Abstract
During hearing, acoustic signals travel up the ascending auditory pathway from the cochlea to auditory cortex; efferent connections provide descending feedback. In human listeners, although auditory and cognitive processing have sometimes been viewed as separate domains, a growing body of work suggests they are intimately coupled. Here we review the effects of hearing loss on neural systems supporting spoken language comprehension, beginning with age-related physiological decline. We suggest that listeners recruit domain general executive systems to maintain successful communication when the auditory signal is degraded, but that this compensatory processing has behavioral consequences: even relatively mild levels of hearing loss can lead to cascading cognitive effects that impact perception, comprehension, and memory, leading to increased listening effort during speech comprehension.
Keywords: listening effort, auditory cortex, speech comprehension, language
Hearing: not all in the ears
“Tout d'abord poussé par ce qui se fait en aviation, j'ai appliqué aux insectes les lois de la résistance de l'air, et je suis arrive…à cette conclusion que leur vol est impossible.”
(Magnan, 1934) [1]
Some 80 years ago, the French etymologist Antoine Magnan, writing about the wing-size to weight ratio of many flying insects—such as the bumblebee—concluded that these physical limitations would make it impossible for them to fly. But how then did they fly? The answer, of course, is that such simple calculations failed to take into account the full complexity of factors related to the structure and movement of insects' wings that ultimately make flight possible [2].
A similar paradox can be found in older adults' speech recognition. Although substantial variability is seen across individuals, the aging brain shows widespread changes in cortical structure [3] and network dynamics that carry cognitive function [4]. The behavioral consequences of these changes appear in a variety of cognitive “fundamentals,” including slowing in perceptual and cognitive operations, a decline in working memory capacity, and reduced efficiency in executive function and inhibition [5]. Also common in adult aging is hearing loss and increased difficulty processing complex auditory signals [6].
Against this backdrop, consider the challenges for comprehension of natural speech as one hears it on a daily basis. Speech rates in everyday conversation average about 150 words per minute (wpm), ranging from a “slow” 90 wpm in thoughtful speech to bursts of over 210 wpm as might be heard from a radio or television newsreader working from a prepared script. Further, although it typically goes unnoticed, everyday speech is surprisingly underarticulated, such that many words would be totally unidentifiable if not heard with the support of acoustic and linguistic context [7,8].1 Adding to the challenges of rapid input rate and variable speech quality, the act of comprehension places a heavy demand on working memory to keep track of a conversation from sentence to sentence and to untangle syntactically complex speech [10].
Given these obstacles one might quite reasonably conclude that speech comprehension by older adults would be, if not impossible, at least severely compromised. And yet, in the absence of advanced neuropathology, comprehension of natural speech is typically well maintained in older age. How then can the bumblebee of speech comprehension in older adults not merely fly, but fly so well?
Successful comprehension is possible because the quality of sensory information is only one element to be considered. Balancing age-related deficits in hearing and cognition are compensatory operations supported by cortical networks that extend far beyond the primary auditory system [11,12]. In this review we trace the impact of hearing loss from the peripheral auditory system to auditory cortex, concluding with its impact on the cognitive processes ultimately required to compensate for the reduced richness of sensory information. Speech comprehension in adult aging is a prime example of systems-level neural flexibility supporting successful behavior.
Changes to the auditory system in adult aging
Although hearing impairment has many etiologies, age-related hearing loss affects 80% of adults over the age of 70 years [13], offering a natural context in which to examine the effects of reduced auditory processing on speech perception. Age-related changes in hearing ability occur at all levels of the auditory system (Figure 1) [14]. We begin with a discussion of age-related changes in auditory physiology, using data from humans and from animal models.
Figure 1.

Hearing loss and age have effects throughout the auditory pathway. (A) Schematic of the ascending human auditory system, available via a CC-BY4.0 license fromhttps://osf.io/u2gxc/. (B) Inner hair cell ribbon counts and spiral ganglion cell (SGC) counts in aging mice decline over the lifespan. Adapted from [18]. (C) Increased auditory brainstem response (ABR) thresholds in Macaque as a function of age. Adapted from [36]. (D) Gray matter volume in human auditory cortex is reduced in listeners with hearing loss. Larger circles indicate multiple participants with the same score. Adapted from [53].
Peripheral and subcortical changes
Detection of auditory signals begins with sound-induced vibration of the eardrum (the tympanic membrane) that sets in motion three articulated bones (the ossicles) in the middle ear. The ossicles mechanically amplify these vibrations, transmitting them to a second membrane (the oval window) that separates the middle ear from the inner ear. Vibration of this second membrane produces motion in a fluid located in the cochlea, a snail-shaped structure about the size of a small pea, containing the basilar membrane that runs along its length. Approximately 12,000 to 15,000 outer hair cells lie along the basilar membrane. Sometimes referred to as cochlear amplifiers, movement of the outer hair cells stimulate some 3,500 inner hair cells that transduce the acoustic energy to electrical nerve signals [15]. It is here that the most prominent peripheral age-related hearing changes occur due to a decrease in the number of outer hair cells. In aging, outer hair cell loss is preferentially seen at the basal end of the basilar membrane responsible for encoding high frequency information [16], contributing to the stereotypical pattern of high-frequency hearing loss seen in older adulthood (Box 1).
Box 1. Measuring age-related hearing loss.
By far the simplest and most common measure of hearing sensitivity is the pure-tone audiogram, in which the softest threshold at which a participant can hear a tone of a particular frequency is established. Thresholds are typically estimated at octave frequencies between 250–8000 Hz (along with some half-octave frequencies). Adult aging is often accompanied by overall poorer audiometric thresholds, and an accented high-frequency hearing loss due to loss of outer hair cells at the basal end of the cochlea (Figure IA). Hearing thresholds can be summarized by averaging over frequencies covering the range of human speech, typically 500, 1000, and 2000 Hz or 1, 2, and 4 kHz in a listener's better ear (a pure tone average;PTA).
A complementary measure of auditory function is the otoacoustic emission (OAE), sounds generated from the action of outer hair cells in the inner ear, measured using a sensitive microphone, which can be spontaneous or evoked. A common method of testing involves distortion product OAEs in which brief tones of two frequencies are played in the ear, and the resulting OAE at unpresented frequencies is measured. OAEs can thus provide a measure of outer hair cell health that does not depend on participant response (and thus particularly useful in infants or other participants who are unable to respond).
Because of the tonotopic arrangement of the hair cells in the cochlea, frequency-specific changes in either PTA or OAE can provide an indication to regional cochlear function without electrophysiological testing.
Finally, the auditory brainstem response (ABR) is an evoked electrophysiological response time-locked to short acoustic stimuli (such as clicks), recorded from electrodes placed on the scalp. Characteristic peaks of the ABR (which begin within the first 10 milliseconds) are evaluated, with the response at different times reflecting different processing stages along the ascending auditory pathway (Figure IB).
Figure I.
