Abstract
There is a strong belief that garlic has medicinal properties and may even reduce the risk of developing certain cancers including those of the gastrointestinal tract. The chemopreventive effects of garlic may be attributed to the anti-inflammatory properties of garlic’s sulfur-containing constituents, which includes diallyl disulfide (DADS). Here we demonstrate that DADS prevented colorectal tumorigenesis in a mouse model of colitis-induced colorectal cancer. Supplementation with 85ppm of DADS (60 mg daily human equivalent dose) in the diet of FVB/N mice treated with chemical carcinogen azoxymethane (AOM) and colonic irritant dextran sodium sulfate (DSS) resulted in the reduction in tumor incidence, tumor number and tumor burden by 21.54%, 47.3%, and 66.4%, respectively. Further analysis revealed that mice fed the DADS supplemented diet resolved the initial DSS-induced inflammation faster than those on the control diet, preventing prolonged inflammation and cellular transformation. Subsequent mechanistic studies in vitro suggest that DADS chemopreventive effects are mediated through NFκB signaling. When SW480 colorectal cancer cells were treated with DADS, NFκB nuclear localization and activity was diminished. Interestingly, NFκB suppression was found to be dependent on DADS inhibition of GSK-3β, a positive regulator of NFκB. Inhibition of GSK-3β and loss of nuclear NFκB activity were also observed in vivo in AOM/DSS treated mice fed a diet supplemented with 85ppm DADS. Our results indicate that DADS can prevent tumorigenesis by suppressing inflammation, a process largely involving GSK-3β inhibition and consequential reduction in NFκB nuclear localization.
Keywords: Diallyl disulfide, colorectal cancer, inflammation, garlic, prevention
Introduction
Colorectal Cancer (CRC) is the third leading cause of cancer related mortality in the United States for both male and female (1). Inflammation has been known to play a pivotal role in the initiation and progression of various tumors including those in the colon (2–4). Individuals with intestinal inflammatory conditions such as Crohn’s disease and ulcerative colitis are at increased risk for developing CRC (4–7).
There are many bioactive food components with anti-inflammatory activity that are being investigated in relation to the management and treatment of inflammatory bowel conditions that may provide a feasible and safe way to reduce CRC risk (8, 9). The sulfur-containing constituents derived from garlic; including diallyl disulfide (DADS) are such examples that have been previously reported to exert anti-inflammatory effects (10). Diallyl disulfide has been demonstrated to alleviate inflammation in pre-clinical models of inflammatory bowel disease by inhibiting the production of pro-inflammatory cytokines, including IL-6 (11).
The nuclear factor kappa B (NFκB) is a pro-inflammatory transcription factor that is hypothesized to promote tumorigenesis by regulating the expression of genes that are involved in cell proliferation, apoptosis and metastasis (12–14). NFκB is constitutively active in patients with inflammatory bowel disease and may in part explain the increased incidence of colorectal cancer observed in these individuals (15). While NFκB has become a promising target for anticancer therapy, the complexity of NFκB regulation has made it challenging to develop agents to suppress its activation. The canonical pathway involves various steps including the degradation of the inhibitor of NFκB (IκB) followed by nuclear translocation of NFκB and activation of gene transcription (16). A variety of agents have been shown to indirectly suppress NFκB by influencing key regulatory elements of the canonical pathway, including kinase subunits of the IKK complex, which is responsible for the degradation of IκBs (17).
Glycogen synthase kinase-3 (GSK-3) is a serine threonine kinase comprising two homologous proteins, GSK-3α and GSK-3β, which are products of highly similar but different genes (18). GSK-3β is better characterized than GSK-3α and possesses both tumor suppressor and tumor promoting activity depending on the cellular context (19, 20). Classically, GSK-3β is regarded primarily as a tumor suppressor due to its role in the Wnt signaling cascade where it phosphorylates β-catenin resulting in its ubiquitin-mediated degradation; thus, preventing its nuclear translocation and subsequent transcription of proto-oncogenes (18). However, emerging evidence has shown that GSK-3β can also promote cancer by activating the NFκB signaling cascade by enhancing the transcriptional activity of NFκB in the nucleus (20). Studies have shown that inhibiting GSK-3β attenuates proliferation and induces apoptosis in colorectal cancer cells (19, 21).
Epidemiological evidence has been suggestive of the benefit of garlic intake in preventing colorectal cancer with at least one interventional study revealing modest benefits of garlic in preventing colorectal adenoma reoccurrence (22). To further investigate the anti-inflammatory activity of garlic and its ability to prevent colorectal cancer we assessed the effect of one of the sulfur containing constituents DADS in a mouse model of colitis-induced CRC. We found that dietary supplementation with DADS significantly reduced colorectal tumorigenesis in mice treated with azoxymethane (AOM) and dextran sulfate sodium (DSS). DADS exerted anti-inflammatory effects resolving DSS-induced inflammation in these mice. DADS also inhibited NFκB activation and nuclear translocation, which we were able to demonstrate, was dependent on the inhibition of GSK-3β in human colorectal cancer cells.
Material and Methods
Animal Studies
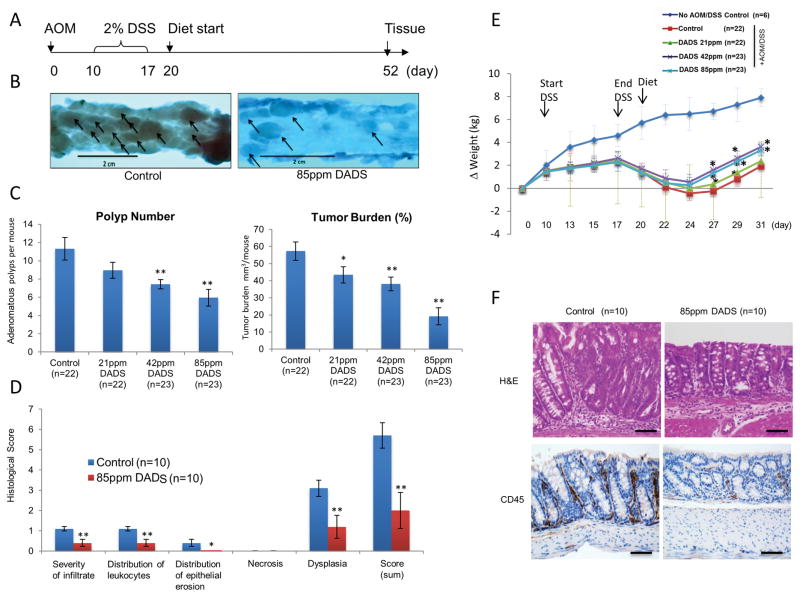
All mouse experiments were conducted according to methods previously described (23) and agreed to and regulated by the Animal Care and Use Committee of the National Cancer Institute (Frederick, MD). Briefly, 6-week old pathogen-free FVB/N mice were injected with AOM (Sigma, St. Louis, MO) intraperitoneally at a dose of 10 mg/kg body weight in 0.1 ml of saline (Day 0, Fig. 1A). Ten days later, mice were treated with 2% DSS (36,000 to 50,000 kDa, MP Biomedicals LL, Solon, OH), dissolved in normal drinking water (reverse osmosis–purified water) for 7 days and then switch back to normal drinking water (Day 17, Fig. 1A). Three days after completion of DSS treatment (Day 20, Fig. 1A) the mice were evenly sorted by change in body weight into 4 diet groups to ensure effects of the DSS were evenly represented in each of the different diets. Mice were single caged and either maintained on AIN-93G purified diet from Harlan Teklad (Madison, WI) (Day 20) or started on an AIN-93G diet supplemented with 21, 42, or 85ppm DADS (Sigma, St. Louis, MO) (Supplementary Table 1). Mice were allowed to eat ad libitum. Food consumption and body weight were measured throughout the entire study. Mice were euthanized on day 52 (Fig. 1A) and colon tissue was harvested and stained with 0.2% methylene blue in PBS. The tumors visualized by staining were counted using dissecting microscope (Fig. 1B).
