Abstract
One of the key purposes of a hemovigilance program is to improve reporting of transfusion related adverse events and subsequent data-driven improvement in blood transfusion (BT) practices. We conducted a study over 3 years to assess the impact of healthcare worker training and an active feedback programme on reporting of adverse reactions to BTs. All hospitalized patients who required a BT were included in the study. Healthcare workers involved in BT to patients were sensitized and trained in adverse reaction reporting by conducting training sessions and meetings. All the transfused patients were ‘actively’ monitored for any acute adverse reaction by using a uniquely coded blood issue form. A total of 18,914 blood components transfused to 5785 different patients resulted in 61 adverse reaction episodes. This incidence of 0.32 % in our study was found to be significantly higher (p < 0.005) than that reported from the same region in the past. Red blood cell units were the most frequently transfused component and thus most commonly involved in an adverse reaction (42.6 %), however apheresis platelets had the highest chance of reaction per unit transfused (0.66 %). There was no mortality associated with the BT during the study period. An active surveillance program significantly improves reporting and management of adverse reactions to BTs.
Keywords: Blood transfusion reactions, Transfusion reactions and India, Hemovigilance, Incidence
Introduction
Hemovigilance may be defined as the collection and analysis of information on the complications of blood transfusion (BT). One of the main purposes of developing a hemovigilance program is to improve reporting of transfusion related adverse events and subsequent data-driven improvement in BT practices [1].It was first introduced in France in 1993 where it was mandatory while first voluntary reporting system was introduced in United Kingdom in 1996. Since then the developed countries have taken a lead in the hemovigilance programme [2], however Asian countries like India are still lacking a well established system for hemovigilance. A hemovigilance program of India was launched in December 2012 with an initial road map for 5 years. One of the key objectives of this program is to create awareness among health care professionals about adverse reactions associated with blood and component transfusions (BT) and monitor these reactions [3]. We thus conducted a study to evaluate the effect of active hemovigilance program on reporting of BT reactions in an Indian hospital setup.
Materials and Methods
This study was conducted in a newly commissioned 250 bedded multispecialty hospital in North India over a period of 3 years from January 2011 to December 2013. All hospitalized patients who required a BT and consented for the same were included in the study. Blood bank issued 4 log leukodepleted packed red blood cells (RBC), while rests of the components issued for the transfusion were non-leukodepleted. Whole BT was provided for exchange transfusion in newborn only and all other patients received blood components. Random donor platelets (RDP) were issued without pooling because it is not permitted by the licensing and regulatory authorities in India. Single donor platelets (SDP) were prepared on automated apheresis devices (Amicus™, Fenwal Inc., Lake Zurich, USA and Trima Accel® Terumo BCT Inc, Lakewood, USA) however leukodepletion achieved was not determined on these SDP units. Alloantibody antibody screening using commercial 3 cell panel (Surgiscreen®, Ortho Clinical Diagnostics, New Jersey, USA) was done on blood samples from all the patients requiring BT. Prolonged storage (beyond 1–2 h) of blood units in the wards, operation theaters and ICUs was discouraged and blood was issued immediately prior to the transfusion.
All the blood and components issued by the blood bank within the hospital were ‘actively’ monitored for any adverse reaction. Patients were monitored before, during and after the BT in line with good transfusion practices [1] and hospital protocols. Patients who experienced a reaction associated with a BT were evaluated and managed by the treating doctors in consultation with the blood bank doctor. It was mandated to immediately report any transfusion reaction to the blood bank.
