Abstract
Objective
to determine the effect of topical corticosteroid (CCS) therapy on intraocular pressure (IOP) in normal cats and cats with primary feline congenital glaucoma (FCG).
Animals studied
5 normal and 11 FCG cats were studied in 2 cohorts.
Procedures
IOP was measured by a single, masked observer, once daily 3–5 days/week throughout the course of CCS treatment and for up to 11 days after treatment discontinuation. One eye per cat was randomly assigned for treatment twice daily with CCS; Balanced salt solution (BSS) applied to the contralateral eye, served as a control. Differences between eyes and between weeks of the study period were calculated for each cat. A positive response to CCS was defined as a consistent >15% or >25% higher IOP in the treated relative to control eye in normal and FCG cats, respectively.
Results
8/11 FCG cats responded to topical CCS after 1–5 weeks of treatment with an increase in IOP relative to the untreated eye (maximum IOP discrepancy of 56 mmHg). 2/5 normal cats responded to topical CCS with appreciable but clinically unimportant increase in IOP in the treated eye (maximum IOP discrepancy of 6.4 mmHg).
Conclusions
our data indicate that the incidence of steroid induced IOP elevation in cats is lower than previously published feline studies suggest. Cats with pre-existing compromise in aqueous humor outflow may show a greater, clinically relevant response to topical CCS than normal cats.
Keywords: cat, corticosteroid, steroid induced ocular hypertension, glaucoma, IOP
Introduction
Steroid induced ocular hypertension (SIOH) and glaucoma are well documented adverse effects of corticosteroid (CCS) administration in humans.[1–3] This phenomenon has also been described in a wide range of other species, including non-human primates,[4–6] mice,[7, 8] rats,[9] rabbits,[10–12] cattle,[13, 14] sheep,[15] dogs,[16] and cats.[17–19] The underlying pathogenic mechanism responsible for SIOH remains poorly understood and is likely multifactorial, with complex alterations in tissues of the aqueous outflow pathways contributing to increased resistance to aqueous drainage.[1, 2, 20] Proposed mechanisms for increase in aqueous outflow resistance and consequent increase in intraocular pressure (IOP) include changes in the microarchitecture of the trabecular meshwork,[21, 22] increased or altered deposition of extracellular matrix material,[23–25] and reduction in protease activity and phagocytic potential of trabecular meshwork cells, all leading to accumulation of substances within the trabecular meshwork.[26–28]
The degree of SIOH is dependent on many factors related to drug pharmacology: including potency; frequency and route of administration; cumulative dose, and duration of treatment[29–36], as well as individual patient susceptibility. Topical ocular CCS administration carries a higher risk for the development of SIOH than either systemic or inhaled routes of drug delivery. [33, 34, 36, 37] Prednisolone, dexamethasone and betamethasone are associated with a relatively higher risk of a clinically significant elevation in IOP,[10, 33, 38–40] as compared to other topical CCS. The increased risk with these agents is attributed to a combination of their high potency and effective absorption into anterior segment.[32, 40–43] In humans with SIOH, elevation in IOP is most commonly observed within the first 2–6 weeks of treatment, with a return to pre-treatment values about one week after discontinuation of CCS administration.[34, 44–46] However, there is pronounced individual variation in the degree and timing of response, and a more acute[47] or chronic[30] time course has been documented. The prevalence and degree of SIOH varies considerably between species. For example, ruminants, or at least the bovine and ovine breeds studied, demonstrate a well-conserved, robust and predictably high response rate, affecting all or most individuals tested.[15] In contrast, other species display a much more variable response rate, as is seen with humans[29, 34, 42, 48–50] and non-human primates.[4, 5]
As topical CCS therapy is routinely used in the management of uveitis and many ocular surface diseases, the study of SIOH is extremely relevant to the field of veterinary ophthalmology. Chronic uveitis is a common clinical presentation in cats, and represents a leading cause of glaucoma in this species.[51, 52] Cats with uveitis are generally managed with topical CCS without major concerns for the potential for SIOH, development or worsening of glaucoma.[53] The incidence and magnitude of SIOH in cats is unknown, as only three relevant studies have been performed in this species.[17–19] Two of these studies reported a clinically unimportant but positive response to CCS in all of the cats in their study populations,[17, 18] while a third study reported a positive response only in a subset of individuals investigated but did not report the proportion of animals that responded.[19]
The effect of CCS on IOP has not been evaluated in cats with pre-existing compromise in aqueous humor outflow. Human primary open-angle glaucoma (POAG) patients, as well as their family members, are more likely to be CCS “responders” as compared to the general population. The response in these individuals also tends to be more rapid and dramatic, and they have a higher risk of developing extreme elevations in IOP resulting in permanent glaucomatous damage.[31, 36, 44, 45, 48] Similar results were also reported in a small study of Beagles with POAG.[16] The objective of this study was to investigate the IOP response to topical CCS administration in in normal cats and in cats with pre-existing aqueous outflow compromise due to primary feline congenital glaucoma (FCG).