Figure 1.
Diallyl disulfide (DADS) inhibited AOM/DSS-induced tumorigenesis. A, Timeline showing experimental time points. Male and female FVB/N mice were treated with a single injection AOM (10mg/kg) on day 0 followed by one cycle of 2% DSS from day 10 to 17. On day 20, mice were grouped by weight loss and randomly started on experimental diets that included AIN93G diet supplemented with 0, 21, 42, or 85ppm of DADS. Mice were euthanized on day 52. B–D, analysis of colorectal tissue collected at day 52. B, Representative macroscopic images of colons stained with 0.2% methylene blue in PBS displaying contours of adenomatous polyps as shown by arrows. Mice supplemented with 85 ppm DADS developed less adenomatous polyps compared to control. C, Adenomatous polyps per mouse were counted and presented with standard error. Tumor burden in mm3/mouse was estimated by geometric mean and expressed with standard error. *P< 0.05, **P < 0.01 indicates a significant difference compared with control group as determined by t-test. D, Histopathology showed a decrease in inflammation in DADS fed mice. Colorectal tissue collected on day 52 were stained with Hematoxylin and Eosin and semi- quantitatively scored for the degree of inflammation and colonic tissue damage produced after AOM/DSS exposure; severity of infiltrate (1=mild, 2=moderate, 3=severe), distribution of leukocytes (1=focal/locally, 2=multifocal, 3=diffuse), Distribution of epithelial erosion (1=focal/locally extensive, 2=multifocal, 3=diffuse), Severity of necrosis (1=mild, 2=moderate, 3=severe), dysplasia (1=low grade unifocal, 2=low grade multifocal, 3=high grade unifocal, 4=high grade multifocal) A score of 0 was assigned for each criterion not represented in the section. *P< 0.05, **P < 0.01 indicates a significant difference compared with control group as determined by t-test. E, Diallyl disulfide (DADS) inhibited inflammation in vivo. Cohorts of AOM/DSS mice on respective experimental diets were weighed approximately every 2–3 days, and the values are expressed as change of weight (kg) from that of the previous measurement. *P< 0.05, **P < 0.01 indicates a significant difference compared with AOM/DSS control group as determined by t-test. F, Photomicrographs (20x) of colon sections at day 52 stained with Hematoxylin and Eosin (Top row) and anti-CD-45 (1:50) immunohistochemistry (Bottom row) revealed decreased inflammation in mice on diet supplemented with 85ppm DADS. Scale bar =100μm
DADS Human Equivalent Dose and Tolerance
The daily human equivalent dose (HED) was calculated using body surface area normalization method in which the animal dose is converted to a human equivalent dose by the following formula (24).
Animal Dose= Diet concentration (mg/kg) x Animal Mass (Kg) /Food Consumption (Kg/week (mean)) / 7.
Km factor for mouse and human is 3, and 37, respectively.
The human equivalent dose of 21, 42, 85ppm dietary DADS was determined to be 15, 30, and 60 mg/kg daily, respectively and was well tolerated with no obvious toxicity.
Histopathology
Colonic tissue was processed utilizing methods previously described (23). For histological analysis colonic sections were stained with hematoxylin–eosin (H&E) and blindly evaluated by a board certified pathologist and diagnosed applying the criteria of a consensus report on murine colon tumors (25). Briefly, tumors were characterized as an adenoma or carcinoma and semi- quantitatively scored on a number of criteria including; severity of infiltrate (1=mild, 2=moderate, 3=severe), distribution of leukocytes (1=focal/locally, 2=multifocal, 3=diffuse), distribution of epithelial erosion (1=focal/locally extensive, 2=multifocal, 3=diffuse), severity of necrosis (1=mild, 2=moderate, 3=severe), dysplasia (1=low grade unifocal, 2=low grade multifocal, 3=high grade unifocal, 4=high grade multifocal). A score of 0 was assigned for each criterion not represented in the section. The summation of the 5 criteria yielded a total disease score per mouse.
Immunohistochemistry
Immunohistochemistry was performed utilizing methods previously described (23). The primary antibodies and dilutions were as follows: pGSK-3αS21βS9 (Cell Signaling Technology, Danvers, MA), 1:50; Ki-67, 1:100; pNFκB P65 S536 (Abcam, Cambridge, MA), 1:100; CD45 (BD biosciences, San Jose, CA, USA), 1: 50. All slides were digitized using Aperio Scanscope CS2 (Leica biosystems, Vista, CA) and evaluated using the Aperio analysis package, which includes highly advanced algorithms for quantifying % of nuclear staining, and staining Intensity quantification ranging from 0 (negative) to 3 (strongly positive).
Cell Culture
Human colorectal cancer SW480 cell line was obtained from American Type Culture Collection (ATCC, Rockville, MD, USA) which prior to distribution were tested and authenticated by supplier using identifiler® STR genotyping. Cells were cultured and stored according to the supplier’s instructions and used between passages 5–10. Once resuscitated, cell lines were cultured in RPMI supplemented with 10% FBS, 100U/ml penicillin and 100mg/ml streptomycin at 37ºC in humidified air with 5% CO2 never passaged over a period exceeding 2 months to ensure authenticity. Diallyl disulfide (>80% purity by high-performance liquid chromatography ~10–20% Diallyl sulfide) and LY294002 (Cell Signaling Technology, Danvers, MA) was used in cell culture experiments.