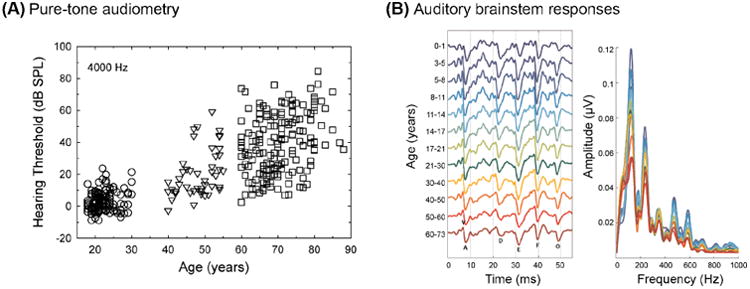
Measuring hearing ability across the lifespan. (A) Pure-tone thresholds at 4000 Hz increase with age. Adapted from [113]. (B) Time and frequency representations of auditory brain stem response (ABR) across different age groups. Adapted from [35].
In addition to hair cell loss, animal models have revealed a more subtle reduction in processing efficiency that stems from synaptic dysfunction and degeneration of cochlear nerve axons. For example, Kujawa and Liberman [17] found that after a single noise exposure cochlear afferent nerve terminals can be weakened even in the absence of hair cell loss or long-term hearing threshold shift [18-21] (Figure 1B). This type of cochlear dysfunction has sometimes been referred to as “hidden hearing loss” because it is not detectable using standard pure-tone audiometry. Among other factors, hidden hearing loss has been linked to difficulty in encoding near-threshold sounds [22] and auditory attention [23,24].
Beyond the cochlea, animal and human data demonstrate age-related changes in function in spiral ganglion neurons [25], cochlear nuclei [26], the superior olivary complex, and other midbrain structures up through the inferior colliculus [27,28]. Age-related changes in the auditory brainstem can yield altered temporal processing ability [29,30], which may be reflected in reduced ability in tasks such as detecting a brief temporal gap in a continuous tone [31]. Age-related differences in temporal processing have been associated with poorer speech perception [32-34] and likely contribute to the challenge of speech comprehension in older adulthood.
The auditory brainstem response (ABR) (see Glossary) is commonly used for evaluating the integrity of the auditory system, reflecting time-locked firing of subcortical auditory nuclei to an acoustic stimulus. Age-related changes in ABRs are routinely seen in both humans [35] and in animal models [36] (Figure 1C). These functional changes may relate to diffusion tensor imaging (DTI) data from humans suggesting age-related reduced fractional anisotropy (FA) values in white matter tracts passing through subcortical nuclei [37,38].
Changes in auditory cortex
Primary auditory cortex (A1) receives information from the ascending auditory pathway and is the gateway to cortical processing of auditory input. Thus, any changes in auditory cortex morphology or function relating to aging and hearing loss are of critical importance.
There is good evidence for age-related molecular change in A1. Interneurons that express the calcium binding protein parvalbumin (PV) are part of a group of cells that play an important role in stimulus selectivity and novel stimulus detection in sensory cortices. With aging comes a reduction in both the number of PV+ neurons and myelin in A1 [39,40]. Aging is also associated with a reduction in the GABA synthetic enzyme GAD in layers II–IV, likely reflecting a reduction in GABA levels [41,42]. Although studies examining GABA in human A1 using magnetic resonance spectroscopy have had mixed results, recent reports suggest reduced GABA in older adults with hearing loss [43,44].
The precision of frequency tuning is important for spectral resolution and perceptually separating speech from background noise. In young adults, A1 layer V neurons show characteristic frequency-selective receptive fields, including a proportion of V- or U-shaped responses. In older rats, fewer neurons with V/U-shaped receptive fields are seen, and neurons with V/U-shaped receptive fields show less firing during stimulus presentation than seen in young animals [45]. Age-related changes in spontaneous and evoked firing in superficial layers of auditory cortex are also observed [46]. Human data are broadly consistent with these animal findings, with a number of experiments suggesting that aging is associated with changes to evoked auditory responses [47,48] (although fine-grained neuroanatomical localization from humanEEG andMEG studies can be challenging).
It is worth noting that many animal studies of A1 function do so in the context of noise-induced hearing loss, and it is natural to ask to what degree these findings might translate to age-related hearing loss. Kamal and colleagues [49] compared responses in primary auditory cortex in noise-exposed young adult rats to those in older rats. They observed that the impact of noise exposure affected brain structure, tuning selectivity, and temporal processing in similar ways to natural aging. (Interestingly, Kamal et al. also found that several of the measured changes showed reversal after returning the young rats to a quiet environment.) Such studies suggest that many of the findings regarding noise-induced hearing loss have implications for normal aging [50-52].
Age-related hearing loss reflected in the structure of human auditory cortex
As we have seen, in animal models peripheral hearing loss leads to numerous changes in subcortical and cortical auditory representations, and changes in PV+ cortical neurons and myelin in auditory cortex. A key question is whether there is evidence for structural reorganization (or atrophy) in human cortical regions as a result of peripheral sensory loss.
To explore this question we asked whether there may be a relationship between auditory sensitivity (assessed by pure-tone audiometry) and gray matter volume in auditory cortex in older adults [53]. Although all of our participants reported themselves to have good hearing, audiometric testing showed significant variability in their hearing levels: better-ear PTAs varied from 10–33.3 dB HL. In this sample hearing loss did not differ between left and right ears, nor did better-ear PTA correlate with age. Using structuralMRI and voxel-based morphometry we found a significant correlation between hearing acuity and gray matter volume in right auditory cortex, such that subjects with poorer hearing acuity also had lower gray matter volume in that region (Figure 1D). Our findings were especially provocative given the relatively good hearing in our sample, as they suggest that even mild levels of hearing loss can lead to structural cortical changes. The intriguing correlation between hearing loss and reduced gray matter in auditory cortex has since been replicated [54,55].
There are two broad accounts that might be proposed for the relationship between hearing sensitivity and gray matter volume in auditory cortex. The first is that a general age-related decline in the nervous system manifests both peripherally and centrally. Inferential support for this general decline position can be seen in the significant correlations between performance on cognitive tests and a range of sensory (audition, vision, olfaction) and motor functions [56]. The possibility that such correlations arise from parallel declines in peripheral and cortical brain regions suggest there may be a common cause underlying these observed relationships [57].
An alternative is that there is a causal relationship between hearing sensitivity and primary auditory cortex, such that the reduced sensory input produces a cascade of events that ultimately affect neural structure. Although longitudinal data relating individual changes in hearing sensitivity and gray matter volume in auditory cortex are needed, our results suggest a link between even mild hearing loss and structural brain integrity which may have downstream implications for speech comprehension.
Beyond classical language areas: Cortical networks supporting degraded speech comprehension
The physiological and behavioral changes reviewed thus far would appear to paint a bleak picture for speech comprehension in older adulthood. Remarkably, however, everyday communication is generally quite good in older age, particularly in the absence of background noise or competing talkers. At the behavioral level, older adults generally make excellent use of preserved linguistic knowledge to mitigate otherwise more serious effects of reduced hearing acuity. This compensation can take the form of semantic context guiding recognition of an acoustically unclear word [58-60] or support from the syntactic structure of an utterance that similarly increases expectancy for certain words over others [61].
