Abstract
Background
Visceral arteries are commonly involved in endovascular repair of complex abdominal aortic aneurysms. To improve repair techniques and reduce long-term complications involving visceral arteries, it is crucial to understand in vivo arterial geometry and the deformations due to visceral organ movement with respiration. This study quantifies deformation of the celiac, superior mesenteric, and renal arteries during respiration, and correlates the deformations with diaphragmatic excursion.
Methods
16 patients with small abdominal aortic aneurysms underwent magnetic resonance angiography during inspiratory and expiratory breathholds. From geometric models of the aorta and visceral arteries, vessel length, branch angle, curvature, and positions were computed, along with degree of diaphragmatic excursion as indicated by kidney translation.
Results
From inspiration to expiration, the celiac artery exhibited axial shortening of 4.8 ± 6.4 % (P< 0.001) and a mean curvature increase of 0.03 ± 0.02 mm−1, greater than other visceral arteries (P< 0.01). With expiration, the superior mesenteric, left and right renal arteries angled upward by −9.8 ± 6.4°, –6.4 ± 6.4°, and −5.2 ± 5.0°, respectively (P< 0.005). All vessels translated superiorly (P< 0.0005) and posteriorly (P< 0.01), and the superior mesenteric artery additionally translated rightward (P< 0.005). The left and right kidneys translated by 22 ± 9 mm and 21 ± 9 mm, mostly superiorly (P< 0.001). Translations of all visceral arteries were moderately correlated to the right kidney (R> 0.50). Correlation of the left renal artery with the left kidney was greater than that of the right renal artery with the right kidney.
Conclusions
The celiac artery exhibited lower branch angle change, and greater axial and curvature deformations than the other visceral arteries, due to the vicinity to the liver and influence of the median arcuate ligament. Correlation between visceral arteries and kidney translations revealed that diaphragmatic excursion affects vessel mobility. Weaker correlation of the right renal artery to the right kidney indicates mechanical shielding from the inferior vena cava.
Keywords: Geometric analysis, Image processing, MRI, Respiration, Visceral arteries, Aneurysms
Introduction
The main visceral arteries, including the celiac artery, superior mesenteric artery (SMA), and left and right renal arteries (LRA and RRA), are major vessels that branch from the abdominal aorta and feed critical visceral organs. During respiration, the liver, pancreas, duodenum, and kidneys translate significantly in the cranio-caudal direction [1–4]. These motions may induce vessel translation or introduce bending and axial deformation with respect to the relatively fixed vessel ostium. It is yet unknown if the respiration-induced deformation differs between vessels, and if the deformation is statistically correlated to respiration depth. Furthermore, the visceral arteries are often involved in endovascular repair via fenestrated or parallel configurations in cases of complex abdominal aortic aneurysm (AAA) disease [5–7]. Such endovascular techniques are effective at preserving target vessel patency initially, but longer-term studies have identified the potential for branch vessel stent-graft instability and renal compromise using either technique, potentially as a result of branch graft dislodgement, migration, or kinking [8–10]. These long-term limitations highlight the need to quantify in vivo visceral vessel geometry and deformation as a function of diaphragmatic excursion and respiratory-related displacement of the abdominal viscera [1,10–12].
We aimed to quantify the geometries of the celiac, SMA, and renal arteries, their deformations due to respiration, and to the correlation of vessel deformation with depth of respiration as indicated by the amount of kidney translation [13,14]. We utilized contrast-enhanced magnetic resonance angiography (MRA) images, acquired during inspiration and expiration breath-holds of patients with small AAAs, and three-dimensional (3D) modeling techniques to quantify visceral vessel geometry, respiratory-induced vessel deformation, and kidney displacement.
Methods
Sixteen male subjects (age 73 ± 7 years) with small infrarenal AAAs (aneurysm diameter 3.6 ± 0.6 cm, range 2.6–4.4 cm) were recruited under a protocol approved by the Institutional Review Board. Informed consent was obtained from each subject. Contrast-enhanced MRA images were acquired from subjects with a 1.5-T MR scanner (GE Signa, GE Healthcare, Waukesha, WI, USA). A timing bolus of MultiHance gadolinium (Bracco Diagnostics, Inc., Milan, Italy) was injected via the left antecubital vein to determine the delay between the injection and the peak image enhancement of the abdominal aorta [15]. With the estimated delay time, contrast was administered at a 0.1 mmol/kg dose with 2-mL/s injection rate. MRA images were acquired during an inspiratory breath-hold, followed by an acquisition during an expiratory breath-hold. Respiratory bellows were used to monitor breath-hold lasting 30–40 s for each acquisition [16]. All image volumes were acquired with 3.0–3.3 ms repetition time, 0.7–0.8 ms echo time, 25 ° flip angle, 80 slices with 3-mm thickness and 1.5-mm overlap, and 40×40×12 cm3 volumetric field of view. The images were reconstructed to 512×512 pixels per slice, with a resultant voxel size of 0.8×0.8×1.5 mm3.