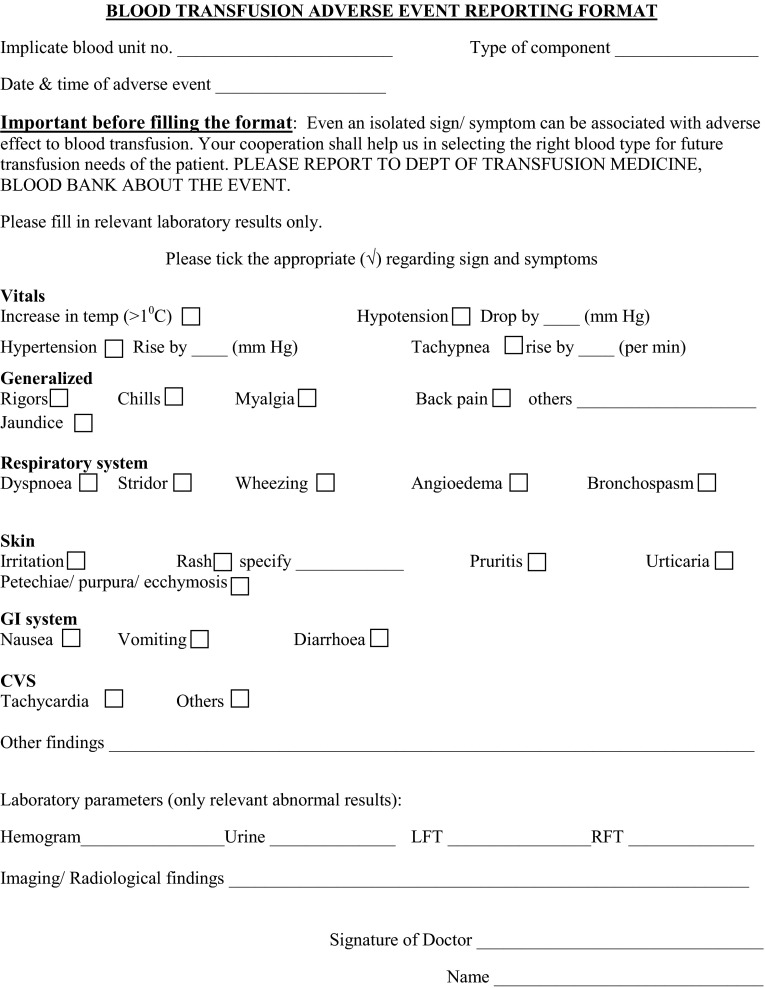
In our study, we sought the feedback on every BT received by the patients and not just adverse reaction cases. For this, all the compatibility cum reaction forms issued with blood units were coded with a unique serial number. This form was asked back by blood bank within 24 h of the transfusion. Serial number allowed tracking of all the compatibility forms and thus blood units issued during the study period. Junior doctors and nurses were trained to fill the reaction form and return it to the blood bank in all the cases irrespective of whether a blood reaction occurred or not. Reporting of the delayed adverse reactions (i.e. those happening after 24 h of a BT) to blood bank was also encouraged. To make reaction reporting objective, uniform as well as sensitive, the blood reaction form had a ‘body system’ vise questionnaire. To encourage compliance, this reaction questionnaire was made in the form of a check-list which could be easily filled in minimum time while capturing all the details (Fig. 1).
Fig. 1.
Blood transfusion reaction questionnaire and reporting format
Compatibility stickers were retained in the patient records. A detailed work-up with relevant investigations was done in all the BT reaction cases as per international standards and local SOP. This comprised a minimum of clerical check, repeat ABO, Rh testing, visual examination and Coomb’s test on pre- and post transfusion samples in all the reaction cases. Additionally other investigation guided by the patient’s reported sign and symptoms were done wherever required. A copy of the reaction work up report with findings, final interpretation and future management of the patient was sent back to the treating unit for filing in the patient record. Transfusion reactions were defined and classified for severity according to the guidelines provided by the International Society of Blood Transfusion working party on hemovigilance (ISBT) [4]. Same guidelines were used to determine the relation of the BT to the adverse transfusion reaction, i.e. imputability of the BT.
Staff Training
It was done as a two tier approach; senior clinicians were sensitized to the BT reactions and its reporting while nurses and junior doctors were trained on all the aspects of reporting of such reactions. The hospital transfusion committee meetings were chosen as a platform to sensitize the senior clinicians and major users of blood. Besides this, at least 1 conference per year and a survey on preferences and practices of senior clinicians was conducted during the study period.
Junior doctors and nursing staff was regularly sensitized and trained on the reporting aspects during the 30 min training session held every 3rd month during the study period. All the newly recruited nurses were trained for the BT reaction reporting in the induction training sessions held for them in the initial few days of their joining.
