Abstract
Purpose
To explore the relationships between the soluble receptor for advanced glycation endproducts (sRAGE) and the outcome parameters following in vitro fertilization-embryo transfer (IVF-ET) in patients with polycystic ovary syndrome (PCOS) and investigate the protective effect of sRAGE in PCOS development regarding inflammation.
Methods
We conducted a prospective analysis of a subsample of 74 participants from the Reproductive Medical Center of the First Affiliated Hospital of Zhengzhou University. We quantified sRAGE, vascular endothelial growth factor (VEGF), tumor necrosis factor (TNF-α), interleukelin-6 (IL-6), and C-reactive protein (CPR) protein levels in the follicular fluid from 39 PCOS and 35 non-PCOS reproductive-age women. sRAGE and VEGF, TNF-α, IL-6, and CRP in follicular fluid aspirated without blood were measured by ELISA.
Results
sRAGE concentrations in the follicular fluid were significantly lower in the PCOS group compared to those in the control group, while VEGF, TNF-α, IL-6, and CRP concentrations were significantly higher in the PCOS group than in the control group (P < 0.05). sRAGE was significantly, inversely correlated with the total dose of gonadotropin (Gn) in the PCOS group undergoing IVF treatment (r = −0.451, P = 0.004). After adjusting for age and Gn dose (in international units used per cycle), sRAGE protein levels in the follicular fluid were significantly, inversely related to VEGF (r = −0.378, P = 0.018), TNF-α (r = −0.450, P = 0.004), IL-6 (r = −0.455, P = 0.004), and CRP (r = −0.375, P = 0.019).
Conclusion
sRAGE in the follicular fluid might exert a protective effect against the inflammatory action of PCOS development.
Keywords: sRAGE, Inflammation, Human reproduction, PCOS, Therapy
Introduction
Polycystic ovarian syndrome (PCOS) is one of the main causes of female infertility with an incidence rate of 5−12 %. PCOS is characterized by hyper-androgenism, polycystic ovaries, oligoovulation, and chronic anovulation [1]. Although the etiology of PCOS is unknown, imbalances between pro-inflammatory and anti-inflammatory pathways play a role in the pathogenesis of PCOS [2].
Among the emerging pro-inflammatory molecules that are elevated in patients with PCOS, advanced glycation endproducts (AGEs) increase due to hyper-glycemia, oxidative stress, and insulin resistance [3, 4]. Many of the effects of AGEs are elicited by their receptor, i.e., the receptor for advanced glycation endproducts (RAGE), which is a member of the immunoglobulin superfamily. RAGE levels are increased in patients with diseases that activate extracellularly regulated protein kinases (ERKs) and nuclear factor kB (NF-kB) pathways and generate pro-inflammatory cytokines, such as tumor necrosis factor (TNF-α), interleukelin-6 (IL-6), and C-reactive protein (CPR) leading to cellular dysfunction and involvement in many pathological processes [5, 6]. The soluble receptor for advanced glycation endproducts (sRAGE) is an extracellular form of RAGE that is missing the cytosolic and transmembrane domains. sRAGE acts as a decoy that interrupts adverse intracellular signaling caused by the AGE-RAGE axis and is not only a biological marker that reflects pathological changes, but is also a protective factor that delays the occurrence of many diseases [7].
The involvement of AGE-RAGE in the inflammatory response results in perturbations of the ovarian microenvironment and PCOS has reached a consensus [8]. But, the relationship of sRAGE and inflammatory factors is controversial. Romero et al. believed that increased amniotic fluid concentrations of sRAGE were positively associated with inflammation in patients with preterm labor [9], while Rzepka et al. [10] observed a negative trend for sRAGE and CRP in plasma, and they concluded that high sRAGE concentrations could avoid premature labor. Additionally, the role of sRAGE in patients with PCOS is unclear. Recently, scholars, such as Merhiet et al., first reported that sRAGE was positively correlated with ovarian reserve [11], while PCOS is accompanied by an inflammatory response and decreased ovarian function. In the present study, we compared sRAGE concentrations in the follicular fluid samples of women with and without PCOS and explored the role of sRAGE in PCOS.
Materials and methods
All patients provided informed consent and the study was approved by the institutional review boards of all of the institutions.
