Abstract
Pancreatic cancers with aberrant expression of macrophage migration inhibitory factor (MIF) are particularly aggressive. To identify key signaling pathways that drive disease aggressiveness in tumors with high MIF expression, we analyzed the expression of coding and non-coding genes in high and low MIF-expressing tumors in multiple cohorts of pancreatic ductal adenocarcinoma (PDAC) patients. The key genes and pathways identified were linked to patient survival and were mechanistically, functionally and clinically characterized using cell lines, a genetically engineered mouse model and PDAC patient cohorts. Here we report evidence of a novel MIF-driven signaling pathway that inhibits the orphan nuclear receptor NR3C2, a previously undescribed tumor suppressor that impacts aggressiveness and survival in PDAC. Mechanistically, MIF upregulated miR-301b which targeted NR3C2 and suppressed its expression. PDAC tumors expressing high levels of MIF displayed elevated levels of miR-301b and reduced levels of NR3C2. Additionally, reduced levels of NR3C2 expression correlated with poorer survival in multiple independent cohorts of PDAC patients. Functional analysis showed that NR3C2 inhibited epithelial-to-mesenchymal transition and enhanced sensitivity to the gemcitabine, a chemotherapeutic drug used in PDAC standard of care. Furthermore, genetic deletion of MIF disrupted a MIF-mir-301b-NR3C2 signaling axis, reducing metastasis and prolonging survival in a genetically engineered mouse model of PDAC. Taken together, our results offer a preclinical proof-of-principle for candidate therapies to target a newly described MIF-miR-301b-NR3C2 signaling axis for PDAC management.
Keywords: MIF, NR3C2, miR-301b, pancreatic cancer, EMT
INTRODUCTION
Pancreatic cancer is a lethal malignancy and ranked as 4th leading cause of death due to cancer (1). Majority of the pancreatic cancer patients are diagnosed at an advanced stage of the disease and are refractory to the available treatments. Therefore, identification of novel therapeutic targets and accelerated assessment of their clinical relevance are desperately needed to improve outcome.
MIF is a proinflammatory cytokine and was initially characterized as a key regulator of innate immune response (2, 3). However, recent investigations have described the presence of a high level of MIF in tumors and provided evidence indicating a role of MIF in human cancer (4, 5). A high level of MIF is found in tumors from PDAC patients (6, 7). MIF-overexpressing tumors are highly aggressive and an elevated MIF in pancreatic tumors predicted poor survival in patients with PDAC indicating its potential biological relevance in disease aggressiveness (7). MIF is produced by a variety of cell type, including immune, inflammatory, and epithelial cells, and can either bind to the cell surface receptor CD74 and CD44 or enter through endocytosis (2, 8, 9). Additionally, tumor cells itself secrete high levels of MIF and thus induce protumorigenic signaling pathway through its autocrine function (10). One of the mechanisms for the enhanced intratumoral expression of MIF in malignant cells is its stabilization by HSP90 chaperone complex by protecting it from degradation (11). Based on the evidence involving MIF in immune regulation and its association with tumor progression, it is hypothesized that MIF is a molecular link between inflammation and cancer (5, 12). However, the exact mechanism is not clear.
Here, we comprehensively investigated the role of MIF in pancreatic cancer progression by applying an integrative analysis of coding and non-coding genes in tumor biology. Based on the analysis of multiple independent cohorts of human PDAC, we identified key characteristics of tumors with aberrantly high MIF expression that link these tumors to enhanced disease aggressiveness and mortality. We also performed functional characterization using in vitro and in vivo models with clinical validation of our findings, which led to the discovery of a novel MIF-driven signaling pathway, involving MIF-miR-301b-NR3C2 axis with potential therapeutic significance in PDAC.
Materials and Methods
Cell Lines, Human Samples and Mouse Model
All the cell lines used in this study were purchased from American Type Culture Collection (ATCC, Rockville, MD) in 2009 and were authenticated by short tandem repeat analysis (STR) analysis in 2015.
Human primary pancreatic tumor tissues from PDAC patients were collected at the University of Maryland Medical System at Baltimore, Maryland and University of Medicine, Gottingen, Germany. The characteristics of the patients in test cohort (N=69) are described in Supplementary Table S1. Patients’ characteristics in validation cohorts (N=41, N=64), and the cohort in The Cancer Genome Atlas (TCGA) (N=69) are shown in Supplementary Table S2, S3 and S4. The tissue microarray was prepared from additional 173 PDAC cases at the Institute of Pathology, University of Heidelberg, Germany (Supplementary Table S5). Use of clinical specimens was reviewed and permitted by the NCI-Office of Human Subject Research Protection (OHSRP, Exempt# 4678) at the National Institutes of Health, Bethesda, MD.
MIF-deficient LSL-KrasG12D;LSL-Trp53R172H/+;Pdx-1-Cre (MKPC) pancreatic cancer mouse model was generated, through cross-breeding of KPC (13) and MIF knockout mice (14) with an approved animal study protocol by Animal Care and Use Committee at NCI, Frederick, MD.
Microarray Expression Profiling
miRNA and mRNA expression profiling was performed at the microarray core facility, NCI, Frederick, MD. The miRNA and mRNA microarray expression data have been deposited in the National Center for Biotechnology Information’s (NCBIs) Gene Expression Omnibus with accession numbers GSE 62498 and GSE 62452. Details are provided in supplementary methods.
Cell proliferation, Migration, and Invasion, Colony formation and Drug Cytotoxicity Assays
These assays were performed using kits from various manufacturers. Details are provided in Supplementary methods.
Statistical Analysis
Student t-test was used to assess the difference in the miRNA and mRNA expression and other end-points including, migration and invasion, colony formation ability, and cell survival among different comparison groups. When more than two groups were compared, a one-way analysis of variance (ANOVA), followed by a Scheffe's multiple comparison test was used. Kaplan-Meier analysis and Log Rank test was performed to compare the difference in survival between two groups of patients using Graphpad Prism 6.0. Pearson's correlation was used for determining any correlation between the expressions of two genes. P<0.05 was considered statistically significant.
