Abstract
We report here a series of ten patients with uncommon presentations and associations of chronic lymphocytic leukemia (CLL) not reported hitherto or occasionally reported in literature. The first two cases describe unusual causes of abdominal distension in CLL and unusual sites infiltration by CLL. The next two cases illustrate occurrence of CLL in association with other hematological malignancies. Cases five and six describe unusual infections and their impact on CLL. Cases seven and eight depict associations of rare non-hematological autoimmune conditions with CLL. The last two cases describe transformation at unusual sites. This series of ten cases illustrates how a common leukemia like CLL can present in different forms and how despite so much progress in understanding of this leukemia so little is known of such presentations.
Keywords: Chronic lymphocytic leukemia, Rare presentation, Infiltration, Infection, Autoimmunity, Transformation
Introduction
Chronic lymphocytic leukemia (CLL) is the commonest leukemia in the west with CLL accounting for nearly 30 % of all adult leukemias. A study from this center had shown that CLL accounted for only 13.56 % of all adult hematological malignancies [1]. We report here a series of ten patients (groups of two each) with uncommon presentations and associations of CLL.
CLL at Unusual Sites
When a case of CLL presents with abdominal distension and pedal edema, it is commonly due to splenomegaly or abdominal lymphadenopathy. The following two cases highlight the fact the CLL presentation may not follow textbook descriptions.
Case 1
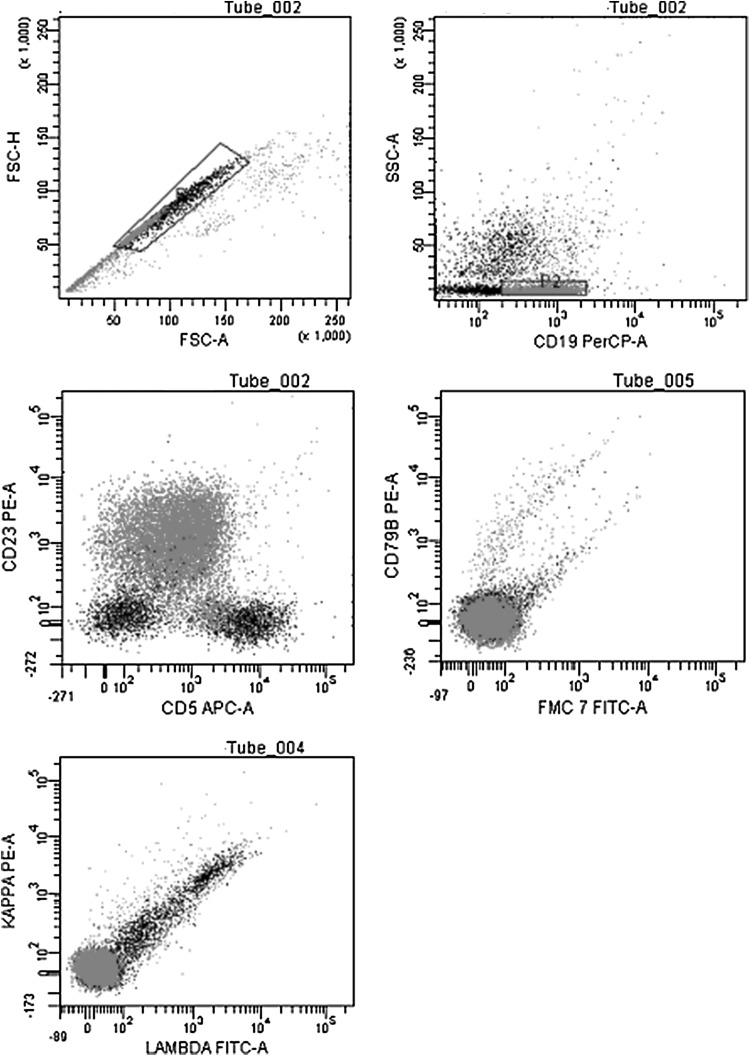
A 68 year old lady known RAI 0 CLL (absolute lymphocyte count 48.37 × 109/L at baseline), Zap70 and CD38 negative, on observation progressed over 2 years. She presented with fatigue, abdominal distension and pedal edema. On clinical examination she had pallor, cervical lymphadenopathy 2 × 2 cm and ascites. Investigations revealed hemoglobin of 84 g/L, platelet count of 64 × 109/L and absolute lymphocyte count of 255 × 109/L. She had massive ascites with spleen of 15 cm on ultrasound. Ascitic fluid analysis revealed a total of 700 cells (80 % lymphocytes), protein of 0.3 gm %, serum ascites albumin gradient of 1.8, adenosine deaminase (ADA) 13 U/L, amylase 63 mg/dL, cholesterol 25 mg/dl, triglycerides 63 mg/dL. Flow cytometry of the ascitic fluid confirmed the CLL immuno-phenotype of the lymphocytes (Fig. 1). She is currently being treated with standard dose CVP.
Fig. 1.
