Abstract
Although our most recent studies have identified Isorhapontigenin (ISO), a novel derivative of stilbene that isolated from a Chinese herb Gnetum cleistostachyum, for its inhibition of human bladder cancer (BC) growth, nothing is known whether ISO possesses an inhibitory effect on BC invasion. Thus, we addressed this important question in current study and discovered that ISO treatment could inhibit mouse invasive BC development following bladder carcinogen N-butyl-N- (4-hydroxybutyl) nitrosamine (BBN) exposure in vivo. We also found that ISO suppressed human BC cell invasion accompanied by up-regulation of the forkhead box class O 1 (FOXO1) mRNA transcription in vitro. Accordingly, FOXO1 was profoundly down-regulated in human BC tissues, and was negatively correlated with BC invasion. Forced expression of FOXO1 specifically suppressed high grade human BC cell invasion, while knockdown of FOXO1 promoted non-invasive BC cells becoming invasive BC cells. Moreover, knockout of FOXO1 significantly increased BC cell invasion and abolished the ISO inhibition of invasion in human BC cells. Further studies showed that the inhibition of STAT1 phosphorylation at Tyr701 was crucial for ISO up-regulation of FOXO1 transcription. Furthermore, this study revealed that metalloproteinase-2 (MMP-2) was a FOXO1 downstream effector, which was also supported by data obtained from mouse model of ISO inhibition BBN-induced mouse invasive BC formation. These findings not only provide a novel insight into the understanding of mechanism of BC’s propensity to invasion, but also identify a new role and mechanisms underlying the natural compound ISO that specifically suppresses such BC invasion through targeting the STAT1-FOXO1-MMP2 axis.
Keywords: Bladder cancer, Isorhapontigenin, cell invasion, FOXO1, STAT-1
Introduction
Bladder cancer (BC) is the sixth most common cancer in the United States and the number one cause of death in patients with urinary system malignancies. The incidence of BC has steadily risen in recent decades. It is estimated that more than 74,690 Americans will be diagnosed with BC and more than 15,580 will die of this disease in 2014 (1). Approximately 20~30% of BC are muscle-invasive, 50% of these patients die from metastasis within 2 years of diagnosis, and the 5-year survival rate for metastatic BC is only 6% (2). The remaining 70%-80% of BC are diagnosed as non-muscular invasion, in which about 20% progress to muscle-invasive BC and have a 43% relative lower 5-year survival rate (2). In addition, BC is one of the most costly cancers as a result of necessary lifetime monitoring and treatment. For these reasons, the identification of new natural compounds that specifically suppress human BC invasion and defining key molecules that are responsible for mediating human BC invasion and metastasis are of extremely importance for improving the clinical outcome of patients with this disease. Isorhapontigenin (ISO) is a novel derivative of stilbene and is isolated and purified from a Chinese herb Gnetum cleistostachyum (3-5), which has been used for centuries as treatment for several cancers including BCs. Recent work by our group has demonstrated that ISO treatment induces cell-cycle arrest at G0/G1 phase and inhibits anchorage-independent cell growth through inhibiting cyclin D1 expression in both RT4 human non-invasive BC cells and UMUC3 human invasive BC cells (4). Our studies have also found that ISO exhibits the anti-cancer activity accompanied by down-regulating X-linked inhibitor of apoptosis protein (XIAP), thus inducing apoptosis in T24T human invasive BC cells in a relative high doses (5). Nevertheless, nothing is known whether ISO is able to inhibit BC invasion at non-cytotoxic doses.
Forkhead box O (FOXO) proteins, which include FOXO1, FOXO3a, FOXO4 and FOXO6 in human, are primarily function as transcription factors in nucleus and act as tumor suppressors (6, 7). FOXO proteins is reported to up-regulate negative regulators of the G1/S transition of the cell cycle, such as p27KIP1, p21WAF1 and p130, and repress positive regulators, such as cyclin D1 and D2 (6). Overexpression of FOXO proteins also enhances Gadd45α and cyclin G2 promoter activity, resulting in cell cycle G2/M arrest (8). Besides, activation of FOXOs triggers apoptosis through binding to the promoters of the pro-apoptotic gene, FasL and Bim, and inducing their expression (9-11). Moreover, FOXO1 has been found to inhibit prostate cancer cell migration and invasion through binding to Runt-domain containing protein Runx2 and repress its transcriptional activity (12). Expression of a constitutive nuclear active form of FOXO1 significantly inhibited matrix metalloproteinase-9 (MMP-9) activation induced by epidermal growth factor (EGF) and prevented cell invasion in glioblastoma cells (13). Although the mRNA and protein expression level of FOXO1 are found to be down-regulated in high grade and invasive BC, nothing is known about the role, mechanisms and the upstream regulator/downstream effectors of FOXO1 in human BC invasion (14). Therefore, we addressed these questions and explored the potential FOXO1-related inhibitory effects of ISO on human BC invasion in the present study both in vitro and in vivo.
Materials and Methods
Plasmids, antibodies, and other reagents
The FLAG-tagged human FOXO1 expression construct, FasL promoter and IGFBG-1 (3×IRS) promoter luciferase reporters were constructed and used in previously studies (15). The shRNA sets for human FOXO1 were purchased from Open Biosystems (Thermo Fisher Scientific, Huntsville, AL). The Clustered Regularly Interspaced Short Palindromic Repeats (CRISPR)/Cas9 system specific targeting FOXO1 was purchased from Applied Biological Materials (ABM) Inc. (Richmond, Canada). Human FOXO1 promoter luciferase reporter was cloned into the pGL3 luciferase assay vector, and was kindly provided by Dr. Jean-Baptiste Demoulin (De Duve Institute, Catholic University of Louvain, BE-1200 Brussels, Belgium) (16). A mutation of STAT1 binding site in the FOXO1 promoter was created using site-directed mutagenesis by the overlap extension PCR method with mutagenic primers 5’-ACAGAAACACTCGAGAAGCGCCATCCAATAATAGAGATCCAAA-3’ (sense), 5’-TTTGGATCTCTATTATTGGATGGCGCTTCTCGAGTGTTTCTGT-3’ (anti-sense), and flanking primers 5’-ATTGAGTAGAATTCCTCGCGGCCGCC-3’ (forward), 5’-TGGAAGATCTCTGCGCTGCTGCCTGTTGAAT-3’ (reverse). GFP-STAT1 Y701F was obtained from Addgene (Cambridge, MA). Human MMP-2 expression construct was kindly provided by Dr. Jian Cao (Department of Medicine, School of Medicine, State University of New York at Stony Brook, Stony Brook, NY) (17). Human MMP-2 promoter luciferase reporter was a gift from Dr. Etty N. Benveniste (Department of Cell Biology, The University of Alabama at Birmingham, Birmingham, Alabama) (18). The antibodies against FOXO1, p-FOXO1, FOXO4, PARP, STAT1, p-STAT1, STAT3, p-STAT3, and CREB were commercially purchased from Cell Signaling Technology (Boston, MA). The antibodies against MMP-2, SOCS1, and GAPDH were bought from Santa Cruz Biotechnology (Santa Cruz, CA). Isorhapontigenin (ISO) with purity greater than 99.9% was purchased from Higher Biotech (Shanghai, China). ISO was dissolved in dimethyl sulfoxide (DMSO, Sigma, St Louis, MO) to make a 20 mM stock solution, and the same concentration of DMSO was also made and used as a vehicle control in all experiments.
Animal experiments
The C57BL/6J male mice at age of 5~6 weeks were randomly divided into three groups, 12 mice in each group, including vehicle-treated control group, N-butyl-N-(4-hydroxybutyl) nitrosamine (BBN)-treated group, and BBN combined with ISO-treated group. Mice in BBN-treated group were received BBN (0.05%) in drinking water for 20 weeks, while mice in BBN combined with ISO-treated group were received BBN and ISO (150 mg/kg/day) in drinking water for 20 weeks. ISO was given to the mice in drinking water on the day of initial exposure to BBN, and continued throughout the tumor induction period. Mice bladder tissues were excised and fixed overnight in 4% paraformaldehyde at 4°C. Fixed tissues were processed for paraffin embedding, and the serial 5 μm thick sections were then stained by Hematoxylin and eosin staining (HE).
Cell culture and transfection
Human invasive BC cell line UMUC3 and non-invasive BC cell line RT4 were provided by Dr. Xue-Ru Wu (Departments of Urology and Pathology, New York University School of Medicine, New York, NY) in 2010, and were described and used in our previous studies (4, 19). The human metastatic BC cell line T24T, which was a lineage-related lung metastatic variant of invasive BC cell line T24 (20-22), was kindly provided by Dr. Dan Theodorescu (Departments of Urology, University of Virginia, Charlottesville, VA) (20) in 2010. All the cell lines were subjected to DNA tests and authenticated in our previous studies (4). The cell lines were regularly authenticated on the basis of viability, recovery, growth, morphology and chemical response as well, and were most recently confirmed 4-6 months before use by using a short tandem repeat method. UMUC3 cells were maintained in Dulbecco’s Modified Eagle Medium (DMEM) supplemented with 10% FBS (HyClone, Logan, UT), 1% penicillin/streptomycin and 2 mM L-glutamine (Life Technologies, Rockville, MD). T24T cells were cultured in DMEM/Ham's F-12 (1:1 volume) mixed medium supplemented with 5% FBS, 1% penicillin/streptomycin and 2 mM L-glutamine. RT4 cells were maintained in McCoy’s 5A supplemented with 10% FBS, 1% penicillin/streptomycin and 2 mM L-glutamine. Transfections were carried out using PolyJetTM DNA In Vitro Transfection Reagent (SignaGen Laboratories, Gaithersburg, MD) according to the manufacturer's instructions. The transfected cells were then respectively selected with G418, hygromycin or puromycin (Life Technologies, Rockville, MD) for 4–6 weeks. Surviving cells were pooled as stable mass transfectants as described in our previous studies (23, 24).