RESULTS
miRNA-Profiling of MIF-high and MIF-low PDAC revealed MIF-associated miRNAs with prognostic significance
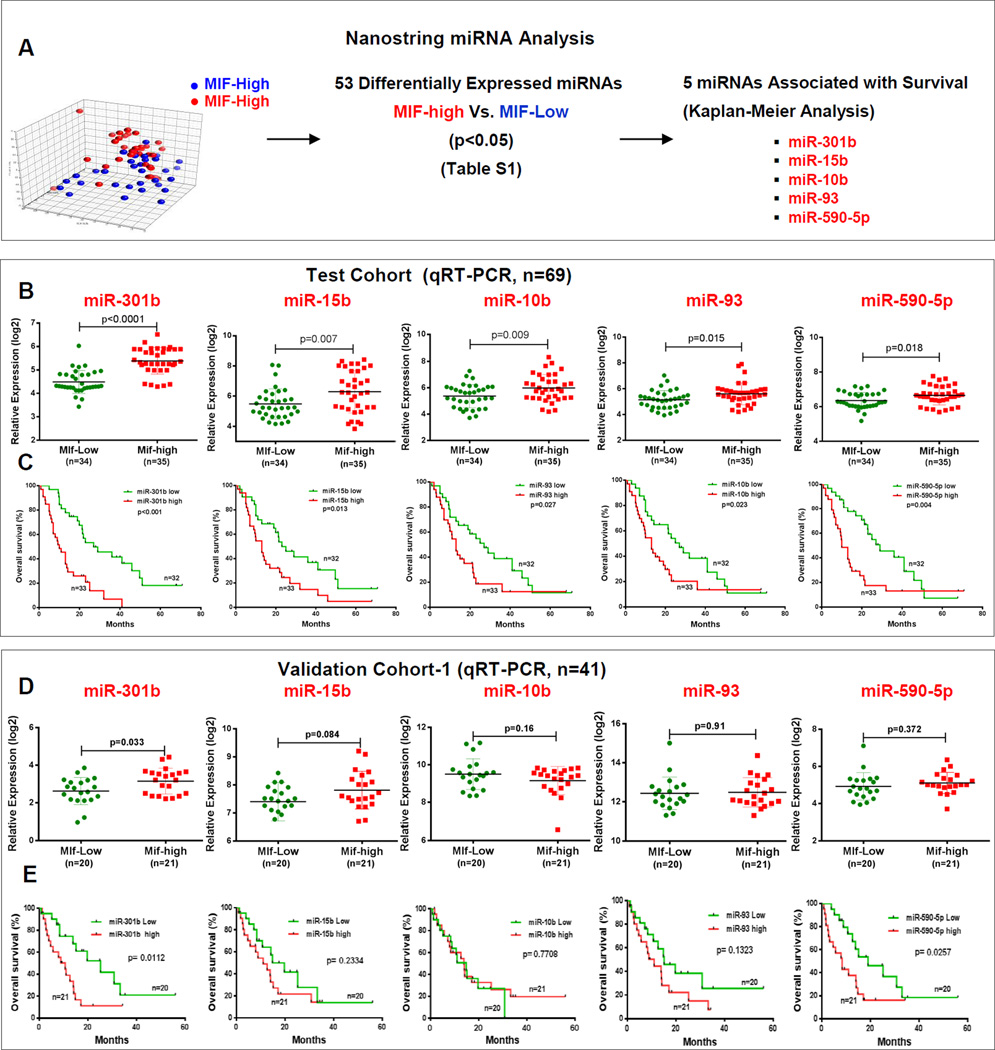
Molecular characterization of MIF-high and MIF-low tumors was performed using miRNA profiling in a test cohort (N=69) of patients with PDAC, using nanostring nCounter miRNA Expression Array, and testing the hypothesis that MIF regulates miRNAs, associated with tumor progression and patient survival. Stratification of PDAC cases into MIF-high and MIF-low groups was based on the MIF expression above the median (high) and below the median (low) values as determined by qRT-PCR. Unsupervised principal component analysis (PCA) of miRNA expression profile revealed distinct groupings for MIF-high and MIF-low tumors indicating a systematic change in global miRNA expression profile by MIF in PDAC (Fig. 1A). Further analysis identified 53 differentially expressed miRNAs in MIF-high vs. MIF-low tumors and a higher expression of five of these miRNAs, miR-301b, miR-15b, miR-10b, miR-93 and miR-590-5p, in MIF-high tumors were also associated with poor survival in PDAC cases (Fig. 1A; Supplementary Fig. S1A and S1B; Supplementary Table S6). Differentially expressed miRNAs in MIF-high and MIF-low tumors also included miR-200b, which is consistent with the earlier report describing its inhibition by MIF. However, the expression of miR-200b does not show any association with patient survival. miRNAs, found to be both differentially expressed in MIF-high versus MIF-low tumors, as well as associated with patient survival, were then validated by using qRT-PCR. Expression of the above mentioned 5 miRNAs (miR-301b, miR-15b, miR-10b, miR-93 and miR-590-5p), as determined by qRT-PCR, and their prediction of prognosis was consistent with the findings from array analysis (Fig. 1B and C). Furthermore, the miRNA expression obtained by miR-array and qRT-PCR were highly correlated (Supplementary Fig. S1C). To ensure that these findings are not restricted to just one group of PDAC used as test cohort, we further validated these results in an independent validation cohort-1 (n=41, Supplementary Table S2) of PDAC. The cases were grouped as MIF-high and MIF-low based on the MIF expression in tumors and dichotomized based on the median value. Expression of miR-301b was elevated in MIF-high tumors as compared to MIF low tumors and a higher miR-301b and miR-590-5p was also associated with poor survival, which is consistent with the findings in the test cohort (Fig. 1D and E). However, the expression of miR-15b, miR-10b, miR-93 and miR-590-5p did not show significant difference between MIF-high and MIF-low groups in the validation cohort. Therefore, we continued our investigation to examine the association between MIF and miR-301b. Examining a panel of eight pancreatic cancer cell lines revealed a positive correlation between MIF and miR-301b expression (r=0.67, P=0.014) (Supplementary Fig. S2A). To assess a potential MIF-mediated regulation of miR-301b, MIF expression was knocked down using lenti-viral mediated small hairpin (shRNA) in pancreatic cancer cell lines, which resulted in a significant reduction in miR-301b (Supplementary Fig. S2B and S2C). These findings were further confirmed by a different approach involving the lenti-viral mediated stable overexpression of MIF, which resulted in a significant increase in miR-301b expression in these pancreatic cancer cells (Supplementary Fig. S2D and S2E). These findings confirmed that MIF positively regulates miR-301b in pancreatic cancer.
Figure 1. miRNA-profiling of MIF-high and MIF-low tumors identified MIF-associated miRNAs with prognostic significance.
(A) Unsupervised principal components analysis, differentially expressed miRNAs and their association with survival in MIF-high and MIF-low tumors. (B–E) miRNA expression as determined by qRT-PCR, and Kaplan-Meier survival analysis in test and validation cohorts.
MIF-induced miR-301b targets nuclear receptor subfamily 3, group C, member 2 (NR3C2)
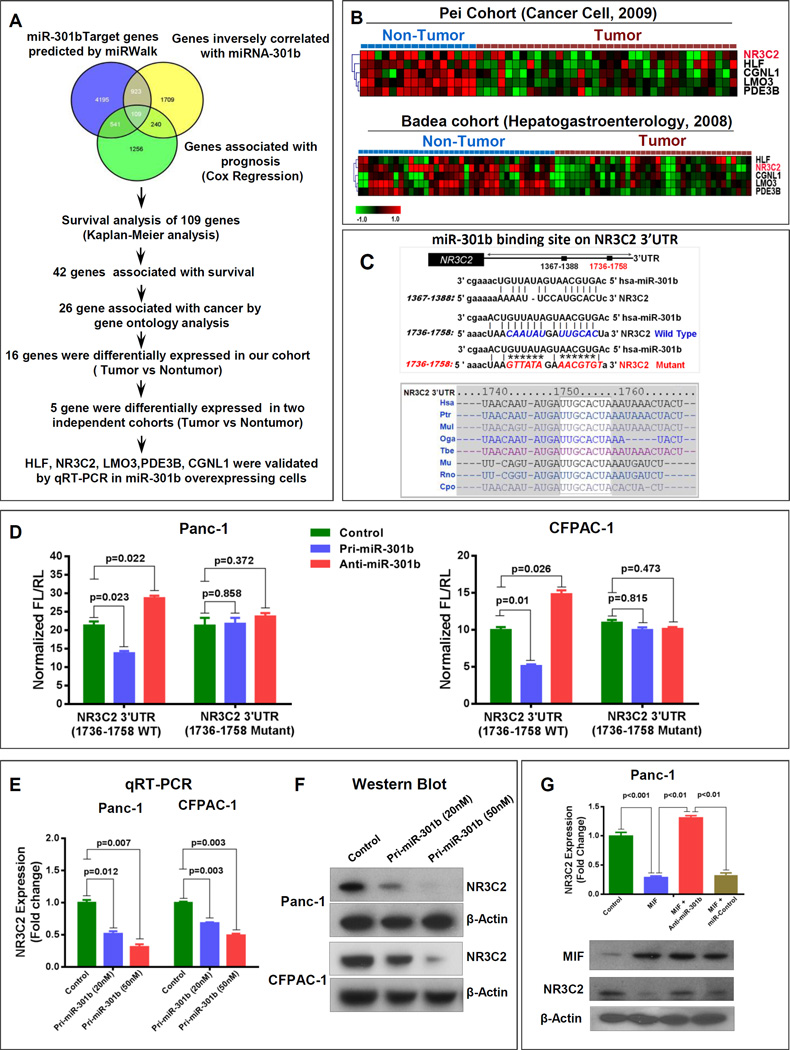
As a next step in our effort to examine MIF-induced signaling, involved in the tumor progression and disease aggressiveness in PDAC, we performed a comprehensive analysis to identify potential targets of MIF-induced miR-301b. By using an integrative strategy, which combines miRWalk-target prediction module (15) with global gene expression profile and Cox-regression analysis in the same cohort of PDAC cases (Fig. 2A). Putative miR-301b target genes predicted by miRWalk were compared with genes that showed inverse correlation with miR-301b through transcriptomic profiling, and genes that were associated with survival by cox regression analysis in our PDAC cohort. This approach identified 109 potential miR-301b target genes (Supplementary Table S7). These potential target genes were further subjected to Kaplan-Meier analysis, gene ontology analysis (Supplementary Fig. S3A), assessment of their differential expressions in