Abstract
Erythrocytes undergo various changes during storage (storage lesion) that in turn reduces their functioning and survival. Oxidative stress plays a major role in the storage lesion and antioxidants can be used to combat this stress. This study elucidates the effects of l-carnitine (LC) on erythrocytes of stored blood. Blood was obtained from male Wistar rats and stored (4 °C) for 20 days in CPDA-1 (citrate phosphate dextrose adenine) solution. Samples were divided into–(i) controls (ii) LC 10 (l-carnitine at a concentration of 10 mM) (iii) LC 30 (l-carnitine at a concentration of 30 mM) and (iv) LC 60 (l-carnitine at a concentration of 60 mM). Every fifth day, the biomarkers (haemoglobin, hemolysis, antioxidant enzymes, lipid peroxidation and protein oxidation products) were analysed in erythrocytes. Hemoglobin and protein sulfhydryls were insignificant during storage indicative of the maintenance of hemoglobin and sulfhydryls in all groups. Superoxide dismutase and malondialdehyde levels increased initially and decreased towards the end of storage. The levels of catalase and glutathione peroxidase were lower in experimentals than controls during storage. l-carnitine assisted the enzymes by scavenging the reactive oxygen species produced. Hemolysis increased in all groups with storage, elucidating that l-carnitine could not completely protect lipids and proteins from oxidative stress. Hence, this study opens up new avenues of using l-carnitine as a component of storage solutions with combinations of antioxidants in order to maintain efficacy of erythrocytes.
Keywords: Erythrocytes, Storage, l-carnitine, Lipid peroxidation, Protein oxidation
Introduction
Erythrocytes undergo various biochemical and structural changes that impair their oxygen-delivering capacity and trigger secondary reactions when stored [1–3]. These changes, collectively constituting the “storage lesion”, are characterized by morphological, physiological, biochemical, metabolic and biomechanical changes. Oxidative stress (OS) has been shown to play a major role in the formation of storage lesion [4–7]. Various mechanisms inherent to erythrocytes combat the damage caused by oxidative stress, primarily involving small molecules that efficiently repair damage to macromolecules [8–10]. A variety of components act against the damage caused by OS, which are of both endogenous and exogenous origin, such as (i) nutrient-derived antioxidants, (ii) antioxidant enzymes and (iii) metal binding proteins [11].
Carnitine is one of the nutrient-derived, non-enzymatic antioxidants [12, 13] that plays an important role in phospholipid fatty acid turnover. l-carnitine, the biologically active stereoisomer, is an endogenous compound derived from the diet or synthesized in the liver from lysine and methionine [14, 15].
Erythrocytes, which do not contain mitochondria, have substantial amounts of l-carnitine and its esters [16]. The effect of l-carnitine on erythrocytes has been studied by Arduini et al. [17] which showed that the uptake of l-carnitine during erythrocyte storage was associated with lower hemolysis and higher erythrocyte-ATP (adenosine triphosphate) levels and by a significantly greater in vivo viability in l-carnitine erythrocytes stored in additive solution-3 (AS-3). l-carnitine or its short-chain esters have demonstrated a positive effect on the stability of erythrocyte membranes under various adverse conditions and they also have been found to affect the molecular dynamics of erythrocyte membrane components [18–22].
Though there are few studies on carnitine and storage [17], the overall effect of l-carnitine on erythrocytes of stored blood has not been evaluated. The storage lesion developed in rats in CPDA-1 (citrate phosphate dextrose adenine) stored for one week is similar to that of human erythrocytes stored for 4 weeks [23]. Hence, this study aims to investigate the influence of l-carnitine on rat erythrocytes during blood storage, by assessing the following biomarkers of OS - hemoglobin, antioxidant enzymes and protein oxidation and lipid peroxidation products.
Materials and Methods
Animal Care and Maintenance
Animal care and maintenance was in accordance with the ethical committee regulations (841/b/04/CPCSEA).
Blood Sampling
Animals were lightly anaesthetized with ether and restrained in dorsal recumbancy as described earlier [6]. In brief, the syringe needle was inserted just below the xyphoid cartilage and slightly to the left of midline. Blood was carefully aspirated from the heart into plastic collecting tubes with CPDA-1 solution.
Experimental Design
Blood was drawn from male Wistar rats (4 months old) and stored over a period of time (20 days) at 4 °C in CPDA-1 solution in polypropylene tubes. Blood was collected from 20 animals and divided into four groups (5 animals each)– (i) Controls (ii) LC 10 (samples with l-carnitine at a concentration of 10 mM) (iii) LC 30 (samples with l-carnitine at a concentration of 30 mM) and (iv) LC 60 (samples with l-carnitine at a concentration of 60 mM Erythrocytes were isolated from the above samples every fifth day and the biomarkers of OS were studied.
Erythrocyte Separation
The erythrocytes were isolated by centrifugation for 20 min at 1000×g at 4 °C. The plasma and buffy coat were removed using a micropipette. The cell pellet was washed and finally suspended in isotonic phosphate buffer [24]. This constituted the erythrocyte suspension.
Hemoglobin Estimation
Hemoglobin (Hb) was measured using Hemocor-D Kit [Coral Clinical Systems, Goa, India], which utilizes cyanomethemoglobin method [25]. Whole blood was incubated with Hb reagent for 3 min at room temperature and absorbance was measured colorimetrically at 540 nm. Hb concentration was represented in terms of g dl−1.
