Abstract
While sporadic pregnancy loss is common, occurring in 15 % of pregnancies, recurrent pregnancy loss (RPL) impacts approximately 5 % of couples. Though multiple causes are known (including structural, hormonal, infectious, autoimmune, and thrombophilic causes), after evaluation, roughly half of all cases remain unexplained. The idiopathic RPL cases pose a challenging therapeutic dilemma in addition to incurring much physical and emotional morbidity. Immunogenetic causes have been postulated to contribute to these cases of RPL. Natural Killer cell, T cell expression pattern changes in the endometrium have both been shown in patients with RPL. Human leukocyte antigen (HLA) and cytokine allelic variations have also been studied as etiologies for RPL. Some of the results have been promising, however the studies are small and have not yet put forth outcomes that would change our current diagnosis and management of RPL. Larger database studies are needed with stricter control criteria before reasonable conclusions can be drawn.
Keywords: Immunogenetics, Recurrent pregnancy loss, T regulatory cell, Human leukocyte antigens, HY-Antibodies
Introduction
Idiopathic recurrent pregnancy loss (RPL) is a condition that leads to both emotional and physical morbidity. Additionally, when these patients do have a successful pregnancy they are at much higher risk for antenatal complications such as growth restriction, preeclampsia, and preterm delivery [1]. The repeated attempts at becoming pregnant with subsequent miscarriages lead many patients to undertake numerous tests and procedures in order to elucidate their individual cause of RPL. While patients may discover a uterine anomaly, hormonal imbalance or karyotype abnormality as the cause of their RPL, about 50 % remain with the diagnosis of idiopathic RPL [2]. Chipping away at this catch-all diagnosis by discovering other causes of RPL is important not just for the scientific community but for the patients themselves; providing them with answers to their condition and, hopefully, leading to new and more targeted therapies.
Diagnostic criteria of recurrent pregnancy loss
Spontaneous pregnancy loss impacts approximately 15 % of pregnancies. It is generally defined as a fetal loss prior to 20 weeks (or below a fetal weight of 500 g). Roughly 1 % of fertile couples will experience a miscarriage, with 12–15 % of women knowingly suffering at least one miscarriage in their lifetime [3–7]. Recurrent pregnancy loss is defined as two or more clinical pregnancy losses. Roughly 5 % of women will experience two pregnancy losses in sequence, while 1 % will experience three or more [3, 8, 9].
All known causes of RPL
RPL is a highly heterogeneous condition. A successful pregnancy is achieved through an appropriate balance of communication between mother and fetus via the placenta and decidua. Disruption or abnormalities in this signaling can result in loss of the pregnancy [10].
Uterine anomalies such as septa, fibroids, bicornuate, and unicornuate uteri are all reasons for RPL. Asherman’s syndrome (intrauterine adhesions) is another intracavitary cause of RPL. While some of these distort the cavity, others have been postulated to cause poor blood supply to the implanted embryo and growing fetus, leading to miscarriage. Chronic medical conditions such as diabetes or thyroid disease have also been noted to increase rates of miscarriage.
Polycystic ovarian syndrome (PCOS) is another cause of RPL, though the reason for this is still unclear. As a cause of infertility, PCOS is well known for causing anovulation. However, once pregnant, it is not clear how PCOS leads to RPL. Various etiologies have been postulated including PCOS. Its elevated levels of insulin and luteinizing hormone (LH) have both been considered plausible reasons for increased numbers of RPL patients in this population.
Luteal phase deficiency, where the corpus luteum degrades at a faster rate than normal (thereby shortening the amount of time it produces progesterone) has also been postulated to be a cause of early pregnancy loss.
Infections can also contribute to early pregnancy loss. Vaginitis, untreated gonorrhea, or chlamydia are all possibilities. While the TORCH (Toxoplasmosis, Other (syphilis, varicella-zoster, parvovirus B19), Rubella, Cytomegalovirus (CMV), and Herpes) infections have been investigated as causes of RPL, they likely do not make up a large percentage. Many infections can lead to endometritis, which is a known cause of RPL. Endometritis has also been seen to occur on its own, in environments with no diagnosable infectious source. Environmental causes can also play a role, including cigarette smoking, obesity, and toxins such as BPA [11].
Autoimmune disease increases a patient’s risk for miscarriage [12]. The lapse of tolerance of self-antigens and increased inflammatory response are both associated with negative pregnancy outcomes [13]. Systemic lupus erythematous (SLE) has long been associated with RPL, as well as antiphospholipid syndrome (APS), which includes RPL as a diagnostic criteria [14]. APS antibodies induce trophoblastic apoptosis, lead to abnormal formation of the spiral arteries, and target the vascular endothelium. APS and autoimmune related thyroid disorders are identifiable by laboratory testing and, if managed appropriately, can be controlled to improve pregnancy outcomes [15]. Those with celiac disease and Crohn’s/ulcerative colitis can also have autoimmune responses, which lead to early pregnancy loss. Those with SLE, inflammatory bowel disease, and autoimmune thyroiditis have a statistically significant increased risk of RPL (odds ratio 1.7–5.3) compared to their controls [16–18]. Other abnormal labs can place the patient at risk for RPL. Women with isolated elevated thyroid stimulating hormone (TSH) have higher incidences of pregnancy loss [2, 19]. Also anyone who tests positive for antinuclear antibody, or anti-thyroid peroxidase, or other autoantibodies even without a confirmed autoimmune disease [13, 20–22]. Studies have shown increased rates of these antibodies in RPL patients [2, 23–26].
Inherited thrombophilias are a controversial etiology of RPL, however many practitioners still test for it. Pregnancy is a known prothrombotic state, but the impact of inherited thrombophilias on RPL is not well established. It is well known that hypercoagulable states impact pregnancy at all stages including increased risk of blood clots, miscarriage, preeclampsia, intrauterine growth restriction, abruption, and stillbirth [2]. Factor V Leiden and Anti-Protein C have both been noted to affect pregnancy. Factor V Leiden mutations can also result in Activated Protein C resistance. Protein C is a key component in the anticoagulant pathway that prevents the actions of clotting factors V and VIII. Resistance to Protein C leads to a hypercoagulable state. The association with pregnancy loss is however stronger in the second trimester than with the first, with most studies finding equivalent rates of Factor V Leiden mutations in RPL populations compared to the general parous population [2]. However the RPL population does have higher rates of Activated Protein C resistance. This could be due to the variety of mutations. In general, those who have had unexplained RPL or a history of other pregnancy complications associated with thrombosis or placental insufficiency may benefit from testing for inherited thrombophilias (including Lupus Anticoagulant, Anti-cardiolipin, and Anti-β2-Glycoprotein, and Factor V Leiden and G20210A Prothrombin Gene Mutations).
However, after the above causes have been ruled out, roughly 50 % of RPL cases are still unexplained [9, 27]. Considerable research has gone into further elucidating other mechanisms for RPL to help treat the patients who fall into the unidentified category. Two major categories of research have surfaced: genetic and immunologic etiologies. Many of these etiologies are thought to be the cause of currently labeled idiopathic RPL. Population-based studies have shown that the frequency of miscarriages is higher amongst siblings with idiopathic RPL compared to the general population, suggesting a possible inheritable cause [28, 29].
Genetic causes
Studies that look at the genetic causes of RPL have multiple aims, two of which are to identify genetic markers (DNA/RNA), which have direct predictive value regarding couples at risk for RPL, and/or to determine the specific pathways, which are involved in implantation and pregnancy [10]. There are three different genetic profiles that can be evaluated: the mother, the father and the fetus/placenta (along with the epigenomes of the fetus and placenta) [10].
Well-known genetic causes of RPL are translocations, both balanced and Robertsonian. Balanced translocations are when two autosomes exchange pieces. Robertsonian translocations occur when the ends of two acrocentric chromosomes merge, loose their short arms, and form a single chromosome at the centromeres. In both cases the carriers are phenotypically normal but can create haploid or unbalanced gametes when they undergo meiosis. Combining these gametes with a chromosomally normal gamete of the partner can lead to an embryo that has an abnormal number (excess or reduction) of chromosomal information. Inversions are less common than translocations and often times have no reproductive implications if they are pericentric [2].
Gross chromosomal defects are a major player in RPL. Over 90 % of the chromosomal abnormalities seen in miscarriages are numerical (polyploidy, aneuploidy). There is a direct correlation between increasing age of the woman and degradation of the cellular controls that manage spindle cell formation and function [2]. This is especially true in women over 35 years old, with trisomy 16 being the most common [30].
Other genetic causes of RPL may involve varying allelic expression, with many studies evaluating different genes and showing mixed results [31]. Those evaluated include ones involved in VEGF expression, homocysteine production (MTHFR gene), cell adhesion, and intracellular signaling, as well as hormone receptors [31–33]. Kosova et al. have found differential expressions of TGF-β, lymphocyte functional associated antigen-1 (LFA-1) (CD56++ NK cells receptor), INPP5D and HAVCR2 (both of which have roles in immunomodulation) in out-of-phase endometrial histologies as well as those who have abnormally elevated levels of endometrial Cyclin E (a possible key genetic regulator during the mid-luteal phase). Left-right determination factor 2 (LEFTY2), important in endometrial bleeding and shown to be associated with implantation failure when overexpressed, was upregulated in out-of-phase endometrium [31].
In evaluating genetic causes, it is clear that some of the genes differentially expressed in PRL patients will have immunologic purposes [31].
Well-known immunologic causes of RPL include all of the aforementioned autoimmune diseases, however, there are also alloimmune causes that have been postulated. While the fetus does exist in a relatively immune-privileged state, there are inflammatory responses that must occur at the appropriate time and strength for human implantation to occur. There are components of the immune system that can either promote or suppress trophoblastic implantation [34, 35]. The entire state of pregnancy is in fact a proinflammatory state with most markers being elevated for the duration of pregnancy, including TNF-α, IL-1, IL-6, and IL-10, along with reduced levels of IL-2 and IFN-γ [36]. These changes are thought to be mediated by estrogen and progesterone. While estrogen is proinflammatory, progesterone is thought to be immunosuppressive, normally resulting in a balance [37].
Acquired alloimmune causes such as anti-paternal lymphocytotoxic antibodies and maternal blocking antibodies are both antibody types created in response to the presence of the fetus but have not been proved to cause RPL.
