Abstract
Cirrhosis is the end-stage liver fibrosis, whereby normal liver architecture is disrupted by fibrotic bands, parenchymal nodules and vascular distortion. Portal hypertension and hepatocyte dysfunction are the end results and give rise to major systemic complications and premature death. Mesenchymal stem cells (MSC) have the capacity of self-renew and to give rise to cells of various lineages, so MSC can be isolated from bone marrow (BM) and induced to differentiate into hepatocyte-like cells. MSC were induced to differentiate into hepatocyte-like cells by hepatotic growth factor (HGF) and fibroblast growth factor-4 (FGF-4). Differentiated cells were examined for the expression of hepatocyte-specific markers and hepatocyte functions. MSC were isolated. Flow cytometry analysis showed that they expressed the MSC-specific markers, reverse transcriptase–polymerase chain reaction (RT-PCR) demonstrated that MSC expressed the hepatocyte-specific marker cytokeratin 18 (CK-18) following hepatocyte induction. This study demonstrates that BM-derived—MSC can differentiate into functional hepatocyte-like cells following the induction of HGF and FGF-4. MSC can serve as a favorable cell source for tissue engineering in the treatment of liver disease.
Keywords: Differentiation, Hepatocyte, Mesenchymal stem cells, Growth factors
Introduction
Cirrhosis is a progressive liver disease and is marked by the gradual destruction of liver tissue over time. Persistent injuries lead to hepatic scarring (fibrosis), which, if unopposed, leads to cirrhosis and demise of liver function. End-stage liver fibrosis is cirrhosis, whereby normal liver architecture is disrupted by fibrotic bands, parenchymal nodules and vascular distortion. Portal hypertension and hepatocyte dysfunction are the end results and give rise to major systemic complications and premature death [1, 2]. The main causes of cirrhosis globally are hepatitis C and B and alcohol abuse. Egypt has the highest prevalence of HCV in the world, up to 20 % in some areas [3].
At the cirrhotic stage, liver disease is considered irreversible and the only solution is orthotopic liver transplantation (OLT). However, the increasing shortage of donor organs restricts liver transplantation. With the widening donor-recipient gap, the increasing incidence of liver disease, life-long dependence on immunosuppression and the poor outcome in patients not supported by liver transplantation. All these drawbacks pave the way for developing new strategies to supplement OLT [2, 4] as stem cell therapies for liver regeneration. Such cells must fulfill two major requirements: (i) they should be capable of proliferating in the recipient’s liver, and (ii) they should differentiate into mature (i.e., functionally competent) hepatocytes. Evidence is growing in support of the role of early pluripotent precursors (stem cells of various origins) as a unique source for transplantation [5].
The first demonstration of the existence of putative liver stem cells in the bone marrow (BM) was reported by Petersen et al. [6]. They showed that BM cells transplanted into lethally irradiated mice engrafted in the recipient’s liver and differentiated into liver stem cells (oval cells) or mature hepatocytes. These in vivo results were confirmed in animal models and in patients who received BM transplantation for hematological disorders [7].
Stem cells are immature cells that have the capacity to self-renew and to give rise to cells of various lineages. Thus, they represent an important paradigm of cell-based therapy for a variety of diseases.
BM contains at least two populations of stem cells, haematopoietic stem cells (HSCs) and mesenchymal stem cells (MSCs), which provide stromal support for HSCs [8]. Both HSCs and MSCs have the ability to trans-differentiate to hepatocytes, but MSCs are the most potent component of BM cells in hepatic differentiation [9]. Their differentiation into functional hepatocyte-like cells has also been demonstrated in vivo [10] and in vitro by continuous exposure to cytokine cocktail [11].This selective differentiation is dependent on specific environmental efforts: usually a combination of growth factors and cytokines supplied in vitro [12].
In our study, we aim to investigate the differentiation of mesenchymal stem cells (MSCs) into hepatocytes by induction of fibroblast growth factor-4 (FGF-4) and or hepatocyte growth factor (HGF), characterization of this population to detect appropriate expression of phenotype-specific markers, gene expression profile, ultrastructure, and functional activity.
Subjects and Methods
Subjects
This study was conducted on 24 BM samples from healthy donors of BM to a related patient from BM Transplantation Unit, Nasser Institute for Research and Treatment.
The donor’s age ranged from 12 to 58 years of both sex and were found healthy in an orienting physical examination and all blood products were negative for common blood-borne pathogens, as detected by standard assays. This study was approved by the Ethical Committee of Faculty of Medicine, Cairo University. After verbal and written consent, 8–10 ml BM was aseptically collected during BMA in a sterile heparinized vacutainers to be handled within 6 h.
Methodology
MSCs Separation and Expansion
Mononuclear Cell Separation
Under complete aseptic conditions (laminar flow work area) the fresh blood was diluted with sterile CMF-PBS in the ratio of 1:1 and they were mixed well. Gently (15 ml) of the diluted blood was layered over one third volume of warm ficoll-hypaque (Biochrome AG) density (1.077 g/L). Then, tubes were centrifuged for 20 min at 2000 rpm at 25 °C. After centrifugation, the mononuclear cells (MNCs) fraction was collected using a Pasteur pipette to carefully transfer the opaque interface into a sterile 50 cc conical centrifuge tube. The cells were washed twice (to avoid platelet contamination) in 15 ml cold sterile PBS and spun at 1500 rpm for 10 min at 4 °C. The PBS was aspirated leaving the cell pellet and immediately was resuspended in 1 ml serum free DMEM-media (Invitrogen) and 100 µ transferred to Eppendorf tube and counted using a hemocytometer.
