Abstract
Purpose
The objective of this meta-analysis is to determine whether there is a higher incidence of preeclampsia (PE) in pregnancies achieved by oocyte donation (OD) compared with pregnancies achieved by in vitro fertilization with autologous oocytes (IVF).
Methods
A systematic review was performed to identify relevant studies published from January 1994 until April 2015 with at least an abstract in English using PubMed, ISI Web of Knowledge, and clinicaltrials.gov. The 11 studies included in this systematic review were retrospective and prospective cohort studies of women reporting results on the association between oocyte donation vs. in vitro fertilization (exposure) and preeclampsia (outcome).
Results
Oocyte donation is a risk factor for the development of PE compared to IVF cycles, with a weighted OR of 3.12 under a fixed effects method (FEM: no heterogeneity between the studies). The weighted OR under a random effects model was 2.9 (REM: heterogeneity between the studies). The meta-regression analysis showed that neither multiple pregnancies (estimate = 0.08; p = 0.19) nor patient age (estimate = −2.29; p = 0.13) significantly explained the variability of the effect of oocyte donation on PE. Q statistic was 12.78 (p = 0.237), suggesting absence of heterogeneity between the studies.
Conclusions
Pregnancies achieved by oocyte donation confer a threefold increase in the likelihood of developing PE than those achieved by in vitro fertilization with own oocytes. Physicians should be aware of this risk in order to both counsel patients and monitor pregnancies accordingly.
Electronic supplementary material
The online version of this article (doi:10.1007/s10815-016-0701-9) contains supplementary material, which is available to authorized users.
Keywords: Induced hypertension, In vitro fertilization, Oocyte donation, Preeclampsia, Pregnancy
Introduction
Preeclampsia (PE), defined as gestational hypertension with either significant proteinuria or end-organ dysfunction after 20 weeks of gestation in a previously normotensive woman, develops in 2–7 % of all pregnancies [1–4], and it is a major contributor to perinatal morbidity and mortality [5].
The involvement of immune mechanisms in the etiology of PE is often suggested. While in normal pregnancy there is a state of tolerance to foreign antigens, in PE, this immunological tolerance may be hampered due to an allogenic mismatch [6]. Oocyte donation is an assisted reproductive treatment with a high success rate ranging from 40 to 60 % [7–9], in which an unnaturally high number of allogenic mismatches have been described [10]. This may explain the higher incidence of PE in oocyte donation pregnancies (OD-P) reported in some series [2, 11–17]. However, whether the transfer of embryos generated from donated oocytes per se represents a risk factor for developing PE is still controversial. Recipients of donated eggs are usually nulliparous, of advanced maternal age, and typically suffer from ovarian failure, all factors independently associated with PE [18–21]. Unfortunately, several studies evaluating PE in OD-P compare its incidence with that of patients who conceived naturally [14, 15, 22, 23], thus weakening the robustness of their findings.
Objective
The aim of this systematic review and meta-analysis is to assess the association between oocyte donation pregnancies and the development of preeclampsia, comparing it with preeclampsia in women pregnant with in vitro fertilization with autologous oocytes (IVF-P).
Methods
Eligibility criteria, information sources, and search strategy
This review was carried out following the guidelines for Meta-analysis Of Observational Studies in Epidemiology (MOOSE) [24]. A systematic search was performed using PubMed, ISI Web of Knowledge, and clinicaltrials.gov to identify relevant studies published from January 1994 until April 2015 in English, French, Spanish, or Italian, using the search queries shown in Supplementary Information 1.
References of relevant publications were manually searched for additional potentially relevant published studies.
The relevance of identified abstract was assessed based on these inclusion criteria by two independent evaluators (A.B. and M.J.L.) blinded to authorship, authors’ institution, and study results. If the studies were considered to be potentially relevant, the full-text article was read. Any disagreement between the two evaluators was resolved by discussion.
Study selection
The criteria for inclusion in this systematic review were cohort studies (retrospective and prospective) which reported in their results on the association between OD-P vs. IVF-P (exposure) and PE (outcome).
In all the studies selected, PE was defined as hypertension with proteinuria after 20 weeks of gestation; therefore, this was the definition of PE that we used to determine the outcome.
Data extraction
The following data were extracted from the selected studies: country where the study was carried out, study period, inclusion and exclusion criteria, sample size, reproductive technique used (oocyte donation vs. IVF with autologous oocytes), patient age, outcome definition, and confounders used in the statistical analysis. Unadjusted and adjusted effect estimates for the association between OD-P (vs. IVF-P) and PE were extracted.
Assessment of risk of bias
Two reviewers (A.B. and MJ.L.) independently assessed the risk of bias using the Newcastle-Ottawa Scale (NOS) [25] for studies included in the systematic review. This instrument assessed three specific domains (selection, comparability, and outcome) for each study included in the review.
