Abstract
The evolution of neurocranial morphology in Homo sapiens is characterized by bulging of the parietal region, a feature unique to our species. In modern humans, expansion of the parietal surface occurs during the first year of life, in a morphogenetic stage which is absent in chimpanzees and Neandertals. A similar variation in brain shape among living adult humans is associated with expansion of the precuneus. Using MRI-derived structural brain templates, we compare medial brain morphology between humans and chimpanzees through shape analysis and geometrical modeling. We find that the main spatial difference is a prominent expansion of the precuneus in our species, providing further evidence of evolutionary changes associated with this area. The precuneus is a major hub of brain organization, a central node of the default-mode network, and plays an essential role in visuospatial integration. Together, the comparative neuroanatomical and paleontological evidence suggest that precuneus expansion is a specialization of Homo sapiens that evolved in the last 150,000 years that may be associated with recent human cognitive specializations.
Keywords: parietal lobes, human evolution, evolutionary neuroanatomy, morphometrics
Introduction
Based on paleontological evidence, it is generally recognized that our species, when compared with other extinct and extant hominoids, is characterized by a rounded braincase (Lieberman et al. 2002; Bookstein et al. 2003). Quantitative analyses revealed that such globularity is not due to a general curvature of the whole neurocranium, but is mostly associated with a specific bulging of the parietal areas (Bruner et al. 2003, 2011; Bruner 2004). According to the functional matrix hypothesis, during cranial morphogenesis the parietal bone is molded by the forces exerted by the growth and development of the corresponding cortical brain areas (Moss and Young 1960). The spatial boundaries between the parietal lobes and the parietal bones may vary at different brain sizes, but their dimensions are correlated and there is a reliable correspondence between their general morphology (Bruner et al., 2015a). Morphological integration, in both brain and skull, is generally based on local influences among contiguous elements (Bruner et al. 2010; Gómez-Robles et al. 2013), and it is therefore likely that the morphological variation described for the parietal bones in the fossil record is mainly due to actual volumetric variation of the underlying parietal cortex. Neurocranial globularity in our species is due to an early postnatal stage of development characterized by expansion of parietal and cerebellar volume (Neubauer et al., 2009). Interestingly, chimpanzees and Neandertals lack this ontogenetic stage, although their subsequent endocranial morphogenesis is similar to the successive modern human pattern (Neubauer et al. 2010; Gunz et al. 2010). Similar parietal changes are also a major source of variation among adult modern humans and, in this case, it is strictly due to variation in the size of the precuneus (Bruner et al. 2014a, 2015b). The similarity between the inter-specific (bone) and intra-specific (brain) spatial variation suggests that these two processes may be related, and explained by common factors (Bruner et al. 2014b). Therefore, precuneus expansion may be responsible for the emergence of neurocranial globularity in anatomically modern humans.
The precuneus is a major hub of brain organization, a central node of the default-mode network, and plays an essential role in visuospatial integration (Cavanna and Trimble 2006; Margulies et al., 2009), suggesting that changes of this area might have been associated with human cognitive specializations. Fossils are the most direct source of evidence about evolutionary history, but inferences about their actual cerebral anatomy must be necessarily based on indirect assumptions. Therefore, an essential complementary source of evidence is the comparative study of human and other living primate brains (Rilling 2006, 2014). In particular, the comparison between humans and our closest living primate relative, the chimpanzee, can provide insights into human brain evolution. Comparisons between human and macaque brains show that there are species-specific differences in the parietal cortex, for example in the structural and functional organization of the intraparietal sulcal cortex (Orban et al., 2006; Scheperjans et al 2008a). However, human-chimpanzee parietal lobe comparisons, and in particular comparisons of the medial parietal surface, are still lacking. In this study, we use structural MRI scans to describe and quantify differences in medial brain morphology between humans and chimpanzees. Human brains are 3–4 times larger than chimpanzee brains (Sherwood et al 2009; Neubauer 2014). Phylogenetic variations in size are often associated with variations in shape due to allometric scaling rules, although in some cases the evolution of novel traits can drive changes in the relative proportions (Rilling, 2006). Either way, these changes would reflect volumetric expansion or contraction of some specific component that occurred after the phylogenetic separation of our two lineages, approximately 5–8 mya (Wood 2000; Strait and Grine 2004).
Materials and Methods
Magnetic Resonance and Imaging
The sample includes ten adult chimpanzees and ten adult humans. Chimpanzee MRI scans were performed on a Siemens 3T Trio scanner with a standard birdcage coil routinely used for human head imaging. Chimpanzees were immobilized with ketamine (2–6 mg/kg, i.m.) prior to being anesthetized with an intravenous propofol drip (10 mg/kg/hr). Animals were under constant observation by the veterinary staff before, during, and after the scan. Head motion was minimized by stabilizing with foam cushions and elastic straps. All procedures were carried out in accordance with protocols approved by the Yerkes National Primate Research Center and the Emory University Institutional Animal Care and Use Committee (approval No. YER-2001206). Human subjects underwent MRI scanning at Emory University on a Siemens 3T scanner with a twelve-channel parallel imaging phase-array coil. Foam cushions were used to minimize head motion. All procedures were carried out in accordance with protocols approved by the Emory University Institutional Review Board (IRB #s: 00000028, 00044782). See online supplementary information for a more detailed description of scanning procedures and templates.