Statistical Calculation
Incidence of BT reaction was calculated as percentage of total units transfused collectively and according to the type of component. Since an active surveillance system was in place from the beginning of the hospital there was no past data for the comparison. Hence, a comparison was made with the incidence reported in the studies published in the indexed medical journals form the same region, i.e. North India [5, 6] to avoid any population bias. A p value less than 0.05 was considered as statistically significant. Chi square test was used to calculate the p value.
Observations and Results
Sensitization of Healthcare Workers
During the study period a total of 8 hospital transfusion committee meetings, 29 teaching sessions and 4 conferences were held to sensitize the doctors and nurses regarding BT reactions. Additionally, one practice and preference survey was also undertaken for the senior clinicians and heads of the major user departments.
Blood Transfusion Reactions
Most of the blood in our hospital was utilized for adult medical and surgical patients receiving blood for the first time. Our hospital did not have in-patient facilities for organ transplant and radiation oncology and there was no separate hemato-oncology unit.
During the study period a total of 61 adverse reactions were noted to an aggregate of 18,914 BT, i.e. an incidence of 0.32 %. These reactions were noted in 58 different patients; three patients reacted twice to BT during the same admission. Age of the patients experiencing an adverse reaction to BT ranged from 5 months to 89 years. A total of 11,776 compatibility cum reaction forms were used to issue these 18,914 blood components to 5785 different patients and 11,643 forms were received back in the blood bank. Almost all the forms which could not be received back in the blood bank were used in the operation theater and were lost in the transit. However no transfusion reaction was noted in the patients who were issued these (missed) forms and same was confirmed by personally communicating with the staff involved in the patient’s care.
Transfusion reactions to various blood and components are summarized in Table 1. Allergic reactions were the most common (60.7 %) type of reactions noted in our study. Although, RBCs were the most commonly implicated (42.6 %) component type for a BT reaction (Table 1), there was no difference in the incidence of reaction due to fresh frozen plasma (FFP) or RBC (0.35 vs 0.31 % respectively; p = 0.7; Table 2). We found that there was a higher than an average chance of a transfusion reaction with a single donor platelet transfusion (0.66 %; p = 0.04) as compared to other blood components. Total components issued and percentage of components which caused an adverse reaction, are shown in Table 2. Pre-transfusion medications to prevent BT reactions were not routinely administered in the hospital.
Table 1.
Types of acute non-infectious transfusion reactions seen with different blood components
| Type of reaction | Type of component | ||||
|---|---|---|---|---|---|
| FFP | RDP | RBC | SDP | Total (%) | |
| Allergic reaction | 18 | 4 | 10 | 5 | 37 (60.7) |
| Febrile non-hemolytic transfusion reactiona | 1 | 1 | 11 | 4 | 17 (27.9) |
| TACO/TADb | 1 | 0 | 3 | 0 | 4 (6.6) |
| Others | 1 | 0 | 2 | 0 | 3 (4.9) |
| Total (%) | 21 (34.4) | 5 (8.2) | 26 (42.6) | 9 (14.8) | 61 (100) |
aOnly rigors and/or chills with or without fever were classified as FHNTR as per definition
bTransfusion associated dyspnea
Table 2.
Reaction percentage according to the type of blood component transfused
| Type of component | Total reactions | Total units issued | Reaction percentage |
|---|---|---|---|
| FFP | 21 | 5970 | 0.35 |
| RDP | 5 | 3298 | 0.15 |
| RBC | 26 | 8276 | 0.31 |
| SDP | 9 | 1370 | 0.66 |
| Total | 61 | 18,914 | 0.32 |
Although it was mandatory to immediately report any adverse transfusion reaction to the blood bank, it was noted that 20 out of total 61 reactions (32.8 %) were not reported. Blood bank came to know of these reactions only through the compatibility cum reaction forms which were solicited back within 24 h of the transfusion. One case of Transfusion associated circulatory overload (TACO) was also not reported and was diagnosed retrospectively. All such un-reported reactions were also worked up in the blood bank and patient records were reviewed by the medical officer of blood bank. Types of unreported reactions are summarized in Table 3.
Table 3.
