Abstract
Purpose
It has been reported single-nucleotide polymorphisms (SNPs) of the IL-10 promoter might be associated with the susceptibility to recurrent pregnancy loss (RPL). Owing to the inconclusive results, we conducted a meta-analysis to systematically summarize and clarify the association between the IL-10 promoter SNPs and RPL risk.
Methods
A systematic search of studies on the association of the three SNPs with RPL was conducted in PubMed and Embase. Odds ratios (ORs) and 95 % confidence intervals (95 % CIs) were used to pool the effect size.
Result
Eleven case–control studies on rs1800896, seven studies on rs1800871, and eight studies on rs1800872 were included. A significant association was identified between IL-10 rs1800896 with RPL risk (G versus A: OR = 1.21, 95 % CI 1.09–1.35). No evidence of association was found between rs1800871 and RPL when restricted to those studies in Hardy–Weinberg equilibrium in controls (T versus C: OR = 1.25, 95 % CI 0.76–2.06). No statistical association was demonstrated between rs1800872 and RPL (C versus A: OR = 1.08, 95 % CI 0.83–1.42).
Conclusions
IL-10 rs1800896 significantly increases the risk of RPL, while rs1800872 is not correlated with RPL risk. No significant association is demonstrated between rs1800871 and RPL risk but this requires further investigation.
Electronic supplementary material
The online version of this article (doi:10.1007/s10815-016-0699-z) contains supplementary material, which is available to authorized users.
Keywords: Gene polymorphism, Interleukin-10, Meta-analysis, Recurrent pregnancy loss
Introduction
Recurrent pregnancy loss (RPL) is defined as more than two to three consecutive miscarriages prior to the 20th week of gestation, which is one of the most common complications of pregnancy, affecting about 1–5 % of pregnant women [1–3]. The causes can be divided into inherited and acquired, including chromosomal numeral and structural abnormalities, immunologic factors and thrombophilia, structural uterine abnormalities, and hormonal and metabolic factors, but the etiology is unknown in approximately 50 % of cases [4, 5]. Intrauterine actions of angiogenesis, immunity, and neuroendocrine regulation are juxtaposed to mechanisms of fetal growth and protection. The interleukin-10 (IL-10) is a key cytokine that plays a vital role in the maintenance of maternal–fetal tolerance by its pleiotropic activities [6, 7]. IL-10 exerts profound effects not only on linking immunity and angiogenesis, but also on suppressing endoplasmic reticulum (ER) stress [8–10]. High levels of IL-10 were detected in normal pregnant women and reduced IL-10 levels were present in women with RPL [11, 12].
Human IL-10 gene is an acid-sensitive homodimeric protein locating on human chromosome 1 (1q31–q32). Several studies have identified single-nucleotide polymorphisms (SNPs) of IL-10 gene can significantly influence the levels of IL-10 production and key obstetric complications, particularly the promoter variants control IL-10 secretion [13–15]. Though a number of studies have been conducted to investigate the association between promoter polymorphisms of IL-10 with RPL, the results are not consistent [14–24]. In addition, three earlier meta-analyses yielded conflicting results regarding the association of the IL-10 promoter SNP (rs1800896: −1802A/G) with RPL [20, 25, 26]. Therefore, we performed a meta-analysis, incorporating recently published studies in, to evaluate the association between the three polymorphisms of IL-10 promoter (rs1800896, rs1800872, rs1800871) and RPL risk.
Methods and materials
Identification of eligible studies
Two independent investigators carried out a systematic search in PubMed and Embase databases, with the last search update on 30 September, 2015. The following terms were used: “interleukin-10” or “cytokine” and “polymorphism” or “polymorphisms” and “habitual abortion” or “recurrent spontaneous abortion” (RSA) or “recurrent pregnancy loss” (RPL) or “recurrent miscarriages” (RM) or “recurrent fetal loss” (RFL) or “idiopathic recurrent miscarriage” (IRM) without any limitation applied. The studies included in the previous meta-analyses were also included. The reference lists of retrieved studies and recent reviews were also manually searched for further relevant studies.
Inclusion and exclusion criteria
Studies in this meta-analysis must meet the following inclusion criteria: (1) the original study was a case–control study; (2) evaluation of the association between IL-10 gene promoter polymorphisms and the RPL; (3) RPL was defined as the occurrence of two or more pregnancy loss in the first two trimesters of pregnancy without any etiology through ultrasonic examination and laboratory tests; (4) the controls were matched women without a history of miscarriage and had at least one successful pregnancy; and (5) detailed genotype data of the case and control groups could be acquired or be calculated. Exclusion criteria: (1) comment, review, and meta-analysis; (2) family-based studies of pedigrees; and (3) duplication of previous publications. Study selection was achieved independently by two investigators according to the inclusion and exclusion criteria by screening the title, abstract, and full-text. Any dispute was solved by discussion.
Data extraction
The data of the eligible studies were extracted by two investigators independently (Gu and Gong). The following contents were collected: name of first author, year of publication, the characteristics of cases and controls, country in which the study was performed, ethnicity of the study population, diagnostic standards of cases in each study, genotyping methods, number of cases and controls, and genotype frequency in cases and controls for IL-10 promoter (rs1800896 A/G, rs1800871 C/T, rs1800872 A/C), respectively, Hardy–Weinberg equilibrium (HWE) if reported in the study. Different ethnicity descents were classified as Caucasian or Asian. Two authors checked the extracted data and reached to consensus on all the data. If a dissent existed, they would recheck the original data of the included studies and have a discussion to reach consensus. If the dissent still existed, the third investigator would be involved to adjudicate the disagreements (Ma).
Methodological quality assessment
The qualities of the included studies were evaluated and scored by two authors respectively according to the methodological quality assessment scale (see Supplement information “Table S1 Scale for methodological quality assessment”), which was adjusted from the Newcastle–Ottawa Assessment Scale for case–control studies (http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp). In this scale, six items including the selection of patients with unexplained recurrent pregnancy loss, source of controls, comparability of cases and controls on the basis of the design or analysis, sample size, quality control of genotyping methods, and HWE were carefully checked. The quality score ranges from 0 to 10, with higher scores indicating a better quality of the study. Two investigators scored the studies independently and solved disagreement through discussion.