Morphometrics
Shape analysis was computed according to the principles of geometric morphometrics (Zelditch et al., 2004). Sixteen landmarks were identified using both homologous neuroanatomical elements and geometrical references from the medial sagittal section crossing the precuneus grey matter (Figure 1a and 1b). Landmarks were selected according to previous analyses (Bruner et al., 2010, 2014a): center of the splenium and genu of the corpus callosum, anterior border of the optic chiasm, central sulcus, marginal ramus of the cingulate sulcus, external and internal extremes of the perpendicular sulcus, central point of the subparietal sulcus, occipito-cerebellar boundary, center of the thalamus, center of the quadrigeminal lamina. Along the frontal curve, from the anterior border of the optic chiasm to the central sulcus, three semilandmarks were sampled at equally distant points (25% of the curve length). Along the occipital curve, between the external point of the perpendicular fissure and the occipito-cerebellar boundary, one semilandmark was sampled at 50% of the curve length. The marginal landmark was sampled where the marginal sulcus is flexed between the precuneus and paracentral lobule, generally generating a minor additional sulcus or else joining the precuneal sulcus. The subparietal landmark was sampled at the center of the subparietal sulcus, generally displaying a form of “H”. Although these two latter landmarks are associated with variable patterns among individuals, they can nonetheless be easily localized in most specimens, and clearly visible in the average templates. In some cases with minor uncertainty, averaging hemispheres further limits any possible bias, which is of a different order of magnitude relative to the observed inter-specific differences considered in this study.
Figure 1.
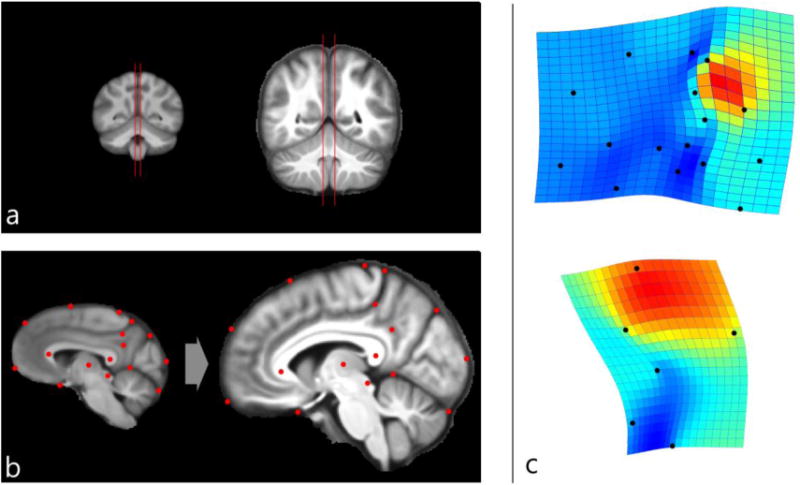
Comparison of average human and chimpanzee MRI templates. a) Using coronal sections, sagittal slices were positioned to intersect the most medial cortical planes in chimpanzees (left) and humans (right). b) The resulting sagittal images were compared according to a geometric model using a set of landmarks. Thin-plate spline deformation grids and expansion maps (red: dilation; blue: compression) were used to visualize shape changes from the chimpanzee template to the human template (c), using the whole configuration (above) or only the landmarks delimiting the precuneus area (below). Both right and left hemispheres are used to compute the averages. Thin-plate spline interpolant function computes the minimum spatial deformation between the two figures. In both cases, the main difference is associated with geometric expansion of the upper precuneus.
Coordinates were superimposed by Procrustes registration, which minimizes and normalizes spatial differences by translation to a common centroid, scaling to unitary size, and rotation so as to minimize the distance between corresponding landmarks (Bookstein, 1991). Residuals have been analyzed by principal component analyses, averaging the right and left hemisphere for each specimen. Means were also compared using only the landmarks associated with precuneus morphology, so as to reveal shape-specific differences of this area, excluding morphological factors from other brain areas, and considering its geometry beyond its overall proportions. Differences between species and along the multivariate vectors were visualized through thin-plate spline interpolant function, showing the minimum deformation necessary to transform one shape into another, and a chromatic scale proportional to the degree of dilation/contraction. Coordinates were sampled with tpsDig 2.17 (Rohlf, 2013), and analyzed with tpsSplin 1.20 (Rohlf, 2004), tpsRelw 1.54 (Rohlf, 2014), MorphoJ 1.06a (Klingenberg, 2011), and PAST 3.05 (Hammer et al., 2001).