Classification of un-reported transfusion reactions
| Type of reaction | Type of component | ||||
|---|---|---|---|---|---|
| FFP | RDP | RBC | SDP | Total (%) | |
| Allergic reaction | 8 | 1 | 2 | 2 | 13 (65) |
| Febrile non-hemolytic transfusion reaction | 1 | 0 | 4 | 1 | 6 (30) |
| TACO | 0 | 0 | 1 | 0 | 1 (5) |
| Total (%) | 9 (45) | 1 (5) | 7 (35) | 3 (15) | 20 (100) |
BT reactions at our centre were compared with those reported in the literature from the same region. On searching the literature only two studies published by premier academic institutions from North India could be found for this comparison (Table 4). Unpublished data from another multi-superspeciality hospital in the same region of Delhi, North India was also obtained for the comparison (personal communication).
Table 4.
Comparison of non-infectious complications of blood transfusion reported in studies from North India
| Current study, Delhi (Jan 2011–Dec 2013) | AIIMS, Delhi Study [5] (Dec 2007–April 2012) | PGIMER, Chandigarh Study [6] (July 2002–July 2003) | Multi- superspeciality hospital, North, Delhib Jan 2012–Dec 2013 | |
|---|---|---|---|---|
| Units transfused | 18,914 | 380,658 | 56,503 | 8487 |
| Patients transfused | 5785 | NA | 29,720 | 2803 |
| RBC used | 4 log leukodepleted | Non-leukodepleted to leukodepleteda | Non-leukodepleted | Leukoreduced using buffy coat method |
| Alloantibody antibody screening done | Yes | No | No | Yes |
| Hemovigilance | Active feedback | Passive reporting | Passive reporting | Passive reporting |
| Number of total reactions | 61 | 196 | 105 | 12 |
| Reactions within 24 h of transfusion (% of total reactions) | 61 (100) | 195 (99.5) | 102 (97.1) | 12 (100) |
| Mortality due to acute transfusion reactions | 0 | NA | 7 (p = 0.04) | 0 |
| Non-immune hemolysis (% of total reactions) | 0 (0) | 5 (2.6) p = 0.21 |
6 (5.7) p = 0.06 |
0 (0) |
| Incidence of adverse reaction per unit transfused (%) | 0.32 | 0.05 p < 0.0001 |
0.19 p = 0.0005 |
0.14 p = 0.007 |
| Incidence of adverse reaction per patient transfused (%) | 1.00c | NA | 0.35 p < 0.0001 |
0.43 p = 0.005 |
NA data not available
aNon-leukodepleted from 2007 to 2009; 2010–2012—leukofiltered; personal communication
bPersonal communication
c61 reactions were seen in 58 different patients
The incidence of BT reactions was found to be significantly higher at our center where an active feedback on all the transfusions was sought (Table 4). All other centers compared in our study relied on a passive reporting of BT reactions to the blood bank. Percentage of reactions per unit as well as per patient transfused was also found to be significantly higher (p = 0.005) at our center.
No delayed transfusion reaction was reported in our study and all the reactions occurred within 24 h of the BT. Similarly other centers also reported nil to negligible delayed reactions. No case of non-immune hemolysis was found at our center. Similarly, there was no BT associated mortality in our study. Only one of the published study from the region reported mortality due to BT reaction and it was significantly higher when compared to that in our study (Table 4).
Although BT at our centre was not associated with any mortality, 10 out of 61 (16.4 %) reactions were classified as sever to life threatening as per ISBT criteria (Table 5). Imputability of the BT to the adverse reactions was possible to definite in approximately 97 % of the cases (Table 6).
Table 5.
Severity classification of adverse transfusion reactions as per ISBT criteria [4]
| Severity grade | Number of reactions (%) |
|---|---|
| 1 (Non severe) | 51 (83.6) |
| (The recipient may have required medical intervention but lack it would not result in permanent damage or impairment of body function) | |
| 2 (Severe) | 6 (9.8) |
| (The recipient required prolongation of hospitalization directly attributable to the reaction/persistent or significant disability/incapacity/reaction necessitated medical or surgical intervention to preclude permanent damage or impairment of body function) | |
| 3 (Life threatening) | 4 (6.6) |
| (The recipient required major intervention following the transfusion to prevent death) | |
| 4 (Death) | 0 (0.0) |
| (The recipient died following an adverse transfusion reaction) | |
| Total | 61 (100.0) |
Table 6.