Cell viability was done using trypan blue (0.04 %) exclusion dye (Sigma) (100 µl cell suspension + 100 µl Trypan blue stain), [13]. This method based on the viable cells do not take up the dyes, whereas dead cells do. Viable cells were counted and the number of viable cells/ml and the total cell number were calculated:
Cell viability was determined by Trypan blue and found to be 95 % for all cases prior to culture.
Isolation, Primary Culture and Subculture of MSCs
The MNC suspensions were plated at a concentration of (1 × 106 cells/mL media) and allow to adhere to tissue culture plastic flasks 25 cm2 (0.2 μm vent cap flasks were used) (Corning®), incubated in fresh complete nutrient medium which was constituted of the following: Low-glucose DMEM (DMEM-LG) with l-glutamine (Lonza) (2 mmol/l l-glutamine), 10 % FBS (Lonza), 2 % Penicillin-streptomycin (10,000 μ/ml) (Sigma), 1 % Fungizone (20 mg/ml) (Bioscience). For proper adherence of the cells, the flasks were incubated in a horizontal position in a humidified incubator at 37 °C and 5 % CO2. The media were examined daily by naked eye to look for signs of microbial contamination or fungal infections. Every 3 days, the flasks was examined by the inverted microscope (100 to 400×) for assessment of the cell morphology (fibroblastiod appearance), viability of cells and microbial contamination. The first change of the media was accomplished at day 7 to remove the non-adherent cells the adherent cells were kept in the flask and were fed by fresh complete nutrient media. Then the media was changed once weekly until reaching 70 to 90 % confluence which is assessed by examination under the inverted microscope, after that the adherent cells were harvested by trypsinization, firstly, the adherent cell layer was washed with 5 ml PBS for 1 min and then incubated in 37 °C for 10 min. The PBS replaced with 2–4 ml trypsin 0.25 % with 0.02 % EDTA and incubated in 37 °C for 7 min. Flasks were shacked by tapping and examined under the inverted microscope to ensure total detachment of the adherent cell layer and after 80–90 % of the cells have become detached the sides of the flask were gently tapped to dislodge any remaining attached cells and 5 ml of RPMI containing 10 % FCS was added to the flasks to stop the action of trypsin. The flasks were rocked back and forth to swirl the media around it, then the cell suspension was transferred into a clean 15 ml conical tube, then the tubes were centrifuged at 200 g for 7 min 20 °C and the supernatant was removed and the pellet was resuspended in appropriate amount of media. Cells were counted using hemocytometer and tested for viability by trypan blue and plated in new flasks.
Evaluation of the Harvested MSCs
Cell count
Cell viability analysis
Flow cytometric characterization of MSCs (Immunophenotyping)
Immunophenotyping was done using CD44, CD90, and CD 105 according the international guidelines for stem cell identification.
Subculture of MSCs
The cells from primary culture are seeded into new 25 cm2 flasks at ratio 1:3 for 1st passage and supplemented by the complete culture media as primary culture, the media changed every 4 day until reaching 80 % confluence.
Induction of MSCs into Hepatocyte Differentiation
At this step, the samples divided into three groups: Group A (8 samples), induced by HGF 20 ng/ml, group B (8 samples), induced by b-FGF 10 ng/ml, and group C (8 samples) induced by HGF + b-FGF. MSCs at 2nd passage are seeded into 35 mm petri dishes at density 1 × 106/dishes. The differentiation media constituted of complete DMEM-LG (2 mmol/l l-glutamine), 10 % FBS, 2 % Penicillin- streptomycin (10,000 μ/ml), 1 % Fungizone (20 mg/ml). Growth factors according to each group, incubated at 37 °C and 5 % CO2. The dishes were examined under inverted microscope for change in morphology and microbial contamination every 4 days. The cells harvested from the dishes and stored at -20o C on RNA cell protect reagent which was further used for RNA extraction for detection of gene expression of cytokeratin 18 (CK18) by RT-PCR.
Gene Expression Analysis
Detection of Gene Expression of CK18 by Reverse Transcription Polymerase Chain Reaction (RT-PCR)
The total cellular RNA was extracted from cells using RNeasy Protect Cell Mini Kit (Qiagen, catalog NO 74624) according to standard protocols. Reverse transcription was performed with The GeneAmp ® Gold RNA PCR core Kit (Applied Biosystems and MicroAmp, U.S. Roche Molecular Systems, Inc) according to the manufacturer’s protocol. PCR amplification was performed in a final volume of 50, 0.5 μl first upstream Primer DM151, 0.5 μl downstream Primer DM152S, 20 μl template cDNA and sterile high-quality water to a final volume of 50 u1. The following primer sequences were used: sense 5′-GAGATCGAGGCTCTCAAGGA-3′, antisense 5′-CAAGCTGGCCTTCAGATTTC-3′. The PCR reaction was carried out in the DNA thermal cycler (Perkin Elmer 9600). Thermal cycler was programmed for the following conditions: hold 95 °C for 10 min; 43 cycles: 94 °C for 20 s (denaturation), 62 °C for 1 min (annealing), 62 °C for 1 min (extension); 1 cycle: 72 °C for 7 min. Then analysis of the product was done by agarose gel electrophoresis (2 %) and ultraviolet light transillumination.