Results
The comparison between human and chimpanzee species-specific mean shapes (Fig. 1c) indicates that the main difference is a longitudinal spatial dilation of the upper part of the precuneus in humans relative to chimpanzees. This longitudinal stretching of the precuneus, between the marginal ramus of the cingulate sulcus and the perpendicular (parieto-occipital) fissure, separates and displaces the frontal and occipital lobes. Other districts do not display any particular change in their relative proportions or shape, except for a change in position induced by expansion of the precuneus and the spatial readjustment and separation of adjacent areas. The result is the same when using the whole mid-sagittal configuration or only the landmarks associated with the precuneus morphology (precuneus and splenial area). This latter comparison further suggests that these differences are associated with the upper and anterior portions of the precuneus, toward the marginal ramus of the cingulate sulcus.
A principal component analysis of the whole sample (Figure 2) shows that the variation is mainly associated with only one dominant component, explaining 57% of the total variance. Each of the other multivariate vectors explains only a very minor percentage of variance, with a marked gap from the first axis, and eigenvalues which are below a threshold of random variation (broken stick model and Jolliffe cutoff value; Wagner 1984; Jolliffe 2002). Accordingly, none of these secondary components are sufficiently stable or reliable to be interpreted in terms of actual morphological patterns, being sensitive to noise and sample size. This structure of the multivariate space suggests that the sample variation is mainly based on a single determinant factor, without evidence of further additional shared patterns. The main vector is associated with the relative proportion of the precuneus, and with a vertical stretching of the posterior parts of the brain. Along this multivariate axis, humans and chimpanzee phenotypes are separated with no overlap in their morphological ranges, with humans displaying dilated precuneus and stretched parieto-occipital areas. Humans appear to be more variable then chimpanzees: comparing the standard deviation of the two species along this vector, the human sample is almost 1.5 times more variable than the chimpanzee sample (144%).
Figure 2.
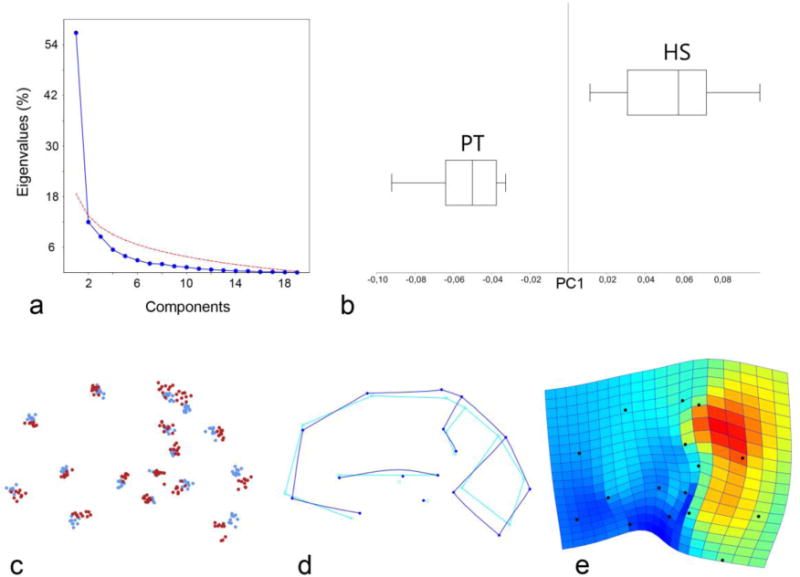
Using the whole-brain configuration, coordinates from ten adult chimpanzees and ten adult humans (averaged hemispheres) were superimposed by Procrustes registration and analyzed through Principal Component Analysis. Above: scree plot (a) showing that there is only one PC above the threshold of random effect (red line), explaining 57% of the variation, and boxplot (b) showing the nonparametric distribution of the values along this vector for Homo sapiens (HS) and Pan troglodytes (PT). Below: scatterplot (c) of each spatial coordinate after superimposition (blue dots: chimps; red dots: humans), wireframe (d) showing the shape changes along PC1 (light blue: lower values; dark blue: higher values), and thin-plate spline deformation grids (e) with expansion map (red: dilation; blue: constriction) showing the associated spatial changes. This morphological component is due to expansion of the precuneus and vertical stretching of the parieto-occipital areas.
The same results were obtained by using the whole brain landmark model, the precuneus landmarks only, or the parieto-occipital landmarks only. A discriminant analysis was also computed to show differences between humans and chimpanzees beyond pairwise mean comparisons and principal components. The discrimination vector was significant but it was associated with the same pattern of the first principal component, adding no further information.
Discussion
The boundaries of the parietal bone and parietal lobe do not show a fixed spatial relationship, but their respective dimensions are nonetheless correlated (Bruner et al. 2015a), and there is a geometrical correspondence of their respective surfaces (Moss and Young 1960; Kobayashi et al. 2014). The globularity of the modern human braincase is primarily determined by the size and shape of the parietal bone, which has a species-specific morphology in Homo sapiens. Midsagittal parietal variation is a major morphological factor in both modern human cranial evolution and modern human brain variability and, in this latter case, the variation is largely due to the size of the precuneus (Bruner et al., 2014b). The current study suggests that the precuneus also shows phylogenetic differences among living hominoids, displaying larger proportions in our species when compared with chimpanzees.