Western Blot Analysis
Approximately, 30 to 80 mg of nuclear or cytoplasmic protein that was isolated from SW480 colorectal cancer cells using NE-PER nuclear and cytoplasmic extraction kit (Thermo Scientific, Waltham, MA) was loaded and separated by SDS-PAGE, transferred to nitrocellulose membrane, and probed with the following antibodies overnight at indicated dilutions. pNFκB P65 S536, 1:1000, NFκB (P65), 1:1000, pGSK-3αS21βS9, 1:1000, pGSK3αY51βY47, 1:2000, GSK-3β, 1:1000, COX-2, 1:1000, pIKBαS32, 1:1000, IKBα, 1:1000, pIKKα/IKKβS176/180, 1:1000, IKKα/IKKβ, 1:1000, pAktS473, 1:2000, Akt, 1:2000, β-Catenin, 1:1000, pβ-CateninS33/37/T41, 1:1000 (Cell Signaling Technology, Danvers, MA); Each membrane was probed with P84 (Gene Tex, Irvine, CA) to ensure consistent loading of nuclear protein and β-actin, 1:5000 (Sigma, St. Louis, MO) to ensure consistent loading of cytoplasmic protein.
Soft Agar Assay
SW480 cells were seeded onto 100mm plates and pre-treated with either DMSO vehicle (<1%) or DADS (5μmol/L) for 24 hours. Cells were trypsinized and seeded at 30,000 cells in 2x RPMI media. Cell suspension was added 1:1 with 0.5% agarose (2ml/well in a 6 well plate) and constitutes the top layer. The bottom layer consisted of 2ml of 1.2% agarose. The cells were maintained in an incubator for 14 days and the colonies were scanned and counted with GelCount (Oxford Optronix Ltd, Oxford United Kingdom).
Cell Proliferation Assay
The MTT [3-(4,5-Dimethylthiazol-2-yl)-2,5-Diphenyltetrazolium Bromide] assay was performed according to methods previously described (26).
Statistical Analyses
All data is presented as the mean ± standard deviation. The significance of the difference between groups was evaluated by one-way analysis of variance (ANOVA) or Student’s t-test, and multiple comparisons with Prism 5.0 software. P<0.05 were considered to be statistically significant.
Results
DADS prevented tumorigenesis in azoxymethane (AOM)/ dextran sulfate sodium (DSS) treated mice
In the dinitrobenzenesulfonic acid (DNBS)-induced colitis mouse model, DADS treatment for 2 days was able to exert an anti-inflammatory effect reducing the expression of pro-inflammatory cytokines (11). To determine whether the anti-inflammatory properties of DADS translate to a decreased risk of developing colorectal cancer, we fed an equal number of FVB/N male and female AOM/DSS treated mice with a diet supplemented with 21, 42, or 85ppm DADS (human equivalent dose of 15, 30, or 60 mg/kg respectively) daily for 5 weeks. FVB/N mice exposed to the chemical carcinogen AOM and gut irritant DSS present with a number of adenomatous lesions localized in the large bowel with the potential to progress to adenocarcinomas (27, 28). Supplementation starting 3 days post-DSS administration with 42 and 85ppm DADS resulted in a significant decrease in the total number of adenomatous polyps by 34.3% (p<0.01) and 47.3% (p<0.01), respectively (Fig. 1C). In addition, 21, 42, 85ppm DADS supplementation resulted in a significant reduction in tumor burden by 24.2% (p<0.05), 33.4% (p<0.01) and 66.4% (p<0.01), respectively (Fig. 1C). While in this study tumorigenesis did not progress to adenocarcinomas as seen in previous studies, histopathological analysis revealed that the lesions in mice treated with 85ppm were less transformed with the dysplasia frequently less than high-grade (p<0.01) (Fig. 1D). Furthermore, 6 out of 23 (24%) of mice treated with 85ppm DADS were tumor-free with normal pathology, compared to 1/22 (4.5%) in control (data not shown). The overall response to DADS did not vary between male and female test mice. Taken together, DADS dietary supplementation prevented the development of colitis-induced colorectal cancer in AOM/DSS mouse model.
DADS shortened the recovery time from DSS-induced weight loss and thereby prevented prolonged inflammation
FVB/N mice exposed to 2% DSS will develop colitis, which has been extensively characterized by various techniques including magnetic resonance imaging (MRI) and the examination of the severity of inflammation correlates with loss in body weight (28, 29). In animals fed the control diet, exposure to 1 cycle of 2% DSS showed a reduction in body weight peaking at 7–9 days post-DSS exposure (Fig. 1E) with a gradual recovery as indicated by positive weight gain. Dietary supplementation with DADS beginning on day 20 resulted in an attenuation of colitis-induced weight loss (not significant). More importantly, mice treated with 42ppm and 85ppm DADS supplemented diets had a significantly (p<0.05) higher recovery rate as measured by weight gain (Fig. 1E, day 27–31). Inflammation can persist several months after DSS administration mediated by several factors, including cyclooxygenase-2 (COX-2) expression and NFκB activation, which parallels the process of tumorigenesis (30). The prolonged inflammation can be visualized histologically by H&E staining and by staining for CD45, a receptor-linked protein tyrosine phosphatase expressed on leukocytes. Histopathological analysis revealed that DADS supplementation at 85ppm decreased the severity and distribution of leukocyte infiltrate (p<0.01), epithelial erosion (p<0.05), and the degree of cell dysplasia (p<0.01) (Fig. 1D), which was confirmed by the regain of structural shape and the decrease in CD45 positive cells in colonic epithelium (Fig. 1F). These results suggest that mice treated with DADS recovered from DSS-induced colitis faster than mice in the control diet preventing prolonged inflammation, which may in part explain the chemopreventive effect of DADS on CRC tumorigenesis.