Antioxidant Enzymes
Superoxide Dismutase [SOD, EC 1.15.1.1]
SOD was measured by the method of Misra and Fridovich [26]. Hemolysate was added to carbonate buffer [0.05 M]. Epinephrine was added to the mixture and measured [ELICO, Model SL 159, India] at 480 nm. SOD activity is expressed as the amount of enzyme that inhibits oxidation of epinephrine by 50 %.
Catalase: [CAT, EC 1.11.1.6]
CAT was determined by the method of Aebi [27]. Briefly, hemolysate with absolute alcohol was incubated at 0 °C. An aliquot was taken up with 6.6 mM H2O2 and decrease in absorbance was measured at 240 nm. An extinction coefficient of 43.6 M cm−1 was used to determine enzyme activity.
Glutathione Peroxidase [GSH-Px, EC.1.11.1.9]
GSH-Px was analyzed by the method of Flohe and Gunzler [28]. Fifty microlitres of 0.1 M phosphate buffer (pH 7.0), 100 µL enzyme sample, 100 µL glutathione reductase (0.24 units), and 100 µL of 10 mM GSH (glutathione) were mixed and pre-incubated for 10 min at 37 °C followed by addition of 100 µL of 1.5 mM nicotinamide adenine dinucleotide phosphate (NADPH) in 0.1 % sodium bicarbonate. The overall reaction was started by adding 100 µL of pre-warmed hydrogen peroxide and the decrease in absorption at 340 nm was monitored for three min. Enzyme activity was expressed as units/g Hb; 1 unit corresponds to 1 mM NADPH oxidized/min.
Malondialdehyde (MDA)
Malondialdehyde (MDA), a product of lipid peroxidation was determined according to the method of Ohkawa et al. [29]. In brief, the hemolysate was added to 8.1 % sodium dodecyl sulphate (SDS), vortexed and incubated at room temperature. This was followed by the addition of 20 % acetic acid and 0.6 % thiobarbituric acid, and placed in boiling water bath. The samples were allowed to cool and butanol-pyridine was added and centrifuged. Absorbance of the coloured layer was measured at 532 nm with 1, 1, 3, 3-tetramethoxy propane as a standard. MDA concentration was expressed as nmol mg−1 protein.
Protein Sulfhydryls (P-SH)
The concentration of P-SH groups in the proteins was measured in lysate as described by Habeeb [30]. In brief, 0.08 mol L−1 sodium phosphate buffer containing 0.5 mg mL−1 of ethylene diamine tetra acetic acid disodium salt (Na2-EDTA), and 2 % SDS were added to each assay tube. 0.1 ml of 5, 5′-dithiobis-2-nitrobenzoic acid (DTNB) was also added. Absorbance was measured at 412 nm. P-SH was calculated from the net absorbance and molar absorptivity, 13,600 M−1 L−1 cm−1.
Hemolysis
A 5 % suspension of packed erythrocytes in buffer was mixed with the equal volume of 8 mM H2O2. The mixtures were incubated at 37 °C in an incubator. Hemolysis was determined by measuring released Hb into the supernatant of the induced samples at 540 nm and expressed on the basis of the maximum absorbance [100 %] in the aliquots of erythrocytes completely hemolysed in distilled water [31].
Protein Determination
Protein was determined in the lysate and membrane by the method of Lowry et al. using bovine serum albumin as the standard [32].
Statistical Analyses
Results are represented as mean ± standard error (SE). Values between the groups were analyzed by two-way analysis of variance (ANOVA) and was considered significant at P < 0.05. Bonferroni’s Post Test was performed for all the assays using GraphPad Prism 5 software. Values between the groups are represented in upper case, while those within the groups are represented in lower case.
Results
Hemoglobin
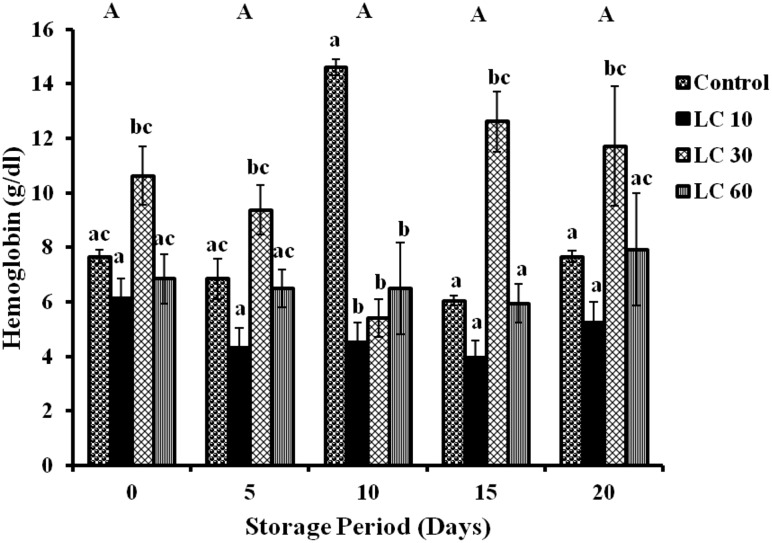
Changes in Hb were insignificant during storage period in all samples, although changes between the subgroups were observed (Fig. 1).
Fig. 1.
