Abstract
Background
Despite a large body of literature evaluating the association between recreational physical activity and epithelial ovarian cancer (EOC) risk, the extant evidence is inconclusive and little is known about the independent association between recreational physical inactivity and EOC risk. We conducted a pooled analysis of nine studies from the Ovarian Cancer Association Consortium (OCAC) to investigate the association between chronic recreational physical inactivity and EOC risk.
Methods
In accordance with the 2008 Physical Activity Guidelines for Americans, women reporting no regular, weekly recreational physical activity were classified as inactive. Multivariable logistic regression was utilized to estimate the odds ratios (OR) and 95% confidence intervals (CI) for the association between inactivity and EOC risk overall and by subgroups based upon histotype, menopausal status, race and body mass index (BMI).
Results
The current analysis included data from 8,309 EOC patients and 12,612 controls. We observed a significant positive association between inactivity and EOC risk (OR=1.34, 95% CI: 1.14-1.57) and similar associations were observed for each histotype.
Conclusions
In this large pooled analysis examining the association between recreational physical inactivity and EOC risk, we observed consistent evidence of an association between chronic inactivity and all EOC histotypes.
Impact
These data add to the growing body of evidence suggesting that inactivity is an independent risk factor for cancer. If the apparent association between inactivity and EOC risk is substantiated, additional work via targeted interventions should be pursued to characterize the dose of activity required to mitigate the risk of this highly fatal disease.
Keywords: ovarian cancer risk, physical inactivity, physical activity, exercise, recreation
INTRODUCTION
It is well established that recreational physical activity is associated with decreased risks of developing breast, colon and endometrial cancers (1, 2) but the association between physical activity and epithelial ovarian cancer (EOC) remains less clear (3, 4). Despite the publication of dozens of individual epidemiological studies, two organizational systematic reviews have concluded that insufficient and inconsistent evidence was available in the current literature to support an association between recreational physical activity and ovarian cancer risk (4, 5).
Inconsistent epidemiological reports of the association between physical activity and EOC risk may be the result of limitations in the physical activity and ovarian cancer literature, or, may be due to a complex dose-response relationship that has not yet been fully elucidated. For example, individual studies of ovarian cancer often have relatively small numbers of case subjects, especially for the less common histotypes, which could limit statistical power to detect significant associations. In fact, the largest individual study to date included 1580 cases, but only 44 patients were diagnosed with mucinous tumors (6). A lack of consistency in the literature could also reflect that only modest decreases in risk are associated with higher levels of activity (7) and that the greatest risk is associated with inactivity (8), a construct which has been scarcely investigated as an independent exposure relative to EOC risk. To our knowledge, all but two EOC studies (9, 10) have examined recreational physical activity using arbitrary cut points of incrementally higher levels of activity, with the low-or no-activity group identified as the reference group. While parameterizing incrementally higher levels of activity exposure is important for detecting dose-response relationships, this approach has been associated with complex and meaningful exposure misclassification (11-13) and has precluded the establishment of a clear public health recommendation specific to ovarian cancer (4, 5). Importantly, this common methodology overlooks recreational physical inactivity as an independent public health exposure of interest (5).
To this end, since the publication of the 2008 Physical Activity Guidelines for Americans (PAGAs), adults have been encouraged to avoid physical inactivity, which is characterized by a lack of regular, weekly, moderate-or vigorous-intensity recreational activity (5). According to the most current data, 25% of Americans (14) and between 10.3% and 51.5% of adult women worldwide (8) are physically inactive. Given the persistence of inactivity at the population level and the hypothesis that the greatest protective benefits can be achieved by increasing activity levels at the low end of the activity continuum (8), inactive individuals could be a particularly important group to study in relationship to disease risk because of the ability for most individuals to increase the amount of activity they perform each week (8). In fact, current estimates suggest that congenital factors contributing to inactivity affect less than one percent of the population globally, implying that most individuals are capable of increasing activity levels (8).
Not only is studying physical inactivity important from a public health perspective (5, 15), it is likely assessed with less exposure misclassification (8, 16) and may also reflect physiological pathways that exert an effect on carcinogenesis separately from pathways associated with physical activity and skeletal muscle contraction (15, 17). Thus, we conducted a pooled analysis of nine population-based case-control studies from OCAC to investigate a novel, well-defined research question. Specifically, we sought to determine if self-reported, chronic, recreational physical inactivity is associated with an increased risk of EOC. We evaluated the association between physical inactivity exposure and EOC risk overall, and according to subgroups based upon EOC histotype, menopause status, race, and body mass index (BMI).
MATERIALS AND METHODS
OCAC Study Population
We obtained individual-level data from nine population-based OCAC case–control studies that had available self-report data on recreational physical inactivity throughout adulthood. Seven of the nine OCAC studies were based in the United States (18-24) and one each were based in Australia (25) and Denmark (26). Additional characteristics of the nine case–control studies included in the present analyses are summarized in Table 1.
Table 1.
Characteristics of the Ovarian Cancer Association Consortium Case-Control Studies Included in the Analyses
| OCAC Study Namea | Study Design |
Case & Control Ascertainment | Year of Diagnosis |
Participation Rates |
Total Nb |
|---|---|---|---|---|---|
| Australian Ovarian Cancer Study/Australian Cancer Study (AUS)(25) |
Population- based |
Cases identified via surgical treatment centers & cancer registries; Controls electoral roll |
2002-2006 | 65% CA 47% CO |
1289 CA 1468 CO 2757 Total |
| Connecticut Ovary Study (CON)(22) |
Population- based |
Cases identified via cancer registries and pathology departments; controls RDD |
1998-2003 | 69% CA 61% CO |
489 CA 545 CO 1034 Total |
| Diseases of the Ovary and their Evaluation (DOV) and (DVE)(23, 71) |
Population- based |
Cases identified via SEER registry; controls RDD |
2002-2009 | 74.2% CA 61.5% CO |
1276 CA 1848 CO 3124 Total |
| Hawaii Ovarian Cancer Case- Control Study (HAW)(19) |
Population- based |
Cases identified via cancer registries; controls selected via Dept. of Health annual survey |
1993-2008 | 78% CA 80% CO |
739 CA 1103 CO 1842 Total |
| Novel Risk Factors & Potential Early Detection Markers for Ovarian Cancer (HOP)(72) |
Population- based |
Cases identified via cancer registries, physician offices, pathology databases; controls RDD |
2003-2014 | 71% CA 68% CO |
604 CA 1800 CO 2404 Total |
| MALignant OVArian cancer (MAL)(26) |
Population- based |
Cases identified via cancer registry and gynecologic departments; controls from population register |
1994-1999 | 81% CA 68% CO |
681 CA 1552 CO 2233 Total |
| New England Case Control Study (NEC)(24) |
Population- based |
Cases identified via hospital tumor boards & cancer registries; controls via RDD & townbook selection |
1992-2008 | 70% CA 72% CO |
1065 CA 1243 CO 2308 Total |
| New Jersey Ovarian Cancer Study (NJO)(18) |
Population- based |
Cases identified via New Jersey State Cancer Registry; controls via RDD if <65 years of age and random selection from insurance lists for women >65 years; also utilized area sampling for women > 55 years of age. |
2004-2008 | 47% CA 40% CO |
195 CA 458 CO 653 Total |
| Los Angeles County Case- Control Studies of Ovarian Cancer-1 & 2 (USC)(73) |
Population- based |
Cases identified through Los Angeles County Cancer Surveillance Program (part of SEER) by rapid case ascertainment. |
1993- current |
80% CA 70% CO |
1971 CA 2595 CO 4566 Total |
Study sites are listed in alphabetical order by OCAC study abbreviation
Total participant numbers reflect the OCAC core data set as of July 2014, including only those who had physical activity data available (N=8,309 cases; N=12,612 controls)
All individual studies obtained institutional review board or research ethics committee approvals and participants for each OCAC study provided written informed consent for all study activities. Approvals for the present analyses were obtained from the OCAC Data Access Coordinating Committee and from individual study site coordinators if additional approvals were required. Data were obtained from 8,309 patients aged 18 years or older with histologically confirmed primary borderline or invasive epithelial ovarian cancer, fallopian tube cancer, or peritoneal cancer. Patients were excluded from the current analyses if they had been diagnosed with non-epithelial ovarian cancers (sarcomas, germ-cell tumors, sex-cord stromal tumors, etc.); if tumor histology was mixed or undifferentiated; or if tumor behavior or histology was missing or unknown. Controls included 12,612 women aged 18 years and older with at least partially intact ovaries and no prior histories of ovarian cancer.