Data synthesis
Extracted results were pooled in the meta-analysis. Both fixed and random effects models (weighting by inverse of variance) were used. A continuity correction was used for cells with zero values. The between-study heterogeneity was assessed using the χ2 (Cochrane Q), H, and I2 statistics. Results were presented using forest plots. An influence analysis was also performed to ascertain the results of the meta-analysis by excluding each of the individual studies. Publication bias was assessed by a funnel plot for meta-analysis and quantified by the Egger method [26]. A meta-regression procedure of the log-OR was performed to evaluate the contribution of multiple pregnancy rates (the prevalence difference between oocyte-donation and IVF groups) and age (the difference in means between oocyte-donation and IVF groups) on the effect.
Statistical analysis were conducted using Stata software v13.0 (Stata Corp., College Station, Texas) (module “meta” [27]) and R v3.1.2 (The R Foundation for Statistical Computing) (package “meta v4.2” [28]).
Results
Study selection
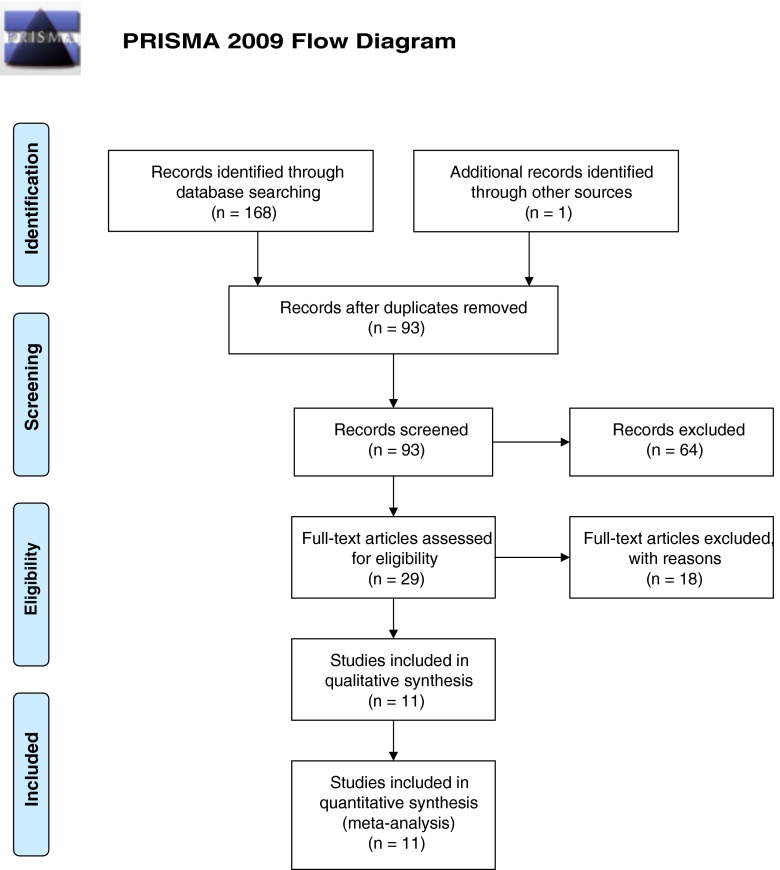
The studies identified through database searching were 168, with an extra one included manually. The references analyzed after removing the duplicates were 93. Sixty-four abstracts were excluded because they did not fulfill the inclusion criteria or were systematic reviews. Of the 29 full-text studies selected for eligibility, 18 were excluded because the final outcome was not PE, or the study population included patient with the Turner syndrome, which could modify the result [29].
Study characteristics
Eleven studies were finally included in the meta-analysis (Fig. 1), the characteristics of which are described in Table 1.
Fig. 1.
PRISMA flow chart
Table 1.
Characteristics of the included studies
| Author | Year of publication | Country | Design | Study period | Inclusion criteria | Exclusion Criteria | Number | Age (average) | |
|---|---|---|---|---|---|---|---|---|---|
| FIV | OD | ||||||||
| Klasky | 2010 | USA | Retrospective cohort | 1998–2005 | Women who deliver during study period: - OD - IVF matched one-to-one for age, singleton/twin |
- Monozygotic twins - Outcome data not provided not reported by obstetrical provider |
158 | 39.8 | 40.2 |
| Krieg | 2008 | USA | Retrospective cohort | Jan 1, 2001–Dec 31, 2005 | Women who delivered during study period: - All OD - IVF > 38 years |
- IVF ≤ 38 years | 179 | 41.3 | 42.7 |
| Le Ray | 2012 | France | Retrospective cohort | Jan 1, 2008–Dec 31, 2010 | Women ≥43 years who delivered during study period: - IVF - OD |
- Women aged <43 years | 144 | 44.0 | 46.2 |
| Levron | 2014 | Israel | Retrospective cohort | 2005–2011 | Women who delivered during study period: - OD - IVF |
- Twins - Pregnancies with congenital anomalies - Pregnancies with chromosomal abnormalities |
265 | 41 | 45 |
| Malchau | 2013 | Denmark | Retrospective cohort | 1995–2010 | Women who delivered during study period: - OD - IVF (IVF + ICSI) |
Women who delivered in Denmark but with OD performed in another country | 24,299 | 33.4 | 37.1 |
| Sekhon | 2014 | USA | Retrospective cohort | June, 2005–June, 2013 | Women who delivered TWINS pregnancies in the study period: - OD - Age and time of delivery-matched IVF (one-to-one) |
- Women >50 years - Monochorionic-monoamniotic placentation - Women diagnosed of hypertension before pregnancy or before 20 w of gestation |