Human tissue specimens
98 pairs of primary BC samples and their paired adjacent normal bladder tissues were obtained from patients who underwent radical cystectomy at Department of Urology of the Union Hospital of Tongji Medical College (Wuhan, China) between 2012 and 2015. All specimens were immediately snap-frozen in liquid nitrogen after surgical removal. Histological and pathological diagnoses were confirmed and the specimens were classified by a certified clinical pathologist according to the 2004 World Health Organization Consensus Classification and Staging System for bladder neoplasms. All specimens were obtained with appropriate informed consent from the patients and a supportive grant obtained from the Medical Ethics Committee of China.
ATP cell viability assay
Cell viability was measured utilizing CellTiter-Glo Luminescent Cell Viability Assay Kit (Promega Corp., Madison, WI) according to the manufacturer’s instructions as described in our previous studies (25). Briefly, Cells were plated in 96-well plates at a density of 10000 cells per well and allowed to adhere overnight. The cell culture medium was then replaced with 0.1% FBS DMEM and cultured for 12 hours. After ISO treatment for the indicated time and doses, 50 μl of CellTiter-Glo assay reagent was added to each well. The contents were mixed on an orbital shaker for 2 minutes to induce cell lysis, and then incubated at room temperature for 10 minutes to stabilize the luminescent signal. Results were read on a microplate luminometer LB 96V (Berthold GmbH & Co. KG, Bad Wildbad, Germany). Cell viability (%) was defined as the relative absorbance of treated samples versus that of the untreated control. All experiments were performed with six wells for each experiment and repeated at least three times.
In vitro cellular migration and invasion assays
In vitro migration and invasion assays were conducted by using transwell chamber (for migration assay) or transwell pre-coated matrigel chamber (for invasion assay) according to the manufacturer's protocol (BD Biosciences, Bedford, MA) as described previously (26, 27). Briefly, 700 μl of medium containing 10% FBS (for UMUC3 and T24T cells) or 40% FBS (for RT4 cells) was added to the lower chambers, while homogeneous single cell suspensions (5×104 cells/well) in 0.1% FBS medium with or without ISO as indicated were added to the upper chambers. The transwell plates were incubated in 5% CO2 incubator at 37°C for 24 hours, and thereafter were washed by PBS, fixed with 4% formaldehyde, and stained with Giemsa stain. The non-migration or non-invading cells were scrapped off on the top of chamber. The migration and invasion rates were quantified by counting the migration and invaded cells at least three random fields under a light microscope (Olympus, Center Valley, PA).
Western blotting
Western blot assay was assessed as previously described (28). Briefly, cells were plated in 6-well plates and cultured in normal FBS medium until 70–80% confluence. The cells were then cultured in 0.1% FBS medium for 12 hours, followed by treatment with different doses of ISO for the indicated time. The cells were washed once with ice-cold phosphate-buffered saline and cell lysates were prepared with a lysis buffer (10 mM Tris-HCl (pH 7.4), 1% SDS, and 1 mM Na3VO4). An equal amount (80 μg) of total protein from each cell lysate was subjected to Western blot with the indicated antibody as described in previous studies (25, 26). Immunoreactive bands were detected by using the alkaline phosphatase-linked secondary antibody and ECF Western blotting system (Amersham Biosciences, Piscataway, NJ). The images were acquired by using Typhoon FLA 7000 imager (GE Healthcare, Pittsburgh, PA).
Nuclear extract preparation
Nuclear extracts were prepared as previously described (23). UMUC3 cells were seeded into 10-cm culture dishes at 70–80% confluence, cultured in 0.1% FBS medium for 12 hours, and then treated either with vehicle (0.1% DMSO) or 10 μM of ISO for 12 hours. The nuclear proteins were extracted by the Nuclear/Cytosol Fractionation Kit (BioVison Technologies, Mountain View, CA) following the manufacturer’s protocols. Equal Protein concentrations were measured by a protein quantification assay kit (Bio-Rad, Hercules, CA). Nuclear extracts were stored at −80°C before they were used.
Reverse transcription-polymerase chain reaction (RT-PCR) and Quantitative RT-PCR
Total RNA was extracted with TRIzol reagent (Invitrogen Corp., Carlsbad, CA) and cDNAs were synthesized with the SuperScript® III First-Strand Synthesis System for RT-PCR (Invitrogen Corp., Carlsbad, CA). A pair of oligonucleotides (Forward: 5′-GATGATCTTGAGGCTGTTGTC-3′ and Reverse: 5′-CAGGGCTGCTTTTAACTCTG-3′) were used to amplify human gapdh cDNA as loading control. The human foxo1 cDNA fragments were amplified by a pair of human foxo1 specific PCR primers (Forward: 5’-AACCTGGCATTACAGTTGGCC-3’; Reverse: 5’-AAATGCAGGAGGCATGACTACGT-3’). The flag-foxo1 fragments were amplified by primers 5’-ACAAGGACGACGATGACAAGGG-3’ (Forward) and 5’-GGCCGAGTTGGACTGGCTAAA-3’ (Reverse). The human mmp-2 cDNA fragments were amplified by 5’-caagtgggacaagaaccaga-3’ (Forward) and 5’-cca aagttgatcatgatgtc-3’ (Reverse). The human mmp-9 cDNA fragments were amplified by 5’-gggacgcagacatcgtcac-3’ (Forward) and 5’-tcgtcatcgtcgaaatggc-3’ (Reverse). The PCR products were separated on 2% agarose gels and stained with ethidium bromide. The images were visualized and scanned with UV lights with FluorChem SP imaging system (Alpha Innotech Inc., San Leandro, CA) as described previously (29). The Quantitative RT–PCR analysis was carried out using the SYBR Green PCR Kit (Qiagen, Santa Clarita, CA) and the 7900HT Fast Real-time PCR system (Applied Biosystems, Carlsbad, CA).
Luciferase assay
As described in our previous studies (4, 5), dual-luciferase reporter assay was performed by using the luciferase assay system (Promega Corp., Madison, WI). Briefly, Human FasL promoter, IGFBG-1 (3×IRS) promoter, FOXO1 promoter, and MMP-2 promoter luciferase reporters were transfected into the indicated human BC cells, respectively. After ISO treatment, cells were extracted with passive lysis buffer [25 mM Tris-phosphate (pH 7.8), 2 mM EDTA, 1% Triton X-100, and 10% glycerol]. The luciferase activity was measured with a microplate luminometer LB 96V (Berthold GmbH & Co. KG, Bad Wildbad, Germany). The Renilla luciferase signal was normalized to the internal firefly luciferase transfection control.
Immunohistochemistry Paraffin (IHC-P) of mice bladder tissues
Mice bladder tissues were immunostained by antibodies specific against FOXO1 (Cell Signaling Technology) and MMP-2 (Santa Cruz Biotechnology), respectively. The resultant immunostaining images were captured using the AxioVision Rel.4.6 computerized image analysis system (Carl Zeiss, Oberkochen, Germany). Protein expression levels were analyzed by calculating the integrated optical density per stained area (IOD/area) using Image-Pro Plus version 6.0 (Media Cybernetics, MD). Briefly, the IHC stained sections were evaluated at 400-fold magnifications, at least 5 representative staining fields of each section were analyzed to calculate the optical density based on typical photographs that had been captured.
Statistical methods
Associations between categorical variables were assessed using the Chi-square test. Student’s t-test was utilized to compare continuous variables, summarized as means ± SD, between different groups. Paired t-test was performed to compare the difference between paired tissues using real-time PCR analyze. P < 0.05 was considered statistically.
Results
ISO treatment inhibited BBN-induced mouse invasive BC formation in vivo and human BC invasion in vitro
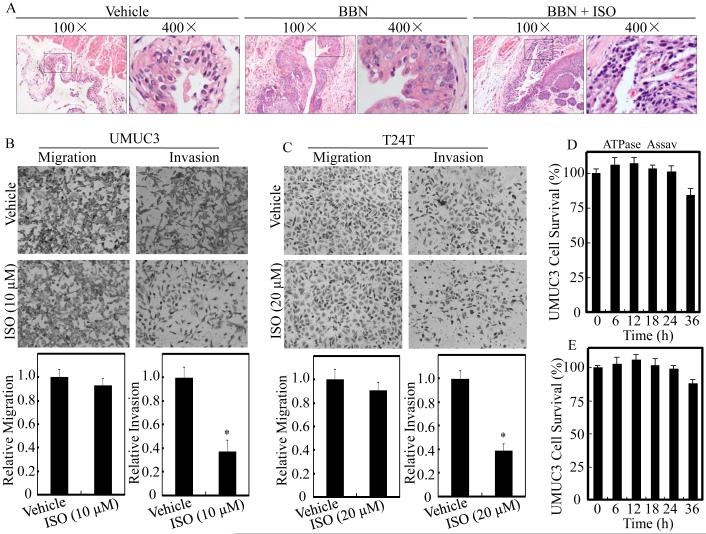
Isorhapontigenin (ISO) has been shown to inhibit growth and induce apoptosis in human BC cells in our recent studies (4, 5). N-butyl-N-(4-hydroxybutyl) nitrosamine (BBN) is a well-characterized bladder carcinogen for its induction of 100% invasive BC in mouse model (30). To explore whether ISO exhibit BC invasion, we first employed BBN-induced invasive BC mouse model and examined the effects of ISO on BBN-induced mouse invasive BC formation. As shown in Table 1 and Figure 1A, none of the vehicle-treated control mice developed BC, whereas BBN induced 100% (12/12) high-grade muscle-invasive BCs formation. Interestingly, only 16.7% (2/12) of the BBN-treated mice developed high-grade muscle-invasive BC while ISO was administrated, with 7 cases of papillomas and 3 cases of low-grade non-muscle-invasive BCs, demonstrating a novel biological activity of ISO as an efficient drug that targets at stage of invasive BC development in vivo (p<0.05). Subsequently, two human invasive BC cell lines UMUC3 and T24T were employed for transwell cancer cell invasion assay in presence of relative low dosages, 10 μM and 20 μM, of ISO treatment, according to the dosages applied in cell proliferation analyses described previously (4, 5). As shown in Figure 1B and 1C, the relative invasion rates of UMUC3 and T24T cells were reduced by 63.1% and 61.2% after 10 μM and 20 μM ISO treatment, respectively, in comparison to the vehicle control, whereas ISO didn’t show observed effects on the BC cell migration under same experimental conditions. To further exclude any possible involvement of cellular toxicity, the cells were treated with the same dosages of ISO as shown in Figure 1D and 1E for different time points from 6 hours to 36 hours, and cell survival rates were analyzed by ATPase assay. As expected, the selected dosages of ISO did not show any observable cellular toxicity to either UMUC3 (Figure 1D) or T24T (Figure 1E) cells at the time point (24 hours) when cell migration and invasion were measured. Taken together, these data, for the first time demonstrate that ISO is a new natural compound that can specifically inhibits mouse invasive BC development in vivo following BBN exposure and suppresses BC cell invasion in vitro.