Strength of relation of BT to adverse reaction as per ISBT criteria [4]
| Imputability | Total reactions (%) |
|---|---|
| Definite (certain) | 39 (63.9) |
| (Conclusive evidence beyond reasonable doubt that the adverse reaction can be attributed to the transfusion) | |
| Probable (likely) | 11 (18.0) |
| (Evidence is clearly in favor of attributing the adverse reaction to the transfusion) | |
| Possible | 9 (14.8) |
| (Evidence is indeterminate for attributing the adverse reaction to the transfusion or an alternate cause) | |
| Unlikely (doubtful) | 2 (3.3) |
| (Evidence is clearly in favor of attributing the adverse reaction to causes other than the transfusion) | |
| Excluded | 0 (0.0) |
| (Conclusive evidence beyond reasonable doubt that the adverse reaction can be attributed to causes other than the transfusion) | |
| Total | 61 (100.0) |
Discussion
Hemovigilance is a relatively recent addition to the concept of blood safety. It is a surveillance procedure covering the complete BT chain—from collection of blood and components to follow up of the recipients. One of the main aims is to identify trends in adverse reactions and events to a BT so that awareness regarding transfusion hazards can be increased and BT practices improved. The landmark hemovigilance scheme of the United Kingdom—Serious hazards of transfusion (SHOT) has already prompted many significant changes in BT practices over 17 years of its existence [7].
A hemovigilance program was recently launched in India also and this program is still in its early infancy. The road map based on local scientific evidences is still under preparation for this program in India. One of the key deciding features of any (hemovigilance) surveillance programme is involvement of its (healthcare) delivery team. We conducted this study to test the feasibility and impact of active surveillance programme on BT reaction reporting in an Indian set up. We then compared the results of our active surveillance system with that of the existing system of passive reporting to blood banks in India.
Mainstay of our surveillance program was active feedback on all the transfused units along with sensitization and training of healthcare workers associated with BT to the patients. All the blood units were tracked for a feedback within 24 h of issue from the blood bank. A standardized and structured reaction form was collected for all the issued units with comments of the doctor involved in that transfusion. Even when no reaction was noticed, this form was mandated to be returned to the blood bank with comments that no reaction occurred to BT. In case of an adverse reaction details of the reaction were to be reported in the structured format.
BT reaction incidence in our study was found to be 0.32 % which was significantly higher (p < 0.001) than that reported in the past from India [5, 6]. Also, this incidence in our study is very similar to that reported by some well developed hemovigilance networks in the developed countries. The incidence reported in the past from Quebec hemovigilance system in Canada, Auckland regional blood centre New Zealand, Washington.
University Missouri United States and French hemovigilance network is 0.35, 0.34, 0.23 and 0.37 % respectively (p > 0.05) [8–11]. Practices at our center like pre-storage leukodepletion of RBC and alloantibody antibody screening on all the patient samples would have expected a lower incidence of transfusion reaction. On the contrary, this incidence was found to be significantly higher than that reported by the other Indian centers compared in our study. This higher incidence in our study can be attributed mainly to the active feedback sought by the blood bank for all the transfused blood and components. It is commonly believed and acknowledged that there is an underreporting of BT reactions in a system of passive reporting. Blood centers in other countries also that relied on a passive reporting system have reported a lesser incidence of adverse reactions. The incidence reported by blood centers in Puerto Rico [12], Pakistan [13] and Serbia [14] was similarly less at a rate of 0.22, 0.12 and 0.13 % respectively (p < 0.05).
We did not rely merely on the voluntary reporting of the BT reactions by the nurses or doctors at the patient bedside. This is evident from the fact that nearly one-third of the reactions were discovered as a result of an active follow up by blood bank and were not voluntarily reported by the healthcare workers at patient bed side.
Underreporting of minor transfusion reactions is a known fact [15]. However, non-reporting of events like febrile non-hemolytic reactions (FNHTR) and TACO, as noticed in our study, can have serious consequences for the transfused patients. Circulatory overload as seen in TACO is one of the most frequent potentially severe complication of RBC transfusion [16]. Unfortunately, it is also one of the most underreported and under-diagnosed complication of BT [17, 18]. An active surveillance system as demonstrated in our study can improve reporting of such complications and prevent such potential morbidities and mortalities due to BT.