Hemoglobin level in erythrocytes of stored blood. Values are expressed as mean ± SE from 5 samples. LC 10—l-carnitine (10 mM); LC 30—l-carnitine (30 mM); LC 60—l-carnitine (60 mM). Changes between groups (across storage) are represented in upper case, while changes within a group (across treatment groups) are represented in lower case. Those not sharing the same letters are significant
Antioxidant Enzymes
Superoxide Dismutase (SOD)
Significant changes in SOD activity were observed in all groups with storage. Increases by 24 and 39 % (on days 5 and 15) and 100 % (day 10) were observed in controls (day 0). SOD activity showed amplifications of 13 and 43 % (days 5 and 10) in LC 10 and 66, 11, 25 and 16 % on days 5, 10, 15 and 20 respectively in LC 30 were observed against the corresponding day 0 samples. LC 60 showed 15 % increase (days 5 and 15) and 15 % decrease (days 10 and 20) with storage (Table 1).
Table 1.
Antioxidant enzymes in erythrocytes of stored blood
| Days | Groups | SOD (Units/g Hb)* | CAT (Units/mg Hb × 10−4)* | GSH-Px (Units/g Hb)* |
|---|---|---|---|---|
| Day 0 | Control | 21674.43 ± 1158.58a | 52.91 ± 13.57a | 85.13 ± 12.09a |
| LC 10 | 25461.98 ± 5569.92a | 49.52 ± 7.72a | 80.34 ± 3.54a | |
| LC 30 | 24732.69 ± 829.26a | 52.57 ± 3.39a | 117.40 ± 12.07a | |
| LC 60 | 30383.70 ± 980.80a | 45.11 ± 5.16a | 102.24 ± 10.92a | |
| Day 5 | Control | 26942.84 ± 4761.74a | 167.05 ± 37.24a | 70.63 ± 43.23a |
| LC 10 | 28817.64 ± 6437.26ac | 15.94 ± 1.57b | 20.31 ± 9.64a | |
| LC 30 | 41145.49 ± 3237.92bc | 66.48 ± 26.57b | 68.55 ± 9.43a | |
| LC 60 | 35921.80 ± 1429.36ac | 45.45 ± 11.64b | 53.50 ± 5.81a | |
| Day 10 | Control | 53978.11 ± 7138.78a | 151.96 ± 19.10a | 271.84 ± 23.07a |
| LC 10 | 36530.04 ± 4546.99b | 46.81 ± 7.20b | 64.94 ± 13.89b | |
| LC 30 | 27412.87 ± 899.94b | 53.59 ± 15.45b | 53.10 ± 5.70b | |
| LC 60 | 25284.77 ± 653.75b | 32.22 ± 11.03b | 50.72 ± 2.73b | |
| Day 15 | Control | 30128.26 ± 3714.96a | 75.64 ± 6.63a | 175.54 ± 19.21a |
| LC 10 | 25228.22 ± 3286.54a | 36.97 ± 3.73a | 86.34 ± 5.86b | |
| LC 30 | 31098.56 ± 2523.21a | 52.23 ± 12.63a | 62.51 ± 9.86b | |
| LC 60 | 33572.79 ± 1366.76a | 31.88 ± 9.20a | 76.88 ± 9.05b | |
| Day 20 | Control | 19748.57 ± 3260.04a | 57.32 ± 12.66a | 11.39 ± 4.51a |
| LC 10 | 27717.28 ± 4212.19a | 15.77 ± 6.57a | 59.64 ± 10.44a | |
| LC 30 | 28574.44 ± 938.067a | 17.64 ± 5.21a | 55.67 ± 5.31a | |
| LC 60 | 26356.16 ± 681.45a | 20.69 ± 3.61a | 56.17 ± 7.34a |
* Changes in SOD, CAT and GSH-Px were significant between the groups. LC 10—l-carnitine (10 mM); LC 30—l-carnitine (30 mM); LC 60—l-carnitine (60 mM).Values are expressed as Mean ± SE from 5 samples
Changes within the groups are represented in lower case. Those not sharing the same letters are significant
Catalase (CAT)
Significant changes in CAT activity with antioxidants and storage were observed in all groups. Increments of 200 % (day 5), 100 % (day 10) and 43 % (day 15) were observed in controls (day 0). LC 10 showed decrements of 68 % on days 5 and 20 and 25 % on day 15. LC 30 showed an initial increase of 26 % on day 5, followed by a decrease of 67 % on day 20. CAT also reduced in LC 60 by 30 % on days 10 and 15 and 54 % on day 20 (Table 1).
Glutathione Peroxidase (GSH-Px)
GSH-Px activity was found to be significant with storage period in all groups. There were elevations of 200 and 100 % on days 10 and 15 respectively and reductions of 17 % (day 5) and 87 % (day 20), in controls (day 0). GSH-Px activity decreased in LC 10 by 75 % (day 5) and 23 % (days 10 and 20); in LC 30 by 50 % (days 5, 10, 15 & 20) and in LC 60 by 50 % (on days 5, 10 and 20) and 25 % (day 15) with respect to day 0 (Table 1).