tumors as compared with nontumor pancreas and validation in two separate cohorts (16, 17) in Oncomine gene expression database. This highly stringent approach identified NR3C2, HLF, LMO3, PDE3B and CGNL1 as the most predicted target of MIF-induced miR-301b in pancreatic cancer (Fig. 2A and B). We then validated these putative targets by overexpressing miR-301b in Panc-1 and Capan-2 pancreatic cancer cell lines. Overexpression of miR-301b significantly reduced NR3C2 expression (Supplementary Fig. S3B). Furthermore, examining 3’UTR of NR3C2 revealed two miR-301b binding sites in the sequence spanning 1367-1388 and 1736-1758 nucleotides with the latter found to be highly conserved in different mammalian species (Fig. 2C). Luciferase reporter-based activity assay showed the interaction of miR-301b to 3’UTR sequence 1736-1758 of NR3C2 resulting in a decrease of reporter activity following the expression of miR-301b. Furthermore, co-transfection with anti-miR-301b enhanced the NR3C2 luciferase reporter activity. However, these effects were abolished by the mutation of miR-301b binding site (Fig. 2D and Supplementary Fig. S3C–S3E). Consistently, the overexpression of pri-miR-301b suppressed both mRNA and protein expression of NR3C2 in a dose dependent manner confirming miR-301b-mediated suppression of NR3C2 (Fig. 2E and F). We then examined, if MIF can down regulate NR3C2 and if this inhibition is dependent on miR-301b. As shown in Fig. 2G, MIF overexpression significantly reduced NR3C2 mRNA and protein levels. However, the MIF-induced suppression of NR3C2 was abolished by the inhibition of miR-301b. Furthermore, endogenous NR3C2 expression was negatively correlated with MIF and miR-301b in a panel of human pancreatic cancer cell lines (Supplementary Fig. S4A and S4B). Taken together these findings show that MIF-induced increase in miR-301b targets and reduces NR3C2 level.
Figure 2. MIF-induced miR-301b targets NR3C2.
(A) Schema depicting integrative approach to identify putative miR-301b target genes. (B) A lower expression of predicted targets of miR-301b in tumors in the publicly available datasets (14, 15). (C) Two miR-301b target sites reside at nucleotides spanning 1367-1388 and 1736-1758 of NR3C2 3’UTR, and 1736-1758 region was highly conserved in different species. (D) Lucifersae reporter assay showing the targeting of 3’UTR sequence of NR3C2 by miR-301b. (E, F) A dose-dependent decrease in endogenous NR3C2 expression by miR-301b in pancreatic cancer cell lines. (G) MIF suppressed NR3C2 expression, which is rescued by co-expression of anti-miR-301b in pancreatic cancer cells.
NR3C2 inhibits proliferation, colony formation, migration, invasion, and enhances sensitivity of pancreatic cancer cells to gemcitabine
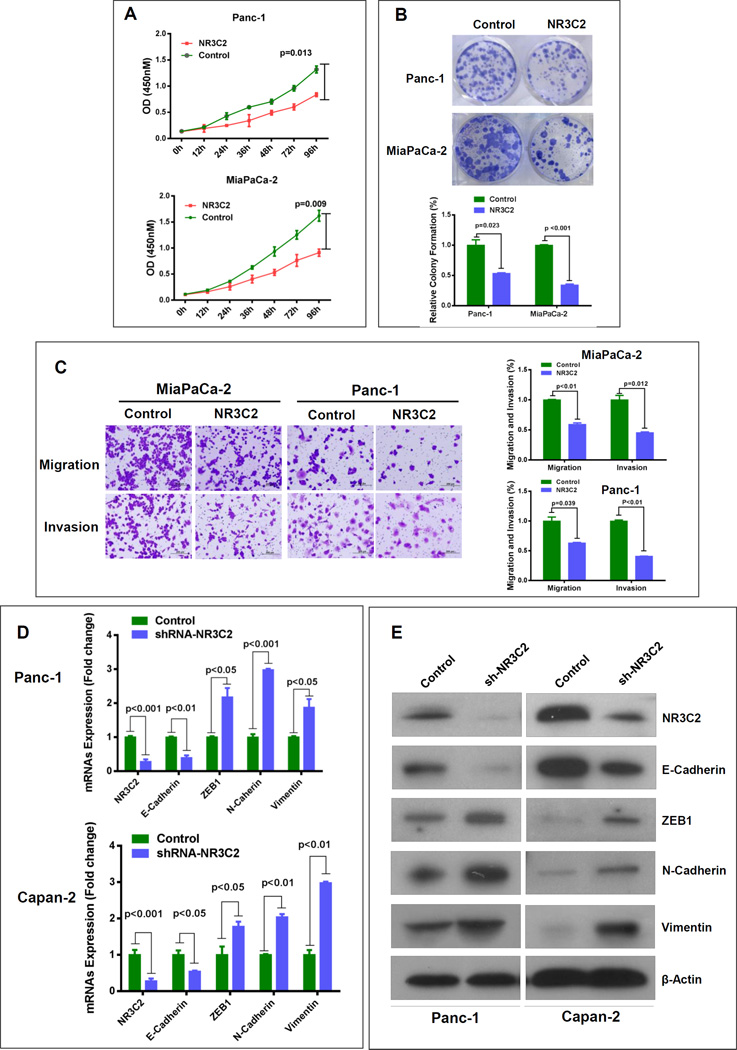
NR3C2 gene encodes a mineralocorticoid receptor and functions as a ligand dependent transcription factor mediating aldosterone action on salt and water balance. Interestingly, a decrease in NR3C2 expression is associated with poor prognosis in lung and colorectal cancer (18, 19). However, the role of NR3C2 has not been described in pancreatic cancer. We investigated the function of NR3C2 in pancreatic cancer by first screening its expression in a panel of pancreatic cancer cell lines. We then generated lentiviral-mediated NR3C2-overexpressing stable pancreatic cancer cell lines, Panc-1 and MiaPaCa-2, which had the lowest endogenous NR3C2 expression (Supplementary Fig. S5A). NR3C2-overexpression resulted in a significant decrease in the proliferation index of these cells (Fig. 3A). The effect of NR3C2 on the growth characteristics was measured by clonogenicity assay, which showed 50-75% reduction in the colony formation (Fig. 3B). We further investigated the potential role of NR3C2 in the mobility of tumor cells, which is essential for invasion and metastasis. NR3C2 overexpression significantly reduced the migration and invasion ability of pancreatic cancer cells (Fig. 3C). Gemcitabine has been used as a first-line standard chemotherapeutic drug against PDAC for more than a decade and recently is being used in combination with other compounds with some success. Therefore, it was examined, if an enhanced level of NR3C2 improves the sensitivity of pancreatic cancer cells to gemcitabine. NR3C2 overexpressing cells showed a significant reduction in survival as compared to control cells following gemcitabine treatment (Supplementary Fig. S5B). These findings were further supported by clonogenicity assay, which showed significantly lower colony forming ability of NR3C2-overexpressing pancreatic cancer cells as compared to control cells following gemcitabine treatment (Supplementary Fig. S5C). On the contrary, stable knockdown of NR3C2 in Capan-2 cells significantly enhanced proliferation and invasiveness, and reduced sensitivity to gemcitabine (Supplementary Fig. S6A–S6D). Taken together these findings support a potential tumor suppressive role of NR3C2 in pancreatic cancer.