112 | 41.9 | 43.0 |
| Söderström-Anttila | 1998 | Finland | Retrospective cohort | Oct. 1991–Dec 1996 | Women who underwent ART during the study period and gave birth: - OD - Time of ART-matched IVF (two-to-one) |
x | 148 | 33.4 | 33.5 |
| Van Dorp | 2014 | The Netherlands | Retrospective cohort | 1992–2009 | Women who underwent ART during the study period: - OD - IVF pregnancies matched by date of embryo transfer (<3 months), maternal age and ZIP code (one-to-one) |
x | 411 | 36.7 | 36.4 |
| Stoop | 2012 | Belgium | Retrospective cohort | Jan. 1999–Dec 2008 | Pregnancies occurred during the study period: - OD - IVF matched in terms of age, ethnicity, parity, and plurality (one-to-one) |
Pregnancies after: - Preimplantation genetic diagnosis - Testicular sperm extraction - Donor sperm |
408 | 36.0 | 36.0 |
| Tranquilli | 2013 | Italy | Retrospective cohort | - OD - IVF consecutive births (two-to-one) |
78 | 37.5 | 42.7 | ||
| Wiggins | 2005 | USA | Retrospective cohort | 1999–May, 2004 | Women who underwent successful ART during study period: - OD - Time-matched IVF (one-to-one) |
100 | 37.7 | 41.9 | |
Risk of bias of included studies
Four studies were considered to have a low risk of bias. Four have a medium risk because the two groups of patients included were comparable only for maternal age or multiple pregnancies, and the last three had a high risk, all of which were due to a lack of comparability in relation both to maternal age and multiple pregnancies (Table 2).
Table 2.
Risk of bias assessment
| Study | Selection | Comparability | Outcome | Stars | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Author | Publication year | Representativeness of the exposed cohort | Selection of the non-exposed cohort | Ascertainment of exposure | Demonstration that outcome of interest was not present at start of study | Study controls for age | Study controls for multiple pregnancies | Assessment of outcome | Long enough follow-up | <10 % lost to follow-up | |
| Klasky | 2010 | * | * | * | * | * | * | * | * | * | 9 |
| Krieg | 2008 | * | * | * | * | * | * | * | 7 | ||
| Le Ray | 2012 | * | * | * | * | * | * | * | 7 | ||
| Levron | 2014 | * | * | * | * | * | * | * | * | 8 | |
| Malchau | 2013 | * | * | * | * | * | * | * | * | 8 | |
| Sekhon | 2014 | * | * | * | * | * | * | * | * | * | 9 |
| Söderström-Anttila | 1998 | * | * | * | * | * | * | * | * | 8 | |
| Van Dorp | 2014 | * | * | * | * | * | * | * | * | * | 9 |
| Stoop | 2012 | * | * | * | * | * | * | * | * | * | 9 |
| Tranquilli | 2013 | * | * | * | * | * | * | * | 7 | ||
| Wiggins | 2005 | * | * | * | * | * | * | * | * | 8 | |
* indicates a star, cells with a star indicate that the corresponding item is addressed satisfactorily in the publication in question
Synthesis of results
Overall, the prevalence of PE in oocyte donation pregnancies (OD-P) was 17.2 % (range 9–29 %) while it was 5.7 % in the in vitro with autologous oocytes pregnancies (IVF-P) (range 0–13 %) (χ2 246.5; p < 0.001). Ten of the 11 articles included in the meta-analysis reported a higher risk of PE in pregnancies achieved after oocyte donation compared with pregnancies achieved with a patient’s autologous oocytes (Table 3). There was only one study where OD-P did not have an increased risk for PE, and this might be due to the small number of participants [30].
Table 3.