Statistics analysis
We conducted our meta-analysis according to the PRISMA checklists and followed the guideline [27]. HWE was evaluated for each study by Chi-square test in control groups, and P < 0.05 was considered a significant deviation from HWE. OR and 95 % CIs were calculated to evaluate the strength of the association between IL-10 promoter polymorphisms (rs1800896: −1802A/G; rs1800871: −819C/T; rs1800872: −592A/C) and susceptibility to RPL. Pooled ORs were performed for allelic comparison (rs1800896: G versus A, rs1800871: T versus C, rs1800872: C versus A), heterozygote model (rs1800896: GA versus AA, rs1800871: TC versus CC, rs1800872: CA versus AA), homozygote model (rs1800896: GG versus AA, rs1800871: TT versus CC, rs1800872: CC versus AA), dominant model (rs1800896: GG + GA versus AA, rs1800871: TT + TC versus CC, rs1800872: CC + CA versus AA), recessive model (rs1800896: GG versus GA + AA, rs1800871: TT versus TC + CC, rs1800872: CC versus CA + AA), respectively. The statistical significant level was determined by Z test with P value less than 0.05. Heterogeneity was evaluated by Q statistic and I2 statistic (greater than 50 % as evidence of significant inconsistency) [28]. Either fixed-effect or random-effects model was used to pool the effect sizes according to the heterogeneity [29]. The studies without deviation from HWE among controls were used to do a supplementary meta-analysis. Sensitivity analysis was also performed to evaluate the effect of each study on the combined ORs by omitting each study in each turn.
Besides, subgroup analyses were stratified by ethnicity and HWE. Potential publication bias was checked by Begg’s funnel plots [30] and Egger’s regression test [31]. An asymmetric plot and the P value of Egger’s test less than 0.05 was considered a significant publication bias. All statistical analyses were performed with Stata 12.0 software (StataCorp, College Station, TX, USA). A two-tailed P < 0.05 was considered significant except for specified conditions, where a certain P value was declared.
Results
Characteristics of studies
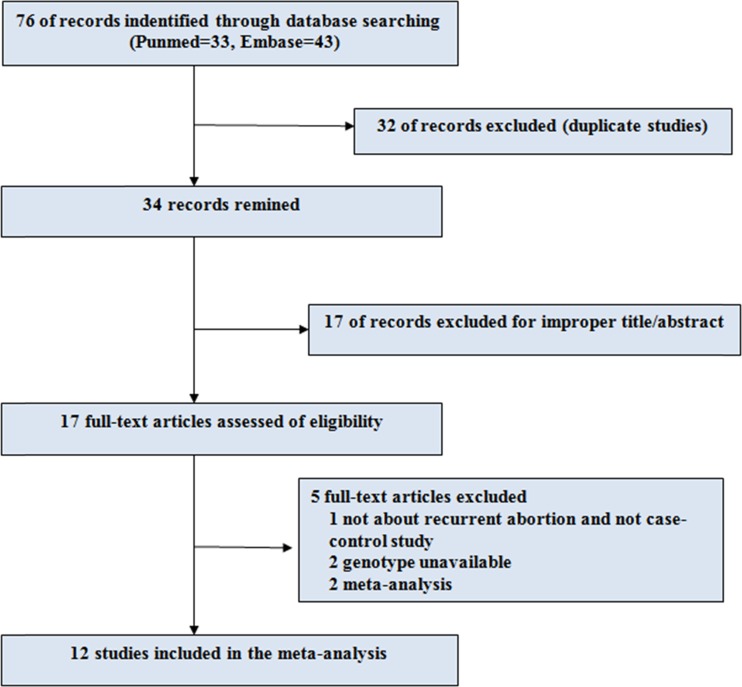
A total of 76 studies were acquired from PubMed and Embase databases (PubMed: 33, Embase: 43). The literature selection process was shown in Fig. 1, 49 articles were excluded, of which 32 were duplicate ones and 17 with no relation to this topic. The remaining 17 studies were full-text reviewed, and five studies were excluded, among which, one was not about recurrent abortion and not a case–control study [13], two were of unavailable genotype [32, 33] and the other two were meta-analysis [25, 26]. Finally, in the current study, 12 eligible case–control studies that meet the inclusion criteria were included in our meta-analysis [14, 16, 17, 19–24, 34–36].
Fig. 1.
Flow chart of study selection
The characteristics of each study were shown in the Table 1. In seven studies genotype frequencies of three SNPs of IL-10 promoter (rs1800896, rs1800871, rs1800872) were presented separately, thus each of them were treated as separate studies. Therefore, among all the twelve included studies, eleven studies containing 1936 cases and 1539 controls for IL-10 rs1800896, seven studies involving 1190 cases and 1213 controls for IL-10 rs1800871, and eight studies involving 1233 cases and 1263 controls for IL-10 rs1800872 were finally analyzed in our meta-analysis. For IL-10 rs1800896, the subjects in seven included studies were of Caucasians and the other four of Asians, and the genotyping distribution was in HWE (P ≥ 0.05) in all studies except for one study. As for IL-10 rs1800871, three studies were carried out in Caucasians and the other four in Asians, three studies were in HWE and the other four deviating from HWE (P < 0.05). For IL-10 rs1800872, three studies were carried out in Caucasians and the other five in Asians, five studies were in HWE and the other three deviating from HWE. Different genotyping methods were utilized, including polymerase chain reaction–restriction fragment length polymorphism (PCR–RFLP), TaqMan, PCR allele-specific amplification (PCR-ASA), PCR-ligation detection reaction (LDR–PCR), and T-ARMS-PCR.
Table 1.