Subjects
The control group consisted of 35 patients who were infertile due to tubal factors, and the PCOS group consisted of 39 patients with PCOS (as diagnosed according to the Rotterdam criteria) [12]. We excluded women with diabetes, chronic kidney disease, and complications, such as chronic metabolic diseases and endometriosis [13–16]. The cycle determinants included age, FSH/LH, body mass index (BMI), total gonadotropin (Gn) dose in international units used per cycle, the number of days of stimulation, the number of oocytes retrieved, the rate of high-quality embryos, and the normal fertilization rate. sRAGE decreases in women older than 35 years old and women with BMIs >25 [17]; thus, only patients <35 years old with BMIs ≥18 and ≤25 were included. The general data in the two groups are shown in Table 1.
Table 1.
BMI and the levels of reproductive hormones and metabolic parameters in the PCOS and control groups (mean ± SD)
| Characteristics | PCOS (n = 39) | Control (n = 35) | P value |
|---|---|---|---|
| Age (year) | 27.670 ± 3.263 | 28.400 ± 2.982 | 0.339 |
| BMI (kg/m2) | 22.049 ± 2.169 | 21.013 ± 1.692 | 0.072 |
| LH/FSH | 1.739 ± 0.671 | 0.841 ± 0.308 | 0.000 |
| T (ng/mL) | 0.217 ± 1.293 | 0.115 ± 0.077 | 0.003 |
| E2 (pg/mL) | 27.836 ± 23.10 | 23.901 ± 19.122 | 0.174 |
| Total dose of Gn (IU) | 1221.154 ± 278.612 | 1916.429 ± 648.710 | 0.008 |
| Oocytes retrieved (n) | 17.770 ± 5.234 | 14.690 ± 8.543 | 0.001 |
| Duration of stimulation (days) | 10.0 ± 1.733 | 11 ± 0.952 | 0.281 |
Note that the values are presented as the means ± SDs. Student’s t or Mann–Whitney U tests were applied for single comparisons between the PCOS and control group. BMI body mass index, Gn gonadotropin
Experimental sample collection and determination
Pituitary desensitization began in the mid-luteal phase via subcutaneous injections of a GnRHagonist (Decapeptyl, Ferring GmbH, Germany). Gn stimulation began after attaining the standards of downregulation with human menopausal Gn (hMG, Livzon, China) and recombinant FSH (Gonal-f, Merck, Germany) and was followed by human chorionic gonadotrophin (hCG, Livzon, China) administration based on follicular size. Oocytes were collected via transvaginal, ultrasound-guided puncture 34–36 h later. The fluid from the first large aspirated follicle without blood was collected for ELISA assessments of sRAGE, vascular endothelial growth factor (VEGF), IL-6, TNF-α, and CRP: Quantikine Human RAGE Immunoassay, Quantikine Human VEGF Immunoassay, Quantikine Human IL-6 Immunoassay, Quantikine Human TNF-α Immunoassay, and Quantikine Human CRP Immunoassay (R&D Systems, Minneapolis, MN, USA). The results are given in picograms per milliliter (pg/mL).
Statistical analysis
The statistical analyses were performed with SPSS12.0 software package (SPSS Inc., Chicago, IL) for Windows. The means and standard deviations (means ± SDs) were used to describe the variables. Student’s t tests or Mann–Whitney U tests were applied for single comparisons between the PCOS and control groups. Pearson’s or Spearman correlation coefficients were used to examine the relationships between the follicular fluid sRAGE levels and clinical parameters, including age, BMI, and the number of stimulation days. Univariate and multivariate linear regressions were performed to examine the relationships between follicular fluid sRAGE levels and the total Gn dose, numbers of oocytes retrieved, normal fertilization rate, rate of high-quality embryos, and experimental data (including VEGF, IL-6, TNF-α, and CRP levels in the follicular fluid). A probability value of P < 0.05 was considered significant.