Table 1.
Effect of ISO treatment ON BBN-induced mouse invasive BC formation
| Group | No. of mice |
Carcinogen | Treatment | No. (%) of Papillomas |
No. (%) of Non-invasive BC |
No. (%) of Invasive BC |
|---|---|---|---|---|---|---|
| 1 | 12 | Vehicle only |
Vehicle only |
0 (0) | 0 (0) | 0 (0) |
| 2 | 12 | BBN | Vehicle only |
0 (0) | 0 (0) | 12 (100)* |
| 3 | 12 | BBN | ISO | 7 (58.3) | 3 (25) | 2 (16.7)# |
Symbol indicates a significant difference between vehicle control group and BBN-treated group (P< 0.05).
Symbol indicates a significant difference between BBN-treated group and BBN combined with ISO-treated group (P< 0.05).
Figure 1. ISO inhibited BC invasion in vivo and in vitro.
(A) Mice were divided into vehicle-treated control group (n=12), BBN-treated group (n=12), and BBN combined with ISO-treated group (n=12). HE staining was performed and the representative images of each group were shown. Human UMUC3 (B) and T24T (C) BC cells were cultured in chamber or pre-coated matrigel chamber and treated with medium containing either vehicle or indicated concentration of ISO for 24 hours. The cells were then fixed and stained. The invasion and migration rates were quantified by counting the relative migrated (transwell) and invaded cells at least three random fields under a light microscope. (D) UMUC3 cells were treated with medium containing either vehicle or 10 µM ISO up to 36 hours for cell viability assessment by ATPase assay. (E) T24T cells were treated with medium containing either vehicle or 20 µM ISO for the indicated time. Cell viability was also evaluated by ATPase assay. Results are the means ± SD of triplicates. Symbol “*” indicates a significant difference between vehicle and ISO-treated group (P < 0.05).
ISO induced FOXO1 protein expression and FOXO1-dependent transcriptional activity in human BC cells
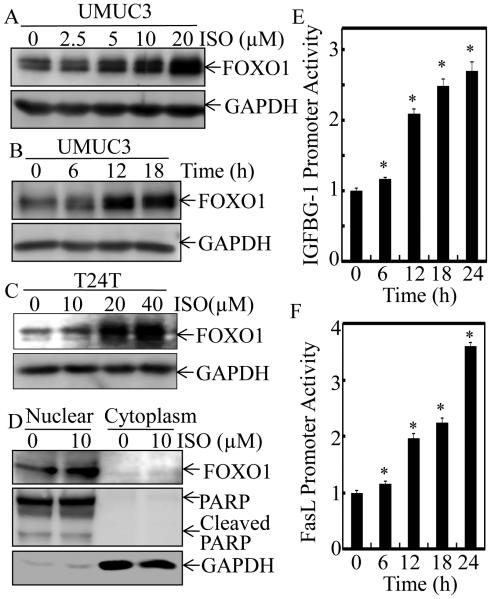
FOXO1 has been proposed as a potential prognostic marker for BC since an increased foxo1 mRNA expression is associated with the reduced BC disease progression and a significantly prolonged survival of the patients (14). To elucidate the mechanisms underlying ISO inhibitory effect on human BC, we investigated whether ISO could regulate the expression of FOXO1 in human invasive BC cell lines, UMUC3 and T24T. As shown in Figure 2A, ISO treatment induced FOXO1 protein expression in a dose-dependent manner in UMUC3 cells. Treatment of UMUC3 cells with 10 µM ISO also resulted in a gradual induction in FOXO1 protein level over various time points (Figure 2B). Consistently, similar FOXO1 protein expression profile was also observed in the metastatic human BC T24T cells upon ISO treatment (Figure 2C). It was noted that ISO treatments at dose of 10 µM for UMUC3 cells and 20 µM for T24T cells could markedly induce FOXO1 protein expression (Figure 2, A-C) with a profoundly specific inhibition of cancer cell invasion without affecting their migration (Figure 1C and 1D). To unravel the function of ISO-induced FOXO1 protein, we determine FOXO1 protein location and its dependent transcriptional activation in ISO-treated cells. As shown in Figure 2D, FOXO1 protein was almost all located in nuclear fraction, while it was not observable in cytoplasmic fraction. Consistent with its location, ISO treatment remarkably enhanced FOXO1-dependent IGFBG-1 promoter and FasL promoter transcription activities (Figure 2E and 2F), strongly demonstrating that ISO-induced FOXO1 protein is not only located in nuclear, but also are functional in human BC cells.
Figure 2. ISO treatment up-regulated FOXO1 expression and enhanced FOXO1-dependent transcriptional activity in human BC cells.
UMUC3 (A & B) and T24T cells (C) were treated with medium containing either vehicle or ISO as the indicated concentrations for 12 hours (A & C) or treated with medium containing either vehicle or 10 μM ISO for 6-18 hours as indicated (B). The whole cell lysates were used for Western blotting. GAPDH was used as protein loading control. (D) The cell nuclear and cytoplasm fractions of UMUC3 cells treated with 10 μM ISO for 12 hours were subjected to SDS-PAGE and immunoblotted with the indicated specific antibodies. The expressions of PARP and GAPDH were respectively used as markers for nuclear and cytoplasm fractions, respectively. (E) UMUC3 cells stably transfected with IGFBG-1 promoter-driven luciferase reporter was treated with 10 μM of ISO for the indicated times. Luciferase activity was determined by the Dual-Luciferase Reporter Assay System. (F) UMUC3 cells were stably transfected with FasL promoter-driven luciferase reporter construct, and was treated with10 μM of ISO for the indicated times to determine the promoter transcriptional activity. Results are the means ± SD of triplicates. Symbol “*” indicates a significant difference between vehicle and ISO-treated group (P < 0.05).
FOXO1 was down-regulated in human BC tissues and acted as an effective suppressor of invasion of human BC cells
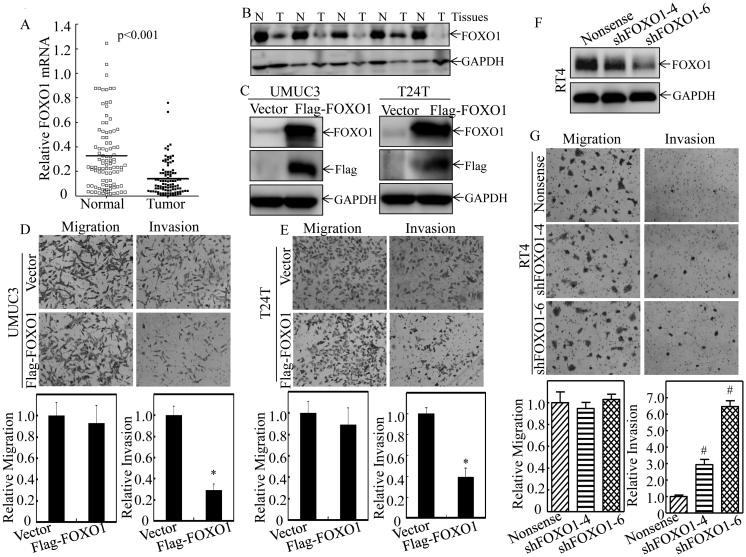
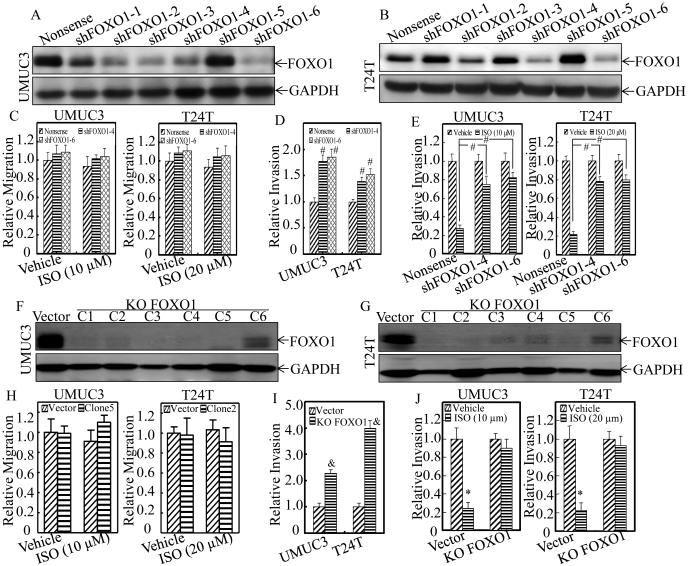
To find out the clinical relevant of therapeutic effect of ISO in human BC, we evaluated FOXO1 expression in 98 pairs of BC tissues and their adjacent normal bladder tissues surgically removed from patients who were diagnosed with BCs. As shown in Figure 3A, a profound reduction of foxo1 mRNA expression was observed in human BC tissues with an overall average 2.4-fold lower relative foxo1 mRNA level in comparison to their adjacent normal bladder tissues (P<0.001). Moreover, a significant negative relationship was observed between foxo1 expression and BC grade, invasion, as well as the lymph nodes metastasis, respectively (P<0.05, Table 2). Consistent with mRNA expression, a significantly decreased FOXO1 protein expression was also observed in human invasive BC tissues (Figure 3B). To clarify the relation of FOXO1 protein to BC invasion, UMUC3 and T24T cells were stably transfected with exogenous Flag-FOXO1. As shown in Figure 3C-3E, ectopic expression of FOXO1 specifically attenuated the cell invasion with no significant inhibition of cell migration in both high grade UMUC3 and T24T BC cells. On the other hand, knockdown of FOXO1(shFOXO1-4 and shFOXO1-6) (Figure 3F) increased invasiveness without affecting migration of non-invasive RT4 BC cells (Figure 3G). These results are consistent to demonstrate that down-regulation of FOXO1 expression plays an important role in the BC invasion, and the FOXO1 induction by ISO treatment might be crucial for its inhibitory effect on human BC invasion.