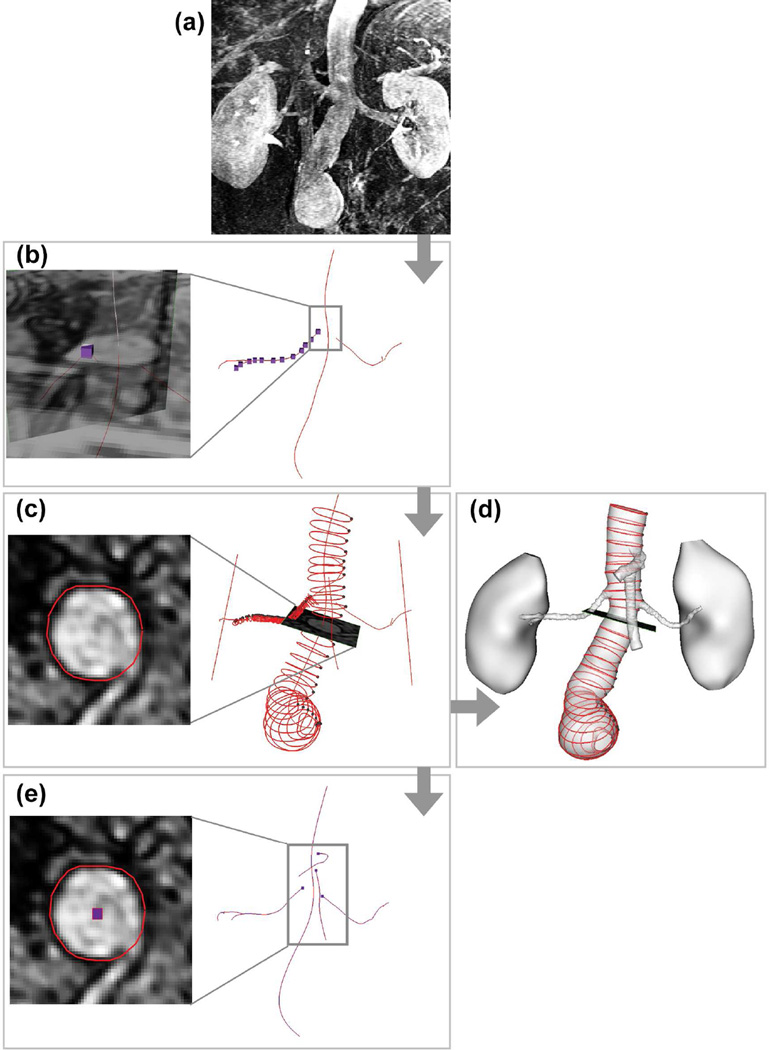
The MRA images were loaded into custom software, SimVascular, to construct lumen models and vessel centerline paths [16–20] (Fig. 1). The abdominal aorta, celiac artery, SMA, LRA, and RRA were segmented by series of lumen contours using a 2D level set segmentation technique [19]. From the segmented contours, arterial centerline paths were formed by connecting the contour centroids, and smoothed with an optimized number of Fourier modes [17]. Arterial centerline paths started at the branch ostia on the abdominal aorta. The centerline path of the celiac artery followed the hepatic artery when it passed the hepato-splenic bifurcation, and terminated at the next bifurcation. The centerline path of the SMA was terminated at the first jejunal branch. The centerline paths of renal arteries terminated at the first bifurcation.
Figure 1.
Image-based model construction and centerline quantification. (a) MRA images were loaded to SimVascular. (b) Lumen paths were constructed along the centers of the vessel lumens. Special attention was paid at the branch ostia to depict the center point from coronal and sagittal image slices of the vessel branch. (c) Lumen cross-section was visualized along each vessel path and segmented to a contour. (d) Lumen contours were lofted to form a 3D lumen model. (e) Mathematical centroids were extracted from lumen contours, connected, and smoothed by Fourier smoothing to form the true centerline path.
Metrics quantified for the visceral vessels were derived from the centerline paths and included axial length, branch angle, curvature, and vessel positions distal from the branch ostia by 10, 20, and 30 mm (Fig. 2). These metrics were measured for inspiration and expiration, and subtracted to quantify the deformations due to respiration. Axial length is the centerline arclength between the branch ostia and distal bifurcation point. Branch angle is the angle between the vectors formed on the centerlines of the abdominal aorta and a branch vessel, with the vector lengths defined as the average diameter of its vessel. Curvature was defined as the inverse of the radius of a circumscribed circle determined by three centerline path points separated by an average vessel radius. Curvature was quantified from 5 to 30-mm distal to the branch point every 0.1 mm on the centerline path. Mean curvature was computed by averaging curvature values over a vessel segment. Maximum curvature changes were computed as the maximum point-to-point curvature difference from inspiration to expiration within the proximal 30 mm of each vessel. Vessel positions 10, 20, and 30 mm distal from the branch ostia were tracked, and vessel translation was computed by subtracting corresponding vessel positions at expiration from that at inspiration.