DADS inhibited proliferation of SW480 colorectal cancer cells in vitro
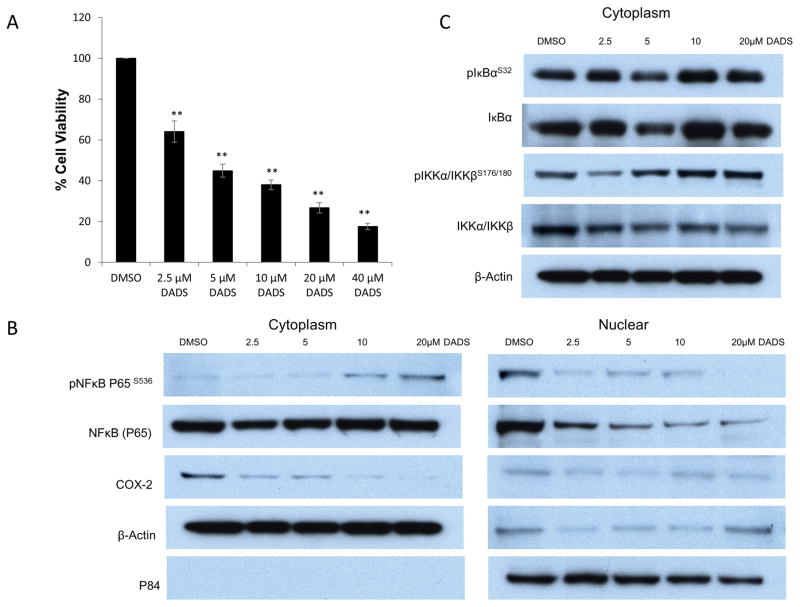
In order to elicit the molecular mechanisms of DADS chemopreventive activity, the human CRC SW480 cell line was treated with varying concentrations of DADS (2.5 μM, 5 μM, 10 μM, 20 μM, 40 μM) for 24hours. The SW480 CRC cell line was chosen based on the observed responses in a previous study with DADS (31) and because the cell line is well characterized in terms of the biochemical and chromosomal properties (32). In addition to the loss of APC on chromosome 5, activating point mutation of Kras and c-myc amplification, SW480 cells harbor mutations in pro-inflammatory mediators, including, transforming growth factor β (TGF-β) (33) and platelet derived growth factor (PDGF) (34), thereby making the SW480 in vitro studies comparable to the AOM/DSS model of inflammatory bowel disease. DADS treatment resulted in a significant concentration-dependent inhibition of cancer cell viability in SW480 cells (Fig. 2A). In contrast, DADS did not inhibit the viability of non-tumor cell line HEK293 (data not shown).
Figure 2.
Diallyl disulfide (DADS) inhibited nuclear localization of NFκB. A; DADS suppressed the proliferation of SW480 colon cancer cells. SW480 cells were seeded into 96-well plates and treated with vehicle (DMSO), 2.5μM, 5μM, 10μM, 20μM, or 40 μM DADS. After 24 hours following treatment the cell viability was determined using the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide assay. Absorbance at 570nm was detected with Micro plate reader. Data presented as mean of three independent experiments. *P< 0.05, **P < 0.01 indicates a significant difference compared with control group by t-test.
B–C, Western blot analyses of cytoplasmic (left) and nuclear (right). SW480 CRC cell lysates probed with NFκB (P65) and p-S536-NFκB (P65), COX-2, p-S32-IκBα, IκBα, p-S175/180-IKKα/IKKβ, and IKKα/IKKβ. Cells were treated with vehicle (DMSO), 2.5μM, 5μM, 10μM, or 20μM DADS for 24hrs. B, DADS induced a dose-dependent decrease in total and S536 phosphorylated nuclear NFkB (P65). C, IκBα and IKKα/IKKβ phosphorylation were unaffected by DADS treatment in both cytoplasm and nucleus (nuclear staining not presented). β-actin was used for a cytoplasmic loading control and p84 displayed as nuclear loading control. Each western analysis shown is a representative example of at least three independent experiments.
DADS inhibited NFκB nuclear translocation and reduced COX-2 expression in vitro
NFκB activation is essential for development of colonic adenomas in AOM/DSS treated mice (35). NFκB contributes to tumorigenesis by inducing genes that are pro-inflammatory such as COX-2 (36). The P65/p50 heterodimer is the most abundant NF-κB complex in the cell and is regulated by the so-called canonical pathway, with phosphorylation of P65 at serine 536 being important for its activity (37, 38). In the current study, we found a dose-dependent reduction in phosphorylated-NFκB (P65) S536 in the nucleus of SW480 cells treated with DADS, which corresponded with a reduction in COX-2 protein expression in the cytoplasm (Fig. 2B). Activation of the canonical NFκB pathway, indicated by phosphorylation of IκBα and IKKβ appears to be unaffected by DADS treatment (Fig. 2C). The DADS dependent reduction of phosphorylated P65 in the nucleus and the corresponding increase in phosphorylated P65 in the cytoplasm suggests DADS treatment is blocking nuclear translocation of P65.
DADS inhibited GSK-3 activity
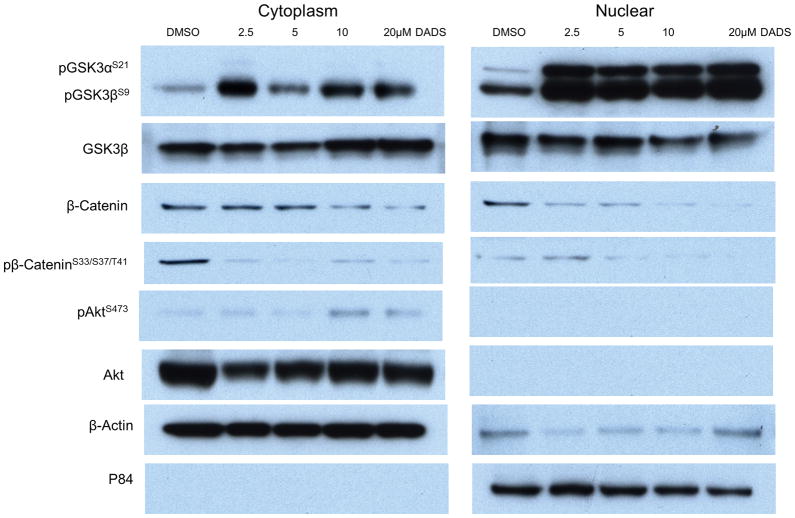
Glycogen synthase kinase-3 (GSK-3) protein that consists of 2 isoforms (α and β) is present in both the cytoplasm and nucleus and is an important regulator of the inflammatory process. GSK-3(α/β) is activated by phosphorylation of Tyrosine (279/216) and is inactivated by phosphorylation of Serine (21/9). GSK-3β knockout mice are embryonically lethal (21). GSK-3β promotes the activation of NFκB leading to the survival of cells exposed to TNF-α or tumor cells in which the NF-κB pathway is constitutively active (39). DADS treatment inhibited GSK-3β in the nucleus as shown by increased expression of pGSK3βS9 in the nuclear fractions (Fig. 3). Furthermore, GSK-3β was fully phosphorylated at Serine 9 even with the lowest dose of DADS (2.5 μM) (Fig. 3) suggesting that the suppressive effects of DADS on GSK-3β activity could be direct and/or highly sensitive.. The inactivation of GSK-3β can occur by a number of kinases most notably by Akt (40). There was a modest increase in Akt activity (pAktS473) in the cytoplasm at the highest dosages of DADS; however, GSK-3β inhibition is seen even at the lowest dose of DADS and therefore it is unlikely that GSK-3β inhibition is mediated through Akt (Fig. 3). Thus, it is possible that another kinase is responsible for GSK3β inhibition or that DADS inhibits GSK-3β through a direct interaction.