Results
Detection of MSCs
Were identified according to the minimal criteria of MSCs identification included the:
First, morphological assay of MSC showed the plastic-adherent cells when maintained in standard culture conditions.
Second, MSC express CD105, and CD90, and lack expression of CD34.
Third, MSC differentiate to osteoblasts, adipocytes and chondroblasts in vitro.
Morphological Assay
Cells were examined every three days, by the inverted microscope (100 to 400×) for assessment of the cell morphology (fibroblastiod appearance), viability of cells and microbial contamination. The first change of the media was accomplished at day 7 to remove the non-adherent cells, the adherent cells were kept in the flask and were fed by fresh complete nutrient media. MSCs were examined until 80 % confluence is reached, the adhered cells were fibroblast-like and grew as a whirlpool. The sub-cultured cells were much purer and fibroblast like, the other cells were disappeared. 100 % of samples in the primary culture reached confluence 10–12 days later, the cells sub-cultured at a ratio of 1:3 reached confluence at 8–10 days later (Figs. 1, 2, 3, 4).
Fig. 1.
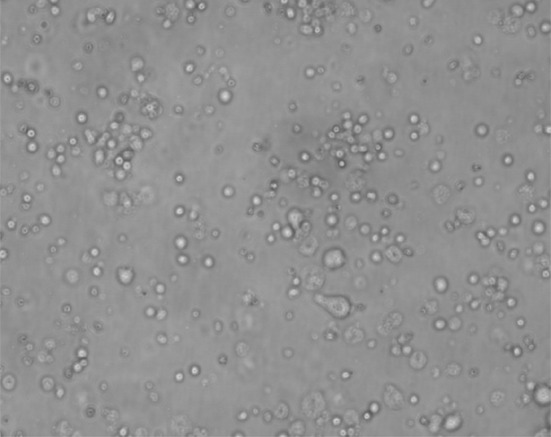
A photomicrograph showing viability of MNCS cells (inverted microscope, ×400)
Fig. 2.
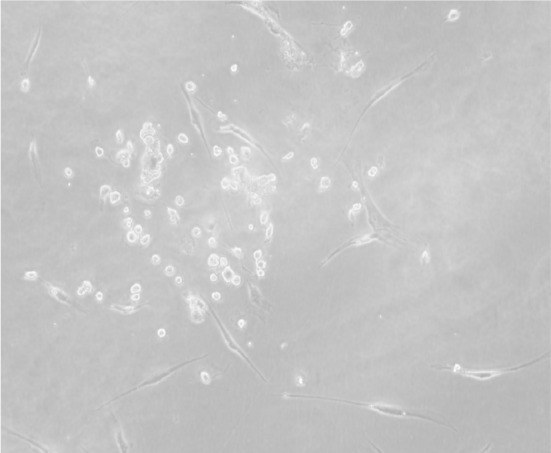
A photomicrograph showing scattered small colonies of mesenchymal stem cells after 3 days (inverted microscope, ×400)
Fig. 3.
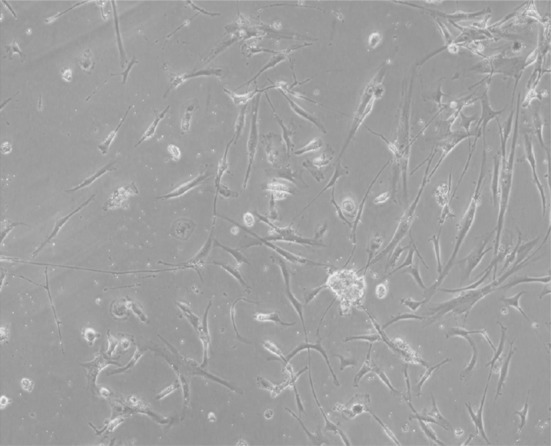
A photomicrograph showing large dense colonies of mesenchymal stem cells (50 % confluence) after 7 days (inverted microscope, ×400)
Fig. 4.

A photomicrograph showing large dense colonies of mesenchymal stem cells (70–90 % confluence) after 12 days (inverted microscope, ×400)
Results of Immunophenotyping of CD105, CD34, and CD44 Positivity
It was found that CD105 ranged from (44.5 to 90) with mean (71.8 ± 11.0), CD34 range from (0.57 to 5.6) with mean (1.76 ± 1.35), and CD90 ranged from (36 to 83) with mean 68.4 ± 11.72.
Evaluation of Hepatic Differentiation
The cultured samples were divided into three groups according to the growth factor used to induce the hepatocytes:
Group A (8 samples), induced by HGF 20 ng/ml.
Group B (8 samples), induced by b-FGF 10 ng/ml.
Group C (8 samples) induced by HGF + b-FGF.
Morphologic Examination (H & E)
Hepatocytes appeared as round or pear shaped, most of them contained one nucleus. However, few cells were binucleated (Fig. 5).
Fig. 5.