Individual odds ratios (and 95 % CI) and relative weights (by inverse of the variance) under fixed and random effects models
| Study | Year | OR | 95 % CI | Fixed effects | Random effects |
|---|---|---|---|---|---|
| Söderström-Anttila | 1998 | 6.09 | 2.58–14.39 | 5.9 | 8.7 |
| Wiggins | 2005 | 3.27 | 0.63–17.07 | 1.6 | 2.7 |
| Krieg | 2007 | 1.5 | 0.47–3.43 | 4.4 | 6.8 |
| Klatsky | 2010 | 3.9 | 1.22–12.58 | 3.2 | 5.2 |
| Le Ray | 2012 | 2.14 | 0.68–6.72 | 3.4 | 5.4 |
| Stoop | 2012 | 1.96 | 0.97–3.96 | 8.8 | 11.8 |
| Malchau | 2013 | 3.76 | 2.82–5.03 | 52.1 | 30.8 |
| Tranquilli | 2013 | 26.86 | 1.4–507.19 | 0.5 | 0.9 |
| Levron | 2014 | 2.06 | 0.76–5.6 | 4.4 | 6.8 |
| Sekhon | 2014 | 2.8 | 1.05–7.47 | 4.6 | 7 |
| Van Dorp | 2014 | 2.04 | 1.09–3.82 | 11.1 | 13.9 |
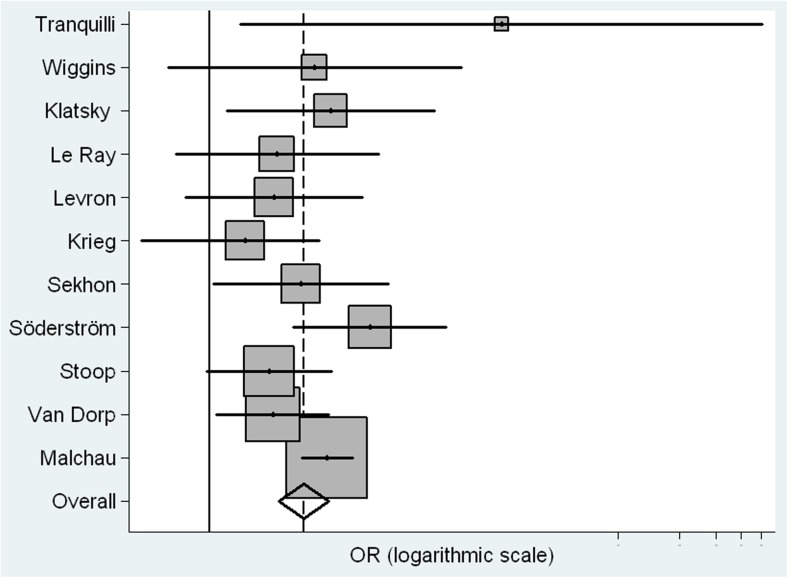
Both fixed and random effects models were used for this meta-analysis. Under the fixed method, the weighted OR of OD-P compared to IVF-P was 3.12 (2.56–3.85) (Table 4). Worthy of note was the fact that the Q statistic was 12.78 (p = 0.23), suggesting absence of heterogeneity, as it also suggests the result of 21.68 (95 % CI 0, 60.7) of the I2, a value that does not depend on the number of the studies included in the analysis. On the other hand, the weighted OR under the random effects model was 2.9 (2.19–3.85); Fig. 2 depicts the forest plot of the ORs extracted from the random effects model.
Table 4.
Meta-analysis of the included studies
| Estimation | p | 95 % CI | ||
|---|---|---|---|---|
| Lower | Upper | |||
| Fixed effects model | ||||
| IoV* weighted OR | 3.05 | <0.001 | 2.48 | 3.74 |
| SE(lnOR) | 0.1068 | |||
| Relative excess H | 1.13 | 0.8 | 1.6 | |
| % variation I 2 due to heterogeneity | 21.68 | 0 | 60.7 | |
| Homogeneity (Q) chi-square | 12.78 | 0.237 | ||
| Random effects model | ||||
| IoV* weighted OR | 2.8 | <0.001 | 2.11 | 3.72 |
| SE(lnOR) | 0.1439 | |||
| Relative excess H | 0.9746 | 0.61 | 1.54 | |
| % variation I 2 due to heterogeneity | 0 | |||
| Homogeneity (Q) chi-square | 9.5 | 0.486 | ||
IoV* inverse of variance
Fig. 2.
Forest plot of the risk of preeclampsia in oocyte donation vs. IVF (weighted by the inverse of the variance under a fixed and random effects)
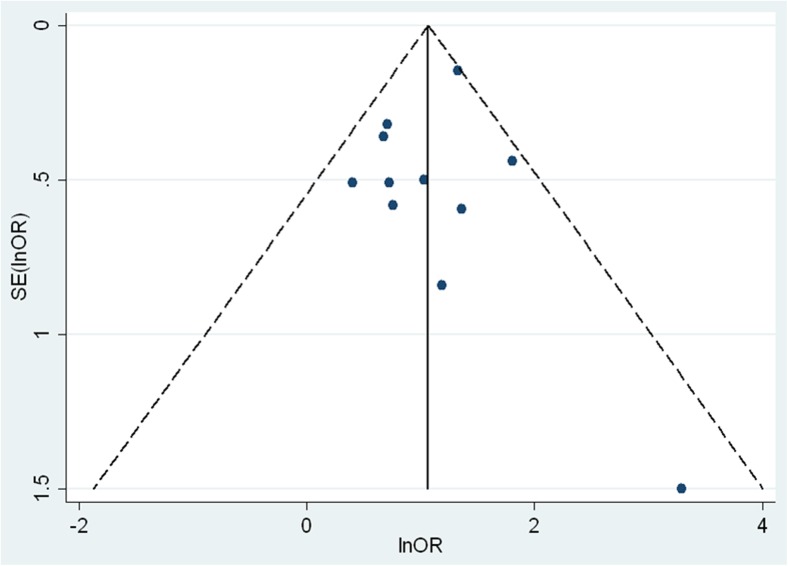
One of the strengths of the meta-analysis is that the exclusion of any of the published studies did not relevantly change the weighted ORs, as evidenced by the influence analysis, also performed under the random effects model (Supplementary Table 1). In addition, the funnel plot, which helps in assessing visually the publication bias (Fig. 3), does not suggest the existence of unpublished studies which were not included in this meta-analysis. Likewise, the Egger k-coefficient calculated to quantify the publication bias was not significant (−0.33, 95 % CI −1.81 to 1.15; p = 0.62).