Analysis Variables
Epidemiological Data
The primary OCAC epidemiological dataset includes information that was collected through self-administered or interviewer-administered questionnaires. Available demographic, lifestyle and clinical variables include age, race, ethnicity, education, tumor behavior, tumor histology, oral contraceptive use, family history of breast and ovarian cancer, age at menarche, number of full-term pregnancies, breastfeeding, age at menopause, hormone therapy use (estrogen-alone or combination therapy), hysterectomy, and several additional epidemiological variables from previously published OCAC pooled analyses [i.e., tubal ligation, smoking history, alcohol use, genital powder use, nonsteroidal anti-inflammatory drug (NSAID) use, recent BMI (1-5 years prior to diagnosis), and personal history of endometriosis] (27-33).
Recreational Physical Inactivity
Recreational physical activity data were directly acquired from each of the nine OCAC studies included in the current analysis. All nine questionnaires assessed recreational activity spanning adulthood up through the reference age, defined as the age of diagnosis among cases and the age of study entry among controls. Specifically, eight of nine questionnaires encompassed the time period spanning all decades of adulthood (i.e., age 20 through the reference date), while one study (DVE) spanned the time period from age 25 through the reference date.
The specific parameters of physical activity were inconsistently measured across studies, precluding the ability to harmonize and parameterize physical activity data in terms of frequency, intensity or duration per session. However, in the current analysis, the exposure of interest was recreational physical inactivity, and all nine questionnaires allowed for the identification of women who self-reported engaging in no regular, weekly moderate-to-vigorous-intensity recreational activity. Questionnaires from most studies (DVE, HAW, HOP, NEC, NJO, USC) utilized a global, dichotomous item assessing ever-participation in regular, weekly, recreational physical activities. For these studies, women answering ‘no’ to the global question were classified as ‘inactive.’ Three studies (AUS, CON, MAL) assessed recreational physical (in)activity based upon pre-specified time periods spanning adulthood (i.e., by decades or a combination of decades ranging from age 20-29 through the reference age). Likewise, women reporting no regular, weekly moderate-or vigorous-intensity recreational activity in all time periods prior to the reference date were classified as inactive. Further, given that the most relevant (in)activity exposure period may be many years before the actual diagnosis of cancer (7), we conducted analyses designed to examine an exposure window encompassing at least two decades of adulthood prior to study entry. Thus, participants with reference dates in their twenties were excluded in sensitivity analyses, yielding a chronic inactivity exposure spanning a minimum of two decades.
Statistical Methods
Identification of Confounding Variables
Based upon the definition of potential confounding (34) and their establishment as factors associated with risk of ovarian cancer, the following variables in the OCAC core data set were pre-specified as important for adjustment when estimating EOC risks: age at reference date, race (White, Black, Asian, other), use of oral contraceptives (ever, never), parity (nulliparous, 1, 2, 3, or ≥ 4 full-term births), family history of breast or ovarian cancer in a first degree relative (yes, no, don’t know), and a personal history of endometriosis (yes, no). In pooled analyses utilizing a combined dataset, we further adjusted all models for study site. We also utilized the ten percent change-in-estimate approach (35) to inform the selection of additional adjustment variables. Based upon this approach, we determined that ethnicity (Hispanic, non-Hispanic), use of hormone therapy (yes or no for estrogen-only or combination estrogen/progesterone), smoking (never, current, former), alcohol use (never, ever, former), education (less than high school, high school, some college, college graduate, graduate school), talc or genital powder use (no use, genital use, non-genital use), NSAIDs use (≥ once per week, <once per week), tubal ligation prior to diagnosis (yes, no), breast-feeding (yes, no, not applicable), hysterectomy prior to diagnosis (yes, no), menopause status (pre or peri, post, don’t know), and recent BMI (underweight, normal weight, overweight, or obese based upon BMI 1-5 years prior to diagnosis) were not relevant confounders. However, because obesity has an established association with physical activity and it is also associated with increased risk of some EOC histotypes, we evaluated models with and without adjustment for BMI, as obesity may be in the causal pathway of interest.
Recreational Physical Inactivity and EOC Risk
To account for between-study heterogeneity, we utilized a meta-analytic approach to examine the association between inactivity and EOC risk overall, and according to EOC endpoints defined by tumor behavior and histology. For each of the nine studies, logistic regression analyses were conducted to estimate study-specific odds ratios (ORs) and 95% confidence intervals (CIs) for the association between chronic physical inactivity and EOC risk. Study-specific ORs and their variances were then combined via meta-analyses to estimate summary ORs and 95% CIs for all EOC endpoints. Meta-analytic analyses were conducted under random-effects or fixed-effects assumptions, depending on measures of between-study heterogeneity, which was assessed and quantified utilizing the Cochran Q-statistic and the I-squared statistic (36). When evidence of significant heterogeneity was observed between studies (Q-statistic p-value <0.05 or I2 value > 50%), we reported a random-effects OR based upon the DerSimonian and Laird method (37) and we conducted further analyses to identify and account for source(s) of heterogeneity. However, when no significant heterogeneity was observed between studies, we reported a fixed-effects OR.
To enable well-powered subgroup analyses, we pooled individual-level data from the nine studies into a combined dataset to examine the association between inactivity and EOC risk by menopause status, race, and BMI classification. We examined associations between inactivity and EOC risk by subgroups of standard categories of BMI (i.e., underweight, normal-weight, overweight, and obese) and by a dichotomous BMI classification (i.e., underweight and normal weight vs. overweight and obese). In pooled analyses, all multivariable models were adjusted by study site and we accounted for the possibility of between-study heterogeneity by testing the significance of a cross product term for site*inactivity in all analyses.
Lastly, we conducted sensitivity analyses designed to address any potential heterogeneity in the observed associations between inactivity and EOC risk that could have resulted from differences in the physical activity questionnaires among OCAC studies. Furthermore, to account for potential cultural or geographical differences in activity patterns, we excluded two studies that were not conducted in the U.S. (AUS & MAL).
RESULTS
The characteristics of the nine OCAC case-control studies included in the analyses are summarized in Table 1. The self-reported prevalence of recreational physical inactivity among the study population is presented in Table 2. Collectively, 23.7% of cases and 20.9% of controls reported a history of inactivity (p<0.001). Inactivity rates varied by study site (10% in HAW and 48.5% in MAL), yielding a combined inactivity prevalence of 22.0% among the total study population. These estimates are very similar to national (25%) and global (10.3 – 51.5%) inactivity estimates (8, 14, 38), thus enhancing the confidence in our characterization of the inactivity exposure.
Table 2.