Malondialdehyde (MDA)
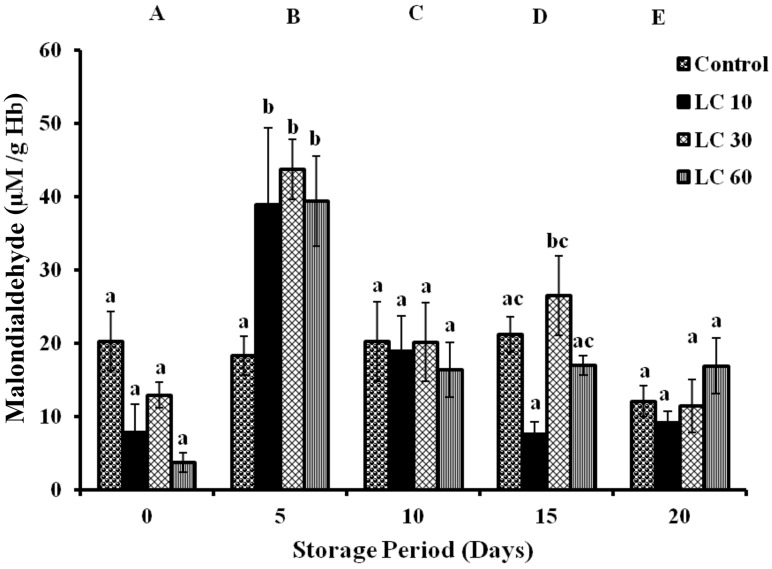
Malondialdehyde (MDA) was assayed as a measure of lipid peroxidation and significant changes were observed between the groups during storage period. Controls showed a decrease of 40 % on day 20. MDA in LC 10 increased by 3 folds and 1 fold (days 5 and 10). In LC 30, MDA elevated by 2 folds (day 5), 56 % (day 10) and 1 fold (day 15) with respect to day 0. LC 60 showed 9 folds (day 5) and 3 folds (days 10, 15 and 20) increments in MDA with storage (Fig. 2).
Fig. 2.
Lipid peroxidation in erythrocytes of stored blood in terms of Malondialdehyde. Values are expressed as Mean ± SE from 5 samples. LC 10—l-carnitine (10 mM); LC 30—l-carnitine (30 mM) LC 60—l-carnitine (60 mM). Changes between groups (across storage) are represented in upper case, while changes within a group (across treatment groups) are represented in lower case. Those not sharing the same letters are significant
Protein Sulfhydryls (P-SH)
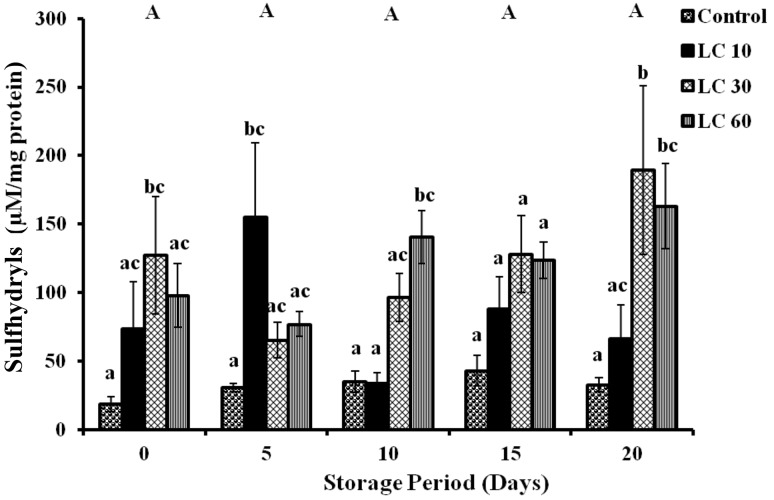
Changes in P-SH were found to be insignificant during storage period in all samples, although changes between the groups of different concentrations of l-carnitine were observed (Fig. 3).
Fig. 3.
Protein sulfhydryls in erythrocytes of stored blood (Lysate). Values are expressed as mean ± SE from 5 samples. LC 10—l-carnitine (10 mM); LC 30—l-carnitine (30 mM) LC 60—l-carnitine (60 mM). Changes between groups (across storage) are represented in upper case, while changes within a group (across treatment groups) are represented in lower case. Those not sharing the same letters are significant
Hemolysis
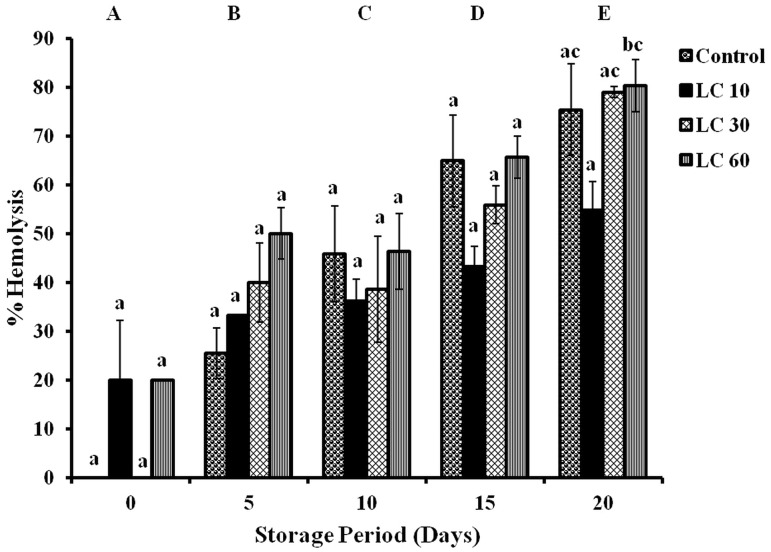
Significant changes in hemolysis were observed in all groups with storage. Hemolysis increased by 26, 46, 65 and 75 % (all days) in controls during storage. Levels of hemolysis increased in LC 10 (15 %—days 5 and 10; 23 %—day 15; 35 %—day 20), in LC 30 (40 %—days 5 and 10; 56 %—day 15; 80 %—day 20) and in LC 60 (30, 26, 46 and 60 %—on all days) with storage (Fig. 4).
Fig. 4.