Characteristics of studies included in the meta-analysis
| First author | Year | Country | Ethnicity | Case | Control | HWE | Quality | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| IL-1802G > A rs800896 | GG | GA | AA | GG | GA | AA | |||||
| Karhukorpi | 2001 | Finland | Caucasian | 9 | 16 | 13 | 23 | 64 | 44 | 0.97 | 5 |
| Babbage | 2001 | UK, | Caucasian | 12 | 23 | 8 | 12 | 41 | 20 | 0.24 | 6 |
| Daher | 2003 | Brazil | Caucasian | 11 | 19 | 13 | 16 | 43 | 45 | 0.29 | 5 |
| Prigoshin | 2004 | Argentina | Caucasian | 6 | 21 | 13 | 9 | 33 | 11 | 0.07 | 6 |
| Kamali-Sarvestani | 2005 | Iran | Asian | 24 | 41 | 62 | 21 | 47 | 62 | 0.02 | 7 |
| Zammiti | 2006 | Tunis | Caucasian | 72 | 185 | 87 | 39 | 107 | 54 | 0.28 | 9.5 |
| Farah Parveen | 2013 | India | Asian | 15 | 99 | 86 | 12 | 108 | 180 | 0.39 | 9 |
| Zastavna | 2014 | Ukraine | Caucasian | 47 | 25 | 28 | 15 | 36 | 22 | 0.97 | 6 |
| Camil L. | 2014 | Romania | Caucasian | 18 | 28 | 7 | 29 | 24 | 11 | 0.14 | 7 |
| Motahareh Bahadori | 2014 | Iran | Asian | 35 | 33 | 17 | 42 | 42 | 20 | 0.12 | 6 |
| R. H. Qaddourah | 2014 | Bahrain | Asian | 58 | 114 | 124 | 33 | 150 | 124 | 0.21 | 9.5 |
| IL-819 C > T rs1800871 | CC | CT | TT | CC | CT | TT | |||||
| Kamali-Sarvestani | 2005 | Iran | Asian | 77 | 49 | 13 | 61 | 56 | 15 | 0.69 | 8 |
| Zammiti | 2006 | Tunis | Caucasian | 182 | 120 | 48 | 124 | 57 | 19 | 0.00 | 9 |
| Farah Parveen | 2013 | India | Asian | 59 | 111 | 30 | 122 | 142 | 36 | 0.59 | 9 |
| Zastavna | 2014 | Ukraine | Caucasian | 39 | 12 | 0 | 57 | 47 | 2 | 0.03 | 5 |
| Camil L. | 2014 | Romania | Caucasian | 28 | 37 | 4 | 43 | 18 | 3 | 0.54 | 7 |
| Motahareh Bahadori | 2014 | Iran | Asian | 7 | 51 | 27 | 14 | 79 | 11 | 0.00 | 5 |
| R. H. Qaddourah | 2014 | Bahrain | Asian | 133 | 124 | 39 | 160 | 113 | 34 | 0.04 | 8.5 |
| IL-592-A > C rs1800872 | AA | AC | CC | AA | AC | CC | |||||
| Kamali-Sarvestani | 2005 | Iran | Asian | 14 | 35 | 83 | 15 | 56 | 61 | 0.69 | 8 |
| Zammiti | 2006 | Tunis | Caucasian | 51 | 93 | 206 | 25 | 41 | 134 | 0.00 | 9 |
| Amrit Kaur | 2011 | India | Asian | 8 | 24 | 18 | 6 | 27 | 17 | 0.34 | 7 |
| Farah Parveen l | 2013 | India | Asian | 30 | 79 | 91 | 36 | 116 | 148 | 0.08 | 9 |
| Zastavna | 2014 | Ukraine | Caucasian | 0 | 12 | 39 | 2 | 47 | 57 | 0.03 | 5 |
| Camil L. | 2014 | Romania | Caucasian | 6 | 33 | 30 | 3 | 26 | 35 | 0.50 | 7 |
| Motahareh Bahadori | 2014 | Iran | Asian | 8 | 34 | 43 | 22 | 49 | 33 | 0.63 | 6 |
| R. H. Qaddourah | 2014 | Bahrain | Asian | 33 | 128 | 135 | 37 | 108 | 162 | 0.01 | 8.5 |
HWE Hardy–Weinberg equilibrium
Association between IL-10 rs1800896 polymorphism and RPL susceptibility
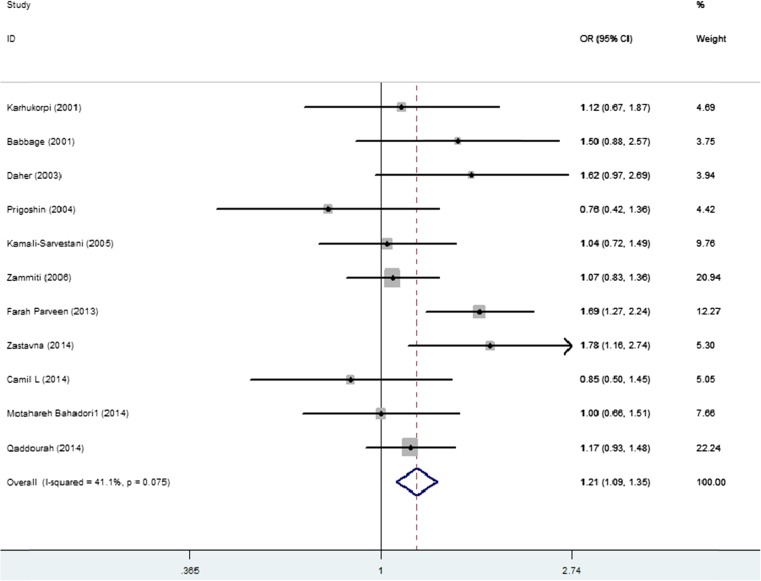
We firstly analyzed the association between IL-10 rs1800896 polymorphism and the susceptibility to RPL. Random-effects model was used in the heterozygote model due to heterogeneity identified by Q test and I-squared statistic (I2 > 50 %), and fixed-effects model was used in the other four models because of no significant heterogeneity (I2 < 50 %). Overall, significant association was identified in four genetic models: the allele model (G versus A: OR = 1.21, 95 % CI 1.09–1.35, I2 = 41 % Fig. 2), homozygote model (GG versus AA: OR = 1.48, 95 % CI 1.18–1.86, I2 = 8 %), dominant model (GA + GG versus AA: OR = 1.18, 95 % CI 1.01–1.38, I2 = 36 %), and recessive model (GG versus GA + AA: OR = 1.44, 95 % CI 1.19–1.75, I2 = 45 %). In the heterozygote model, no significant association was found (GA versus AA: OR = 1.03, 95 % CI 0.78–1.35, I2 = 52 %) (Table 2).
Fig. 2.
Forest plot shows the odds ratio for the association between the IL-10 rs1800896 and the risk of PRL in overall comparison (G versus A). Horizontal lines represent 95 % CIs and vertical lines the value of the summary OR
Table 2.
