. 2010;16:4532–4542. doi: 10.1158/1078-0432.CCR-10-0523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Innocenti F, Undevia SD, Iyer L, Chen PX, Das S, Kocherginsky M, et al. Genetic variants in the UDP-glucuronosyltransferase 1A1 gene predict the risk of severe neutropenia of irinotecan. J Clin Oncol. 2004;22:1382–1388. doi: 10.1200/JCO.2004.07.173. [DOI] [PubMed] [Google Scholar]
- 30.Iyer L, Das S, Janisch L, Wen M, Ramirez J, Karrison T, et al. UGT1A1*28 polymorphism as a determinant of irinotecan disposition and toxicity. Pharmacogenomics J. 2002;2:43–47. doi: 10.1038/sj.tpj.6500072. [DOI] [PubMed] [Google Scholar]
- 31.Camptosar (irinotecan hydrochloride injection) drug package insert. NY: Pfizer Inc, NY; 2012. Jul, Revised. [Google Scholar]
- 32.Wiegand R, Wu J, Sha X, LoRusso P, Li J. Simultaneous determination of ABT-888, a poly (ADP-ribose) polymerase inhibitor, and its metabolite in human plasma by liquid chromatography/tandem mass spectrometry. J Chromatography B. 2010;878:333–339. doi: 10.1016/j.jchromb.2009.11.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Poujol S, Pinguet F, Malosse F, Astre C, Ychou M, Culine S, et al. Sensitive HPLC-fluorescence method for irinotecan and four major metabolites in human plasma and saliva: application to pharmacokinetic studies. Clin Chem. 2003;49:1900–1908. doi: 10.1373/clinchem.2003.023481. [DOI] [PubMed] [Google Scholar]
- 34.Kinders RJ, Hollingshead M, Khin S, Rubinstein L, Tomaszewski JE, Doroshow JH, et al. Preclinical modeling of a phase 0 clinical trial: qualification of a pharmacodynamic assay of poly (ADP-ribose) polymerase in tumor biopsies of mouse xenografts. Clin Cancer Res. 2008;14:6877–6885. doi: 10.1158/1078-0432.CCR-08-0214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kinders RJ, Hollingshead M, Lawrence S, Ji J, Tabb B, Bonner WM, et al. Development of a validated immunofluorescence assay for gammaH2AX as a pharmacodynamic marker of topoisomerase I inhibitor activity. Clin Cancer Res. 2010;16:5447–5457. doi: 10.1158/1078-0432.CCR-09-3076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.de Jonge MJ, Verweij J, de Bruijn P, Brouwer E, Mathijssen RH, van Alphen RJ, et al. Pharmacokinetic, metabolic, and pharmacodynamic profiles in a dose-escalating study of irinotecan and cisplatin. J Clin Oncol. 2000;18:195–203. doi: 10.1200/JCO.2000.18.1.195. [DOI] [PubMed] [Google Scholar]
- 37.Pommier Y, Barcelo JM, Rao VA, Sordet O, Jobson AG, Thibaut L, et al. Repair of topoisomerase I-mediated DNA damage. Prog Nucleic Acid Res Mol Biol. 2006;81:179–229. doi: 10.1016/S0079-6603(06)81005-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pommier Y. Drugging topoisomerases: lessons and challenges. ACS Chem Biol. 2013;8:82–95. doi: 10.1021/cb300648v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Murai J, Huang SY, Das BB, Dexheimer TS, Takeda S, Pommier Y. Tyrosyl-DNA phosphodiesterase 1 (TDP1) repairs DNA damage induced by topoisomerases I and II and base alkylation in vertebrate cells. J Biol Chem. 2012;287:12848–12857. doi: 10.1074/jbc.M111.333963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Das BB, Huang SY, Murai J, Rehman I, Ame JC, Sengupta S, et al. PARP1-TDP1 coupling for the repair of topoisomerase I-induced DNA damage. Nucleic Acids Res. 2014;42:4435–4449. doi: 10.1093/nar/gku088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ray Chaudhuri A, Hashimoto Y, Herrador R, Neelsen KJ, Fachinetti D, Bermejo R, et al. Topoisomerase I poisoning results in PARP-mediated replication fork reversal. Nat Struct Mol Biol. 2012;19:417–423. doi: 10.1038/nsmb.2258. [DOI] [PubMed] [Google Scholar]
- 42.Zhang YW, Regairaz M, Seiler JA, Agama KK, Doroshow JH, Pommier Y. Poly(ADP-ribose) polymerase and XPF-ERCC1 participate in distinct pathways for the repair of topoisomerase I-induced DNA damage in mammalian cells. Nucleic Acids Res. 2011;39:3607–3620. doi: 10.1093/nar/gkq1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Celeste A, Fernandez-Capetillo O, Kruhlak MJ, Pilch DR, Staudt DW, Lee A, et al. Histone H2AX phosphorylation is dispensable for the initial recognition of DNA breaks. Nat Cell Biol. 2003;5:675–679. doi: 10.1038/ncb1004. [DOI] [PubMed] [Google Scholar]
- 44.Hussain M, Carducci MA, Slovin S, Cetnar J, Qian J, McKeegan EM, et al. Targeting DNA repair with combination veliparib (ABT-888) and temozolomide in patients with metastatic castration-resistant prostate cancer. Invest New Drugs. 2014;32:904–912. doi: 10.1007/s10637-014-0099-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kurzrock R, Galanis E, Johnson DR, Kansra V, Wilcoxen K, Mcclure T, et al. A phase I study of niraparib in combination with temozolomide (TMZ) in patients with advanced cancer. J Clin Oncol. 2014;32(suppl):A2092. [abstract] [Google Scholar]
- 46.Khan OA, Gore M, Lorigan P, Stone J, Greystoke A, Burke W, et al. A phase I study of the safety and tolerability of olaparib (AZD2281, KU0059436) and dacarbazine in patients with advanced solid tumours. Br J Cancer. 2011;104:750–755. doi: 10.1038/bjc.2011.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.