The goal of this paper is to discuss the immunologic causes of RPL that are genetically linked. While there are many studies out there from case reports to meta-analysis which describe the various possible immunogenetic causes of RPL, the goal of this review is to synthesize the main findings currently in the literature and discuss areas where further investigation may be warranted.
Immunogenetic causes
The endometrium of the uterus is in a constantly dynamic state [12]. The receptive stage for pregnancy during a menstrual cycle is usually cycle days 16–20, the mid-luteal phase (assuming a 28-day menstrual cycle) when the cells are most receptive to implantation. During pregnancy this is known as decidualization [38, 39]. During this time, the endometrium must undergo critical changes for successful implantation [40]. During decidualization, mushroom-shaped projections from the endometrium, called pinopods, form tight junctions with the blastocyst tissue. This allows for the communication and recruitment of immune cells. Immune cells from the maternal side can interact with the fetal cells throughout the syncytiotrophoblast which covers the placental villi as well as the implantation site where the extravillous trophoblasts invade into the decidua [12].
Kosova et al. have shown allelic variance as well as varying expression of immune cells including leukocytes, lymphocytes, and T cell activation in patients with RPL [31], The balance between the immune promotion and suppression is postulated to be necessary for the development of the fetus [12, 41]. Here we discuss various types of immune cells, antigens, and cytokines and their genetic variation in RPL.
Natural killer cells
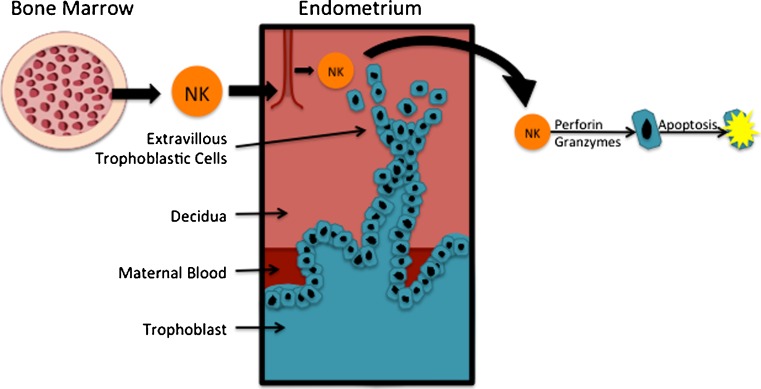
Uterine natural killer (NK) cells are cytokine producers presenting early in pregnancy in the decidua in response to trophoblast antigens [42]. They are the dominant lymphocyte in the uterus, representing roughly 70 % of all present lymphocytes [13]. They are regulated by both hormonal changes and trophoblast invasion, migrating from the bone marrow (Fig. 1) to the uterus in response to progesterone and estrogen [2]. NK cells get rid of cells by detecting altered MHC class I proteins and destroying them using perforin and granzymes, causing apoptosis of the extracellular villous trophoblast cells. They can be activated by interacting with human leukocyte antigen (HLA)-G, HLA-E, or HLA-C antigens (which will be discussed later) on the placental surface. When functioning properly, HLA-G and HLA-E should down regulate NK function [43]. They are essential for trophoblastic migration and invasion, changing character and cytokine expression during the menstrual cycle. They can secrete IFN-γ to induce local angiogenesis and spiral artery formation [42, 44, 45]. However, when activated by HLA-G or HLA-C, they can lead to defective proliferation and invasion of the feto-maternal surface [45–53].
Fig. 1.
Natural killer cells. NK cells migrate from the bone marrow to the uterus in response to progesterone and estrogen [17]. NK cells detect altered MHC class 1 proteins and use perforin and granzymes to destroy extravillous trophoblastic cells [42]
Mouse models have shown excessive levels of NK cells in the decidua are seen in mice with RPL. This has also been observed in women with RPL though the etiology remains unclear as to whether these are the result or cause of RPL [2].
Certain phenotypes of NK cells are found more commonly in successful pregnancy. CD56bright NK cells are noted to release high numbers of cytokines but have low cytotoxicity for the pregnancy [12, 13, 46, 54]. CD56bright NK cells exist in higher numbers in fertile patients’ endometrium than their RPL counterparts with lower CD56bright/CD56dim ratios in the latter patients. They also have lower ratios in their peripheral blood [54]. CD56bright NK cells were found to have decreased numbers in the decidua of chromosomally normal miscarriages compared to aneuploid miscarriages. Peripheral blood NK cells in the presence of the uterus may also be a cause of RPL. They have been shown to produce embryotoxic levels of cytokines when stimulated by trophoblastic antigens [48].
CD56bright NK cells express LFA-1 receptors, which are necessary for successful implantation, and, as stated above, are shown to be downregulated in patients with RPL [31, 55, 56].
However, practical assessment of NK cells in patients is difficult. Christiansen et al. have noted that NK cell evaluation in endometrial tissue is prone to errors. Peripheral blood evaluation is much more objective. However, as stated above, peripheral levels are not indicative of uterine cells [13].
Mast cells
Mast cells are also important mediators of implantation, being moderated by estrogen and progesterone. They exist in the peripheral circulation and the uterus and during normal pregnancy are found to be elevated in the areas around vessels at the maternal-fetal surface. They are important in regulating placentation, tissue remodeling, spiral artery formation, and angiogenesis. Studies in mice have shown that those who lack mast cells have impaired implantation with dysregulation of TGF-β expression and defective spiral artery formation, with intrauterine growth restriction for those pregnancies that do survive. This is resolved by restoration of bone marrow-derived mast cells [57, 58].
Macrophages
Macrophages are also known to accumulate at the site of implantation. Their quantities increase in the endometrium during the late secretory phase and help eliminate pathogens [59]. They have immunosuppressive roles in the decidua, however studies have shown that they secrete both proinflammatory and anti-inflammatory cytokines, which could assist with the balance of establishing pregnancy [60–62].
Polymorphonuclear neutrophils
Polymorphonuclear neutrophils (PMNs) are active during tissue remodeling, promoting angiogenesis especially in the setting of infection [63]. They produce defensins, which promote endometritis, which is a current known cause of RPL [9, 27, 64–67].
T cells
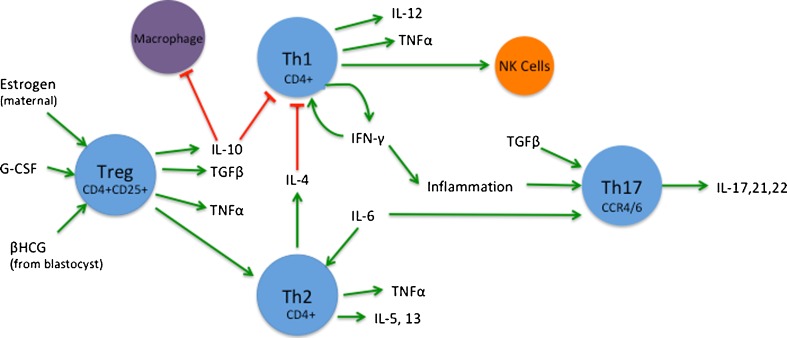
T cells differentiate into multiple subsets in response to internal and external signals (Fig. 2).
Fig. 2.
T cells. T cell expression and regulation is an important process in the establishment and maintenance of early pregnancy. There are still many unknowns regarding their mechanisms of action. This diagram gives a general overview. Estrogen, βHCG, and G-CSF are all known to induce expression of Treg cells and migration to the endometrium [68–71]. There they express IL-10, TGFβ, and TNFα [10]. TNF-α is produced by all CD4+ T cells [11, 72–75]. IL-10 inhibits macrophages and Th1 cell proliferation [76, 77]. Th1 cells both produce and are promoted by IFN-γ [11, 72, 73, 78, 79]. They secrete IL-12 as well [41]. They can induce Th17 and NK cells. Th2 cells produce IL-4, -5, -13 [41]. IL-4 is known to inhibit Th1 [76]. IL-6 is known to promote Th2 cells [80]. Th17 are derived in response to TGFβ and IL-6 as well as inflammation [41]. They secrete IL-17, -21, -22 [41, 81–86]
T regulatory cells
T regulatory (Treg) cells (identified by their CD4 + CD25+ phenotype as well as intracellular FoxP3) function to prevent autoimmunity by adapting in response to self and nonself antigen exposure. They regulate both Th1 and Th2 cells and secrete TGF-β and IL-10 [42]. They are key in the early recognition of fetal tissue and development of tolerance. Even on the fetal side, maternal cells, which cross the placenta, have been shown to reside in the lymph nodes, induce fetal Treg cells, which then suppress fetal anti-maternal immunity. The maternal Treg cells migrate in response to maternally secreted estrogen and blastocyst secreted bHCG, and peak in the endometrium during implantation. They also proliferate in response to granulocyte colony stimulating factor (G-CSF). Some have postulated that this action of G-CSF may reduce the incidence of miscarriage as they can recognize HLA-C on extravillous cytotrophoblasts [68–70, 87].
Dysfunction of Treg cells can lead to known complications of autoimmune diseases such as SLE and type I Diabetes. Altered Treg function is noted in abnormal pregnancies [68, 87]. The levels of Treg cells in RPL patients have been noted to be lower in the decidua and peripheral serum than of their control counterparts [71, 88, 89]. Using foxP3 as a marker of Treg function, a two-fold reduction was noted in its expression in the endometrium of patients with primary infertility [71].
CTLA-4, an inhibitor of T cell proliferation, is involved in autoimmune disease. Dysregulated forms of this are theorized to be involved in miscarriages, however there is little data out there currently on this [81].
And given that Treg cells function to regulate both Th1 and Th2 cells, it is possible that changes in expression of Treg cells can lead to alterations in expression of Th1 and Th2 cells.
T-helper lymphocyte-1 cells
T-helper lymphocyte-1 (Th1) cells are produced from exposure to INF-γ. They secrete IL12 and IFN-γ and have the primary purpose of fighting intracellular infections [42]. Th1 type immunity is thought to possibly be harmful to the fetus [42]. Approximately 15–20 % of women with unexplained RPL have abnormal Th1 cell response to trophoblast antigens in vitro [2]. Th1 cells can induce TH17 and NK cells which have both been implicated in cellular dysregulation leading to RPL.
T-helper lymphocyte-2 cells
T-helper lymphocyte-2 (Th2) cells produce IL-4, IL-5, and IL-13. Their primary functions are elimination of helminth infections and management of allergic disease [42]. Th1 and Th2 cells inhibit one another and are both regulated by Treg cells. An overexpression of Th1 immunity has been thought to play a role in RPL. Counter to that, serologic shifts which show greater Th2 immunity have also been correlated with pregnancies that progress longer [90, 91].