Prevalence of Inactivity Among the Study Population including Nine Participating Ovarian Cancer Association Consortium Studies
| Study sitea | Prevalence of inactivity in the combined study population |
Prevalence of inactivity in cases (n/%) |
Prevalence of inactivity in controls (n/%) |
Chi-square p -valueb |
|---|---|---|---|---|
| MAL | 1082/2233 (48.5%) | 348/681 (51.1%) | 734/1552 (48.4%) | 0.097 |
| CON | 358/1034 (34.6%) | 189/489 (38.7%) | 169/545 (31.0%) | 0.010 |
| NEC | 695/2308 (30.1%) | 366/1065 (34.4%) | 329/1243 (26.5%) | <0.001 |
| NJO | 106/653 (16.2%) | 56/195 (28.7%) | 50/458 (10.9%) | <0.001 |
| DOV | 654/3124 (20.9%) | 279/1276 (21.9%) | 375/1848 20.30%) | 0.288 |
| HOP | 469/2404 (19.5%) | 119/604 (19.7%) | 350/1800 (19.4%) | 0.890 |
| AUS | 512/2757 (18.6%) | 253/1289 (19.6%) | 259/1468 (17.6%) | 0.005 |
| USC | 543/4566 (11.9%) | 269/1971 (13.6%) | 274/2595 (10.6%) | 0.001 |
| HAW | 184/1842 (10%) | 94/739 (12.7%) | 90/1103 (8.2%) | 0.001 |
Study sites are listed in descending order by prevalence of physical inactivity among cases.
p-value represents differences in the distribution of inactivity between cases vs. controls.
In meta-analyses, we observed a significant positive association between self-reported, chronic physical inactivity and all EOC endpoints (Table 3). Specifically, among inactive women, we observed a 34% increased risk of EOC overall (OR= 1.34, 95% CI: 1.14-1.57) (Figure 1a); a 35% increased risk of invasive tumors (OR =1.35, 95% CI 1.14-1.60) (Figure 1b); and a 27% increased risk of borderline tumors (OR =1.27, 95% CI 1.10-1.46) (Figure 1c). Among the five invasive histotypes, we observed increased risks ranging between 29-54% among inactive women (Figures 2a-e). Physical inactivity was also associated with a 27% and 31% increased risk of borderline serous and borderline mucinous tumors, respectively (Figures 3a and b). Importantly, adding BMI to multivariable models did not change these estimates appreciably (Table 3).
Table 3.
Weighted Odds Ratios and 95% Confidence Intervals Representing the Association between Physical Inactivity and Epithelial Ovarian Cancer among Case-Control Studies included in the Meta-analysesa
| Epithelial Ovarian Cancer (EOC) Histological Type |
Modelb | Summary ORc |
95% CI | Heterogeneity | |||
|---|---|---|---|---|---|---|---|
| Lower | Upper | Q-statistic | P-value | I2 | |||
|
Overall EOC
(all histological types combined) |
Multivariable | 1.34 (RE) | 1.14 | 1.57 | 33.77 | <0.001 | 76.31% |
| Add BMI | 1.32 (RE) | 1.12 | 1.56 | 34.43 | <0.001 | 76.76% | |
|
Invasive EOC
(all invasive cases combined) |
Multivariable | 1.35 (RE) | 1.14 | 1.60 | 34.23 | <0.001 | 76.63% |
| Add BMI | 1.33 (RE) | 1.12 | 1.58 | 33.13 | <0.001 | 75.85% | |
| Invasive High-grade Serous | Multivariable | 1.30 (RE) | 1.10 | 1.54 | 23.40 | 0.003 | 65.82% |
| Add BMI | 1.30 (RE) | 1.08 | 1.53 | 22.60 | 0.004 | 64.60% | |
| Invasive Low-grade Serous | Multivariable | 1.39 (FE) | 1.06 | 1.83 | 15.02 | 0.059 | 46.72% |
| Add BMI | 1.33 (FE) | 1.01 | 1.76 | 13.90 | 0.084 | 42.44% | |
| Invasive Endometrioid | Multivariable | 1.29 (RE) | 1.01 | 1.65 | 17.79 | 0.023 | 55.03% |
| Add BMI | 1.26 (RE) | 0.98 | 1.63 | 18.29 | 0.019 | 56.23 | |
| Invasive Mucinous | Multivariable | 1.54 (FE) | 1.22 | 1.95 | 5.40 | 0.715 | 0.00% |
| Add BMI | 1.50 (FE) | 1.17 | 1.10 | 5.78 | 0.672 | 0.00% | |
| Invasive Clear Cell | Multivariable | 1.45 (FE) | 1.16 | 1.80 | 15.46 | 0.051 | 48.24% |
| Add BMI | 1.40 (FE) | 1.11 | 1.74 | 13.59 | 0.093 | 41.13% | |
|
Borderline EOC
(serous & mucinous combined) |
Multivariable | 1.27 (FE) | 1.10 | 1.46 | 7.84 | 0.250 | 23.50% |
| Add BMI | 1.20 (FE) | 1.04 | 1.39 | 7.64 | 0.265 | 21.51% | |
| Borderline Serous | Multivariable | 1.27 (FE) | 1.07 | 1.52 | 6.59 | 0.360 | 8.95% |
| Add BMI | 1.20 (FE) | 1.01 | 1.44 | 5.59 | 0.470 | 0.00% | |
| Borderline Mucinous | Multivariable | 1.31 (FE) | 1.06 | 1.61 | 9.69 | 0.138 | 38.08% |
| Add BMI | 1.25 (FE) | 1.01 | 1.55 | 9.88 | 0.130 | 39.30% | |
For each EOC endpoint, studies with >10 cases are included in meta-analyses
Study specific multivariable models are adjusted by age, race, parity, oral contraceptive use, a personal history of endometriosis, and a family history of breast or ovarian cancer
Random-effects (RE) ORs reported when Q-statistic is significant (p<0.05) or when I2 is greater than 50%; Fixed-effects (FE) models reported when no significant heterogeneity was observed.
Figure 1.
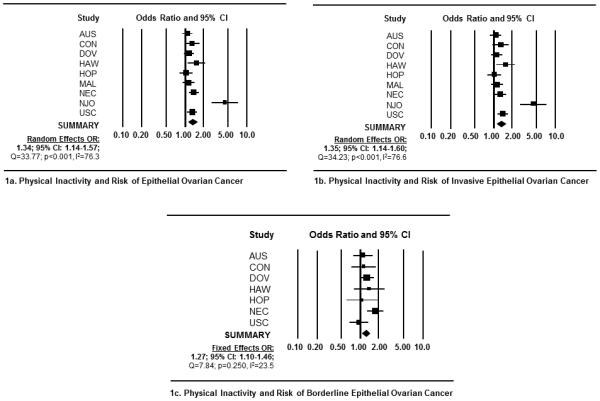
Forest plots depicting study-specific and summary ORs and 95% CIs representing the association between recreational physical inactivity and risk of epithelial ovarian cancer (EOC). Forest plots include measures of association for (1a) all EOC histological types combined; (1b) all invasive tumors combined; and (1c) all borderline tumors combined. Study specific ORs and 95% CI were estimated utilizing logistic regression models adjusted for age, race, parity, oral contraceptive use, history of breast or ovarian cancer in first-degree relative, and a personal history of endometriosis. Summary ORs were generated via random-effects models if significant heterogeneity was detected (i.e., Q-statistic p-value <0.05 or I-squared >50%). Each square represents study-specific ORs and the lines represent the width of the 95% CIs. The size of the square is proportionate with the size of each study. The weighted, summary OR and 95% confidence interval is represented by the black diamond in each figure.
Figure 2.