Hemolysis in erythrocytes of stored blood. Values are expressed as mean ± SE from 5 samples. LC 10—l-carnitine (10 mM); LC 30—l-carnitine (30 mM) LC 60—l-carnitine (60 mM). Changes between groups (across storage) are represented in upper case, while changes within a group (across treatment groups) are represented in lower case. Those not sharing the same letters are significant
Discussion
Erythrocytes are particularly susceptible to oxidative damage because (i) being oxygen carriers, they are exposed uninterruptedly to high oxygen tension [33, 34] and (ii) hemoglobin is susceptible to autoxidation and their membrane components to lipid peroxidation [35, 36]. l-carnitine did not play a major role in maintaining Hb.
The antioxidant enzymes have a major role in maintaining oxidative stress in erythrocytes. SOD catalyzes the disproportionation reaction of superoxide radicals into H2O2 and O2. The superoxide radical can dismutate into hydrogen peroxide and react with Hb to generate additional potent oxidation by products [36, 37]. Increase in SOD activity is indicative of increased generation of superoxide radicals [38]. Our results of increased SOD during storage in controls (till day 10), is in correlation with increase in reactive oxygen species (ROS) [39]. SOD decreased towards the end of storage to the initial day 0 values, indicative of the effective antioxidant system in erythrocytes which scavenged the free radicals. In the l-carnitine groups, on day 10, the levels of SOD were found to be lower than that of the control. This can be attributed to the antioxidant activity and modulation of SOD by l-carnitine, to counteract the excess free radicals produced during storage [40]. l-carnitine and its derivatives have been shown to prevent the formation of ROS [39], scavenge free radicals (superoxide and hydrogen peroxide) and protect cells from peroxidative stress [12, 41–44].
Catalase and GSH-Px act on the same substrate, H2O2, at high and low concentrations respectively [35, 45, 46]. In accordance with the SOD activity, CAT and GSH-Px were activated initially during storage. The levels of CAT increased in controls on days 5 & 10, while GSH-Px showed increases on days 10 and 15 corresponding to the SOD activity, in turn indicative of the H2O2 produced. However, the levels of CAT and GSH-Px in the experimentals were lower than those in controls as storage progressed. This can be attributed to (i) the initial scavenging of superoxide by l-carnitine, hence reducing the H2O2 formed and (ii) the H2O2 scavenging activity of l-carnitine [12, 40].
During storage, OS causes various changes in the erythrocyte membrane, affecting proteins and lipids. Free radicals and intermediate peroxidation products damage the integrity and functioning of the membrane [47–49]. Lipids (cholesterol, polyunsaturated fatty acids (PUFA)) are the main targets of oxidative attack and this leads to the formation and the accumulation of lipid peroxidation (LPO) products [50–52].
Malondialdehyde (MDA) is a marker of membrane LPO resulting from the interaction of ROS and the cellular membrane. The levels of MDA decreased in controls towards the end of storage, indicative of the effective antioxidant system in the erythrocytes. The decrease in MDA can also be attributed to microvesiculation which occurs in vitro [53] In the l-carnitine groups, peaks were observed on day 5 due to increase in ROS. The decrease in MDA in the LC groups to the control levels can be attributed to the combined effect of the initial scavenging of free radicals by l-carnitine [54] and the inherent antioxidant system of the erythrocytes. Although l-carnitine reduces MDA levels by facilitating fatty acid transport thereby lowering the availability of lipids for peroxidation [55], in our study l-carnitine has helped to maintain MDA levels by breaking the chain of ROS formation.
Protein oxidation is defined as the covalent modification of a protein induced either directly by ROS or indirectly by reaction with secondary by-products of oxidative stress [56]. ROS can lead to oxidation of amino acid residue side chains, formation of protein–protein cross-linkages, and oxidation of the protein backbone resulting in protein fragmentation [57]. Protein sulfhydryls are sites of reversible and irreversible oxidative modifications. Sulfhydryls can be reversibly oxidized to disulfides, S-thiolated, S-nitrosylated, and sulfenic acid forms of cysteines or irreversibly converted to sulfinic and sulfonic acid forms. The absence of sufficient antioxidant protection may cause irreversible damage to these reactive sites [58]. Sulfhydryls maintain cellular integrity and are important to cellular functions. In this study, l-carnitine assisted in maintaining sulfhydryls in the reduced state throughout storage.
Hemolysis caused by free radicals can be characterized by two key events: (i) lipid peroxidation and (ii) redistribution of oxidized band 3 within the membrane [59]. Hemolysis increased in all groups, with progression in storage, indicating that l-carnitine could not protect lipids and proteins from oxidative damage.
Our findings show that the addition of l-carnitine assisted SOD, CAT and GSH-Px by scavenging superoxide and hydrogen peroxide while maintaining Hb and sulfhydryls with storage. The different concentrations of l-carnitine have not shown significant variations. As this study has focussed on the changes in the oxidative stress markers in rat erythrocytes with l-carnitine, there is a need to assess the biochemical changes associated with storage. Subsequently, the effects of l-carnitine on human erythrocytes during storage should be determined. The results obtained using l-carnitine, clearly elucidates the need to explore the effectiveness of l-carnitine in combination with other antioxidants, to successfully combat the oxidative stress during storage.
In conclusion, l-carnitine has been successful as the first line of defence against free radicals during storage. Hence, this study opens up new avenues of using l-carnitine as a component of storage solutions with other antioxidants in order to maintain efficacy of erythrocytes.
Acknowledgments
The authors acknowledge Dr. Leela Iyengar, Manasa K and Jain University for their support. The authors also acknowledge the award of INSPIRE-Fellowship from the Department of Science and Technology (DST), India to Ms. Soumya.