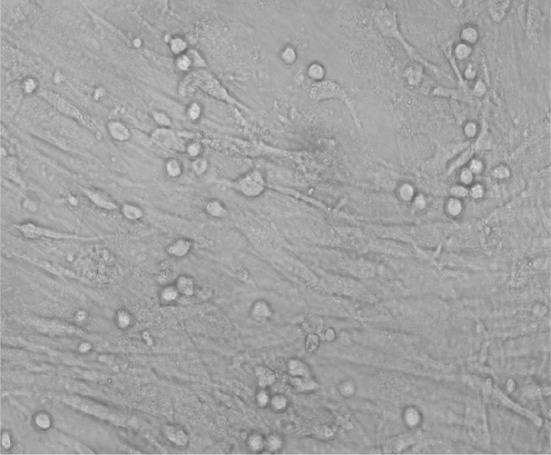
Photomicrograph of culture MSC differentiated into hepatocyte, (inverted microscope, ×400)
Genetic Analysis
Results of CK-18 gene detection by RT-PCR within group A, CK-18 gene was detected in sample 3,4,6,7, and 8, in day 4, day 8, and day 16, while in sample 1 and 2CK-18 gene was not detected in day 4, day 8 or day 16, on other hand in sample 5 CK-18 was detected in day 4 and day 8 but was not detected in day 16. Percentage of CK-18 gene detection within group A was the same in day 4 and day 8 which was 75 % (CK-18 gene was detected in 6 samples out of the 8 samples), while in day 16 it was 62.5 % (CK-18 gene was detected in 5 samples out of the 8 samples).
Results of CK-18 gene detection within group, CK-18 gene was detected in all samples in day 4, day 8, and day 16. Percentage of CK-18 gene detection within group B, the differentiation was 100 % (CK-18 gene was detected in all 8 samples) in day 4, day 8 and day 16.
Results of CK-18 gene detection within group C, CK-18 was detected in all samples in day 4, day 8, and day 16 except sample number 3, CK-18 gene was not detected at all. Percentage of CK-18 gene detection within group C, the differentiation was the same in day 4, day 8 and day 16 which was 87.5 % (CK-18 gene was detected in 7 samples out of 8 samples). Table 1.
Table 1.
Comparison between Percentage of CK-18 gene within Group A, Group B and Group C in Day 4, Day 8 and Day 16
| Item | Day 4 (%) | Day 8 (%) | Day 16 (%) |
|---|---|---|---|
| Percentage of CK-18 gene within Group A | 75 | 75 | 62.5 |
| Percentage of CK-18 gene within Group B | 100 | 100 | 100 |
| Percentage of CK-18 gene within Group C | 87.5 | 87.5 | 87.5 |
Samples showed that MSCs could express this hepatocyte-specific gene in a time-dependent manner. mRNA expression for tested gene appeared to be significantly promoted in differentiating medium containing b-FGF or HGF or both (b-FGF + HGF) Figs. 6, 7.
Fig. 6.
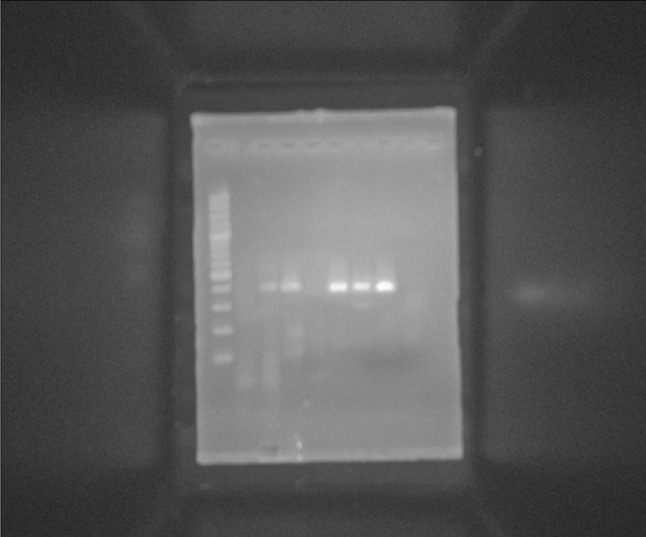
RT-PCR analyses of the temporal expression pattern of hepatocyte-specific marker (CK-18) after hepatic differentiation of MSCs. M PCR marker (100–1000 bp), Lane 1 non induced day 4 (negative control), Lane 2 CK-18 gene detected in day 4 induced by HGF, Lane 3 CK-18 gene detected in day 8 induced by HGF, Lane 4 CK-18 gene was not detected in day 16 induced by HGF, Lane 5 CK-18 gene detected in day 4 induced by b-FGF, Lane 6 CK-18 gene detected in day 8 induced by b-FGF, Lane 7 CK-18 gene detected in day 16 induced by b-FGF
Fig. 7.