Fig. 3.
Funnel plot with pseudo 95 % confidence limits
The meta-regression procedure showed that neither multiple pregnancies (estimate = 0.08; p = 0.19) nor patient age (estimate = −2.29; p = 0.13) significantly contributed to the effect of OD-P on PE. Supplementary Figures 1 and 2 show the bubble graph with the fitted meta-regression line of the multiple pregnancy rates (prevalence difference between oocyte donation and IVF groups) and age (difference between OD-P and IVF-P means). There was a non-significant trend towards a greater association between PE and OD-P as the difference in age between IVF-P and OD increased. On the other hand, there was also a non-significant trend towards a smaller association as the proportion of multiple pregnancies in the OD-P group increases with respect to the IVF-P group.
Discussion
Main findings
With 26.302 cases analyzed, this meta-analysis shows an association between OD-P and PE. This is of a special interest, as fertility treatments with donated oocytes are growing steadily worldwide. In the USA alone, cycles where embryos derived from donated oocytes are transferred account for 15.4 % of the cycles initiated in the country [9], mainly due to the increase in maternal age [31–33].
Strengths and limitations
To our knowledge, this is the first meta-analysis to have focused specifically on PE and including solely cohort studies comparing cycles of in vitro fertilization with OD-P vs. IVF-P. Since we avoided the comparison with natural conception pregnancies, we exclude by design the bias of the assisted reproductive technique per se.
Another relevant strength of our review is the absence of heterogeneity (Supplementary Table 1) and publication bias between the included articles (Fig. 3).
A limitation of this review is the lack of information in the included studies about the cause of infertility that has lead to assisted reproductive technology (ART) with either autologous or donor oocytes, because the underlying type of infertility leading to one or the other treatment might itself contribute to the pathophysiology of PE. The factors causing the need of ART certainly vary from patients that will require donated oocytes from the ones that will perform an IVF with autologous oocytes (for instance, tubal infertility is typically a cause of IVF with autologous oocytes, while a premature ovarian failure will most likely lead to OD), thus making it difficult to determine if it is the reception of the oocytes or the cause of its necessity that is associated with the increase in PE incidence.
Another limitation is the lack of detailed information in the included studies on severity or gestational age at onset of PE: the association with OD would be more clinically relevant in early or severe PE, since it is more amenable to prevention by aspirin than mild and late-onset disease [34].
Comparison with existing literature
There are numerous studies demonstrating a relation between ART and hypertensive complications [11, 14, 16, 17, 35, 36]. Several differences between natural conception gestations and ART gestations can explain this association: the effects on the endometrium due to the controlled ovarian stimulation therapy, the different implantation due to the transfer of the embryo, and the fact that the process of the formation of the trophoblast begins in vitro instead of in vivo. To establish the risk of PE in OD-P, and thus avoid the bias of the ART, it should be an IVF-P control group. Other factors that can confound the results of the analysis are the advanced maternal age and multiple pregnancies, as both are associated with PE and with the need to undergo oocyte reception. Our review includes a meta-regression procedure that accounts for such potential confounding. Although our analysis did not reach significance on the association between age and the effect of OD-P on PE, it seems that there is a trend towards it. The difference in the ages between groups in some of the studies and the small number of included studies could explain this limitation of our analysis.
The etiological relationship between OD-P and PE remains unclear. Despite the fact that the cause of developing PE seems to be multifactorial, OD-P is associated with an increased incidence of this disorder. An immunological theory, based on the allogenicity of the fetus to the mother, has been postulated: early on during implantation, the decidua is invaded by trophoblastic cells expressing HLA-C, a ligand for the immunoglobulin receptor (KIR) on the uterine natural killer cells (uNK). The uNK provide regulation of the neovascularization of the decidua, via proangiogenic and endothelial factors, which in turn modulate the spiral arteries. When executed correctly, this process guarantees proper blood flow to the developing fetus. The fetal HLA-C differs from the maternal HLA-C because it also expresses paternal HLA-C alleles. When donated oocytes are used, the trophoblastic HLA-C is less recognizable for the immunological system of the mother because it is completely allogeneic. Speculatively, this can lead to an altered functionality of the uNK and consequently to an altered maternal blood supply to the placenta, facilitating disorders like PE and fetal growth restriction [2, 37, 38] Also, T cells have been involved in the immune down-regulation that allows the fetus to develop in the maternal allogeneic environment [39]. In a study of 26 placentas of oocyte donation pregnancies, an infiltrate of macrophages was found in placentas of pregnancies uncomplicated by PE and not in the placentas of pregnancies with PE. This lesion in the chorionic plate was associated with intervillositis, chronic deciduitis, higher expression of CD14+ macrophages, and fetal HLA-C2. All these data suggested an immunological protection of the mother to the fetus, which was absent in PE gestations [40].