Compliance with Ethical Standards
Conflict of interest
All authors declare no personal or financial conflict of interests.
References
- 1.Hess JR. An update on solutions for red cell storage. Vox Sang. 2006;91:13–19. doi: 10.1111/j.1423-0410.2006.00778.x. [DOI] [PubMed] [Google Scholar]
- 2.Kucukakin B, Kocak V, Lykkesfeldt J, Nielsen HJ, Magnussen K, Rosenberg J, Gogenur I. Storage-induced increase in biomarkers of oxidative stress and inflammation in red blood cell components. Scand J Clin Lab Investig. 2011;71:299–303. doi: 10.3109/00365513.2011.563789. [DOI] [PubMed] [Google Scholar]
- 3.Tinmouth A, Fergusson D, Yee IC, Hebert PC. Clinical consequences of red cell storage in the critically ill. Transfusion. 2006;46:2014–2027. doi: 10.1111/j.1537-2995.2006.01026.x. [DOI] [PubMed] [Google Scholar]
- 4.Karon BS, van Buskirk CM, Jaben EA, Hoyer JD, Thomas DD. Temporal sequence of major biochemical events during blood bank storage of packed red blood cells. Blood Tranfus. 2012;10:453–461. doi: 10.2450/2012.0099-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chaudhary R, Katharia R. Oxidative injury as contributory factor for red cells storage lesion during twenty eight days of storage. Blood Transfus. 2011;10:59–62. doi: 10.2450/2011.0107-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vani R, Koshy AA, Koushik AK, Kaur H, Kumari K, Agrawal M, Priyanka Ramya, Khatai S, Gowda V, Kumar V. The efficacy of erythrocytes isolated from blood stored under blood bank conditions. Transfus Apher Sci. 2012;47:359–364. doi: 10.1016/j.transci.2012.06.014. [DOI] [PubMed] [Google Scholar]
- 7.Kanias T, Acker JP. Biopreservation of red blood cells—the struggle with hemoglobin oxidation. FEBS J. 2010;277:343–356. doi: 10.1111/j.1742-4658.2009.07472.x. [DOI] [PubMed] [Google Scholar]
- 8.Valko M, Leibfritz D, Moncol J, Cronin MTD, Mazur M, Telser J. Free radicals and antioxidants in normal physiological functions and human disease. Int J Biochem Cell Biol. 2007;39:44–84. doi: 10.1016/j.biocel.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 9.Yu BP. Cellular defenses against damage from reactive oxygen species. Immunol Rev. 1994;14:139–162. doi: 10.1152/physrev.1994.74.1.139. [DOI] [PubMed] [Google Scholar]
- 10.Kohen R, Nyska A. Oxidation of biological systems: oxidative stress phenomena, antioxidants, redox reactions and methods for their quantification. Toxicol Pathol. 2002;30:620–650. doi: 10.1080/01926230290166724. [DOI] [PubMed] [Google Scholar]
- 11.Percival M (1998) Antioxidants. Clin Nutr Insights 1/96:10/98
- 12.Bieber LL. Carnitine. Annu Rev Biochem. 1988;57:261–283. doi: 10.1146/annurev.bi.57.070188.001401. [DOI] [PubMed] [Google Scholar]
- 13.Vanella A, Russo A, Acquaviva R, Campisi A, Di Giacomo C, Sorrenti V, Barcellona ML. l-propionyl-carnitine as superoxide scavenger, antioxidant, and DNA cleavage protector. Cell Biol Toxicol. 2000;16:99–104. doi: 10.1023/A:1007638025856. [DOI] [PubMed] [Google Scholar]
- 14.Rebouche CJ, Paulson DJ. Carnitine metabolism and function in humans. Annu Rev Nutr. 1986;6:41–66. doi: 10.1146/annurev.nu.06.070186.000353. [DOI] [PubMed] [Google Scholar]
- 15.Bremer J. Carnitine-metabolism and functions. Physiol Rev. 1983;63:1420–1480. doi: 10.1152/physrev.1983.63.4.1420. [DOI] [PubMed] [Google Scholar]
- 16.Cooper MB, Forte CA, Jones DA. Carnitine and acetylcarnitine in red blood cells. Biochim Biophys Acta. 1988;959:100–105. doi: 10.1016/0005-2760(88)90020-3. [DOI] [PubMed] [Google Scholar]
- 17.Arduini A, Holmne S, Sweeney JD, Dottori S, Sciarroni AF, Caluani M. Addition of l-carnitine to additive solution-suspended red cells stored at 4 °C reduces in vitro hemolysis and improves in vivo viability. Transfusion. 1997;37:166–174. doi: 10.1046/j.1537-2995.1997.37297203519.x. [DOI] [PubMed] [Google Scholar]
- 18.Kobayashi A, Watanabe H, Fujisawa S, Yamamoto T, Yamazaki N. Effects of l-carnitine and palmitoylcarnitine on membrane fluidity of human erythrocytes. Biochim Biophys Acta. 1989;986:83–88. doi: 10.1016/0005-2736(89)90275-7. [DOI] [PubMed] [Google Scholar]
- 19.Watanabe H, Kobayashi A, Hayashi H, Yaniazaki N. Effects of long-chain acyl carnitine on membrane fluidity of human erythrocytes. Biochim Biophys Acta. 1989;980:315–318. doi: 10.1016/0005-2736(89)90318-0. [DOI] [PubMed] [Google Scholar]