Figure 3. NR3C2 inhibits proliferation, colony formation, invasiveness and epithelial-to-mesenchymal transition.
(A) NR3C2 overexpression suppresses the proliferation of Panc-1 and MiaPaCa-2 cells. (B) NR3C2-overexpressing cells showed reduced colony formation as compared with control cells. (C) NR3C2 significantly attenuated cell motility and invasiveness in Panc-1 and MiaPaCa-2 cell lines. (D, E) NR3C2-knockdown resulted in a marked decrease in E-cadherin and increase in zeb-1, N-cadherin and vimentin.
NR3C2 inhibits epithelial-to-mesenchymal transition (EMT)
Acquiring features of epithelial-to-mesenchymal transition (EMT) is a critical component in the ability of cancer cells to metastasize. Inflammatory mediators enhance metastatic ability of pancreatic tumors and pre-neoplastic cells (20) and MIF-overexpressing pancreatic tumors are highly metastatic (7). To further examine the role of NR3C2 in pancreatic cancer, we hypothesized that NR3C2 inhibits EMT. A lower endogenous NR3C2 expression was associated with EMT phenotype (Supplementary Fig. S7A and S7B). We further showed that NR3C2 inhibition by lentiviral-mediated shRNA (Supplementary Fig. S8A) resulted in the acquisition of mesenchymal features in pancreatic cancer cells. NR3C2 inhibition led to the reduction in E-cadherin and increase in zeb1, N-cadherin and vimentin mRNA and protein expression in pancreatic cancer cell lines (Fig. 3D and E; Supplementary Fig. S8B–S8E). These findings show that NR3C2 inhibits EMT in pancreatic cancer cells and support its potential tumor suppressive role in pancreatic cancer.
MIF enhances tumor cell invasiveness by targeting NR3C2 through the upregulation of miR-301b
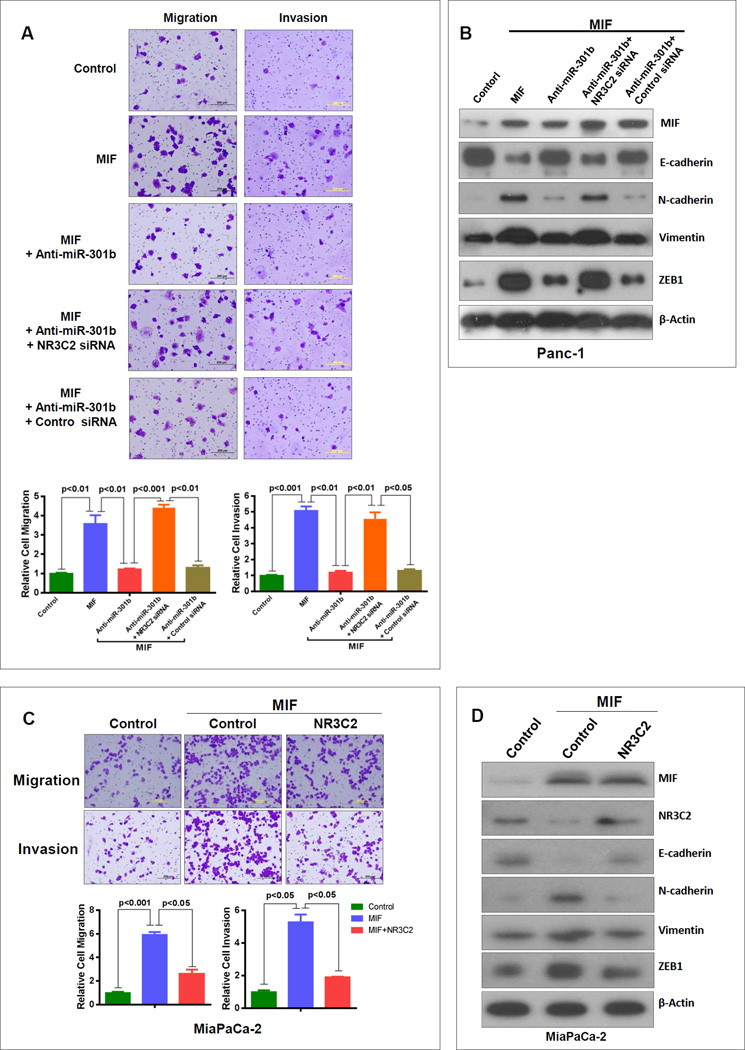
MIF-induced signaling leading to enhanced tumor cell aggressiveness is not clearly understood. An enhanced migration and invasion in MIF-overexpressing stable pancreatic cancer cells were attenuated, when miR-301b was inhibited (Fig. 4A, Supplementary Fig. S9). However, this inhibition was abrogated by siRNA-mediated depletion of NR3C2 (Fig. 4A). These results showed that MIF-induced migration and invasion is mediated by targeting NR3C2 through an increase in miR-301b. We then examined, if the MIF-induced suppression of NR3C2 and the resulting enhancement of migration and invasion in pancreatic cancer cells is achieved through EMT. Pancreatic cancer cells with stable overexpression of MIF showed a decrease in E-cadherin and increase in N-cadherin, vimentin and zeb1 expression (Fig. 4B). However, inhibition of miR-301b in these cells enhanced E-cadherin and reduced the expression of N-cadherin, vimentin and zeb1. We then examined, if this reversion in the expression of EMT marker proteins following miR-301b inhibition is due to an increase in NR3C2. Suppressing both miR-301b and NR3C2 resulted in a decrease in E-cadherin and increase in N-cadherin, vimentin and zeb1 expression. These results showed that inhibition of NR3C2 by MIF through upregulation of miR-301b mediated the MIF-induced invasiveness and EMT (Fig. 4B). We then validated these findings using a complimentary approach of overexpressing NR3C2 in pancreatic cancer cells with stable high expression of MIF. NR3C2-overexpression led to a decrease in MIF-induced migration and invasion (Fig. 4C). Furthermore, NR3C2 overexpression resulted in an increase in E-cadherin and decrease in N-cadherin, vimentin and zeb1 (Fig. 4D). Taken together these findings showed that MIF-induced tumor cell invasiveness and EMT involves the upregulation of miR-301b and subsequent targeting and down regulation of NR3C2.
Figure 4. MIF enhances tumor cell invasiveness by targeting NR3C2 through the upregulation of miR-301b.
(A) MIF-induced increase in tumor cell migration and invasion is suppressed by the inhibition of miR-301b. However, the suppression of migration and invasion following miR-301b inhibition were abrogated by knocking-down NR3C2. (B) MIF overexpression decreases E-cadherin and increases N-cadherin, vimentin and zeb1 protein levels in pancreatic cancer cells. Whereas, miR-301b inhibition in the MIF overexpressing cells is associated with an increase in E-cadherin, and a decrease in N-cadherin, vimentin and zeb1, these effects were abolished when NR3C2 was inhibited as well. (C) MIF-induced increase in tumor cell invasiveness is suppressed by overexpressing NR3C2. (D) The effect of MIF on the expression of EMT-associated markers was attenuated by NR3C2.
