Abstract
Purpose
The aim of this study is to investigate the effect of acteoside, an antioxidant, on in vitro maturation (IVM) of oocytes to improve early parthenogenetic embryonic developmental competence.
Methods
Porcine immature oocytes (total 770) were cultured in IVM medium with acteoside at various concentrations, 0 (control), 10, 30, and 50 μM. Each group was assessed for maturation and subsequent development rates, reactive oxygen species (ROS) level (15 oocytes per group and four independent experiments performed), ultrastructure observation (15 oocytes per group), mitochondrial activity (30 oocytes per groups and three independent experiments performed), and expression patterns of apoptosis-related genes (100 expended parthenogenetic embryos per group and three independent experiment performed). Main outcome measures were the rates of IVM, blastocyst formation, ROS, mitochondria, and expression of apoptosis-related genes in oocytes treated with acteoside.
Result(s)
Addition of acteoside during IVM did not change the maturation efficiency of oocytes but improved the rate of blastocyst formation with significantly decreased ROS level. Moreover, in acteoside-treated oocytes, cytoplasmic maturation was improved with morphologically uniform distribution of mitochondria and lipid droplets in cytoplasm. Acteoside supplementation also increased the mRNA expression levels of antiapoptotic genes and reduced those of pro-apoptotic genes.
Conclusion(s)
Acteoside supplementation in IVM medium improves the oocyte quality and subsequent development of pre-implantation embryos that would eventually contribute to produce embryos with high embryonic development competence.
Electronic supplementary material
The online version of this article (doi:10.1007/s10815-016-0729-x) contains supplementary material, which is available to authorized users.
Keywords: Acteoside, IVM, ROS, Mitochondria, Apoptosis-related genes
Introduction
Assisted reproductive techniques (ART) such as in vitro fertilization (IVF) and intracytoplasmic sperm injection (ICSI) have been used as important tools for increased productivity in animal agriculture and human infertility treatment. In vitro maturation (IVM) is the initial step of ART that is highly correlated to further successful pre-implantation embryo development in vitro. IVM has also been recognized as an important step for achieving complete meiosis in embryos produced in vitro. In humans, IVM has been proposed as a strategy to optimize or improve the development of healthier oocytes [1–4]. Various methods have been suggested to improve the efficiency of IVM [5–10], which actually contributed to increasing the rate of IVM. However, the developmental competence of oocytes matured in vitro is still known to be unstable; the cytoplasmic maturation was frequently found to be incomplete even after treatment with various chemical supplementations or substances such as hormones, vitamins, and cytokines [11–13].
Reactive oxygen species (ROS) such as superoxide anions (O2−), hydroxyl radicals (OH−), and hydrogen peroxide (H2O2) are produced during regular metabolism of oxygen, and play important roles in cell signaling [14]. However, excessive ROS can induce lipid peroxidation and enzyme inactivation by hydroxyl radical formation, finally resulting in cell apoptosis [15, 16]. In the case of oocytes and embryos, endogenous ROS are produced by metabolic pathways and enzymes [17]. Overproduction of ROS in oocytes during IVM causes lipid peroxidation [18] and DNA fragmentation, which affect both RNA transcription and protein synthesis [19]. It has been reported that antioxidant supplementation of the IVM medium improves the oocyte quality and embryo developmental efficiency by enhancing nuclear and cytoplasmic maturation through regulation of ROS production and gene expression [20–23].
Acteoside (verbascoside) is a typical phenylethanoid glycoside, which is extracted from various plants including Syringa vulgaris. Acteoside has been known to have various biological functions including antioxidative, antiinflammatory, and antihypertensive activities and has been used pharmaceutically [24–27]. It has been well known as an especially powerful antioxidant that acts either by directly scavenging the ROS and nitrogen species or by acting as a chain-breaking peroxyl radical scavenger [28]. However, to date, little is known about the effect of acteoside on oocytes during in vitro maturation and pre-implantation embryo development.
Mitochondria generate cellular energy to support metabolism in oocytes [29, 30] via the production of ATP by oxidative phosphorylation [31, 32]. They are involved in apoptosis [33], calcium signaling [34], ROS generation, and production of intermediary metabolites [35]. Mitochondria also play an important role in oocyte maturation and subsequent embryonic development. The intracellular distribution of mitochondria and their metabolic activity undergo substantial changes during oocyte maturation and embryonic development in many species [36–40]. For example, in a previous study, the enhancement of mitochondrial activity and lipid β-oxidation improved the oocyte developmental competence in mouse [41]. However, the relationship between mitochondria and ROS was not fully understood during oocyte maturation.