Summary of polled ORs in the meta-analysis
| N | OR | I 2 (%) | OR | I 2 (%) | OR | I 2 (%) | OR | I 2 (%) | OR | I 2 (%) | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| rs1800896 A/G | G/A | GA/AA | GG/AA | GA + GG/AA | GG/GA + AA | ||||||
| Overall | 11 | 1.21 (1.09–1.35) | 41 | 1.03 (0.78–1.35) | 52 | 1.48 (1.18–1.86) | 8 | 1.18 (1.01–1.38) | 36 | 1.441 (1.19–1.75) | 45 |
| Ethnicity | |||||||||||
| Caucasian | 7 | 1.18 (1.01–1.38) | 40 | 1.00 (0.76–1.31) | 14 | 1.43 (1.04–1.97) | 12 | 1.14 (0.88–1.47) | 0 | 1.41 (0.91–1.79) | 57 |
| Asian | 4 | 1.22 (0.97–1.55) | 55 | 1.06 (0.64–1.76) | 78 | 1.54 (1.11–2.12) | 24 | 1.18 (0.77–1.45) | 72 | 1.53 (1.15–2.05) | 26 |
| rs1800871 C/T | T/C | TC/CC | TT/CC | TT + TC/CC | TT/TC + CC | ||||||
| Overall | 7 | 1.21 (0.92–1.57) | 74 | 1.20 (0.82–1.77) | 75 | 1.52 (1.15–2.00) | 36 | 1.24 (0.84–1.82) | 77 | 141 (1.09–1.83) | 39 |
| Ethnicity | |||||||||||
| Caucasian | 3 | 1.12 (0.53–2.39) | 68 | 1.19 (0.82–1.73) | 88 | 1.47 (1.06–2.05) | 0 | 1.20 (0.44–3.28) | 88 | 1.41 (0.85–2.36) | 0 |
| Asian | 4 | 1.22 (0.93–1.60) | 85 | 1.20 (0.43–3.34) | 56 | 1.54 (0.86–2.74) | 62 | 1.24 (0.84–1.83) | 64 | 1.46 (0.85–2.52) | 67 |
| HWE | |||||||||||
| ≥0.05 | 3 | 1.25 (0.76–2.06) | 80 | 1.47 (0.68–3.17) | 84 | 1.31 (0.83–2.05) | 44 | 1.46 (0.68–3.10) | 85 | 1.13 (0.74–1.71) | 0 |
| <0.05 | 4 | 1.18 (0.81–1.71) | 77 | 1.04 (0.63–1.72) | 71 | 1.66 (1.16–2.37) | 42 | 1.1 (0.66–1.85) | 76 | 1.72 (0.95–3.11) | 58 |
| rs1800872 A/C | C/A | CA/AA | CC/AA | CA + CC/AA | CC/CA + AA | ||||||
| Overall | 8 | 1.08 (0.83–1.42) | 75 | 1.03 (0.79–1.36) | 0 | 0.97 (0.75–1.26) | 43 | 0.99 (0.77–1.27) | 8 | 1.14 (0.79–1.63) | 77 |
| Ethnicity | |||||||||||
| Caucasian | 4 | 1.02 (0.54–1.91) | 80 | 1.03 (0.60–1.78) | 0 | 0.75 (0.46–1.21) | 0 | 0.82 (0.51–1.32) | 0 | 1.04 (0.46–2.34) | 82 |
| Asian | 4 | 1.41 (0.82–1.57) | 76 | 1.03 (0.76–1.42) | 16 | 1.18 (0.71–1.96) | 57 | 1.06 (0.79–1.42) | 33 | 1.21 (0.78–1.89) | 78 |
| HWE | |||||||||||
| ≥0.05 | 5 | 1.12 (0.77–1.63) | 75 | 0.88 (0.61–1.29) | 0 | 1.13 (0.56–2.21) | 62 | 1.00 (0.71–1.42) | 40 | 1.22 (0.76–1.97) | 73 |
| <0.05 | 3 | 1.03 (0.66–1.59) | 78 | 1.23 (0.83–1.83) | 0 | 0.86 (0.60–1.24) | 0 | 0.98 (0.77–1.27) | 0 | 1.02 (0.56–1.86) | 82 |
Numbers in italics indicate ORs with statistical significance
N number of studies included, OR odds ratio and 95 % CI, I 2 value for heterogeneity
HWE P ≥ 0.05 in Hardy–Weinberg equilibrium, P < 0.05 for those that deviated from the Hardy–Weinberg equilibrium
Next, subgroup analysis was conducted according to ethnicity. In Caucasians, significant heterogeneity was identified in the recessive model, and in Asians, heterogeneity was identified in the allele, heterozygote, and dominant models, so that the random-effects model was used. Fixed-effects model was used in other genetic models. In Caucasians, significant association was found in the allele and homozygote models (G versus A: OR = 1.18, 95 % CI 1.01–1.38, I2 = 40 %; GG versus AA: OR = 1.43, 95 % CI 1.04–1.97, I2 = 12 %), and no significant association was found in other models. In Asians, significant association was found in the homozygote and recessive models (GG versus AA: OR = 1.54, 95 % CI 1.11–2.12, I2 = 24 %; GG versus GA + AA: OR = 1.52, 95 % CI 1.15–2.05, I2 = 26 %) and no significant association was found in other models (Table 2).
Association between IL-10 rs1800871 polymorphism and RPL susceptibility
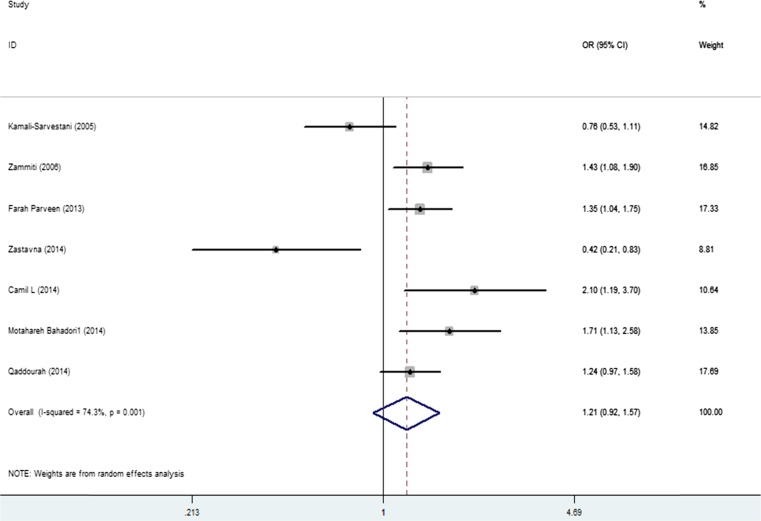
The association between IL-10 rs1800871 polymorphism and the risk of RPL was analyzed in seven independent studies. Fixed-effects model was used in the homozygote and recessive models due to no heterogeneity and the random-effects model was used in the other three genetic models. Significant association was identified in the homozygote and recessive models (TT versus CC: OR = 1.52, 95 % CI 1.15–2.00, I2 = 36 %; TT versus TC + CC: OR = 1.41, 95 % CI 1.09–1.83, I2 = 39 %), and no statistical association was identified in other three models (T versus C: OR = 1.21, 95 % CI 0.92–1.57, I2 = 74 % (Fig. 3); TC versus CC: OR = 1.20, 95 % CI 0.82–1.77, I2 = 75 %; TT + TC versus CC: OR = 1.24, 95 % CI 0.84–1.82, I2 = 77 %) (Table 2).
Fig. 3.
Forest plot shows the odds ratio for the association between the IL-10 rs1800871 and the risk of PRL in overall comparison (T versus C). Horizontal lines represent 95 % CIs and vertical lines the value of the summary OR
Four out of seven studies were deviated from HWE (P < 0.05) and other three studies were in HWE (P ≥ 0.05) in control subjects. Subgroup analysis was assessed according to HWE. The fixed-effects model was used in three genetic models due to no significant heterogeneity: the homozygote and recessive models of the HWE group and the homozygote model of those that deviated from the HWE group. The random-effects model was used in all other genetic models of both subgroups. Overall, no significant association was identified (T versus C: OR = 1.25, 95 % CI 0.76–2.06, I2 = 80 %; TC versus CC: OR = 1.47, 95 % CI 0.68–3.17, I2 = 84 %; TT versus CC: OR = 1.31, 95 % CI 0.83–2.05, I2 = 44 %; TT + TC versus CC: OR = 1.46, 95 % CI 0.68–1.85, I2 = 85 %; TT versus TC + CC: OR = 1.13, 95 % CI 0.74–1.71, I2 = 0 %) (Table 2) except for the homozygote model of those that deviated from the HWE group (TT versus CC: OR = 1.66, 95 % CI 1.16–2.37, I2 = 42 %).