Flow cytometry of the asctic fluid revealed lymphocyte immunophenotype corresponding to the CLL cells
Case 2
A 50 year old lady known RAI 0 CLL (absolute lymphocyte count 25.5 x 109/L at baseline), Zap70 negative and CD38 positive, on clinical observation presented 2 years later with a history of abdominal distension and pedal edema. Investigations revealed she had mild ascites, bilateral renal enlargement, with a mass at the upper pole of right kidney. Her ALC at this time was 197.2 × 109/L; hemoglobin was 90 g/L and proteinuria (8.7 gm/24 h), serum albumin 2.6 mg/dL, total cholesterol 405 mg/dL. A renal biopsy to investigate for the cause of nephrotic syndrome showed membranoproliferative glomerulonephritis (MPGN) (Fig. 2), while the fine needle aspirate from the renal mass at the upper pole of right kidney revealed infiltration by CLL. Considering the possibility of the paraneoplastic manifestation of nephrotic syndrome associated with CLL, she was treated with four cycles of bendamustine (100 mg/m2) and rituximab (375 mg/m2). Following this the proteinuria resolved (0.26 gm/24 h), the renal mass regressed. She is asymptomatic for the past 1 year and continues to be in complete remission.
Fig. 2.
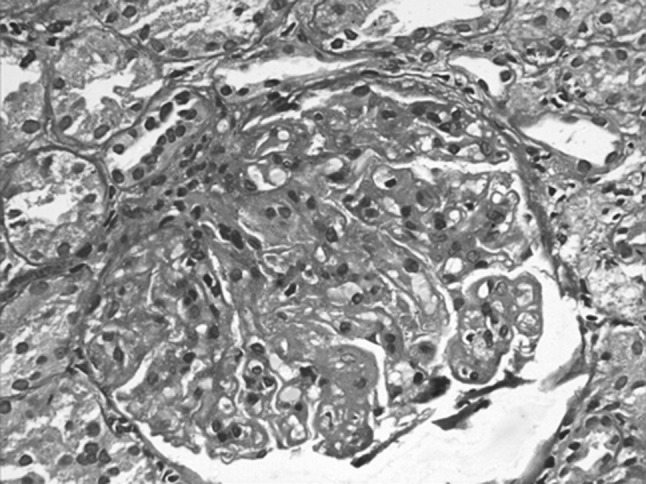
Membranoproliferative glomerulonephritis in a case of CLL
Review of literature showed few isolated cases reporting these unusual presentations. In a case of CLL presenting with abdominal distension and pedal edema the following differential diagnosis should be considered.
Abdominal lymphadenopathy and splenomegaly (Most common).
Nephrotic syndrome [2] Although various case reports have shown that any glomerular pathology can accompany CLL, most common is membranoproliferative glomerulonephritis [3]. Few have proposed this to be a paraneoplastic manifestation of CLL [4].
- Ascites various causes of ascites in CLL have been proposed. Chylous ascites may be due to lymphatic obstruction, Transudative ascites may be due to hepatosplenomegaly and portal hypertension, hepatic venous obstruction. Lastly infiltration of the peritoneum may be an explanation for malignant ascites.
- Chylous [5].
Gastrointestinal infiltration [12, 13] CLL infiltration has been described in all parts of the GI tract from the stomach to the colon, however the clinical presentation is as an occult or evident GI bleed and rarely as a mass in the abdomen.
CLL as a Surprise
Though CLL is known to be associated with a higher risk for secondary malignancies, most of these are solid cancers [14] and therapy related myeloid neoplasms are seen in reference to the chemotherapy given for treatment of CLL [15]. The following two cases are interesting as to what the pathogenesis might be for the development of a clonal lymphoid disorder in a known case of clonal myeloid and plasma cell disorder.
Case 3
A 45 year old lady diagnosed accelerated phase chronic myeloid leukemia in 2011, on imatinib 600 mg initially, hiked to 800 mg/day for failure to achieve complete hematologic response. A year and a half later she presents with cervical lymphadenopathy, anemia (Hb 82 g/L), absolute lymphocyte count 36.9 × 109/L and thrombocytopenia (platelet 35 × 109/L). A bone marrow at this time point showed flow cytometry consistent with CLL. Zap70 was negative and CD38 was positive. Bone marrow cytogenetics showed CML was in complete cytogenetic response (0/30) and Rq-PCR for Bcr-Abl was major molecular response (0.95 %). In view of severe cytopenias the imatinib dose was reduced to 400 mg/day. She was also started on oral prednisolone at a dose of 1 mg/kg considering the possibility of autoimmune cytopenias associated with CLL. Prednisolone was tapered to 5 mg over 6 weeks and stopped after 6 months. This case was reported by the authors at this time point [16]. The last Rq-PCR for Bcr-Abl is below detection limits (IS). She is currently symptomatic with fatigue and lymphocyte doubling time less than 6 months (Hb = 91 g/L, platelet = 68 × 103/L, ALC = 84.1 × 109/L). She is planned for treatment with bendamustine and rituximab.