Figure 3.
Diallyl disulfide (DADS) inhibited GSK-3. Western blot analysis of cytoplasmic (left) and nuclear (right) SW480 CRC cell lysates probed with Abs against p-S21/9-GSK-3α/β, GSK-3β, total β-catenin, phosphorylated β-catenin at S33/S37/T41, Akt, and p-S473-Akt. Cells were treated with vehicle (DMSO), 2.5μM, 5μM, 10μM, or 20μM DADS for 24hrs. DADS induced inhibition of GSK-3 as shown by increased phosphoylation at S21/9 which was more pronounced in the nuclear fraction. DADS also reduced expression and phosphorylation at S33/S37/T41 of β-catenin, a major substrate of GSK-3β, confirming the suppressive effect of DADS on GSK-3β activity. β-actin was used a cytoplasmic loading control and p84 displayed as nuclear loading control. Each western analysis shown is a representative example of at least three independent experiments.
DADS inhibition of NFκB and COX-2 was GSK-3β dependent
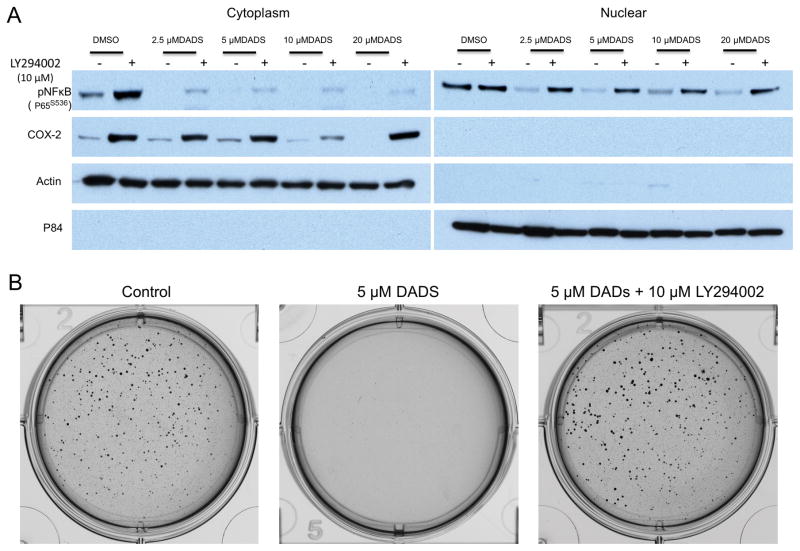
To determine if DADS suppression of NFκB activity is dependent on GSK-3β inhibition, we treated SW480 cells with DADS together with a GSK-3β activator, LY294002. When cells were treated both with LY294002 and DADS, the decreased phosphorylation and nuclear translocation of P65-NFκB, as well as the COX-2 inhibition, previously observed with DADS only treatment, was attenuated (Fig. 4A). These results suggested that DADS mediated NFκB inhibition is GSK-3β-dependent. To evaluate whether the GSK-3β-dependent inhibition of NFκB correlated with a decrease in tumorigencity, we employed the use of a soft agar assay to monitor anchorage-independent three-dimensional colony formation. This method can deliver results that are comparable to those obtained when injecting tumorigenic cells into nude mice (41). Treatment with 5μM DADS was sufficient to prevent the ability of SW480 cells to form colonies in soft agar. The simultaneous treatment with a GSK-3β activator LY294002 (10 μM) and DADS (5 μM) abolished anti-tumorigenic effects of DADS (Fig. 4B). These results show that DADS prevents tumorigencity in vitro, which appears to be dependent of GSK-3β inhibition and likely to be contributed to the inhibition of the NFκB pathway.
Figure 4.
Diallyl Disulfide (DADS) inhibition of NFκB nuclear localization was dependent on DADS inhibition of GSK-3. A, Western blot analysis of cytoplasmic (left) and nuclear (right) SW480 CRC cell lysates probed with p-S536-NFκB (P65), and COX-2. Cells were treated with vehicle (DMSO), 2.5μM, 5μM, 10μM, or 20μM DADS followed by exposure to LY294002 (10μM), a GSK-3β activator, or vehicle indicated by + or −, respectively. B, Soft agar assay measuring anchorgage-independent growth. SW480 CRC cells were pre-treated with vehicle or 5μM DADS +/− 10μM LY294002, GSK-3β activator for 24 hours and grown for 10days. Photomicrographs shown are representative of triplicate samples.
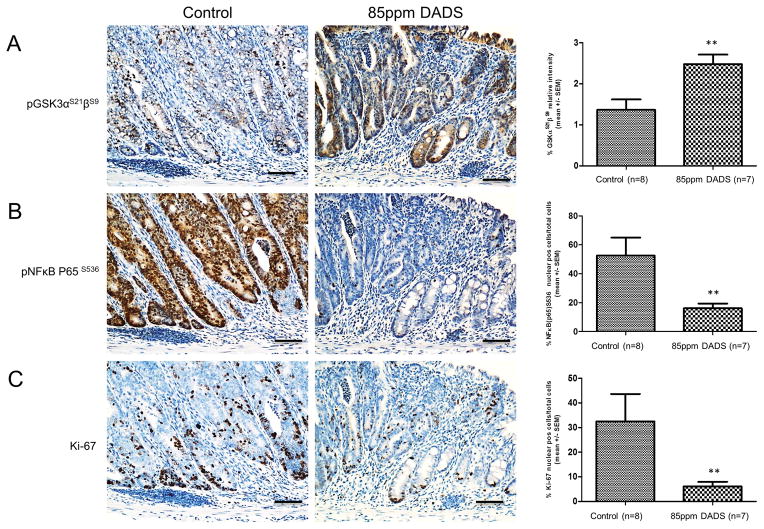
DADS inhibited GSK-3β and NFκB in vivo
To determine if the effects of DADS on GSK-3β inhibition and NFκB localization seen in SW480 cells can be translated in vivo, we measured using immunohistochemistry phosphorylated GSK-3(α/β) at Serine (21/9) and nuclear localization of phosphorylated NFκB (P65) Serine 536 in the colons of AOM/DSS treated mice. Similar to the SW480 cells, mice that consumed a diet supplemented with 85ppm of DADS had a significantly higher level of inactivated GSK-3(α/β) compared to mice on the control diet and a significantly lower nuclear localization of phosphorylated NFκB (P65) (Fig. 5A and B). Furthermore, the changes correlated with an 86.2% decrease in Ki-67 expression, a marker of cellular proliferation (Fig. 5C). These results further confirm that DADS inhibits GSK-3β and suppresses the activation and nuclear localization of NFκB, which may in part contribute to the chemopreventive activity of DADS.