Reconciling the paradox of preserved performance in the context of significant declines in auditory processing lies in understanding the extended cortical networks supporting spoken language processing—that is, the systems involved in extracting meaning from the acoustic signal beyond primary auditory cortex.
Early “classical” models of language function based on neuropsychological patients of Broca, Wernicke, and others, focused almost exclusively on a broad perisylvian region in the left hemisphere in right handed (and most left handed) individuals, in which damage to the ventral inferior frontal cortex is most strongly associated with deficits in language production and damage to the posterior lateral temporal cortex with deficits in language comprehension [62]. Aided by functional imaging, contemporary neuroanatomical models of speech processing now implicate a large-scale neural network that includes bilateral temporal cortex and left inferior frontal gyrus in the context of a hierarchical dual stream model with parallel ventral and dorsal pathways (Figure 2A) [63-66].
Figure 2.

Acoustic challenge alters the networks supporting speech comprehension. (A) A core speech network comprised of bilateral temporal cortex is active during speech perception, supplemented by left ventral inferior frontal gyrus during sentence comprehension. When speech is acoustically challenging, additional regions are recruited: (B) Increased activity listening to single words in background babble includes portions of the cingulo-opercular network, adapted from [69]. (C) Increased activity in the left hemisphere related to perceptual effort when listening to noise-vocoded words, adapted from [77]; (D) Increased activity when listening to degraded sentences, adapted from [64]. The top rendering shows increased activation in left hemisphere regions related to acoustic degradation. The bottom rendering shows the degree to which this increased activity depended on the specific acoustic form of the sentences: activity in regions near auditory cortex depends on the type of acoustic manipulation, whereas activity further away does not.
Current data suggest these models can be further extended to encompass the neural processing necessary when the acoustic signal is degraded, as in the case of hearing impairment. One strong candidate is the cingulo-opercular network, comprised of bilateral anterior frontal opercula and dorsal anterior cingulate, an attention network involved in top-down task maintenance and cognitive control [67,68]. Cingulo-opercular activity is regularly seen when participants listen to speech that is acoustically degraded (Figure 2B) [69-72]. Critically, cingulo-opercular activity depends on a listener's attention [73] and predicts success on the next trial [74], indicating that its involvement in speech comprehension is more than epiphenomenal.
A second candidate system for processing acoustically degraded speech is found in premotor cortex. Left premotor cortex has been the center of a heated debate regarding the involvement of the motor system in speech perception [75,76]. Although the degree to which motor activity is necessary during speech perception is still disputed, there is a consensus that activity in premotor cortex is often observed when listening to speech, particularly when speech is acoustically degraded [64,77] (Figure 2C-D). There are two common explanations for the function served by premotor activity. The first appeals to some aspects of the motor theory of speech recognition: Given that premotor cortex is involved in articulatory representations [78], it could be that listeners make use of stored motor plans when comprehending difficult speech. A second possible explanation appeals to the role of premotor cortex in verbal short-term memory [79,80], and suggests that increased rehearsal of verbal information is required when the acoustic signal is degraded [81].
Thus, when acoustic detail is lacking—as occurs with age-related hearing loss—we expect core speech processing regions to be complemented by additional networks [82]. The cingulo-opercular network and premotor cortex are appealing candidates in this regard, but they do not form an exhaustive list. A challenge for continuing research is to identify the linguistic, acoustic, and cognitive conditions under which these various additional systems are activated, as well as the degree to which task effects drive activations [83].
How might top-down mechanisms improve speech perception?
Although we have focused largely on the ascending (afferent) auditory pathway, efferent connections occur at every level of the auditory system [84] and allow higher-level cortical regions to modify auditory peripheral processing [85-88]. At the cortical level, ongoing oscillations in auditory cortex track the acoustic speech signal [89,90], a process which can be modulated by directed attention (for example, to focus on a target talker instead of a competing talker) [91-94]. Observational and interventional training studies also suggest that higher-level mechanisms can act to aid auditory processing. These include work suggesting that trained musicians show more resilience to the effects of noise masking on speech perception [95] and have more robust subcortical auditory representations [96,97]. In intervention studies, auditory training can shape neural representations in both animals [39] and humans [98]. Thus, one potential mechanism of top-down influence is altering the sensitivity or spectrotemporal selectivity of the auditory system, making it more effective at processing speech-relevant acoustic cues.
However, in addition to the shaping of auditory processing, top-down modulation of the speech signal is also found in numerous perceptual and cognitive contexts. For example, non-auditory information (such as semantic context, visual information, or lexical/phonological constraints) exert strong effects on speech recognition [99], with neural evidence indicating that activity in frontal cortex shapes responses in temporal cortex [100]. It is therefore unlikely that dissociable domain-general cortical systems always act to improve comprehension in the same way. For example, the cingulo-opercular network is likely involved in overall task maintenance and vigilance, whereas premotor cortex may act to support rehearsal mechanisms of verbal working memory. These are complementary cognitive functions that act to improve the overall comprehension performance at levels beyond the auditory system, supplementing the ability of top-down processes to sharpen auditory filters. It is important to note that the involvement of at least some of these cognitive systems depends on the level of linguistic processing required [101]: for example, lexical constraints will not come into play during isolated phoneme perception, sentence-level syntactic processing is not a factor in single word perception, and so on. Therefore, the type of top-down constraints available depends not only on an individual listener's hearing and cognitive ability, but on the type of speech processing they are performing.
Consequences of compensation
Although speech comprehension in older adults with hearing impairment may be successful, there is now considerable evidence that the additional cognitive effort required for this success can have negative downstream consequences for behavior. For example, memory is typically poorer for acoustically degraded words [102-105] and stories [106,107] than for acoustically clear versions. Importantly, these memory deficits occur even when it can be demonstrated that the words themselves have been successfully recognized.
Descriptively, one could say that the cognitive processing needed for successful front-end perception of a degraded signal may draw resources that would ordinarily be available for encoding what had been heard in memory [108] or for comprehension of the meanings of sentences [109]. Episodic memory deficits for degraded speech are consistent with computational models in which acoustic challenge affects a short-term memory buffer that interferes with memory encoding and storage [110]. Thus, although speech comprehension can be quite good in listeners with hearing loss, the extra cognitive processing may have other, more subtle, consequences for behavior.
Such findings also add a cautionary note for assessment of cognitive function among older adults: Listening effort may affect test performance even when an individual assures the tester that spoken instructions can be heard, mirroring similar concerns in cases of degraded vision [111].
Concluding remarks
The past two decades have seen an exponential growth in our understanding of the two sides of the cognitive aging coin: biological declines one the one side, and compensatory mechanisms on the other. As this work has progressed, we have moved beyond the classical views of spoken language comprehension as involving limited areas of the left hemisphere, and models that gave little attention to the widespread effects of listening effort. Today we recognize that speech comprehension engages large-scale neural networks that involve connected activity across numerous cortical and subcortical areas, with the level and pattern of activity affected when dealing with speech whose richness is limited by hearing impairment. Indeed, in many ways research on aging—both healthy aging and in the context of neurodegenerative disease—is forcing us to push the boundaries of our present knowledge of neural organization for speech comprehension.