Methods
Procedures involving animals were conducted in accordance with the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research, and were approved by the Institutional Animal Care and Use Committee at the University of Wisconsin-Madison. All cats were housed in a research colony maintained at the University of Wisconsin-Madison, under standard laboratory conditions with a consistent 12-h light/dark cycle (6 am/6 pm). The study utilized two separate cohorts of cats, each including both male and female cats. The first cohort consisted of five clinically normal cats and five glaucomatous cats known to be homozygous for a completely penetrant, autosomal recessive form of inherited FCG (Kuehn, M.H., et al.; manuscript in review). The second cohort consisted of 6 FCG cats. Ages of cats in both cohorts ranged from 8 months to 4 years of age. Disease status was determined by lineage and confirmed by ophthalmic examination, performed by a board certified veterinary ophthalmologist (GM). Cats were determined to be normal based on IOP history and the absence of any ocular lesions on ophthalmic examination. All affected PGC cats exhibited bilaterally symmetric disease, with consistently high IOP (mean = 34 mmHg +/− 16 mmHg standard deviation), buphthalmos and elongated ciliary processes. Aside from elevated IOP and the characteristic clinical features of FCG described above, the FCG cats used in this study were free of other, potentially confounding ocular abnormalities such as lens luxation or corneal disease.
During the study all IOP measurements were acquired using rebound tonometry (TonoVet; Icare Finland Oy, Helsinki, Finland)[54, 55] by a single, masked observer, once daily, 5 days/week or 3 days/week for cohorts 1 and 2, respectively, throughout the study. Three consistent IOP values (no error bar on the instrument display) were recorded per eye and averaged to provide daily IOP values. All cats were acclimated to rebound tonometry and were routinely handled for IOP measurements and ocular exams for at least 50 days prior to the study. During this acclimation period IOPs were recorded, and then baseline values established for each cat. All IOP measurements were obtained at a consistent time of day (between 7 am and 10 am) to limit variation due to circadian rhythm.[56, 57] The cats were gently restrained in a sternal upright position, avoiding pressure on the neck or eyelids.
One eye per cat was randomly assigned for treatment with a topical corticosteroid (CCS), and balanced salt solution (BSS) was applied to the contralateral eye, which served as a control. In cohort 1 animals, one drop (35–40 μl) of 0.1% dexamethasone sodium phosphate solution [DEX] (Bausch & Lomb Inc., Rochester, NY, USA) was applied to the treated eye twice daily, approximately 8 h apart, for four weeks. The CCS applied was then switched to 1% prednisolone acetate (PRED) suspension 1% prednisolone acetate suspension (Alcon Laboratories, Inc., Fort Worth, TX, USA for an additional four weeks. In cohort 2 animals, PRED was applied as described for cohort 1, for a total of five weeks. At the end of each cohort’s treatment phase, all ophthalmic drops were discontinued, and IOP was recorded until it returned to pre-treatment baseline. All cats were carefully observed throughout the study for clinical signs of ocular irritation, such as squinting, redness, or discharge, or development of additional ocular abnormalities, attributable to either their underlying disease process or to topical CCS administration.
Using IOP measurements that were taken weekly for 11 weeks prior to the study initiation, a baseline difference between eyes was calculated for each cat. During the study period, the difference (percent) in IOP between the CCS-treated and the control eyes was calculated and averaged by week for each cat. For the normal cats, a positive response to topical CCS was defined as a positive difference of at least 15% between the treated and the control eye for at least 2 consecutive weeks of the 5–8 week treatment phase. For the FCG cats, due to a greater observed variability in IOP between the two eyes during the pre-treatment phase, a positive response to topical CCS was defined as a positive difference of at least 25% between the CCS-treated and the control eye for at least 2 consecutive weeks of the 5–8 week treatment phase.
Results
Rebound tonometry was well tolerated by the cats, allowing rapid and repeatable measurements to be collected throughout the duration of the study period. No adverse effects (such as ocular irritation, keratitis or conjunctivitis) were noted in either eye of any cat at any time point.
Normal cats
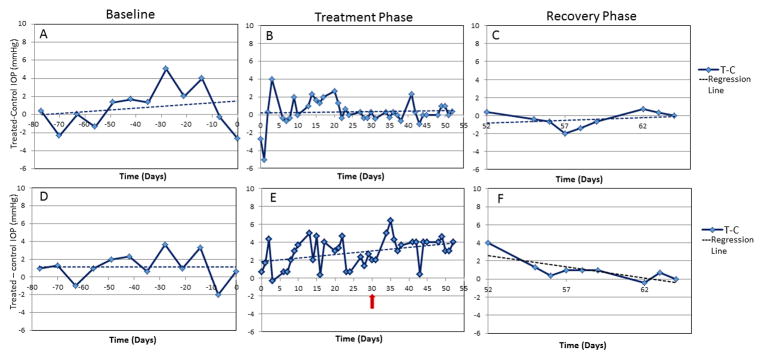
Two of the five normal cats in cohort one exhibited a positive, clinically unimportant elevation in IOP in response to topical CCS. The elevation was considered mild in each case as all recorded IOPs remained within the normal reference range for rebound tonometry in cats. The maximum recorded IOP discrepancy between eyes was 6.4 mmHg and IOP recorded in the placebo-treated control eyes during the treatment period showed no consistent increase from the baseline IOP data collected prior to treatment. Once the topical CCS was withdrawn, the difference between the IOP of the treated and control eyes returned to baseline within about seven days (Fig. 1A–C). In the three remaining normal cats there was no change in difference in IOP between the treated eye and the control eyes during the study period as compared to baseline data (Fig. 1D–F). Additionally, neither the control eyes nor the treated eyes showed a clinically important increase in IOP relative to baseline IOP data collected prior to CCS treatment.
Figure 1.