Results
Follicular fluid sRAGE concentrations in the PCOS group were lower than those of controls
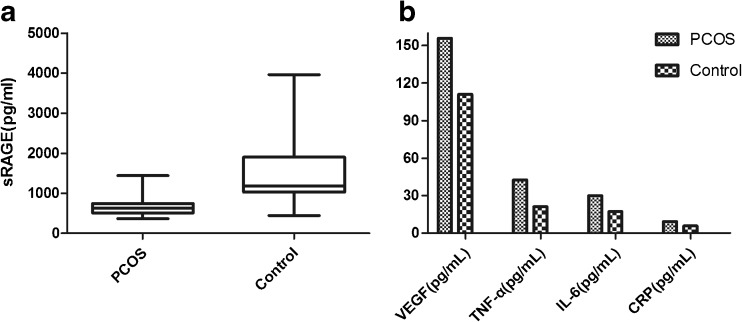
The follicular fluid concentrations of sRAGE in the PCOS and control groups are presented in Fig. 1. The follicular fluid sRAGE concentrations were measured in 35 participants in the control group (mean age 28.40 ± 2.982 years, mean BMI 21.013 ± 1.692) and 39 participants in the PCOS group (mean age 27.670 ± 3.263 years, mean BMI 22.049 ± 2.169). The follicular fluid sRAGE concentrations were significantly different between the two groups (P < 0.005; Student’s t test followed by an F test, 666.753 ± 233.153 pg/mL, n = 39 in the PCOS group, and 1518.608 ± 872.130 pg/mL, n = 35 in the control group).
Fig. 1.
The follicular fluid concentrations of sRAGE, VEGF, TNF-α, IL-6, and CRP in the PCOS and the control groups. a sRAGE concentrations in the follicular fluid were measured with ELISA. The sRAGE concentrations in the follicular fluid were lower in the PCOS group than in the controls (666.753 ± 233.153 vs. 1518.608 ± 872.130; P = 0.000). b VEGF, TNF-α, IL-6, and CRP levels in the follicular fluid were measured with ELISA. VEGF, TNF-α, IL-6, and CRP concentrations in the follicular fluid were higher in the PCOS group than in the controls (155.761 ± 23.314 vs. 110.955 ± 21.838, 42.636 ± 7.726 vs. 21.136 ± 9.266, 29.932 ± 5.148 vs. 17.327 ± 6.495, and 9.249 ± 1.385 vs. 5.839 ± 1.382, respectively; P < 0.05). sRAGE soluble isoform of the receptor for advanced glycation endproducts, VEGF vascular endothelial growth factor, TNF-α tumor necrosis factor α, IL-6 interleukelin-6, and CRP C-reactive protein
The follicular fluid concentrations of VEGF, TNF-α, IL-6, and CRP in the PCOS and control groups are presented in Fig. 1. The concentrations of these factors were significantly higher in the PCOS group compared to the control group (P < 0.005; Student’s t tests followed by F tests, VEGF 155.761 ± 23.314 vs. 110.955 ± 21.838, TNF-α 42.636 ± 7.726 vs. 21.136 ± 9.266, IL-6 29.932 ± 5.148 vs. 17.327 ± 6.495, and CRP 9.249 ± 1.385 vs.5.839 ± 1.382).
The follicular fluid sRAGE levels in the PCOS group were inversely correlated with the Gn dose
We determined whether sRAGE levels in the follicular fluid in the PCOS group were correlated with the age, BMI, total Gn dose, number of days of stimulation, number of oocytes retrieved, and number of high-quality embryos. The results are presented in Table 2.
Table 2.
Correlations between the follicular fluid sRAGE protein concentration and other clinical parameters
| Variable | r | P value | |
|---|---|---|---|
| FF sRAGE (pg/mL) | Age | 0.134 | 0.417 |
| BMI | −0.192 | 0.242 | |
| Days of stimulation | −0.104 | 0.523 | |
| Total Gn dose | −0.451 | 0.004 | |
| Oocytes retrieved | −0.037 | 0.831 | |
| Normal fertilization rate | 0.026 | 0.739 | |
| Number of high-quality embryos | 0.119 | 0.407 |
Note that Pearson’s or Spearman’s rank correlation coefficients were calculated using the follicular fluid sRAGE levels and each clinical parameter, such as age or BMI. Univariate and multivariate linear regressions were performed to examine the relationships between follicular fluid sRAGE level and total Gn dose, number of days of stimulation, number of oocytes retrieved, and number of high-quality embryos. BMI body mass index, Gn gonadotropin
Pearson’s and Spearman’s correlation coefficients were calculated to determine whether follicular fluid sRAGE levels were correlated with clinical parameters, such as age, BMI, and days of stimulation. Follicular fluid sRAGE concentration was not correlated with age.