Some authors hypothesize that a patient needing oocyte donation might have, in addition, an immunologically based condition that predisposes to PE [12, 41]. Circulating antibodies against granulosa cells and zona pellucida have been detected in patients with ovarian failure [42], and as mentioned earlier, it seems that an autoregulation of the immune response by the mother is needed to provide a good placentation and to avoid PE. It is suggested that some autoantibodies or immune dysregulation can cause an injury to the trophoblastic cells invading the decidua.
Conclusions and implications
The rising number of pregnancies achieved by oocyte donation and PE morbidity confers importance to the results of this meta-analysis and necessitates an increase in our understanding of the biochemical and immunological causes of PE in order to develop possible preventive strategies, such as the selection of the oocyte donor immunologically matched to the HLA of the recipient [10, 43]. Nonetheless, the absence of prospective studies from the scientific literature limits the strength of the results of this meta-analysis, which should be interpreted with caution.
Meanwhile, patients undergoing OD cycles should be aware of the possible increased risk of developing PE, and physicians should provide strict obstetrical surveillance for these women, in order to perform early diagnosis and management if the case.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Influence analysis under the random effect model. Of note, the exclusion of any of the studies did not relevantly change the weighted ORs. (DOCX 189 kb)
Bubble graph with fitted meta-regression line of multiple pregnancy rate (prevalence difference between oocyte donation and IVF groups) against log-OR for PE. (GIF 12 kb)
Bubble graph with fitted meta-regression of age (difference between oocyte donation and IVF means) against log-OR for PE. (GIF 11 kb)
The search equation used in this systematic review. (DOCX 13.5 kb)
Acknowledgments
The authors would like to thank M.J. Lopez of Clínica EUGIN, Barcelona 08029, Spain, for the help in selecting the studies included.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Sources of funding
None.
Footnotes
Capsule Pregnancies achieved by oocyte donation confer a threefold increase in the likelihood of developing PE than those achieved by in vitro fertilization with own oocytes.
References
- 1.Smith GN, Walker M, Tessier JL, Millar KG. Increased incidence of preeclampsia in women conceiving by intrauterine insemination with donor versus partner sperm for treatment of primary infertility. Am J Obstet Gynecol. 1997;177(2):455–8. doi: 10.1016/S0002-9378(97)70215-1. [DOI] [PubMed] [Google Scholar]
- 2.Klatsky PC, Delaney SS, Caughey AB, Tran ND, Schattman GL, Rosenwaks Z. The role of embryonic origin in preeclampsia: a comparison of autologous in vitro fertilization and ovum donor pregnancies. Obstet Gynecol. 2010;116(6):1387–92. doi: 10.1097/AOG.0b013e3181fb8e59. [DOI] [PubMed] [Google Scholar]
- 3.Kyrou D, Kolibianakis EM, Devroey P, Fatemi HM. Is the use of donor sperm associated with a higher incidence of preeclampsia in women who achieve pregnancy after intrauterine insemination? Fertil Steril. 2010;93(4):1124–7. doi: 10.1016/j.fertnstert.2008.12.021. [DOI] [PubMed] [Google Scholar]
- 4.American College of O, Gynecologists, Task Force on Hypertension in P. Hypertension in pregnancy. Report of the American College of Obstetricians and Gynecologists' Task Force on Hypertension in Pregnancy. Obstet Gynecol. 2013;122(5):1122–31. doi:10.1097/01.AOG.0000437382.03963.88. [DOI] [PubMed]
- 5.Flenady V, Koopmans L, Middleton P, Froen JF, Smith GC, Gibbons K, et al. Major risk factors for stillbirth in high-income countries: a systematic review and meta-analysis. Lancet. 2011;377(9774):1331–40. doi: 10.1016/S0140-6736(10)62233-7. [DOI] [PubMed] [Google Scholar]
- 6.Redman CW, Sargent IL. Immunology of pre-eclampsia. Am J Reprod Immunol. 2010;63(6):534–43. doi: 10.1111/j.1600-0897.2010.00831.x. [DOI] [PubMed] [Google Scholar]
- 7.Garrido N, Bellver J, Remohi J, Alama P, Pellicer A. Cumulative newborn rates increase with the total number of transferred embryos according to an analysis of 15,792 ovum donation cycles. Fertil Steril. 2012;98(2):341–6 e1-2. doi: 10.1016/j.fertnstert.2012.04.039. [DOI] [PubMed] [Google Scholar]
- 8.Group ECW Failures (with some successes) of assisted reproduction and gamete donation programs. Hum Reprod Update. 2013;19(4):354–65. doi: 10.1093/humupd/dmt007. [DOI] [PubMed] [Google Scholar]