Representative plots from one normal cat designated as a non-responder (A–C) and one normal cat designated as a mild responder (D–F), before (A,D), during (B,E) and after (C,F) topical corticosteroid therapy. Baseline IOP data shows consistency in IOP between the right and left eyes (A and D). The red arrow highlights the onset of the prednisolone acetate arm. Following withdrawal of topical corticosteroid treatment (C and F) IOP rapidly returned to baseline after cessation of corticosteroid treatment (F).
Glaucomatous cats
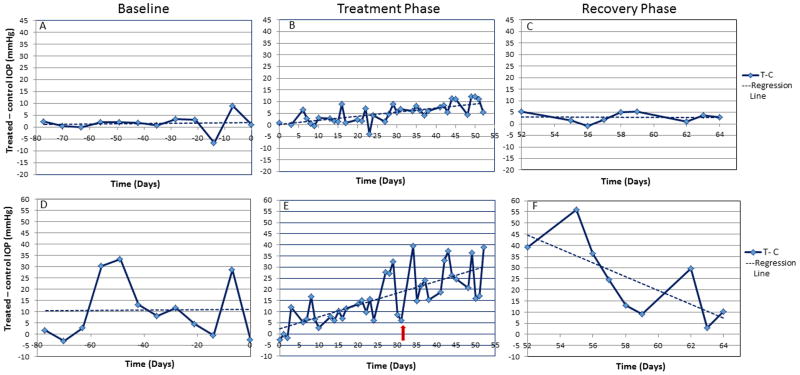
Eight of the 11 FCG cats (4/5 in cohort 1 and 4/6 in cohort 2) exhibited a positive response to topical CCS (Fig. 2). The steroid-induced elevation in IOP ranged from mild in 3 cats (<15 mmHg increase in IOP relative to control eye; Fig. 2A–C), moderate in 2 cats (15–25mmHg), and marked in 3 cats (> 25 mmHg increase in IOP relative to control eye; Fig. 2D–F). In the most extreme instance there was a maximum recorded discrepancy in IOP of 56 mmHg between the treated and control eyes (Fig. 2F). Once established, the difference in IOP between the treated and the control eyes persisted until CCS treatment was discontinued. Once the topical CCS was withdrawn, the difference in IOP between the treated and control eyes returned to baseline in all cats within 7–10 days (Fig. 2C, F). The IOP recorded in the control eye during the treatment period showed no increase relative to baseline IOP data collected prior to treatment initiation.
Figure 2.
Representative plots from one FCG cat characterized as a weakly positive or mild responder (<15 mmHg increase in IOP in the treated relative to control eye) and one FCG cat characterized as a markedly positive responder ( >25mmHg increase in IOP in the treated relative to control eye) before (A,C), during (B,D) and after (E) topical corticosteroid therapy. Baseline IOP data illustrates the relative consistency in IOP between eyes despite marked fluctuations in IOP that characterize feline congenital glaucoma. The red arrow highlights the onset of the prednisolone acetate arm. IOP rapidly returned to baseline after cessation of corticosteroid treatment (E).
The remaining 3/11 FCG cats showed no difference in the IOP recorded in the treated eye as compared to the control eye over the course of the CCS treatment phase, or after CCS treatment was withdrawn. One of these three cats did exhibit a clear upward trend in IOP in the treated eye as compared to the control eye during the study period as compared to baseline, but the difference did not meet the criteria for designation as a positive responder. During collection of this cat’s baseline IOP data the “treated eye” consistently had a lower IOP as compared to the “control eye.” This then resulted in a negative pre-treatment difference in IOP between eyes, meaning that the subsequent magnitude of the positive difference in IOP following CCS treatment for this cat represented a greater net IOP change than in the other cats whose baseline measurements between eyes were either near zero or were positive.
Dexamethasone versus prednisolone treatment
In the first cohort in this study, topical dexamethasone (DEX) and topical prednisolone acetate (PRED) were not associated with differences in incidence of positive response or in the degree of IOP elevation recorded. In the cats that responded positively to DEX, no additional or intensified elevation in IOP was observed in either eye following initiation of PRED treatment (Fig. 1 and 2). There was no significant change in the slope of the regression line created by plotting the difference in IOP between eyes against day for the DEX arm of the study compared to the PRED arm. The transition from DEX to PRED did not unveil any additional “CCS-responders,” i.e. no cats that showed a lack of response to DEX subsequently demonstrated a positive response to PRED. In both normal and FCG groups, cats that failed to respond to DEX also showed a lack of IOP-response to PRED.
Discussion
In contrast to previous studies in cats,[17, 18] IOP increase in response to topical CCS administration was not a consistent finding in all cats in our study. This discrepancy may reflect differences in both study design and data interpretation. Previous studies presented their IOP data in the form of group averages which may have limited the ability of these prior studies to address the concept of individual “non-responders.” The results of our study highlight both a need to consider likely individual variability in response to therapy, and a need to exercise caution when interpreting averaged responses of a group of normal cats to a given drug, when planning the clinical management of individual patients in a practice setting. A third, more recently published study performed using normal cats also reported an elevation in IOP in response to topical 0.1% dexamethasone in an unspecified proportion of the animals that were evaluated,[19] however IOP values recorded during that study and the total number of animals evaluated were not reported.
All previous studies designed to investigate IOP in response to topical corticosteroids in cats, [17–19] measured IOP using applanation pneumotonographs, only one of which had been investigated for use in this species specifically.[58] Appropriate tonometer selection is a critical aspect of any study design involving the measurement of IOP. Many commercially available tonometers underestimate the IOP especially as the pressure exceeds the normal range.[58–60] Rebound tonometry using the TonoVet has been shown in cats to retain accuracy at higher levels of IOP,[54, 55]making it more appropriate for a study involving ocular hypertension and glaucomatous subjects.