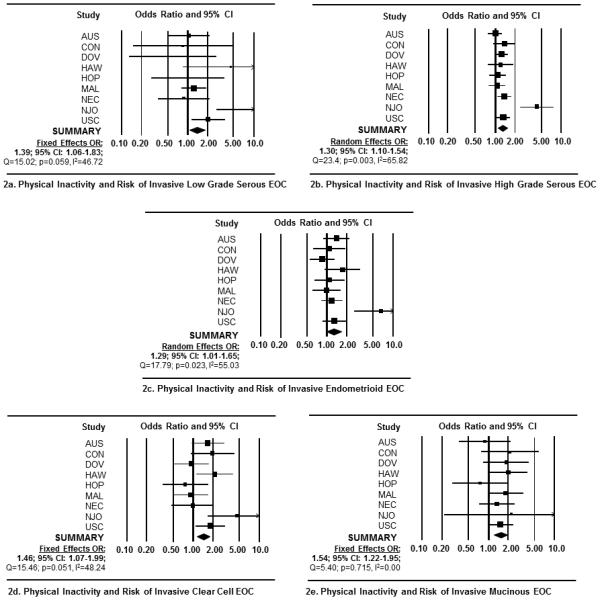
Forest plots depicting the study-specific and summary ORs and 95% CIs representing the association between recreational physical inactivity and risk of invasive epithelial ovarian cancer. Forest plots represent measures of association for (2a) invasive low-grade serous tumors; (2b) invasive high-grade serous tumors; (2c) invasive endometrioid tumors; (2d) invasive clear cell tumors; and (2e) invasive mucinous tumors. Study specific ORs and 95% CI were estimated using logistic regression models adjusted by age, race, parity, oral contraceptive use, family history of breast or ovarian cancer, and personal history of endometriosis. Summary ORs were calculated via fixed-effects models when no significant heterogeneity was detected between studies (i.e., Q-statistic p-value >0.05) and random-effects models were reported if significant heterogeneity was detected (i.e., Q-statistic p-value <0.05). Each square represents the ORs and the lines represent the width of the 95% CIs. The size of the square is proportionate with the size of each study. The weighted, summary OR and 95% confidence interval is represented by the black diamond in each figure.
Figure 3.
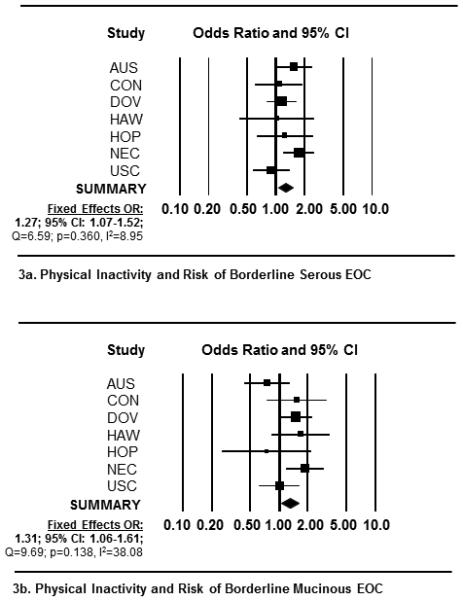
Forest plots depicting the study-specific and weighted summary ORs and 95% CIs representing the association between recreational physical inactivity and risk of borderline epithelial ovarian cancer by OCAC study site. Forest plots include measures of association for (3a) borderline serous tumors and by (3b) borderline mucinous tumors. Study specific ORs and 95% CI were estimated using logistic regression models adjusted by age, race, parity, oral contraceptive use, history of breast or ovarian cancer in first-degree relative and a personal history of endometriosis. Fixed-effects weighted ORs are reported because no significant heterogeneity was detected for either borderline histological type. In the figures above, each square represents the study-specific ORs and the lines represent the width of the 95% CIs. The size of the square is proportionate with the size of each study. The weighted, summary OR and 95% confidence interval is represented by the black diamond in each figure.
Significant between-study heterogeneity was noted for EOC overall, invasive tumors, high-grade serous tumors, and endometrioid tumors (Table 3). The observed heterogeneity was explained by one study (NJO) and we accounted for between-study heterogeneity by presenting random-effects summary ORs. Upon excluding NJO in sensitivity analyses, associations between inactivity and EOC risk for EOC overall, invasive tumors, high-grade serous tumors and endometrioid tumors were of comparable magnitude and remained statistically significant. For example, the OR and 95% CI for EOC overall was (OR=1.25, 95% CI: 1.16-1.35), p-for-heterogeneity=0.467.
After excluding NJO, we further compared the associations between inactivity and EOC risk based upon the questionnaire format utilized among the OCAC studies included in the analysis. We observed no significant heterogeneity in weighted point estimates among those studies utilizing one global item to assess (in)activity throughout adulthood (OR=1.27, 95% CI:1.16-1.39) vs. the studies utilizing multiple pre-specified time periods throughout adulthood (OR=1.20, 95% CI:1.04-1.40), yielding an overall fixed-effects OR=1.25, (95% CI: 1.16-1.35), p-for-heterogeneity=0.523. Importantly, even when adding NJO back into the analyses, there was no significant heterogeneity observed between the two formats incorporated herein (OR=1.27, 95% CI: 1.16-1.39), p-for-heterogeneity=0.154. Lastly in additional sensitivity analyses excluding two studies conducted outside the U.S. (AUS, MAL), the observed associations between inactivity and EOC risk were strengthened (OR=1.43, 95% CI: 1.17-1.76) and the association remained significant after excluding NJO (OR=1.27, 95% CI: 1.16-1.39).
In subgroup analyses, we observed no convincing evidence of effect modification by menopause status (p-for-interaction=0.483) (Supplemental Table S1); race (p-for-interaction=0.337) (Supplemental Table S2); or by standard BMI categories (p-for-interaction=0.082) (Supplemental Table S3). Lastly, when the association between inactivity and EOC risk was examined utilizing a dichotomous BMI variable (BMI <25 or BMI ≥ 25), we observed a significant, positive association between inactivity and EOC risk among underweight/normal-weight women and overweight/obese women: (OR=1.33, 95% CI: 1.19-1.49) and (OR=1.21, 95% CI: 1.09-1.34), respectively (p-for-interaction=0.041) (Supplemental Table S4).
DISCUSSION
In the current analysis, we observed consistent evidence of a statistically significant positive association between self-reported, chronic recreational physical inactivity and all histotypes of EOC. While published data describing the association between recreational physical inactivity and EOC risk are scant, two recent prospective studies reported the association between EOC risk and the most inactive group of women, in comparison to a reference group of women engaging in moderate amounts of activity (9, 10). Leitzmann et al. reported an increased risk of early-stage and fatal EOC among the most inactive women (RR=1.29, 95% CI: 0.55-3.04 and RR=1.20, 95% CI 0.71-2.03, respectively) and Huang et al. reported a 29% increased risk of EOC among women who were the least active during pre-menopausal years (HR=1.29, 95% CI: 0.95-7.75). Although these estimates did not reach statistical significance, they are of similar magnitude to each other and to the point estimates reported for overall EOC in our primary and sensitivity analyses.
Our analyses did not account for explicitly sedentary behaviors such as sitting, television watching, or computer use, yet there is mounting epidemiological evidence demonstrating a positive association between sedentary behaviors and EOC risk (39-43). Although sedentary behavior is a separate behavioral construct independent of recreational physical (in)activity, these collective findings add to the growing body of evidence that behavior patterns indicative of a sedentary lifestyle, either by way of prolonged sitting or a lack of recreational physical activity, appear to be associated with increased EOC risk. Further, it is plausible that the underlying biological mechanisms responsible for an apparent association of sedentary behavior and recreational physical inactivity with EOC risk are likely similar.