Case 4
A 45 year old lady diagnosed plasma cell myeloma in July 2012 (BM: 70 % plasma cells), ISS stage III (b2 m = 5.95 mg/L, albumin = 3.38 mg/dL), Immunofixation IgM/kappa (M = 4.03), with related organ or tissue impairment in the form of anemia and skeletal lytic lesions. She was treated with 12 cycles of lenalidomide (15 mg) and dexamethasone (20 mg/week) and monthly ibandronate. She achieved a VGPR (M = 0.79), however was not transplanted due to financial limitations. At the end of 12 cycles of therapy she started developing lymphocytosis (ALC = 16.35 × 109/L), BM and flow confirmed the diagnosis of CLL. Zap70 and CD38 were negative. She continues to be in VGPR from myeloma point of view, however she has pure red cell aplasia (Hb = 63 g/L), immune thrombocytopenia (platelet = 80 × 109/L) and rapid lymphocyte doubling time (−3 months). She is planned for lenalidomide and rituximab based therapy.
There are isolated cases reporting the occurrence of concurrent lymphoproliferative and myeloproliferative malignancies. Most of these reports had CLL preceding CML, few occulting simultaneously, but only three cases where CLL followed CML [17–19]. Cytogenetic studies have shown that the CLL was derived from the Ph negative clone [18].
Similarly there have been isolated case reports of concurrent CLL and multiple myeloma. Some studies have suggested separate clonal origin based on different cytogenetics [20], light chain restriction and IgHV rearrangement [21, 22], while some studies have shown same clonal origin based on common idiotypes and IgHV rearrangement to both these malignancies [23, 24]. Unfortunately we did not have cytogenetic profiles and IgHV status in both our cases and hence were not able to demonstrate the clonal origin to infer whether the concurrence of these cases was either coincidental or related.
With Unusual Infections
Patients with CLL are predisposed to a wide spectrum of infections due to inherent defects in cellular and humoral immunity and therapy related immunosuppression [25].
Case 5
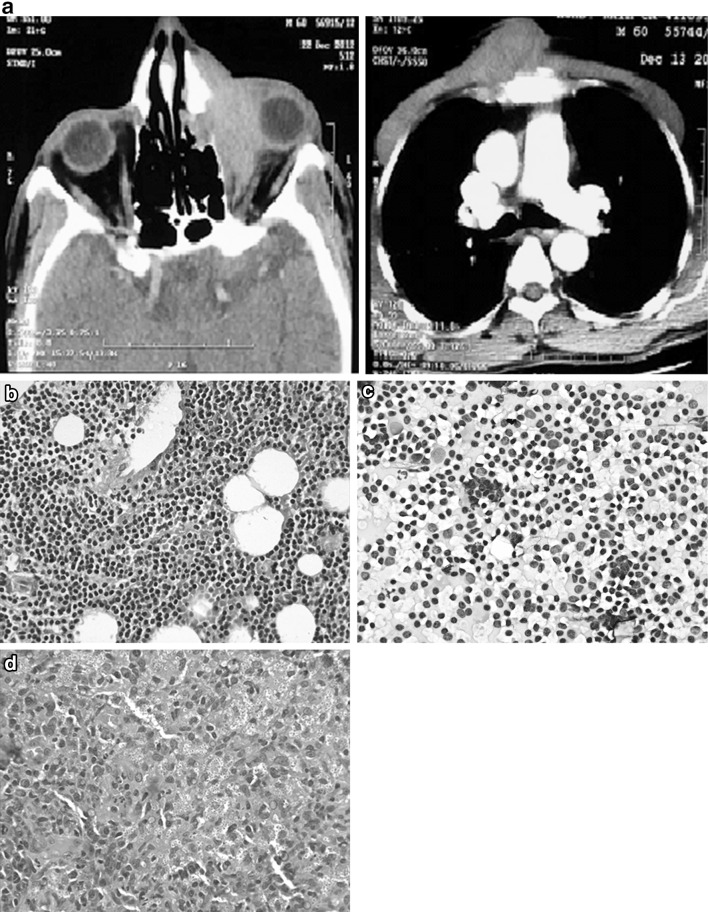
A 60 year old gentleman presented with 6 × 8 cm, painless, ulcero-nodular midline chest swelling, left eye proptosis, external ophthalmoplegia and left parotid swelling for 3 months. On examination he had cervical and inguinal lymphadenopathy. Peripheral blood revealed lymphocytosis (ALC = 81.2 × 109/L), flow cytometry of the bone marrow confirmed CLL, Zap70 and CD38 were negative. CECT imaging showed a soft tissue mass in the left orbit eroding the medial wall with extension to the lateral wall of nasal cavity and a chest wall mass eroding the sternum (Fig. 3a). While the biopsy from the nasal aspect of the orbital mass and fine needle aspiration of the parotid swelling showed infiltration by CLL (Fig. 3b, c), the biopsy from the sternal mass showed histoplasmosis (Fig. 3d). He also had panhypogammaglobulinemia. For cutaneous histoplasmosis he was treated with oral itraconazole 200 mg bid for 3 months followed by once a day for 3 more months. His sternal mass healed leaving behind a scar. In view of vision threatening involvement (left eye light perception only) by CLL infiltration of the orbit he was initially treated with chlorambucil (45 mg/m2) for three cycles. As there was no response he was treated with six cycles of CVP. He had recurrent chest infections during this period requiring treatment interruptions. His left eye proptosis has resolved and his vision restored (6/12). He is asymptomatic currently and continues to be on observation for CLL.