Univariate and multivariate linear regressions were performed to examine the relationships between follicular fluid sRAGE levels and total Gn dose, number of days of stimulation, number of oocytes retrieved, and number of high-quality embryos. The multivariate analysis was adjusted for age, BMI, and day 3 FSH. Higher follicular fluid sRAGE protein levels in the PCOS participants were associated with lower total Gn doses (international units used per cycle; r = −0.451, P = 0.004). After adjusting for age, BMI, day 3 FSH, and Gn dose (international units used per cycle), the follicular fluid sRAGE concentration did not correlate with the number of oocytes retrieved (r = −0.037, P = 0.831), the normal fertilization rate (r = 0.026, P = 0.739), or the rate of high-quality embryos (r = 0.119, P = 0.407).
Follicular fluid sRAGE concentration was inversely correlated with follicular fluid VEGF, TNF-α, IL-6, and CRP protein concentrations
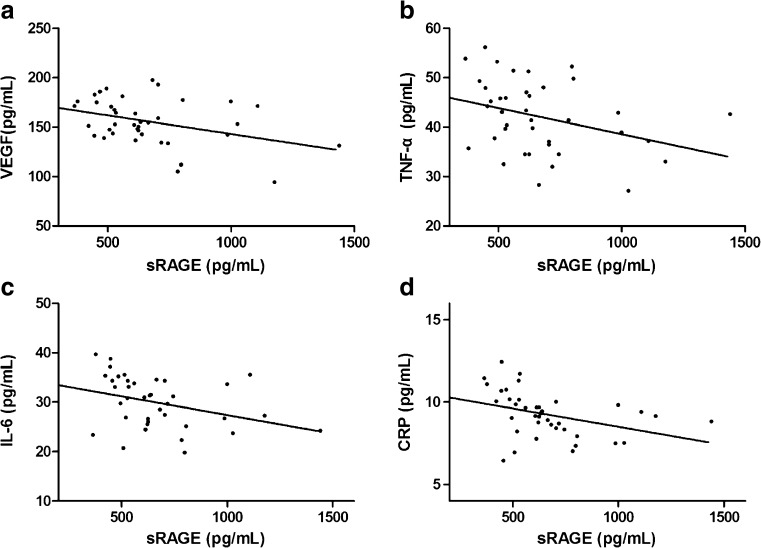
The relationships between follicular fluid sRAGE in the PCOS group and VEGF, TNF-α, IL-6, and CRP protein are presented in Fig. 2. After adjusting for age and Gn dose (international units used per cycle), the follicular fluid sRAGE protein levels were significantly, inversely correlated with VEGF (r = −0.378, P = 0.018; Fig. 2a), TNF-α (r = −0.450, P = 0.004; Fig. 2b), IL-6 (r = −0.455, P = 0.004; Fig. 2c), and CRP (r = −0.375, P = 0.019).
Fig. 2.
The relationships between sRAGE and VEGF, TNF-α, IL-6, and CRP in the follicular fluid from the PCOS group. Follicular fluid sRAGE, VEGF, TNF-α, IL-6, and CRP concentrations in follicular fluid were measured with ELISA. sRAGE was significantly, inversely correlated with VEGF (r = −0.378, P = 0.018) (a), TNF-α (r = −0.450, P = 0.004) (b), IL-6 (r = −0.455, P = 0.004) (c), and CRP (r = −0.375, P = 0.019) (d). sRAGE soluble isoform of the receptor for advanced glycation endproducts, VEGF vascular endothelial growth factor, TNF-α tumor necrosis factor α, IL-6 interleukelin-6, CRP C-reactive protein
Discussion
In the present study, we quantified the follicular fluid sRAGE protein concentrations and determined whether sRAGE correlated with inflammatory proteins in patients with PCOS. Inflammation is involved in the pathogenesis of PCOS and its associated complications [18]. Follicular fluid sRAGE was inversely correlated with follicular fluid VEGF, TNF-α, IL-6, and CRP protein. We also analyzed the relationship between follicular fluid sRAGE and IVF-ET outcome parameters, such as the number of oocytes retrieved, the normal fertilization rate, and the number of high-quality embryos, among the PCOS participants. The results were not significant with one exception. Higher follicular fluid sRAGE protein levels were associated with lower total Gn doses (international units used per cycle).