T-helper lymphocyte-17 cells
T-helper lymphocyte-2 (Th17) cells are derived from naïve CD4+ T cells in response to TGF-beta and IL-6 (during inflammation or infection) [42]. They co-express the chemokine receptor (CCR) 4 and CCR6, which allows them to migrate to mucosal areas and provide protection against bacteria and fungi. In the mucosal membranes they secrete IL-17, as well as IL-21 and IL-22. IL-17, in conjunction with neutrophils, works to eradicate extracellular organisms. IL-17 also works synergistically with IL-1 and TGF-α to induce matrix metalloproteinase (MMPs), which are known to be key in the development of early pregnancy. Dysregulation of MMPs can lead to miscarriage. Increased levels of Th17 cells have been found in the decidua and peripheral blood of patients with RPL compared to their controls. They have a negative impact on the early stages of pregnancy [42, 76, 82–85, 89].
Gamma-T lymphocyte cells
Gamma-T lymphocyte (ϒ-T) cells are also found in peripheral blood and in the decidua during pregnancy. Decidual γ-T cells are CD4-CD8-Tcells that have cytolytic properties through the production of Granzyme A and perforin. They are capable of inducing autoantibodies through autologous B cells [86, 92, 93].
Human leukocyte antigens
The placenta allows for interaction of immune-competent cells from the fetus to the mother and vice versa, resulting in the maternal tolerance to the fetus. HLA alleles are expressed on the extravillous trophoblast at the base of anchoring villi in proximity to maternal immune-competent cells. HLA-C, -E and -G are expressed on the decidua [13].
It is postulated that HLA sharing depresses the maternal immune response that is necessary for implantation [41, 94]. Postulations have been made that inbred populations (such as the Hutterites) have major histocompatibility complex HLAs shared between mother and father which can lead to RPL [2]. Other studies have supported this theory with higher rates of RPL seen in couples with HLA compatibility [41]. One study found increased frequencies of identical HLA-A and HLA-B alleles in families with higher rates of RPL [95]. HLA alleles have been previously shown to exhibit positive linkage disequilibrium [95]. One study reviewed a series of RPL patients and their HLA typing, found strong positive linkage disequilibrium between HLA-G14 insertion polymorphism, and HLA-A*01, -A*11, -A*31, -B*08, and DRB1*03. A strong negative linkage equilibrium was found between HLA G14 insertion and HLA-A*02, -A*03, and -A*24. The frequency of the genotypes with the insertion inherited from the mother was significantly increased in patients with RPL [94, 96].
HLA alleles are on chromosome 6. They are the human versions of MHC genes. Class I HLAs present peptides from within the cell. Class II present antigens outside of the cell.
HLA-G
HLA-G is an MHC Class I antigen and is expressed on the fetal placental cells. It binds to killer cell receptors, blocking their activity [2, 97]. They can bind to NK cells in the serum or in the trophoblast [52]. They have both immunosuppressant and immunotolerant features in the development of the fetus [98]. HLA-G is monomorphic at the protein level, so allele variations will likely not alter NK cell/HLA-G interactions. However, allele differences, leading to lower solubility of HLA-G, likely do alter NK functions. There are various polymorphisms of this gene and those which lead to lower production levels have been considered as a cause of RPL [2, 98, 99]. Homozygosity for a 14-base pair insertion in the HLA-G gene exon 8 produces low soluble HLA-G [13]. One study noted increased miscarriage rates in polymorphisms of the HLA-G promoter region, finding the -725C/G allele to carry increased risks for miscarriage [97]. Other studies have shown that HLA-G polymorphisms in conjunction with H-Y Antibodies (male-specific minor antigen) in a male fetus can lead to RPL, as well as a significantly decreased birth weight in a male offspring [12].
HLA-C
HLA-C also interacts with NK cells, and is responsible for the autologous recognition of the fetal tissue. They are expressed on the extravillous trophoblast and can bind to NK cells via the killer immunoglobulin like receptors (KIRs) and have been postulated to mediate trophoblast invasion. HLA-C has the most polymorphisms of the HLAs expressed on the trophoblast [13]. Defective HLA-C polymorphisms can have a strong inhibitory action on NK cells, which leads to defective trophoblast proliferation and invasion, leading to RPL and possibly growth restriction [13, 52, 100, 101]. There are two main allotype groups, which have been identified. HLA-C1 allotypes inhibit KIR2DL2/3 and activate HIR2DS2 receptors. HLA-C2 allotypes inhibit KIR2DL1 and activate KIR2DS1 receptors [13]. In one study, HLA-C1 was found in greater frequency of RPL patients compared to HLA-C2 [53]. Another study noted women who had a history of RPL and preeclampsia had higher chances (compared to their normal peers) of carrying KIR genotypes (AA genotypes) with increased levels of inhibitory receptors in combination with paternal HLA-C2 expression on the trophoblast. This combination is postulated to lead to higher rates of uterine NK cell inhibition and decreased cytokine levels at the maternofetal interface, which can lead to defective proliferation [102]. Hiby et al. also noted that variations in HLA-C2 genotypes independently lead to RPL, especially when this occurs on the paternally derived HLA-C2 [52]. However, a pooled analysis of studies regarding sharing of HLA-C alleles showed no increased risk of RPL [103].
HLA-E
Studies show conflicting evidence regarding HLA-E alleles and RPL. One study showed no association with RPL and HLA-E compatibility, however, another did note that certain genotypes had higher frequencies in the maternal genotypes of RPL patient populations. All of these studies were small and localized to one ethnic group [104–106].
MHC class II alleles have more recently been studied for their link to RPL. While some will be discussed later in the Anti-HY section, we touch on them here. There are other Class II antigens, which are being explored but have not had much research to date.
HLA-DP
HLA-DP antigens were also explored as an etiology of RPL with one study showing increased levels of certain alleles in RPL patients; but clear linkage has not been shown [107].
HLA-DQ
Mueleman et al. did find that HLA-DQ sharing had an OR that trended in favor of association with RPL between HLA-DQB1 and HLA-DQ [103]. Two small studies suggest a linkage between RPL and HLA-DQB1 allele [108, 109].
HLA-DR
The meta-analysis by Muelman et al. showed an increased risk of RPL in couples with HLA-DR sharing [103]. Another meta-analysis has looked at HLA-DR1 and -DR3 and its links to RPL. It found HLA-DR1 to have statistically significant links to RPL (odds of RPL 1.29, 95%CI 1.05–1.58 p < 0.05), whereas no association was found for HLA-DR3 [110]. Significant associations were observed for phenotypic frequencies of HLA-DRB1*4, HLA-DRB1*13, HLA-DRB1*14 and HLA-DRB1*15 with RPL. However, after correcting for multiple testing none of these were statistically significant [103].
Further studies are needed to look at the role of HLA compatibility from mother to fetus. Currently, the majority focus on HLA compatibility within couples but do not address the intersection of mother and fetus as it relates to RPL [41]. Most studies are also still relatively small, with much heterogeneity between studies, leading to difficulty in drawing consistent conclusions. The meta-analysis by Meuleman et al. aimed to attempt to address this, however even they still found it hard to assess [103].
HY-antibodies
HY-Antibodies are male-specific minor antigens. They are ubiquitously expressed on the Y chromosome. HY-antibodies have been found in approximately 30 % of females, but in few males [111]. They are found primarily in patients with secondary RPL who had a firstborn male. Studies have shown reduced probabilities of subsequent live births with the majority of successful ones being female offspring [13, 96, 112]. When compared to those with similar genotype who had a firstborn girl, the latter statistically did not suffer from the same complications [13, 100, 101, 112].
HLA class II alleles seem to be the ones related to Anti-HY immunity. Nielsen et al. noted HLA-DRB1*15, HLA-DQB1*05:01/02 and HLA–DRB3*03:01 (which are often termed HY-restricting) were found to have increased rates of expression in patients with secondary RPL who had a firstborn boy that was stillborn or low birth weight, however these increased rates were not noted in those with a firstborn boy with a normal weight [113].
Christiansen et al. studied a cohort of women with unexplained secondary RPL and followed them in their subsequent pregnancy, finding that there was a 46 % rate of miscarriage in the patients with a firstborn boy and 24 % in patients with a firstborn girl (p < 0.0001). Carrier status of HY-restricting HLA class I alleles did not alter pregnancy prognosis between these two groups. However patients carrying HY-restricting HLA class II alleles reduced the risk of pregnancy in the firstborn boy cohort by roughly 50 %, while carrying two alleles further reduced it to roughly 20% compared to patients with firstborn boys not carrying HY-restricting HLA class II alleles. However, if the child, but not the mother, carried the HY-restricting HLA class II alleles, there was no impact on future pregnancies of the mother [112].
However, the study by Nielsen et al. regarding HLA antibody presence in patients with secondary RPL who had delivered a boy, found both HLA class I and II alleles with higher frequency in these patients compared to those who had a firstborn girl or those with primary RPL, with the majority being HLA class I. When they looked at the timing of expression, they noted that patients with early presence of HLA antibodies during pregnancy were more likely to have a miscarriage compared to those without early presentation, however, when in pregnancy this Early presentation could have started, or how rapidly it might progress to signal miscarriage risk, is unclear [101].
The pathogenesis for this has multiple theories. One, it is thought to be related to the mechanism by which HY-antibodies are presented. One thought is that the HY peptides are presented via maternal antigen-presenting cells to CD4+ T cells in the presence of HY-restricting HLA class II molecules. However, CD4+ T cells with anti-HY specificity have not yet been isolated. Another mechanism may be B cell production of anti-HY-antibodies. In addition, the pro inflammatory profile of late pregnancy may lead to increased exposure of HY-antigens in a male fetus. Given that, Nielsen at al. noted higher rates of RPL after a firstborn boy who was stillborn or lower weight; this may be due to the increased cytokine profile of those pregnancies. All the above theories surmise that with the next pregnancy the adaptive immune response against the HY antigen would result in increased miscarriage or low birth weight against male fetus’ and bias towards female fetuses [13, 112].
Cytokines
Cytokines are proteins that are important in intercellular communication especially in things like inflammation. Patients with recurrent miscarriages have increased expressions of cytokines in the placental tissue [2]. Here, we will touch on some of the ones most commonly studied in RPL.