Fig. 3.
a CT showing orbital mass and chest mass eroding the sternum, b orbital infiltration by CLL, c parotid FNAC showing infiltration by CLL, d histopathology of the sternal mass showing histoplasmosis
Case 6
A 63 year old lady at the time of presentation in 2011 with community acquired pneumonia was found to have peripheral blood lymphocytosis (ALC = 113.9 × 109/L). She also had anemia (Hb = 95 gm/L). Bone marrow and flow confirmed the diagnosis of CLL with PRCA. Zap70 and CD38 were negative. She was started on steroids 0.5 mg/kg for PRCA during which she had a partial response with Hb rising to above 100 g/L. However 3 months later she developed pulmonary tuberculosis (Brochoalveolar lavage AFB positive). She was given 6 months of anti-tuberculous therapy. During the time she was on ATT her ALC decreased progressively to 2.5 × 109/L, hemoglobin increased to 121 g/L. She is asymptomatic currently and continues to be on regular follow-up.
There are at least 12 case reports of the occurrence of disseminated histoplasmosis in CLL patients, however only one case reporting cutaneous histoplasmosis in CLL [26]. Though orbital infiltration by CLL has been reported previously [27–29], this case is the first report of CLL infiltrating the parotid gland. The second case is interesting in the fact that though there are case series documenting spontaneous remissions (SR) in CLL [30–33], this is the first report where SR has been seen after treatment for tuberculosis. This patient was not on any other concomitant anti-hypertensive [30] or complementary and alternative medications [34] which have been known to induce remissions in CLL. Immunological causes have been proposed for SR seen after vaccinations, viral or bacterial infections, where activation of the host cellular and humoral immune responses leading to release of cytokines, tumor necrosis factor, interferon may lead to CLL cells apoptosis and regression [35]. It would be difficult to prove whether anti-tuberculous therapy or tuberculosis itself caused CLL regression in this case.
Non-hematological Autoimmune Condition
Case 7
A 50 year old gentleman, reformed smoker (Smoking Index = 200) presented in 2005 with a history of intermittent claudication. During evaluation he was found to have absent right brachial, radial, femoral and bilateral popliteal, dorsalis pedis and posterior tibial pulsations. He also had a right subclavian and bilateral renal artery bruit. CT-angiography confirmed the stenosis of bilateral subclavian, renal artery and right common iliac artery stenosis. Considering the diagnosis of Takayasu arteritis he was managed with antihypertensives and antiplatelets. However he was lost to follow-up for the next 8 years and presented again in 2013 with intermittent claudication for which internal iliac artery stenting was done. At this time he was incidentally found to have lymphocytosis (ALC = 33.7 × 109/L), without any lymphadenopathy or splenomegaly. Flow cytometry confirmed the diagnosis of CLL. He had improvement in his claudication symptoms post stenting of the common iliac artery; however had renal dysfunction (creatinine 2 mg/dL) related to bilateral renal artery stenosis. He remains asymptomatic and on observation from CLL point of view (Rai 0).
Case 8
A 60 year old gentleman had presented in 2006 with bullous skin lesions. Direct immuno-fluorescence of the skin biopsy was negative for deposition of IgA, IgG, IgM and C3. His ALC at this time was 4.5 × 109/L. He was managed as a case of paraneoplastic pemphigus with steroids and dapsone following which his skin lesions resolved. Six years later he presented with a papular skin eruption, lymphocytosis (ALC = 17 × 109/L) and hepatosplenomegaly. Flow cytometry confirmed the diagnosis of CLL (Rai II). Prognostic marker CD38 was positive and Zap70 was negative. Skin biopsy this time revealed perivascular and periadnexal lymphomononuclear infiltrate which was CD5+ suggesting infiltration by CLL (Fig. 4). This skin eruption also responded to oral steroids. It waxes and wanes as steroids are stopped and re-introduced. Currently his ALC is 59.6 × 109/L, is asymptomatic and on observation for CLL.
Fig. 4.
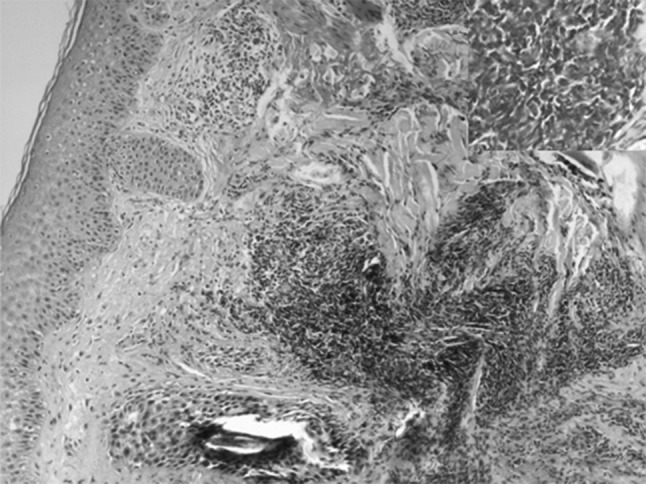
Cutaneous (perivascular and periadnexal) infiltration by CLL cells
Non-hematological autoimmune complications are very rare in CLL. Of these paraneoplastic pemphigus and glomerulonephritis are known to be associated with CLL [36]. Vasculitis has not been described commonly with CLL. There are few case reports showing the co-occurrence of CLL with giant cell arteritis [37], Polyarteritis nodosa [38] and leukocytoclastic vasculitis [39]. Case seven is the first report to the best of our knowledge of the occurrence of CLL in a case of Takayasu arteritis. These autoimmune conditions are known to precede the development of CLL [40]. Cutaneous infiltration by CLL is caused by perivascular and periadnexal infiltration by CLL cells [41] as seen in case eight. It can be differentiated from cutaneous lymphocytic vasculitis; a paraneoplastic condition caused due perivascular infiltration by non-malignant T-cells [42]. This case is unique in the way that paraneoplastic pemphigus preceded CLL and the presentation of CLL was with cutaneous infiltration by malignant B-cells rather than paraneoplastic vasculitis.