MIF-mediated regulation of miR-301b and NR3C2 involves PI3K/Akt pathway
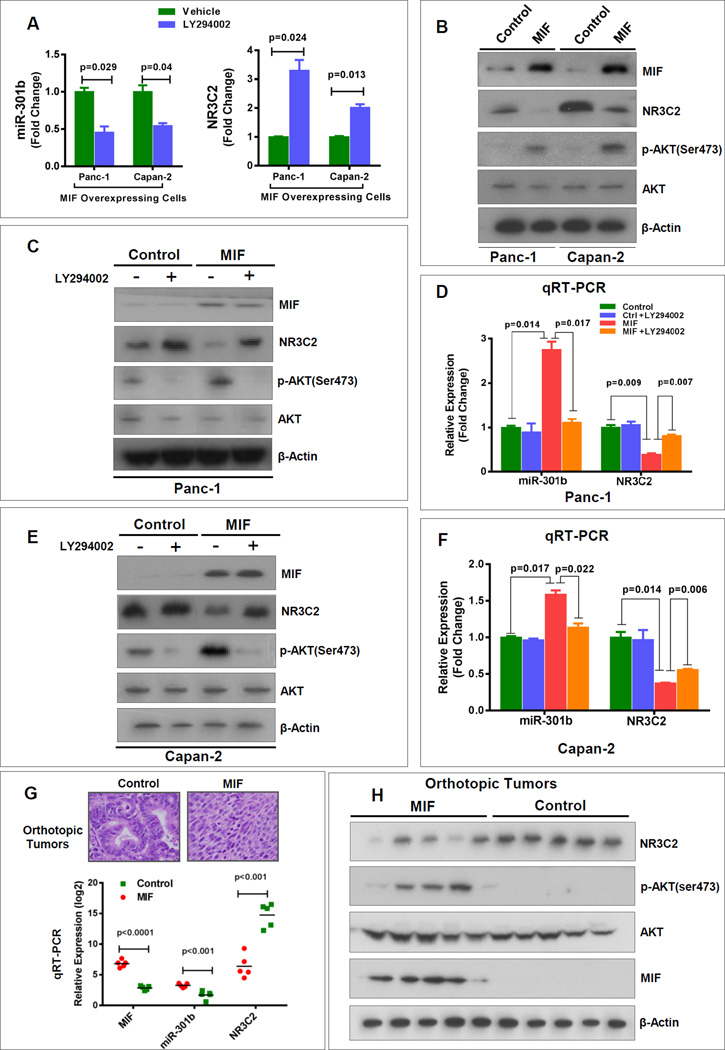
MIF activates PI3K/Akt and MEK/MAP kinase signaling pathways, which plays a role in tumorigenesis (10, 21, 22). We investigated if the MIF-induced upregulation of miR-301b and subsequent targeting and down regulation of NR3C2 involves the activation of PI3K/Akt and MEK/MAPK signaling. Treatment of MIF-overexpressing pancreatic cancer cells with PI3K/Akt inhibitor LY294002 resulted in the significant decline in miR-301b and enhancement of NR3C2 expression (Fig. 5A). However, treatment with AZD6244, an inhibitor of MEK, did not have any effect on miR-301b or NR3C2 expression levels (Supplementary Fig. S10). Consistently, MIF-overexpression showed an increase in the level of phosphorylated AKT (p-AKTser473) and a decrease in NR3C2 protein level determined by western blot analysis (Fig. 5B). We then confirmed if the treatment with LY294002 inhibits phosphorylation of AKT, and affect the expression of miR-301b and NR3C2. LY294002-treatment of MIF-overexpressing cells markedly reduced p-AKT ser473, which resulted in a decline in miR-301b expression and increase in mRNA and protein level of NR3C2 (Fig. 5C–F). These in vitro findings show that MIF-induced upregulation of miR-301b and subsequent targeting and down regulation of NR3C2 is accomplished by activation of PI3K/Akt signaling. To validate these findings in vivo, we examined the orthotopic pancreatic tumors that were earlier generated from MIF-overexpressing or control human pancreatic cancer cells (7). Examination of MIF-overexpressing orthotopic tumors showed an elevated miR-301b and a marked decline in NR3C2 expressions as compared with control (Fig. 5G and H). Furthermore, an elevated expression of p-AKTser473 in MIF-overexpressing tumors confirmed the activation of PI3K/Akt signaling pathway in these tumors (Fig. 5H).
Figure 5. MIF-mediated regulation of miR-301b and NR3C2 involves AKT pathway.
(A) Treatment of MIF-expressing stable Panc-1 and Capan-2 cells with Akt inhibitor, LY294002, inhibited miR-301b and enhanced the expression of NR3C2. Cells were treated with LY294002 for 48 hrs. (B) Stable MIF-overexpressing cells expressed a higher level of phosphorylated Akt (p-AktSer473) and a lower level of NR3C2. (C–F) Inhibition of Akt by LY294002 increased NR3C2 and decreased miR-301b level in MIF-overexpressing cells as compared with control cells. (G) MIF-expressing orthotopic tumors showed an increase in miR-301b and decrease in NR3C2 expression as compared with control tumors. (H) MIF activated AKT pathway and decreased NR3C2 level in MIF-expressing orthotopic tumors. MIF-expressing orthotopic tumors showed an enhanced level of p-Akt Ser473 and reduced level of NR3C2 as compared with control tumors.
Genetic-ablation of MIF disrupted MIF/miR-301b/NR3C2 axis, enhanced survival and reduced metastasis in KPC mouse model of pancreatic cancer
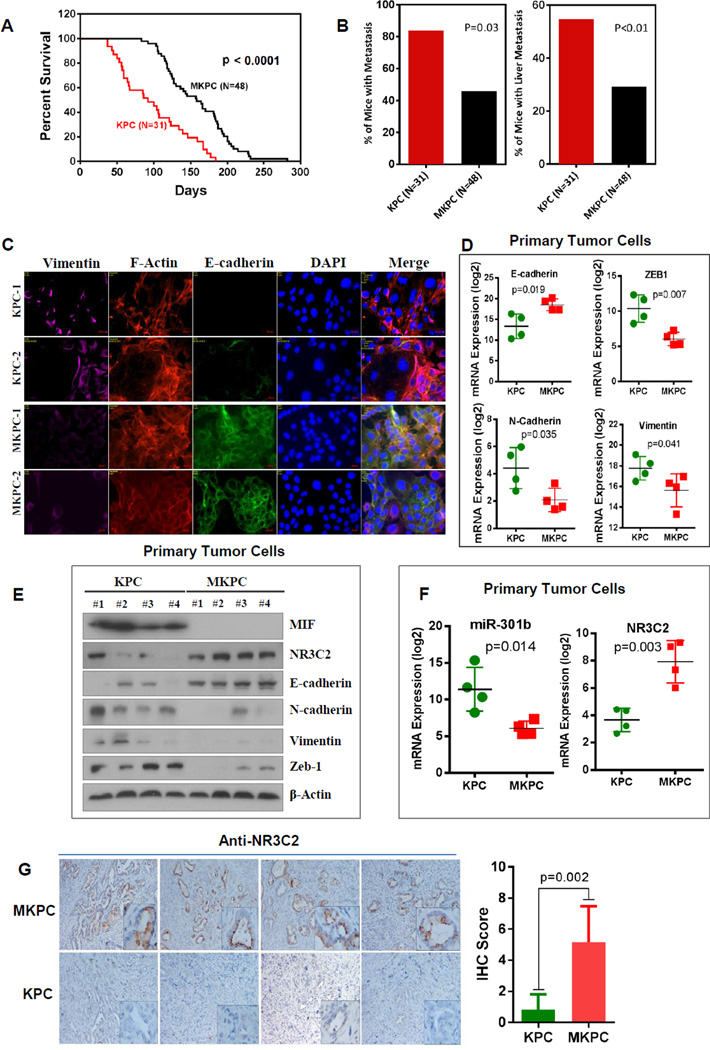
To examine, if MIF-signaling is a potential therapeutic target in PDAC, we used a genetic strategy to target MIF in KPC mice, a genetically engineered mouse model of PDAC. Kaplan-Meier analysis showed that MIF-deficient KPC (MKPC) mice survived significantly longer than that of KPC mice with wild-type MIF (P<0.0001) (Fig. 6A). Additionally, MKPC mice showed a significant reduction in metastatic burden (P<0.01) (Fig. 6B). A majority of these metastasis occurred in liver of both KPC and MKPC mice. Furthermore, primary tumor cells isolated from MKPC mice showed an enhanced expression of E-cadherin and reduced expression of vimentin, zeb1 and N-cadherin at both the protein and mRNA levels, as compared with primary tumor cells from KPC mice suggesting an attenuation of EMT in MKPC tumors (Fig. 6C–E). Next, we examined if MIF-deficiency affected MIF-miR-301b-NR3C2 signaling pathway in this pre-clinical model of PDAC. Primary tumor cells from MKPC mice showed a reduced expression of miR-301b and an enhanced expression of NR3C2 as compared to KPC mice (Fig. 6E and F). Additionally, immunohistochemical analysis of pancreatic tumors showed a significantly higher expression of NR3C2 in MKPC mice as compared to KPC mice (Fig. 6G). Taken together, these findings provide in vivo evidence that targeting MIF-miR-301b-NR3C2 signaling pathway reduce metastasis and enhance survival in KPC mouse model of pancreatic cancer.