Figure 5.
Diallyl disulfide (DADS) induced GSK-3α/β phosphorylation at S21/9, inhibited nuclear localization NFkB (P65) phosphylated at S536, and Ki-67 expression in vivo. A–C, IHC analysis p-S21/9 GSK-3α/β (1:100), p-S536-NFκB (P65) (1:100), and Ki-67 (1:100) in colonic epithelium at day 52 from mice from AOM/DSS control group or 85ppm DADS group. A, DADS induced inhibition of GSK-3α/β as shown by increased phosphorylation at S21/9 with both cytoplasmic and nuclear localization. B, DADS reduced expression of p-S536-NFκB (P65) in nucleus. C, DADS inhibited proliferation as shown by staining with Ki-67. Scale bar= 100μm. Right panels are quantitation of staining using Aperio Scanscope nuclear staining program. **P < 0.01 indicates a significant difference compared with control group as determined by t-test.
Discussion
Epidemiological evidence has shown an association between garlic intake and decreased risk of developing colorectal cancer (42, 43). Likewise, considerable experimental evidence has shown that garlic sulfur-containing constituents, such as diallyl disulfide (DADS), can reduce the growth of colon tumor cells in animals (44, 45). To our knowledge, the underlying mechanism explaining the anti-tumorigenic effects of DADS has yet to be elucidated. In the present study, we examined the hypothesis that DADS exerts its protective effects against colorectal tumors by suppressing inflammation, utilizing the colitis-induced colorectal cancer AOM/DSS mouse model. Our results indicated that dietary supplementation with 42 and 85 ppm DADS (human equivalent dose of 30 and 60 mg/daily, respectively) for 32 days resulted in a significantly shortened recovery time from the DSS-induced inflammatory insult and significantly reduced number and size of colorectal tumors in AOM/DSS-treated mice.
In vitro studies using the human CRC SW480 cell line revealed that the tumor suppressive effect of DADS may, in part, be mediated by GSK-3β-dependent NFκB inhibition.
Constitutive NFκB activation in inflammatory bowel disease is associated with an increased risk of developing colorectal cancer (15). The NFκB heterodimeric Rel-A (P65)-p50 complex is sequestered in the cytoplasm under non-stimulated conditions by IκB. Pro- inflammatory cytokines within the tumor microenvironment, such as, tumor necrosis factor-1 and interleukin-1 induce phosphorylation of IκB by IKK complexes, which allows the P65:p50 heterodimer to be released and translocate to the nucleus where they activate various target genes (16). IKKs and IκB inhibitors are the mainstay of targeting NFkB, however, because of their harmful side effects, these agents are often not used clinically (46). In SW480 CRC cells that were used in this study, NFκB is constitutively phosphorylated by activated IKK. Unlike aspirin and other NSAIDs that suppress NFκB activity by inhibiting IKK activity, increasing concentrations of DADS had no effect on either IKK or IκB, indicating the upstream canonical pathway is not involved in DADS inhibition of NFκB. There was however a decrease in phosphorylated P65 (NFκB-P65S536) in the nucleus with a corresponding increase in the cytoplasm, suggesting that the suppressive effect of DADS on NFκB activity is likely due to its ability to inhibit the nuclear translocation of the phosphorylated P65. This finding was consistent with the decrease in NFkB phosphorylation in colon tissue of DADS fed mice.
Glycogen synthase kinase-3 (GSK-3) is a serine/threonine kinase that has diverse cellular functions and numerous substrates. In our study, DADS, a major anti-inflammatory component in garlic, inactivated GSK-3 (α/β) by blocking its phosphorylation of serine residue 21/9 and this event was determined to inhibit NFκB nuclear translocation. As mentioned previously, NFκB transcription family members associate as homodimers and heterodimers complexes, of which the RelA (P65)/p50 is the most abundant (38). The p50 protein is produced from constitutive processing of p105 through ubiquitin-mediated proteolysis, a process mediated by IKK (47). In addition, the precursor protein p105 is responsible for the cytoplasmic retention of NFκB heterodimers (48). Interestingly, GSK-3 has been shown to phosphorylate p105 in vitro, accelerating p105 proteolysis to p50 (39). Furthermore, down-regulation of GSK-3 in fibroblasts was shown to inhibit NFκB induction (20). Thus, it is possible that DADS inhibition of GSK-3 is disrupting the distribution of p105 and p50, resulting in reduced nuclear expression and translocation of the active RelA P65/p50 NFκB heterodimer. Our notion that DADS inhibition of GSK-3 is responsible for the observed anti-inflammatory effects, including the inhibition of NFκB is further supported by an in-vivo study that demonstrated that administration of GSK-3 inhibitors could effectively down-regulate NFκB activity and significantly attenuate TNBS-induced colitis in rats (49).
In conclusion, we have provided evidence that garlic constituent DADS interferes with nuclear translocation of pro-inflammatory transcription factor NFκB and the expression of tumorigenic enzyme COX2 via GSK-3 (α/β) inactivation. Future studies will be needed to determine the exact mechanism of DADS-induced GSK-3 (α/β) inactivation. In addition, there are over thirty known organosulfur compounds in garlic (50) that should also be explored further. The importance of dietary components including DADS and metabolic enzymes such as GSK-3 (α/β) in maintaining homeostasis of intracellular microenvironment to prevent perpetual inflammatory pathways therefore cannot be overemphasized.
Supplementary Material
Acknowledgments
Financial Support: All co-authors were supported by the National Cancer Institute, and by the National Institutes of Health intramural program, ZIA BC 011159. In addition, the China Postdoctoral Science Foundation also supported W. Li; Grant number: 20110490559; 2012T50199.
This humble work is dedicated in memory of the late Dr. John A. Milner who pioneered the notion that nutrition can provide a preventative touch in the fight against cancer.