Figure 3. FOXO1 was down-regulated in human BC tissues and acted as a regulator of human BC cell invasion.
Total RNA and protein lysates were prepared from human normal (N) and paired cancerous (T) tissues among 98 patients diagnosed with BCs, and subjected to Quantitative RT-PCR and Western blotting analyses for determining FOXO1 mRNA (A) and protein (B) expression profiles, respectively. Flag-FOXO1 expression construct was used to stably transfected into UMUC3 and T24T cells, respectively. The stable transfectants, UMUC3(Flag-FOXO1) and T24T(Flag-FOXO1) were identified by Western blotting (C), and then used for determination of their abilities of cell invasion and migration as compared with their vector control transfectants (D & E) as described in “Materials and Methods” section. (F) RT4 cells were stably transfected with Nonsense shRNA or two FOXO1 shRNA constructs (shFOXO1-4, shFOXO1-6), respectively, and the knockdown efficiency of FOXO1 protein was evaluated by Western Blot. (G) FOXO1 stably knockdown transfectants, RT4(shFOXO1-4) and RT4(shFOXO1-6), as well as Nonsense transfectant RT4(Nonsense) were then used for determination of their migration and invasion abilities. Results are the means ± SD of triplicates. Symbol “*” indicates a significant difference between vector control and FOXO1 overexpression group (P < 0.05). Symbol “#” indicates a significant difference between Nonsense transfectant and shRNA transfectants (P < 0.05).
Table 2.
Correlation between foxo1 mRNA level and clinic pathological factors
| Parameters | Group | Total | foxo1 expression |
P value |
|
|---|---|---|---|---|---|
| Low | high | ||||
| Gender | Male | 79 | 60 | 19 | |
| Female | 19 | 13 | 6 | 0.561 | |
|
| |||||
| Age (years) | <55 | 17 | 14 | 3 | |
| ≥55 | 81 | 59 | 22 | 0.548 | |
|
| |||||
| Histological grade | Low | 32 | 19 | 13 | |
| High | 66 | 54 | 12 | 0.017* | |
|
| |||||
| Tumor stage | Tis,Ta,T1 | 39 | 22 | 17 | |
| T2,T3,T4 | 59 | 51 | 8 | 0.001* | |
|
| |||||
| Tumor size | <3.0cm | 40 | 31 | 9 | |
| ≥3.0cm | 58 | 42 | 16 | 0.570 | |
|
| |||||
| Tumor multiplicity | Unifocal | 25 | 19 | 6 | |
| Multifocal | 73 | 54 | 19 | 0.841 | |
|
| |||||
| Lymph nodes metastasis | Absent | 65 | 45 | 20 | |
| Present | 33 | 30 | 3 | 0.008* | |
P<0.05
ISO up-regulated FOXO1 expression by increasing its mRNA transcription in human BC cells
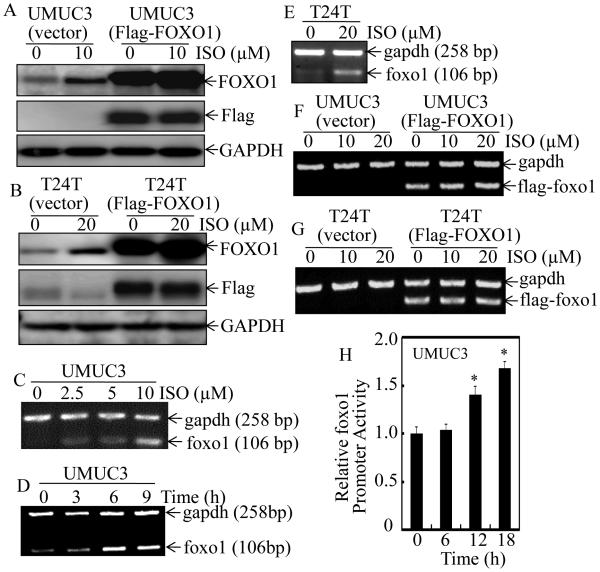
It is well-known that FOXO1 expression can be regulated through its nuclear exportation and degradation (6). To address whether ISO treatment alternates FOXO1 protein degradation, both transfectants of UMUC3(vector) and UMUC3(Flag-FOXO1) were employed. As shown in Figure 4A and 4B, ISO treatment only increased endogenous FOXO1 protein expression in UMUC3(vector) cells, whereas it did not affect exogenous Flag-FOXO1 expression, excluding the possibility that FOXO1 protein induction by ISO is at protein degradation level. We, therefore, next evaluated the mRNA levels of foxo1 in the same BC cells. Consistent with the results obtained from protein levels, endogenous foxo1 mRNA expression was markedly up-regulated upon ISO treatment in both UMUC3 and T24T cells (Figure 4C-4E), whereas the flag-tagged exogenous flag-foxo1 mRNA was not affected by ISO treatment (Figure 4F and 4G). These results suggest that ISO-induced FOXO1 expression may be regulated at mRNA transcription level. To test this notion, the foxo1 promoter-driven luciferase reporter was transfected into UMUC3 cells and foxo1 promoter activity was significantly up-regulated in a time-dependent manner upon ISO treatment (Figure 4H). Taken together, these data strongly reveal that ISO-induced FOXO1 protein expression is regulated at mRNA transcriptional level in human BC cells.
Figure 4. ISO up-regulated FOXO1 expression at mRNA transcriptional level in human BC cells.
UMUC3(Vector) and UMUC3(Flag-FOXO1) (A) or T24T(Vector) and T24T(Flag-FOXO1) (B) cells were treated with medium containing either vehicle or ISO as the indicated concentrations for 12 hours. The cell extracts were subjected to Western Blotting for determination of ISO on protein expression of FOXO1 and Flag-FOXO1 as indicated. UMUC3 (C & D) and T24T (E) cells were treated with ISO for 6 hours at indicated concentrations (C & E) or 10 μM ISO for the indicated time (D), and foxo1 mRNA expression was determined by RT-PCR. The gapdh mRNA level was used as loading control. UMUC3(Vector) and UMUC3(Flag-FOXO1) (F) or T24T (Vector) and T24T (Flag-FOXO1) (G) cells were treated with medium containing either vehicle or ISO as the indicated concentrations for 6 hours. The Flag-foxo1 mRNA levels were evaluated by RT-PCT as indicated. (H) UMUC3 cells stably transfected with foxo1 promoter-driven luciferase reporter was treated with 10 μM ISO for the indicated times. Luciferase activity was evaluated by the Dual-Luciferase Reporter Assay System. Results are the means ± SD of triplicates. Symbol “*” indicates a significant difference between vehicle and ISO-treated group (P < 0.05).
FOXO1 expression was required for ISO inhibition of BC cell invasion
To address the contribution of FOXO1 induction by ISO to its inhibition of BC invasion, we transfected various FOXO1 shRNAs into UMUC3 and T24T, respectively, and the stable FOXO1 knockdown transfectants were identified by Western Blotting as shown in Figure 5A and 5B. The stable transfectants, UMUC3(shFOXO1-4), UMUC3(shFOXO1-6), T24T(shFOXO1-4) and T24T(shFOXO1-6), were used to determine invasion and migration abilities as compared with their Nonsense control transfectants, UMUC3(Nonsense) and T24T(Nonsense), respectively. As shown in Figure 5C and Supplemental Figure 1A and 1B, knockdown of FOXO1 by its shRNAs(shFOXO1-4 and shFOXO1-6) showed no effects on cell migration, but significantly promoted invasion in both UMUC3 and T24T cells (Figure 5D and Supplemental Figure 1A and 1B), which consistently support that FOXO1 protein exhibits specific inhibition of cancer invasion. Importantly, suppression of FOXO1 protein expression markedly reduced ISO inhibition of cell invasion in both UMUC3 and T24T cells (Figure 5E and Supplemental Figure 1A and 1B). To further verify the role of FOXO1 in ISO suppression of BC invasion, CRISPR/Cas9 systems were used to knockout FOXO1 gene in both UMUC3 and T24T cells. The single clone stable FOXO1 knockout transfectant, UMUC3(KO FOXO1, clone 2) and T24T(KO FOXO1, clone 5), were selected for our studies (Figure 5F and 5G). As shown in Figure 5H and Supplemental Figure 2A and 2B, BC migration was not affected upon FOXO1 knockout, while the invasion of UMUC3 and T24T cells was further increased (Figure 5I and Supplemental Figure 2A and 2B). Moreover, knockout of FOXO1 successfully diminished the effects of ISO on BC invasion (Figure 5J and Supplemental Figure 2A and 2B). These results greatly demonstrate that FOXO1 protein induction by ISO is crucial for its inhibition of BC invasion.
Figure 5. Knockdown and knockout of FOXO1 reversed ISO inhibition of BC cell invasion.
(A-E) UMUC3 (A) and T24T (B) cells were stably transfected with Nonsense shRNA or a set of six various FOXO1 shRNA (shFOXO1) constructs, respectively. The knockdown efficiency of FOXO1 protein was assessed by western blotting. FOXO1 stably knockdown transfectants, including UMUC3(shFOXO1-4), UMUC3(shFOXO1-6), T24T(shFOXO1-4) and T24T(shFOXO1-6), as well as Nonsense transfectants were then used for determination of their cell migration (C) and invasion (D & E) in presence of either vehicle or the indicated concentration of ISO treatment for 24 hours. (F-J) CRISPR/Cas9 systems were then applied to knockout FOXO1 gene, and the single clone stable FOXO1 knockout transfectant, UMUC3(KO FOXO1, C1-C6) and T24T(KO FOXO1, C1-C6), were identified by Western Blot (F & G). FOXO1 stably knockout transfectants, including UMUC3(KO FOXO1, clone5) and T24T(KO FOXO1, clone2), as well as Vector control transfectants were used for determination of their cell migration (H) and invasion (I & J) in presence of either vehicle or the indicated concentration of ISO treatment for 24 hours. Results are the means ± SD of triplicates. Symbol “#” indicates a significant difference between Nonsense transfectant and shRNA transfectants (P < 0.05). Symbol “&” indicates a significant difference between scramble vector control and FOXO1 knockout transfectants (P<0.05). Symbol “*” indicates a significant difference between vehicle and ISO-treated group (P < 0.05).