Figure 2.
Quantification of the vessel axial length, curvature, branch angle, and relative vessel position of the celiac artery, superior mesenteric artery (SMA), and left and right renal arteries (LRA and RRA). (a) The axial length is the arclength of the vessel centerline path between the branch point and bifurcation point. (b) The branch angle is the angle between two vectors, one from the projection point of the branch along the aortic path, and another from the branch point along the vessel path. (c) Curvature is the inverse of the radius, R, of a circumscribed circle on the centerline path. The circle was formed by three points on the vessel path such that the points were apart by the average vessel radius. (d) Relative vessel position is the position of a vessel centerline point 10, 20, and 30 mm distal from the branch point.
Kidney position was defined as the mid-point between the most superior and inferior points of the kidney cortex (Fig. 3). Respiration-induced kidney translation was computed as the positional difference of the mid-point from inspiration to expiration. Aorta-kidney distance was quantified as the distance between the renal artery ostia and the mid-point of each kidney.
Figure 3.
Quantification of kidney position during inspiration and expiration. For the left and right kidneys, the superior and inferior extremities of the cortex were picked from the image volume. The mid-point of the kidney was computed as the center between the superior and inferior extremities. Aorta-kidney distance was quantified as the distance between the renal artery ostia and the kidney mid-point.
We reported average population data as mean ± standard deviation. Two-tailed paired t-tests were performed for comparisons. Statistical significance was defined as P< 0.05 for single comparisons and adjusted by Bonferroni-Holm correction for multiple comparisons [21]. Pearson’s correlation coefficient (R) was computed to examine the correlation between vessel deformations and kidney translations. Coefficient 0.5≤R<0.8 indicates moderate correlation, and R≥ 0.8 indicates strong correlation.
Results
Vessel axial length, branch angle, and their changes from inspiration to expiration are shown in Table I. The celiac artery exhibited axial shortening of 4.8 ± 6.4 % from inspiration to expiration (P< 0.001). With expiration, the SMA (−9.8 ± 6.4°) and renal (Left = −6.4 ± 6.4°, Right = −5.2 ± 5.0°) branches angled upward with decreased branch angles (P< 0.005). The celiac branch angle changed significantly less than those of the other vessels (P< 0.04). Mean curvature and max curvature change are shown in Table II. The celiac artery exhibited the greatest mean curvature at inspiration (0.08 ± 0.03 mm−1) and expiration (0.11 ± 0.03 mm−1), as well as the greatest increase of mean curvature from inspiration to expiration (0.03 ± 0.02 mm−1) (P< 0.01). Furthermore, the max curvature change of the celiac artery (0.10 ± 0.07 mm−1) was greater than the SMA (0.05 ± 0.02 mm−1) and RRA (0.04 ± 0.03 mm−1) (P< 0.005). The SMA exhibited the lowest curvatures at inspiration (0.03 ± 0.02 mm−1) and expiration (0.05 ± 0.02 mm−1) (P< 0.01). The RRA curvature was greater than the LRA at inspiration and expiration (P< 0.04), but their curvature changes were not significantly different (P> 0.05).
Table I.
Axial length and branch angle of the celiac artery, SMA, LRA, and RRA at inspiration and expiration, and changes from inspiration to expiration.
| Measurement | Celiac artery | SMA | LRA | RRA |
|---|---|---|---|---|
| Axial length (mm) | ||||
| Inspiration | 33.8 ± 5.5†,§ | 54.3 ± 10.9†,[] | 40.3 ± 14.2[] | 49.4 ± 16.9§ |
| Expiration | 32.4 ± 6.5†,§ | 52.6 ± 10.6†,[] | 39.7 ± 14.4[] | 49.5 ± 17.3§ |
| Axial strain (%) | −4.8 ± 6.4* | −3.1 ± 6.0 | −1.7 ± 4.7 | −0.3 ± 3.3 |
| Branch angle (°) | ||||
| Inspiration | 126.4 ± 17.8 | 118.8 ± 10.8 | 115.0 ± 13.6 | 122.2 ± 14.8 |
| Expiration | 125.0 ± 19.9†,‡ | 109.0 ± 13.4† | 108.7 ± 12.0‡ | 117.0 ± 16.7 |
| Difference | 1.4 ± 8.6†,‡,§ | −9.8 ± 6.4*,† | −6.4 ± 6.4*,‡ | −5.2 ± 5.0*,§ |
Values are mean ± standard deviation. Axial strain is percent change of the axial length computed by (Expiration − Inspiration) / Inspiration × 100.
indicates significant changes due to respiration.
indicates significant difference between celiac and SMA.
indicates significant difference between celiac and LRA.
indicates significant difference between celiac and RRA.
indicates significant difference between SMA and LRA.