While research has established that the incidence of certain types of tumors are reduced by regular physical activity, the biological mechanisms relating physical (in)activity and cancer risk are not entirely understood (44). The most commonly cited mediators of these associations include changes in body fat, changes in circulating reproductive hormone levels, alterations in inflammatory cytokine and growth factor milieus and alterations in immune function (44). Inasmuch as the current leading hypotheses about the etiology of EOC each share inflammation as an underlying mechanism (45), it is possible that inactivity increases EOC risk by way of increased systemic inflammation, decreased immune function, and increased circulating levels of sex hormones. For example, both acute and chronic physical activity produce an anti-inflammatory effect via reduced levels of inflammatory markers such as C-reactive protein and tumor necrosis factor (46). Additionally, both mechanistic and epidemiological evidence suggests a role for dysregulated adiponectin (an anti-inflammatory adipokine) and leptin (a pro-inflammatory adipokine) in epithelial ovarian carcinogenesis (47-52). There is also evidence suggesting a dysregulated adipokine milieu promotes an immunosuppressive environment via myeloid derived suppressor cell (MDSC) induction and FoxP3+ T-regulatory cell recruitment (53), an area that has been under intense investigation in relationship to ovarian cancer etiology and prognosis (54-57). Lastly, there are endocrine-related hypotheses that support the plausibility of an inverse association between activity and EOC risk by way of decreased levels of circulating estrogens and androgens, and in fact, most studies seem to suggest that exercise decreases the availability of biologically active estrogens and androgens (58).
Emerging evidence also supports the hypotheses that physical inactivity and sedentary behaviors are exposures with distinct metabolic consequences which could be independent of physical activity and obesity-related mechanisms (15, 17, 59, 60). Our data also imply that the observed association between physical inactivity and EOC risk is mostly independent of BMI. Although adding BMI to our multivariable models yielded a slight attenuation in the observed ORs, an independent, consistent and significant effect of inactivity remained.
Furthermore, while there appears to be a borderline statistically significant interaction between BMI classification (i.e., underweight, normal-weight, overweight, obese) and inactivity in relation to EOC risk, the apparent stronger association among underweight women, and weaker association among obese women were accompanied by CIs that included the null. This suggests there may be imprecision in estimating EOC risk at the extreme distribution of BMI and inactivity when they are considered jointly. Lastly, when the association between inactivity and EOC risk was examined in two subgroups of BMI (i.e., <25 or ≥25), we observed a statistically significant BMI*inactivity interaction for EOC overall (p=0.041). Although the interaction p-value for EOC overall suggests this association may not be due to chance alone, we believe the associations between inactivity and EOC risk are qualitatively comparable across BMI stratum.
A key strength of our study is that our analyses were conducted with individual-level data from well-designed population-based epidemiological investigations, yielding the first substantial analysis of the association between recreational physical inactivity and EOC risk. Furthermore, our ability to adjust for well-established risk and protective factors associated with EOC risk decreased the likelihood that the observed associations were the result of confounding. Additionally, the observed associations between inactivity and EOC risk remained significant and of similar magnitude in all sensitivity analyses designed to reduce potential sources of bias. Lastly, our use of chronic inactivity as the exposure of interest reduced the likelihood of reverse causation bias as a potential explanation for the observed associations reported herein.
The potential measurement error associated with self-report physical activity data categorized as a dichotomous variable is an important limitation to the current analysis. Although the dichotomous nature of the exposure variable assumes a homogenous group of activity and does not allow for an examination of the dose-response association with physical activity exposure, there are important advantages to our approach. First, while misreporting of inactivity does occur, we assume the misclassification across incremental categories of activity would be greater (8, 11, 12, 16) and more influential with respect to biasing observed associations of interest. In fact, previous research has demonstrated that the greatest concordance between direct and self-report measures of activity is found at the lowest ends of the activity continuum, with more measurement error surrounding measures of mid-to-upper levels of exposure (8, 11, 12). Utilizing a dichotomous variable avoids a potentially misleading level of accuracy in categorizing incremental levels of self-reported physical activity (8). Second, there is a body of evidence demonstrating that the use of one global question is a validated method for identifying inactive individuals (13, 61-66). Third, although it is impossible to know whether (in)activity misclassification was differential by case-control status, one tactic is to compare our findings with those from cohort studies where self-reported (in)activity wouldn’t be subjected to recall bias (7). Among the two prospective studies providing risk estimates for inactivity (9, 10), associations were similar to those reported herein, arguing against a bias in case-control studies due to differential misclassification. Importantly, non-differential misclassification with a dichotomous exposure variable would likely result in an underestimate of the true association between inactivity and EOC risk(67).
We also recognize that our findings may be limited by the potential for a higher prevalence of healthier women to have volunteered as controls. If so, this would have inflated our observed estimates of association between inactivity and EOC risk. Likewise, associations between EOC risk and other lifestyle factors, such as smoking, alcohol consumption and obesity, would also show inflated risk estimates. Yet, previously published OCAC pooled analyses utilizing data from the same studies have yielded no evidence of an association between alcohol consumption and EOC risk (28) and evidence of associations between EOC risk and smoking and obesity has been restricted to specific histotypes (27, 29). It is also possible that additional unmeasured factors that may parallel physical activity (or inactivity) in lifestyle patterns could contribute to an observed association between physical inactivity and EOC risk.
Although the goal of the current study was to examine the association between recreational physical inactivity and EOC risk, it is worth noting that previous epidemiological studies of physical activity and EOC risk have yielded inconsistent findings based upon the type of observational study. While the first published meta-analysis of the association between physical activity and EOC risk reported similar risk estimates for case-control studies (OR=0.79, 95% CI: 0.70-0.85) and cohort studies (OR=0.81, 95% 0.72-0.92) (6), a more recent meta-analysis reported a significant inverse association between activity and EOC risk among case-control studies (OR=0.86, 95% CI: 0.80-0.93) but reported no association among cohort studies (OR=1.03, 95% CI:0.87-1.20) (68).
It is also important to highlight that there are competing hypotheses regarding the shape of the dose-response physical activity curve in relationship to chronic disease risk (i.e., linear vs. non-linear) (8). In fact, prospective studies have yielded data suggesting a significant positive association between vigorous physical activity and EOC risk (9, 69, 70). While there is biological plausibility for a positive association between excessive vigorous exercise and increased EOC risk by way of impaired immune function (44) and exercise-induced increases in gonadotropin and androgen secretion (70), researchers have cautioned that observations of a direct association between activity and EOC risk could be due to chance (69), small cell sizes (69) and detection bias (10, 69). Importantly, non-differential misclassification of self-reported physical activity parameterized in more than two categories can result in biased estimates away from the null (67).
In conclusion, in the first substantial analysis designed to examine chronic recreational physical inactivity as an independent exposure of interest, we observed evidence of a significant positive association between recreational inactivity and EOC risk that was consistently observed among all EOC histotypes. These data add to the growing body of literature demonstrating that physical inactivity is associated with a plethora of unfavorable health outcomes, including an increased risk for early death, heart disease, stroke, type 2 diabetes and certain cancers including breast, colon, and endometrial tumors (5, 8, 14). Additional prospective epidemiological studies are warranted to further elucidate the dose-response association between recreational physical (in)activity and EOC risk. If the apparent association between inactivity and EOC risk is substantiated, then additional work via targeted intervention studies should be pursued to characterize the dose of recreational physical activity required to mitigate the risk of this highly fatal disease.