Synchronous DLBCL or Richter’s Transformation
Case 9
This 55 year old gentleman incidentally diagnosed CLL in 2012 (ALC = 17.6x109/L, Hb = 120gm/L, platelet = 218 × 109/L). Prognostic marker CD38 was positive, Zap70 was negative. Cytogenetics showed 13q14 deletion. He presented 6 months later with holocranial headache, sudden onset vision loss and altered sensorium. He had accelerated hypertension (BP = 190/130 mmHg). MRI brain showed lacunar infarct in left thalamus. CSF showed 20 polymorphs with normal sugar and proteins. He was treated for hypertensive encephalopathy and recovered sensorium and normal vision. Since he was asymptomatic, he was kept on observation for CLL. 3 months later he again presented with similar history of headache, giddiness and transient visual obscurations. He was normotensive this time. He had bilateral papilledema. A repeat MRI brain showed lacunar infarcts and bulky optic nerves. CSF showed 3,200 cells, all lymphocytes, proteins 140 mg/dL, sugars 45 mg/dL, ADA = 6 U. CSF malignant cytology showed infiltration by DLBCL (Fig. 5). He was treated with R-DHAP protocol.
Fig. 5.
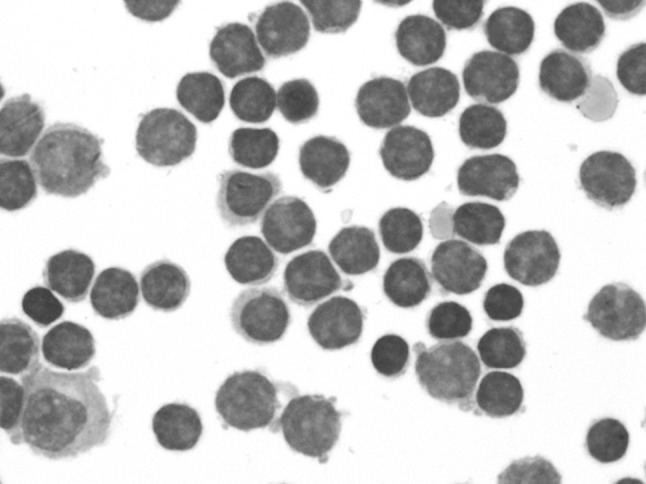
CSF cytospin smears showing infiltration by large lymphoid cells. Flow was consistent with mature B-cell lymphoma
Case 10
A 65 year old gentleman had presented in the year 2012 with left sided tonsillar enlargement and dysphagia. Hemogram revealed lymphocytosis (ALC = 29.78 × 109/L), Hb = 119 g/L, platelet = 1.39 × 109/L. Bone marrow flow cytometry confirmed the diagnosis of CLL, ZAP70 and CD38 was negative. He had no lymphadenopathy or hepato-splenomegaly. He was observed initially as he had no other symptoms related to CLL. However as the tonsillar swelling increased crossing over to the midline over the next 4 months, a tonsillectomy was done. Histopathological examination of the tonsillar mass revealed diffuse large B cell lymphoma (DLBCL) (Fig. 6). He was treated with six cycles of R-CHOP-21 following which he achieved a CR (ALC = 2.34 × 109/L). He was offered maintenance rituximab every 3 months. However he relapsed within 6 months (ALC = 21.4 × 109/L). Currently he has rapid lymph-node and lymphocyte doubling time. Cervical LN biopsy was suggestive of CLL/SLL and did not show high grade morphology. He is planned for therapy with BR.
Fig. 6.