- 20.Arduini A, Rossi M, Mancinelli G, Belfiglio M, Scurti R, Radatti G, Shohet SB. Effect of l-carnitine and acetyl-l-carnitine on the human erythrocyte membrane stability and deformability. Life Sci. 1990;47:2395–2400. doi: 10.1016/0024-3205(90)90483-8. [DOI] [PubMed] [Google Scholar]
- 21.Arduini A, Gorbunov N, Arrigoni-Martelli E, Dottori S, Molajoni F, Russo F, Federici G. Effects of l-carnitine and its acetate and propionate esters on the molecular dynamics of human erythrocyte membrane. Biochim Biophys Acta. 1993;1146:229–235. doi: 10.1016/0005-2736(93)90360-C. [DOI] [PubMed] [Google Scholar]
- 22.Wanic-Kossowska M, Kaźmierski M, Pawliczak E, Kobelski M. Combined therapy with l-carnitine and erythropoietin of anemia in chronic kidney failure patients undergoing hemodialysis. Pol Arch Med Wewn. 2007;117:14–19. [PubMed] [Google Scholar]
- 23.d’Almeida MS, Gray D, Martin C, Ellis CG, Yee IC. Effect of prophylactic transfusion of stored RBCs on oxygen reserve in response to acute isovolemic hemorrhage in a rodent model. Transfusion. 2001;41:950–956. doi: 10.1046/j.1537-2995.2001.41070950.x. [DOI] [PubMed] [Google Scholar]
- 24.Dodge JT, Mitchell C, Hanahan DJ. The preparation and chemical characteristics of hemoglobin-free ghosts of human erythrocytes. Arch Biochem Biophys. 1983;100:119–130. doi: 10.1016/0003-9861(63)90042-0. [DOI] [PubMed] [Google Scholar]
- 25.Oser BL. Hawk’s physiological chemistry. London: Tata McGraw Hill Publication; 1965. [Google Scholar]
- 26.Misra HP, Fridovich I. The role of superoxide anion in the auto oxidation of epinephrine and a simple assay for superoxide dismutase. J Biol Chem. 1972;247:3170–3175. [PubMed] [Google Scholar]
- 27.Aebi H. Catalase in vitro. Methods Enzymol. 1984;105:121–126. doi: 10.1016/S0076-6879(84)05016-3. [DOI] [PubMed] [Google Scholar]
- 28.Flohe L, Gunzler WA. Assays for glutathione peroxidase. Methods Enzymol. 1984;105:114–121. doi: 10.1016/S0076-6879(84)05015-1. [DOI] [PubMed] [Google Scholar]
- 29.Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxidation in animal tissues by thiobarbituric acid reaction. Anal Biochem. 1979;95:351–358. doi: 10.1016/0003-2697(79)90738-3. [DOI] [PubMed] [Google Scholar]
- 30.Habeeb AFSA. Reaction of protein sulfhydryl groups with Ellman’s reagent. Methods Enzymol. 1972;34:457–464. doi: 10.1016/S0076-6879(72)25041-8. [DOI] [PubMed] [Google Scholar]
- 31.Senturk UK, Gunduz F, Kuru O, Aktekin MR, Kipmen D, Yalcin O, Bor-Kukukatay M, Yesilkaya A, Baskurt OK. Exercise-induced oxidative stress affects erythrocyte in sedentary rats but not exercise-trained rats. J Appl Physiol. 2001;91:1999–2004. doi: 10.1152/jappl.2001.91.5.1999. [DOI] [PubMed] [Google Scholar]
- 32.Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin Phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 33.Minetti M, Malorni W. Redox control of red blood cell biology: the red blood cell as a target and source of prooxidant species. Antioxid Redox Signal. 2006;8:1165–1169. doi: 10.1089/ars.2006.8.1165. [DOI] [PubMed] [Google Scholar]
- 34.Cimen MYB. Free radical metabolism in human erythrocytes. Clin Chim Acta. 2008;390:1–11. doi: 10.1016/j.cca.2007.12.025. [DOI] [PubMed] [Google Scholar]
- 35.Kurata M, Suzuki M, Agar NS. Antioxidant systems and erythrocyte life-span in mammals. Comp Biochem Physiol B. 1993;106:477–487. doi: 10.1016/0305-0491(93)90121-k. [DOI] [PubMed] [Google Scholar]
- 36.Nagababu E, Rifkind JM. The origin of red cell fluorescence caused by hydrogen peroxide treatment. Free Radic Biol Med. 2000;29:659–663. doi: 10.1016/S0891-5849(00)00348-8. [DOI] [PubMed] [Google Scholar]
- 37.Sztiller M, Puchala M, Kowalczyk A, Bartosz G. The influence of ferrylhemoglobin and methemoglobin on the human erythrocyte membrane. Redox Rep. 2006;11:263–271. doi: 10.1179/135100006X155012. [DOI] [PubMed] [Google Scholar]
- 38.Gürlek A, Tutar E, Akçil E, Dinçer I, Erol C, Kocatürk PA, Oral D. The effects of l-carnitine treatment on left ventricular function and erythrocyte superoxide dismutase activity in patients with ischemic cardiomyopathy. Eur J Heart Fail. 2000;2:189–193. doi: 10.1016/S1388-9842(00)00064-7. [DOI] [PubMed] [Google Scholar]
- 39.Soumya R, Vani R. CUPRAC–BCS and antioxidant activity assays as reliable markers of antioxidant capacity in erythrocytes. Hematology. 2015;20:165–174. doi: 10.1179/1607845414Y.0000000177. [DOI] [PubMed] [Google Scholar]