The present study aimed to investigate the effect of acteoside on oocyte maturation and embryo development, particularly in relation to mitochondrial distribution and apoptosis. Porcine IVM medium was supplemented with acteoside, and then mitochondrial distribution and expression of apoptosis-related genes were observed.
Materials and methods
Chemicals and reagents
Acteoside was purchased from Chengdu Biopurify Phytochemicals Ltd. (China). Other chemicals and reagents used in this study were purchased from Sigma-Aldrich Co. (USA), unless otherwise stated.
The basic medium used for oocyte IVM was tissue culture medium-199 (TCM-199) supplemented with 2.5 mM fructose, 0.4 mM L-cysteine, 1 mM sodium pyruvate, 0.13 mM kanamycin, 10 % (v/v) porcine follicular fluid, 10.0 ng/mL epidermal growth factor (EGF), and 500 IU/mL gonadotropin hormone.
The medium used for in vitro culture was porcine zygote medium-3 (PZM-3) consisting of 108.0 mM NaCl, 0.4 mM MgSO4°7H2O, 10.0 mM KCl, 0.35 mM KH2PO4, 2 % basal medium Eagle essential amino acids, 1 % minimum essential medium non-essential amino acids, 5.0 mM hypotaurine, 25.0 mM NaHCO3, 1.0 mM L-glutamine, 0.2 mM sodium-pyruvate, 2.0 mM Ca(lactate)2°5H2O, 0.3 % bovine serum albumin (BSA), and 0.13 mM kanamycin (pH 7.3 and 285 Osm).
Oocyte collection and in vitro maturation
Porcine ovaries were collected from a local slaughterhouse and transported to the laboratory in physiological saline containing 1 % penicillin/streptomycin (approximately 25 °C) within 3 h. The ovaries were washed three times with physiological saline. Antral follicles of 3–8 mm diameter were aspirated using an 18-gauge needle attached to a 10 mL disposable syringe. The aspirated follicular fluid was precipitated in a 37 °C water bath. The sediments were washed twice with physiological saline. Compact cumulus-oocyte complexes (COCs) surrounded by at least three layers of cumulus cells were selected. The selected COCs were washed with IVM medium. After washing, approximately 50 COCs per well were cultured in maturation medium supplemented with 0, 10, 30, and 50 μM of acteoside for 22 h at 39 °C in 5 % CO2 in air. They were then transferred into the IVM medium without hormones, and cultured for an additional 22 h under the same conditions. After IVM for 44 h, cumulus cells were removed by repeated gentle pipetting in 0.1 % hyaluronidase. For calculating the rate of maturation, oocytes that displayed the first polar body were considered in the nuclear maturation stage metaphase II (MII).
Measurement of ROS level
After IVM, a total of 60 oocytes were collected to measure ROS level (repeated four times). The ROS level in each oocyte was measured by Image-iT™ LIVE Green ROS detection kit (Invitrogen, USA). Briefly, mature oocytes were washed in Dulbecco’s physiological buffered saline (DPBS) supplemented with 0.10 g/L CaCl2 and 0.10 g/L MgCl2·6H2O. After washing, oocytes were transferred into PZM-3 containing 100 μM tert-butyl hydroperoxide at 39 °C in 5 % CO2 in air. After 60–90 min of incubation, oocytes were transferred into DPBS containing 25 μM 5-(and 6)-carboxy-2′,7′-dichlorodihydrofluorescein diacetate (carboxy-H2DCFDA) and were incubated for 30 min at 39 °C in 5 % CO2 in air, protected from light. Oocytes were then washed in DPBS. The fluorescent emissions from the oocytes were recorded using the camera attached to a fluorescence microscope with filters at 495 nm for excitation and at 529 nm for emission. The recorded fluorescent images were analyzed for intensity using NIS-Elements BRsoftware 3.00 (Nikon, Japan).