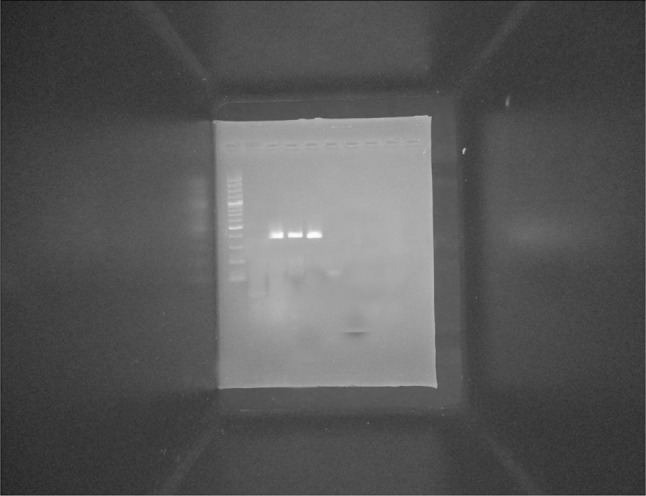
RT-PCR analyses of the temporal expression pattern of hepatocyte-specific marker (CK-18) after hepatic differentiation of MSCs. M PCR marker (100–1000 bp), Lane 1 non induced day 16 (negative control), Lane 2 (HGF + b-FGF) day 4, Lane 3 (HGF + b-FGF) day 8, Lane 4 (HGF + b-FGF) day 16. CK-18 gen was detected in: Lane 2, 3 and 4
Discussion
BM derived stem cells express the property of plasticity. This means that these cells have the potential to transdifferentiate into cells of other organs [14].
Many methods can isolate MNCs from BM [15–17] In this study, we used the Ficoll (1.077 g/ml) to isolate MNCs from BM, we combined the gradient density centrifugation with plastic adherence and changed the medium many times to purify MNC after gradient intensity centrifugation. This method is relatively simply, and can easily yield pure MSC [16].
In the present study, MSCs were isolated from MNCs by conventional plastic adherence. Tondreau et al. reported that different techniques were used to isolate MSCs: plastic adhesion, negative selection (magnatic beads CD45/Gly-A, and RosetteSep) or positive selection (CD44, CD90, CD29 and CD105) [17].
The culture done in this work was on DMEM media supplemented with 20 % FCS that was in agreement with Hung et al., Shadhafar et al. and Montzka et al. [18–20].
In this study, MSCs were detected after 3 days culture and become more confluent after 7 days. They were seen by inverted microscope and were proven by their immunophenotyping profile (negative for CD 34), and positive for CD44, CD105). The international society for cellular therapy had defined MSCs, following isolation and culture expansion, by being plastic adherent cells that have trilineage differentiation potential towards osteoblast, adipocytes, and chondroblasts. In addition, they are known by their expression of various molecules including CD44, CD90, CD105 and CD73 and absence of markers like CD34, CD45, and CD 14 [20].
A variety of culture protocols have been developed for promoting the differentiation of adult stem cells into hepatocytes. Most protocols employ growth factors important for liver development or regeneration. Among the factors implied in the embryonic liver development, fibroblast growth factors (FGFs), produced by cardiac mesodermal cells, are involved at an initial stage of endodermal patterning to induce hepatic fate [21–23], Oncostatin M (OSM), a member of the interleukin-6 cytokine family produced by hematopoietic cells, is required from the mid-fetal to the neonatal stages [24], and apparently coordinates liver development and hematopoiesis in the fetus [25], finally, several extracellular signals including epidermal growth factor (EGF), hepatic growth factor (HGF), OSM, FGFs, glucocorticoids and insulin are involved in the late maturation stage leading to an increase in liver-specific gene expressions, and their effects on differentiation vary as a function of gestation age [26]. Corticosteroids, HGF and EGF play important roles in hepatic biology [27]. HGF is a more potent proliferating factor for human hepatocytes in culture than EGF [28, 29], and plays an important role in liver development and regeneration in humans [30, 31]. The differentiation of BMSCs into hepatocyte-like phenotypes in vitro by induction with HGF has been reported [30, 32]. Other reports showed differentiation of BM-derived MAPC toward hepatocyte-like cells induced by FGF-4, however the degree of differentiation was higher when cells were also treated with HGF [33]. This is consistent with the fact that FGF-4 may play a role in endoderm specification [22], and that HGF induces differentiation of hepatocytes that are not actively proliferating [30]. BM cells cultured with HGF and EGF showed morphologic and phenotypic characteristics of mature hepatocytes [34]. Also, bFGF is required to induce a hepatic fate in the foregut endoderm [33]. In our study, we use HGF 20 ng/ml, and b-FGF 10 ng/ml and a combination of both growth factors. The morphology of the cells changed after 5 days culture where round or polygonal-shaped cells with moderate cytoplasm and a medium-sized nucleus were observed. This was matching with many authors who stated that, while a morphological change is common after in vitro cell conditioning, the morphology is rarely similar to mature hepatocytes and authors should rather talk about a hepatocyte like morphology in differentiating cells [35]. Also Snykers et al. stated that epithelioid cells appeared in culture from day 6 on. Furthermore, more than 85 % of these epithelioid cells expressed liver-associated genes and proteins. The presence of both morphologic and phenotypic features, similar to that of primary hepatocytes, does, however, not fully prove the differentiation of BMSC into mature hepatocytes [36].
In our study, hepatocyte differentiation was evaluated for (CK18) differentiation markers, after 4, 8 and 16 days. Cells treated with 10 ng/ml bFGF alone, HGF alone, or a combination of both, almost all samples expressed CK-18 from the day 4 and continue to express it on day 8 and day 16.