We believe that the next step will approach what many consider a fundamental principle in circuit neurobiology: how a surprisingly wide range of network parameters can produce similar behavioral outcomes [112] (see Outstanding Questions). This notion of multiple solutions may represent a framework for successful speech comprehension in the older adult. As in animal network models, some neural solutions may be more effective than others, particularly under perturbation. Such a case applies in aging, where the comprehension success for simple sentences frequently breaks down when the task is made more challenging through the use of complex syntax or rapid speech, all of which is exacerbated by poor hearing acuity. Closely related to the focus on multiple solutions is a concern that the traditional search for general principles has had the unintended consequence of leading to a focus on averages while treating variability as “noise”. This is especially critical in the case of older adulthood, where interindividual variability is a hallmark of the aging process.
We have discussed evidence from multiple sources that yield several fundamental principles governing speech comprehension in the aging brain and auditory system. First, even mild hearing loss has profound effects on neural processing, from the auditory periphery through to cognitive systems engaged by human listeners during speech comprehension. Second, views of language comprehension focusing on temporal cortex and left inferior frontal gyrus must be expanded to recognize the full cortical engagement that occurs during spoken language comprehension, which we have emphasized here in the context of acoustic challenge. Finally, although cortical compensation can frequently result in successful comprehension, it is not without consequence for further operations, such as remembering what we have heard.
Hearing loss thus has cascading influences from the auditory periphery through higher-level executive systems governing human behavior. The increasing recognition of the dynamic interaction between sensory and cognitive function in speech comprehension and the mechanisms underlying the changes in adult aging can serve as a model for aging research beyond audition, and offer guide posts for hearing aid and cochlear implant development that take into account the effects of cognitive challenge in outcome measures.
Box 2. Hearing loss and dementia.
A number of studies have shown a small but a significant correlation between hearing acuity and the incidence of dementia [114,115], as well as between hearing acuity and cognitive function in non-demented individuals [56,116-118] (Figure II). The relationship between hearing and cognition appears to hold even after the data are statistically controlled for such variables as age, gender, race, education, presence of diabetes, smoking history and hypertension.
There is no question that hearing loss can lead to social isolation and depression that may exacerbate any appearance of cognitive decline, and that the constant perceptual effort resulting from reduced hearing acuity can be a source of stress and mental fatigue [119]. An important challenge for future studies is to elucidate whether there is a causal link between hearing loss and cognitive function, or whether these two are simply complementary dependent measures of a common neurological decline [120]. It is also critical to consider the effect sizes of the additional risk explained by hearing level relative to that experienced in normal aging: in epidemiological studies with large samples statistical significance is frequently not difficult to achieve, and so understanding the relative change to risk is particularly important.
Figure II.

The risk for dementia increases proportionally with hearing loss (pure-tone thresholds) in older age. The hazard ratio (increased likelihood of developing dementia) is shown by the red line, with the gray area indicating the 95% confidence interval. Clinical levels of hearing loss are included below the x-axis. Adapted from [114].
How do task demands impact the neural systems engaged during speech comprehension?
Do individual listeners rely on different patterns of systems-level balance between neural systems during speech comprehension?
To what degree do individual differences in hearing and cognitive ability determine the neural systems listeners use to understand spoken language?
How do measures of human brain activity relate to subjective effort during speech comprehension?
What are the mechanisms linking age-related hearing loss to cognitive decline?
Healthy aging is associated with neurophysiological changes at every stage of the human auditory system including the cochlea, spiral ganglion neurons, cochlear nuclei, and other midbrain structures up through the auditory cortex.
Despite widespread declines in hearing ability, speech comprehension in older adulthood is generally quite good.
To maintain high levels of speech comprehension success, hearing impaired listeners recruit systems outside the canonical speech processing network to compensate for a poor auditory signal.
The additional cognitive effort required when listening to a degraded speech signal can impact other operations, such as remembering what has been heard.
Acknowledgments
This work was supported by the National Institutes of Health under awards R01AG038490, R01AG019714, R01DC014281, and the Dana Foundation.
Glossary
- A1
Primary auditory cortex
- ABR
Auditory brainstem response, an electrophysiological signature of auditory processing measured using EEG
- DTI
Diffusion tenor imaging, a type of structural MRI particularly sensitive to white matter
- EEG
Electroencephalography, a noninvasive electrophysiological technique for recording the brain's electrical activity from the scalp
- Listening effort
Additional cognitive resources required to understand acoustically-degraded speech
- MRI
Magnetic resonance imaging
- OAE
Otoacoustic emissions, sounds generated via the hair cells of the inner ear and used to assess cochlear function
- PTA
Pure tone average, a summary measure of hearing thresholds (often reported for a listener's better-hearing ear), and averaged over frequencies important for speech (either 500, 1000, 2000 Hz, or 1, 2, 4 kHz). A higher number reflects poorer hearing sensitivity
Footnotes
Interestingly, speakers seem to factor this knowledge into their utterances, with speech tending to show a functional adaptation—a principle of least effort—in which the more probable a word is in an utterance, the less carefully we articulate it [9].