Association between IL-10 rs1800872 polymorphism and RPL susceptibility
For the association between IL-10 rs1800872 polymorphism and RPL risk, eight independent studies were analyzed. Fixed-effects model was used in the heterozygote, homozygote, and dominant models due to no significant heterogeneity and the random-effects model was used in the other two genetic models. Overall, no significant association was identified in any genetic models (C versus A: OR = 1.08, 95 % CI 0.83–1.42, I2 = 75 % ; CA versus AA: OR = 1.03, 95 % CI 0.79–1.36, I2 = 0 %; CC versus AA: OR = 0.97, 95 % CI 0.75–1.26, I2 = 43 %; CA + CC versus AA: OR = 0.99, 95 % CI 0.77–1.27, I2 = 8 %; CC versus CA + AA: OR = 1.14, 95 % CI 0.79–1.63, I2 = 77 %).
Three out of eight studies were deviated from HWE and other five studies were in agreement with HWE in control subjects. Subgroup analysis was also assessed according to HWE, but no significant statistical association was identified in both subgroups (Table 2 and Fig. 4).
Fig. 4.
Forest plot shows the odds ratio for the association between the IL-10 rs1800872 and the risk of PRL in stratification according to HWE (C versus A). (1: in Hardy–Weinberg equilibrium, 0: deviation from Hardy–Weinberg equilibrium) Horizontal lines represent 95 % CIs and vertical lines the value of the summary OR
Sensitivity analysis
Sensitivity analysis was performed to examine the influence set by individual study on the pooled ORs for IL-10 rs1800896, rs1800871, and rs1800872 by deleting each study once in every genetic model. The results revealed no evidence of any individual study having excessive influence on the summary effect under any genetic model (data not published). Furthermore, sensitivity analysis was also carried out by excluding the study in which the control group’s genotypes deviated from HWE in IL-10 rs1800896. Consistently, the pooled estimate of the remaining studies showed no influence on the summary effect. Subgroup analysis was performed according to HWE in IL-10 rs1800871 and rs1800872.
Publication bias
No publication bias for the association between IL-10 rs1800896 and RPL susceptibility was identified by Begg’s funnel plot (P = 0.755, G versus A) or Egger’s regression test (P = 0.599, G versus A). Symmetrical funnel plots were obtained in all the genetic models (unpublished data). Begg’s funnel plot and Egger’s regression test was also not identified publication bias between IL-10 rs1800871 and rs1800872 with RPL susceptibility (rs1800871 T versus C: Begg’s P = 1.000, Egger’s P = 0.426; rs1800872 C versus A: Begg’s P = 0.266, Egger’s P = 0.298) (Fig. 5).
Fig. 5.
Begg’s funnel plot for publication bias analysis for IL-10. a rs1800896 G versus A, b rs1800871 T versus C, c: rs1800872 A versus C
Discussion
Pregnancy is a well-choreographed physiological process that involves intricate interplay of inflammatory and anti-inflammatory milieu, and cellular and molecular events at the maternal–fetal interface. IL-10 was first reported as an inhibitor of inflammatory, which is secreted by both immune and non-immune cells in an autocrine and paracrine manner [37, 38]. In recent years, immense amounts of research have investigated about the actions of IL-10 during pregnancy and shown some profound and interesting findings. Firstly, as the most temporal immunosuppressive and anti-inflammatory molecule, IL-10 maintains the balance of the utero-placental produced pro-inflammatory and anti-inflammatory factors [8]. Secondly, recent studies showed that IL-10 promotes de novo angiogenesis at the maternal–fetal interface through inducing production of vascular endothelial growth factor, consequently improves implantation and placental development [9, 39]. In addition, IL-10 emerges as a novel modulator of the endoplasmic reticulum (ER) stress, which is essential to protein synthesis and energy balance. A higher level of ER stress leads to the activation of pro-inflammatory pathways and disadvantages of placental development [10, 40, 41]. Furthermore, increased IL-10 expression is associated with successful reproduction and IL-10 deficiency is associated with many adverse pregnancy outcomes such as PRL, preterm birth, and preeclampsia [11, 42–44]. Animal experimental studies also show that administration of recombinant IL-10 reverses or alleviates symptoms of many adverse pregnancy outcomes [45–48]. Polymorphic change in the human IL-10 gene promoter are thought to contribute to reduced IL-10 production and to the onset and severity of autoimmune, neoplastic, and infectious disorders such as systemic lupus erythematosus and Alzheimer’s disease [49–51]. Evidence has also been found for the associated of the IL-10 gene promoter polymorphisms with other adverse pregnancy outcomes [52–54].
In this meta-analysis, involving 1,936 cases and 1,539 controls, the pooled results indicated that the IL-10 rs1800896 is significantly associated with the risk of RPL. The result is consist with the earlier meta-analysis by Medica et al. [26], but inconsistent with previous meta-analyses by Bombell et al. and Preveen et al. [20, 25]. The first, including six studies involving 635 RPL cases and 691 controls, documented a significant association between the mutant type allele and RPL under the dominant model [26]. Although included the same six studies as the first meta-analysis, the second analysis documented no significant association between the IL-10 rs1800896 and RPL [25]. The more recent meta-analysis, the date for 835 patients and 991 control subjects, which included the studies included in the two earlier meta-analyses as well as their own results on North Indian women, demonstrated an RPL-protective nature of the IL-10 rs1800896 variant [20]. These discrepancies can likely be attributed to ethnic differences, sample size, and differences in interpretation of the association of rs1800896 with RPL risk. In our meta-analysis, not only a larger sample size was included, but also multiples genetic models and subgroups comparing was employed to identify the result. Our results showed that the IL-10 rs1800896 polymorphism is associated with RPL risk. The rs1800896 A/G polymorphisms in the IL-10 promoter have been found at -1802 bp upstream from the transcription start site. Moreover, the gene promoter polymorphisms have been also described to influence transcriptional and functional characteristics of IL-10 gene [55, 56]. Combining with the results of our meta-analysis, we think that the IL-10 rs1800896 A to G nucleotide exchange may result in decreased promoter activity and reduced IL-10 gene production. However, more convincing evidence, such as large sample size, number of studies, and ethnicities, is required to consolidate the conclusion.