- 9.Kupka MS, Ferraretti AP, de Mouzon J, Erb K, D'Hooghe T, Castilla JA, et al. Assisted reproductive technology in Europe, 2010: results generated from European registers by ESHRE. Hum Reprod. 2014;29(10):2099–113. doi: 10.1093/humrep/deu175. [DOI] [PubMed] [Google Scholar]
- 10.van der Hoorn ML, Lashley EE, Bianchi DW, Claas FH, Schonkeren CM, Scherjon SA. Clinical and immunologic aspects of egg donation pregnancies: a systematic review. Hum Reprod Update. 2010;16(6):704–12. doi: 10.1093/humupd/dmq017. [DOI] [PubMed] [Google Scholar]
- 11.Gundogan F, Bianchi DW, Scherjon SA, Roberts DJ. Placental pathology in egg donor pregnancies. Fertil Steril. 2010;93(2):397–404. doi: 10.1016/j.fertnstert.2008.12.144. [DOI] [PubMed] [Google Scholar]
- 12.Keegan DA, Krey LC, Chang HC, Noyes N. Increased risk of pregnancy-induced hypertension in young recipients of donated oocytes. Fertil Steril. 2007;87(4):776–81. doi: 10.1016/j.fertnstert.2006.08.105. [DOI] [PubMed] [Google Scholar]
- 13.Le Ray C, Scherier S, Anselem O, Marszalek A, Tsatsaris V, Cabrol D, et al. Association between oocyte donation and maternal and perinatal outcomes in women aged 43 years or older. Hum Reprod. 2012;27(3):896–901. doi: 10.1093/humrep/der469. [DOI] [PubMed] [Google Scholar]
- 14.Salha O, Sharma V, Dada T, Nugent D, Rutherford AJ, Tomlinson AJ, et al. The influence of donated gametes on the incidence of hypertensive disorders of pregnancy. Hum Reprod. 1999;14(9):2268–73. doi: 10.1093/humrep/14.9.2268. [DOI] [PubMed] [Google Scholar]
- 15.Wolff KM, McMahon MJ, Kuller JA, Walmer DK, Meyer WR. Advanced maternal age and perinatal outcome: oocyte recipiency versus natural conception. Obstet Gynecol. 1997;89(4):519–23. doi: 10.1016/S0029-7844(97)00051-3. [DOI] [PubMed] [Google Scholar]
- 16.Soderstrom-Anttila V, Tiitinen A, Foudila T, Hovatta O. Obstetric and perinatal outcome after oocyte donation: comparison with in-vitro fertilization pregnancies. Hum Reprod. 1998;13(2):483–90. doi: 10.1093/humrep/13.2.483. [DOI] [PubMed] [Google Scholar]
- 17.Levron Y, Dviri M, Segol I, Yerushalmi GM, Hourvitz A, Orvieto R, et al. The ‘immunologic theory’ of preeclampsia revisited: a lesson from donor oocyte gestations. Am J Obstet Gynecol. 2014;211(4):19. doi: 10.1016/j.ajog.2014.03.044. [DOI] [PubMed] [Google Scholar]
- 18.Myatt L, Redman CW, Staff AC, Hansson S, Wilson ML, Laivuori H, et al. Strategy for standardization of preeclampsia research study design. Hypertension. 2014;63(6):1293–301. doi: 10.1161/HYPERTENSIONAHA.113.02664. [DOI] [PubMed] [Google Scholar]
- 19.Woldringh GH, Frunt MHA, Kremer JAM, Spaanderman MEA. Decreased ovarian reserve relates to pre-eclampsia in IVF/ICSI pregnancies. Hum Reprod. 2006;21(11):2948–54. doi: 10.1093/humrep/del155. [DOI] [PubMed] [Google Scholar]
- 20.Paulson RJ, Boostanfar R, Saadat P, Mor E, Tourgeman DE, Slater CC, et al. Pregnancy in the sixth decade of life: obstetric outcomes in women of advanced reproductive age. JAMA. 2002;288(18):2320–3. doi: 10.1001/jama.288.18.2320. [DOI] [PubMed] [Google Scholar]
- 21.Yogev Y, Melamed N, Bardin R, Tenenbaum-Gavish K, Ben-Shitrit G, Ben-Haroush A. Pregnancy outcome at extremely advanced maternal age. Am J Obstet Gynecol. 2010;203(6). doi:10.1016/j.ajog.2010.07.039. [DOI] [PubMed]
- 22.Porreco RP, Harden L, Gambotto M, Shapiro H. Expectation of pregnancy outcome among mature women. Am J Obstet Gynecol. 2005;192(1):38–41. doi: 10.1016/j.ajog.2004.07.035. [DOI] [PubMed] [Google Scholar]
- 23.Shrim A, Levin I, Mallozzi A, Brown R, Salama K, Gamzu R, et al. Does very advanced maternal age, with or without egg donation, really increase obstetric risk in a large tertiary center? J Perinat Med. 2010;38(6):645–50. doi: 10.1515/jpm.2010.084. [DOI] [PubMed] [Google Scholar]
- 24.Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA. 2000;283(15):2008–12. doi: 10.1001/jama.283.15.2008. [DOI] [PubMed] [Google Scholar]
- 25.Wells G, Shea B, O'Connell D, Peterson J, Welch V, Losos M et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. [http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp]. Accessed 19/2/2015.
- 26.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629–34. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sterne J. Meta-analysis in Stata: an updated collection from the Stata Journal. College Station (Texas): Stata Press; 2009. [Google Scholar]
- 28.Schwarzer G. Meta-analysis. The R Foundation for Statistical Computing. 2007.