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Magnan A. Le vol des insectes. Hermann: 1934. [Google Scholar]
- 2.Sane SP. Steady or unsteady? Uncovering the aerodynamic mechanisms of insect flight. J Exp Biol. 2011;214:349–351. doi: 10.1242/jeb.048330. [DOI] [PubMed] [Google Scholar]
- 3.Raz N, Lindenberger U, Rodrigue KM, Kennedy KM, Head D, Williamson A, et al. Acker JD. Regional brain changes in aging healthy adults: General trends, individual differences and modifiers. Cereb Cortex. 2005;15:1676–1689. doi: 10.1093/cercor/bhi044. [DOI] [PubMed] [Google Scholar]
- 4.Burke SN, Barnes CA. Neural plasticity in the ageing brain. Nat Rev Neurosci. 2006;7:30–40. doi: 10.1038/nrn1809. [DOI] [PubMed] [Google Scholar]
- 5.Salthouse TA. Selective review of cognitive aging. Journal of the International Neuropsychological Society. 2010;16:754–760. doi: 10.1017/S1355617710000706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Humes LE, Dubno JR. Factors affecting speech understanding in older adults. In: Gordon-Salant S, et al., editors. The aging auditory system. Springer; 2010. pp. 211–258. [Google Scholar]
- 7.Pollack I, Pickett JM. The intelligibility of excerpts from conversation. Language and Speech. 1963;6:165–171. [Google Scholar]
- 8.Wingfield A, Alexander AH, Cavigelli S. Does memory constrain utilization of top-down information in spoken word recognition? Evidence from normal aging. Language and Speech. 1994;37:221–235. doi: 10.1177/002383099403700301. [DOI] [PubMed] [Google Scholar]
- 9.Lindblom B, Brownlee S, Davis B, Moon SJ. Speech transforms. Speech Comm. 1992;11:357–368. [Google Scholar]
- 10.Wingfield A, Amichetti NM, Lash A. Cognitive aging and hearing acuity: Modeling spoken language comprehension. Frontiers in Psychol. 2015;6:684. doi: 10.3389/fpsyg.2015.00684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Peelle JE, Troiani V, Wingfield A, Grossman M. Neural processing during older adults' comprehension of spoken sentences: Age differences in resource allocation and connectivity. Cereb Cortex. 2010;20:773–782. doi: 10.1093/cercor/bhp142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tyler LK, Shafto MA, Randall B, Wright P, Marslen-Wilson WD, Stamatakis EA. Preserving syntactic processing across the adult life span: The modulation of the frontotemporal language system in the context of age-related atrophy. Cereb Cortex. 2010;20:352–364. doi: 10.1093/cercor/bhp105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cruickshanks KJ, Wiley TL, Tweed TS, Klein BE, Klein R, Mares-Perlman JA, Nondahl DM. Prevalence of hearing loss in older adults in Beaver Dam, Wisconsin: The epidemiology of hearing loss study. Am J Epidemiol. 1998;148:879–886. doi: 10.1093/oxfordjournals.aje.a009713. [DOI] [PubMed] [Google Scholar]
- 14.Gates GA, Mills JH. Presbycusis. Lancet. 2005;366:1111–1120. doi: 10.1016/S0140-6736(05)67423-5. [DOI] [PubMed] [Google Scholar]
- 15.Ashmore J. Cochlear outer hair cell motility. Physiol Rev. 2008;88:173–210. doi: 10.1152/physrev.00044.2006. [DOI] [PubMed] [Google Scholar]
- 16.Merchant SN, Nadol JB. Schuknecht's pathology of the inner ear. People's Publishing House; 2010. [Google Scholar]
- 17.Kujawa SG, Liberman MC. Adding insult to injury: Cochlear nerve degeneration after “temporary” noise-induced hearing loss. J Neurosci. 2009;29:14077–14085. doi: 10.1523/JNEUROSCI.2845-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sergeyenko Y, Lall K, Liberman MC, Kujawa SG. Age-related cochlear synaptopathy: An early-onset contributor to auditory functional decline. J Neurosci. 2013;33:13686–13694. doi: 10.1523/JNEUROSCI.1783-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Viana LM, O'Malley JT, Burgess BJ, Jones DD, Oliveira CACP, Santos F, et al. Liberman MC. Cochlear neuropathy in human presbycusis: Confocal analysis of hidden hearing loss in post-mortem tissue. Hearing Research. 2015;327:78–88. doi: 10.1016/j.heares.2015.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kujawa SG, Liberman MC. Synaptopathy in the noise-exposed and aging cochlea: Primary neural degeneration in acquired sensorineural hearing loss. Hearing Research. 2015;330:191–199. doi: 10.1016/j.heares.2015.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fernandez KA, Jeffers PWC, Lall K, Liberman MC, Kujawa SG. Aging after noise exposure: Acceleration of cochlear synaptopathy in “recovered” ears. J Neurosci. 2015;35:7509–7520. doi: 10.1523/JNEUROSCI.5138-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bharadwaj HM, Verhulst S, Shaheen L, Liberman MC, Shinn-Cunningham BG. Cochlear neuropathy and the coding of supra-threshold sound. Front Syst Neurosci. 2014;8:26. doi: 10.3389/fnsys.2014.00026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kujala T, Shtyrov Y, Winkler I, Saher M, Tervaniemi M, Sallinen M, et al. Näätänen R. Long-term exposure to noise impairs cortical sound processing and attention control. Psychophysiol. 2004;31:875–881. doi: 10.1111/j.1469-8986.2004.00244.x. [DOI] [PubMed] [Google Scholar]
- 24.Plack CJ, Barker D, Prendergast G. Perceptual consequences of “hidden” hearing loss. Trends Hear. 2014;18:1–11. doi: 10.1177/2331216514550621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bao J, Ohlemiller KK. Age-related loss of spiral ganglion neurons. Hearing Research. 2010;264:93–97. doi: 10.1016/j.heares.2009.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gray DT, Engle JR, Recanzone GH. Age-related neurochemical changes in the rhesus macaque cochlear nucleus. Journal of Comparative Neurology. 2014;522:1527–1541. doi: 10.1002/cne.23479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Caspary DM, Ling L, Turner JG, Hughes LF. Inhibitory neurotransmission, plasticity and aging in the mammalian central auditory system. J Exp Biol. 2008;211:1781–1791. doi: 10.1242/jeb.013581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Engle JR, Gray DT, Turner H, Udell JB, Recanzone GH. Age-related neurochemical changes in the rhesus macaque inferior colliculus. Front Aging Neurosci. 2014;6:73. doi: 10.3389/fnagi.2014.00073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Walton JP, Simon H, Frisna RD. Age-related alterations in the neural coding of envelope periodicities. J Neurophysiol. 2002;88:565–578. doi: 10.1152/jn.2002.88.2.565. [DOI] [PubMed] [Google Scholar]
- 30.Strouse A, Ashmead DH, Ohde RN, Grantham DW. Temporal processing in the aging auditory system. J Acoust Soc Am. 1998;104:2385–2399. doi: 10.1121/1.423748. [DOI] [PubMed] [Google Scholar]
- 31.Schneider BA, Pichora-Fuller MK, Kowalchuk D, Lamb M. Gap detection and the precedence effect in young and old adults. J Acoust Soc Am. 1994;95:980–991. doi: 10.1121/1.408403. [DOI] [PubMed] [Google Scholar]
- 32.Walton JP. Timing is everything: Temporal processing deficits in the aged auditory brainstem. Hearing Research. 2010;264:63–69. doi: 10.1016/j.heares.2010.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pichora-Fuller MK, Souza PE. Effects of aging on auditory processing of speech. Int J Audiol. 2003;42:11–16. [PubMed] [Google Scholar]