True ocular hypertension in response to topical CCS was not documented in any normal cat, at any time point in the study. All IOPs in normal cats remained below the published mean TonoVet-derived IOP value for normal cats (20.74 ±0.49 mmHg[55]). Although ruminants display a robust, highly conserved and predictable response to CCS between individuals,[13, 15] it would appear that this is not the case in normal cats. In human studies, about 5% of the population exhibits a high or marked response to topical CCS and about 30% exhibit a moderate or intermediate response.[61–63] The majority, therefore, are “non-responders” or “poor-responders” to topical CCS treatment. An increase in IOP of >10 mmHg from baseline is considered clinically significant.[3, 42, 63,64] Applying similar standards to this study, all five normal cats would fall into the “poor-“or “non-responder” categories. Thus, our results suggest that the use of topical CCS in cats with ocular surface disease, in the absence of significant intraocular disease, carries a relatively low risk of inducing a clinically significant elevation in IOP. Furthermore, it would appear that the veterinary clinician can prescribe topical CCS without major risk of SIOH in cats with uveitis, provided there is no evidence of pre-existing aqueous outflow compromise, in the face of low or normal IOP. However, our results should be interpreted with caution, given the limitations of the small study population involved, as it is possible that a larger study may have identified a subset of normal cats that are “high responders.”
FCG cats more frequently exhibited a positive response to topical CCS treatment than normal cats in this study. The magnitude of IOP increase observed in those FCG cats that responded was striking, with a discrepancy between eyes of one individual of up to 56 mmHg. This has the clear potential to significantly worsen potentially blinding ocular damage associated with glaucoma. A similar relationship between underlying primary glaucoma and a higher prevalence of steroid induced glaucoma has been well described in both human patients,[31, 34, 44, 45, 48] and has been documented in Beagles.[16] To our knowledge, this study is the first to document a similar effect of topical CCS on IOP in cats with underlying, spontaneous glaucoma, in which aqueous outflow pathways demonstrate pre-existing abnormalities including a narrowed ciliary cleft and paucity of aqueous outflow channels[57](Kuehn, M.H. et al, manuscript in review). Careful monitoring of IOP in cats with glaucoma and clinical signs of uveitis, which is a leading cause of secondary glaucoma in cats,[51, 52] is strongly recommended when CCS therapy is prescribed. As IOP is the most significant risk factor for disease progression in both humans and animals with glaucoma, topical CCS must be used with caution in these patients. When topical CCS are necessary in the management of the glaucomatous patient, frequent reevaluation to detect evidence of poor IOP regulation and worsening of underlying disease is indicated. As glaucomatous cats have been shown to display dramatic fluctuations in IOP,[65, 66] a single normal IOP measurement in isolation may confound the early diagnosis of steroid-induced elevations in IOP. Therefore, concurrent monitoring for other signs of glaucomatous damage, including optic nerve head cupping, progressive globe enlargement and Haab’s striae, as indicators of poor IOP regulation and disease progression is also important in affected animals.[51, 53]
In this study, there was no significant difference in the response rate or degree of response to topical 0.1% dexamethasone or 1% prednisolone acetate, which is in agreement to a previous study conducted in normal cats.[17] Dexamethasone and prednisolone are both potent CCS, capable of achieving a high concentration in the anterior segment of the eye when administered topically. While their greater potency and higher intraocular concentrations achieved make dexamethasone and prednisolone more effective in the management of anterior uveitis, these topical agents also may put patients at higher risk for developing SIOH and glaucoma, and careful IOP monitoring and frequent ocular examination is warranted for the duration of topical treatment. Both dexamethasone and prednisolone are associated with a higher rate of SIOH following topical use in humans, as compared to e.g. loteprednol, rimexolone, and fluorometholone, due to differences in potency, absorption, and type of CCS.[3, 38, 40, 43] This was also suggested in a small study of cats, in which IOP increases in response to topical application of these latter CCS were less than those observed in response to topical dexamethasone or prednisolone.[17]
The underlying pathophysiology of SIOH and glaucoma is complex and poorly understood. In humans, traditional anti-glaucoma medications, such as carbonic anhydrase inhibitors and beta-blockers, can be used to decrease the magnitude of IOP elevation but neither prevent the initial positive response, nor entirely eliminate the risk of glaucomatous damage.[2] Therapies directed more specifically at the underlying mechanism of disease may be more successful; CCS receptor blockers and CCS antagonists,[67–70] as well as synthetic cortisone derivatives[71–75] have all shown promise in the prevention of SIOH. In addition to competitive inhibition at the receptor site, these agents may be effective due to a direct alteration of CCS-induced changes in the trabecular meshwork.[76] Agents intended to alter protease activity, specifically of the matrix-metalloproteinase (MMP) family,[77],[75] have also shown early promise, and may represent an avenue for future studies.
Conclusion
In summary, marked elevation in IOP in response to topical CCS has the potential to contribute to worsening of glaucomatous damage in cats with pre-existing compromise in their aqueous outflow pathways. Our findings also indicate that while not all cats demonstrate an IOP elevation in response to topical CCS administration, and SIOH or glaucoma is unlikely to be a major concern in cats being treated with topical CCS for ocular surface disease, the potential for a marked response exists. The highly variable nature of CCS-induced IOP responses in cats highlights the need for close monitoring of each individual patient’s response to treatment.