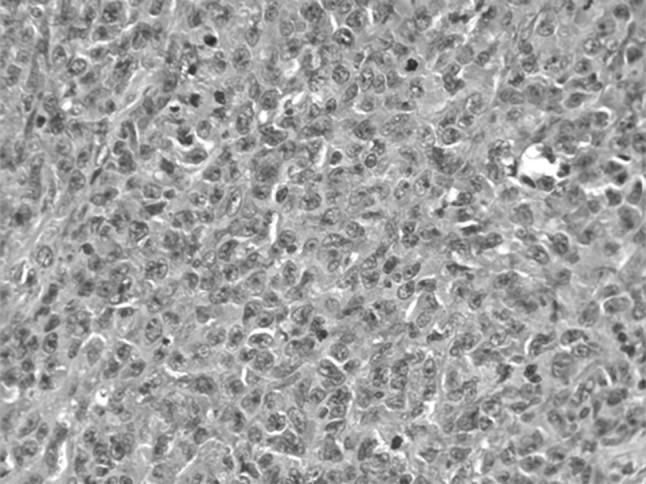
Tonsil histopathology shows diffuse large B-cell lymphoma in a case of CLL
Though CNS involvement by CLL is rare with less than 100 cases reported ante mortem, Richter’s transformation of the CNS is even rarer with only about 17 cases reported in literature [43]. CNS Richter’s can involve the brain parenchyma, leptomeninges as seen in our case or the dura mater. Optic nerve infiltration associated with leukemia meningitis has also been described in CLL [44]. Diagnosis can be confirmed by CSF immunophenotyping as was done in our case. Treatment may include radiotherapy or intrathecal chemotherapy with or without systemic chemo-immunotherapy. There is a report of sustained response with intrathecal liposomal cytarabine [45]. Whether case ten represents Richter’s transformation of CLL in the tonsils or concurrent DLBCL and CLL remains a matter of debate. There are no cases in literature reporting either occurrence to the best of our knowledge.
Acknowledgments
Conflict of interest
All the authors declare that they have no competing interests.
Footnotes
Deepesh Lad, Pankaj Malhotra, Neelam Varma, Manupdesh Singh Sachdeva, Ashim Das, Radhika Srinivasan, Amanjit Bal, Alka Khadwal, Gaurav Prakash, Vikas Suri, Savita Kumari, Sanjay Jain and Subhash Varma have contributed equally to the manuscript.
References
- 1.Kumar N, Varma N, Varma S, Malhotra P. Study of diagnostic and prognostic parameters (including flow cytometry) in chronic lymphoproliferative disorders: the Indian perspective. Anal Quant Cytol Histol. 2009;31:296–303. [PubMed] [Google Scholar]
- 2.Da’as N, Polliack A, Cohen Y, et al. Kidney involvement and renal manifestations in non-Hodgkin’s lymphoma and lymphocytic leukemia: a retrospective study in 700 patients. Eur J Haematol. 2001;67:158–164. doi: 10.1034/j.1600-0609.2001.5790493.x. [DOI] [PubMed] [Google Scholar]
- 3.Mutluay R, Aki SZ, Erten Y, et al. Membranoproliferative glomerulonephritis and light-chain nephropathy in association with chronic lymphocytic leukemia. Clin Nephrol. 2008;70:527–531. doi: 10.5414/CNP70527. [DOI] [PubMed] [Google Scholar]
- 4.Aslam N, Nseir NI, Viverett JF, Bastacky SI, Johnson JP. Nephrotic syndrome in chronic lymphocytic leukemia: a paraneoplastic syndrome? Clin Nephrol. 2000;54:492–497. [PubMed] [Google Scholar]
- 5.Davis MN, Alloy AM, Chiesa JC, Pecora AA. Chronic lymphocytic leukemia presenting with massive chylous ascites. Am J Gastroenterol. 1990;85:593–596. [PubMed] [Google Scholar]
- 6.Yonal I, Nazligul E, Tas G, Agan MR, Yenerel MN, Nalcaci M. A case of chronic lymphocytic leukemia with massive ascites. Rare Tumors. 2012;4:13. doi: 10.4081/rt.2012.e51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Siddiqui N, Al-Amoudi S, Aleem A, Arafah M, Al-Gwaiz L. Massive ascites as a presenting manifestation of chronic lymphocytic leukemia. World J Gastroenterol. 2008;14:3594–3597. doi: 10.3748/wjg.14.3594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mamode C, Beauregard P, Langevin S, Mongeau CJ. Chronic lymphoid leukemia complicated with ascites. Can J Gastroenterol. 2000;14:181D–184D. doi: 10.1155/2000/759693. [DOI] [PubMed] [Google Scholar]
- 9.Case records of the Massachusetts General Hospital Weekly clinicopathological exercises. Case 31-1983. Chronic lymphocytic leukemia with the recent development of hepatosplenomegaly and ascites. N Engl J Med. 1983;309:297–305. doi: 10.1056/NEJM198308043090508. [DOI] [PubMed] [Google Scholar]
- 10.May JT, Costanzi JJ. Ascites in chronic leukemia: a case report and review of the literature. Oncology. 1982;39:55–58. doi: 10.1159/000225605. [DOI] [PubMed] [Google Scholar]
- 11.Mouly S, Cochand-Priollet B, Halimi C, Bergmann JF. Portal hypertension caused by intra-hepatic block in chronic lymphoid leukemia. Presse Med. 1996;25:497–498. [PubMed] [Google Scholar]
- 12.Kuse R, Lueb H. Gastrointestinal involvement in patients with chronic lymphocytic leukemia. Leukemia. 1997;11:S50–S51. [PubMed] [Google Scholar]