In a study to differentiate MAPCs from the BM into functional hepatocyte like cells; cells treated with 10 ng/ml FGF-4 alone, 20 ng/ml HGF alone, or a combination of both. After 7 days, few cells stained positive for CK18. After 14 and 21 days, more than 90 % of epithelioid cells stained positive for CK18 [33].
In li et al. study, he reported the successful induction of functional hepatocytes from induced pluripotent stem cells by the sequential addition of DMSO and sodium butyrate in the culture which expressed a panel of the hepatic lineage markers, CK7, CK8, CK18, CK19, AFP, ALB, and Cyp7a1, and exhibited functional hepatic characteristics [35].
Zhang et al. study demonstrated that HGF and FGF-4 can effectively promote UC MSC to differentiate into hepatocyte-like cells; the growth medium without HGF and FGF-4 did not have any effect on hepatocyte differentiation, also they demonstrated that ALB and CK-18 protein expression is first detected on day 14 following hepatocyte differentiation and the expression levels are similar or higher on day 21 compared with day 14, suggesting that cells on days 14 and 21 following differentiation contain more mature hepatocytes than on day 7 [31].
Song et al. checked in their study the expression of AFP, ALB and CK18 at day 0 in the Undifferentiated iPS cells and found negative staining. However, the differentiated cells expressed hepatic genes including AFP, ALB, CK8, CK18, CK19 and PEPCK and the liver enriched transcription factors HNF4α, HNF6, CEBPα, GATA4 and HEX at the mRNA level, as detected by RTPCR, while these genes were not expressed in the undifferentiated iPS cells and hES cells [37].
Moreover, Theise et al. and Alison et al. reported that hepatocytes were derived from BM cells during the 28 days of differentiation, with GF-4 and HGF induced MSCs into cells with morphological and functional characteristics of hepatocytes. And cells that differentiated into hepatocyte-like cells could produce urea, secrete albumin, AFP and Ck-18, and store glycogens [38].
In Kang et al. study, their results showed UCB-derived MSCs When were cultured with FGF-4 and HGF Cells first expressed CK-18 on day 16 through immunocytochemistry analysis. RT-PCR analysis showed that differentiated cells could express a number of hepatocyte-specific genes in a time-dependent manner, Gene expressions of albumin, AFP and CK-18 were readily detectable from day 8 and substantially increased thereafter during the maturation process [39].
The present study indicates that under certain defined inducing conditions, MSC, isolated from BM, can differentiate toward a hepatic phenotype in vitro. In addition to hepatic biochemical functions, as shown in a previous report.
In summary, we have presented in vitro production of functional and transplantable hepatocytes from BM derived MSCs. Our findings, combined with the development of tissue engineering technologies, may support stem cell-based therapy for liver injuries and for the establishment of a bioartificial liver.
The concept of this study may indeed be considered as a future hypothetical option for patients who might benefit from stem cells therapy. However, given these preliminary results, testing in vivo the regenerative potential of this cell population in animal models, including large animals, will be the next logical step.
Compliance with Ethical Standards
Conflict of Interest
No conflict of interest
References
- 1.Svegliati-Baroni G, De Minicis S, Marzioni M. Hepatic fibrogenesis in response to chronic liver injury: novel insights on the role of cell-to-cell interaction and transition. Liver Int. 2008;28(8):1052–1064. doi: 10.1111/j.1478-3231.2008.01825.x. [DOI] [PubMed] [Google Scholar]
- 2.Forbes JS. Stem cell therapy for liver disease-choosing the right tools for the job. Gut. 2008;57:153–155. doi: 10.1136/gut.2007.134247. [DOI] [PubMed] [Google Scholar]
- 3.Darwish MA, Faris R, Darwish N, Shouman A, Gadallah M, El-Sharkawy MS, Edelman R, Grumbach K, Rao MR, Clemens JD. Hepatitis C and cirrhotic liver disease in the Nile delta of Egypt: a community-based study. Am J Trop Med Hyg. 2001;64:147–153. doi: 10.4269/ajtmh.2001.64.147. [DOI] [PubMed] [Google Scholar]
- 4.Freeman RB, Steffick DE, Guidinger MK, Farmer DG, Berg CL, Merion RM. Liver and intestine transplantation in the United States, 1977–2006. Am J Transplant. 2008;8(Part 2):958–976. doi: 10.1111/j.1600-6143.2008.02174.x. [DOI] [PubMed] [Google Scholar]
- 5.Sukhikh GT, Shtil AA. Stem cell transplantation for treatment of liver diseases: from biological foundations toclinical experience (review) Int J Mol Med. 2003;3:395–400. [PubMed] [Google Scholar]