This morphological difference could be the result of a brain size effect associated with positive allometry of the parietal proportions, or else a species-specific character. The current evidence suggests that an allometric effect is unlikely, for two main reasons. First, endocranial morphogenesis in Homo sapiens and Pan troglodytes differs most prominently due to a parietal bulging stage in humans (Neubauer et al. 2010). The fact that chimpanzees lack this discrete stage is difficult to explain in terms of allometry, and it is likely that this specific stage newly evolved in modern humans. All living apes lack this parietal bulging stage (Scott et al. 2014), and hence the modern pattern is likely to be the derived one. Second, despite having a cranial capacity similar to or even larger than modern humans, Neandertals also lack this parietal bulging stage (Gunz et al. 2010) and do not display bulging parietal bones or lobes (Bruner et al. 2003, 2011; Bruner 2004). Therefore, an allometric effect of the brain size is unlikely to explain the human-chimpanzee differences we observe in precuneus proportions. Given that modern humans display a specific ontogenetic stage of parietal expansion when compared with chimpanzees, and given that there is also a patent difference in the proportions of the precuneus between the two species, it is reasonable to hypothesize that the ontogenetic stage characterizing the endocranial ontogeny of our species may be associated with the development of the medial parietal cortex. Because similar morphological variation also characterizes the main neurocranial difference between modern humans and the extinct human species, there may well have been a major expansion of the precuneus in recent human evolution.
In humans, there is an inverse correlation between the parietal and occipital bone morphology, and the bulging of the former involves the flattening of the latter (Gunz and Harvati 2007). Conversely, there is no correlation between parietal and occipital cortical volume among modern humans, but instead parietal cortex shows an inverse correlation with the volume of the frontal lobes (Allen et al., 2002). It remains to be evaluated whether the phylogenetic expansion of the precuneus described in this study, beyond a spatial displacement of the frontal areas, involved a relative reduction of the frontal cortex along the modern human lineage. However, it should also be noted that intra-specific and inter-specific variability can be based on different and independent rules (e.g., Martin and Barbour, 1989), and the morphological changes associated with larger precuneus in our species must be further investigated taking into consideration structural and functional differences between brain and bone components, and between intra- and inter-specific phenotypic variation.
The parietal lobes display subtle parcellations, with cytoarchitecture and connectivity largely conserved in humans and monkeys (Scheperjans et al. 2008b; Caspers et al., 2011; Mars et al. 2011; Caminiti et al. 2015). Because of this stable organization, it has been hypothesized that possible differences in humans may reflect expansion and functional modification of existing subdivisions, more-so than the addition of novel elements. The precuneus is the junction of three cortical territories: somatosensory cortex anteriorly, posterior cingulate cortex ventrally, and medial parietal cortex, dorsally (Margulies et al. 2009). The species difference we observe here is probably concentrated in medial parietal and somatosensory cortex. The medial parietal cortex is involved in higher-order cognitive processing (Cavanna and Trimble 2006; Margulies et al., 2009). It is a hub of cortical connectivity (Hagmann et al. 2008; Li et al. 2013) and a component of the default-mode network (Utevsky et al. 2014). Humans and chimpanzees, along with macaque monkeys, appear to possess a default-mode network comprised of a set of homologous areas (Rilling et al. 2007; Mantini et al. 2011), although this does not imply that the cognitive processes associated with these networks are identical across species. The medial parietal cortex is also implicated in higher cognitive processes such as autobiographical memory retrieval, theory of mind and self-reflection (Cavanna and Trimble 2006; Schneider et al. 2014). The precuneus is also essential in visuospatial functions which are central for the integration between brain, body, and environment, as for managing internal cognitive models (Land 2014; Bruner and Iriki 2015; Peer et al. 2015). Current perspectives in cognitive sciences suggest that the integration between body and environment is also crucial to coordinate spatial and chronological processes with social perception (Hills et al. 2015; Maister et al 2015). It is likely that some of these capacities underwent selection in recent human evolution.
A final note concerns metabolism. Despite its high glucose metabolic rate, the precuneus is thought to have high energy efficiency (i.e., high degree of connectivity relative to its metabolic rate; Tomasi et al. 2013). Enlarging a precuneus hub could therefore minimize total energy requirements associated with increases in cortical connectivity. The expanded parietal region includes cortex that is highly metabolically active (Cavanna and Trimble 2006; Sotero and Iturria-Medina 2011). These same areas are associated, only in modern humans and not in other extinct hominids, with expanded vascular networks (Bruner et al. 2011; Rangel de Lázaro et al. 2015), and are close to the thermal core of the brain (Bruner et al., 2012). All these changes may therefore suggest a relationship between morphological complexity, encephalization, vascular adaptations, and thermoregulation (Bruner et al. 2014b). Beyond this difference in medial structures, chimpanzees and humans also differ in their relative temporal lobe volume (Rilling and Seligman 2002). Interestingly, Alzheimer’s Disease, which is particularly prevalent in our species, is associated with early metabolic impairments of the precuneus and later damage to the temporal lobe (Bruner and Jacobs 2013). These areas, both especially developed in modern humans, are connected through the default-mode network, suggesting probable evolutionary factors influencing sensitivity to neurodegenerative processes.