- 34.Gordon-Salant S, Yeni-Komshian GH, Fitzgibbons PJ, Barrett J. Age-related differences in identification and discrimination of temporal cues in speech segments. J Acoust Soc Am. 2006;119:2455–2466. doi: 10.1121/1.2171527. [DOI] [PubMed] [Google Scholar]
- 35.Skoe E, Krizman J, Anderson S, Kraus N. Stability and plasticity of auditory brainstem function across the lifespan. Cereb Cortex. 2015;25:1415–1426. doi: 10.1093/cercor/bht311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ng CW, Navarro X, Engle JR, Recanzone GH. Age-related changes of auditory brainstem responses in non-human primates. J Neurophysiol. 2015;114:455–467. doi: 10.1152/jn.00663.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chang Y, Lee SH, Lee YJ, Hwang MJ, Bae SJ, Kim MN, et al. Kang DS. Auditory neural pathway evaluation on sensorineural hearing loss using diffusion tensor imaging. NeuroReport. 2004;15:1699–1703. doi: 10.1097/01.wnr.0000134584.10207.1a. [DOI] [PubMed] [Google Scholar]
- 38.Lin Y, Wang J, Wu C, Wai Y, Yu J, Ng S. Diffusion tensor imaging of the auditory pathway in sensorineural hearing loss: Changes in radial diffusivity and diffiusion anisotropy. J Magn Reson Imaging. 2008;28:598–603. doi: 10.1002/jmri.21464. [DOI] [PubMed] [Google Scholar]
- 39.de Villers-Sidani E, Alzghoul L, Zhou X, Simpson KL, Lin RCS, Merzenich MM. Recovery of functional and structural age-related changes in the rat primary auditory cortex with operant training. Proc Nat Acad Sci USA. 2010;107:13900–13905. doi: 10.1073/pnas.1007885107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.del Campo HNM, Measor KR, Razak KA. Parvalbumin immunoreactivity in the auditory cortex of a mouse model of presbycusis. Hearing Research. 2012;294:31–39. doi: 10.1016/j.heares.2012.08.017. [DOI] [PubMed] [Google Scholar]
- 41.Ling LL, Hughes LF, Caspary DM. Age-related loss of the GABA synthetic enzyme glutamic acid decarboxylase in rat primary auditory cortex. Neuroscience. 2005;132:1103–1113. doi: 10.1016/j.neuroscience.2004.12.043. [DOI] [PubMed] [Google Scholar]
- 42.Burianova J, Ouda L, Profant O, Syka J. Age-related changes in GAD levels in the central auditory system of the rat. Exp Gerontol. 2009;44:161–169. doi: 10.1016/j.exger.2008.09.012. [DOI] [PubMed] [Google Scholar]
- 43.Profant O, Balogová Z, Dezortová M, Wagnerová D, Hájek M, Syka J. Metabolic changes in the auditory cortex in presbycusis demonstrated by MR spectroscopy. Exp Gerontol. 2013;48:795–800. doi: 10.1016/j.exger.2013.04.012. [DOI] [PubMed] [Google Scholar]
- 44.Gao F, Wang G, Ma W, Ren F, Li M, Dong Y, et al. Edden RAE. Decreased auditory GABA+ concentrations in presbycusis demonstrated by edited magnetic resonance spectroscopy. NeuroImage. 2015;106:311–316. doi: 10.1016/j.neuroimage.2014.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Turner JG, Hughes LF, Caspary DM. Affects of aging on receptive fields in rat primary auditory cortex layer V neurons. J Neurophysiol. 2005;94:2738–2747. doi: 10.1152/jn.00362.2005. [DOI] [PubMed] [Google Scholar]
- 46.Hughes LF, Turner JG, Parrish JL, Caspary DM. Processing of broadband stimuli across A1 layers in young and aged rats. Hearing Research. 2010;264:79–85. doi: 10.1016/j.heares.2009.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Alain C, Roye A, Salloum C. Effects of age-related hearing loss and background noise on neuromagnetic activity from auditory cortex. Front Syst Neurosci. 2014;8:8. doi: 10.3389/fnsys.2014.00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tremblay KL, Piskosz M, Souza P. Effects of age and age-related hearing loss on the neural representation of speech cues. Clinical Neurophysiology. 2003;114:1332–1343. doi: 10.1016/s1388-2457(03)00114-7. [DOI] [PubMed] [Google Scholar]
- 49.Kamal BS, Holman C, de Villers-Sidani E. Shaping the aging brain: Role of auditory input patterns in the emergence of auditory cortical impairments. Front Syst Neurosci. 2013;7:52. doi: 10.3389/fnsys.2013.00052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Henry KS, Kale S, Heinz MG. Noise-induced hearing loss increases the temporal precision of complex envelope coding by auditory-nerve fibers. Front Syst Neurosci. 2014;8:20. doi: 10.3389/fnsys.2014.00020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Keating P, King AJ. Developmental plasticity of spatial hearing following asymmetric hearing loss: context-dependent cue integration and its clinical implications. Front Syst Neurosci. 2013;7:123. doi: 10.3389/fnsys.2013.00123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Butler BE, Lomber SG. Functional and structural changes throughout the auditory system following congenital and early-onset deafness: implications for hearing restoration. Front Syst Neurosci. 2013;7:92. doi: 10.3389/fnsys.2013.00092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Peelle JE, Troiani V, Grossman M, Wingfield A. Hearing loss in older adults affects neural systems supporting speech comprehension. J Neurosci. 2011;31:12638–12643. doi: 10.1523/JNEUROSCI.2559-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Eckert MA, Cute SL, Vaden KI, Jr, Kuchinsky SE, Dubno JR. Auditory cortex signs of age-related hearing loss. JARO. 2012;13:703–713. doi: 10.1007/s10162-012-0332-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lin FR, Ferrucci L, An Y, Goh JO, Doshi J, Metter EJ, et al. Resnick SM. Association of hearing impairment with brain volume changes in older adults. NeuroImage. 2014;90:84–92. doi: 10.1016/j.neuroimage.2013.12.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Humes LE, Busey TA, Craig J, Kewley-Port D. Are age-related changes in cognitive function driven by age-related changes in sensory processing? Atten Percept Psycho. 2013;75:508–524. doi: 10.3758/s13414-012-0406-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Li KZH, Lindenberger U. Relations between aging sensory/sensorimotor and cognitive functions. Neurosci Biobehav R. 2002;26:777–783. doi: 10.1016/s0149-7634(02)00073-8. [DOI] [PubMed] [Google Scholar]
- 58.Benichov J, Cox LC, Tun PA, Wingfield A. Word recognition within a linguistic context: Effects of age, hearing acuity, verbal ability and cognitive function. Ear Hear. 2012;32:250–256. doi: 10.1097/AUD.0b013e31822f680f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rogers CS, Wingfield A. Stimulus-independent semantic bias misdirects word recognition in older adults. J Acoust Soc Am. 2015;138:EL26. doi: 10.1121/1.4922363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lash A, Rogers CS, Zoller A, Wingfield A. Expectation and entropy in spoken word recognition: Effects of age and hearing acuity. Experimental Aging Research. 2013;39:235–253. doi: 10.1080/0361073X.2013.779175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.DeCaro R, Peelle JE, Grossman M, Wingfield A. The two sides of sensory-cognitive interactions: Effects of age, hearing acuity, and working memory span on sentence comprehension. Frontiers in Psychol. 2016;7:236. doi: 10.3389/fpsyg.2016.00236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Goodglass H, Wingfield A. The changing relationship between anatomic and cognitive explanation in the neuropsychology of language. Journal of Psycholinguistic Research Special Issue: Celebrating a quarter century of (the Journal of) Psycholinguistic Research. 1998;27:147–165. doi: 10.1023/a:1023293814792. [DOI] [PubMed] [Google Scholar]