During the past two decades, emerging evidence has supported the essential contribution of AGE-RAGE to increased oxidative stress and inflammation. These processes perturbate the ovarian microenvironment and may cause infertility [2]. According to Merhiet et al. [11], high follicular fluid sRAGE correlates with elevated ovarian reserve as measured by AMH [19]. This finding corroborates the notion that sRAGE might play a protective role in the follicular environment [20]. However, other scholars have reached the opposite conclusion. In one study, intrafollicular sRAGE was inversely associated with embryo development [21]. In present study, the mean sRAGE concentrations in the control group were more than double the concentrations in the PCOS patients. The follicular fluid sRAGE levels were significantly decreased in the PCOS group, but the mechanism and effects remained unclear.
The present study recruited 35 reproductive aged women with tubal factor infertility. Patients with tubal inflammation cannot be excluded, which may affect the levels of inflammation in follicular fluid. As the results showed that sRAGE protein levels in follicular fluid were significantly, inversely related to the inflammatory factor, we speculated that the follicular fluid sRAGE levels were lower in patients with tubal inflammation. We found the mean sRAGE concentrations in the control group were more than double the concentrations in PCOS patients, so the difference might be more pronounced between PCOS patients and non-tubal factor patients. sRAGE decreased in women with a BMI of greater than 25 [17], so only patients with BMIs ≤25 were included. As there is a documented negative correlation between the sRAGE levels and BMI [22], sRAGE might be lower in the obese PCOS patients. Therefore, even though the present results may not be generalizable to all PCOS patients, the sRAGE differential between PCOS and non-PCOS patients may be greater than indicated. As decreased sRAGE is involved in the development of many diseases [23–25], we predict that decreased sRAGE plays a role in the mechanism of PCOS.
After adjusting for age, BMI, day 3 FSH, and Gn dose (international units used per cycle), follicular fluid sRAGE was not associated with the number of oocytes retrieved, the normal fertilization rate, or the number of high-quality embryos, but higher follicular fluid sRAGE protein levels in the PCOS group were associated with lower total Gn doses (international units used per cycle). Our previous research showed that follicular fluid sRAGE protein levels were negatively related to the total dose of Gn international units used per cycle and positively related to the number of oocytes retrieved in patients without PCOS. This suggested that sRAGE may be beneficial to the reproductive environment and, hence, reproductive potential. In PCOS, higher sRAGE levels were associated with lower total Gn doses, predicting that higher sRAGE still supported good reproductive potential in PCOS patients. As we know, the units of Gn used for patients with lean PCOS have to be reduced to avoid ovarian hyperstimulation syndrome (OHSS). Another possible explanation for this result could be that decreased sRAGE plays a role in the causes and mechanism of PCOS. Even if the levels of sRAGE were decreased, higher sRAGE still predicted good ovarian function.
Elevated sRAGE concentrations can reduce the expression of VEGF [26]. However, Goova et al. found that sRAGE enhances the expression of VEGF and accelerates skin wound healing [27]. But, the relationship between sRAGE and VEGF in follicular fluid of patients with PCOS remains unclear. In the present study, follicular fluid sRAGE protein levels were significantly, inversely associated with VEGF. Accumulating evidence indicates that VEGF is increased during PCOS and might play a major role in PCOS pathogenesis and the vascular changes that occur [28]. Additionally, elevated concentrations of follicular fluid VEGF reduces the successful pregnant rate in assisted reproductive technology [29]. Therefore, the present results provide additional evidence of the protective role of sRAGE to PCOS and follicular environment.