- 13.Malhotra P, Singh M, Kochhar R, et al. Leukemia infiltration of bowel in chronic lymphocytic leukemia. Gastrointest Endosc. 2005;62:614–615. doi: 10.1016/S0016-5107(05)01644-5. [DOI] [PubMed] [Google Scholar]
- 14.Hisada M, Biggar RJ, Greene MH, Fraumeni JF, Jr, Travis LB. Solid tumors after chronic lymphocytic leukemia. Blood. 2001;98:1979–1981. doi: 10.1182/blood.V98.6.1979. [DOI] [PubMed] [Google Scholar]
- 15.Zhou Y, Tang G, Medeiros LJ, et al. Therapy-related myeloid neoplasms following fludarabine, cyclophosphamide, and rituximab (FCR) treatment in patients with chronic lymphocytic leukemia/small lymphocytic lymphoma. Mod Pathol. 2012;25:237–245. doi: 10.1038/modpathol.2011.158. [DOI] [PubMed] [Google Scholar]
- 16.Kumar N, Ahluwalia J, Malhotra P, Sachdeva MU. Chronic lymphocytic leukemia developing in a case of chronic myelogenous leukemia–accelerated phase: a rare case with review of the literature. Indian J Pathol Microbiol. 2013;56:303–305. doi: 10.4103/0377-4929.120406. [DOI] [PubMed] [Google Scholar]
- 17.Salim R, Wang L, Lin K, Clark RE. Chronic lymphocytic leukaemia developing in the course of chronic myeloid leukaemia. Leuk Lymphoma. 2002;43:2225–2227. doi: 10.1080/1042819021000016140a. [DOI] [PubMed] [Google Scholar]
- 18.Gargallo P, Cacchione R, Chena C, et al. Chronic lymphocytic leukemia developing in a patient with chronic myeloid leukemia: evidence of distinct lineage-associated genomic events. Cancer Genet Cytogenet. 2005;161:74–77. doi: 10.1016/j.cancergencyto.2005.01.008. [DOI] [PubMed] [Google Scholar]
- 19.Bhagavathi S, Borromeo V, Desai H, Crisan D. Case report and literature review: a rare patient with chronic myeloid leukemia and chronic lymphocytic leukemia. Ann Clin Lab Sci. 2008;38:405–409. [PubMed] [Google Scholar]
- 20.Chang H, Wechalekar A, Li L, Reece D. Molecular cytogenetic abnormalities in patients with concurrent chronic lymphocytic leukemia and multiple myeloma shown by interphase fluorescence in situ hybridization: evidence of distinct clonal origin. Cancer Genet Cytogenet. 2004;148:44–48. doi: 10.1016/S0165-4608(03)00217-6. [DOI] [PubMed] [Google Scholar]
- 21.Brouet JC, Fermand JP, Laurent G, et al. The association of chronic lymphocytic leukaemia and multiple myeloma: a study of eleven patients. Br J Haematol. 1985;59:55–66. doi: 10.1111/j.1365-2141.1985.tb02963.x. [DOI] [PubMed] [Google Scholar]
- 22.Browett PJ, Leber BF, Coustan-Smith E, Norton JD. Independent clonal origin of coexisting chronic lymphocytic leukaemia and multiple myeloma. Br J Haematol. 1988;70:126–127. doi: 10.1111/j.1365-2141.1988.tb02446.x. [DOI] [PubMed] [Google Scholar]
- 23.Fermand JP, James JM, Herait P, Brouet JC. Associated chronic lymphocytic leukemia and multiple myeloma: origin from a single clone. Blood. 1985;66:291–293. [PubMed] [Google Scholar]
- 24.Saltman DL, Ross JA, Banks RE, Ross FM, Ford AM, Mackie MJ. Molecular evidence for a single clonal origin in biphenotypic concomitant chronic lymphocytic leukemia and multiple myeloma. Blood. 1989;74:2062–2065. [PubMed] [Google Scholar]
- 25.Morrison VA. Infectious complications of chronic lymphocytic leukaemia: pathogenesis, spectrum of infection, preventive approaches. Best Pract Res Clin Haematol. 2010;23:145–153. doi: 10.1016/j.beha.2009.12.004. [DOI] [PubMed] [Google Scholar]
- 26.Johnston CA, Tang CK, Jiji RM. Histoplasmosis of skin and lymph nodes and chronic lymphocytic leukemia. Arch Dermatol. 1979;115:336–337. doi: 10.1001/archderm.1979.04010030044017. [DOI] [PubMed] [Google Scholar]
- 27.Kiratli H, Tarlan B, Uzun S, Tanas O, Uner A. Orbital richter syndrome. Orbit. 2013;32:381–383. doi: 10.3109/01676830.2013.815223. [DOI] [PubMed] [Google Scholar]
- 28.Skinnider LF, Romanchuk KG. Orbital involvement in chronic lymphocytic leukemia. Can J Ophthalmol. 1984;19:142–144. [PubMed] [Google Scholar]
- 29.Ramkissoon YD, Lee RW, Malik R, Hsuan JD, Potts MJ. Bilateral infiltrative disease of the extraocular muscles: a rare clinical presentation of early stage chronic lymphocytic leukemia. Orbit. 2008;27:293–295. doi: 10.1080/01676830802222878. [DOI] [PubMed] [Google Scholar]