No significant differences were found between SMA and RRA or LRA and RRA. Significance threshold (P<0.05) was adjusted by Bonferroni-Holm correction for multiple comparisons between vessels.
Table II.
Mean curvature of the celiac artery and its maximum point-to-point curvature change from inspiration to expiration, in comparison with the curvatures of SMA, LRA, and RRA [20].
| Curvature (mm−1) | Celiac artery | SMA | LRA | RRA |
|---|---|---|---|---|
| Mean curvature | ||||
| Inspiration | 0.08 ± 0.03†,‡ | 0.03 ± 0.02†,[],‖ | 0.05 ± 0.02‡,[],¶ | 0.07 ± 0.02‖,¶ |
| Expiration | 0.11 ± 0.03†,‡,§ | 0.05 ± 0.02†,[],‖ | 0.06 ± 0.01‡,[],¶ | 0.08 ± 0.02§,‖,¶ |
| Difference | 0.03 ± 0.02*,†,‡,§ | 0.02 ± 0.01*,† | 0.01 ± 0.01*,‡ | 0.01 ± 0.01*,§ |
| Max. curvature change | 0.10 ± 0.07†,§ | 0.05 ± 0.02† | 0.07 ± 0.03 | 0.04 ± 0.03§ |
Values are mean ± standard deviation.
indicates significant changes due to respiration.
indicates significant difference between celiac and SMA.
indicates significant difference between celiac and LRA.
indicates significant difference between celiac and RRA.
indicates significant difference between SMA and LRA.
indicates significant difference between SMA and RRA.
indicates significant difference between LRA and RRA.
Significance threshold (P<0.05) was adjusted by Bonferroni-Holm correction for multiple comparisons between vessels.
From inspiration to expiration, all visceral arteries translated superiorly, as shown in an example of vessel morphology changes accompanied by kidney translation (Fig. 4). Moreover, the SMA translated rightward and the renal arteries translated posteriorly. For quantitative comparison, centerline points 10, 20, and 30-mm distal from the branch ostia were tracked from inspiration to expiration (Table III). With expiration, all vessels translated superiorly (P< 0.0005), the SMA translated rightward at all points (P< 0.005), and the celiac and renal arteries translated posteriorly at the 20 and 30-mm points (P< 0.01). In the celiac, SMA, and LRA, superior translation was greater than that of other directions at all points (P< 0.02). In the RRA, superior translation was greater than that of other directions, at the 30-mm point only (P< 0.01). Regarding the net displacement, the LRA translated less than the SMA and RRA at the 10-mm point (P≤ 0.03), and the RRA translated less than the celiac and LRA at the 30-mm point (P≤ 0.03).
Figure 4.
Lumen morphology of two subjects during inspiration (light grey) and expiration (dark grey) in coronal, axial, and sagittal views. The abdominal aorta is depicted during inspiration (light grey). Note the kidney translation accompanying morphological changes of the celiac artery, superior mesenteric artery (SMA) and left and right renal arteries (LRA and RRA) due to respiration.
Table III.
Three-dimensional translation of celiac artery, SMA, LRA, and RRA with respect to the branch ostia from inspiration to expiration.
| Displacement (mm) | Celiac artery | SMA | LRA | RRA |
|---|---|---|---|---|
| 10-mm point | ||||
| Rightward | 0.1 ± 1.9 | 0.8 ± 0.9* | −0.4 ± 0.7* | 0.8 ± 0.9* |
| Posterior | −0.2 ± 0.6 | −0.2 ± 0.5 | 0.3 ± 0.8 | 0.9 ± 1.1* |
| Superior | 1.2 ± 1.0* | 2.3 ± 1.4* | 1.2 ± 0.7* | 1.2 ± 0.9* |
| Net displacement | 2.1 ± 1.2* | 2.6 ± 1.5*,‡ | 1.6 ± 0.9*,‡, § | 2.2 ± 1.0*,§ |
| 20-mm point | ||||
| Rightward | 1.4 ± 2.4* | 2.0 ± 2.1* | −0.7 ± 1.7 | 0.4 ± 1.6 |
| Posterior | 1.1 ± 1.6* | 0.1 ± 1.7 | 1.8 ± 1.3* | 2.6 ± 1.8* |
| Superior | 5.3 ± 2.2* | 5.4 ± 3.9* | 4.7 ± 2.4* | 3.3 ± 1.6* |
| Net displacement | 6.2 ± 2.3*,† | 6.3 ± 4.0* | 5.4 ± 2.7* | 4.7 ± 1.9*,† |
| 30-mm point | ||||
| Rightward | 2.1 ± 3.8 | 3.3 ± 3.3* | −0.2 ± 2.7 | −0.4 ± 1.5 |
| Posterior | 4.2 ± 2.2* | 0.3 ± 2.2 | 3.2 ± 2.0* | 3.4 ± 2.2* |
| Superior | 9.2 ± 3.4* | 7.1 ± 6.0* | 8.6 ± 4.5* | 5.5 ± 2.3* |
| Net displacement | 11.1 ± 3.4*,† | 8.6 ± 6.2* | 9.6 ± 4.7*,§ | 6.9 ± 2.5*,†,§ |
Values are mean ± standard deviation. The net displacement combines three dimensional components.
indicates significant changes due to respiration.
indicates significant difference of the net displacement between celiac and RRA.
indicates significant difference of the net displacement between SMA and LRA.
indicates significant difference of the net displacement between LRA and RRA.