IL-1
IL-1 is an important inflammatory marker in early pregnancy, but increased expression later on, in conjunction with its receptor IL-1R, could have deleterious effects on the pregnancy [114]. There are various polymorphisms of IL-1. The most studied is IL-1β. Studies are conflicting regarding its association with RPL. Multiple studies have not yet shown any significant association between RPL and genetic polymorphisms of IL-1β, however ones have found significant associations between them (pooled odds ratio (OR) 2.12 (95 % confidence interval (CI) 1.04 to 4.33)) [77, 115, 116]. One study also noted a relationship between RPL and IL-1RA, IL1RN*2, and IL1RN*3 [115].
IL-4
IL-4 is key in developing Th2 cells. IL-4 has also been thought to be protective for pregnancy as it inhibits Th1 activation [91]. While studied in the past, so far no significant association between IL-4 genetic polymorphisms and RPL has been found [117, 118].
IL-10
IL-10 is thought to be important during embryonic development by inhibiting Th1 and macrophage function [91, 114]. One meta-analysis performed did not show any significant association between RPL and genetic polymorphisms of IL-10, however other individual studies have noted a statistically significant associations (especially with IL-10 alleles -1082 and -592) [77, 115, 117, 119, 120]. Overall, the role of IL-10 in RPL still needs further exploration.
IL-6
IL-6 is a cytokine known to promote the differentiation of Th2 cells and subsequent suppression of Th1 cells in conjunction with IL-4 [121]. There are conflicting results on significance of IL-6 genetic polymorphism impact on RPL. Various studies noted that there were increased levels of IL-6 as well as its receptor in RPL patients [80, 114], while others have noted decreased expression levels [114, 122]. A meta-analysis showed that there was no impact on RPL of the IL-6 (-174G) polymorphism however there was a statistically significant association between RPL and those carrying IL-6 (-634) polymorphism in more than one study [77, 115]. Another study, which evaluated the genotypes of RPL patients with ethnically matched controls, did not find any association between RPL and IL-6 polymorphisms [119].
IL-8
IL-8 is involved in leukocyte recruitment and proliferation. It has not been studied greatly, however in patients with euploid miscarriages, upregulation of the proinflammatory IL-8 in the decidua has been seen [123].
IFN-γ
IFN-ɣ is key in developing Th1 cells. These cells also release IFN-γ to promote inflammation. It has been shown to be elevated in RPL patients [13, 72, 78, 79, 124]. Significant associations between RPL and the IFN-γ(+874) polymorphisms have been found [115, 119, 120]. However, a meta-analysis performed did not show any significant association between RPL and any genetic polymorphisms of IFN-γ [77].
TNF-α
TNF-α is involved in inflammation and is released by multiple cells including CD4+ T-cells, NK cells and macrophages. TNF-α has been shown to be increased in euploid compared to aneuploid miscarriages, as well as increased compared to the euploid pregnancies that survive [13, 72–74, 79]. This increase has also been noted in their peripheral blood. Another study showed a trend for association between RPL and certain TNF-α genotypes (−308 A?A and A/G (OR = 1.61; p = 0.18)) [119]. However, two studies performed did not show any significant association between RPL and genetic polymorphisms of TNF-α [77, 120]. If there is a relationship it is possibly a weak one. Increased levels of both TNF-α and IFN-γ have been linked to increased levels of NK cell, as well as macrophage, activation [91].
Other cytokines
Other molecules that participate in immune response have been studied as well. Mannan-binding lectin (MBL) participates in the innate immune system and has also been postulated to be involved with RPL. It binds to the surface of microorganisms and activates the lectin pathway of the compliment system, enhancing phagocytosis. It may also work to clear apoptotic cells, debris, and immune complexes. A deficiency of MBL may lead to decreased clearance of particles resulting in inflammation. Christiansen et al. noted increased rates of MBL deficiency in women with unexplained second trimester losses, as well as those with miscarriages as a result of cervical insufficiency. MBL deficiency may be caused by variations in the MBL2 gene, which has three known polymorphisms in the exon 1 region [13]. While two other large studies showed low levels of MBL in the PRL population, it is also true that the majority of patients with low MBL do not experience RPL [75, 125, 126].
Conclusions
There are a few overarching questions that are raised by the above data.
The first is whether the above immune responses cause or are in response to the pregnancy loss.
Second is whether or not there is one immunogenetic cause that would rise above the others as a prominent etiology of RPL. As eutherian (placental) mammals, humans evolved this ability to undergo decidualization of the endometrial stroma, with direct implantation of the fetus. During this time, the maternal immune system must recognize the pregnancy and develop maternal immunotolerance of the fetal allograft, which allows for survival of the pregnancy. Kosova et al. postulated that the genes involved in immune expression, which evolved as a function of eutherian pregnancy, are preferentially dysregulated in the endometrium of patients with RPL [31]. The dysregulation of any one of these genes may lead to failed implantation and thus could benefit from future research. This could include understanding the cascade effect of the immune system. For example, alterations in T cell expressions including decreased Treg and Th2, and increased TH17 and Th1 could promote an inflammatory state promoting expression of dysregulated cytokines that is toxic to the early pregnancy. Ole Christiansen has postulated that immune mechanisms protecting the fetus are likely redundant given the important need for reproductive success in a species. Thus there are likely both innate and adaptive immune systems at work to grow and protect the developing pregnancy. Given this, he believes that individual failures of one system will not inherently lead to miscarriage, but rather combinations of failures of the innate and adaptive immune systems at the trophoblast are what can lead to RPL [13].
Treatment
So how could this data impact treatment?
Steroid therapy has been used in the past with autoimmune diseases and RPL and may be beneficial for unknown etiologies as it has been shown to increase Treg function [127, 128]. Prednisolone has also been shown to reduce uterine NK cell levels in patients with RPL [129].
Progesterone could be a treatment for RPL by normalizing the expressions of genes involved in implantation. Some of those that Kosava et al. noted dysregulated in the patients with out-of-phase endometrium or abnormal Cyclin E levels are also controlled by progesterone and thus could have changes in their expression patterns by its administration [31]. While there are currently ongoing randomized control trials to evaluate the use of progesterone in RPL, current literature is conflicting and would overall not support routine use [130].
Paternal leukocyte immunization has been trialed as a treatment to reduce maternal NK cell response to fetal circulating antigens. The theory stems from successful treatment of transplant patients with third-party leukocytes on allograft rejection. However, studies done in patients with RPL have yielded conflicting results. The largest trials have shown no benefit [131, 132].
Intravenous immunoglobulins (IVIG) have also been studied as a means of suppressing the maternal immune response. Results of multiple studies have failed to show benefit in improving pregnancy outcomes in patients with RPL [132–134]. One randomized study noted no difference in birth outcomes of patients taking IVIG versus placebo [135]. However, there is evidence that IVIG does reduce levels of NK and NKT [136, 137].
TNF-inhibiting factor treatment is another controversial treatment in early study. Risks of this treatment include, but are not limited to, developing granulomatous disease, lymphoma, and demyelinating diseases [126].
But what about identifying these underlying polymorphisms?
Main treatment goals for patients with RPL still stem from identifying and remedying underlying causes. These include weight loss, thyroid correction, and diabetes management. Jauniaux et al. have also recommended that parental karyotyping only be performed when the probability of carrier status is >2.2 % (roughly those who are under 39 with a family history of RPL and a personal history of two miscarriages, or a negative family history with three or more miscarriages, and those 36 and younger with two or more miscarriages) [126].
Testing is difficult for many immunologic cell lines and cytokines. Testing for peripheral NK cell levels gives little to no useful information regarding the numbers of uterine NK cells [138].
Currently, there is insufficient evidence to support the inclusion of cytokine polymorphisms in the routine workup of RSA patients [11].
Future studies
Future studies in the field of genetics and immunological causes of RPL would benefit from larger database studies, as many of the current studies are still small (Table 1), which focus on the genetics of the mother/father and fetus/placenta as well as controls from viable pregnancies from these couples, if they exist. This would allow for more specific analysis of allele specific expression and/or epigenetic modifications. Most current studies regarding genetic causes suffer from one or more of the following issues: focusing on the genetics of the mother rather than couples, low statistical power, ethnic/population variations, lack of control for environmental and/or lifestyle factors, and/or lack of control for secondary pathways which affect the protein translation and metabolism which can lead to differences in genotype expressions [10, 98].
Table 1.