ISO treatment enhanced FOXO1 transcription through inhibition of STAT1 phosphorylation at Tyr701
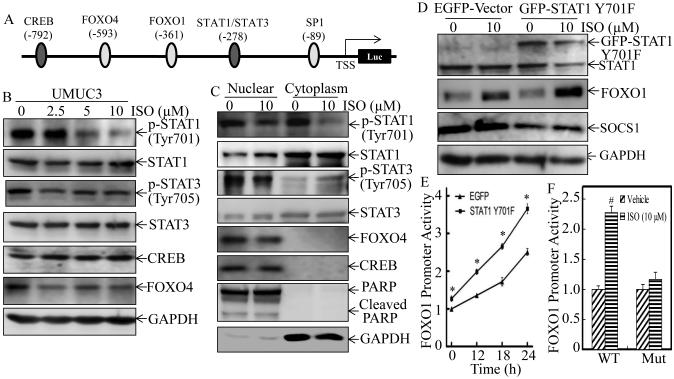
The above results demonstrate that ISO inhibition of human BC invasion was dependent on FOXO1 induction via up-regulating FOXO1 gene promoter transcription activity. To identify the transcription factor that is responsible for ISO up-regulation of FOXO1 transcription, TFANSFAC® Transcription Factor Binding Sites Software (Biological Database, Wolfenbutel, Germany) was used for bioinformatics analysis of the FOXO1 promoter region. The results revealed that an approximate 800bp promoter region of the human FOXO1 gene contained the putative DNA-binding sites of various transcription factors, including CREB, FOXO4, STAT1, and STAT3 (Figure 6A). We next examined the changes in both expression and the nuclear translocation of these factors following ISO treatment for 12 hours. As shown in Figure 6B, treatment of UMUC3 cells with ISO resulted in a dramatic inhibition of STAT1 phsphorylation at Tyr701 in a dose-dependent manner without effecting total protein expression, while there was only a slightly effect on phsphorylation of STAT3 and expression of FOXO4 and CREB. The results obtained from distributions of the transcription factors between nuclear and cytoplasm fractions consistently indicated that ISO treatment mainly targeted on STAT1 phsphorylation at Tyr701 (Figure 6C). To investigate the role of STAT1 phosphorylation in up-regulation of FOXO1 by ISO, a dominant negative mutant form of STAT1(GFP-STAT1 Y701F) was ectopically expressed in UMUC3 cells and the dominant negative effect was confirmed by its inhibition of a STAT1-regulated SOCS1 expression (31) (Figure 6D). The results showed that transfection of dominant negative STAT1 markedly enhanced ISO-induced FOXO1 protein expression (Figure 6D) as well as FOXO1 promoter-driven transcription activity (Figure 6E), indicating that suppression of STAT1 activity could mimic ISO treatment. In the contrast, mutation of STAT1 binding site effectively diminished the increased FOXO1 promoter activity induced by ISO (Figure 6F). Thus, these data suggest that phosphorylated STAT1 at Tyr701 can bind to the FOXO1 promoter to inhibit its transcription, and ISO-induced up-regulation of FOXO1 transcription is specifically mediated through ISO suppression of STAT1 phosphorylation at Tyr701.
Figure 6. ISO promoted FOXO1 transcription by inhibition of STAT1 phosphorylation at Tyr701.
(A) Schematic representation of transcription factor binding sites of human FOXO1 gene promoter. (B) UMUC3 cells were treated with either vehicle or ISO as the indicated concentrations for 12 hours. Expression of the related transcription factors in the whole cell lysates was determined by Western blotting, and GAPDH was used as protein loading control. (C) The UMUC3 cells were treated with either vehicle or 10 μM of ISO for 12 hours. The cell extracts were used to isolate cell nuclear and cytoplasm fractions and then subjected to Western Blotting with the specific antibodies as indicated. PARP and GAPDH were used as markers for nuclear and cytoplasm fractions, respectively. (D) UMUC3 cells were stably transfected with dominant negative STAT1, GFP-STAT1 Y701F, and the stable transfectants were treated with either vehicle or 10 μM ISO for 12 hours. The expression of FOXO1 and SOCS1 was determined by Western blotting. GAPDH was used as protein loading control. (E) FOXO-1 promoter-driven luciferase reporter was tranfected into UMUC3(EGFP) and UMUC3(STAT1 Y701F) cells, and the stable transfectants were then treated with 10 μM of ISO for the indicated times. Luciferase activity was evaluated by the Dual-Luciferase Reporter Assay System. (F) UMUC3 cells were stably transfected with FOXO-1 promoter-driven luciferase or the STAT1 binding site mutant, and the stable transfectants were treated with 10 μM of ISO for 18 hours. Dual-Luciferase Reporter Assay System was performed to determine the Luciferase activity. Results are the means ± SD of triplicates. Symbol “*” indicates a significant difference between UMUC3(EGFP-vector) and UMUC3(GFP-STAT1 Y701F) in the same time point upon ISO treatment (P <0.05). Symbol “#”indicates a significant difference between vehicle control and ISO–treated group (P< 0.05).
FOXO1 mediated ISO inhibition of BC invasion through down-regulation of MMP-2 expression
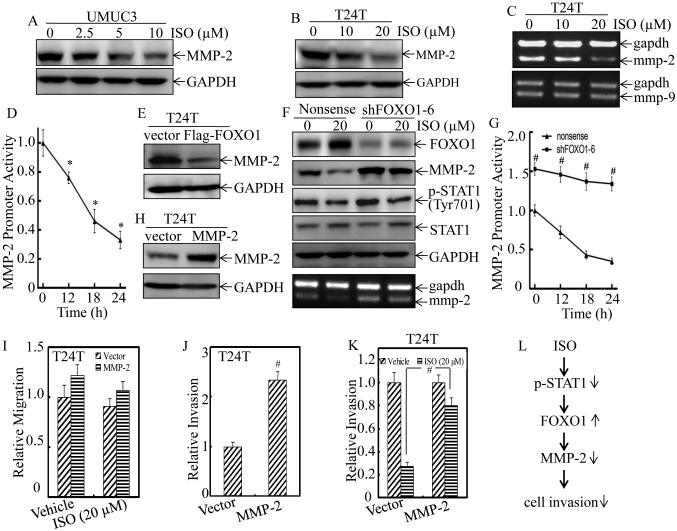
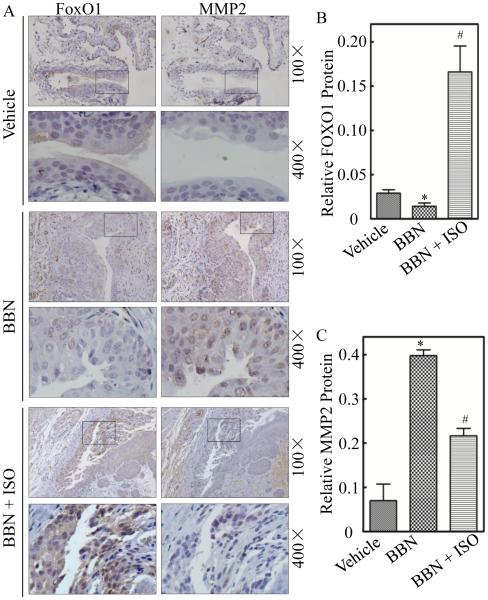
Extensive studies have identified matrix metalloproteinases (MMPs) as the key players in cancer cell invasion by degrading cellular matrix components and the basement membrane (32). Elevated MMP-2 levels have been reported to be of independent prognostic value in patients with BCs (33), while the other essential MMP member, such as MMP9 expression, is not correlated with tumor grade, stage, or overall survival of the patients with BC (34). Our previous study has also confirmed that up-regulation of MMP-2, but not MMP9, participated in mediation of BC invasion (22). To determine whether MMP-2 is associated with ISO inhibition of human BC invasion, we evaluated the effect of ISO on MMP-2 protein expression. The results showed that ISO treatment profoundly inhibited MMP-2 protein expression in both UMUC3 and T24T cells (Figure 7A and 7B). Moreover, MMP-2 mRNA level and its promoter-driven luciferase reporter activity was also attenuated by ISO treatment (Figure 7C and 7D), suggesting that the down-regulation of MMP-2 expression by ISO occurred at transcriptional level. Meanwhile, the mRNA level of MMP-9 was not affected upon ISO treatment (Figure 7C). To test the potential relation of FOXO1 induction to MMP2 inhibition following ISO treatment in BC cells, we next evaluated the effects of FOXO1 overexpression and knockdown on MMP-2 expression in T24T cells. As shown in Figure 7E, enforced expression of FOXO1 impaired MMP-2 expression, while knockdown of FOXO1 up-regulated the basal levels of MMP-2 protein and mRNA, and effectively reversed ISO down-regulation of MMP-2 expression (Figure 7F). Moreover, the inhibition of STAT1 phosphorylation at Tyr701 by ISO was comparable between T24T(Nonsense) and T24T(shFOXO1) transfectants. Accordingly, the basal level of MMP-2 promoter activity in T24T(shFOXO1) transfectant was also significantly increased in comparison to T24T(Nonsense) transfectant, and ISO inhibition of MMP-2 promoter activity was impaired in T24T(shFOXO1) transfectant (Figure 7G). It was important to note that ectopic expression of MMP-2 (Figure 7H) in T24T cells did not affect cell migration (Figure 7I, Supplemental Figure 3), but specifically enhanced the basal level of cell invasion (Figure 7J, Supplemental Figure 3), while it effectively attenuated ISO inhibition of T24T cell invasion (Figure 7K, Supplemental Figure 3). These results revealed that MMP2 acted as FOXO1 downstream being responsible for ISO inhibition of BC invasion and this novel finding was greatly supported by our results obtained from BBN-induced mouse invasive BCs in vivo showing that ISO treatment down-regulated FOXO1 and up-regulated MMP-2 expression in mouse bladder tissues (Figure 8, A, B and C). Taken together, our results demonstrate that FOXO1 mediates ISO inhibition of MMP-2 transcription and protein expression, by which inhibits BC invasion in vitro and invasive BC development in vivo as diagramed in Figure 7L.