- 29.Hagman A, Loft A, Wennerholm U-B, Pinborg A, Bergh C, Aittomaki K, et al. Obstetric and neonatal outcome after oocyte donation in 106 women with Turner syndrome: a Nordic cohort study. Hum Reprod. 2013;28(6):1598–609. doi: 10.1093/humrep/det082. [DOI] [PubMed] [Google Scholar]
- 30.Krieg SA, Henne MB, Westphal LM. Obstetric outcomes in donor oocyte pregnancies compared with advanced maternal age in in vitro fertilization pregnancies. Fertil Steril. 2008;90(1):65–70. doi: 10.1016/j.fertnstert.2007.06.014. [DOI] [PubMed] [Google Scholar]
- 31.de Graaff AA, Land JA, Kessels AG, Evers JL. Demographic age shift toward later conception results in an increased age in the subfertile population and an increased demand for medical care. Fertil Steril. 2011;95(1):61–3. doi: 10.1016/j.fertnstert.2010.05.013. [DOI] [PubMed] [Google Scholar]
- 32.Blickstein I. Motherhood at or beyond the edge of reproductive age. Int J Fertil Women's Med. 2003;48(1):17–24. [PubMed] [Google Scholar]
- 33.Pal L, Santoro N. Age-related decline in fertility. Endocrinol Metab Clin N Am. 2003;32(3):669–88. doi: 10.1016/S0889-8529(03)00046-X. [DOI] [PubMed] [Google Scholar]
- 34.Roberge S, Villa P, Nicolaides K, Giguere Y, Vainio M, Bakthi A, et al. Early administration of low-dose aspirin for the prevention of preterm and term preeclampsia: a systematic review and meta-analysis. Fetal Diagn Ther. 2012;31(3):141–6. doi: 10.1159/000336662. [DOI] [PubMed] [Google Scholar]
- 35.Thomopoulos C, Tsioufis C, Michalopoulou H, Makris T, Papademetriou V, Stefanadis C. Assisted reproductive technology and pregnancy-related hypertensive complications: a systematic review. J Hum Hypertens. 2013;27(3):148–57. doi: 10.1038/jhh.2012.13. [DOI] [PubMed] [Google Scholar]
- 36.Hernandez-Diaz S, Werler MM, Mitchell AA. Gestational hypertension in pregancies supported by infertility treatments: role of infertility, treatments, and multiple gestations. Fertil Steril. 2007;88(2):438–45. doi: 10.1016/j.fertnstert.2006.11.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Madeja Z, Yadi H, Apps R, Boulenouar S, Roper SJ, Gardner L, et al. Paternal MHC expression on mouse trophoblast affects uterine vascularization and fetal growth. Proc Natl Acad Sci U S A. 2011;108(10):4012–7. doi: 10.1073/pnas.1005342108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hiby SE, Walker JJ, O'Shaughnessy KM, Redman CW, Carrington M, Trowsdale J, et al. Combinations of maternal KIR and fetal HLA-C genes influence the risk of preeclampsia and reproductive success. J Exp Med. 2004;200(8):957–65. doi: 10.1084/jem.20041214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tilburgs T, Roelen DL, van der Mast BJ, de Groot-Swings GM, Kleijburg C, Scherjon SA, et al. Evidence for a selective migration of fetus-specific CD4 + CD25bright regulatory T cells from the peripheral blood to the decidua in human pregnancy. J Immunol. 2008;180(8):5737–45. doi: 10.4049/jimmunol.180.8.5737. [DOI] [PubMed] [Google Scholar]
- 40.Schonkeren D, Swings G, Roberts D, Claas F, de Heer E, Scherjon S. Pregnancy close to the edge: an immunosuppressive infiltrate in the chorionic plate of placentas from uncomplicated egg cell donation. PLoS One. 2012;7(3):27. doi: 10.1371/journal.pone.0032347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pecks U, Maass N, Neulen J. Oocyte donation: a risk factor for pregnancy-induced hypertension: a meta-analysis and case series. Dtsch Arztebl Int. 2011;108(3):23–31. doi: 10.3238/arztebl.2011.0023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kelkar RL, Meherji PK, Kadam SS, Gupta SK, Nandedkar TD. Circulating auto-antibodies against the zona pellucida and thyroid microsomal antigen in women with premature ovarian failure. J Reprod Immunol. 2005;66(1):53–67. doi: 10.1016/j.jri.2005.02.003. [DOI] [PubMed] [Google Scholar]
- 43.Alecsandru D, Garrido N, Vicario JL, Barrio A, Aparicio P, Requena A, et al. Maternal KIR haplotype influences live birth rate after double embryo transfer in IVF cycles in patients with recurrent miscarriages and implantation failure. Hum Reprod. 2014;29(12):2637–43. doi: 10.1093/humrep/deu251. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Influence analysis under the random effect model. Of note, the exclusion of any of the studies did not relevantly change the weighted ORs. (DOCX 189 kb)
Bubble graph with fitted meta-regression line of multiple pregnancy rate (prevalence difference between oocyte donation and IVF groups) against log-OR for PE. (GIF 12 kb)
Bubble graph with fitted meta-regression of age (difference between oocyte donation and IVF means) against log-OR for PE. (GIF 11 kb)
The search equation used in this systematic review. (DOCX 13.5 kb)