Conclusions
A longitudinal parietal expansion is a key feature of modern human evolution, a major source of intra-specific variation in living adults, and a main difference between humans and chimpanzees. In the last two cases, brain imaging has revealed that the size of the precuneus is the factor involved in such morphological variation. These differences in medial brain morphology between humans and chimpanzees therefore have important implications for both comparative primate neurobiology and paleoanthropology. In terms of living species, they demonstrate a different anatomical organization of the medial parietal cortex in humans and apes. In terms of human evolution, they further point to a role for the precuneus in explaining brain differences between modern humans and extinct human species. Fossils associated with the modern human lineage dating back to 150–200 thousand years ago do not show a modern-like parietal expansion (Bruner and Pearson, 2013). This suggests that expansion of the medial parietal cortex in the modern human lineage may have occurred after the origin of our species.
Integrating evidence from paleoneurology and comparative neuroanatomy, we hypothesize a conspicuous enlargement of the precuneus associated with our recent evolutionary history, which may be associated with specific human cognitive specializations. Further research is needed to investigate the changes in tissue organization and connectivity that underlie the observed macroscopic change, and their possible functional significance. In humans, brain morphology shows greater phenotypic plasticity than in chimpanzees (Gómez-Robles et al., 2015), and the medial parietal lobe is influenced by both genetic (Chen et al., 2012) and environmental (Iriki and Taoka, 2012) factors, including culture. The contribution of these two components to the overall morphological differences among individuals and among species remains to be evaluated.
Supplementary Material
Acknowledgments
This work was supported by National Institutes of Health Grants P01AG026423 and National Center for Research Resources P51RR165 (superceded by the Office of Research Infrastructure Programs/OD P51OD11132), by the John Templeton Foundation (award 40463), and by the Center for Behavioral Neuroscience. EB is funded by the Spanish Government (CGL2012-38434-C03-02/03) and by the Italian Institute of Anthropology. We are grateful to an anonymous reviewer for comments and suggestions on an earlier version of this article.
References
- Allen JS, Damasio H, Grabowski TJ. Normal neuroanatomical variation in the human brain: an MRI-Volumetric Study. Am J Phys Anthropol. 2002;118:341–358. doi: 10.1002/ajpa.10092. [DOI] [PubMed] [Google Scholar]
- Barks SK, Parr LA, Rilling JL. The default mode network in chimpanzees (Pan troglodytes) is similar to that of humans. Cereb Cortex. 2015;25:538–544. doi: 10.1093/cercor/bht253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bookstein F. Landmark methods for forms without landmarks: morphometrics of group differences in outline shape. Med Image Anal. 1997;1:225–243. doi: 10.1016/s1361-8415(97)85012-8. [DOI] [PubMed] [Google Scholar]
- Bookstein FL, Gunz P, Mitteroecker P, Prossinger H, Schaefer K, Seidler H. Cranial integration in Homo: singular warps analysis of the midsagittal plane in ontogeny and evolution. J Hum Evol. 2003;44:167–87. doi: 10.1016/s0047-2484(02)00201-4. [DOI] [PubMed] [Google Scholar]
- Bookstein FL. Morphometric tools for landmark data. Cambridge University Press; Cambridge: 1991. [Google Scholar]
- Bruner E. Geometric morphometrics and paleoneurology: brain shape evolution in the genus Homo. J Hum Evol. 2004;47:279–303. doi: 10.1016/j.jhevol.2004.03.009. [DOI] [PubMed] [Google Scholar]
- Bruner E, Jacobs HIL. Alzheimer’s Disease: the downside of a highly evolved parietal lobe? J Alz Dis. 2013;35:227–240. doi: 10.3233/JAD-122299. [DOI] [PubMed] [Google Scholar]
- Bruner E, Pearson O. Neurocranial evolution in modern humans: the case of Jebel Irhoud 1. Anthropol Sci. 2013;121:31–41. [Google Scholar]
- Bruner E, Iriki A. Extending mind, visuospatial integration, and the evolution of the parietal lobes in the human genus. Quat Int. 2015 doi: 10.1016/j.quaint.2015.05.019. [DOI] [Google Scholar]