- 40.Dokmeci D, Akpolat M, Aydogdu N, Uzal C, Doganay L, Turan FN. The protective effect of l-carnitine on ionizing radiation-induced free oxygen radicals. Scand J Lab Anim Sci. 2006;33:75–83. [Google Scholar]
- 41.Luo X, Reichetzer B, Trines J, Benson LN, Lehotay DC. l-carnitine attenuates doxorubicin- induced lipid peroxidation in rats. Free Radic Biol Med. 1999;26:1158–1165. doi: 10.1016/S0891-5849(98)00303-7. [DOI] [PubMed] [Google Scholar]
- 42.Arockia Rani PJ, Panneerselvam C. Carnitine as a free radical scavenger in aging. Exp Gerontol. 2001;36:1713–1726. doi: 10.1016/S0531-5565(01)00116-4. [DOI] [PubMed] [Google Scholar]
- 43.Sener G, Paskaloglu K, Satiroglu H, Alican H, Kacmaz A, Sakarcan A. l-carnitine ameliorates oxidative damage due to chronic renal failure in rats. J Cardiovasc Pharmacol. 2004;43:698–705. doi: 10.1097/00005344-200405000-00013. [DOI] [PubMed] [Google Scholar]
- 44.Dokmeci D. Oxidative stress, male infertility and the role of carnitines. Folia Med (Plovdiv) 2005;47:26–30. [PubMed] [Google Scholar]
- 45.Gaetani GF, Ferraris AM, Rolfo M, Mangerini R, Arena S, Kirkman HN. Predominant role of catalase in the disposal of hydrogen peroxide within human erythrocytes. Blood. 1996;87:1569–1599. [PubMed] [Google Scholar]
- 46.Mueller S, Riedel HD, Stremmel W. Direct evidence for catalase as the predominant H2O2 removing enzyme in human erythrocytes. Blood. 1997;90:4973–4978. [PubMed] [Google Scholar]
- 47.Wagner GM, Chiu DT, Qju JH, Heath RH, Lubin BH. Spectrin oxidation correlates with membrane vasculation in stored RBC. Blood. 1987;69:1777–1781. [PubMed] [Google Scholar]
- 48.Kriebardis AG, Antonelou MH, Stamoulis KE, Economou- Petersen E, Margaritis LH, Papassideri IS. RBC-derived vesicles during storage: ultrastructure, protein composition, oxidation, and signalling components. Transfusion. 2008;48:1943–1953. doi: 10.1111/j.1537-2995.2008.01794.x. [DOI] [PubMed] [Google Scholar]
- 49.Kriebardis AG, Antonelou MH, Stamoulis KE, Economou- Petersen E, Margaritis LH, Papassideri IS. Progressive oxidation of cytoskeletal proteins and accumulation of denatured hemoglobin in stored red cells. J Cell Mol Med. 2007;11:148–155. doi: 10.1111/j.1582-4934.2007.00008.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sies H. Oxidative stress: from basic research to clinical application. Am J Med. 1991;91:S31–S38. doi: 10.1016/0002-9343(91)90281-2. [DOI] [PubMed] [Google Scholar]
- 51.Sies H. Oxidative stress: oxidants and antioxidants. Exp Physiol. 1997;82:291–295. doi: 10.1113/expphysiol.1997.sp004024. [DOI] [PubMed] [Google Scholar]
- 52.Negre-Salvayre A, Auge N, Ayala V, Basaga H, Boada J, Brenke R, Chapple S, Cohen G, Feher J, Grune T, Lengyel G, Mann GE, Pamplona R, Poli G, Portero-otin M, Riahi Y, Salvayre R, Sasson S, Serrano J, Shamni O, Siems W, Siow RCM, Wiswedel I, Zarkovic K, Zarkovic N. Pathological aspects of lipid peroxidation. Free Radic Res. 2010;44:1125–1171. doi: 10.3109/10715762.2010.498478. [DOI] [PubMed] [Google Scholar]
- 53.Greenwalt TJ. The how and why of exocytic vesicles. Transfusion. 2006;46:143–152. doi: 10.1111/j.1537-2995.2006.00692.x. [DOI] [PubMed] [Google Scholar]
- 54.Gülçin I. Antioxidant and antiradical activities of l-carnitine. Life Sci. 2006;78:803–811. doi: 10.1016/j.lfs.2005.05.103. [DOI] [PubMed] [Google Scholar]
- 55.Rajasekar P, Kaviarasan S, Anuradha CV. l-carnitine administration prevents oxidative stress in high fructose-fed insulin resistant rats. Diabetol Croat. 2005;34:21–28. [Google Scholar]
- 56.Shacter E. Quantification and significance of protein oxidation in biological samples. Drug Metabol Rev. 2000;32:307–326. doi: 10.1081/DMR-100102336. [DOI] [PubMed] [Google Scholar]
- 57.Berlett BS, Stadtman ER. Protein oxidation in aging, disease and oxidative stress. J Biol Chem. 1997;272:20313–20316. doi: 10.1074/jbc.272.33.20313. [DOI] [PubMed] [Google Scholar]
- 58.Thomas JA, Mallis RJ. Aging and oxidation in protein sulfhydryls. Exp Gerontol. 2001;36:1519–1526. doi: 10.1016/S0531-5565(01)00137-1. [DOI] [PubMed] [Google Scholar]
- 59.Sato Y, Kamo S, Takahashi T, Suzuki Y. Mechanism of free radical-induced hemolysis of human erythrocytes: hemolysis by water-soluble radical initiator. Biochemistry. 1995;34:8940–8949. doi: 10.1021/bi00028a002. [DOI] [PubMed] [Google Scholar]