Study size. Selection of studies reviewed and their sizes as well as overall outcomes (were the levels in the RPL patients increased, decreased relative to controls; were there allelic variations; or was there no association between RPL status and that cell/protein)
| Study | Sample sizea | Levels in RPL patients (relative to controls) | |
|---|---|---|---|
| NK cellsb | Sheshadri et al. [45] | Meta-analysis f22 studies | Increased |
| Park et al. [46] | 22 | Increased | |
| Thum et al. [47] | 138 | Increased | |
| Polgar et al. [48] | 32 | Increased | |
| Hiby et al. [52] | 12 | Allelic variation | |
| Faridi et al. [53] | 177 | Allelic variation | |
| King et al. [54] | 147 | Increased | |
| Kodama et al. [54] | 42 | Increased | |
| Fukui et al. [56] | 82 | Increased | |
| Mast cells | Woidaki et al. [57] | 42 | Decreased |
| Macrophages | Houser et al. [62] | 8 | Allelic variation |
| T cells | |||
| T Reg | Jasper et al. [71] | 22 | Decreased |
| Jin et al. [88] | 32 | Decreased | |
| CTLA-4 | Tsai et al. [81] | 60 | Allelic variation |
| TH2 | Kwak-Kim et al. [90] | 47 | Decreased |
| TH17 | Wang et al. [76] | 30 | Increased |
| Szereday et al. [84] | 8+ (control values not stated) | Increased | |
| Saifi et al. [85] | 40 | Increased | |
| HLA | |||
| HLA Sharing | Beydoun et al. [41] | Meta-analysis 40 studies | Increased/decreased/no association |
| Kolte et al. [96] | 276 | Increased/allelic variation | |
| Moghraby et al. [94] | 253 | No association | |
| Christiansen et al. [95] | 29 | Allelic variation | |
| HLA-G | Hviid et al. [99] | 183 | Allelic variation |
| Ober et al. [97] | 403 | Increased/allelic variation | |
| HLA-C | Hiby et al. [52] | 12 | Allelic variation |
| HLA-C1 | Faridi et al. [53] | 177 | Increased |
| HLA-C2 | Faridi et al. [53] | 177 | Decreased |
| HLA-E | Kanai et al. [104] | 30 | No association |
| Mosaad et al. [105] | 228 | Allelic variation | |
| Steffensen et al. [106] | 232 | No association | |
| HLA-DP | Takakuwa et al. [107] | 60 | Allelic variation |
| HLA-DQ Sharing | Meuleman et al. [103] | Meta-analysis 41 studies | Increased |
| HLA-DQB1 | Aruna et al. [108] | 293 | Increased |
| Steck et al. [109] | 57 | Increased | |
| HLA-DR Sharing | Mueleman et al. [103] | Meta-analysis 41 studies | Increased |
| HLA-DR1 | Christiansen et al. [110] | Meta-analysis 18 studies | Increased |
| HLA-DR3 | Christiansen et al. [110] | Meta-analysis 18 studies | No association |
| HLA-DRB | Mueleman et al. [103] | Meta-analysis 41 studies | Increased |
| HY-antibodies | Christiansen et al. [112] | 358 | Increased |
| Nielsen et al. [101] | 93 | Increased | |
| Cytokines | |||
| IL-1β | Bombell et al. [77] | Meta-analysis 16 studies | Increased |
| Choi et al. [115] | Meta-analysis 5 studies | Increased/allelic variation | |
| IL-1R | Traina et al. [116] | 280 | No association |
| Choi et al. [115] | Meta-analysis 4 studies | Increased/allelic variation | |
| IL-4 | Kamali-Sarvestani et al. [117] | 282 | No association |
| Saijoet al. [118] | 319 | No association | |
| IL-10 | Bombell et al. [77] | Meta-analysis 16 studies | No association |
| Choi et al. [115] | Meta-analysis 5 studies | Increased/allelic variation | |
| Kamali-Sarvestani et al. [117] | 282 | Allelic variation | |
| Daher et al. [119] | 156 | Increased | |
| Deihl et al. [120] | 95 | No association | |
| IL-6 | Arruvito et al. [80] | 137 | Allelic variation |
| Koumantaki et al. [122] | 141 | Decreased | |
| Bombell et al. [77] | Meta-analysis 16 studies | No Association | |
| Choi et al. [115] | Meta-analysis 4 studies | Increased/allelic variation | |
| Daher et al. [119] | 156 | No association | |
| IL-8 | Krieg et al. [123] | 16 | Increased |
| Koumantaki et al. [122] | 141 | Decreased | |
| IFN-gamma | Christiansen et al. [13] | DELETE | Increased |
| Kalu et al. [124] | 28 | Increased | |
| Calleja-Agius et al. [79] | 106 | Increased | |
| Calleja-Agius et al. [72] | 35 | Increased | |
| Choi et al. [115] | Meta-analysis 4 studies | Increased/allelic variation | |
| Daher et al. [119] | 156 | Increased | |
| Deihl et al. [120] | 95 | Increased | |
| Bombell et al. [77] | Meta-analysis 16 studies | No association | |
| TNF-α | Calleja-Agius et al. [79] | 106 | Increased |
| Calleja-Agius et al. [72] | 35 | Increased | |
| Mueller-Eckhardt et al. [74] | 52 | Increased | |
| Daher et al. [119] | 156 | Increased | |
| Bombell et al. [77] | Meta-analysis 16 studies | No association | |
| Deihl et al. [120] | 95 | No association | |
| MBL | Kruse et al. [75] | 532 | Increased |
| Kilpatrick et al. [125] | 243 | Decreased | |
aWhen the sample size was based on couples, the number of couples is provided
bCertain studies addressed specific alleles only, please see text for more in depth description
Future studies could also benefit from stricter criteria regarding patients included. Mueleman et al. recommended restricting criteria to patients with three miscarriages and also ensuring that those with other explanatory factors be excluded [103].
It will also be important to ensure that if/when cytokine analysis are used for patients that genotype frequencies be clearly understood, as many of the above studies show prevalences specific to one ethnic group and may not be extractable to larger populations [32].
Compliance with ethical standards
Funding sources
None.
Conflict of interest
The authors declare that there are no conflicts of interest.
Footnotes
Capsule
Immunogenetic causes of recurrent pregnancy loss are promising areas of research that with further study could lead to improved diagnostic and treatment modalities.
References
- 1.Jivraj S, et al. Obstetric and neonatal outcome in women with a history of recurrent miscarriage: a cohort study. Hum Reprod. 2001;16(1):102–6. doi: 10.1093/humrep/16.1.102. [DOI] [PubMed] [Google Scholar]
- 2.Fritz MA, Speroff L. Clinical gynecologic endocrinology and infertility. 8. Philadelphia: Lippincott Williams and Wilkins; 2010. [Google Scholar]
- 3.Stephenson M, Kutteh W. Evaluation and management of recurrent early pregnancy loss. Clin Obstet Gynecol. 2007;50(1):132–45. doi: 10.1097/GRF.0b013e31802f1c28. [DOI] [PubMed] [Google Scholar]
- 4.Stirrat GM. Recurrent miscarriage. II: clinical associations, causes, and management. Lancet. 1990;336(8717):728–33. doi: 10.1016/0140-6736(90)92215-4. [DOI] [PubMed] [Google Scholar]
- 5.Berry CW, et al. The Euro-Team Early Pregnancy (ETEP) protocol for recurrent miscarriage. Hum Reprod. 1995;10(6):1516–20. doi: 10.1093/HUMREP/10.6.1516. [DOI] [PubMed] [Google Scholar]
- 6.Boklage CE. Survival probability of human conceptions from fertilization to term. Int J Fertil. 1990;35(2):75. [PubMed] [Google Scholar]
- 7.Wilcox AJ, et al. Incidence of early loss of pregnancy. N Engl J Med. 1988;319(4):189–94. doi: 10.1056/NEJM198807283190401. [DOI] [PubMed] [Google Scholar]
- 8.Branch DW, Gibson M, Silver RM. Clinical practice. Recurrent miscarriage. N Engl J Med. 2010;363(18):1740–7. doi: 10.1056/NEJMcp1005330. [DOI] [PubMed] [Google Scholar]
- 9.Practice Committee of American Society for Reproductive, M Definitions of infertility and recurrent pregnancy loss: a committee opinion. Fertil Steril. 2013;99(1):63. doi: 10.1016/j.fertnstert.2012.09.023. [DOI] [PubMed] [Google Scholar]
- 10.Rull K, Nagirnaja L, Laan M. Genetics of recurrent miscarriage: challenges, current knowledge, future directions. Front Genet. 2012;3:34. doi: 10.3389/fgene.2012.00034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.American College of, O. and Gynecologists ACOG practice bulletin. Management of recurrent pregnancy loss. Number 24, February 2001. (Replaces Technical Bulletin Number 212, September 1995). American College of Obstetricians and Gynecologists. Int J Gynaecol Obstet. 2002;78(2):179–90. doi: 10.1016/S0020-7292(02)00197-2. [DOI] [PubMed] [Google Scholar]
- 12.Krieg S, Westphal L. Immune function and recurrent pregnancy loss. Semin Reprod Med. 2015;33(4):305–12. doi: 10.1055/s-0035-1554917. [DOI] [PubMed] [Google Scholar]
- 13.Christiansen OB. Reproductive immunology. Mol Immunol. 2013;55(1):8–15. doi: 10.1016/j.molimm.2012.08.025. [DOI] [PubMed] [Google Scholar]
- 14.Miyakis S, et al. International consensus statement on an update of the classification criteria for definite antiphospholipid syndrome (APS) J Thromb Haemost. 2006;4(2):295–306. doi: 10.1111/j.1538-7836.2006.01753.x. [DOI] [PubMed] [Google Scholar]
- 15.Reid SM, et al. Interventions for clinical and subclinical hypothyroidism pre-pregnancy and during pregnancy. Cochrane Database Syst Rev. 2013;5:CD007752. doi: 10.1002/14651858.CD007752.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Petri M, Allbritton J. Fetal outcome of lupus pregnancy: a retrospective case–control study of the Hopkins Lupus Cohort. J Rheumatol. 1993;20(4):650–6. [PubMed] [Google Scholar]
- 17.Hardy CJ, et al. Pregnancy outcome and family size in systemic lupus erythematosus: a case–control study. Rheumatology (Oxford) 1999;38(6):559–63. doi: 10.1093/rheumatology/38.6.559. [DOI] [PubMed] [Google Scholar]