- Bruner E, Manzi G, Arsuaga JL. Encephalization and allometric trajectories in the genus Homo: evidence from the Neanderthal and modern lineages. Proc Natl Acad Sci USA. 2003;100:15335–15340. doi: 10.1073/pnas.2536671100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruner E, Martin-Loeches M, Colom R. Human midsagittal brain shape variation: patterns, allometry and integration. J Anat. 2010;216:589–599. doi: 10.1111/j.1469-7580.2010.01221.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruner E, De la Cuétara JM, Holloway R. A bivariate approach to the variation of the parietal curvature in the genus Homo. Anat Rec. 2011a;294:1548–1556. doi: 10.1002/ar.21450. [DOI] [PubMed] [Google Scholar]
- Bruner E, Mantini S, Musso F, de la Cuétara JM, Ripani M, Sherkat S. The evolution of the meningeal vascular system in the human genus: from brain shape to thermoregulation. Am J Hum Biol. 2011b;23:35–43. doi: 10.1002/ajhb.21123. [DOI] [PubMed] [Google Scholar]
- Bruner E, de la Cuétara JM, Masters M, Amano H, Ogihara N. Functional craniology and brain evolution: from paleontology to biomedicine. Front Neuroanat. 2014a;8:19. doi: 10.3389/fnana.2014.00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruner E, Rangel de Lázaro G, de la Cuétara JM, Martín-Loeches M, Colom R, Jacobs HIL. Midsagittal brain variation and MRI shape analysis of the precuneus in adult individuals. J Anat. 2014b;224:367–376. doi: 10.1111/joa.12155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruner E, Amano H, de la Cuétara JM, Ogihara N. The brain and the braincase: a spatial analysis on the midsagittal profile in adult humans. J Anat. 2015a;227:268–276. doi: 10.1111/joa.12355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruner E, Román FJ, de la Cuétara JM, Martin-Loeches M, Colom R. Cortical surface area and cortical thickness in the precuneus of adult humans. Neurosci. 2015b;286:345–352. doi: 10.1016/j.neuroscience.2014.11.063. [DOI] [PubMed] [Google Scholar]
- Caminiti R, Innocenti GM, Battaglia-Mayer A. Organization and evolution of parieto-frontal processing streams in macaque monkeys and humans. Neuosci Biobehav Rev. 2015;56:73–96. doi: 10.1016/j.neubiorev.2015.06.014. [DOI] [PubMed] [Google Scholar]
- Caspers S, Eickhoff SB, Rick T, von Kapri A, Kuhlen T, Huang R, Shah NJ, Zilles K. Probabilistic fiber tract analysis of cytoarchitectonically defined human inferior parietal lobule areas reveals similarities to macaques. Neuroimage. 2011;58:362–380. doi: 10.1016/j.neuroimage.2011.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavanna AE, Trimble MR. The precuneus: a review of its functional anatomy and behavioural correlates. Brain. 2006;129:564–583. doi: 10.1093/brain/awl004. [DOI] [PubMed] [Google Scholar]
- Chen CH, Gutierrez ED, Thompson W, Panizzon MS, Jernigan TL, Eyler LT, Fennema-Notestine C, Jak AJ, Neale MC, Franz CE, Lyons MJ, Grant MD, Fischl B, Seidman LJ, Tsuang MT, Kremen WS, Dale AM. Hierarchical genetic organization of human cortical surface area. Science. 2012;335:1634–1636. doi: 10.1126/science.1215330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gómez-Robles A, Hopkins WD, Sherwood CC. Increased morphological asymmetry, evolvability and plasticity in human brain evolution. Proc R Sci B. 2013;280:20130575. doi: 10.1098/rspb.2013.0575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gómez-Robles A, Hopkins WD, Schapiro SJ, Sherwood CC. Relaxed genetic control of cortical organization in human brains compared with chimpanzees. Proc Natl Acad Sci USA. 2015 doi: 10.1073/pnas.1512646112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunz P, Harvati K. The Neanderthal “chignon”: variation, integration, and homology. J Hum Evol. 2007;52:262–274. doi: 10.1016/j.jhevol.2006.08.010. [DOI] [PubMed] [Google Scholar]
- Gunz P, Neubauer S, Maureille B, Hublin JJ. Brain development after birth differs between Neanderthals and modern humans. Curr Biol. 2010;20:R921–R922. doi: 10.1016/j.cub.2010.10.018. [DOI] [PubMed] [Google Scholar]
- Hagmann P, Cammoun L, Gigandet X, Meuli R, Honey CJ, Wedeen VJ, Sporns O. Mapping the structural core of human cerebral cortex. PLOS Biol. 2008;6:e159. doi: 10.1371/journal.pbio.0060159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammer Ø, Harper DAT, Ryan PD. PAST: paleontological statistics software package for education and data analysis. Palaentol Electron. 2001;4:1–9. [Google Scholar]