Figure 6. MIF-deficiency increased NR3C2, enhanced survival and reduced metastasis in KPC mouse model of pancreatic cancer.
(A) Kaplan-Meier analysis showing a significantly longer survival of MIF-deficient KPC mice (MKPC, N=48) as compared with KPC mice (N=31) (P<0.0001, Log-Rank test). (B) A significantly lower percentage of MKPC mice showed metastasis as compared with KPC mice. (C) Immunoflorescence assay showing an enhanced expression of E-cadherin and reduced expression of vimentin in the primary tumor cells isolated from MKPC mice as compared with the KPC mice. (D, E) MIF deficiency resulted in a marked increase in E-cadherin and decrease in zeb-1, N-cadherin and vimentin at the mRNA and protein level in primary tumor cells. The reduction in the level of EMT markers was associated with an increase in NR3C2 expression in primary tumor cells from MKPC mice as compared to KPC mice. (F) MIF-deficiency resulted in an increased NR3C2 and a decrease in miR-301b expression in primary tumor cells from MKPC mice as compared to KPC mice. (G) Immunohistochemical staining of tumors, showing a higher NR3C2 expression in MKPC as compared with KPC mice (Students t-test, p=0.002).
Clinical validation of MIF/miR-301b/NR3C2 axis in human pancreatic cancer
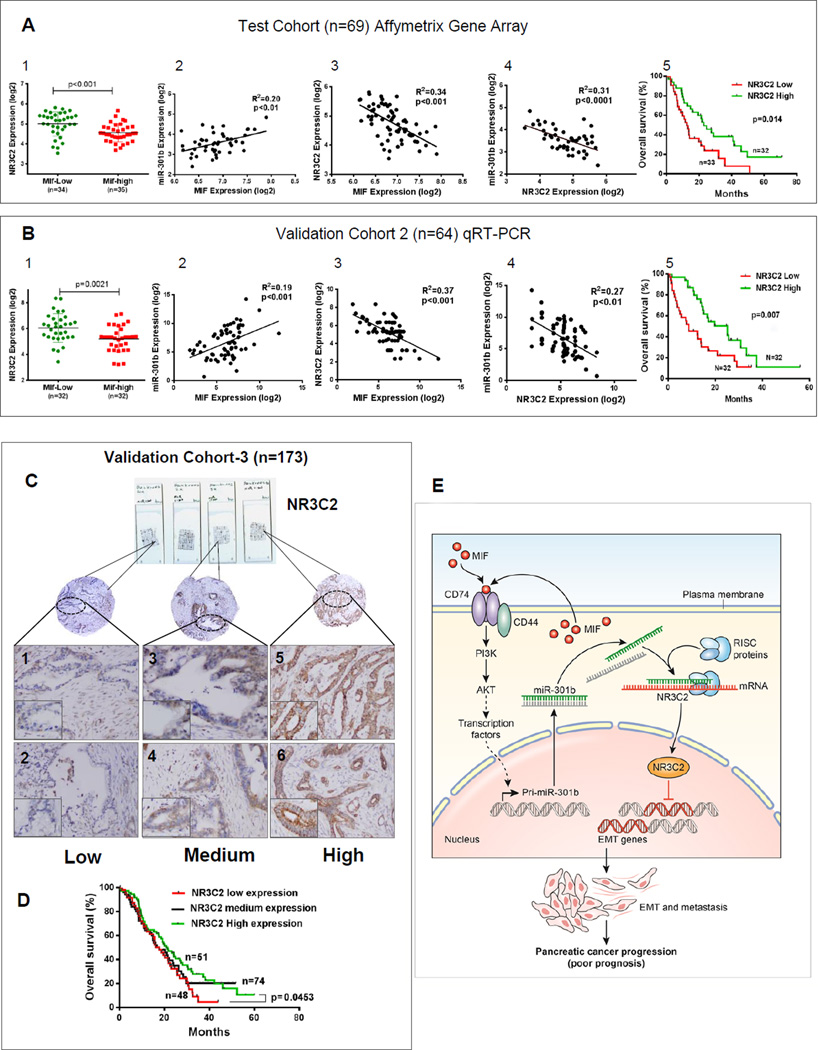
Next, we examined the relevance of MIF/miR-301b/NR3C2 axis in the disease aggressiveness in human pancreatic cancer using multiple independent cohorts of PDAC. MIF-high tumors showed a reduced expression of NR3C2 in test cohort (N=69) (Fig. 7A, Panel 1). Furthermore, MIF expression in the tumors was positively correlated with miR-301b and negatively correlated with NR3C2 expression (Fig. 7A, Panel 2-3). Additionally, a negative correlation was found between miR-301b and NR3C2 expression in these tumors (Fig. 7A, Panel 4). Moreover, a lower expression of NR3C2 in tumors was associated with poorer survival of patients in this cohort (Fig. 7, Panel 5). We then validated these findings in our validation cohort-2 (n=64) (Supplementary Table S3). Adding to our confidence, all the findings in validation cohort were consistent with the test cohort (Fig. 7B). Furthermore, a reduced NR3C2 expression was found in tumors as compared to adjacent nontumor pancreas in both of these cohorts (Supplementary Fig. S11A and 11B). Next, immunohistochemical analysis of tissue microarray (TMA) in a second independent validation cohort-3 of PDAC (n=173) further validated that a lower expression of NR3C2 is associated with poorer patient survival (Fig. 7C and D; Supplementary Table S5). Additionally, a third validation could be achieved by examining the gene expression profile of PDAC patient cohorts in “The Cancer Genome Atlas” (TCGA) (www.cancergenome.nih.gov). Tumors from non-metastatic PDAC cases showed an elevated level of NR3C2 as compared with metastatic cases in TCGA cohort (Supplementary Fig. 12A). Furthermore, a lower expression of NR3C2 in tumors correlated with poorer survival of patients in this cohort as well (Supplementary Fig. 12B). Finally, a lower expression of NR3C2 was validated in metastatic tumors as compared with primary tumors in a recently published large gene expression dataset (GSE71729, Supplementary Fig. S12C) (23). A lower expression of NR3C2 in these tumors also predicted poorer survival (Supplementary Fig. S12D). Taken together these findings and their multiple validations demonstrate the clinical relevance of MIF/miR-301b/NR3C2 signaling pathway in disease aggressiveness and survival in patients with PDAC.