For the IL-10 rs1800871 C/T polymorphism, no statistical association was observed in three studies in HWE when subgroups analysis is performed [20, 22, 34]. Although Hardy–Weinberg disequilibrium is not necessarily invalidate the results of an association study, the inability to ascertain the reason for the disequilibrium necessitates a cautious approach to these studies [57]. Therefore, when the different result was found in subgroups analysis according to HWE, we think the result of restricting in the HWE group would be more valid—no significant association between IL-10 rs1800871 and RPL risk. This consists of an earlier meta-analysis by Bombell et al. [25]. However, this result should be considered with caution and well-designed studies with a larger sample size are needed to validate. For IL-10 rs1800872 A/C polymorphism, the summery pooled result demonstrated no significant association with the risk of RPL in all five genetic models. In subgroups analysis according to HWE, also no significant association was found in both subgroups. So we further conducted a subgroup analysis focus on the ethnicity regardless of HWE (data not published), with three studies carried out in Caucasians and the other five in Asians, and no significant association was found in both subgroups. We believe that IL-10 rs1800872 A/C polymorphism would not increase the susceptibility to RPL in either Asians or Caucasians.
This meta-analysis has several strengths. First of all, we investigated the association of IL-10 promoter polymorphisms with the RPL risk in the appropriate method to obtain a more precise conclusion. In this meta-analysis, more recently studies were included and supplementary analysis including subgroup and sensitivity analysis were performed. In addition, all the included studies had high qualities according to the methodological quality assessment. Moreover, no publication bias was identified by either Begg’s funnel plot or Egger’s regression test. Finally, no limitation was made in literature search, thus selection bias was well controlled.
In spite of the considerable efforts to explore the possible relationship between the three SNPs and RPL risk, some limitations should be considered. Firstly, several studies did not conform to HWE expectations. However, restricted to those studies in HWE was further analyzed. Secondly, both primary and secondary RPL was included in this meta-analysis, so we could not improve that is there a difference if only primary RPL is included. Thirdly, our study only included articles published in English, which might limit the results of the meta-analysis. Finally, this meta-analysis is all about Caucasians and Asians and does not apply to all populations.
In summary, this meta-analysis highlights the important role of IL-10 promoter polymorphisms as potential risk factors for RPL. Significant association is demonstrated in IL-10 rs1800896 A/G polymorphism with RPL but not in rs1800872 A/C polymorphism. No significantly association is demonstrated between rs1800871 C/T polymorphism with RPL risk when excluded studies deviating from HWE in controls, but the result should be interpreted with caution. Well-designed studies with a larger sample size are required to validate the results identified in the current meta-analysis and to determine if screening and analysis of IL-10 rs1800896 A/G polymorphism mutations may be included in routine workups of patients with RPL.
Electronic supplementary material
Below is the link to the electronic supplementary material.
(DOC 35.5 kb)
Acknowledgments
This work was supported by the National Basic Research Program of China (973 Program, 2012CB947600) and the National Natural Science Foundation of China (31070676, 90919006, and 31300961). The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Footnotes
Capsule IL-10 rs1800896 significantly increases the risk of RPL, while rs1800872 is not correlated with RPL risk. No significant association is demonstrated between rs1800871 and RPL risk but this requires further investigation.
References
- 1.Saravelos SH, Regan L. Unexplained recurrent pregnancy loss. Obstet Gynecol Clin N Am. 2014;41(1):157–66. doi: 10.1016/j.ogc.2013.10.008. [DOI] [PubMed] [Google Scholar]
- 2.Practice Committee of American Society for Reproductive M Definitions of infertility and recurrent pregnancy loss: a committee opinion. Fertil Steril. 2013;99(1):63. doi: 10.1016/j.fertnstert.2012.09.023. [DOI] [PubMed] [Google Scholar]
- 3.Bardos J, Hercz D, Friedenthal J, Missmer SA, Williams Z. A national survey on public perceptions of miscarriage. Obstet Gynecol. 2015;125(6):1313–20. doi: 10.1097/AOG.0000000000000859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rai R, Regan L. Recurrent miscarriage. Lancet. 2006;368(9535):601–11. doi: 10.1016/S0140-6736(06)69204-0. [DOI] [PubMed] [Google Scholar]
- 5.Yang CJ, Stone P, Stewart AW. The epidemiology of recurrent miscarriage: a descriptive study of 1214 prepregnant women with recurrent miscarriage. Aust N Z J Obstet Gynaecol. 2006;46(4):316–22. doi: 10.1111/j.1479-828X.2006.00599.x. [DOI] [PubMed] [Google Scholar]
- 6.Denney JM, Nelson EL, Wadhwa PD, Waters TP, Mathew L, Chung EK, et al. Longitudinal modulation of immune system cytokine profile during pregnancy. Cytokine. 2011;53(2):170–7. doi: 10.1016/j.cyto.2010.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cheng SB, Sharma S. Interleukin-10: a pleiotropic regulator in pregnancy. Am J Reprod Immunol. 2015;73(6):487–500. doi: 10.1111/aji.12329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Samanna M, Chaouat G, Moron AF, Freitas V, Kulay L, Frydman R, et al. The emerging role of decidual NK cells for regulation of trophoblast IL-10 synthesis in early pregnancy. Fertil Steril. 2004;82:S60. doi: 10.1016/j.fertnstert.2004.07.153. [DOI] [Google Scholar]