- 6.Petersen BE, Bowen WC, Patrene KD, Mars WM, Sullivan AK, Murase N, Boggs SS, Greenberger JS, Goff JP. Bone marrow as a potential source of hepatic oval cells. Science. 1999;284:1168–1170. doi: 10.1126/science.284.5417.1168. [DOI] [PubMed] [Google Scholar]
- 7.Alison MR, Poulsom R, Jeffrey R, Dhillon AP, Quaglia A, Jacob J, Novelli M, Prentice G, Williamson J, Wright NA. Hepatocytes from non-hepatic stem cells. Nature. 2000;405:257. doi: 10.1038/35018642. [DOI] [PubMed] [Google Scholar]
- 8.Kale S. Bone marrow stem cells contribute to repair of the ischemically injured renal tubule. J Clin Invest. 2003;112:42–49. doi: 10.1172/JCI17856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Thiese ND. Emergent themes of liver stem cell biology: niche, quiescence, self-renewal, and plasticity. Am J Physiol Gastrointest Liver Physiol. 2006;290:189–193. doi: 10.1152/ajpgi.00041.2005. [DOI] [PubMed] [Google Scholar]
- 10.Fang B, Shi M, Liao L, Yang S, Liu Y, Zhao RC. Systemic infusion of FLK1 mesenchymal stem cells ameliorates carbontetrachloride-induced liver fibrosis in mice. Transplantation. 2004;78:83–88. doi: 10.1097/01.TP.0000128326.95294.14. [DOI] [PubMed] [Google Scholar]
- 11.Sarah S, Tamora V, Peggy P, Aernout L, Yuehuga J. Sequential exposure to cytokines reflecting embryogenesis: the key for in vitro differentiation of adult bone marrow stem cells into functional hepatocyte-like cells. Toxicol Sci. 2006;94(2):330–341. doi: 10.1093/toxsci/kfl058. [DOI] [PubMed] [Google Scholar]
- 12.Rebelatto CK, Aguiar AM, Moretao MP, Senegaglia AC, Hansen P, Barchiki F, Oliveira J, Martins J, Kuligovski C, Mansur F, Christofis A, Amaral VF, Brofman PS, Goldenberg S, Nakao LS, Correa A. Dissimilar differentiation of mesenchymal stem cells from bone marrow, umbilical cord blood, and adipose tissue. Exp Biol Med. 2008;233:901–913. doi: 10.3181/0712-RM-356. [DOI] [PubMed] [Google Scholar]
- 13.Freshney RI. Culture of animal cells: a manual of basic technique. 3. New York: Willy-Liss; 2009. pp. P105–P148. [Google Scholar]
- 14.Wagers AJ, Weissman IL. Plasticity of adult stem cells. Cell. 2004;116(5):639–648. doi: 10.1016/S0092-8674(04)00208-9. [DOI] [PubMed] [Google Scholar]
- 15.Schwinger W, Benesch M, Lackner H, Kerbl R, Walcher P, Urban C. Comparison of different methods for separation and ex = ivo expansion of cord blood progenitor cells. Ann Hematol. 1999;78:364–370. doi: 10.1007/s002770050530. [DOI] [PubMed] [Google Scholar]
- 16.Yang SE, Ha CW, Jung M, Jin HJ, Lee M, Song H, Choi S, Oh W, Yang PS. Mesenchymal stem/progenitor cells developed in cultures from UC blood. Cytotherapy. 2004;6:476–486. doi: 10.1080/14653240410005041. [DOI] [PubMed] [Google Scholar]
- 17.Tondreau T, Meuleman N, Delforge A, et al. Mesenchymal Stem Cells Derived from CD133-positive cells in mobilized peripheral blood and cord blood: proliferation, oct4 expression, and plasticity. Stem Cells. 2005;23:1105–1112. doi: 10.1634/stemcells.2004-0330. [DOI] [PubMed] [Google Scholar]
- 18.Huang S-C, Cheng H, Pan C-Y, et al. In-vitro differentiation of size-sieved stem cells into electrically active neural cells. Stem Cells. 2002;20:522–529. doi: 10.1634/stemcells.20-6-522. [DOI] [PubMed] [Google Scholar]
- 19.Shahdfar A, Fronsdal K, Haug T, et al. In vitro expansion of human mesenchymal stem cells: choice of serum is a determinant of cell proliferation, differentiation, gene expression, and transcriptome stability. Stem Cells. 2005;23:1357–1366. doi: 10.1634/stemcells.2005-0094. [DOI] [PubMed] [Google Scholar]
- 20.Montzka K, Lassonczyk N, Tschöke B, Neuss S, Führmann T, Franzen R, Smeets R, Brook GA, Wöltje M. Neural differentiation potential of human bone marrow-derived mesenchymal stromal cells: misleading marker gene expression. BMC Neurosci. 2009;10:16–28. doi: 10.1186/1471-2202-10-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jung J, Zheng M, Goldfarb M, Zaret KS. Initiation of mammalian liver development from endoderm by fibroblast growth factors. Science. 1999;284:1998–2003. doi: 10.1126/science.284.5422.1998. [DOI] [PubMed] [Google Scholar]
- 22.Wells JM, Melton DA. Early mouse endoderm is patterned by soluble factors from adjacent germ layers. Development. 2000;127:1563–1572. doi: 10.1242/dev.127.8.1563. [DOI] [PubMed] [Google Scholar]