No significant differences were found between celiac and LRA, SMA and RRA, or LRA and RRA. For multiple comparisons, significance threshold (P<0.05) was adjusted by Bonferroni-Holm correction.
Translations of the left and right kidneys from inspiration to expiration are shown in Table IV. With expiration, both kidneys translated significantly superiorly and posteriorly (P< 0.0001), and the left kidney also translated rightward (P< 0.025). On both sides, superior translation was dominant (P< 0.001), comprising approximately 90 % of the net displacement. The aorta-kidney distance at inspiration was 64.7 ± 7.3 mm and 78.4 ± 7.4 mm for the left and right kidneys, respectively. From inspiration to expiration, the distance decreased significantly for the left kidney (Δ= 1.9 ± 3.5 mm, P< 0.05), and insignificantly for the right kidney (Δ= 0.9 ± 4.5 mm, P> 0.05). The aorta-kidney distance was greater on the right side during both inspiration and expiration (P< 0.0001).
Table IV.
Translation of the left and right kidneys from inspiration to expiration.
| Displacement (mm) | Left kidney | Right kidney |
|---|---|---|
| Rightward | 1.6 ± 3.1*,†,‡ | 0.4 ± 4.4†,‡ |
| Posterior | 8.4 ± 4.5*,†,§ | 7.8 ± 4.7*,†,§ |
| Superior | 19.7 ± 8.7*,‡,§ | 19.1 ± 8.3*,‡,§ |
| Net displacement | 21.9 ± 9.2* | 21.3 ± 9.0* |
Values are mean ± standard deviation of displacement of the mid-point on each kidney. The net displacement combines three dimensional components.
indicates significant changes due to respiration.
indicates significant difference between the rightward and posterior displacement.
indicates significant difference between the rightward and superior displacement.
indicates significant difference between the posterior and superior displacement.
No significant difference was found between the left and right kidneys. For multiple comparisons, significance threshold (P<0.05) was adjusted by Bonferroni-Holm correction.
Correlation between the net displacement of kidneys and vessel curvature change was examined. The celiac artery exhibited the strongest correlation between its mean curvature change and the net displacement of both kidneys (R= 0.51 and 0.57 for the left and right kidneys, respectively). Curvature changes in other vessels were not correlated. Correlation between the net displacement of kidneys and net displacement of the 10, 20, and 30-mm points on each vessel are shown in Figure 5. Translations of the celiac and SMA were moderately correlated to that of the right kidney (R> 0.50 at the 20 and 30-mm points on the celiac, and all points on the SMA). Translation of each renal artery was moderately or strongly correlated to that of its corresponding kidney (R> 0.50), with greater correlation on the left side.
Figure 5.
Linear regression between kidney translation (net displacement) and translation of the 10-mm (light grey circle), 20-mm (dark grey square), and 30-mm (black triangle) centerline points along celiac artery, superior mesentery artery (SMA), and left and right renal arteries (LRA and RRA) starting from the branch ostia. Numbers on the right side of the trend lines indicate R-value (10-mm point in light grey, 20-mm point in dark grey, and 30-mm point in black). Celiac artery and SMA translations were more highly correlated to the right kidney as compared to the left. Each renal artery’s translation correlated moderately to the translation of its corresponding kidney, but the correlation was stronger on the left side.
Discussion
Marked differences in the respiratory-induced deformations were observed between the celiac, SMA, and renal arteries. While the celiac artery exhibited significant axial shortening but no change in branch angle due to expiration, other visceral arteries experienced significant upward angling but no significant changes in axial length with expiration. In addition, the celiac artery exhibited the greatest curvature, as well as the greatest change of curvature, as compared to the other arteries. These differences are likely due to the differing anatomic environments between the arteries. The anterior-perpendicular orientation of the celiac artery with respect to the aorta, combined with the posterior movement of the abdominal wall with expiration, may explain the significant shortening of the celiac artery with expiration. Conversely, the posterior motion of the abdominal wall does not as strongly affect the axial lengths of the anterior-downward oriented SMA and the laterally oriented renal arteries. Regarding branch angle change, the celiac trunk (before branching into the common hepatic, splenic, and left gastric arteries) appears to be relatively static, stabilizing the celiac trunk branch angle. This is likely due to the constraint of the median arcuate ligament. Conversely, the branching of the SMA and renal arteries are relatively free to angle upwards during expiration as the diaphragm and viscera move superiorly.