Inflammation causes disorders by affecting the hypothalamus–pituitary–gonadal axis results in follicular atresia [30]. Recent studies suggest that chronic inflammation is involved in the pathogenesis of and long-term risk associated with PCOS. In the present study, TNF-α, IL-6, and CRP levels were significantly higher in the PCOS group compared to the control group, and these results support the findings from many other studies [31, 32]. sRAGE inhibits the effect of RAGE, which can inhibit the expression of inflammatory cells and inhibit the inflammatory reaction mediated by an injury [16, 33]. Romero et al. believed that patients with preterm labor have increased amniotic fluid concentrations of sRAGE, and these levels are positively associated with intraamniotic infection and inflammation [9]. However, there are many reports which do not support this. A study by Malickova et al. [34] showed that women with significantly higher sRAGE were more likely to conceive. Rzepka et al. [10] observed a negative trend for sRAGE and CRP plasma levels in a group with preterm premature rupture of membranes, and they concluded that high sRAGE concentration can be a favorable prognostic factor in the presence of symptoms of threatened premature labor. In the present study, follicular fluid sRAGE protein levels were significantly, inversely related to TNF-α, IL-6, and CRP. Accordingly, we surmise that sRAGE may act as a protective factor (from the perspective of inflammation) to prevent the occurrence of PCOS.
In conclusion, sRAGE concentrations were decreased in the follicular fluid samples from PCOS patients, and these concentrations were inversely associated with VEGF, TNF-α, IL-6, and CRP protein levels. Next, we will investigate the effects of sRAGE during direct interventions affecting inflammatory factors in granulosa cells, tissues, and whole animals. We will also verify whether sRAGE is protective against the occurrence and development of PCOS. From the new perspective of sRAGE, we want to reveal the mechanism of the occurrence and development of PCOS and provide further new ideas for the prevention and treatment of this syndrome.
Acknowledgments
This work was supported by grant 81571409 from the National Natural Science Foundation, The mechanism of sRAGE in the regulation of VEGF production in granulosa cells of PCOS.
Compliance with ethical standards
Competing interests
The authors declare that they have no competing interests.
Footnotes
Capsule sRAGE could be a potential target in the pathophysiology of PCOS in human
BiJun Wang and MengMeng Hao contributed equally to this work.
References
- 1.Hart R, Doherty DA. The potential implications of a PCOS diagnosis on a woman’s long-term health using data linkage. J Clin Endocrinol Metab. 2015;100(3):911–9. doi: 10.1210/jc.2014-3886. [DOI] [PubMed] [Google Scholar]
- 2.Liu M, Gao J, Zhang Y, Li P, Wang H, Ren X, et al. Serum levels of TSP-1, NF-kappaB and TGF-beta1 in polycystic ovarian syndrome (PCOS) patients in northern China suggest PCOS is associated with chronic inflammation. Clin Endocrinol. 2015;83(6):913–22. doi: 10.1111/cen.12951. [DOI] [PubMed] [Google Scholar]
- 3.Pertynska-Marczewska M, Diamanti-Kandarakis E, Zhang J, Merhi Z. Advanced glycation end products: a link between metabolic and endothelial dysfunction in polycystic ovary syndrome? Metab Clin Exp. 2015;64(11):1564–73. doi: 10.1016/j.metabol.2015.08.010. [DOI] [PubMed] [Google Scholar]
- 4.Hu H, Jiang H, Ren H, Hu X, Wang X, Han C. AGEs and chronic subclinical inflammation in diabetes: disorders of immune system. Diabetes Metab Res Rev. 2015;31(2):127–37. doi: 10.1002/dmrr.2560. [DOI] [PubMed] [Google Scholar]