Supplementary Material
Acknowledgments
Financial support:
AUS (G. Chenevix-Trench/P.M. Webb): U.S. Army Medical Research and Materiel Command (DA17-01-1-0729), National Health & Medical Research Council of Australia, Cancer Councils of New South Wales, Victoria, Queensland, South Australia and Tasmania, Cancer Foundation of Western Australia; National Health and Medical Research Council of Australia (199600 and 400281). The grant numbers for AOCS Cancer Council funding are as follows- Multi-State Application Numbers 191, 211 and 182;
CON (H.A. Risch): National Institutes of Health (R01-CA074850; R01-CA080742);
DOV (M.A. Rossing): National Institutes of Health R01-CA112523 and R01-CA87538;
HAW (M.T. Goodman): U.S. National Institutes of Health (R01-CA58598, N01-CN-55424 and N01-PC-67001);
HOP (F. Modugno, R.B. Ness, K.B. Moysich): DOD: DA17-02-1-0669 and NCI: K07-CA080668, R01-CA95023, P50-CA159981
MAL (S.K. Kjaer): Funding for this study was provided by research grant R01- CA61107 from the National Cancer Institute, Bethesda, ; research grant 94 222 52 from the Danish Cancer Society, Copenhagen, Denmark; and the Mermaid I project;
MAY/MAC (E.L. Goode): R01-CA122443
NEC (D.W. Cramer, K.L. Terry): National Institutes of Health R01-CA54419 and P50-CA105009 and Department of Defense W81XWH-10-1-02802;
NJO (E.V. Bandera) National Cancer Institute (NIH-K07 CA095666, R01-CA83918, NIH-K22-CA138563, and P30-CA072720) and the Cancer Institute of New Jersey; (S.H. Olson) National Cancer Institute CCSG award (P30-CA008748);
USC (C.L. Pearce): P01CA17054, P30CA14089, R01CA61132, N01PC67010, R03CA113148, R03CA115195, N01CN025403, and California Cancer Research Program (00-01389V-20170, 2II0200).
Footnotes
Conflict of Interest Statement:
D. Cramer reports a financial relationship with Beasley Allen Crow; P. Webb reports a relationship with BUPA; E. Goode reports a relationship with Johnson & Johnson; there are no additional conflicts of interest
REFERENCES
- 1.Friedenreich CM, Neilson HK, Lynch BM. State of the epidemiological evidence on physical activity and cancer prevention. Eur J Cancer. 2010;46:2593–604. doi: 10.1016/j.ejca.2010.07.028. [DOI] [PubMed] [Google Scholar]
- 2.Kruk J, Czerniak U. Physical activity and its relation to cancer risk: updating the evidence. Asian Pac J Cancer Prev. 2013;14:3993–4003. doi: 10.7314/apjcp.2013.14.7.3993. [DOI] [PubMed] [Google Scholar]
- 3.Cannioto RA, Moysich KB. Epithelial Ovarian Cancer and Recreational Physical Activity: A Review of the Epidemiological Literature and Implications for Exercise Prescription. Gynecol Oncol. 2015 doi: 10.1016/j.ygyno.2015.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.WCRF/AICR Food, Nutrition, Physical Activity, and the Prevention of Ovarianc Cancer 2014 [electronic article] Continuous Update Project Report Ovarian Cancer 2014 Summary. Advance Access: June 23, 2014. [Google Scholar]
- 5.USDHHS . 2008 Physical Activity Guidelines for Americans. Office of Disease Prevention and Health Promotion; Washington, D.C.: 2008. [Google Scholar]
- 6.Olsen CM, Bain CJ, Jordan SJ, Nagle CM, Green AC, Whiteman DC, et al. Recreational physical activity and epithelial ovarian cancer: a case-control study, systematic review, and meta-analysis. Cancer Epidemiol Biomarkers Prev. 2007;16:2321–30. doi: 10.1158/1055-9965.EPI-07-0566. [DOI] [PubMed] [Google Scholar]
- 7.Moorman PG, Jones LW, Akushevich L, Schildkraut JM. Recreational physical activity and ovarian cancer risk and survival. Ann Epidemiol. 2011;21:178–87. doi: 10.1016/j.annepidem.2010.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bull FC, Armstrong TP, Dixon T, Ham S, Neiman A, Pratt M. Physical Inactivity. In: WHO, editor. Comparitive Quantification of Health Risks: Global and Regional Burden of Disease Attributable to selected Major Risk Factors. WHO; 2004. pp. 729–881. [Google Scholar]
- 9.Huang T, Eliassen AH, Hankinson SE, Okereke OI, Kubzansky LD, Wang M, et al. A prospective study of leisure-time physical activity and risk of incident epithelial ovarian cancer: Impact by menopausal status. Int J Cancer. 2016;138:843–52. doi: 10.1002/ijc.29834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Leitzmann MF, Koebnick C, Moore SC, Danforth KN, Brinton LA, Hollenbeck AR, et al. Prospective study of physical activity and the risk of ovarian cancer. Cancer Causes Control. 2009;20:765–73. doi: 10.1007/s10552-008-9291-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Troiano R. Limitations of Self-Report in Physical Activity and Obesity Research. In: Bouchard CK, editor. Physical Activity and Obesity. 2nd. Human Kinetics; Champaign, IL: 2010. pp. 34–7. PT. [Google Scholar]
- 12.Troiano RD. Differences between objective and self-report measures of physical activity. What do they mean? J Kor Soc Meas Eval. 2008;10:31–42. KW. [Google Scholar]
- 13.Tudor-Locke CE, Myers AM. Challenges and opportunities for measuring physical activity in sedentary adults. Sports Med. 2001;31:91–100. doi: 10.2165/00007256-200131020-00002. [DOI] [PubMed] [Google Scholar]
- 14.Moore LV, Harris CD, Carlson SA, Kruger J, Fulton JE. Trends in no leisure-time physical activity--United States, 1988-2010. Res Q Exerc Sport. 2012;83:587–91. doi: 10.1080/02701367.2012.10599884. [DOI] [PubMed] [Google Scholar]
- 15.Sanchis-Gomar F, Lucia A, Yvert T, Ruiz-Casado A, Pareja-Galeano H, Santos-Lozano A, et al. Physical inactivity and low fitness deserve more attention to alter cancer risk and prognosis. Cancer Prev Res (Phila) 2015;8:105–10. doi: 10.1158/1940-6207.CAPR-14-0320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Celis-Morales CA, Perez-Bravo F, Ibanez L, Salas C, Bailey ME, Gill JM. Objective vs. self-reported physical activity and sedentary time: effects of measurement method on relationships with risk biomarkers. PLoS One. 2012;7:e36345. doi: 10.1371/journal.pone.0036345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fiuza-Luces C, Garatachea N, Berger NA, Lucia A. Exercise is the real polypill. Physiology (Bethesda) 2013;28:330–58. doi: 10.1152/physiol.00019.2013. [DOI] [PubMed] [Google Scholar]
- 18.Bandera EV, King M, Chandran U, Paddock LE, Rodriguez-Rodriguez L, Olson SH. Phytoestrogen consumption from foods and supplements and epithelial ovarian cancer risk: a population-based case control study. BMC Womens Health. 2011;11:40. doi: 10.1186/1472-6874-11-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Goodman MT, Lurie G, Thompson PJ, McDuffie KE, Carney ME. Association of two common single-nucleotide polymorphisms in the CYP19A1 locus and ovarian cancer risk. Endocr Relat Cancer. 2008;15:1055–60. doi: 10.1677/ERC-08-0104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ness RB, Dodge RC, Edwards RP, Baker JA, Moysich KB. Contraception methods, beyond oral contraceptives and tubal ligation, and risk of ovarian cancer. Ann Epidemiol. 2011;21:188–96. doi: 10.1016/j.annepidem.2010.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pike MC, Pearce CL, Peters R, Cozen W, Wan P, Wu AH. Hormonal factors and the risk of invasive ovarian cancer: a population-based case-control study. Fertil Steril. 2004;82:186–95. doi: 10.1016/j.fertnstert.2004.03.013. [DOI] [PubMed] [Google Scholar]