Figure 7. Clinical validation of MIF/miR-301b/NR3C2 axis in human pancreatic cancer.
(A) MIF-high tumors expressed a higher level of NR3C2 as compared with MIF-low tumors in PDAC cases (N=69). Dot plots represent the normalized log 2 transformed NR3C2 expression values obtained by Affymetrix gene expression microarray analysis (Panel 1); Pearson correlation analysis between MIF, miR-301b and NR3C2 expression level in the same cohort (Panel 2–4); Kaplan-Meier analysis showing poor survival in patients with lower NR3C2 expression in tumors (Panel 5). (B) Validation of MIF/miR-301b/NR3C2 axis in an independent cohort of PDAC cases (N=64). (C) Tissue-microarray-based immunohistochemical analysis of NR3C2 in an independent validation cohort-3 (N=173). (D) Kaplan–Meier analysis showing different survival in groups of PDAC patients with low and high NR3C2 expression levels in tumors. (E) Schematic representation of the regulation of NR3C2 by MIF in pancreatic cancer progression and disease aggressiveness.
DISCUSSION
Inflammatory mediators are critical components of pancreatic tumor biology, and their paracrine and autocrine functions significantly contributes to tumor progression and disease aggressiveness in patients with PDAC (20, 24). MIF was primarily described as a pro-inflammatory cytokine, however, recent evidence of a high level of MIF expression in many tumor types and its association with prognosis implicates it in the development and progression of human cancer. Pancreatic tumors with an aberrant MIF expression are highly aggressive and a higher MIF expression is associated with poorer survival in patients with PDAC (7). Recently, exosomal-MIF is linked with the formation of pre-metastatic niche in liver and described as a potential biomarker of liver metastasis in PDAC (25). In this study, we performed molecular characterization of MIF-high and MIF-low pancreatic tumors to examine the underlying mechanism of the highly aggressive behavior of pancreatic tumors with an aberrantly higher MIF expression in patients with PDAC, by applying an integrative analysis of coding and non-coding gene expression profiles. Our findings demonstrated that a novel MIF-miR-301b-NR3C2 signaling pathway is a key driver of disease aggressiveness and may be therapeutically targeted to improve survival in PDAC.
Several pro-inflammatory cytokines regulate miRNAs that are associated with the development and progression of human cancer, which may constitute one of the mechanisms underlying the role of cytokines in tumorigenesis (26). Based on this premise, we hypothesized that MIF regulates miRNAs, associated with tumor progression and disease aggressiveness in PDAC. The presence of an elevated level of miR-301b in MIF-high tumors as compared with MIF-low tumors in this study suggested a potential regulation of miR-301b by MIF in patients with PDAC, which was then confirmed through overexpression or depletion of MIF in genetically engineered pancreatic cancer cell lines and MIF-overexpressing orthotopic tumors, showing that MIF induces the upregulation of miR-301b and is mediated through the activation of PI3K/Akt pathway. MIF has been earlier shown to activate PI3K/Akt and ERK/MAPK signaling pathways. However, inhibition of ERK/MAPK signaling did not have any effect on MIF-induced increase in miR-301b (Fig. S10) in pancreatic cancer cells. miR-301b overexpression enhances migration and invasion in pancreatic cancer cell lines (27). However, its implication in disease outcome in patients with PDAC is not described. An association between the enhanced miR-301b expression and poorer survival in multiple independent cohorts of PDAC patients analyzed in our study suggested that MIF-induced upregulation of miR-301b may contribute to enhanced disease aggressiveness.
Several miRNAs are implicated in the development and progression of pancreatic cancer (28). The aberrant expression of these miRNAs alters the expression of critical target genes responsible for maintaining cellular homeostasis. Our comprehensive analyses to identify the key target of MIF-induced miR-301b, which mechanistically enhances pancreatic tumor progression and disease aggressiveness, resulted in the discovery of NR3C2. NR3C2 is a mineralocorticoid receptor gene encoding mineralocorticoid receptor (MR), which functions as a transcription factor and plays a crucial role in regulating electrolyte balance (29). Interestingly, a lower expression of MR predicted poor prognosis in lung and colon cancer patients (18,19). To our knowledge the role of NR3C2 has not been described in pancreatic cancer. The discovery and validation of NR3C2 as a novel target of MIF-signaling, and the poorer survival of patients with a lower expression of NR3C2 in tumors from multiple independent cohorts in our study, suggested its potential tumor suppressive role in PDAC. The functional role of NR3C2 in tumorigenesis is not clear. An association between lower NR3C2 expression and increased angiogenesis has been described in colon cancer (19). EMT is a critical event in the progression of pancreatic cancer. In a recent study, ataxia telangiectasia group D complementing gene (ATDC)-induced EMT critically led to the acquisition of invasive phenotype in KRAS-induced pancreatic tumorigenesis (30). The mechanistic and functional characterization of NR3C2 in our study revealed, for the first time, that it inhibits EMT and significantly reduced the metastatic potential of pancreatic cancer cells. These findings are consistent with a potential tumor suppressive role of NR3C2 in pancreatic cancer. Consistently, we found that genetic deletion of MIF enhanced NR3C2 expression, reduced metastasis and significantly prolonged survival in genetically engineered KPC mouse model of PDAC (Fig. 6). Aligned with these findings, a positive correlation between MIF and miR-301b, and negative correlations between MIF and NR3C2, and also NR3C2 and miR-301b expressions in human PDAC provided a clinical validation of MIF/miR-301b/NR3C2 axis, which enhances disease aggressiveness in these patients (Fig. 7E). Collectively, these data suggest that MIF-induced signaling pathway leading to the inhibition of NR3C2 is a key driver of disease aggressiveness in patients with PDAC.
Taken together, the findings in this study revealed a novel MIF-driven signaling pathway, which mechanistically links MIF-high tumors to enhanced disease aggressiveness and mortality, and also provide proof-of-principle that therapies targeting MIF/miR-301b/NR3C2 axis may improve disease outcome in patients with PDAC.
Supplementary Material
Acknowledgments
Authors thank Ms. Elise Bowman for the management of clinical samples, and the personnel at University of Maryland Medical System for collection of clinical biospecimens under NCI-UMD resource contract. Availability of data generated by the TCGA Research Network (http://cancergenome.nih) for validation of individual gene is highly appreciated. This work was supported by the Intramural Research Program of the Center for Cancer Research, NCI, NIH.