- 63.Hickok G, Poeppel D. The cortical organization of speech processing. Nat Rev Neurosci. 2007;8:393–402. doi: 10.1038/nrn2113. [DOI] [PubMed] [Google Scholar]
- 64.Davis MH, Johnsrude IS. Hierarchical processing in spoken language comprehension. J Neurosci. 2003;23:3423–3431. doi: 10.1523/JNEUROSCI.23-08-03423.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Peelle JE, Johnsrude IS, Davis MH. Hierarchical processing for speech in human auditory cortex and beyond. Front Hum Neurosci. 2010;4:51. doi: 10.3389/fnhum.2010.00051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Rauschecker JP, Scott SK. Maps and streams in the auditory cortex: nonhuman primates illuminate human speech processing. Nat Neurosci. 2009;12:718–724. doi: 10.1038/nn.2331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Neta M, Miezin FM, Nelson SM, Dubis JW, Dosenbach NUF, Schlaggar BL, Petersen SE. Spatial and temporal characteristics of error-related activity in the human brain. J Neurosci. 2015;35:253–266. doi: 10.1523/JNEUROSCI.1313-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Power JD, Petersen SE. Control-related systems in the human brain. Current Opinion in Neurobiology. 2013;23:223–228. doi: 10.1016/j.conb.2012.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Vaden KI, Jr, Kuchinsky SE, Ahlstrom JB, Dubno JR, Eckert MA. Cortical activity predicts which older adults recognize speech in noise and when. J Neurosci. 2015;35:3929–3937. doi: 10.1523/JNEUROSCI.2908-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Adank P. The neural bases of difficult speech comprehension and speech production: Two Activation Likelihood Estimation (ALE) meta-analyses. Brain Lang. 2012;122:42–54. doi: 10.1016/j.bandl.2012.04.014. [DOI] [PubMed] [Google Scholar]
- 71.Erb J, Henry MJ, Eisner F, Obleser J. The brain dynamics of rapid perceptual adaptation to adverse listening conditions. J Neurosci. 2013;33:10688–10697. doi: 10.1523/JNEUROSCI.4596-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Eckert MA, Menon V, Walczak A, Ahlstrom J, Denslow S, Horwitz A, Dubno JR. At the heart of the ventral attention system: The right anterior insula. Hum Brain Mapp. 2009;30:2530–2541. doi: 10.1002/hbm.20688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wild CJ, Yusuf A, Wilson D, Peelle JE, Davis MH, Johnsrude IS. Effortful listening: The processing of degraded speech depends critically on attention. J Neurosci. 2012;32:14010–14021. doi: 10.1523/JNEUROSCI.1528-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Vaden KI, Jr, Kuchinsky SE, Cute SL, Ahlstrom JB, Dubno JR, Eckert MA. The cingulo-opercular network provides word-recognition benefit. J Neurosci. 2013;33:18979–18986. doi: 10.1523/JNEUROSCI.1417-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lotto AJ, Hickok GS, Holt LL. Reflections on mirror neurons and speech perception. Trends in Cognitive Sciences. 2009;13:110–114. doi: 10.1016/j.tics.2008.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.D'Ausillo A, Pulvermüller F, Salmas P, Bufalari I, Begliomini C, Fadiga L. The motor somatotopy of speech perception. Curr Biol. 2009;19:381–385. doi: 10.1016/j.cub.2009.01.017. [DOI] [PubMed] [Google Scholar]
- 77.Hervais-Adelman A, Carlyon RP, Johnsrude IS, Davis MH. Brain regions recruited for the effortful comprehension of noise-vocoded words. Language and Cognitive Processes. 2012;27:1145–1166. [Google Scholar]
- 78.Evans S, Davis MH. Hierarchical organization of auditory and motor representations in speech perception: Evidence from searchlight similarity analysis. Cereb Cortex. 2015;25:4772–4788. doi: 10.1093/cercor/bhv136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Chein JM, Fiez JA. Evaluating models of working memory through the effects of concurrent irrelevant information. J Exp Psychol Gen. 2010;139:117–137. doi: 10.1037/a0018200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Szenkovits G, Peelle JE, Norris D, Davis MH. Individual differences in premotor and motor recruitment during speech perception. Neuropsychologia. 2012;50:1380–1392. doi: 10.1016/j.neuropsychologia.2012.02.023. [DOI] [PubMed] [Google Scholar]
- 81.Rönnberg J, Lunner T, Zekveld A, Sörqvist P, Danielsson H, Lyxell B, et al. Rudner M. The ease of language understanding (ELU) model: theoretical, empirical, and clinical advances. Front Syst Neurosci. 2013;7:31. doi: 10.3389/fnsys.2013.00031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wingfield A, Grossman M. Language and the aging brain: Patterns of neural compensation revealed by functional brain imaging. J Neurophysiol. 2006;96:2830–2839. doi: 10.1152/jn.00628.2006. [DOI] [PubMed] [Google Scholar]
- 83.Davis SW, Zhuang J, Wright P, Tyler LK. Age-related sensitivity to task-related modulation of language-processing networks. Neuropsychologia. 2014;63:107–115. doi: 10.1016/j.neuropsychologia.2014.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.The mammalian auditory pathway: Neuroanatomy. Springer-Verlag; 1992. [Google Scholar]
- 85.Maison S, Micheyl C, Collet L. Influence of focused auditory attention on cochlear activity in humans. Psychophysiol. 2001;38:35–40. [PubMed] [Google Scholar]
- 86.Elhilali M, Xiang J, Shamma SA, Simon JZ. Interaction between attention and bottom-up saliency mediates the representation of foreground and background in an auditory scene. PLoS Biology. 2009;7:e1000129. doi: 10.1371/journal.pbio.1000129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Lakatos P, Musacchia G, O'Connel MN, Falchier AY, Javitt DC, Schroeder CE. The specrotemporal filter mechanism of auditory selective attention. Neuron. 2013;77:750–761. doi: 10.1016/j.neuron.2012.11.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Srinivasan S, Keil A, Stratis K, Woodruff Carr KL, Smith DW. Effects of cross-modal selective attention on the sensory periphery: Cochlear sensitivity is altered by selective attention. Neuroscience. 2012;223:325–332. doi: 10.1016/j.neuroscience.2012.07.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Peelle JE, Davis MH. Neural oscillations carry speech rhythm through to comprehension. Frontiers in Psychol. 2012;3:320. doi: 10.3389/fpsyg.2012.00320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Giraud AL, Poeppel D. Cortical oscillations and speech processing: Emerging computational principles and operations. Nat Neurosci. 2012;15:511–517. doi: 10.1038/nn.3063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Zion Golumbic E, Ding N, Bickel S, Lakatos P, Schevon CA, McKhann GM, et al. Schroeder CE. Mechanisms underlying selective neuronal tracking of attended speech at a “cocktail party”. Neuron. 2013;77:980–991. doi: 10.1016/j.neuron.2012.12.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Mesgarani N, Chang EF. Selective cortical representation of attended speaker in multi-talker speech perception. Nature. 2012;485:233–237. doi: 10.1038/nature11020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kerlin JR, Shahin AJ, Miller LM. Attentional gain control of ongoing cortical speech representations in a “cocktail party”. J Neurosci. 2010;30:620–628. doi: 10.1523/JNEUROSCI.3631-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Rimmele JM, Zion Golumbic E, Schröger E, Poeppel D. The effects of selective attention and speech acoustics on neural speech-tracking in a multi-talker scene. Cortex. 2015;68:144–154. doi: 10.1016/j.cortex.2014.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Zendel BR, Alain C. Musicians experience less age-related decline in central auditory processing. Psychol Aging. 2012;27:410–417. doi: 10.1037/a0024816. [DOI] [PubMed] [Google Scholar]