- 18.Naganuma M, et al. Conception and pregnancy outcome in women with inflammatory bowel disease: a multicentre study from Japan. J Crohns Colitis. 2011;5(4):317–23. doi: 10.1016/j.crohns.2011.02.003. [DOI] [PubMed] [Google Scholar]
- 19.Stagnaro-Green A, et al. Detection of at-risk pregnancy by means of highly sensitive assays for thyroid autoantibodies. JAMA. 1990;264(11):1422–5. doi: 10.1001/jama.1990.03450110068029. [DOI] [PubMed] [Google Scholar]
- 20.Imaizumi M, et al. Pregnancy and murine thyroiditis: thyroglobulin immunization leads to fetal loss in specific allogeneic pregnancies. Endocrinology. 2001;142(2):823–9. doi: 10.1210/endo.142.2.7966. [DOI] [PubMed] [Google Scholar]
- 21.Imaizumi M, et al. Non-MHC driven exacerbation of experimental thyroiditis in the postpartum period. Autoimmunity. 2001;34(2):95–105. doi: 10.3109/08916930109001957. [DOI] [PubMed] [Google Scholar]
- 22.Iijima T, et al. Effects of autoantibodies on the course of pregnancy and fetal growth. Obstet Gynecol. 1997;90(3):364–9. doi: 10.1016/S0029-7844(97)00283-4. [DOI] [PubMed] [Google Scholar]
- 23.Christiansen OB, et al. Multifactorial etiology of recurrent miscarriage and its scientific and clinical implications. Gynecol Obstet Investig. 2008;66(4):257–67. doi: 10.1159/000149575. [DOI] [PubMed] [Google Scholar]
- 24.Ticconi C, et al. Antinuclear autoantibodies in women with recurrent pregnancy loss. Am J Reprod Immunol. 2010;64(6):384–92. doi: 10.1111/j.1600-0897.2010.00863.x. [DOI] [PubMed] [Google Scholar]
- 25.Meroni PL, et al. Pathogenesis of antiphospholipid syndrome: understanding the antibodies. Nat Rev Rheumatol. 2011;7(6):330–9. doi: 10.1038/nrrheum.2011.52. [DOI] [PubMed] [Google Scholar]
- 26.Qureshi F, et al. Anti-DNA antibodies cross-reacting with laminin inhibit trophoblast attachment and migration: implications for recurrent pregnancy loss in SLE patients. Am J Reprod Immunol. 2000;44(3):136–42. doi: 10.1111/j.8755-8920.2000.440302.x. [DOI] [PubMed] [Google Scholar]
- 27.Practice Committee of the American Society for Reproductive, M Evaluation and treatment of recurrent pregnancy loss: a committee opinion. Fertil Steril. 2012;98(5):1103–11. doi: 10.1016/j.fertnstert.2012.06.048. [DOI] [PubMed] [Google Scholar]
- 28.Andersen AMN, et al. Maternal age and fetal loss: population based register Linkage study. Br Med J. 2000;320(7251):1708–12. doi: 10.1136/bmj.320.7251.1708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kolte AM, et al. A genome-wide scan in affected sibling pairs with idiopathic recurrent miscarriage suggests genetic linkage. Mol Hum Reprod. 2011;17(6):379–85. doi: 10.1093/molehr/gar003. [DOI] [PubMed] [Google Scholar]
- 30.Marquard K, et al. Etiology of recurrent pregnancy loss in women over the age of 35 years. Fertil Steril. 2010;94(4):1473–7. doi: 10.1016/j.fertnstert.2009.06.041. [DOI] [PubMed] [Google Scholar]
- 31.Kosova G, et al. Evolutionary forward genomics reveals novel insights into the genes and pathways dysregulated in recurrent early pregnancy loss. Hum Reprod. 2015;30(3):519–29. doi: 10.1093/humrep/deu355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Daher S, et al. Genetic polymorphisms and recurrent spontaneous abortions: an overview of current knowledge. Am J Reprod Immunol. 2012;67(4):341–7. doi: 10.1111/j.1600-0897.2012.01123.x. [DOI] [PubMed] [Google Scholar]
- 33.Nelen WL, et al. Hyperhomocysteinemia and recurrent early pregnancy loss: a meta-analysis. Fertil Steril. 2000;74(6):1196–9. doi: 10.1016/S0015-0282(00)01595-8. [DOI] [PubMed] [Google Scholar]
- 34.Haller-Kikkatalo K, et al. Autoimmune activation toward embryo implantation is rare in immune-privileged human endometrium. Semin Reprod Med. 2014;32(5):376–84. doi: 10.1055/s-0034-1376356. [DOI] [PubMed] [Google Scholar]
- 35.Park DW, Yang KM. Hormonal regulation of uterine chemokines and immune cells. Clin Exp Reprod Med. 2011;38(4):179–85. doi: 10.5653/cerm.2011.38.4.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Laird SM, et al. A review of immune cells and molecules in women with recurrent miscarriage. Hum Reprod Update. 2003;9(2):163–74. doi: 10.1093/humupd/dmg013. [DOI] [PubMed] [Google Scholar]
- 37.Lessey BA, Young SL. Homeostasis imbalance in the endometrium of women with implantation defects: the role of estrogen and progesterone. Semin Reprod Med. 2014;32(5):365–75. doi: 10.1055/s-0034-1376355. [DOI] [PubMed] [Google Scholar]
- 38.Singh M, Chaudhry P, Asselin E. Bridging endometrial receptivity and implantation: network of hormones, cytokines, and growth factors. J Endocrinol. 2011;210(1):5–14. doi: 10.1530/JOE-10-0461. [DOI] [PubMed] [Google Scholar]
- 39.Lockwood CJ, et al. The role of decidualization in regulating endometrial hemostasis during the menstrual cycle, gestation, and in pathological states. Semin Thromb Hemost. 2007;33(1):111–7. doi: 10.1055/s-2006-958469. [DOI] [PubMed] [Google Scholar]
- 40.Ruiz-Alonso M, Blesa D, Simon C. The genomics of the human endometrium. Biochim Biophys Acta. 2012;1822(12):1931–42. doi: 10.1016/j.bbadis.2012.05.004. [DOI] [PubMed] [Google Scholar]
- 41.Beydoun H, Saftlas AF. Association of human leucocyte antigen sharing with recurrent spontaneous abortions. Tissue Antigens. 2005;65(2):123–35. doi: 10.1111/j.1399-0039.2005.00367.x. [DOI] [PubMed] [Google Scholar]
- 42.Bansal AS. Joining the immunological dots in recurrent miscarriage. Am J Reprod Immunol. 2010;64(5):307–15. doi: 10.1111/j.1600-0897.2010.00864.x. [DOI] [PubMed] [Google Scholar]
- 43.Blaschitz A, Hutter H, Dohr G. HLA class I protein expression in the human placenta. Early Pregnancy. 2001;5(1):67–9. [PubMed] [Google Scholar]
- 44.Teles A, Zenclussen AC. How cells of the immune system prepare the endometrium for implantation. Semin Reprod Med. 2014;32(5):358–64. doi: 10.1055/s-0034-1383735. [DOI] [PubMed] [Google Scholar]
- 45.Seshadri S, Sunkara SK. Natural killer cells in female infertility and recurrent miscarriage: a systematic review and meta-analysis. Hum Reprod Update. 2014;20(3):429–38. doi: 10.1093/humupd/dmt056. [DOI] [PubMed] [Google Scholar]
- 46.Park DW, et al. Peripheral blood NK cells reflect changes in decidual NK cells in women with recurrent miscarriages. Am J Reprod Immunol. 2010;63(2):173–80. doi: 10.1111/j.1600-0897.2009.00777.x. [DOI] [PubMed] [Google Scholar]
- 47.Thum MY, et al. An increase in the absolute count of CD56dimCD16 + CD69+ NK cells in the peripheral blood is associated with a poorer IVF treatment and pregnancy outcome. Hum Reprod. 2004;19(10):2395–400. doi: 10.1093/humrep/deh378. [DOI] [PubMed] [Google Scholar]
- 48.Polgar K, Hill JA. Identification of the white blood cell populations responsible for Th1 immunity to trophoblast and the timing of the response in women with recurrent pregnancy loss. Gynecol Obstet Investig. 2002;53(1):59–64. doi: 10.1159/000049413. [DOI] [PubMed] [Google Scholar]
- 49.Wilkens J, et al. Uterine NK cells regulate endometrial bleeding in women and are suppressed by the progesterone receptor modulator asoprisnil. J Immunol. 2013;191(5):2226–35. doi: 10.4049/jimmunol.1300958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.King A, Loke YW, Chaouat G. NK cells and reproduction. Immunol Today. 1997;18(2):64–6. doi: 10.1016/S0167-5699(97)01001-3. [DOI] [PubMed] [Google Scholar]
- 51.Boyton RJ, Altmann DM. Natural killer cells, killer immunoglobulin-like receptors and human leucocyte antigen class I in disease. Clin Exp Immunol. 2007;149(1):1–8. doi: 10.1111/j.1365-2249.2007.03424.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hiby SE, et al. Maternal activating KIRs protect against human reproductive failure mediated by fetal HLA-C2. J Clin Invest. 2010;120(11):4102–10. doi: 10.1172/JCI43998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Faridi RM, Agrawal S. Killer immunoglobulin-like receptors (KIRs) and HLA-C allorecognition patterns implicative of dominant activation of natural killer cells contribute to recurrent miscarriages. Hum Reprod. 2011;26(2):491–7. doi: 10.1093/humrep/deq341. [DOI] [PubMed] [Google Scholar]
- 54.King K, et al. Detailed analysis of peripheral blood natural killer (NK) cells in women with recurrent miscarriage. Hum Reprod. 2010;25(1):52–8. doi: 10.1093/humrep/dep349. [DOI] [PubMed] [Google Scholar]
- 55.Kodama T, et al. Characteristic changes of large granular lymphocytes that strongly express CD56 in endometrium during the menstrual cycle and early pregnancy. Hum Reprod. 1998;13(4):1036–43. doi: 10.1093/humrep/13.4.1036. [DOI] [PubMed] [Google Scholar]
- 56.Fukui K, et al. Leukocyte function-associated antigen-1 expression on decidual natural killer cells in patients with early pregnancy loss. Mol Hum Reprod. 1999;5(11):1083–8. doi: 10.1093/molehr/5.11.1083. [DOI] [PubMed] [Google Scholar]
- 57.Woidacki K, et al. Mast cells rescue implantation defects caused by c-kit deficiency. Cell Death Dis. 2013;4:e462. doi: 10.1038/cddis.2012.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Woidacki K, Jensen F, Zenclussen AC. Mast cells as novel mediators of reproductive processes. Front Immunol. 2013;4:29. doi: 10.3389/fimmu.2013.00029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Eidukaite A, Tamosiunas V. Endometrial and peritoneal macrophages: expression of activation and adhesion molecules. Am J Reprod Immunol. 2004;52(2):113–7. doi: 10.1111/j.1600-0897.2004.00201.x. [DOI] [PubMed] [Google Scholar]