A well designed system for detecting BT reactions and training of staff involved in BT therapy are critical for accurate collection of such data [19]. Standardization of the reporting system is recommended to improve the monitoring of BT reactions. Active surveillance system not only improves reporting of adverse BT events, it may also decrease the incidence of non-immune hemolysis and resultant mortality. Bhattacharya et al. had reported [5] that non-immune hemolysis occurred mainly because of prolonged storage of RBC units outside blood bank.
Active feedback on all issued units within a stipulated time may tackle this issue of prolonged storage of blood units outside blood bank and thus decrease such incidents.
Most common reactions were allergic and anaphylactoid type in our study (60.7 %), a percentage similar to that by Kumar et al. from AIIMS, Delhi (60.2 %) [5] and Bhattacharya et al. from PGIMER, Chandigarh (37.8 %) [6]. Other studies also have reported a similar incidence of these non-infectious transfusion reactions [20, 21]. Therefore, while commonest type of reactions reported in our study were similar to that reported earlier from India, overall incidence was much higher in our study.
Characteristics of the transfused patient determine the chances of a BT reaction. Therefore, reaction per patient rather than the overall incidence seems to be a better predictor of the risk [21] and thus should be reported in all such studies. As our hospital was a new facility, software implementation was initially patchy and patient demographics could not be captured seamlessly.
We thus could not analyze the characteristics of the transfused population in terms of gender ratio, specialty and diagnosis. However, we found that 1 out of every 100 transfused patient experienced an adverse reaction to BT in our study population. This figure was again significantly higher than that reported from India (Table 4). Considering the increasingly large number of patients requiring BT every year [22], a large number of patients can be presumed to be exposed to this risk. However, most blood centers and hospitals lack a proper educational and counseling program on these risks for the BT recipients. Such an educational program will not only alleviate the BT recipient’s apprehensions, it will also further improve reporting of such BT reactions.
Conclusion
An active feedback on all the transfused blood units rather than mere passive reporting of the adverse BT reactions is an essential pre-requisite for an active hemovigilance programme. Healthcare worker sensitization and training is an equally important aspect of such a program. We showed in our study that an active surveillance program significantly improves the reporting and thus morbidity and mortality due to adverse BT reactions. We also demonstrated that this active surveillance can be implemented within the existing framework of blood banking in India.
Acknowledgments
We would like to thank Dr. Kabita Chatterjee, Head of the department, Transfusion Medicine, AIIMS, Delhi, India for providing the unpublished details of her study and encouraging words.
Contributor Information
Naveen Agnihotri, Phone: +91 11 45302282, Email: naveenagnihotri@gmail.com.
Ajju Agnihotri, Email: ajjuagnihotri@gmail.com.
References
- 1.Mazzei CA, Popovsky MA, Kopko PM. Noninfectious complications of blood transfusion. In: Roback JD, editor. Technical Manual. 17. Bethesda: American Association of Blood Banks; 2011. pp. 727–758. [Google Scholar]
- 2.Faber JC. Haemovigilance procedure in transfusion medicine. Haematol J. 2004;5:S74–S82. doi: 10.1038/sj.thj.6200427. [DOI] [PubMed] [Google Scholar]
- 3.Bisht A, Singh S, Marwaha N. Hemovigilance Program—India. Asian J Transfus Sci. 2013;7:73–74. doi: 10.4103/0973-6247.106744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Popovsky MA, Robillard P, Schipperus M, Stainsby D, Tissot JD and Wiersum J (2006). ISBT Working Party on Hemovigilance. Proposed standard definitions for surveillance of noninfectious adverse transfusion reactions. http://www.ihn1org.com/wp-content/uploads/2011/06/ISBT-definitions-for-non-infectious-transfusionreactions. pdf. Accessed 1 May 2013