The relatively stable celiac trunk is also the cause of the greater celiac curvature and respiratory-induced change in curvature as compared to the other visceral arteries. The greatest mean curvature of the celiac artery is located at the sharp curve where the celiac artery branches into the common hepatic artery, where it is also constrained by the median arcuate ligament [22,23]. This constrained zone, combined with the superior motion of the diaphragm, liver, spleen, and stomach, also causes the celiac artery to experience greater mean and maximum curvature change as compared to the other arteries. Also, the inferior-superior translation of the liver is 10–26 mm during respiration, and is greater than that of the kidneys and lower viscera since the liver is in direct contact with the right diaphragm [24–26]. To accommodate the more mobile liver and relatively static celiac trunk, the celiac artery exhibits larger curvature changes than the other visceral arteries.
Kidney displacement has been shown to be an indicator of diaphragmatic excursion in previous studies using respiratory-resolved computed tomography; the cranio-caudal mobility of the kidneys were correlated to the respiratory phase, lung volumes, and diaphragm displacements [13,14]. In our results, kidney translation due to respiration occurred mostly in the inferior-superior direction, and the net translation distance (21.9 ± 9.2 and 21.3 ± 9.0 mm for the left and right kidneys, respectively) is within the range of previously reported measurements [1,3,13,25]. We observed that not only is renal artery translation correlated to the kidney translation, but celiac artery and SMA translations are also correlated to right kidney translation, but not left kidney translation. Siva et al. observed a stronger correlation between the displacements of the right kidney versus the right diaphragm, as compared to the left side, possibly due to the rightward dominated liver influencing the motion of the right kidney [13]. The left kidney, on the other hand, may be in an environment with a greater degree of freedom, which may lead to dissociation from the respiratory-induced excursion.
Correlation of RRA translation versus the right kidney was weaker than that of LRA translation versus the left kidney. The 10, 20, and 30-mm point displacements of the RRA were correlated to the right kidney displacement with R-values of 0.50, 0.71, and 0.79, respectively. On the other hand, the 20 and 30-mm point displacements of the LRA were strongly correlated to the left kidney displacement with R-values exceeding 0.90 (Fig. 5). This may be due to the fact that: 1) the right kidney is more distant from the abdominal aorta as compared to the left kidney, and 2) RRA motion may be partially shielded from the right kidney’s influence by the presence of the inferior vena cava (IVC). The RRA was in contact with the IVC 20 to 34-mm distal to the aorta, explaining why the RRA translated significantly less than the LRA at the 30-mm point (P≤ 0.03) due to respiration. This evidence supports previous findings that the IVC’s contact with the RRA provides mechanical support [10,12]. Due to this combination of characteristics, the RRA translation within the proximal 30-mm of length appears to be less correlated to kidney translation as compared to the LRA.
Currently, in endovascular repair of complex AAAs, all of the visceral arteries are treated with similar branching devices and techniques for fenestrated and snorkel / chimney grafts. However, visceral artery-specific motions presented in this study suggest that stent design, coverage length, and attachment technique may benefit from branch-specific consideration to reduce the frequency of stent fractures, kinking, and dislodgement. Moreover, we believe that respiratory-gated CT or MRI can be used to help physicians make treatment decisions when considering different options for complex EVAR (e.g. fenestrated vs. snorkel / chimney vs. branched endografts). Furthermore, the magnitude of visceral artery motion may be able to help predict the longevity of endovascular repairs.
In addition to this study which was focused on native arterial motion, in a different study we observed respiratory-induced motion of stented visceral arteries after fenestrated and snorkel / chimney endovascular aneurysm repair. Stented visceral arteries exhibit negligible branch angulation due to the mechanical support of stents, but experienced significant deformation distal to the stents [27].
Our findings are based on the patients with small AAAs, and it is uncertain if patients with larger AAAs exhibit similar motion of visceral arteries. The large aneurysmal volume may moderate the influence of diaphragmatic movement on viscera. Stronger statistical findings may result from larger study populations, and the behavior of the visceral arteries in female subjects, patients with larger or ruptured AAAs, or patients after endovascular aneurysm repair may differ. The variation of the posture from supine to upright may also lead to the different vascular morphology, which was not considered in this study. Cardiac- and respiratory-gated imaging modalities with more refined spatial resolution may improve the accuracy of motion data by separating cardiac- and respiratory-induced motion, and eliminating involuntary diaphragmatic motion during breath-holds [28,29].
In conclusion, this study provides in-depth quantification of visceral artery geometry and their changes due to respiration. From inspiration to expiration, all vessels translated superiorly and posteriorly accompanied by branch angle decrease and curvature increase. The celiac artery exhibited the most severe deformation including significant shortening and bending due to the motions of the diaphragm and liver, and proximal constraint from the median arcuate ligament. Comparison of visceral artery and kidney translations revealed that respiration depth is correlated to abdominal vessel mobility. The correlation of the right renal artery and the right kidney was weaker than that of the left side, possibly due to its longer length and the IVC support on the right side.