- 60.Tachi C, Tachi S. Macrophages and implantation. Ann N Y Acad Sci. 1986;476:158–82. doi: 10.1111/j.1749-6632.1986.tb20929.x. [DOI] [PubMed] [Google Scholar]
- 61.Miller L, Hunt JS. Sex steroid hormones and macrophage function. Life Sci. 1996;59(1):1–14. doi: 10.1016/0024-3205(96)00122-1. [DOI] [PubMed] [Google Scholar]
- 62.Houser BL, et al. Two unique human decidual macrophage populations. J Immunol. 2011;186(4):2633–42. doi: 10.4049/jimmunol.1003153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lin YJ, et al. Neutrophils and macrophages promote angiogenesis in the early stage of endometriosis in a mouse model. Endocrinology. 2006;147(3):1278–86. doi: 10.1210/en.2005-0790. [DOI] [PubMed] [Google Scholar]
- 64.Strzemienski PJ, Dyer RM, Kenney RM. Effect of estradiol and progesterone on antistaphylococcal activity of neutrophils from ovariectomized mares. Am J Vet Res. 1987;48(11):1638–41. [PubMed] [Google Scholar]
- 65.Strzemienski PJ, et al. Bactericidal activity of peripheral blood neutrophils during the oestrous cycle and early pregnancy in the mare. J Reprod Fertil. 1987;80(1):289–93. doi: 10.1530/jrf.0.0800289. [DOI] [PubMed] [Google Scholar]
- 66.Wiesenfeld HC, et al. Association between elevated neutrophil defensin levels and endometritis. J Infect Dis. 2002;186(6):792–7. doi: 10.1086/342417. [DOI] [PubMed] [Google Scholar]
- 67.Amsalem H, et al. Identification of a novel neutrophil population: proangiogenic granulocytes in second-trimester human decidua. J Immunol. 2014;193(6):3070–9. doi: 10.4049/jimmunol.1303117. [DOI] [PubMed] [Google Scholar]
- 68.La Rocca C, et al. The immunology of pregnancy: regulatory T cells control maternal immune tolerance toward the fetus. Immunol Lett. 2014;162(1 Pt A):41–8. doi: 10.1016/j.imlet.2014.06.013. [DOI] [PubMed] [Google Scholar]
- 69.Wurfel W. Treatment with granulocyte colony-stimulating factor in patients with repetitive implantation failures and/or recurrent spontaneous abortions. J Reprod Immunol. 2015;108:123–35. doi: 10.1016/j.jri.2015.01.010. [DOI] [PubMed] [Google Scholar]
- 70.Rahmati M, et al. Granulocyte-colony stimulating factor related pathways tested on an endometrial ex-vivo model. PLoS One. 2014;9(9):e102286. doi: 10.1371/journal.pone.0102286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Jasper MJ, Tremellen KP, Robertson SA. Primary unexplained infertility is associated with reduced expression of the T-regulatory cell transcription factor Foxp3 in endometrial tissue. Mol Hum Reprod. 2006;12(5):301–8. doi: 10.1093/molehr/gal032. [DOI] [PubMed] [Google Scholar]
- 72.Calleja-Agius J, Jauniaux E, Muttukrishna S. Placental villous expression of TNFalpha and IL-10 and effect of oxygen tension in euploid early pregnancy failure. Am J Reprod Immunol. 2012;67(6):515–25. doi: 10.1111/j.1600-0897.2012.01104.x. [DOI] [PubMed] [Google Scholar]
- 73.Choudhury SR, Knapp LA. Human reproductive failure I: immunological factors. Hum Reprod Update. 2001;7(2):113–34. doi: 10.1093/humupd/7.2.113. [DOI] [PubMed] [Google Scholar]
- 74.Mueller-Eckhardt G, et al. Immunogenetic and serological investigations in nonpregnant and in pregnant women with a history of recurrent spontaneous abortions. German RSA/IVIG Study Group. J Reprod Immunol. 1994;27(2):95–109. doi: 10.1016/0165-0378(94)90026-4. [DOI] [PubMed] [Google Scholar]
- 75.Kruse C, et al. Low serum level of mannan-binding lectin is a determinant for pregnancy outcome in women with recurrent spontaneous abortion. Am J Obstet Gynecol. 2002;187(5):1313–20. doi: 10.1067/mob.2002.126846. [DOI] [PubMed] [Google Scholar]
- 76.Wang WJ, et al. Increased prevalence of T helper 17 (Th17) cells in peripheral blood and decidua in unexplained recurrent spontaneous abortion patients. J Reprod Immunol. 2010;84(2):164–70. doi: 10.1016/j.jri.2009.12.003. [DOI] [PubMed] [Google Scholar]
- 77.Bombell S, McGuire W. Cytokine polymorphisms in women with recurrent pregnancy loss: meta-analysis. Aust N Z J Obstet Gynaecol. 2008;48(2):147–54. doi: 10.1111/j.1479-828X.2008.00843.x. [DOI] [PubMed] [Google Scholar]
- 78.Choudhury SR, Knapp LA. Human reproductive failure II: immunogenetic and interacting factors. Hum Reprod Update. 2001;7(2):135–60. doi: 10.1093/humupd/7.2.135. [DOI] [PubMed] [Google Scholar]
- 79.Calleja-Agius J, et al. Investigation of systemic inflammatory response in first trimester pregnancy failure. Hum Reprod. 2012;27(2):349–57. doi: 10.1093/humrep/der402. [DOI] [PubMed] [Google Scholar]
- 80.Arruvito L, et al. IL-6 trans-signaling and the frequency of CD4 + FOXP3+ cells in women with reproductive failure. J Reprod Immunol. 2009;82(2):158–65. doi: 10.1016/j.jri.2009.04.010. [DOI] [PubMed] [Google Scholar]
- 81.Tsai AF, et al. Transmission disequilibrium of maternally-inherited CTLA-4 microsatellite alleles in idiopathic recurrent miscarriage. J Reprod Immunol. 1998;40(2):147–57. doi: 10.1016/S0165-0378(98)00073-4. [DOI] [PubMed] [Google Scholar]
- 82.Wang WJ, et al. Regulation of the expression of Th17 cells and regulatory T cells by IL-27 in patients with unexplained early recurrent miscarriage. J Reprod Immunol. 2013;99(1–2):39–45. doi: 10.1016/j.jri.2013.04.002. [DOI] [PubMed] [Google Scholar]
- 83.Bansal AS, Bajardeen B, Thum MY. The basis and value of currently used immunomodulatory therapies in recurrent miscarriage. J Reprod Immunol. 2012;93(1):41–51. doi: 10.1016/j.jri.2011.10.002. [DOI] [PubMed] [Google Scholar]
- 84.Szereday L, et al. Commitment of decidual haematopoietic progenitor cells in first trimester pregnancy. Am J Reprod Immunol. 2012;67(1):9–16. doi: 10.1111/j.1600-0897.2011.01029.x. [DOI] [PubMed] [Google Scholar]
- 85.Saifi B, et al. Th17 cells and related cytokines in unexplained recurrent spontaneous miscarriage at the implantation window. Reprod Biomed Online. 2014;29(4):481–9. doi: 10.1016/j.rbmo.2014.06.008. [DOI] [PubMed] [Google Scholar]
- 86.Bansal RR, et al. IL-21 enhances the potential of human gammadelta T cells to provide B-cell help. Eur J Immunol. 2012;42(1):110–9. doi: 10.1002/eji.201142017. [DOI] [PubMed] [Google Scholar]
- 87.Alijotas-Reig J, Llurba E, Gris JM. Potentiating maternal immune tolerance in pregnancy: a new challenging role for regulatory T cells. Placenta. 2014;35(4):241–8. doi: 10.1016/j.placenta.2014.02.004. [DOI] [PubMed] [Google Scholar]
- 88.Jin LP, et al. The CD4 + CD25 bright regulatory T cells and CTLA-4 expression in peripheral and decidual lymphocytes are down-regulated in human miscarriage. Clin Immunol. 2009;133(3):402–10. doi: 10.1016/j.clim.2009.08.009. [DOI] [PubMed] [Google Scholar]
- 89.Saito S, et al. Th1/Th2/Th17 and regulatory T-cell paradigm in pregnancy. Am J Reprod Immunol. 2010;63(6):601–10. doi: 10.1111/j.1600-0897.2010.00852.x. [DOI] [PubMed] [Google Scholar]
- 90.Kwak-Kim JY, et al. Increased T helper 1 cytokine responses by circulating T cells are present in women with recurrent pregnancy losses and in infertile women with multiple implantation failures after IVF. Hum Reprod. 2003;18(4):767–73. doi: 10.1093/humrep/deg156. [DOI] [PubMed] [Google Scholar]
- 91.Chaouat G. The Th1/Th2 paradigm: still important in pregnancy? Semin Immunopathol. 2007;29(2):95–113. doi: 10.1007/s00281-007-0069-0. [DOI] [PubMed] [Google Scholar]
- 92.Szekeres-Bartho J, et al. The role of gamma/delta T cells in the feto-maternal relationship. Semin Immunol. 2001;13(4):229–33. doi: 10.1006/smim.2000.0318. [DOI] [PubMed] [Google Scholar]
- 93.Mincheva-Nilsson L. Pregnancy and gamma/delta T cells: taking on the hard questions. Reprod Biol Endocrinol. 2003;1:120. doi: 10.1186/1477-7827-1-120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Moghraby JS, et al. HLA sharing among couples appears unrelated to idiopathic recurrent fetal loss in Saudi Arabia. Hum Reprod. 2010;25(8):1900–5. doi: 10.1093/humrep/deq154. [DOI] [PubMed] [Google Scholar]
- 95.Christiansen OB, et al. Association of maternal HLA haplotypes with recurrent spontaneous abortions. Tissue Antigens. 1989;34(3):190–9. doi: 10.1111/j.1399-0039.1989.tb01736.x. [DOI] [PubMed] [Google Scholar]
- 96.Kolte AM, et al. Study of the structure and impact of human leukocyte antigen (HLA)-G-A, HLA-G-B, and HLA-G-DRB1 haplotypes in families with recurrent miscarriage. Hum Immunol. 2010;71(5):482–8. doi: 10.1016/j.humimm.2010.02.001. [DOI] [PubMed] [Google Scholar]
- 97.Ober C, et al. Variation in the HLA-G promoter region influences miscarriage rates. Am J Hum Genet. 2003;72(6):1425–35. doi: 10.1086/375501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Cecati M, et al. HLA-G and pregnancy adverse outcomes. Med Hypotheses. 2011;76(6):782–4. doi: 10.1016/j.mehy.2011.02.017. [DOI] [PubMed] [Google Scholar]
- 99.Hviid TV, et al. Association between human leukocyte antigen-G genotype and success of in vitro fertilization and pregnancy outcome. Tissue Antigens. 2004;64(1):66–9. doi: 10.1111/j.1399-0039.2004.00239.x. [DOI] [PubMed] [Google Scholar]