Figure 7. ISO inhibited BC cell invasion through down-regulation of MMP-2 in a FOXO1-denpendent manner.
UMUC3 (A) and T24T (B) cells were treated with either vehicle or ISO as the indicated concentrations for 18 hours. The protein level of MMP-2 was determined by Western blotting, and GAPDH was used as protein loading control. (C) T24T cells were treated with either vehicle or the indicated concentrations of ISO for 18 hours. The mmp-2 and mmp-9 mRNA levels were evaluated by RT-PCR, and gapdh mRNA was used as the internal loading control. (D) T24T cells were stably transfected with MMP-2 promoter-driven luciferase reporter and the stable transfectants were treated with 20 μM of ISO for the indicated times. Dual-Luciferase Reporter Assay System was used to detect the Luciferase activity. Results are the means ± SD of triplicates. Symbol “*” indicates a significant difference between vehicle control and ISO-treated groups (P < 0.05). (E) The expression of FOXO1 in T24T(Vector) and T24T(Flag-FOXO1) stable transfectants were detected by Western blotting. (F) T24T(shFOXO1-6) and T24T(Nonsense) cells were treated with vehicle or 20 μM of ISO for 18 hours. Cell lysates were subjected to Western blotting with the specific antibodies indicated. (G) T24T(shFOXO1-6) and T24T(Nonsense) cells were tranfected with MMP-2 promoter-driven luciferase reporter, and the stable transfectants were then treated with 20 μM of ISO for the indicated times, and cells were then subjected to determine luciferase activity using Dual-Luciferase Reporter Assay. Results are the means ± SD of triplicates. Symbol “#” indicates a significant difference between T24T(shFOXO1-6) and T24T(Nonsense) cells in the same time point upon ISO treatment (P < 0.05). (H) MMP-2 was stably transfected into T24T cells and the stable transfectant was identified by verification of ectopic expression of MMP-2 protein by Western blotting. The MMP-2 stable transfectant and its vector control transfectant were subjected to cell migration (I) and invasion (J & K) assay in presence of either vehicle or 20 μM of ISO for 24 hours. Results are the means ± SD of triplicates. Symbol “#” indicates a significant difference between Vector transfectant and MMP-2 stable transfectant (P<0.05). (L) The proposed schematic for the cascade underlying ISO inhibition of human BC cell invasion through down-regulation of MMP-2 in FOXO1-dependent manner.
Figure 8. ISO reversed the BBN-induced down-regulation of FOXO1 and up-regulation of MMP-2 in mice.
Mice were divided into vehicle-treated control group (n=12), BBN-treated group (n=12), and BBN combined with ISO-treated group (n=12). (A) IHC-P staining by antibodies specific against FOXO1 and MMP-2 were performed. FOXO1 (B) and MMP-2 (C) protein expression levels were analyzed by calculating the integrated optical density per stained area (IOD/area) using Image-Pro Plus version 6.0. Results are the means ± SD of 12 mice in each group. Symbol “*” indicates a significant difference between vehicle control group and BBN-treated group (P < 0.05). Symbol “#” indicates a significant difference between BBN-treated group and BBN combined with ISO-treated group (P < 0.05).
Discussion
Muscular invasion of BC causes 100% death and represents a major therapeutic challenge of this disease. Therefore, an endeavor to identify new anti-cancer compounds with strong inhibition of cancer invasion and understand the mechanisms underlying their inhibitory effect on BC invasion is the key steps to discover the unmet future medicines against the invasive malignant disease. ISO is a new derivative isolated from a Chinese herb Gnetum Cleistostachyum, which has been used to treat BC for many years (3). Our recent in vivo pharmacokinetic study shows that the concentration of ISO in serum can reach to 47.9 µmol/L without any observed toxicity to experimental mice (4). Here, our results obtained from in vivo animal studies show that ISO treatment inhibits BBN-induced mouse invasive BC formation, for first time demonstrating the chemopreventive effects of ISO to the best of our knowledge. We also find that ISO at in vivo relevant concentrations of 10 to 20 µmol/L specifically represses BC invasion by promoting FOXO1 transcription through inhibition of STAT1 phosphorylation at Tyr701. Our study also identify that MMP-2 is a downstream target of FOXO1 for its mediation of ISO inhibition of BC invasion. Our results clearly demonstrate that the natural compound ISO specifically inhibits human BC invasion through targeting STAT1-FOXO1-MMP2 axis, which is completely distinct from its induction of cancer cell apoptosis and inhibition of cancer cell anchorage-independent growth. Prevention and therapeutic intervention by phytochemicals represents a newer dimension in cancer management. Silymarin, a mixture of flavonoids isolated mainly from milk thistle, has been extensively studied in pre-clinical models as well as clinically for its efficacy against variety of cancers including BC, and it has been shown to effectively inhibit cell proliferation, angiogenesis, epithelial mesenchymal transition (EMT), metastasis, as well as promote cell apoptosis (35, 36). Whether ISO exhibits other anti-cancer effects apart from targeting cell growth, apoptosis, and invasion, are deserved our near further investigation.
Our recent studies have revealed that ISO effectively attenuates transcription factor specific protein 1 (Sp1) expression and transactivation (4, 5), which results in the decreased bindings of Sp1 to promoter region of its regulated genes, cyclin D1 and XIAP, in turn leading to the down-regulation of these gene expression, and consequently resulting in suppression of cancer cell anchorage-independent growth and induction of apoptosis in BC cells (4, 5). Although our group has reported above mechanism underlying anticancer effects of ISO compound, evaluation whether ISO is a new agent with strong activity for inhibition of cancer invasion and understand the potential mechanism involved in its inhibition of BC invasion is tremendous importance for ISO potentially therapeutic clinical application. In the present study, we demonstrate that ISO at relevant applicable concentrations show a great inhibition of BC invasion in vitro and mouse invasive BC development in vivo. Accordingly, treatment of ISO significantly promotes FOXO1 expression, whereas suppression of FOXO1 expression completely abolishes ISO inhibition of BC invasion in both UMUC3 and T24T cells. Thus, ISO is an effective therapeutic agent for inhibiting BC invasion, and up-regulation of FOXO1 plays a critical role in this biological effect. It is highlighted in the previous reports that activated epidermal growth factor receptor (EGFR) signaling serves as a potential therapeutic target for muscle-invasive BCs (37, 38). Given that FOXO1 can restore chemosensitivity to anti-EGFR–based therapies both in vitro and in vivo (39), the combination of ISO with anti-EGFR–based therapy may result in improved treatment response through up-regulation of FOXO1 expression. FOXO1 has been found to inhibit invasion of others cancers, such as prostate cancer and glioblastoma (12, 13), indicating treatment of ISO may result in similar therapeutic effects in these cancers. Collectively, our present study provides valuable information for the design of more effective strategies for utilizing ISO or for the synthesis of other novel conformation-constrained derivatives to treat BCs and other cancers.
FOXO1, which primarily functions as transcription factor, regulates a large spectrum of cancer-related gene expression, thus plays important biological roles as tumor suppressor in regulating cell cycle arrest, apoptosis, DNA damage repair, and/or oxidative stress resistance (6, 7). Nevertheless, the FOXO1 signaling in regulation of cell cancer cell invasion is differential, depending on the cancer types and cellular context. Forced expression of FOXO1 has been reported to suppress Runx2-promoted prostate cancer cell migration and invasion (12). Inhibition of FOXO1 nuclear exclusion prevents the metastatic factor MMP-9 activation and cell invasion induced by EGF in Glioblastoma (13). However, FOXO1 has been shown to directly regulate transcription of MMP1 and is thought to promote breast cancer cell metastasis (40). Moreover, FOXO1 is required for keratinocyte migration and wound healing, which involves up-regulation of transforming growth factor β1 (TGF-β1) and its downstream targets (41). To explore the role of FOXO1 in BC invasion, the expression of FOXO1 is examined in human BC tissues as well as the adjacent normal bladder tissues surgically removed 98 patients diagnosed with BCs. We find that FOXO1 was profoundly down-regulated in human BC tissues, and a significant negative relationship was found between foxo1 mRNA expression and BC invasion, which is consistently supported by the previous study that FOXO1 expression is reduced in the progressed BC tissues as compared with non-progressed patients (14). Moreover, we demonstrate that ectopic expression of FOXO1 suppresses cancer cell invasion in both UMUC3 and T24T human invasive BC cells. Furthermore, knockdown of FOXO1 further enhances BC invasion in absence of ISO treatment, whereas it impairs ISO inhibition of BC invasion. Thus, our results demonstrate that down-regulation of FOXO1 expression contributes to BC invasion, and that ISO-induced FOXO1 expression plays a crucial role in its inhibition of BC invasion.
FOXO1 itself is subject to multiple levels of regulation via various signaling pathways, while regulation of its post-translational modifications and subcellular localization has been intensively investigated (7). Phosphorylation of FOXO1 by protein kinases such as Akt and cyclin-dependent kinase 2 (CDK2) results in nuclear export, reduced DNA-binding ability and degradation of this transcription factor (42, 43). Although hyper-activation of the phosphoinositol-3-kinase (PI3K)-Akt cascade is a common feature in many cancers including BC (44), FOXO1 was still mainly located in the nuclear of BC cells as demonstrated in the present study, suggesting post-translational modification by AKT probably is not major player in the ISO regulation of FOXO1 expression in BC cells. This notion is greatly supported by the results showing that the exogenous FOXO1 protein is not affected by ISO treatment. Meanwhile, the expressions of endogenous protein and mRNA, as well as promoter activity of FOXO1 are significantly induced upon ISO treatment, demonstrating that the increased nuclear-located FOXO1 were regulated at mRNA transcriptional level.