Footnotes
Conflicting Interest: None
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA: a cancer journal for clinicians. 2015;65:5–29. doi: 10.3322/caac.21254. [DOI] [PubMed] [Google Scholar]
- 2.Janakiram NB, Rao CV. The role of inflammation in colon cancer. Advances in experimental medicine and biology. 2014;816:25–52. doi: 10.1007/978-3-0348-0837-8_2. [DOI] [PubMed] [Google Scholar]
- 3.Mariani F, Sena P, Roncucci L. Inflammatory pathways in the early steps of colorectal cancer development. World journal of gastroenterology : WJG. 2014;20:9716–31. doi: 10.3748/wjg.v20.i29.9716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Scarpa M, Castagliuolo I, Castoro C, Pozza A, Scarpa M, Kotsafti A, et al. Inflammatory colonic carcinogenesis: a review on pathogenesis and immunosurveillance mechanisms in ulcerative colitis. World journal of gastroenterology : WJG. 2014;20:6774–85. doi: 10.3748/wjg.v20.i22.6774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jess T, Rungoe C, Peyrin-Biroulet L. Risk of colorectal cancer in patients with ulcerative colitis: a meta-analysis of population-based cohort studies. Clinical gastroenterology and hepatology : the official clinical practice journal of the American Gastroenterological Association. 2012;10:639–45. doi: 10.1016/j.cgh.2012.01.010. [DOI] [PubMed] [Google Scholar]
- 6.Kim ER, Chang DK. Colorectal cancer in inflammatory bowel disease: the risk, pathogenesis, prevention and diagnosis. World journal of gastroenterology : WJG. 2014;20:9872–81. doi: 10.3748/wjg.v20.i29.9872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shenoy AK, Fisher RC, Butterworth EA, Pi L, Chang LJ, Appelman HD, et al. Transition from colitis to cancer: high Wnt activity sustains the tumor-initiating potential of colon cancer stem cell precursors. Cancer research. 2012;72:5091–100. doi: 10.1158/0008-5472.CAN-12-1806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Abuajah CI, Ogbonna AC, Osuji CM. Functional components and medicinal properties of food: a review. Journal of food science and technology. 2015;52:2522–9. doi: 10.1007/s13197-014-1396-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.DiMarco-Crook C, Xiao H. Diet-based strategies for cancer chemoprevention: the role of combination regimens using dietary bioactive components. Annual review of food science and technology. 2015;6:505–26. doi: 10.1146/annurev-food-081114-110833. [DOI] [PubMed] [Google Scholar]
- 10.Schafer G, Kaschula CH. The immunomodulation and anti-inflammatory effects of garlic organosulfur compounds in cancer chemoprevention. Anti-cancer agents in medicinal chemistry. 2014;14:233–40. doi: 10.2174/18715206113136660370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fasolino I, Izzo AA, Clavel T, Romano B, Haller D, Borrelli F. Orally administered allyl sulfides from garlic ameliorate murine colitis. Molecular nutrition & food research. 2015;59:434–42. doi: 10.1002/mnfr.201400347. [DOI] [PubMed] [Google Scholar]
- 12.Erstad DJ, Cusack JC., Jr Targeting the NF-kappaB pathway in cancer therapy. Surgical oncology clinics of North America. 2013;22:705–46. doi: 10.1016/j.soc.2013.06.011. [DOI] [PubMed] [Google Scholar]
- 13.Hoesel B, Schmid JA. The complexity of NF-kappaB signaling in inflammation and cancer. Molecular cancer. 2013;12:86. doi: 10.1186/1476-4598-12-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jing H, Lee S. NF-kappaB in cellular senescence and cancer treatment. Molecules and cells. 2014;37:189–95. doi: 10.14348/molcells.2014.2353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Atreya I, Atreya R, Neurath MF. NF-kappaB in inflammatory bowel disease. Journal of internal medicine. 2008;263:591–6. doi: 10.1111/j.1365-2796.2008.01953.x. [DOI] [PubMed] [Google Scholar]
- 16.Solt LA, May MJ. The IkappaB kinase complex: master regulator of NF-kappaB signaling. Immunologic research. 2008;42:3–18. doi: 10.1007/s12026-008-8025-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gupta SC, Sundaram C, Reuter S, Aggarwal BB. Inhibiting NF-kappaB activation by small molecules as a therapeutic strategy. Biochimica et biophysica acta. 2010;1799:775–87. doi: 10.1016/j.bbagrm.2010.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Doble BW, Patel S, Wood GA, Kockeritz LK, Woodgett JR. Functional redundancy of GSK-3alpha and GSK-3beta in Wnt/beta-catenin signaling shown by using an allelic series of embryonic stem cell lines. Developmental cell. 2007;12:957–71. doi: 10.1016/j.devcel.2007.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ghosh JC, Altieri DC. Activation of p53-dependent apoptosis by acute ablation of glycogen synthase kinase-3beta in colorectal cancer cells. Clinical cancer research : an official journal of the American Association for Cancer Research. 2005;11:4580–8. doi: 10.1158/1078-0432.CCR-04-2624. [DOI] [PubMed] [Google Scholar]
- 20.Hoeflich KP, Luo J, Rubie EA, Tsao MS, Jin O, Woodgett JR. Requirement for glycogen synthase kinase-3beta in cell survival and NF-kappaB activation. Nature. 2000;406:86–90. doi: 10.1038/35017574. [DOI] [PubMed] [Google Scholar]
- 21.Shakoori A, Mai W, Miyashita K, Yasumoto K, Takahashi Y, Ooi A, et al. Inhibition of GSK-3 beta activity attenuates proliferation of human colon cancer cells in rodents. Cancer science. 2007;98:1388–93. doi: 10.1111/j.1349-7006.2007.00545.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Millen AE, Subar AF, Graubard BI, Peters U, Hayes RB, Weissfeld JL, et al. Fruit and vegetable intake and prevalence of colorectal adenoma in a cancer screening trial. The American journal of clinical nutrition. 2007;86:1754–64. doi: 10.1093/ajcn/86.5.1754. [DOI] [PubMed] [Google Scholar]
- 23.Saud SM, Young MR, Jones-Hall YL, Ileva L, Evbuomwan MO, Wise J, et al. Chemopreventive activity of plant flavonoid isorhamnetin in colorectal cancer is mediated by oncogenic Src and beta-catenin. Cancer research. 2013;73:5473–84. doi: 10.1158/0008-5472.CAN-13-0525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Reagan-Shaw S, Nihal M, Ahmad N. Dose translation from animal to human studies revisited. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2008;22:659–61. doi: 10.1096/fj.07-9574LSF. [DOI] [PubMed] [Google Scholar]