- 30.Del Giudice I, Chiaretti S, Tavolaro S, et al. Spontaneous regression of chronic lymphocytic leukemia: clinical and biologic features of 9 cases. Blood. 2009;114:638–646. doi: 10.1182/blood-2008-12-196568. [DOI] [PubMed] [Google Scholar]
- 31.Thomas R, Ribeiro I, Shepherd P, et al. Spontaneous clinical regression in chronic lymphocytic leukaemia. Br J Haematol. 2002;116:341–345. doi: 10.1046/j.1365-2141.2002.03286.x. [DOI] [PubMed] [Google Scholar]
- 32.Nakhla PS, Butera JN, Treaba DO, Castillo JJ, Quesenberry PJ. Spontaneous regression of chronic lymphocytic leukemia to a monoclonal B-lymphocytosis or to a normal phenotype. Leuk Lymphoma. 2013;54:1647–1651. doi: 10.3109/10428194.2012.753449. [DOI] [PubMed] [Google Scholar]
- 33.Herishanu Y, Solar I, Ben-Ezra J, et al. Complete spontaneous regression of chronic lymphocytic leukemia. J Clin Oncol. 2012;30:29. doi: 10.1200/JCO.2011.40.7536. [DOI] [PubMed] [Google Scholar]
- 34.D’Arena G, Laurenti L, Coscia M, et al. Complementary and alternative medicine use in patients with chronic lymphocytic leukemia: an Italian multicentric survey. Leuk Lymphoma. 2013;17:17. doi: 10.3109/10428194.2013.803223. [DOI] [PubMed] [Google Scholar]
- 35.Hoption Cann SA, van Netten JP, van Netten C. Dr William Coley and tumour regression: a place in history or in the future. Postgrad Med J. 2003;79:672–680. [PMC free article] [PubMed] [Google Scholar]
- 36.Hamblin TJ. Autoimmune complications of chronic lymphocytic leukemia. Semin Oncol. 2006;33:230–239. doi: 10.1053/j.seminoncol.2006.01.011. [DOI] [PubMed] [Google Scholar]
- 37.Gonzalez-Gay MA, Blanco R, Gonzalez-Lopez MA. Simultaneous presentation of giant cell arteritis and chronic lymphocytic leukemia. J Rheumatol. 1997;24(2):407–408. [PubMed] [Google Scholar]
- 38.Branagan NM, Higgins SP, Halim SA, Le TH. Systemic polyarteritis nodosa mimicking pyoderma gangrenosum in a rare association with small lymphocytic leukaemia/chronic lymphocytic leukaemia. Clin Exp Dermatol. 2009;34:1365–2230. doi: 10.1111/j.1365-2230.2008.03152.x. [DOI] [PubMed] [Google Scholar]
- 39.Lulla P, Bandeali S, Baker K. Fatal paraneoplastic systemic leukocytoclastic vasculitis as a presenting feature of chronic lymphocytic leukemia. Clin Lymphoma Myeloma Leuk. 2011;11:29. doi: 10.1016/j.clml.2011.03.030. [DOI] [PubMed] [Google Scholar]
- 40.Polliack A, Lugassy G. Autoimmunity and auto-immune syndromes associated with and preceding the development of lymphoproliferative disorders. Leukemia. 1992;4:152–154. [PubMed] [Google Scholar]
- 41.Cerroni L, Zenahlik P, Hofler G, Kaddu S, Smolle J, Kerl H. Specific cutaneous infiltrates of B-cell chronic lymphocytic leukemia: a clinicopathologic and prognostic study of 42 patients. Am J Surg Pathol. 1996;20:1000–1010. doi: 10.1097/00000478-199608000-00009. [DOI] [PubMed] [Google Scholar]
- 42.Pavlidis NA, Klouvas G, Tsokos M, Bai M, Moutsopoulos HM. Cutaneous lymphocytic vasculopathy in lymphoproliferative disorders–a paraneoplastic lymphocytic vasculitis of the skin. Leuk Lymphoma. 1995;16:477–482. doi: 10.3109/10428199509054437. [DOI] [PubMed] [Google Scholar]
- 43.Lopes da Silva R. Spectrum of neurologic complications in chronic lymphocytic leukemia. Clin Lymphoma Myeloma Leuk. 2012;12:164–179. doi: 10.1016/j.clml.2011.10.005. [DOI] [PubMed] [Google Scholar]
- 44.Saenz-Frances F, Calvo-Gonzalez C, Reche-Frutos J, et al. Bilateral papilledema secondary to chronic lymphocytic leukaemia. Arch Soc Esp Oftalmol. 2007;82:303–306. doi: 10.4321/S0365-66912007000500010. [DOI] [PubMed] [Google Scholar]
- 45.Calvo-Villas JM, Fernandez JA, de la Fuente I, Godoy AC, Mateos MC, Poderos C. Intrathecal liposomal cytarabine for treatment of leptomeningeal involvement in transformed (Richter’s syndrome) and non-transformed B-cell chronic lymphocytic leukaemia in Spain: a report of seven cases. Br J Haematol. 2010;150(5):618–620. doi: 10.1111/j.1365-2141.2010.08238.x. [DOI] [PubMed] [Google Scholar]