- 5.Palimeri S, Palioura E, Diamanti-Kandarakis E. Current perspectives on the health risks associated with the consumption of advanced glycation end products: recommendations for dietary management. Diabetes, Metab Syndr Obes: Targets Ther. 2015;8:415–26. doi: 10.2147/DMSO.S63089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang YS, Li YY, Wang LH, Kang Y, Zhang J, Liu ZQ, et al. Tanshinone IIA attenuates chronic pancreatitis-induced pain in rats via downregulation of HMGB1 and TRL4 expression in the spinal cord. Pain Physician. 2015;18(4):E615–28. [PubMed] [Google Scholar]
- 7.Lazo M, Halushka MK, Shen L, Maruthur N, Rebholz CM, Rawlings AM, et al. Soluble receptor for advanced glycation end products and the risk for incident heart failure: the atherosclerosis risk in communities study. Am Heart J. 2015;170(5):961–7. doi: 10.1016/j.ahj.2015.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Merhi Z. Advanced glycation end products and their relevance in female reproduction. Human reproduction (Oxford, England). 2014; 29(1): 135–45. doi:10.1093/humrep/det383. [DOI] [PubMed]
- 9.Romero R, Espinoza J, Hassan S, Gotsch F, Kusanovic JP, Avila C, et al. Soluble receptor for advanced glycation end products (sRAGE) and endogenous secretory RAGE (esRAGE) in amniotic fluid: modulation by infection and inflammation. J Perinat Med. 2008;36(5):388–98. doi: 10.1515/JPM.2008.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rzepka R, Dolegowska B, Rajewska A, Kwiatkowski S, Salata D, Budkowska M, et al. Soluble and endogenous secretory receptors for advanced glycation end products in threatened preterm labor and preterm premature rupture of fetal membranes. BioMed Res Int. 2015;2015:568042. doi: 10.1155/2015/568042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Merhi Z, Irani M, Doswell AD, Ambroggio J. Follicular fluid soluble receptor for advanced glycation end-products (sRAGE): a potential indicator of ovarian reserve. J Clin Endocrinol Metab. 2014;99(2):E226–33. doi: 10.1210/jc.2013-3839. [DOI] [PubMed] [Google Scholar]
- 12.Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome (PCOS). Human reproduction (Oxford, England). 2004; 19(1): 41–7. [DOI] [PubMed]
- 13.Wu TL, Tsai CC, Wang YY, Ho KY, Wu YM, Hung HC, et al. The association between the RAGE G82S polymorphism, sRAGE and chronic periodontitis in Taiwanese individuals with and without diabetes. J Periodontal Res. 2015;50(6):881–9. doi: 10.1111/jre.12282. [DOI] [PubMed] [Google Scholar]
- 14.Sharma I, Dhawan V, Saha SC, Rashmi B, Dhaliwal LK. Implication of the RAGE-EN-RAGE axis in endometriosis. Int J Gynaecol Obstet: Off Organ Int Fed Gynaecol Obstet. 2010;110(3):199–202. doi: 10.1016/j.ijgo.2010.03.037. [DOI] [PubMed] [Google Scholar]
- 15.Yamagishi S, Matsui T. Soluble form of a receptor for advanced glycation end products (sRAGE) as a biomarker. Front biosci (Elite edition) 2010;2:1184–95. doi: 10.2741/e178. [DOI] [PubMed] [Google Scholar]
- 16.Lee EJ, Park EY, Mun H, Chang E, Ko JY, do Kim Y, et al. Soluble receptor for advanced glycation end products inhibits disease progression in autosomal dominant polycystic kidney disease by down-regulating cell proliferation. FASEB J: Off Publ Fed Am Soc Exp Biol. 2015;29(8):3506–14. doi: 10.1096/fj.15-272302. [DOI] [PubMed] [Google Scholar]
- 17.He CT, Lee CH, Hsieh CH, Hsiao FC. Soluble form of receptor for advanced glycation end products is associated with obesity and metabolic syndrome in adolescents. Int J Endocrinol. 2014;2014:657607. doi: 10.1155/2014/657607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gonzalez F. Nutrient-induced inflammation in polycystic ovary syndrome: role in the development of metabolic aberration and ovarian dysfunction. Semin Reprod Med. 2015;33(4):276–86. doi: 10.1055/s-0035-1554918. [DOI] [PubMed] [Google Scholar]
- 19.Peluso C, Fonseca FL, Rodart IF, Cavalcanti V, Gastaldo G, Christofolini DM, et al. AMH: an ovarian reserve biomarker in assisted reproduction. Clin Chim Acta: Int J Clin Chem. 2014;437:175–82. doi: 10.1016/j.cca.2014.07.029. [DOI] [PubMed] [Google Scholar]