- 100.Moffett A, Hiby SE, Sharkey AM. The role of the maternal immune system in the regulation of human birthweight. Philos Trans R Soc Lond B Biol Sci. 2015;370(1663):20140071. doi: 10.1098/rstb.2014.0071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Nielsen HS, et al. The presence of HLA-antibodies in recurrent miscarriage patients is associated with a reduced chance of a live birth. J Reprod Immunol. 2010;87(1–2):67–73. doi: 10.1016/j.jri.2010.05.006. [DOI] [PubMed] [Google Scholar]
- 102.Hiby SE, et al. Combinations of maternal KIR and fetal HLA-C genes influence the risk of preeclampsia and reproductive success. J Exp Med. 2004;200(8):957–65. doi: 10.1084/jem.20041214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Meuleman T, et al. HLA associations and HLA sharing in recurrent miscarriage: a systematic review and meta-analysis. Hum Immunol. 2015;76(5):362–73. doi: 10.1016/j.humimm.2015.02.004. [DOI] [PubMed] [Google Scholar]
- 104.Kanai T, et al. Polymorphism of human leukocyte antigen-E gene in the Japanese population with or without recurrent abortion. Am J Reprod Immunol. 2001;45(3):168–73. doi: 10.1111/j.8755-8920.2001.450308.x. [DOI] [PubMed] [Google Scholar]
- 105.Mosaad YM, et al. Association between HLA-E *0101 homozygosity and recurrent miscarriage in Egyptian women. Scand J Immunol. 2011;74(2):205–9. doi: 10.1111/j.1365-3083.2011.02559.x. [DOI] [PubMed] [Google Scholar]
- 106.Steffensen R, et al. HLA-E polymorphism in patients with recurrent spontaneous abortion. Tissue Antigens. 1998;52(6):569–72. doi: 10.1111/j.1399-0039.1998.tb03088.x. [DOI] [PubMed] [Google Scholar]
- 107.Takakuwa K, et al. Possible susceptibility of the HLA-DPB1*0402 and HLA-DPB1*04 alleles to unexplained recurrent abortion: analysis by means of polymerase chain reaction-restricted fragment length polymorphism method. Am J Reprod Immunol. 1999;42(4):233–9. doi: 10.1111/j.1600-0897.1999.tb00096.x. [DOI] [PubMed] [Google Scholar]
- 108.Aruna M, et al. Novel alleles of HLA-DQ and -DR loci show association with recurrent miscarriages among South Indian women. Hum Reprod. 2011;26(4):765–74. doi: 10.1093/humrep/der024. [DOI] [PubMed] [Google Scholar]
- 109.Steck T, et al. HLA-DQA1 and HLA-DQB1 haplotypes in aborted fetuses and couples with recurrent spontaneous abortion. J Reprod Immunol. 1995;29(2):95–104. doi: 10.1016/0165-0378(95)00937-G. [DOI] [PubMed] [Google Scholar]
- 110.Christiansen OB, et al. Association between HLA-DR1 and -DR3 antigens and unexplained repeated miscarriage. Hum Reprod Update. 1999;5(3):249–55. doi: 10.1093/humupd/5.3.249. [DOI] [PubMed] [Google Scholar]
- 111.Miklos DB, et al. Antibody responses to H-Y minor histocompatibility antigens correlate with chronic graft-versus-host disease and disease remission. Blood. 2005;105(7):2973–8. doi: 10.1182/blood-2004-09-3660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Christiansen OB, Steffensen R, Nielsen HS. The impact of anti-HY responses on outcome in current and subsequent pregnancies of patients with recurrent pregnancy losses. J Reprod Immunol. 2010;85(1):9–14. doi: 10.1016/j.jri.2009.12.008. [DOI] [PubMed] [Google Scholar]
- 113.Nielsen HS, et al. Association of HY-restricting HLA class II alleles with pregnancy outcome in patients with recurrent miscarriage subsequent to a firstborn boy. Hum Mol Genet. 2009;18(9):1684–91. doi: 10.1093/hmg/ddp077. [DOI] [PubMed] [Google Scholar]
- 114.Saini V, et al. Cytokines in recurrent pregnancy loss. Clin Chim Acta. 2011;412(9–10):702–8. doi: 10.1016/j.cca.2011.01.002. [DOI] [PubMed] [Google Scholar]
- 115.Choi YK, Kwak-Kim J. Cytokine gene polymorphisms in recurrent spontaneous abortions: a comprehensive review. Am J Reprod Immunol. 2008;60(2):91–110. doi: 10.1111/j.1600-0897.2008.00602.x. [DOI] [PubMed] [Google Scholar]
- 116.Traina E, et al. Polymorphisms in VEGF, progesterone receptor and IL-1 receptor genes in women with recurrent spontaneous abortion. J Reprod Immunol. 2011;88(1):53–7. doi: 10.1016/j.jri.2010.07.006. [DOI] [PubMed] [Google Scholar]
- 117.Kamali-Sarvestani E, et al. Cytokine gene polymorphisms and susceptibility to recurrent pregnancy loss in Iranian women. J Reprod Immunol. 2005;65(2):171–8. doi: 10.1016/j.jri.2005.01.008. [DOI] [PubMed] [Google Scholar]
- 118.Saijo Y, et al. Interleukin-4 gene polymorphism is not involved in the risk of recurrent pregnancy loss. Am J Reprod Immunol. 2004;52(2):143–6. doi: 10.1111/j.1600-0897.2004.00193.x. [DOI] [PubMed] [Google Scholar]
- 119.Daher S, et al. Associations between cytokine gene polymorphisms and recurrent pregnancy loss. J Reprod Immunol. 2003;58(1):69–77. doi: 10.1016/S0165-0378(02)00059-1. [DOI] [PubMed] [Google Scholar]
- 120.Prigoshin N, et al. Cytokine gene polymorphisms in recurrent pregnancy loss of unknown cause. Am J Reprod Immunol. 2004;52(1):36–41. doi: 10.1111/j.1600-0897.2004.00179.x. [DOI] [PubMed] [Google Scholar]
- 121.Diehl S, Rincon M. The two faces of IL-6 on Th1/Th2 differentiation. Mol Immunol. 2002;39(9):531–6. doi: 10.1016/S0161-5890(02)00210-9. [DOI] [PubMed] [Google Scholar]
- 122.Koumantaki Y, et al. Detection of interleukin-6, interleukin-8, and interleukin-11 in plasma from women with spontaneous abortion. Eur J Obstet Gynecol Reprod Biol. 2001;98(1):66–71. doi: 10.1016/S0301-2115(01)00289-5. [DOI] [PubMed] [Google Scholar]
- 123.Krieg SA, et al. Global alteration in gene expression profiles of deciduas from women with idiopathic recurrent pregnancy loss. Mol Hum Reprod. 2012;18(9):442–50. doi: 10.1093/molehr/gas017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Kalu E, et al. Serial estimation of Th1:th2 cytokines profile in women undergoing in-vitro fertilization-embryo transfer. Am J Reprod Immunol. 2008;59(3):206–11. doi: 10.1111/j.1600-0897.2007.00565.x. [DOI] [PubMed] [Google Scholar]
- 125.Kilpatrick DC, Bevan BH, Liston WA. Association between mannan binding protein deficiency and recurrent miscarriage. Hum Reprod. 1995;10(9):2501–5. doi: 10.1093/oxfordjournals.humrep.a136330. [DOI] [PubMed] [Google Scholar]
- 126.Jauniaux E, et al. Evidence-based guidelines for the investigation and medical treatment of recurrent miscarriage. Hum Reprod. 2006;21(9):2216–22. doi: 10.1093/humrep/del150. [DOI] [PubMed] [Google Scholar]
- 127.Dao Nguyen X, Robinson DS. Fluticasone propionate increases CD4CD25 T regulatory cell suppression of allergen-stimulated CD4CD25 T cells by an IL-10-dependent mechanism. J Allergy Clin Immunol. 2004;114(2):296–301. doi: 10.1016/j.jaci.2004.04.048. [DOI] [PubMed] [Google Scholar]
- 128.Peek EJ, et al. Interleukin-10-secreting “regulatory” T cells induced by glucocorticoids and beta2-agonists. Am J Respir Cell Mol Biol. 2005;33(1):105–11. doi: 10.1165/rcmb.2005-0100OC. [DOI] [PubMed] [Google Scholar]
- 129.Quenby S, et al. Prednisolone reduces preconceptual endometrial natural killer cells in women with recurrent miscarriage. Fertil Steril. 2005;84(4):980–4. doi: 10.1016/j.fertnstert.2005.05.012. [DOI] [PubMed] [Google Scholar]
- 130.Whitley KA, Ural SH. Treatment modalities in recurrent miscarriages without diagnosis. Semin Reprod Med. 2014;32(4):319–22. doi: 10.1055/s-0034-1375185. [DOI] [PubMed] [Google Scholar]
- 131.Ober C, et al. Mononuclear-cell immunisation in prevention of recurrent miscarriages: a randomised trial. Lancet. 1999;354(9176):365–9. doi: 10.1016/S0140-6736(98)12055-X. [DOI] [PubMed] [Google Scholar]
- 132.Scott JR. Immunotherapy for recurrent miscarriage. Cochrane Database Syst Rev. 2003;1:CD000112. doi: 10.1002/14651858.CD000112. [DOI] [PubMed] [Google Scholar]
- 133.Worldwide collaborative observational study and meta-analysis on allogenic leukocyte immunotherapy for recurrent spontaneous abortion. Recurrent Miscarriage Immunotherapy Trialists Group. Am J Reprod Immunol, 1994. 32(2): p. 55–72. [PubMed]
- 134.Porter TF, Scott JR. Alloimmune causes of recurrent pregnancy loss. Semin Reprod Med. 2000;18(4):393–400. doi: 10.1055/s-2000-13729. [DOI] [PubMed] [Google Scholar]
- 135.Christiansen OB, et al. Intravenous immunoglobulin treatment for secondary recurrent miscarriage: a randomised, double-blind, placebo-controlled trial. BJOG. 2015;122(4):500–8. doi: 10.1111/1471-0528.13192. [DOI] [PubMed] [Google Scholar]
- 136.Heilmann L, Schorsch M, Hahn T. CD3-CD56 + CD16+ natural killer cells and improvement of pregnancy outcome in IVF/ICSI failure after additional IVIG-treatment. Am J Reprod Immunol. 2010;63(3):263–5. doi: 10.1111/j.1600-0897.2009.00790.x. [DOI] [PubMed] [Google Scholar]
- 137.van den Heuvel MJ, et al. Decline in number of elevated blood CD3(+) CD56(+) NKT cells in response to intravenous immunoglobulin treatment correlates with successful pregnancy. Am J Reprod Immunol. 2007;58(5):447–59. doi: 10.1111/j.1600-0897.2007.00529.x. [DOI] [PubMed] [Google Scholar]
- 138.Moffett A, Regan L, Braude P. Natural killer cells, miscarriage, and infertility. BMJ. 2004;329(7477):1283–5. doi: 10.1136/bmj.329.7477.1283. [DOI] [PMC free article] [PubMed] [Google Scholar]