- 22.Risch HA, Bale AE, Beck PA, Zheng W. PGR +331 A/G and increased risk of epithelial ovarian cancer. Cancer Epidemiol Biomarkers Prev. 2006;15:1738–41. doi: 10.1158/1055-9965.EPI-06-0272. [DOI] [PubMed] [Google Scholar]
- 23.Rossing MA, Cushing-Haugen KL, Wicklund KG, Doherty JA, Weiss NS. Menopausal hormone therapy and risk of epithelial ovarian cancer. Cancer Epidemiol Biomarkers Prev. 2007;16:2548–56. doi: 10.1158/1055-9965.EPI-07-0550. [DOI] [PubMed] [Google Scholar]
- 24.Terry KL, De Vivo I, Titus-Ernstoff L, Shih MC, Cramer DW. Androgen receptor cytosine, adenine, guanine repeats, and haplotypes in relation to ovarian cancer risk. Cancer Res. 2005;65:5974–81. doi: 10.1158/0008-5472.CAN-04-3885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Merritt MA, Green AC, Nagle CM, Webb PM. Talcum powder, chronic pelvic inflammation and NSAIDs in relation to risk of epithelial ovarian cancer. Int J Cancer. 2008;122:170–6. doi: 10.1002/ijc.23017. [DOI] [PubMed] [Google Scholar]
- 26.Glud E, Kjaer SK, Thomsen BL, Hogdall C, Christensen L, Hogdall E, et al. Hormone therapy and the impact of estrogen intake on the risk of ovarian cancer. Arch Intern Med. 2004;164:2253–9. doi: 10.1001/archinte.164.20.2253. [DOI] [PubMed] [Google Scholar]
- 27.Faber MT, Kjaer SK, Dehlendorff C, Chang-Claude J, Andersen KK, Hogdall E, et al. Cigarette smoking and risk of ovarian cancer: a pooled analysis of 21 case-control studies. Cancer Causes Control. 2013;24:989–1004. doi: 10.1007/s10552-013-0174-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kelemen LE, Bandera EV, Terry KL, Rossing MA, Brinton LA, Doherty JA, et al. Recent alcohol consumption and risk of incident ovarian carcinoma: a pooled analysis of 5,342 cases and 10,358 controls from the Ovarian Cancer Association Consortium. BMC Cancer. 2013;13:28. doi: 10.1186/1471-2407-13-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Olsen CM, Nagle CM, Whiteman DC, Ness R, Pearce CL, Pike MC, et al. Obesity and risk of ovarian cancer subtypes: evidence from the Ovarian Cancer Association Consortium. Endocr Relat Cancer. 2013;20:251–62. doi: 10.1530/ERC-12-0395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pearce CL, Templeman C, Rossing MA, Lee A, Near AM, Webb PM, et al. Association between endometriosis and risk of histological subtypes of ovarian cancer: a pooled analysis of case-control studies. Lancet Oncol. 2012;13:385–94. doi: 10.1016/S1470-2045(11)70404-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sieh W, Salvador S, McGuire V, Weber RP, Terry KL, Rossing MA, et al. Tubal ligation and risk of ovarian cancer subtypes: a pooled analysis of case-control studies. Int J Epidemiol. 2013;42:579–89. doi: 10.1093/ije/dyt042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Terry KL, Karageorgi S, Shvetsov YB, Merritt MA, Lurie G, Thompson PJ, et al. Genital powder use and risk of ovarian cancer: a pooled analysis of 8,525 cases and 9,859 controls. Cancer Prev Res (Phila) 2013;6:811–21. doi: 10.1158/1940-6207.CAPR-13-0037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Trabert B, Ness RB, Lo-Ciganic WH, Murphy MA, Goode EL, Poole EM, et al. Aspirin, nonaspirin nonsteroidal anti-inflammatory drug, and acetaminophen use and risk of invasive epithelial ovarian cancer: a pooled analysis in the Ovarian Cancer Association Consortium. J Natl Cancer Inst. 2014;106:djt431. doi: 10.1093/jnci/djt431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Szklo M, Nieto J. Indentifying Non causal Associations: Confounding. In: Szklo MN, editor. Epidemiology: Beyond the Basics. Jones & Bartlett Learning; Sudbury, MA: 2007. pp. 151–82. J. [Google Scholar]
- 35.Maldonado G, Greenland S. Simulation study of confounder-selection strategies. Am J Epidemiol. 1993;138:923–36. doi: 10.1093/oxfordjournals.aje.a116813. [DOI] [PubMed] [Google Scholar]
- 36.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–60. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–88. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 38.Hallal PC, Andersen LB, Bull FC, Guthold R, Haskell W, Ekelund U. Global physical activity levels: surveillance progress, pitfalls, and prospects. Lancet. 2012;380:247–57. doi: 10.1016/S0140-6736(12)60646-1. [DOI] [PubMed] [Google Scholar]
- 39.Patel AV, Hildebrand JS, Campbell PT, Teras LR, Craft LL, McCullough ML, et al. Leisure-Time Spent Sitting and Site-Specific Cancer Incidence in a Large U.S. Cohort. Cancer Epidemiol Biomarkers Prev. 2015;24:1350–9. doi: 10.1158/1055-9965.EPI-15-0237. [DOI] [PubMed] [Google Scholar]
- 40.Patel AV, Rodriguez C, Pavluck AL, Thun MJ, Calle EE. Recreational physical activity and sedentary behavior in relation to ovarian cancer risk in a large cohort of US women. Am J Epidemiol. 2006;163:709–16. doi: 10.1093/aje/kwj098. [DOI] [PubMed] [Google Scholar]
- 41.Xiao Q, Yang HP, Wentzensen N, Hollenbeck A, Matthews CE. Physical activity in different periods of life, sedentary behavior, and the risk of ovarian cancer in the NIH-AARP diet and health study. Cancer Epidemiol Biomarkers Prev. 2013;22(11):2000–8. doi: 10.1158/1055-9965.EPI-13-0154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhang M, Xie X, Lee AH, Binns CW. Sedentary behaviours and epithelial ovarian cancer risk. Cancer Causes Control. 2004;15:83–9. doi: 10.1023/B:CACO.0000016633.47025.2a. [DOI] [PubMed] [Google Scholar]
- 43.Hildebrand JS, Gapstur SM, Gaudet MM, Campbell PT, Patel AV. Moderate-to-vigorous physical activity and leisure-time sitting in relation to ovarian cancer risk in a large prospective US cohort. Cancer Causes Control. 2015 doi: 10.1007/s10552-015-0656-7. [DOI] [PubMed] [Google Scholar]
- 44.Walsh NP, Gleeson M, Shephard RJ, Gleeson M, Woods JA, Bishop NC, et al. Position statement. Part one: Immune function and exercise. Exerc Immunol Rev. 2011;17:6–63. [PubMed] [Google Scholar]
- 45.Ness RB, Cottreau C. Possible role of ovarian epithelial inflammation in ovarian cancer. J Natl Cancer Inst. 1999;91:1459–67. doi: 10.1093/jnci/91.17.1459. [DOI] [PubMed] [Google Scholar]
- 46.Kasapis C, Thompson PD. The effects of physical activity on serum C-reactive protein and inflammatory markers: a systematic review. J Am Coll Cardiol. 2005;45(10):1563–9. doi: 10.1016/j.jacc.2004.12.077. [DOI] [PubMed] [Google Scholar]