Signal transducer and activator of transcription 1 (STAT1) is a cytoplasmic protein that is activated via phosphorylation at Tyr701 by interferons (IFNs) and other cell signals in lymphocytes (45). Following activation, STAT1 is translocated to bind specific regulatory sequences to activate or repress transcription of its targeted genes (45). As IFN-induced STAT1 signaling is primarily implicated in mediating immune antiviral and/or antipathogen functions, suppression of cell proliferation, and the induction of apoptosis in lymphocytes, which is the concept of STAT1 as a tumor-suppressive function (45, 46). Nevertheless, the tumor suppressor recognition of STAT1 has been shattered by emerging evidence that constitutively activated STAT1 signaling is involved in the resistance to DNA-damaging therapeutic agents of epithelial cancers (45-47). In line with these observations, muscle-invasive BC tissues were reported to be characterized by constitutively nuclear expression of phosphorylated STAT1 (48). STAT1 has been thought to exhibit a negative regulatory effect on FOXO1 transcription in pancreatic β-cells (49), however, this notion has never been explored in any cancer cells. In addition, nothing is known about the mechanism underlying STAT1 regulation of FOXO1 transcription. In the present study, we demonstrate that ISO treatment increases FOXO1 transcription accompanied with repression of STAT1 phsphorylation at Tyr701 in BC cells. The inhibition of STAT1 activity by overexpression of dominant negative STAT1 could mimic the biological effects of ISO treatment, while the mutation of STAT1 binding site in FOXO1 promoter region reversed the increased FOXO1 transcription activity induced by ISO treatment. These results reveals a novel function of phsphorylated STAT1 at Tyr701 for its direct binding to FOXO1 promoter resulting in suppression of FOXO1 transcription, which is also the mechanisms for ISO up-regulation of FOXO1 transcription and expression, and in turn inhibiting BC invasion in vitro and mouse invasive BC formation in vivo, and providing new information for using ISO as a new agent targeting STAT1 and FOXO1 for the BC therapy.
FOXO proteins could not only act as transcription factors in the nucleus through sequence-specific interaction with DNA binding sites, but can also cooperate with or titrate away specific transcription factors or cofactors, and subsequently activate or repress the transcription of the downstream genes that lack consensus binding sequences for FOXO factors (50). FOXO1 has been found to physically interact with transcription factor Runx2 and down-regulate the transcription of Runx2-targeted endogenous genes, such as the osteopontin (OP), vascular endothelial growth factor (VEGF), and MMP-13, thereby inhibiting prostate cancer cell migration and invasion (12). The present study shows that transcription of MMP-2, a key player in many cancer cell invasion, is down-regulated upon ISO treatment. Moreover, knockdown of FOXO1 up-regulates the basal expression level of MMP-2, and effectively reverses ISO inhibition of MMP-2 expression and BC invasion as well, suggesting that FOXO1 plays a negative role in regulation of MMP-2 transcription. Accordingly, the inhibition of BBN-induced mice invasive BC formation by ISO is accompanied by up-regulation of FOXO1 and down-regulation of MMP-2 expression. As MMP-2 promoter lacks consensus binding site for FOXO1, we anticipate that FOXO1 probably represses MMP-2 transcription indirectly through interacting with other specific transcription factors. Further elucidating of this hypothesis is currently undergoing in our laboratory.
In conclusion, we demonstrate that FOXO1 acts as a tumor suppressor by inhibiting BC invasion, and the novel derivative anti-cancer drug ISO specifically represses BBN-induced invasive BC formation in vivo and human BC invasion in vitro through up-regulation of FOXO1 expression at transcription level. Moreover, the STAT1 binding site in FOXO1 promoter is critical for its suppression of FOXO1 transcription, and ISO-induced FOXO1 expression is mediated through dephosphorylated of STAT1 at Tyr701. In addition, down-regulation of MMP-2 mRNA transcription by FOXO1 is responsible for ISO inhibition of BC invasion. These findings not only provide a novel insight into understanding of underlying mechanism of BC’s propensity to invasion, but also identify natural compound ISO acting as a new agent for inhibition of invasive BC formation, which could be used for preventive strategies or to prevent recurrence and progress after Transurethral Resection of Bladder Tumor (TURBT) of non-muscle invasive tumors.
Supplementary Material
Acknowledgments
We thank Dr. Jean-Baptiste Demoulin from Catholic University of Louvain for generous gift of human FOXO1 promoter luciferase reporter; Dr. Jian Cao from State University of New York at Stony Brook for the gift of human MMP-2 expression construct; and Dr. Etty N. Benveniste from The University of Alabama at Birmingham for providing human MMP-2 promoter luciferase reporter.
Funding: This work was partially supported by grants from NIH/NCI CA177665 (C. Huang), CA165980 (C. Huang), CA112557 (C. Huang), NIH/NIEHS ES000260 (C. Huang), Natural Science Foundation of China (NSFC81229002) (C. Huang), and Key Project of Science and Technology Innovation Team of Zhejiang Province (2013TD10) (C. Huang), as well as Wenzhou Science and Technology Bureau (Y20150008) (H. Huang).
Footnotes
Disclosure of Potential Conflict of Interest: The authors disclose no potential conflicts of interest
References
- 1.Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA Cancer J Clin. 2014;64:9–29. doi: 10.3322/caac.21208. [DOI] [PubMed] [Google Scholar]
- 2.Kaufman DS, Shipley WU, Feldman AS. Bladder cancer. Lancet. 2009;374:239–49. doi: 10.1016/S0140-6736(09)60491-8. [DOI] [PubMed] [Google Scholar]
- 3.Huang KS, Wang YH, Li RL, Lin M. Stilbene dimers from the lianas of Gnetum hainanense. Phytochemistry. 2000;54:875–81. doi: 10.1016/s0031-9422(00)00151-5. [DOI] [PubMed] [Google Scholar]
- 4.Fang Y, Cao Z, Hou Q, Ma C, Yao C, Li J, et al. Cyclin d1 downregulation contributes to anticancer effect of isorhapontigenin on human bladder cancer cells. Mol Cancer Ther. 2013;12:1492–503. doi: 10.1158/1535-7163.MCT-12-0922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fang Y, Yu Y, Hou Q, Zheng X, Zhang M, Zhang D, et al. The Chinese herb isolate isorhapontigenin induces apoptosis in human cancer cells by down-regulating overexpression of antiapoptotic protein XIAP. J Biol Chem. 2012;287:35234–43. doi: 10.1074/jbc.M112.389494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Huang H, Tindall DJ. Dynamic FoxO transcription factors. J Cell Sci. 2007;120:2479–87. doi: 10.1242/jcs.001222. [DOI] [PubMed] [Google Scholar]
- 7.Fu Z, Tindall DJ. FOXOs, cancer and regulation of apoptosis. Oncogene. 2008;27:2312–9. doi: 10.1038/onc.2008.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tran H, Brunet A, Grenier JM, Datta SR, Fornace AJ, Jr., DiStefano PS, et al. DNA repair pathway stimulated by the forkhead transcription factor FOXO3a through the Gadd45 protein. Science. 2002;296:530–4. doi: 10.1126/science.1068712. [DOI] [PubMed] [Google Scholar]
- 9.Brunet A, Bonni A, Zigmond MJ, Lin MZ, Juo P, Hu LS, et al. Akt promotes cell survival by phosphorylating and inhibiting a Forkhead transcription factor. Cell. 1999;96:857–68. doi: 10.1016/s0092-8674(00)80595-4. [DOI] [PubMed] [Google Scholar]
- 10.Stahl M, Dijkers PF, Kops GJ, Lens SM, Coffer PJ, Burgering BM, et al. The forkhead transcription factor FoxO regulates transcription of p27Kip1 and Bim in response to IL-2. J Immunol. 2002;168:5024–31. doi: 10.4049/jimmunol.168.10.5024. [DOI] [PubMed] [Google Scholar]
- 11.Gilley J, Coffer PJ, Ham J. FOXO transcription factors directly activate bim gene expression and promote apoptosis in sympathetic neurons. J Cell Biol. 2003;162:613–22. doi: 10.1083/jcb.200303026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang H, Pan Y, Zheng L, Choe C, Lindgren B, Jensen ED, et al. FOXO1 inhibits Runx2 transcriptional activity and prostate cancer cell migration and invasion. Cancer Res. 2011;71:3257–67. doi: 10.1158/0008-5472.CAN-10-2603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen J, Huang Q, Wang F. Inhibition of FoxO1 nuclear exclusion prevents metastasis of Glioblastoma. Tumour Biol. 2014 doi: 10.1007/s13277-014-1913-1. [DOI] [PubMed] [Google Scholar]
- 14.Kim TH, Jo SW, Lee YS, Kim YJ, Lee SC, Kim WJ, et al. Forkhead box O-class 1 and forkhead box G1 as prognostic markers for bladder cancer. J Korean Med Sci. 2009;24:468–73. doi: 10.3346/jkms.2009.24.3.468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lu H, Liu P, Pan Y, Huang H. Inhibition of cyclin-dependent kinase phosphorylation of FOXO1 and prostate cancer cell growth by a peptide derived from FOXO1. Neoplasia. 2011;13:854–63. doi: 10.1593/neo.11594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Essaghir A, Dif N, Marbehant CY, Coffer PJ, Demoulin JB. The transcription of FOXO genes is stimulated by FOXO3 and repressed by growth factors. J Biol Chem. 2009;284:10334–42. doi: 10.1074/jbc.M808848200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cao J, Rehemtulla A, Pavlaki M, Kozarekar P, Chiarelli C. Furin directly cleaves proMMP-2 in the trans-Golgi network resulting in a nonfunctioning proteinase. J Biol Chem. 2005;280:10974–80. doi: 10.1074/jbc.M412370200. [DOI] [PubMed] [Google Scholar]