- Hills TT, Todd PM, Lazer D, Redish AD, Couzin ID, the Cognitive Search Research Group Exploration versus exploitation in space, mind, and society. Trends Cogn Sci. 2015;19:46–54. doi: 10.1016/j.tics.2014.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iriki A, Taoka M. Triadic (ecological, neural, cognitive) niche construction: a scenario of human brain evolution extrapolating tool use and language from the control of reaching actions. Philos Trans R Soc London B Biol Sci. 2012;367:10–23. doi: 10.1098/rstb.2011.0190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jolliffe IT. Principal Component Analysis. Springer; Berlin: 2002. [Google Scholar]
- Klingenberg CP. MorphoJ: an integrated software package for geometric morphometrics. Mol Ecol Resour. 2011;11:353–357. doi: 10.1111/j.1755-0998.2010.02924.x. [DOI] [PubMed] [Google Scholar]
- Kobayashi Y, Matsui T, Haizuka Y, Ogihara N, Hirai N, Matsumura G. Cerebral sulci and gyri observed on macaque endocasts. In: Akazawa T, Ogihara N, Tanabe HC, Terashima H, editors. Dynamics of Learning in Neanderthals and Modern Humans. Vol. 2. Springer; Japan: 2014. pp. 131–137. [Google Scholar]
- Kojima T, Onoe H, Hikosaka K, Tsutsui K, Tsukada H, Watanabe M. Default mode of brain activity demonstrated by positron emission tomography imaging in awake monkeys: higher rest-related than working memory-related activity in medial cortical areas. J Neurosci. 2009;29:14463–14471. doi: 10.1523/JNEUROSCI.1786-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Land MF. Do we have an internal model of the outside world? Phil Trans R Soc B. 2014;369:20130045. doi: 10.1098/rstb.2013.0045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Hu X, Preuss TM, Glasser MF, Damen FW, Qiu Y, Rilling J. Mapping putative hubs in human, chimpanzee and rhesus macaque connectomes via diffusion tractography. Neuroimage. 2013;80:462–474. doi: 10.1016/j.neuroimage.2013.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieberman DE, McBratney BM, Krovitz G. The evolution and development of cranial form in Homo sapiens. Proc Natl Acad Sci USA. 2002;99:1134–1139. doi: 10.1073/pnas.022440799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maister L, Slater M, Sanchez-Vives MV, Tsakiris M. Changing bodies changes minds: owning another body affects social cognition. Trends Cogn Sci. 2015;19:6–12. doi: 10.1016/j.tics.2014.11.001. [DOI] [PubMed] [Google Scholar]
- Mantini D, Gerits A, Nelissen K, Durand JB, Joly O, Simone L, Sawamura H, Wardak C, Orban GA, Buckner RL, Vanduffel W. Default mode of brain function in monkeys. J Neurosci. 2011;31:12954–12956. doi: 10.1523/JNEUROSCI.2318-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margulies DS, Vincent JL, Kelly C, Lohmann G, Uddin LQ, Biswal BB, Villringer A, Castellanos FX, Milham MP, Petrides M. Precuneus shares intrinsic functional architecture in humans and monkeys. Proc Natl Acad Sci USA. 2009;106:20069–20074. doi: 10.1073/pnas.0905314106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin R, Barbour AD. Aspects of line-fitting in bivariate allometric analyses. Folia Primatol. 1989;53:65–81. doi: 10.1159/000156409. [DOI] [PubMed] [Google Scholar]
- Mars R, Jbabdi S, Sallet J, O’Reilly JX, Croxson PL, Olivier E, Noonan MAP, Bergmann C, Mitchell AS, Baxter MG, Behrens TEJ, Johansen-Berg H, Tomassini V, Miller KL, Rushworth MFS. Diffusion-weighted imaging tractography-based parcellation of the human parietal cortex and comparison with human and macaque resting-state functional connectivity. J Neurosci. 2011;31:4087–4100. doi: 10.1523/JNEUROSCI.5102-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moss ML, Young RW. A functional approach to craniology. Am J Phys Anthropol. 1960;18:281–292. doi: 10.1002/ajpa.1330180406. [DOI] [PubMed] [Google Scholar]
- Neubauer S. Endocasts: possibilities and limitations for the interpretation of human brain evolution. Brain Behav Evol. 2014;84:117–134. doi: 10.1159/000365276. [DOI] [PubMed] [Google Scholar]
- Neubauer S, Gunz P, Hublin JJ. The pattern of endocranial ontogenetic shape changes in humans. J Anat. 2009;215:240–255. doi: 10.1111/j.1469-7580.2009.01106.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neubauer S, Gunz P, Hublin JJ. Endocranial shape changes during growth in chimpanzees and humans: a morphometric analysis of unique and shared aspects. J Hum Evol. 2010;59:555–566. doi: 10.1016/j.jhevol.2010.06.011. [DOI] [PubMed] [Google Scholar]