- 9.Kalkunte SS, Mselle TF, Norris WE, Wira CR, Sentman CL, Sharma S. Vascular endothelial growth factor C facilitates immune tolerance and endovascular activity of human uterine NK cells at the maternal-fetal interface. J Immunol. 2009;182(7):4085–92. doi: 10.4049/jimmunol.0803769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hasnain SZ, Tauro S, Das I, Tong H, Chen ACH, Jeffery PL, et al. IL-10 promotes production of intestinal mucus by suppressing protein misfolding and endoplasmic reticulum stress in goblet cells. Gastroenterology. 2013;144(2):357. doi: 10.1053/j.gastro.2012.10.043. [DOI] [PubMed] [Google Scholar]
- 11.Raghupathy R, Makhseed M, Azizieh F, Hassan N, Al-Azemi M, Al-Shamali E. Maternal Th1-and Th2-type reactivity to placental antigens in normal human pregnancy and unexplained recurrent spontaneous abortions. Cell Immunol. 1999;196(2):122–30. doi: 10.1006/cimm.1999.1532. [DOI] [PubMed] [Google Scholar]
- 12.Bates MD, Quenby S, Takakuwa K, Johnson PM, Vince GS. Aberrant cytokine production by peripheral blood mononuclear cells in recurrent pregnancy loss? Hum Reprod. 2002;17(9):2439–44. doi: 10.1093/humrep/17.9.2439. [DOI] [PubMed] [Google Scholar]
- 13.Cochery-Nouvellon E, Nguyen P, Attaoua R, Cornillet-Lefebvre P, Mercier E, Vitry F, et al. Interleukin 10 gene promoter polymorphisms in women with pregnancy loss: preferential association with embryonic wastage. Biol Reprod. 2009;80(6):1115–20. doi: 10.1095/biolreprod.108.072215. [DOI] [PubMed] [Google Scholar]
- 14.Qaddourah RH, Magdoud K, Saldanha FL, Mahmood N, Mustafa FE, Mahjoub T, et al. IL-10 gene promoter and intron polymorphisms and changes in IL-10 secretion in women with idiopathic recurrent miscarriage. Hum Reprod. 2014;29(5):1025–34. doi: 10.1093/humrep/deu043. [DOI] [PubMed] [Google Scholar]
- 15.Mormann M, Rieth H, Hua TD, Assohou C, Roupelieva M, Hu SL, et al. Mosaics of gene variations in the interleukin-10 gene promoter affect interleukin-10 production depending on the stimulation used. Genes Immun. 2004;5(4):246–55. doi: 10.1038/sj.gene.6364073. [DOI] [PubMed] [Google Scholar]
- 16.Daher S, Shulzhenko N, Morgun A, Mattar R, Rampim GF, Camano L, et al. Associations between cytokine gene polymorphisms and recurrent pregnancy loss. J Reprod Immunol. 2003;58(1):69–77. doi: 10.1016/S0165-0378(02)00059-1. [DOI] [PubMed] [Google Scholar]
- 17.Prigoshin N, Tambutti M, Larriba J, Gogorza S, Testa R. Cytokine gene polymorphisms in recurrent pregnancy loss of unknown cause. Am J Reprod Immunol. 2004;52(1):36–41. doi: 10.1111/j.1600-0897.2004.00179.x. [DOI] [PubMed] [Google Scholar]
- 18.Kamali-Sarvestani E, Zolghadri J, Gharesi-Fard B, Sarvari J. Cytokine gene polymorphisms and susceptibility to recurrent pregnancy loss in Iranian women. J Reprod Immunol. 2005;65(2):171–8. doi: 10.1016/j.jri.2005.01.008. [DOI] [PubMed] [Google Scholar]
- 19.Zammiti W, Mtiraoui N, Cochery-Nouvellon E, Mahjoub T, Almawi WY, Gris JC. Association of -592C/A, -819C/T and -1082A/G interleukin-10 promoter polymorphisms with idiopathic recurrent spontaneous abortion. Mol Hum Reprod. 2006;12(12):771–6. doi: 10.1093/molehr/gal084. [DOI] [PubMed] [Google Scholar]
- 20.Parveen F, Shukla A, Agarwal S. Cytokine gene polymorphisms in northern Indian women with recurrent miscarriages. Fertil Steril. 2013;99(2):433–40. doi: 10.1016/j.fertnstert.2012.09.025. [DOI] [PubMed] [Google Scholar]
- 21.Zastavna D, Sosnina K, Terpylyak O, Huleyuk N, Bezkorovayna H, Mikula M, et al. Cytogenetic and immunogenetic analysis of recurrent pregnancy loss in women. TSitologiia i genetika. 2014;48(4):44–50. [PubMed] [Google Scholar]
- 22.Kamali-Sarvestani B, Zolghadri J, Gharesi-Fard B, Sarvari J. Cytokine gene polymorphisms and susceptibility to recurrent pregnancy loss in Iranian women. J Reprod Immunol. 2005;65(2):171–8. doi: 10.1016/j.jri.2005.01.008. [DOI] [PubMed] [Google Scholar]
- 23.Babbage SJ, Arkwright PD, Vince GS, Perrey C, Pravica V, Quenby S, et al. Cytokine promoter gene polymorphisms and idiopathic recurrent pregnancy loss. J Reprod Immunol. 2001;51(1):21–7. doi: 10.1016/S0165-0378(01)00069-9. [DOI] [PubMed] [Google Scholar]
- 24.Kaur A, Kaur A. Recurrent pregnancy loss: TNF-α and IL-10 polymorphisms. J Hum Reprod Sci. 2011;4(2):91–4. doi: 10.4103/0974-1208.86090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bombell S, McGuire W. Cytokine polymorphisms in women with recurrent pregnancy loss: meta-analysis. Aust N Z J Obstet Gyn. 2008;48(2):147–54. doi: 10.1111/j.1479-828X.2008.00843.x. [DOI] [PubMed] [Google Scholar]
- 26.Medica I, Ostojic S, Pereza N, Kastrin A, Peterlin B. Association between genetic polymorphisms in cytokine genes and recurrent miscarriage—a meta-analysis. Reprod Biomed Online. 2009;19(3):406–14. doi: 10.1016/S1472-6483(10)60176-9. [DOI] [PubMed] [Google Scholar]
- 27.Moher D, Liberati A, Tetzlaff J, Altman DG, Grp P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med. 2009;151(4):264–W64. doi: 10.7326/0003-4819-151-4-200908180-00135. [DOI] [PubMed] [Google Scholar]
- 28.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21(11):1539–58. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 29.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7(3):177–88. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 30.Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50(4):1088–101. doi: 10.2307/2533446. [DOI] [PubMed] [Google Scholar]
- 31.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629–34. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Alkhuriji AE, Alhimaidi AR, Babay ZA, Wary AS. The relationship between cytokine gene polymorphism and unexplained recurrent spontaneous abortion in Saudi females. Saudi Med J. 2013;34(5):484–9. [PubMed] [Google Scholar]
- 33.Costeas PA, Koumouli A, Giantsiou-Kyriakou A, Papaloizou A, Koumas L. Th2/Th3 cytokine genotypes are associated with pregnancy loss. Hum Immunol. 2004;65(2):135–41. doi: 10.1016/j.humimm.2003.11.007. [DOI] [PubMed] [Google Scholar]
- 34.C LB, V ER. Interleukin-6 and interleukin-10 gene polymorphisms and recurrent pregnancy loss in Romanian population. Iran J Reprod Med. 2014;12(9):617–22. [PMC free article] [PubMed]