Acknowledgments
The authors are grateful to Drs. Ellison Bentley and Paul Miller for their helpful discussions during data analysis and manuscript preparation. The authors would also like to thank the students who assisted with this study, in particular Mary E. Mohr and Daniel Shinsako. This work was supported by NIH grants K08 EY018609 and P30 EY0016665; New faculty startup funds from the University of Wisconsin-Madison, and unrestricted funds to the Department of Ophthalmology and Visual Sciences, University of Wisconsin-Madison from Research to Prevent Blindness.
References
- 1.Jones R, Rhee DJ. Corticosteroid-induced ocular hypertension and glaucoma: a brief review and update of the literature. Current Opinion in Ophthalmology. 2006;17:163–167. doi: 10.1097/01.icu.0000193079.55240.18. [DOI] [PubMed] [Google Scholar]
- 2.Kersey JP, Broadway DC. Corticosteroid-induced glaucoma: a review of the literature. Eye (London) 2006;20:407–416. doi: 10.1038/sj.eye.6701895. [DOI] [PubMed] [Google Scholar]
- 3.Pleyer U, Ursell PG, Rama P. Intraocular pressure effects of common topical steroids for post-cataract inflammation: are they all the same? Ophthalmology and Therapy. 2013;2:55–72. doi: 10.1007/s40123-013-0020-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Armaly MF. Aqueous outflow facility in monkeys and the effect of topical corticoids. Investigative Ophthalmology. 1964;3:534–538. [PubMed] [Google Scholar]
- 5.DeSantis L, Garthwaite C, Knepper P. Dexamthesone-induction of ocular hypertension in the primate [AFVD abstract] Investigative Ophthalmology and Visual Science. 1990;31:488. [Google Scholar]
- 6.Fingert JH, Clark AF, Craig JE, et al. Evaluation of the myocilin (MYOC) glaucoma gene in monkey and human steroid-induced ocular hypertension. Investigative Ophthalmology and Visual Science. 2001;42:145–152. [PubMed] [Google Scholar]
- 7.Kumar S, Shah S, Tang HM, et al. Tissue plasminogen activator in trabecular meshwork attenuates steroid induced outflow resistance in mice. PLoS One. 2013;8:e72447. doi: 10.1371/journal.pone.0072447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Overby DR, Bertrand J, Tektas OY, et al. Ultrastructural Changes Associated with Dexamethasone-Induced Ocular Hypertension in Mice. Investigative Ophthalmology and Visual Science. 2014;55(8):4922–4933. doi: 10.1167/iovs.14-14429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Miyara N, Shinzato M, Yamashiro Y, et al. Proteomic analysis of rat retina in a steroid-induced ocular hypertension model: potential vulnerability to oxidative stress. Japanese Journal of Ophthalmology. 2008;52:84–90. doi: 10.1007/s10384-007-0507-5. [DOI] [PubMed] [Google Scholar]
- 10.Qin Y, Lam S, Yam GH, et al. A rabbit model of age-dependant ocular hypertensive response to topical corticosteroids. Acta Ophthalmologica. 2012;90:559–563. doi: 10.1111/j.1755-3768.2010.02016.x. [DOI] [PubMed] [Google Scholar]
- 11.Ticho U, Lahav M, Berkowitz S, Yoffe P. Ocular changes in rabbits with corticosteroid-induced ocular hypertension. British Journal of Ophthalmology. 1979;63:646–650. doi: 10.1136/bjo.63.9.646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.François J, Benozzi G, Victoria-Troncoso V, Bohyn W. Ultrastructural and morphometric study of corticosteroid glaucoma in rabbits. Ophthalmic Research. 1984;16:168–178. doi: 10.1159/000265313. [DOI] [PubMed] [Google Scholar]
- 13.Gerometta R, Podos SM, Candia OA, et al. Steroid-induced ocular hypertension in normal cattle. Archives of Ophthalmology. 2004;122:1492–1497. doi: 10.1001/archopht.122.10.1492. [DOI] [PubMed] [Google Scholar]
- 14.Danias J, Gerometta R, Ge Y, et al. Gene expression changes in steroid-induced IOP elevation in bovine trabecular meshwork. Investigative Ophthalmology and Visual Science. 2011;52:8636–8645. doi: 10.1167/iovs.11-7563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gerometta R, Podos SM, Danias J, Candia OA. Steroid-induced ocular hypertension in normal sheep. Investigative Ophthalmology and Visual Science. 2009;50:669–673. doi: 10.1167/iovs.08-2410. [DOI] [PubMed] [Google Scholar]
- 16.Gelatt KN, Mackay EO. The ocular hypertensive effects of topical 0. 1% dexamethasone in beagles with inherited glaucoma. Journal of Ocular Pharmacology and Therapeutics. 1998;14:57–66. doi: 10.1089/jop.1998.14.57. [DOI] [PubMed] [Google Scholar]
- 17.Bhattacherjee P, Paterson CA, Spellman JM, et al. Pharmacological validation of a feline model of steroid-induced ocular hypertension. Archives of Ophthalmology. 1999;117:361–364. doi: 10.1001/archopht.117.3.361. [DOI] [PubMed] [Google Scholar]