- 23.Taléns-Visconti R, Bonora A, Jover R, Mirabet V, Carbonell F, Castell JV, Gómez-Lechón MJ. Hepatogenic differentiation of human mesenchymal stem cells from adipose tissue in comparison with bone marrow mesenchymal stem cells. World J Gastroenterol. 2006;12(36):5834–5845. doi: 10.3748/wjg.v12.i36.5834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kamiya A, Kinoshita T, Ito Y, Matsui T, Morikawa Y, Senba E, Nakashima K, Taga T, Yoshida K, Kishimoto T, Miyajima A. Fetal liver development requires a paracrine action of oncostatin M through the gp130 signal transducer. EMBO J. 1999;18:2127–2136. doi: 10.1093/emboj/18.8.2127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Miyajima A, Kinoshita T, Tanaka M, Kamiya A, Mukouyama Y, Hara T. Role of oncostatin M in hematopoiesis and liver development. Cytokine Growth Factor Rev. 2000;11:177–183. doi: 10.1016/S1359-6101(00)00003-4. [DOI] [PubMed] [Google Scholar]
- 26.Hamazaki T, Iiboshi Y, Oka M, Papst PJ, Meacham AM, Zon LI, Terada N. Hepatic maturation in differentiating embryonic stem cells in vitro. FEBS Lett. 2001;497:15–19. doi: 10.1016/S0014-5793(01)02423-1. [DOI] [PubMed] [Google Scholar]
- 27.Michalopoulos GK, Bowen WC, Mule K, Luo J. HGF-, EGF-, and dexamethasone-induced gene expression patterns during formation of tissue in hepatic organoid cultures. Gene Expr. 2003;11:55–75. doi: 10.3727/000000003108748964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Strain AJ, Ismail T, Tsubouchi H, Arakaki N, Hishida T, Kitamura N, Daikuhara Y, McMaster P. Native and recombinant human hepatocyte growth factors are highly potent promoters of DNA synthesis in both human and rat hepatocytes. J Clin Invest. 1991;87:1853–1857. doi: 10.1172/JCI115207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gomez-Lechon MJ, Castelli J, Guillen I, OConnor E, Nakamura T, Fabra R, Trullenque R. Effects of hepatocyte growth factor on the growth and metabolism of human hepatocytes in primary culture. Hepatology. 1995;21:1248–1254. doi: 10.1002/hep.1840210506. [DOI] [PubMed] [Google Scholar]
- 30.Oh SH, Miyazaki M, Kouchi H, Inoue Y, Sakaguchi M, Tsuji T, Shima N, Higashio K, Namba M. Hepatocyte growth factor induces differentiation of adult rat bone marrow cells into a hepatocyte lineage in vitro. Biochem Biophys Res Commun. 2000;279:500–504. doi: 10.1006/bbrc.2000.3985. [DOI] [PubMed] [Google Scholar]
- 31.Zhang Y-N, Lie P, Wei X. Differentiation of mesenchymal stromal cells derived from umbilical cord Wharton’s jelly into hepatocyte-like cells. Cytotherapy. 2009;11(5):548–558. doi: 10.1080/14653240903051533. [DOI] [PubMed] [Google Scholar]
- 32.Wang PP, Wang JH, Yan ZP, Hu MY, Lau GK, Fan ST, Luk JM. Expression of hepatocyte-like phenotypes in bone marrow stromal cells after HGF induction. Biochem Biophys Res Commun. 2004;320:712–716. doi: 10.1016/j.bbrc.2004.05.213. [DOI] [PubMed] [Google Scholar]
- 33.Schwartz RE, Reyes M, Koodie L, Jiang Y, Blackstad M, Lund T, Lenvik T, Johnson S, Hu WS, Verfaillie CM. Multipotent adult progenitor cells from bone marrow differentiate into functional hepatocyte-like cells. J Clin Invest. 2002;109:1291–1302. doi: 10.1172/JCI0215182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nishizaki T, Takenaka K, Yoshizumi T, Yanaga K, Soejima Y, Shirabe K, Sugimachi K. Alteration in levels of human hepatocyte growth factor following hepatectomy. J Am Coll Surg. 1995;181:6–10. [PubMed] [Google Scholar]
- 35.Li W, Wang D, Qin J, Liu C, Zhang Q, Zhang X, Yu X, Lahn BT, Mao FF, Xiang AP. Generation of functional hepatocytes from mouse induced pluripotent stem cells. J Cell Physiol. 2010;222(3):492–501. doi: 10.1002/jcp.22000. [DOI] [PubMed] [Google Scholar]
- 36.Snykers S, Vanhaecke T, Becker A, et al. Chromatin remodeling agent trichostatin A: a key-factor in the hepatic differentiation of human mesenchymal stem cells derived of adult bone marrow. BMC Dev Biol. 2007;7:24. doi: 10.1186/1471-213X-7-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Song Z, Cai J, Liu Y, Zhao D, Yong J, Duo S, Song X, Guo Y, Zhao Y, Qin H, Yin X, Wu C, Che J, Lu S, Ding M, Deng H. Efficient generation of hepatocyte-like cells from human induced pluripotent stem cells. Cell Res. 2009;19(11):1233–1242. doi: 10.1038/cr.2009.107. [DOI] [PubMed] [Google Scholar]
- 38.Theise ND, Badve S, Saxena R, Henegariu O, Sell S, Crawford JM, et al. Derivation of hepatocytes from bone marrow cells in mice after radiation-induced myeloablation. Hepatology. 2000;31:235–240. doi: 10.1002/hep.510310135. [DOI] [PubMed] [Google Scholar]
- 39.Kang XQ, Zang WJ, Bao LJ, Li DL, Xu XL, Yu XJ. Differentiating characterization of human umbilical cord blood-derived mesenchymal stem cells in vitro. Cell Biol Int. 2006;30(7):569–575. doi: 10.1016/j.cellbi.2006.02.007. [DOI] [PubMed] [Google Scholar]