Footnotes
Conflict of Interest: None
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015;65(1):5–29. doi: 10.3322/caac.21254. [DOI] [PubMed] [Google Scholar]
- 2.Calandra T, Roger T. Macrophage migration inhibitory factor: a regulator of innate immunity. Nat Rev Immunol. 2003;3(10):791–800. doi: 10.1038/nri1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bernhagen J, Calandra T, Mitchell RA, Martin SB, Tracey KJ, Voelter W, et al. MIF is a pituitary-derived cytokine that potentiates lethal endotoxaemia. Nature. 1993;365(6448):756–759. doi: 10.1038/365756a0. [DOI] [PubMed] [Google Scholar]
- 4.Tomiyasu M, Yoshino I, Suemitsu R, Okamoto T, Sugimachi K. Quantification of macrophage migration inhibitory factor mRNA expression in non-small cell lung cancer tissues and its clinical significance. Clin Cancer Res. 2002;8(12):3755–3760. [PubMed] [Google Scholar]
- 5.Bucala R, Donnelly SC. Macrophage migration inhibitory factor: a probable link between inflammation and cancer. Immunity. 2007;26(3):281–285. doi: 10.1016/j.immuni.2007.03.005. [DOI] [PubMed] [Google Scholar]
- 6.Denz A, Pilarsky C, Muth D, Ruckert F, Saeger HD, Grutzmann R. Inhibition of MIF leads to cell cycle arrest and apoptosis in pancreatic cancer cells. J Surg Res. 2010;160(1):29–34. doi: 10.1016/j.jss.2009.03.048. [DOI] [PubMed] [Google Scholar]
- 7.Funamizu N, Hu C, Lacy C, Schetter A, Zhang G, He P, et al. Macrophage Migration Inhibitory Factor (MIF) induces epithelial to mesenchymal transition, enhances tumor aggressiveness and predicts clinical outcome in resected pancreatic ductal adenocarcinoma. Int J Cancer. 2012 doi: 10.1002/ijc.27736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shi X, Leng L, Wang T, Wang W, Du X, Li J, et al. CD44 is the signaling component of the macrophage migration inhibitory factor-CD74 receptor complex. Immunity. 2006;25(4):595–606. doi: 10.1016/j.immuni.2006.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Leng L, Metz CN, Fang Y, Xu J, Donnelly S, Baugh J, et al. MIF signal transduction initiated by binding to CD74. J Exp Med. 2003;197(11):1467–1476. doi: 10.1084/jem.20030286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lue H, Thiele M, Franz J, Dahl E, Speckgens S, Leng L, et al. Macrophage migration inhibitory factor (MIF) promotes cell survival by activation of the Akt pathway and role for CSN5/JAB1 in the control of autocrine MIF activity. Oncogene. 2007;26(35):5046–5059. doi: 10.1038/sj.onc.1210318. [DOI] [PubMed] [Google Scholar]
- 11.Schulz R, Marchenko ND, Holembowski L, Fingerle-Rowson G, Pesic M, Zender L, et al. Inhibiting the HSP90 chaperone destabilizes macrophage migration inhibitory factor and thereby inhibits breast tumor progression. J Exp Med. 2012;209(2):275–289. doi: 10.1084/jem.20111117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cordon-Cardo C, Prives C. At the crossroads of inflammation and tumorigenesis. J Exp Med. 1999;190(10):1367–1370. doi: 10.1084/jem.190.10.1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hingorani SR, Wang L, Multani AS, Combs C, Deramaudt TB, Hruban RH, et al. Trp53R172H and KrasG12D cooperate to promote chromosomal instability and widely metastatic pancreatic ductal adenocarcinoma in mice. Cancer Cell. 2005;7(5):469–483. doi: 10.1016/j.ccr.2005.04.023. [DOI] [PubMed] [Google Scholar]
- 14.Bozza M, Satoskar AR, Lin G, Lu B, Humbles AA, Gerard C, et al. Targeted disruption of migration inhibitory factor gene reveals its critical role in sepsis. J Exp Med. 1999;189(2):341–346. doi: 10.1084/jem.189.2.341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dweep H, Sticht C, Pandey P, Gretz N. miRWalk--database: prediction of possible miRNA binding sites by "walking" the genes of three genomes. Journal of biomedical informatics. 2011;44(5):839–847. doi: 10.1016/j.jbi.2011.05.002. [DOI] [PubMed] [Google Scholar]
- 16.Pei H, Li L, Fridley BL, Jenkins GD, Kalari KR, Lingle W, et al. FKBP51 affects cancer cell response to chemotherapy by negatively regulating Akt. Cancer cell. 2009;16(3):259–266. doi: 10.1016/j.ccr.2009.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Badea L, Herlea V, Dima SO, Dumitrascu T, Popescu I. Combined gene expression analysis of whole-tissue and microdissected pancreatic ductal adenocarcinoma identifies genes specifically overexpressed in tumor epithelia. Hepatogastroenterology. 2008;55(88):2016–2027. [PubMed] [Google Scholar]
- 18.Jeong Y, Xie Y, Xiao G, Behrens C, Girard L, Wistuba II, et al. Nuclear receptor expression defines a set of prognostic biomarkers for lung cancer. PLoS Med. 2010;7(12):e1000378. doi: 10.1371/journal.pmed.1000378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tiberio L, Nascimbeni R, Villanacci V, Casella C, Fra A, Vezzoli V, et al. The decrease of mineralcorticoid receptor drives angiogenic pathways in colorectal cancer. PLoS One. 2013;8(3):e59410. doi: 10.1371/journal.pone.0059410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rhim AD, Mirek ET, Aiello NM, Maitra A, Bailey JM, McAllister F, et al. EMT and Dissemination Precede Pancreatic Tumor Formation. Cell. 2012;148(1–2):349–361. doi: 10.1016/j.cell.2011.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Oliveira CS, de Bock CE, Molloy TJ, Sadeqzadeh E, Geng XY, Hersey P, et al. Macrophage migration inhibitory factor engages PI3K/Akt signalling and is a prognostic factor in metastatic melanoma. BMC cancer. 2014;14:630. doi: 10.1186/1471-2407-14-630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fan H, Hall P, Santos LL, Gregory JL, Fingerle-Rowson G, Bucala R, et al. Macrophage migration inhibitory factor and CD74 regulate macrophage chemotactic responses via MAPK and Rho GTPase. Journal of immunology. 2011;186(8):4915–4924. doi: 10.4049/jimmunol.1003713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Moffitt RA, Marayati R, Flate EL, Volmar KE, Loeza SG, Hoadley KA, et al. Virtual microdissection identifies distinct tumor- and stroma-specific subtypes of pancreatic ductal adenocarcinoma. Nature genetics. 2015;47(10):1168–1178. doi: 10.1038/ng.3398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gukovsky I, Li N, Todoric J, Gukovskaya A, Karin M. Inflammation, autophagy, and obesity: common features in the pathogenesis of pancreatitis and pancreatic cancer. Gastroenterology. 2013;144(6):1199–1209. e4. doi: 10.1053/j.gastro.2013.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Costa-Silva B, Aiello NM, Ocean AJ, Singh S, Zhang H, Thakur Basant K, et al. Pancreatic cancer exosomes initiate pre-metastatic niche formation in the liver. Nature Cell Biology. 2015 doi: 10.1038/ncb3169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hussain SP, Harris CC. Inflammation and cancer: an ancient link with novel potentials. Int J Cancer. 2007;121(11):2373–2380. doi: 10.1002/ijc.23173. [DOI] [PubMed] [Google Scholar]
- 27.Funamizu N, Lacy CR, Parpart ST, Takai A, Hiyoshi Y, Yanaga K. MicroRNA-301b promotes cell invasiveness through targeting TP63 in pancreatic carcinoma cells. International journal of oncology. 2014;44(3):725–734. doi: 10.3892/ijo.2014.2243. [DOI] [PubMed] [Google Scholar]
- 28.Bloomston M, Frankel WL, Petrocca F, Volinia S, Alder H, Hagan JP, et al. MicroRNA expression patterns to differentiate pancreatic adenocarcinoma from normal pancreas and chronic pancreatitis. JAMA. 2007;297(17):1901–1908. doi: 10.1001/jama.297.17.1901. [DOI] [PubMed] [Google Scholar]
- 29.Horisberger JD, Rossier BC. Aldosterone regulation of gene transcription leading to control of ion transport. Hypertension. 1992;19(3):221–227. doi: 10.1161/01.hyp.19.3.221. [DOI] [PubMed] [Google Scholar]
- 30.Wang L, Yang H, Abel EV, Ney GM, Palmbos PL, Bednar F, et al. ATDC induces an invasive switch in KRAS-induced pancreatic tumorigenesis. Genes Dev. 2015;29(2):171–183. doi: 10.1101/gad.253591.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.