- 18.Zhan GL, Miranda OC, Bito LZ. Steroid glaucoma: corticosteroid-induced ocular hypertension in cats. Experimental Eye Research. 1992;54:211–218. doi: 10.1016/s0014-4835(05)80210-6. [DOI] [PubMed] [Google Scholar]
- 19.Daull P, Paterson CA, Kuppermann BD, Garrigue JS. A preliminary evaluation of dexamethasone palmitate emulsion: a novel intravitreal sustained delivery of corticosteroid for treatment of macular edema. Journal of Ocular Pharmacology and Therapeautics. 2013;29:258–269. doi: 10.1089/jop.2012.0044. [DOI] [PubMed] [Google Scholar]
- 20.Overby DR, Clark AF. Animal models of glucocorticoid-induced glaucoma. Experimental Eye Research. 2015;141:15–22. doi: 10.1016/j.exer.2015.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Clark AF, Wilson K, McCartney MD, et al. Glucocorticoid-induced formation of cross-linked actin networks in cultured human trabecular meshwork cells. Investigative Ophthalmology and Visual Science. 1994;35:281–294. [PubMed] [Google Scholar]
- 22.Clark AF, Brotchie D, Read AT, et al. Dexamethasone alters F-actin architecture and promotes cross-linked actin network formation in human trabecular meshwork tissue. Cell Motility and Cytoskeleton. 2005;60:83–95. doi: 10.1002/cm.20049. [DOI] [PubMed] [Google Scholar]
- 23.Wilson K, McCartney MD, Miggans ST, Clark AF. Dexamethasone induced ultrastructural changes in cultured human trabecular meshwork cells. Current Eye Research. 1993;12:783–793. doi: 10.3109/02713689309020383. [DOI] [PubMed] [Google Scholar]
- 24.Yue BY. The extracellular matrix and its modulation in the trabecular meshwork. Survey of Ophthalmology. 1996;40:379–390. doi: 10.1016/s0039-6257(96)80066-x. [DOI] [PubMed] [Google Scholar]
- 25.Johnson DH, Bradley JM, Acott TS. The effect of dexamethasone on glycosaminoglycans of human trabecular meshwork in perfusion organ culture. Investigative Ophthalmology and Visual Science. 1990;31:2568–2571. [PubMed] [Google Scholar]
- 26.Zhang X, Ognibene CM, Clark AF, Yorio T. Dexamethasone inhibition of trabecular meshwork cell phagocytosis and its modulation by glucocorticoid receptor beta. Experimental Eye Research. 2007;84:275–284. doi: 10.1016/j.exer.2006.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Matsumoto Y, Johnson DH. Dexamethasone decreases phagocytosis by human trabecular meshwork cells in situ. Investigative Ophthalmology and Visual Science. 1997;38:1902–1907. [PubMed] [Google Scholar]
- 28.Wordinger RJ, Clark AF. Effects of glucocorticoids on the trabecular meshwork: towards a better understanding of glaucoma. Progress in Retina and Eye Research. 1999;18:629–667. doi: 10.1016/s1350-9462(98)00035-4. [DOI] [PubMed] [Google Scholar]
- 29.François J, Heintz-de Bree CH, Tripathi RC. The cortisone test and the heredity of primary open-angle glaucoma. American Journal of Ophthalmology. 1966;62:844–852. doi: 10.1016/0002-9394(66)91908-8. [DOI] [PubMed] [Google Scholar]
- 30.François J. Corticosteroid glaucoma. Ophthalmologica. 1984;188:76–81. doi: 10.1159/000309345. [DOI] [PubMed] [Google Scholar]
- 31.Becker B, Ballin N. Glaucoma and corticosteroid provocative testing. Archives of Ophthalmology. 1965;74:621–624. doi: 10.1001/archopht.1965.00970040623007. [DOI] [PubMed] [Google Scholar]
- 32.Spaeth GL. Effects of topical dexamethasone on intraocular pressure and the water drinking test. Archives of Ophthalmology. 1966;76:772–783. doi: 10.1001/archopht.1966.03850010774003. [DOI] [PubMed] [Google Scholar]
- 33.Renfro L, Snow JS. Ocular effects of topical and systemic steroids. Dermatology Clinics. 1992;10:505–512. [PubMed] [Google Scholar]
- 34.Armaly MF. Effect of corticosteroids on intraocular pressure and fluid dynamics. I. The effect of dexamethasone in the normal eye. Archives of Ophthalmology. 1963;70:482–491. doi: 10.1001/archopht.1963.00960050484010. [DOI] [PubMed] [Google Scholar]
- 35.Bernstein HN, Mills DW, Becker B. Steroid-induced elevation of intraocular pressure. Archives of Ophthalmology. 1963;70:15–18. doi: 10.1001/archopht.1963.00960050017005. [DOI] [PubMed] [Google Scholar]
- 36.Diotallevi M, Bocci N. Effect of systemically administered corticosteroids on intraocular pressure and fluid dynamics. Acta Ophthalmologica (Copenhagen) 1965;43:524–527. doi: 10.1111/j.1755-3768.1965.tb03687.x. [DOI] [PubMed] [Google Scholar]
- 37.Garbe E, LeLorier J, Boivin JF, Suissa S. Inhaled and nasal glucocorticoids and the risks of ocular hypertension or open-angle glaucoma. Journal of the American Medical Association. 1997;277:722–727. [PubMed] [Google Scholar]