Acknowledgments
This work was supported by a research gift from Medtronic Vascular (Santa Rosa, California), W. L. Gore & Associates (Flagstaff, Arizona), and by National Institutes of Health Grants (P50 HL083800 and P41 RR09784). We acknowledge Stanford University Medical Center; the Lucas Center for Magnetic Resonance Imaging at Stanford University; Veterans Affairs Palo Alto Health Care System. We thank Dr. Andrea S. Les, Dr. Adam Tenforde, Mary McElrath, and Teresa Nelson for their support with recruitment and imaging, and all patients for their participation.
Abbreviations
- SMA
superior mesenteric artery
- LRA
left renal artery
- RRA
right renal artery
- IVC
inferior vena cava
- MRA
magnetic resonance angiography
- 3D
three-dimensional
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Draney MT, Zarins CK, Taylor CA. Three-dimensional analysis of renal artery bending motion during respiration. J Endovasc Ther. 2005;12:380–386. doi: 10.1583/05-1530.1. [DOI] [PubMed] [Google Scholar]
- 2.Langen KM, Jones DTL. Organ motion and its management. Int J Rad Oncol Biol Phys. 2001;50:265–278. doi: 10.1016/s0360-3016(01)01453-5. [DOI] [PubMed] [Google Scholar]
- 3.Moerland MA, van den Bergh ACM, Bhagwandien R, Janssen WM, Bakker CJG, Lagendijk JJW, et al. The influence of respiration induced motion of the kidney on the accuracy of radiotherapy treatment planning, a magnetic resonance imaging study. Radiother Oncol. 1994;30:150–154. doi: 10.1016/0167-8140(94)90045-0. [DOI] [PubMed] [Google Scholar]
- 4.Suramo I, Päivänsalo M, Myllylä V. Cranio-caudal movements of the liver, pancreas and kidneys in respiration. Acta Radiol Diag. 1984;25:129–131. doi: 10.1177/028418518402500208. [DOI] [PubMed] [Google Scholar]
- 5.Diehm N, Baum S, Benenati JF. Fenestrated and branched endografts: why we need them now. J Vasc Interv Radiol. 2008;19:S63–S67. doi: 10.1016/j.jvir.2008.01.019. [DOI] [PubMed] [Google Scholar]
- 6.Chuter TAM, Hiramoto JS, Park K, Reilly LM. The transition from custom-made to standardized multibranch thoracoabdominal stent-grafts. J Vasc Surg. 2011;54:660–668. doi: 10.1016/j.jvs.2011.03.005. [DOI] [PubMed] [Google Scholar]
- 7.Lee JT, Greenberg JI, Dalman RL. Early experience with the snorkel technique for juxtarenal aneurysms. J Vasc Surg. 2012;55:935–946. doi: 10.1016/j.jvs.2011.11.041. [DOI] [PubMed] [Google Scholar]
- 8.Casey K, Hernandez-Boussard T, Mell MW, Lee JT. Readmissions after AAA repair: Differences between open repair and EVAR. J Vasc Surg. 2013;57:89–95. doi: 10.1016/j.jvs.2012.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Haddad F, Greenberg RK, Walker E, Nally J, O’Neill S, Kolin G, et al. Fenestrated endovascular grafting: The renal side of the story. J Vasc Surg. 2005;41:181–190. doi: 10.1016/j.jvs.2004.11.025. [DOI] [PubMed] [Google Scholar]
- 10.Robertson SW, Jessup DB, Boero IJ, Cheng CP. Right renal artery in vivo stent fracture. J Vasc Interv Radiol. 2008;19:439–442. doi: 10.1016/j.jvir.2007.11.014. [DOI] [PubMed] [Google Scholar]
- 11.Wang LC, Scott DJ, Clemens MS, Hislop SJ, Arthurs ZM. Mechanism of stent failure in a patient with fibromuscular dysplasia following renal artery stenting. Ann Vasc Surg. 2015;19:123.e19–123.e21. doi: 10.1016/j.avsg.2014.08.002. [DOI] [PubMed] [Google Scholar]
- 12.Suh G-Y, Choi G, Herfkens RJ, Dalman RL, Cheng CP. Respiration-induced deformations of the superior mesenteric and renal arteries in patients with abdominal aortic aneurysms. J Vasc Interv Radiol. 2013;24:1035–1042. doi: 10.1016/j.jvir.2013.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Siva S, Pham D, Gill S, Bressel M, Dang K, Devereux T, et al. An analysis of respiratory induced kidney motion on four-dimensional computed tomography and its implications for stereotactic kidney radiotherapy. Rad Oncol. 2013;8:248–255. doi: 10.1186/1748-717X-8-248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Van Sörnsen de Koste JR, Senan S, Kleynen CE, Slotman BJ, Lagerwaard FJ. Renal mobility during uncoached quiet respiration: an analysis of 4DCT scans. Int J Radiation Oncol Biol Phys. 2006;64:799–803. doi: 10.1016/j.ijrobp.2005.09.012. [DOI] [PubMed] [Google Scholar]