- 96.White-Schwoch T, Carr KW, Anderson S, Strait DL, Kraus N. Older adults benefit from music training early in life: Biological evidence for long-term training-driven plasticity. J Neurosci. 2013;33:17667–17674. doi: 10.1523/JNEUROSCI.2560-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Weiss MW, Bidelman GM. Listening to the brainstem: Musicianship enhances intelligibility of subcortical representations for speech. J Neurosci. 2015;35:1687–1691. doi: 10.1523/JNEUROSCI.3680-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Anderson S, White-Schwoch T, Choi HJ, Kraus N. Training changes processing of speech cues in older adults with hearing loss. Front Syst Neurosci. 2013;7:97. doi: 10.3389/fnsys.2013.00097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Davis MH, Johnsrude IS. Hearing speech sounds: Top-down influences on the interface between audition and speech perception. Hearing Research. 2007;229:132–147. doi: 10.1016/j.heares.2007.01.014. [DOI] [PubMed] [Google Scholar]
- 100.Sohoglu E, Peelle JE, Carlyon RP, Davis MH. Predictive top-down integration of prior knowledge during speech perception. J Neurosci. 2012;32:8443–8453. doi: 10.1523/JNEUROSCI.5069-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Peelle JE. The hemispheric lateralization of speech processing depends on what “speech” is: A hierarchical perspective. Front Hum Neurosci. 2012;6:309. doi: 10.3389/fnhum.2012.00309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.McCoy SL, Tun PA, Cox LC, Colangelo M, Stewart R, Wingfield A. Hearing loss and perceptual effort: Downstream effects on older adults' memory for speech. Quarterly Journal of Experimental Psychology. 2005;58:22–33. doi: 10.1080/02724980443000151. [DOI] [PubMed] [Google Scholar]
- 103.Rabbitt PMA. Channel capacity, intelligibility and immediate memory. Quarterly Journal of Experimental Psychology. 1968;20:241–248. doi: 10.1080/14640746808400158. [DOI] [PubMed] [Google Scholar]
- 104.Heinrich A, Schneider BA. Elucidating the effects of ageing on remembering perceptually distorted word pairs. Quarterly Journal of Experimental Psychology. 2011;64:186–205. doi: 10.1080/17470218.2010.492621. [DOI] [PubMed] [Google Scholar]
- 105.Piquado T, Cousins KAQ, Wingfield A, Miller P. Effects of degraded sensory input on memory for speech: Behavioral data and a test of biologically constrained computational models. Brain Research Bulletin. 2010;1365:48–65. doi: 10.1016/j.brainres.2010.09.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Piquado T, Benichov JI, Brownell H, Wingfield A. The hidden effect of hearing acuity on speech recall, and compensatory effects of self-paced listening. Int J Audiol. 2012;51:576–583. doi: 10.3109/14992027.2012.684403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Ward CM, Rogers CS, Van Engen KJ, Peelle JE. Effects of age, acoustic challenge, and verbal working memory on recall of narrative speech. Experimental Aging Research. 2016;42:126–144. doi: 10.1080/0361073X.2016.1108785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Wingfield A, Tun PA, McCoy SL. Hearing loss in older adulthood: What it is and how it interacts with cognitive performance. Current Directions in Psychological Science. 2005;14:144–148. [Google Scholar]
- 109.Wingfield A, McCoy SL, Peelle JE, Tun PA, Cox C. Effects of adult aging and hearing loss on comprehension of rapid speech varying in syntactic complexity. Journal of the American Academy of Audiology. 2006;17:487–497. doi: 10.3766/jaaa.17.7.4. [DOI] [PubMed] [Google Scholar]
- 110.Cousins KAQ, Dar H, Wingfield A, Miller P. Acoustic masking disrupts time-dependent mechanisms of memory encoding in word-list recall. Memory and Cognition. 2014;42:622–638. doi: 10.3758/s13421-013-0377-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Toner CK, Reese BE, Neargardner S, Riedel TM, Gilmore GC, Cronin-Golomb A. Vision-fair neuropsychological assessment in normal aging, Parkinson's disease and Alzheimer's disease. Psychol Aging. 2012;27:785–790. doi: 10.1037/a0026368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Marder E, Goaillard JM. Variability, compensation and homeostasis in neuron and network function. Nat Rev Neurosci. 2006;7:563–574. doi: 10.1038/nrn1949. [DOI] [PubMed] [Google Scholar]
- 113.Humes LE, Kewley-Port D, Fogerty D, Kinney D. Measures of hearing threshold and temporal processing across the adult lifespan. Hearing Research. 2010;264:30–40. doi: 10.1016/j.heares.2009.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Lin FR, Metter J, O'Brien RJ, Resnick SM, Zonderman AB, Ferrucci L. Hearing loss and incident dementia. Archives of Neurology. 2011;68:214–220. doi: 10.1001/archneurol.2010.362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Gates GA, Anderson ML, McCurry SM, Feeney MP, Larson EB. Central auditory dysfunction as a harbinger of Alzheimer dementia. Arch Otolaryngol. 2011;137:390–395. doi: 10.1001/archoto.2011.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Lin FR. Hearing loss and cognition among older adults in the United States. J Gerontol A Biol Sci Med Sci. 2011;66A:1131–1136. doi: 10.1093/gerona/glr115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Lin FR, Yaffe K, Xia J, Xue QL, Harris TB, Purchase-Helzner E, et al. Simonsick EM. Hearing loss and cognitive decline in older adults. JAMA Intern Med. 2013;173:293–299. doi: 10.1001/jamainternmed.2013.1868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Lin FR, Ferrucci L, Metter EJ, An Y, Zonderman AB, Resnick SM. Hearing loss and cognition in the Baltimore Longitudinal Study of Aging. Neuropsychology. 2011;25:763–770. doi: 10.1037/a0024238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Fellinger J, Holzinger D, Gerich J, Goldberg D. Mental distress and quality of life in the hard of hearing. Acta Psychiatr Scand. 2007;115:243–245. doi: 10.1111/j.1600-0447.2006.00976.x. [DOI] [PubMed] [Google Scholar]
- 120.Wayne RV, Johnsrude IS. A review of causal mechanisms underlying the link between age-related hearing loss and cognitive decline. AGeing Res Rev. 2015;23 doi: 10.1016/j.arr.2015.06.002. [DOI] [PubMed] [Google Scholar]