- Orban GA, Claeys K, Nelissen K, Smans R, Sunaert S, Todd JT, Wardak C, Durand JB, Vanduffel W. Mapping the parietal cortex of human and non-human primates. Neuropsychologia. 2006;44:2647–67. doi: 10.1016/j.neuropsychologia.2005.11.001. [DOI] [PubMed] [Google Scholar]
- Peer M, Salomon R, Goldberg I, Blanke O, Arzy S. Brain system for mental orientation in space, time, and person. Proc Natl Acad Sci USA. 2015;112:11072–11077. doi: 10.1073/pnas.1504242112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rangel de Lázaro G, de la Cuétara JM, Píšová H, Lorenzo C, Bruner E. Diploic vessels and computed tomography: segmentation and comparison in modern humans and fossil hominids. Am J Phys Anthropol. 2015 doi: 10.1002/ajpa.22878. [DOI] [PubMed] [Google Scholar]
- Rilling JK. Human and non-human primate brains: are they allometrically scaled versions of the same design? Evol Anthropol. 2006;15:65–67. [Google Scholar]
- Rilling JK. Comparative primate neuroimaging: insights into human brain evolution. Trends Cogn Sci. 2014;18:45–55. doi: 10.1016/j.tics.2013.09.013. [DOI] [PubMed] [Google Scholar]
- Rilling JK, Barks SK, Parr LA, Preuss TM, Faber TL, Pagnoni G, Bremner JD, Votaw JR. A comparison of resting-state brain activity in humans and chimpanzees. Proc Natl Acad Sci USA. 2007;104:17146–17151. doi: 10.1073/pnas.0705132104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rilling JK, Seligman RA. A quantitative morphometrics comparative analysis of the primate temporal lobe. J Hum Evol. 2002;42:505–533. doi: 10.1006/jhev.2001.0537. [DOI] [PubMed] [Google Scholar]
- Rohlf JF. tpsSplin 1.20. Department of Ecology and Evolution, SUNY, Stony Brook; New York: 2004. [Google Scholar]
- Rohlf JF. tpsDig 2.17. Department of Ecology and Evolution, SUNY, Stony Brook; New York: 2013. [Google Scholar]
- Rohlf JF. tpsRelw 1.54. Department of Ecology and Evolution, SUNY, Stony Brook; New York: 2014. [Google Scholar]
- Scheperjans F, Hermann K, Eickhoff SB, Amunts K, Schleicher A, Zilles K. Observer-independent cytoarchitectonic mapping of the human superior parietal cortex. Cerebral Cortex. 2008a;18:846–867. doi: 10.1093/cercor/bhm116. [DOI] [PubMed] [Google Scholar]
- Scheperjans F, Eickhoff SB, Hömke L, Mohlberg H, Hermann K, Amunts K, Zilles K. Probabilistic maps, morphometry, and variability of cytoarchitectonic areas in the human superior parietal cortex. Cerebral Cortex. 2008b;18:2141–2157. doi: 10.1093/cercor/bhm241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider D, Slaughter VP, Becker SI, Dux PE. Implicit false-belief processing in the human brain. Neuroimage. 2014;101:268–275. doi: 10.1016/j.neuroimage.2014.07.014. [DOI] [PubMed] [Google Scholar]
- Scott N, Neubauer S, Hublin JJ, Gunz P. A shared pattern of postnatal endocranial development in extant hominoids. Evol Biol. 2014;41:572–594. [Google Scholar]
- Sherwood CC, Rilling JK, Holloway RL, Hof PR. Evolution of the brain in humans – specializations in a comparative perspective. In: Binder MD, Hirokawa N, Windhorst U, Hirsch MC, editors. Encyclopedia of Neuroscience. Springer-Verlag; Berlin: 2009. pp. 1334–1338. [Google Scholar]
- Sotero RC, Iturria-Medina Y. From blood oxygenation level dependent (BOLD) signals to brain temperature maps. B Math Biol. 2011;73:2731–2747. doi: 10.1007/s11538-011-9645-5. [DOI] [PubMed] [Google Scholar]
- Strait DS, Grine FE. Inferring hominoid and early hominid phylogeny using craniodental characters: the role of fossil taxa. J Hum Evol. 2004;47:399–452. doi: 10.1016/j.jhevol.2004.08.008. [DOI] [PubMed] [Google Scholar]
- Tomasi D, Wang GJ, Volkow ND. Energetic cost of brain functional connectivity. Proc Natl Acad Sci USA. 2013;110:13642–13647. doi: 10.1073/pnas.1303346110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Utevsky AV, Smith DV, Huettel SA. Precuneus is a functional core of the default-mode network. J Neurosci. 2014;34:932–940. doi: 10.1523/JNEUROSCI.4227-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner GP. On the eigenvalue distribution of genetic and phenotypic dispersion matrices: evidence for a nonrandom organization of quantitative character variation. J Math Biol. 1984;21:77–95. [Google Scholar]
- Wood B. Investigating human evolutionary history. J Anat. 2000;197:3–17. doi: 10.1046/j.1469-7580.2000.19710003.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zelditch ML, Swidersky DL, Sheets HD, Fink WL. Geometric morphometrics for biologists. Elsevier; San Diego: 2004. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