- 15.Wilman AH, Riederer SJ, King BF, Debbins JP, Rossman PJ, Ehman RL. Fluoroscopically triggered contrast-enhanced three-dimensional MR angiography with elliptical centric view order: application to the renal arteries. Radiology. 1997;205:137–146. doi: 10.1148/radiology.205.1.9314975. [DOI] [PubMed] [Google Scholar]
- 16.Suh G-Y, Choi G, Draney MT, Herfkens RJ, Dalman RL, Cheng CP. Respiration-induced 3D deformations of the renal arteries quantified with geometric modeling during inspiration and expiration breath-holds of magnetic resonance angiography. J Magn Reson Imaging. 2013;38:1325–1332. doi: 10.1002/jmri.24101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Choi G, Cheng CP, Wilson NM, Taylor CA. Methods for quantifying three-dimensional deformation of arteries due to pulsatile and nonpulsatile forces: implications for the design of stents and stent grafts. Ann Biomed Eng. 2009;37:14–33. doi: 10.1007/s10439-008-9590-0. [DOI] [PubMed] [Google Scholar]
- 18.Taylor CA, Draney MT, Ku JP, Parker D, Steele BN, Wang K, et al. Predictive medicine: Computational techniques in therapeutic decision-making. Comp Aided Surg. 1999;4:231–247. doi: 10.1002/(SICI)1097-0150(1999)4:5<231::AID-IGS1>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 19.Wang KC. Ph.D. thesis. Stanford CA: Stanford University Press; 2001. Level set methods for computational prototyping with application to hemodynamic modeling. [Google Scholar]
- 20.Wilson N, Wang K, Dutton RW, Taylor CA. A software framework for creating patient specific geometric models from medical imaging data for simulation based medical planning of vascular surgery. Lect Notes Comput Sci. 2001;2208:449–456. [Google Scholar]
- 21.Holm A. A simple sequentially rejective multiple test procedure. Scand J Stat. 1979;6:65–70. [Google Scholar]
- 22.Douard R, Ettore GM, Chevallier J-M, Delmas V, Cugnenc P-H, Belghiti J. Celiac trunk compression by arcuate ligament and living-related liver transplantation: a two-step strategy for flow-induced enlargement of donor hepatic artery. Surg Radiol Anat. 2002;24:327–331. doi: 10.1007/s00276-002-0073-y. [DOI] [PubMed] [Google Scholar]
- 23.Reilly LM, Ammar AD, Stoney RJ, Ehrenfeld WK. Late results following operative repair for celiac artery compression syndrome. J Vasc Surg. 1985;2:79–91. [PubMed] [Google Scholar]
- 24.Brandner ED, Wu A, Chen H, Heron D, Kalnicki S, Komanduri K, et al. Abdominal organ motion measured using 4D CT. Int J Radiation Oncol Biol Phys. 2006;65:554–560. doi: 10.1016/j.ijrobp.2005.12.042. [DOI] [PubMed] [Google Scholar]
- 25.Bussels B, Goethals L, Feron M, Bielen D, Dymarkowski S, Suetens P, et al. Respiration-induced movement of the upper abdominal organ: a pitfall for the three-dimensional conformal radiation treatment of pancreatic cancer. Radiother Oncol. 2003;68:69–74. doi: 10.1016/s0167-8140(03)00133-6. [DOI] [PubMed] [Google Scholar]
- 26.Clifford MA, Banovac F, Levy E, Cleary K. Assessment of hepatic motion secondary to respiration for computer assisted interventions. Comp Aided Surg. 2009;7:291–299. doi: 10.1002/igs.10049. [DOI] [PubMed] [Google Scholar]
- 27.Ullery BW, Suh G-Y, Lee JT, Liu B, Stineman R, Dalman RL, et al. Geometry and respiratory-induced deformation of abdominal branch vessels following complex EVAR. J Vasc Surg. 2015;61:875–884. doi: 10.1016/j.jvs.2014.11.075. [DOI] [PubMed] [Google Scholar]
- 28.Holland AE, Goldfarb JW, Edelman RR. Diaphragmatic and cardiac motion during suspended breathing: preliminary experience and implications for breath-hold MR imaging. Radiology. 1998;209:483–489. doi: 10.1148/radiology.209.2.9807578. [DOI] [PubMed] [Google Scholar]
- 29.Kaandrop DW, Boudewijn G, Vasbinder C, de Haan MW, Kemerink GJ, van Engelshoven JMA. Motion of the proximal renal artery during the cardiac cycle. J Magn Reson Imaging. 2000;12:924–928. doi: 10.1002/1522-2586(200012)12:6<924::aid-jmri16>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]