- 20.Irani M, Merhi Z. Role of vitamin D in ovarian physiology and its implication in reproduction: a systematic review. Fertil Steril. 2014;102(2):460–8.e3. doi: 10.1016/j.fertnstert.2014.04.046. [DOI] [PubMed] [Google Scholar]
- 21.Bonetti TC, Borges E, Jr, Braga DP, Iaconelli A, Jr, Kleine JP, Silva ID. Intrafollicular soluble receptor for advanced glycation end products (sRAGE) and embryo quality in assisted reproduction. Reprod Biomed Online. 2013;26(1):62–7. doi: 10.1016/j.rbmo.2012.10.001. [DOI] [PubMed] [Google Scholar]
- 22.Guclu M, Ali A, Eroglu DU, Buyukuysal SO, Cander S, Ocak N. Serum levels of sRAGE are associated with body measurements, but not glycemic parameters in patients with prediabetes. Metab Syndr Relat Disord. 2016;14(1):33–9. doi: 10.1089/met.2015.0078. [DOI] [PubMed] [Google Scholar]
- 23.Uribarri J, del Castillo MD, de la Maza MP, Filip R, Gugliucci A, Luevano-Contreras C et al. Dietary advanced glycation end products and their role in health and disease. 2015. [DOI] [PMC free article] [PubMed]
- 24.Schmidt AM. Soluble RAGEs—prospects for treating & tracking metabolic and inflammatory disease. Vasc Pharmacol. 2015;72:1–8. doi: 10.1016/j.vph.2015.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stewart C, Cha S, Caudle RM, Berg K, Katz J. Decreased levels of soluble receptor for advanced glycation end products in patients with primary Sjogren’s syndrome. Rheumatol Int. 2008;28(8):771–6. doi: 10.1007/s00296-008-0529-4. [DOI] [PubMed] [Google Scholar]
- 26.Chen CG, Tang P, Yu ZT. Effect of HMGB1 on the VEGF-C expression and proliferation of esophageal squamous cancer cells. Zhonghua zhong liu za zhi Chin J Oncol. 2012;34(8):566–70. doi: 10.3760/cma.j.issn.0253-3766.2012.08.002. [DOI] [PubMed] [Google Scholar]
- 27.Goova MT, Li J, Kislinger T, Qu W, Lu Y, Bucciarelli LG, et al. Blockade of receptor for advanced glycation end-products restores effective wound healing in diabetic mice. Am J Pathol. 2001;159(2):513–25. doi: 10.1016/S0002-9440(10)61723-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tal R, Seifer DB, Grazi RV, Malter HE. Follicular fluid placental growth factor is increased in polycystic ovarian syndrome: correlation with ovarian stimulation. Reprod Biol Endocrinol: RB&E. 2014;12:82. doi: 10.1186/1477-7827-12-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Manau D, Balasch J, Jimenez W, Fabregues F, Civico S, Casamitjana R, et al. Follicular fluid concentrations of adrenomedullin, vascular endothelial growth factor and nitric oxide in IVF cycles: relationship to ovarian response. Hum Reprod (Oxford, England) 2000;15(6):1295–9. doi: 10.1093/humrep/15.6.1295. [DOI] [PubMed] [Google Scholar]
- 30.Wu R, Van der Hoek KH, Ryan NK, Norman RJ, Robker RL. Macrophage contributions to ovarian function. Hum Reprod Update. 2004;10(2):119–33. doi: 10.1093/humupd/dmh011. [DOI] [PubMed] [Google Scholar]
- 31.Gonzalez F. Inflammation in polycystic ovary syndrome: underpinning of insulin resistance and ovarian dysfunction. Steroids. 2012;77(4):300–5. doi: 10.1016/j.steroids.2011.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Escobar-Morreale HF, Luque-Ramirez M, Gonzalez F. Circulating inflammatory markers in polycystic ovary syndrome: a systematic review and metaanalysis. Fertil Steril. 2011;95(3):1048–58.e1-2. doi: 10.1016/j.fertnstert.2010.11.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cataldegirmen G, Zeng S, Feirt N, Ippagunta N, Dun H, Qu W, et al. RAGE limits regeneration after massive liver injury by coordinated suppression of TNF-alpha and NF-kappaB. J Exp Med. 2005;201(3):473–84. doi: 10.1084/jem.20040934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Malickova K, Jarosova R, Rezabek K, Fait T, Masata J, Janatkova I, et al. Concentrations of sRAGE in serum and follicular fluid in assisted reproductive cycles—a preliminary study. Clin Lab. 2010;56(9–10):377–84. [PubMed] [Google Scholar]