- 25.Washington MK, Powell AE, Sullivan R, Sundberg JP, Wright N, Coffey RJ, et al. Pathology of rodent models of intestinal cancer: progress report and recommendations. Gastroenterology. 2013;144:705–17. doi: 10.1053/j.gastro.2013.01.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li W, Hua B, Saud SM, Lin H, Hou W, Matter MS, et al. Berberine regulates AMP-activated protein kinase signaling pathways and inhibits colon tumorigenesis in mice. Molecular carcinogenesis. 2014 doi: 10.1002/mc.22179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tanaka T, Kohno H, Suzuki R, Yamada Y, Sugie S, Mori H. A novel inflammation-related mouse colon carcinogenesis model induced by azoxymethane and dextran sodium sulfate. Cancer science. 2003;94:965–73. doi: 10.1111/j.1349-7006.2003.tb01386.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Young MR, Ileva LV, Bernardo M, Riffle LA, Jones YL, Kim YS, et al. Monitoring of tumor promotion and progression in a mouse model of inflammation-induced colon cancer with magnetic resonance colonography. Neoplasia (New York, NY) 2009;11:237–46. doi: 10.1593/neo.81326. 1p following 46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kitajima S, Takuma S, Morimoto M. Changes in colonic mucosal permeability in mouse colitis induced with dextran sulfate sodium. Experimental animals/Japanese Association for Laboratory Animal Science. 1999;48:137–43. doi: 10.1538/expanim.48.137. [DOI] [PubMed] [Google Scholar]
- 30.Karin M. Nuclear factor-kappaB in cancer development and progression. Nature. 2006;441:431–6. doi: 10.1038/nature04870. [DOI] [PubMed] [Google Scholar]
- 31.Liao QJ, Su J, Zhou XT, Tang HL, Song Y, Su Q. Inhibitory effect of diallyl disulfide on proliferation of human colon cancer cell line SW480 in nude mice. Ai zheng = Aizheng = Chinese journal of cancer. 2007;26:828–32. [PubMed] [Google Scholar]
- 32.Ahmed D, Eide PW, Eilertsen IA, Danielsen SA, Eknaes M, Hektoen M, et al. Epigenetic and genetic features of 24 colon cancer cell lines. Oncogenesis. 2013;2:e71. doi: 10.1038/oncsis.2013.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Coffey RJ, Jr, Goustin AS, Soderquist AM, Shipley GD, Wolfshohl J, Carpenter G, et al. Transforming growth factor alpha and beta expression in human colon cancer lines: implications for an autocrine model. Cancer research. 1987;47:4590–4. [PubMed] [Google Scholar]
- 34.Anzano MA, Rieman D, Prichett W, Bowen-Pope DF, Greig R. Growth factor production by human colon carcinoma cell lines. Cancer research. 1989;49:2898–904. [PubMed] [Google Scholar]
- 35.Greten FR, Arkan MC, Bollrath J, Hsu LC, Goode J, Miething C, et al. NF-kappaB is a negative regulator of IL-1beta secretion as revealed by genetic and pharmacological inhibition of IKKbeta. Cell. 2007;130:918–31. doi: 10.1016/j.cell.2007.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Charalambous MP, Lightfoot T, Speirs V, Horgan K, Gooderham NJ. Expression of COX-2, NF-kappaB-p65, NF-kappaB-p50 and IKKalpha in malignant and adjacent normal human colorectal tissue. British journal of cancer. 2009;101:106–15. doi: 10.1038/sj.bjc.6605120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ghosh S, Karin M. Missing pieces in the NF-kappaB puzzle. Cell. 2002;109(Suppl):S81–96. doi: 10.1016/s0092-8674(02)00703-1. [DOI] [PubMed] [Google Scholar]
- 38.Hayden MS, Ghosh S. Signaling to NF-kappaB. Genes & development. 2004;18:2195–224. doi: 10.1101/gad.1228704. [DOI] [PubMed] [Google Scholar]
- 39.Demarchi F, Bertoli C, Sandy P, Schneider C. Glycogen synthase kinase-3 beta regulates NF-kappa B1/p105 stability. The Journal of biological chemistry. 2003;278:39583–90. doi: 10.1074/jbc.M305676200. [DOI] [PubMed] [Google Scholar]
- 40.Wang H, Brown J, Martin M. Glycogen synthase kinase 3: a point of convergence for the host inflammatory response. Cytokine. 2011;53:130–40. doi: 10.1016/j.cyto.2010.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Freedman VH, Shin SI. Cellular tumorigenicity in nude mice: correlation with cell growth in semi-solid medium. Cell. 1974;3:355–9. doi: 10.1016/0092-8674(74)90050-6. [DOI] [PubMed] [Google Scholar]
- 42.Giovannucci E, Rimm EB, Stampfer MJ, Colditz GA, Ascherio A, Willett WC. Intake of fat, meat, and fiber in relation to risk of colon cancer in men. Cancer research. 1994;54:2390–7. [PubMed] [Google Scholar]
- 43.Steinmetz KA, Kushi LH, Bostick RM, Folsom AR, Potter JD. Vegetables, fruit, and colon cancer in the Iowa Women’s Health Study. American journal of epidemiology. 1994;139:1–15. doi: 10.1093/oxfordjournals.aje.a116921. [DOI] [PubMed] [Google Scholar]
- 44.Sundaram SG, Milner JA. Diallyl disulfide suppresses the growth of human colon tumor cell xenografts in athymic nude mice. The Journal of nutrition. 1996;126:1355–61. doi: 10.1093/jn/126.5.1355. [DOI] [PubMed] [Google Scholar]
- 45.Wargovich MJ. Diallyl sulfide, a flavor component of garlic (Allium sativum), inhibits dimethylhydrazine-induced colon cancer. Carcinogenesis. 1987;8:487–9. doi: 10.1093/carcin/8.3.487. [DOI] [PubMed] [Google Scholar]
- 46.Gamble C, McIntosh K, Scott R, Ho KH, Plevin R, Paul A. Inhibitory kappa B Kinases as targets for pharmacological regulation. British journal of pharmacology. 2012;165:802–19. doi: 10.1111/j.1476-5381.2011.01608.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Senftleben U, Cao Y, Xiao G, Greten FR, Krahn G, Bonizzi G, et al. Activation by IKKalpha of a second, evolutionary conserved, NF-kappa B signaling pathway. Science (New York, NY) 2001;293:1495–9. doi: 10.1126/science.1062677. [DOI] [PubMed] [Google Scholar]
- 48.Heissmeyer V, Krappmann D, Wulczyn FG, Scheidereit C. NF-kappaB p105 is a target of IkappaB kinases and controls signal induction of Bcl-3-p50 complexes. The EMBO journal. 1999;18:4766–78. doi: 10.1093/emboj/18.17.4766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Whittle BJ, Varga C, Posa A, Molnar A, Collin M, Thiemermann C. Reduction of experimental colitis in the rat by inhibitors of glycogen synthase kinase-3beta. British journal of pharmacology. 2006;147:575–82. doi: 10.1038/sj.bjp.0706509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Amagase H. Clarifying the real bioactive constituents of garlic. The Journal of nutrition. 2006;136:716s–25s. doi: 10.1093/jn/136.3.716S. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.