- 18.Qin H, Sun Y, Benveniste EN. The transcription factors Sp1, Sp3, and AP-2 are required for constitutive matrix metalloproteinase-2 gene expression in astroglioma cells. J Biol Chem. 1999;274:29130–7. doi: 10.1074/jbc.274.41.29130. [DOI] [PubMed] [Google Scholar]
- 19.Zhang R, Wang Y, Li J, Jin H, Song S, Huang C. The Chinese herb isolate yuanhuacine (YHL-14) induces G2/M arrest in human cancer cells by up-regulating p21 protein expression through an p53 protein-independent cascade. J Biol Chem. 2014;289:6394–403. doi: 10.1074/jbc.M113.513960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gildea JJ, Golden WL, Harding MA, Theodorescu D. Genetic and phenotypic changes associated with the acquisition of tumorigenicity in human bladder cancer. Genes Chromosomes Cancer. 2000;27:252–63. doi: 10.1002/(sici)1098-2264(200003)27:3<252::aid-gcc5>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 21.Titus B, Frierson HF, Jr., Conaway M, Ching K, Guise T, Chirgwin J, et al. Endothelin axis is a target of the lung metastasis suppressor gene RhoGDI2. Cancer Res. 2005;65:7320–7. doi: 10.1158/0008-5472.CAN-05-1403. [DOI] [PubMed] [Google Scholar]
- 22.Jin H, Yu Y, Hu Y, Lu C, Li J, Gu J, et al. Divergent behaviors and underlying mechanisms of cell migration and invasion in non-metastatic T24 and its metastatic derivative T24T bladder cancer cell lines. Oncotarget. 2015;6:522–36. doi: 10.18632/oncotarget.2680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Song L, Li J, Zhang D, Liu ZG, Ye J, Zhan Q, et al. IKKbeta programs to turn on the GADD45alpha-MKK4-JNK apoptotic cascade specifically via p50 NF-kappaB in arsenite response. J Cell Biol. 2006;175:607–17. doi: 10.1083/jcb.200602149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Song L, Li J, Ye J, Yu G, Ding J, Zhang D, et al. p85alpha acts as a novel signal transducer for mediation of cellular apoptotic response to UV radiation. Mol Cell Biol. 2007;27:2713–31. doi: 10.1128/MCB.00657-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang D, Wang Y, Liang Y, Zhang M, Wei J, Zheng X, et al. Loss of p27 upregulates MnSOD in a STAT3-dependent manner, disrupts intracellular redox activity and enhances cell migration. J Cell Sci. 2014;127:2920–33. doi: 10.1242/jcs.148130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu J, Zhang D, Luo W, Yu Y, Yu J, Li J, et al. X-linked inhibitor of apoptosis protein (XIAP) mediates cancer cell motility via Rho GDP dissociation inhibitor (RhoGDI)-dependent regulation of the cytoskeleton. J Biol Chem. 2011;286:15630–40. doi: 10.1074/jbc.M110.176982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yu J, Zhang D, Liu J, Li J, Yu Y, Wu XR, et al. RhoGDI SUMOylation at Lys-138 increases its binding activity to Rho GTPase and its inhibiting cancer cell motility. J Biol Chem. 2012;287:13752–60. doi: 10.1074/jbc.M111.337469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ouyang W, Ma Q, Li J, Zhang D, Liu ZG, Rustgi AK, et al. Cyclin D1 induction through IkappaB kinase beta/nuclear factor-kappaB pathway is responsible for arsenite-induced increased cell cycle G1-S phase transition in human keratinocytes. Cancer Res. 2005;65:9287–93. doi: 10.1158/0008-5472.CAN-05-0469. [DOI] [PubMed] [Google Scholar]
- 29.Zhang D, Li J, Costa M, Gao J, Huang C. JNK1 mediates degradation HIF-1alpha by a VHL-independent mechanism that involves the chaperones Hsp90/Hsp70. Cancer Res. 2010;70:813–23. doi: 10.1158/0008-5472.CAN-09-0448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Miyamoto H, Yang Z, Chen YT, Ishiguro H, Uemura H, Kubota Y, et al. Promotion of bladder cancer development and progression by androgen receptor signals. J Natl Cancer Inst. 2007;99:558–68. doi: 10.1093/jnci/djk113. [DOI] [PubMed] [Google Scholar]
- 31.Baus D, Nonnenmacher F, Jankowski S, Doring C, Brautigam C, Frank M, et al. STAT6 and STAT1 are essential antagonistic regulators of cell survival in classical Hodgkin lymphoma cell line. Leukemia. 2009;23:1885–93. doi: 10.1038/leu.2009.103. [DOI] [PubMed] [Google Scholar]
- 32.Gialeli C, Theocharis AD, Karamanos NK. Roles of matrix metalloproteinases in cancer progression and their pharmacological targeting. FEBS J. 2011;278:16–27. doi: 10.1111/j.1742-4658.2010.07919.x. [DOI] [PubMed] [Google Scholar]
- 33.Kanayama H, Yokota K, Kurokawa Y, Murakami Y, Nishitani M, Kagawa S. Prognostic values of matrix metalloproteinase-2 and tissue inhibitor of metalloproteinase-2 expression in bladder cancer. Cancer. 1998;82:1359–66. [PubMed] [Google Scholar]
- 34.Kumar B, Koul S, Petersen J, Khandrika L, Hwa JS, Meacham RB, et al. p38 mitogen-activated protein kinase-driven MAPKAPK2 regulates invasion of bladder cancer by modulation of MMP-2 and MMP-9 activity. Cancer Res. 2010;70:832–41. doi: 10.1158/0008-5472.CAN-09-2918. [DOI] [PubMed] [Google Scholar]
- 35.Kavitha CV, Deep G, Gangar SC, Jain AK, Agarwal C, Agarwal R. Silibinin inhibits prostate cancer cells- and RANKL-induced osteoclastogenesis by targeting NFATc1, NF-kappaB, and AP-1 activation in RAW264.7 cells. Mol Carcinog. 2014;53:169–80. doi: 10.1002/mc.21959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tyagi A, Raina K, Singh RP, Gu M, Agarwal C, Harrison G, et al. Chemopreventive effects of silymarin and silibinin on N-butyl-N-(4-hydroxybutyl) nitrosamine induced urinary bladder carcinogenesis in male ICR mice. Mol Cancer Ther. 2007;6:3248–55. doi: 10.1158/1535-7163.MCT-07-2006. [DOI] [PubMed] [Google Scholar]
- 37.Yang X, Kessler E, Su LJ, Thorburn A, Frankel AE, Li Y, et al. Diphtheria toxin-epidermal growth factor fusion protein DAB389EGF for the treatment of bladder cancer. Clin Cancer Res. 2013;19:148–57. doi: 10.1158/1078-0432.CCR-12-1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rebouissou S, Bernard-Pierrot I, de Reynies A, Lepage ML, Krucker C, Chapeaublanc E, et al. EGFR as a potential therapeutic target for a subset of muscle-invasive bladder cancers presenting a basal-like phenotype. Sci Transl Med. 2014;6:244ra91. doi: 10.1126/scitranslmed.3008970. [DOI] [PubMed] [Google Scholar]
- 39.Sangodkar J, Dhawan NS, Melville H, Singh VJ, Yuan E, Rana H, et al. Targeting the FOXO1/KLF6 axis regulates EGFR signaling and treatment response. J Clin Invest. 2012;122:2637–51. doi: 10.1172/JCI62058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Feng X, Wu Z, Wu Y, Hankey W, Prior TW, Li L, et al. Cdc25A regulates matrix metalloprotease 1 through Foxo1 and mediates metastasis of breast cancer cells. Mol Cell Biol. 2011;31:3457–71. doi: 10.1128/MCB.05523-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ponugoti B, Xu F, Zhang C, Tian C, Pacios S, Graves DT. FOXO1 promotes wound healing through the up-regulation of TGF-beta1 and prevention of oxidative stress. J Cell Biol. 2013;203:327–43. doi: 10.1083/jcb.201305074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Huang H, Regan KM, Lou Z, Chen J, Tindall DJ. CDK2-dependent phosphorylation of FOXO1 as an apoptotic response to DNA damage. Science. 2006;314:294–7. doi: 10.1126/science.1130512. [DOI] [PubMed] [Google Scholar]
- 43.Biggs WH, 3rd, Meisenhelder J, Hunter T, Cavenee WK, Arden KC. Protein kinase B/Akt-mediated phosphorylation promotes nuclear exclusion of the winged helix transcription factor FKHR1. Proc Natl Acad Sci U S A. 1999;96:7421–6. doi: 10.1073/pnas.96.13.7421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Knowles MA, Platt FM, Ross RL, Hurst CD. Phosphatidylinositol 3-kinase (PI3K) pathway activation in bladder cancer. Cancer Metastasis Rev. 2009;28:305–16. doi: 10.1007/s10555-009-9198-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Khodarev NN, Roizman B, Weichselbaum RR. Molecular pathways: interferon/stat1 pathway: role in the tumor resistance to genotoxic stress and aggressive growth. Clin Cancer Res. 2012;18:3015–21. doi: 10.1158/1078-0432.CCR-11-3225. [DOI] [PubMed] [Google Scholar]
- 46.Wong GS, Lee JS, Park YY, Klein-Szanto AJ, Waldron TJ, Cukierman E, et al. Periostin cooperates with mutant p53 to mediate invasion through the induction of STAT1 signaling in the esophageal tumor microenvironment. Oncogenesis. 2013;2:e59. doi: 10.1038/oncsis.2013.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Greenwood C, Metodieva G, Al-Janabi K, Lausen B, Alldridge L, Leng L, et al. Stat1 and CD74 overexpression is co-dependent and linked to increased invasion and lymph node metastasis in triple-negative breast cancer. J Proteomics. 2012;75:3031–40. doi: 10.1016/j.jprot.2011.11.033. [DOI] [PubMed] [Google Scholar]
- 48.Sun Y, Cheng MK, Griffiths TR, Mellon JK, Kai B, Kriajevska M, et al. Inhibition of STAT signalling in bladder cancer by diindolylmethane: relevance to cell adhesion, migration and proliferation. Curr Cancer Drug Targets. 2013;13:57–68. [PubMed] [Google Scholar]
- 49.Fiorentino L, Cavalera M, Menini S, Marchetti V, Mavilio M, Fabrizi M, et al. Loss of TIMP3 underlies diabetic nephropathy via FoxO1/STAT1 interplay. EMBO Mol Med. 2013;5:441–55. doi: 10.1002/emmm.201201475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Barthel A, Schmoll D, Unterman TG. FoxO proteins in insulin action and metabolism. Trends Endocrinol Metab. 2005;16:183–9. doi: 10.1016/j.tem.2005.03.010. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.