- 38.Podos SM, Krupin T, Asseff C, Becker B. Topically administered corticosteoid preparations. Comparison of intraocular pressure effects. Archives of Ophthalmology. 1971;86:251–254. doi: 10.1001/archopht.1971.01000010253002. [DOI] [PubMed] [Google Scholar]
- 39.Mindel JS, Tavitian HO, Smith H, Walker EC. Comparative ocular pressure elevation by medrysone, fluorometholone, and dexamethasone phosphate. Archives of Ophthalmology. 1980;98:1577–1578. doi: 10.1001/archopht.1980.01020040429006. [DOI] [PubMed] [Google Scholar]
- 40.Cantrill HL, Palmberg PF, Zink HA, et al. Comparison of in vitro potency of corticosteroids with ability to raise intraocular pressure. American Journal of Ophthalmology. 1975;79:1012–1017. doi: 10.1016/0002-9394(75)90687-x. [DOI] [PubMed] [Google Scholar]
- 41.Becker B, Mills DW. Elevated intraocular pressure following corticosteroid eye drops. Journal of the American Medical Association. 1963;185:884–886. doi: 10.1001/jama.1963.03060110088027. [DOI] [PubMed] [Google Scholar]
- 42.Becker B. Intraocular pressure response to topical corticosteroids. Investigative Ophthalmology. 1965;4:198–205. [PubMed] [Google Scholar]
- 43.Awan MA, Agarwal PK, Watson DG, et al. Penetration of topical and subconjunctival corticosteroids into human aqueous humour and its therapeutic significance. British Journal of Ophthalmology. 2009;93:708–713. doi: 10.1136/bjo.2008.154906. [DOI] [PubMed] [Google Scholar]
- 44.Armaly MF. Effect of corticosteroids on intraocular pressure and fluid dynamics. II. The effect of dexamethasone in the glaucomatous eye. Archives of Ophthalmology. 1963;70:492–499. doi: 10.1001/archopht.1963.00960050494011. [DOI] [PubMed] [Google Scholar]
- 45.Becker B, Mills DW. Corticosteroids and intraocular pressure. Archives of Ophthalmology. 1963;70:500–507. doi: 10.1001/archopht.1963.00960050502012. [DOI] [PubMed] [Google Scholar]
- 46.Lewis JM, Priddy T, Judd J, et al. Intraocular pressure response to topical dexamethasone as a predictor for the development of primary open-angle glaucoma. American Journal of Ophthalmology. 1988;106:607–612. doi: 10.1016/0002-9394(88)90595-8. [DOI] [PubMed] [Google Scholar]
- 47.Weinreb RN, Polansky JR, Kramer SG, Baxter JD. Acute effects of dexamethasone on intraocular pressure in glaucoma. Investigative Ophthalmology and Visual Science. 1985;26:170–175. [PubMed] [Google Scholar]
- 48.Kitazawa Y, Horie T. The prognosis of corticosteroid-responsive individuals. Archives of Ophthalmology. 1981;99:819–823. doi: 10.1001/archopht.1981.03930010819005. [DOI] [PubMed] [Google Scholar]
- 49.Armaly MF. Statistical attributes of the steroid hypertensive response in the clinically normal eye. I. The demonstration of three levels of response. Investigative Ophthalmology. 1965;4:187–197. [PubMed] [Google Scholar]
- 50.Palmberg PF, Mandell A, Wilensky JT, et al. The reproducibility of the intraocular pressure response to dexamethasone. American Journal of Ophthalmology. 1975;80:844–856. doi: 10.1016/0002-9394(75)90282-2. [DOI] [PubMed] [Google Scholar]
- 51.McLellan GJ, Miller PE. Feline glaucoma--a comprehensive review. Veterinary Ophthalmology. 2011;14(Suppl 1):15–29. doi: 10.1111/j.1463-5224.2011.00912.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Peiffer RL, Wilcock BP. Histopathologic study of uveitis in cats: 139 cases (1978–1988) Journal of the American Veterinary Medical Association. 1991;198:135–138. [PubMed] [Google Scholar]
- 53.McLellan GJ, Teixeira LB. Feline Glaucoma. Veterinary Clinics of North America Small Animal Practice. 2015;45:1307–1333. doi: 10.1016/j.cvsm.2015.06.010. [DOI] [PubMed] [Google Scholar]
- 54.McLellan GJ, Kemmerling JP, Kiland JA. Validation of the TonoVet® rebound tonometer in normal and glaucomatous cats. Veterinary Ophthalmology. 2013;16:111–118. doi: 10.1111/j.1463-5224.2012.01038.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rusanen E, Florin M, Hässig M, Spiess BM. Evaluation of a rebound tonometer (Tonovet) in clinically normal cat eyes. Veterinary Ophthalmology. 2010;13:31–36. doi: 10.1111/j.1463-5224.2009.00752.x. [DOI] [PubMed] [Google Scholar]
- 56.Del Sole MJ, Sande PH, Bernades JM, et al. Circadian rhythm of intraocular pressure in cats. Veterinary Ophthalmology. 2007;10:155–161. doi: 10.1111/j.1463-5224.2007.00525.x. [DOI] [PubMed] [Google Scholar]
- 57.Gomes FE, Bentley E, Lin TL, McLellan GJ. Effects of unilateral topical administration of 0. 5% tropicamide on anterior segment morphology and intraocular pressure in normal cats and cats with primary congenital glaucoma. Veterinary Ophthalmology. 2011;14(Suppl 1):75–83. doi: 10.1111/j.1463-5224.2011.00927.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hammond BR, Bhattacherjee P. Calibration of the Alcon applanation pneumatonograph and Perkins tonometer for use in rabbits and cats. Current Eye Research. 1984;3:1155–1158. doi: 10.3109/02713688409000816. [DOI] [PubMed] [Google Scholar]