- 35.Karhukorpi J, Laitinen T, Karttunen R, Tiilikainen AS. The functionally important IL-10 promoter polymorphism (-1082G→A) is not a major genetic regulator in recurrent spontaneous abortions. Mol Hum Reprod. 2001;7(2):201–3. doi: 10.1093/molehr/7.2.201. [DOI] [PubMed] [Google Scholar]
- 36.Bahadori M, Zarei S, Zarnani AH, Zarei O, Idali F, Hadavi R, et al. IL-6, IL-10 and IL-17 gene polymorphisms in Iranian women with recurrent miscarriage. Iran J Immunol: IJI. 2014;11(2):97–104. [PubMed] [Google Scholar]
- 37.Moore KW, O’Garra A, de Waal MR, Vieira P, Mosmann TR. Interleukin-10. Annu Rev Immunol. 1993;11:165–90. doi: 10.1146/annurev.iy.11.040193.001121. [DOI] [PubMed] [Google Scholar]
- 38.Ouyang W, Rutz S, Crellin NK, Valdez PA, Hymowitz SG. Regulation and functions of the IL-10 family of cytokines in inflammation and disease. Annu Rev Immunol. 2011;29:71–109. doi: 10.1146/annurev-immunol-031210-101312. [DOI] [PubMed] [Google Scholar]
- 39.Didion SP, Kinzenbaw DA, Schrader LI, Chu Y, Faraci FM. Endogenous interleukin-10 inhibits angiotensin II-induced vascular dysfunction. Hypertension. 2009;54(3):619–U331. doi: 10.1161/HYPERTENSIONAHA.109.137158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Burton GJ, Yung HW. Endoplasmic reticulum stress in the pathogenesis of early-onset pre-eclampsia. Pregnancy Hypertens. 2011;1(1-2):72–8. doi: 10.1016/j.preghy.2010.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shkoda A, Ruiz PA, Daniel H, Kim SC, Rogler G, Sartor RB, et al. Interleukin-10 blocked endoplasmic reticulum stress in intestinal epithelial cells: impact on chronic inflammation. Gastroenterology. 2007;132(1):190–207. doi: 10.1053/j.gastro.2006.10.030. [DOI] [PubMed] [Google Scholar]
- 42.von Wolff M, Thaler CJ, Strowitzki T, Broome J, Stolz W, Tabibzadeh S. Regulated expression of cytokines in human endometrium throughout the menstrual cycle: dysregulation in habitual abortion. Mol Hum Reprod. 2000;6(7):627–34. doi: 10.1093/molehr/6.7.627. [DOI] [PubMed] [Google Scholar]
- 43.Hennessy A, Pilmore HL, Simmons LA, Painter DM. A deficiency of placental IL-10 in preeclampsia. J Immunol. 1999;163(6):3491–5. [PubMed] [Google Scholar]
- 44.Hanna N, Bonifacio L, Weinberger B, Reddy P, Murphy S, Romero R, et al. Evidence for interleukin-10-mediated inhibition of cyclo- oxygenase-2 expression and prostaglandin production in preterm human placenta. Am J Reprod Immunol. 2006;55(1):19–27. doi: 10.1111/j.1600-0897.2005.00342.x. [DOI] [PubMed] [Google Scholar]
- 45.Terrone DA, Rinehart BK, Granger JP, Barrilleaux PS, Martin JN, Jr, Bennett WA. Interleukin-10 administration and bacterial endotoxin-induced preterm birth in a rat model. Obstet Gynecol. 2001;98(3):476–80. doi: 10.1016/s0029-7844(01)01424-7. [DOI] [PubMed] [Google Scholar]
- 46.Tinsley JH, South S, Chiasson VL, Mitchell BM. Interleukin-10 reduces inflammation, endothelial dysfunction, and blood pressure in hypertensive pregnant rats. Am J Physiol Regul Integr Comp Physiol. 2010;298(3):R713–9. doi: 10.1152/ajpregu.00712.2009. [DOI] [PubMed] [Google Scholar]
- 47.Lai Z, Kalkunte S, Sharma S. A critical role of interleukin-10 in modulating hypoxia-induced preeclampsia-like disease in mice. Hypertension. 2011;57(3):505–14. doi: 10.1161/HYPERTENSIONAHA.110.163329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lin Y, Liu X, Shan B, Wu J, Sharma S, Sun Y. Prevention of CpG-induced pregnancy disruption by adoptive transfer of in vitro-induced regulatory T cells. PLoS One. 2014;9(4):e94702. doi: 10.1371/journal.pone.0094702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lin MT, Storer B, Martin PJ, Tseng LH, Gooley T, Chen PJ, et al. Relation of an interleukin-10 promoter polymorphism to graft-versus-host disease and survival after hematopoietic-cell transplantation. N Engl J Med. 2003;349(23):2201–10. doi: 10.1056/NEJMoa022060. [DOI] [PubMed] [Google Scholar]
- 50.Costa GC, Rocha MOD, Moreira PR, Menezes CAS, Silva MR, Gollob KJ, et al. Functional IL-10 gene polymorphism is associated with Chagas disease cardiomyopathy. J Infect Dis. 2009;199(3):451–4. doi: 10.1086/596061. [DOI] [PubMed] [Google Scholar]
- 51.Gezen-Ak D, Dursun E, Hanagasi H, Bilgic B, Lohman E, Araz OS, et al. BDNF, TNFalpha, HSP90, CFH, and IL-10 serum levels in patients with early or late onset Alzheimer’s disease or mild cognitive impairment. J Alzheimers Dis: JAD. 2013;37(1):185–95. doi: 10.3233/JAD-130497. [DOI] [PubMed] [Google Scholar]
- 52.Makris A, Xu B, Yu B, Thornton C, Hennessy A. Placental deficiency of interleukin-10 (IL-10) in preeclampsia and its relationship to an IL-10 promoter polymorphism. Placenta. 2006;27(4-5):445–51. doi: 10.1016/j.placenta.2005.05.003. [DOI] [PubMed] [Google Scholar]
- 53.Daher S, Sass N, Oliveira LG, Mattar R. Cytokine genotyping in preeclampsia. Am J Reprod Immunol. 2006;55(2):130–5. doi: 10.1111/j.1600-0897.2005.00341.x. [DOI] [PubMed] [Google Scholar]
- 54.Sowmya S, Ramaiah A, Sunitha T, Nallari P, Jyothy A, Venkateshwari A. Role of IL-10 -819(t/c) promoter polymorphism in preeclampsia. Inflammation. 2014;37(4):1022–7. doi: 10.1007/s10753-014-9824-2. [DOI] [PubMed] [Google Scholar]
- 55.Murphy SP, Sharma S. IL-10 and pregnancy. In Immunology of Pregnancy. 2006; G Mor (ed.):26-36.
- 56.Sharma S, Stabila J, Pietras L, Singh AR, McGonnigal B, Ernerudh J, et al. Haplotype-dependent differential activation of the human IL-10 gene promoter in macrophages and trophoblasts: implications for placental IL-10 deficiency and pregnancy complications. Am J Reprod Immunol. 2010;64(3):179–87. doi: 10.1111/j.1600-0897.2010.00854.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Thakkinstian A, McElduff P, D’Este C, Duffy D, Attia J. A method for meta-analysis of molecular association studies. Stat Med. 2005;24(9):1291–306. doi: 10.1002/sim.2010. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOC 35.5 kb)