- 5.Kumar P, Thapliyal R, Coshic P, Chatterjee K. Retrospective evaluation of adverse transfusion reactions following blood product transfusion from a tertiary care hospital: a preliminary step towards hemovigilance. Asian J Transfus Sci. 2013;7:109–115. doi: 10.4103/0973-6247.115564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bhattacharya P, Marwaha N, Dhawan HK, Roy P, Sharma RR. Transfusion related adverse events at the tertiary care center in North India: an institutional hemovigilance effort. Asian J Transfus Sci. 2011;5:164–170. doi: 10.4103/0973-6247.83248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bolton-Maggs PHB, Cohen H. Serious hazards of transfusion (SHOT) hemovigilance and progress is improving transfusion safety. BJH. 2013;163:303–314. doi: 10.1111/bjh.12547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Robillard P, Nawej KI, Jochem K. The Quebec hemovigilance system: description and results from the first two years. Transfus Apher Sci. 2004;31:111–122. doi: 10.1016/j.transci.2004.07.005. [DOI] [PubMed] [Google Scholar]
- 9.Henderson RA, Pinder L. Acute transfusion reactions. N Z Med J. 1990;103:509–511. [PubMed] [Google Scholar]
- 10.Uhlmann EJ, Isgriggs E, Wallhermfechtel M, Goodnough LT. Prestorage universal WBC reduction of RBC units does not affect the incidence of transfusion reactions. Transfusion. 2001;41:997–1000. doi: 10.1046/j.1537-2995.2001.41080997.x. [DOI] [PubMed] [Google Scholar]
- 11.Frère MC, Rapaille A, Bouillenne C, Gérard C, Sondag D, Verhees A. Analysis of 516 reports of reactions after the transfusion of labile blood products. Transfus Clin Biol. 2001;8:333–342. doi: 10.1016/S1246-7820(01)00185-9. [DOI] [PubMed] [Google Scholar]
- 12.Climent-Peris C, Vélez-Rosario R. Immediate transfusion reactions. P R Health Sci J. 2001;20:229–235. [PubMed] [Google Scholar]
- 13.Karim F, Moiz B, Shamsuddin N, Naz S, Khurshid M (2013). Root cause analysis of noninfectious transfusion complications and the lessons learnt. Transfus Apher Sci (Epub ahead of print) [DOI] [PubMed]
- 14.Grujić J, Gulan Z, Budakov Z. Importance of hemovigilance and reports on transfusion reaction in blood component therapy. Med Pregl. 2012;65:50–53. doi: 10.2298/MPNS1202050G. [DOI] [PubMed] [Google Scholar]
- 15.Narvios AB, Lichtiger B, Neumann JL. Underreporting of minor transfusion reactions in cancer patients. Med Gen Med. 2004;6:17. [PMC free article] [PubMed] [Google Scholar]
- 16.Kleinman S, Chan P, Robillard P. Risks associated with transfusion of cellular blood components in Canada. Transfus Med Rev. 2003;17:120–162. doi: 10.1053/tmrv.2003.50009. [DOI] [PubMed] [Google Scholar]
- 17.Narick C, Triulzi DJ, Yazer MH. Transfusion-associated circulatory overload after plasma transfusion. Transfusion. 2012;52:160–165. doi: 10.1111/j.1537-2995.2011.03247.x. [DOI] [PubMed] [Google Scholar]
- 18.Popovsky MA, Taswell RF (2002). Circulatory overload: An underdiagnosed consequence of transfusion. Vox Sang 83:469 (Abstr suppl)
- 19.Bennardello F, Fidone C, Spadola V, Cabibbo S, Travali S, Garozzo G, et al. The prevention of adverse reactions to transfusions in patients with hemoglobinopathies: a proposed algorithm. Blood Transfus. 2013;11:377–384. doi: 10.2450/2013.0017-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Karim F, Moiz B, Shamsuddin N, Naz S, Khurshid M (2013). Root cause analysis of noninfectious transfusion complications and the lessons learnt. Transfus Apher Sci 31 (Epub ahead of print) [DOI] [PubMed]
- 21.Kato H, Uruma M, Okuyama Y, Fujita H, Handa M, Tomiyama Y, et al. Incidence of transfusion-related adverse reactions per patient reflects the potential risk of transfusion therapy in Japan. Am J Clin Pathol. 2013;140:219–224. doi: 10.1309/AJCP6SBPOX0UWHEK. [DOI] [PubMed] [Google Scholar]
- 22.Choudhury N. Blood transfusion in borderless South Asia. Asian J Transfus Sci. 2011;5:117–120. doi: 10.4103/0973-6247.83234. [DOI] [PMC free article] [PubMed] [Google Scholar]