- 47.Dupont J, Reverchon M, Cloix L, Froment P, Rame C. Involvement of adipokines, AMPK, PI3K and the PPAR signaling pathways in ovarian follicle development and cancer. Int J Dev Biol. 2012;56:959–67. doi: 10.1387/ijdb.120134jd. [DOI] [PubMed] [Google Scholar]
- 48.Mor G, Visintin I, Lai Y, Zhao H, Schwartz P, Rutherford T, et al. Serum protein markers for early detection of ovarian cancer. Proc Natl Acad Sci U S A. 2005;102(21):7677–82. doi: 10.1073/pnas.0502178102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Visintin I, Feng Z, Longton G, Ward DC, Alvero AB, Lai Y, et al. Diagnostic markers for early detection of ovarian cancer. Clin Cancer Res. 2008;14:1065–72. doi: 10.1158/1078-0432.CCR-07-1569. [DOI] [PubMed] [Google Scholar]
- 50.Cuello-Fredes M, Kato S, Abarzua-Catalan L, Delpiano A, Trigo C, Garcia K, et al. Leptin promotes a more aggresive behavior of ovarian cancer cells: a potential explanation for a worse prognosis in obese ovarian cancer patients: igcs-0095 ovarian cancer. Int J Gynecol Cancer. 2015;25(Suppl 1):67. doi: 10.1097/00009577-201505001-00052. [DOI] [PubMed] [Google Scholar]
- 51.Diaz ES, Karlan BY, Li AJ. Obesity-associated adipokines correlate with survival in epithelial ovarian cancer. Gynecol Oncol. 2013;129:353–7. doi: 10.1016/j.ygyno.2013.02.006. [DOI] [PubMed] [Google Scholar]
- 52.Wu MM, Chen HC, Chen CL, You SL, Cheng WF, Chen CA, et al. A prospective study of gynecological cancer risk in relation to adiposity factors: cumulative incidence and association with plasma adipokine levels. PLoS One. 2014;9:e104630. doi: 10.1371/journal.pone.0104630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Okwan-Duodu D, Umpierrez GE, Brawley OW, Diaz R. Obesity-driven inflammation and cancer risk: role of myeloid derived suppressor cells and alternately activated macrophages. Am J Cancer Res. 2013;3:21–33. [PMC free article] [PubMed] [Google Scholar]
- 54.Charbonneau B, Moysich KB, Kalli KR, Oberg AL, Vierkant RA, Fogarty ZC, et al. Large-scale evaluation of common variation in regulatory T cell-related genes and ovarian cancer outcome. Cancer Immunol Res. 2014;2:332–40. doi: 10.1158/2326-6066.CIR-13-0136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Godoy HE, Khan AN, Vethanayagam RR, Grimm MJ, Singel KL, Kolomeyevskaya N, et al. Myeloid-derived suppressor cells modulate immune responses independently of NADPH oxidase in the ovarian tumor microenvironment in mice. PLoS One. 2013;8:e69631. doi: 10.1371/journal.pone.0069631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Govindaraj C, Scalzo-Inguanti K, Madondo M, Hallo J, Flanagan K, Quinn M, et al. Impaired Th1 immunity in ovarian cancer patients is mediated by TNFR2+ Tregs within the tumor microenvironment. Clin Immunol. 2013;149:97–110. doi: 10.1016/j.clim.2013.07.003. [DOI] [PubMed] [Google Scholar]
- 57.Obermajer N, Muthuswamy R, Odunsi K, Edwards RP, Kalinski P. PGE(2)-induced CXCL12 production and CXCR4 expression controls the accumulation of human MDSCs in ovarian cancer environment. Cancer Res. 2011;71:7463–70. doi: 10.1158/0008-5472.CAN-11-2449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Nock N, Berger NA. Obesity and Cancer: Overview of Mechanisms. In: Berger N, editor. Cancer and Energy Balance, Epidemiology and Overview. Springer; 2010. pp. 129–79. [Google Scholar]
- 59.Byers T. Physical activity and gastric cancer: so what? An epidemiologist's confession. Cancer Prev Res (Phila) 2014;7:9–11. doi: 10.1158/1940-6207.CAPR-13-0400. [DOI] [PubMed] [Google Scholar]
- 60.Lynch BM. Sedentary behavior and cancer: a systematic review of the literature and proposed biological mechanisms. Cancer Epidemiol Biomarkers Prev. 2010;19:2691–709. doi: 10.1158/1055-9965.EPI-10-0815. [DOI] [PubMed] [Google Scholar]
- 61.Li S, Carlson E, Holm K. Validation of a single-item measure of usual physical activity. Percept Mot Skills. 2000;91:593–602. doi: 10.2466/pms.2000.91.2.593. [DOI] [PubMed] [Google Scholar]
- 62.Milton K, Bull FC, Bauman A. Reliability and validity testing of a single-item physical activity measure. Br J Sports Med. 2011;45:203–8. doi: 10.1136/bjsm.2009.068395. [DOI] [PubMed] [Google Scholar]
- 63.Rose S, Elley CR, Lawton BA, Dowell AC. A single question reliably identifies physically inactive women in primary care. The New Zealand Medical Journal. 2008;121:U2897. [PubMed] [Google Scholar]
- 64.Schechtman KB, Barzilai B, Rost K, Fisher EB., Jr Measuring physical activity with a single question. Am J Public Health. 1991;81:771–3. doi: 10.2105/ajph.81.6.771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Smith BJ, Marshall AL, Huang N. Screening for physical activity in family practice: evaluation of two brief assessment tools. Am J Prev Med. 2005;29:256–64. doi: 10.1016/j.amepre.2005.07.005. [DOI] [PubMed] [Google Scholar]
- 66.Weiss TW, Slater CH, Green LW, Kennedy VC, Albright DL, Wun CC. The validity of single-item, self-assessment questions as measures of adult physical activity. J Clin Epidemiol. 1990;43:1123–9. doi: 10.1016/0895-4356(90)90013-f. [DOI] [PubMed] [Google Scholar]
- 67.Rothman K, Greenland S, Lash TL. Validity in Epidemiologic Studies. In: Rothman K, Greenland S, Lash TL, editors. Modern Epidemiology. 3rd Wolters Kluwer/Lippincott Williams & Wilkins; Philadelphia, PA: 2012. pp. 128–47. [Google Scholar]
- 68.Zhong S, Chen L, Lv M, Ma T, Zhang X, Zhao J. Nonoccupational physical activity and risk of ovarian cancer: a meta-analysis. Tumour Biol. 2014 doi: 10.1007/s13277-014-2385-z. [DOI] [PubMed] [Google Scholar]
- 69.Anderson JP, Ross JA, Folsom AR. Anthropometric variables, physical activity, and incidence of ovarian cancer: The Iowa Women's Health Study. Cancer. 2004;100:1515–21. doi: 10.1002/cncr.20146. [DOI] [PubMed] [Google Scholar]
- 70.Chionh F, Baglietto L, Krishnan K, English DR, MacInnis RJ, Gertig DM, et al. Physical activity, body size and composition, and risk of ovarian cancer. Cancer Causes Control. 2010;21:2183–94. doi: 10.1007/s10552-010-9638-y. [DOI] [PubMed] [Google Scholar]
- 71.Bodelon C, Cushing-Haugen KL, Wicklund KG, Doherty JA, Rossing MA. Sun exposure and risk of epithelial ovarian cancer. Cancer Causes Control. 2012;23(12):1985–94. doi: 10.1007/s10552-012-0076-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lo-Ciganic WH, Zgibor JC, Bunker CH, Moysich KB, Edwards RP, Ness RB. Aspirin, nonaspirin nonsteroidal anti-inflammatory drugs, or acetaminophen and risk of ovarian cancer. Epidemiology. 2012;23:311–9. doi: 10.1097/EDE.0b013e3182456ad3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wu AH, Pearce CL, Tseng CC, Pike MC. African Americans and Hispanics Remain at Lower Risk of Ovarian Cancer Than Non-Hispanic Whites after Considering Nongenetic Risk Factors and Oophorectomy Rates. Cancer Epidemiol Biomarkers Prev. 2015;24:1094–100. doi: 10.1158/1055-9965.EPI-15-0023. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