- 59.Miller PE, Pickett JP, Majors LJ, Kurzman ID. Evaluation of two applanation tonometers in cats. American Journal of Veterinary Research. 1991;52:1917–1921. [PubMed] [Google Scholar]
- 60.Miller PE. Clinical comparison of the Mackay-Marg and Tono-Pen applanation tonometers in the dog. Progress in Veterinary and Comparative Ophthalmology. 1991;1:171–176. [Google Scholar]
- 61.Tripathi RC, Parapuram SK, Tripathi BJ, et al. Corticosteroids and glaucoma risk. Drugs Aging. 1999;15:439–450. doi: 10.2165/00002512-199915060-00004. [DOI] [PubMed] [Google Scholar]
- 62.Becker B, Hahn KA. Topical corticosteroids and heredity in primary open-angle glaucoma. American Journal of Ophthalmology. 1964;57:543–551. doi: 10.1016/0002-9394(64)92500-0. [DOI] [PubMed] [Google Scholar]
- 63.Armaly MF. Genetic factors related to glaucoma. Annals of the New York Academy of Sciences. 1968;151:861–875. doi: 10.1111/j.1749-6632.1968.tb48270.x. [DOI] [PubMed] [Google Scholar]
- 64.Armaly MF. The heritable nature of dexamethasone-induced ocular hypertension. Archives of Ophthalmology. 1966;75:32–35. doi: 10.1001/archopht.1966.00970050034007. [DOI] [PubMed] [Google Scholar]
- 65.Sigle KJ, Camaño-Garcia G, Carriquiry AL, et al. The effect of dorzolamide 2% on circadian intraocular pressure in cats with primary congenital glaucoma. Veterinary Ophthalmology. 2011;14(Suppl 1):48–53. doi: 10.1111/j.1463-5224.2011.00913.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.McDonald JE, Kiland JA, Kaufman PL, et al. Effect of topical latanoprost 0.005% on intraocular pressure and pupil diameter in normal and glaucomatous cats. Veterinary Ophthalmology. 2015 doi: 10.1111/vop.12292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Green K, Phillips CI, Gore SM, et al. Ocular fluid dynamics response to topical RU486, a steroid blocker. Current Eye Research. 1985;4:605–612. doi: 10.3109/02713688508999992. [DOI] [PubMed] [Google Scholar]
- 68.Phillips CI, Green K, Gore SM, et al. Eye drops of RU 486–6, a peripheral steroid blocker, lower intraocular pressure in rabbits. Lancet. 1984;1:767–768. doi: 10.1016/s0140-6736(84)91279-0. [DOI] [PubMed] [Google Scholar]
- 69.Southren AL, l’Hommedieu D, Gordon GG, Weinstein BI. Intraocular hypotensive effect of a topically applied cortisol metabolite: 3 alpha, 5 beta-tetrahydrocortisol. Investigative Ophthalmology and Visual Science. 1987;28:901–903. [PubMed] [Google Scholar]
- 70.Southren AL, Wandel T, Gordon GG, Weinstein BI. Treatment of glaucoma with 3 alpha, 5 beta-tetrahydrocortisol: a new therapeutic modality. Journal of Ocular Pharmacology. 1994;10:385–391. doi: 10.1089/jop.1994.10.385. [DOI] [PubMed] [Google Scholar]
- 71.Robin AL, Clark AF, Covert DW, et al. Anterior juxtascleral delivery of anecortave acetate in eyes with primary open-angle glaucoma: a pilot investigation. American Journal of Ophthalmology. 2009;147:45–50.e2. doi: 10.1016/j.ajo.2008.07.039. [DOI] [PubMed] [Google Scholar]
- 72.Robin AL, Suan EP, Sjaarda RN, et al. Reduction of intraocular pressure with anecortave acetate in eyes with ocular steroid injection-related glaucoma. Archives of Ophthalmology. 2009;127:173–178. doi: 10.1001/archophthalmol.2008.595. [DOI] [PubMed] [Google Scholar]
- 73.Prata TS, Tavares IM, Mello PA, et al. Hypotensive effect of juxtascleral administration of anecortave acetate in different types of glaucoma. Journal of Glaucoma. 2010;19:488–492. doi: 10.1097/IJG.0b013e3181c4b0e8. [DOI] [PubMed] [Google Scholar]
- 74.Candia OA, Gerometta R, Millar JC, Podos SM. Suppression of corticosteroid-induced ocular hypertension in sheep by anecortave. Archives of Ophthalmology. 2010;128:338–343. doi: 10.1001/archophthalmol.2009.387. [DOI] [PubMed] [Google Scholar]
- 75.Clark AF. AL-3789: a novel ophthalmic angiostatic steroid. Expert Opinion and Investigational Drugs. 1997;6:1867–1877. doi: 10.1517/13543784.6.12.1867. [DOI] [PubMed] [Google Scholar]
- 76.Clark AF, Lane D, Wilson K, et al. Inhibition of dexamethasone-induced cytoskeletal changes in cultured human trabecular meshwork cells by tetrahydrocortisol. Investigative Ophthalmology and Visual Science. 1996;37:805–813. [PubMed] [Google Scholar]
- 77.Gerometta R, Spiga M, Candia O, Borra’s T. Treatment of steroid-induced ocular hypertension by inducible gene transfer of MMP1 in sheep. Investigative Ophthalmology and Visual Science. 2009 doi: 10.1167/iovs.09-4920. ARVO abstract 5722. [DOI] [PMC free article] [PubMed] [Google Scholar]