Parthenogenetic activation and in vitro culture
The electrical activation of the oocytes was executed according to the general method described in previous reports [7]. Briefly, denuded oocytes were allocated and equilibrated in electrical activation medium containing 0.3 M mannitol, 0.05 % BSA, 0.1 mM MgSO4, and 0.1 mM CaCl2. Equilibrated oocytes were placed between two electrodes overlaid with electrical activation medium; a direct-current (DC) pulse of 1.8 kV/cm for 30 μs was applied for single pulse, using a BTX electro-Cell Manipulator 2001. After activation by a single DC pulse, oocytes were washed thrice in PZM-3, and then cultured in PZM-3 containing 1.9 mM N-6-dimethylaminopurine (6-DMAP) for 3 h. The activated oocytes were again washed three times in PZM-3. Fifteen to 20 embryos were cultured in 20 μL of PZM-3 medium droplets overlaid with mineral oil in a 35 × 9 mm Petri dish for 7 days at 39 °C in 5 % CO2 in air. The cleavage and development status were evaluated at 2 and 7 days after electrical induction of parthenogenesis.
Oocyte ultrastructure observation by transmission electron microscopy
The ultrastructure of oocytes in maturation process was observed by transmission electron microscopy (TEM). Briefly, immature (0 h), maturing (22 h), and matured (44 h) oocytes were collected (15 oocytes per each group). For ultrastructural observation, oocytes were fixed with 2 % glutaraldehyde in PBS for 2 h at 4 °C. Fixed oocytes were washed five times with 0.1 M cacodylate buffer containing 0.1 % CaCl2 at 4 °C. Oocytes were then post-fixed with 1 % OsO4 in 0.1 M cacodylate buffer (pH 7.2) containing 0.1 % CaCl2 for 1 h at 4 °C. After rinsing with cold distilled water, oocytes were dehydrated slowly with ethanol immersion series and propylene oxide (50, 75, and 100 % for 5 min at 4 °C). The samples were embedded in EMbed-812 (EMS, USA). After polymerization of the resin at 60 °C for 36 h, serial sections (thickness ranging from 80 to 100 nm) were obtained from the mid part of oocytes with a diamond knife on an LEICA EM UC6 ultramicrotome (Leica, Austria) and mounted on formvar-coated slot grids. Sections were stained with 4 % uranyl acetate and lead citrate and examined under a Tecnai G2 Spirit Twin transmission electron microscope (FEI Company, USA) and a JEM ARM 1300S high-voltage electron microscope (JEOL, Japan). The number of total and swollen mitochondria was determined from the TEM images of 5 sections of 15 oocytes from each group. Counting the number of mitochondria was performed at random areas of each section as representative samples.
Evaluation of mitochondrial distribution
For evaluation of mitochondrial distribution, 30 oocytes were stained with MitoTracker Red TM (Invitrogen, USA) according to the manufacturer’s instructions. A stock solution of MitoTracker Red was dissolved in dimethyl sulfoxide (DMSO) to a final concentration of 1 mM and stored at −20 °C. After IVM, mature oocytes were incubated for 45 min in PZM-3 containing 100 nM of MitoTracker Red TM at 39 °C in 5 % CO2 in air. Following staining, oocytes were washed twice with PBS for 10 min, and then observed under a laser scanning confocal microscope (Carl Zeiss, Germany). Images were captured using Zeiss LSM5 Live Release ver. 4.2. and analyzed using SP1 Image Browser software (Carl Zeiss, Germany).
RT-PCR
After culturing for 7 days following electrical parthenogenetic activation, expanded blastocysts from each group were collected and stored at −80 °C for further analysis. Total RNA was extracted from 100 blastocysts using TRIzol reagent (Invitrogen, USA) according to the manufacturer’s instructions. RNA samples were primed with oligo dT primer to generate complementary DNA (cDNA) using iScript reverse transcriptase (BIO-RAD Laboratories, Hercules, CA, USA). Specific sequences of anti- and pro-apoptotic genes were prepared for reverse transcription polymerase chain reaction (RT-PCR). Primers are listed in Table 1. The total reaction mixture in 20 μL volume consisted of 10 μL premix Taq™ (Takara, Japan), 1 μL each of forward and reverse primers (10 mol/L), 2 μL of cDNA, and 6 μL double-distilled water. RT-PCR consisted of 3 min denaturation at 94 °C, followed by 45 cycles of denaturation at 94 °C, annealing at 58 °C, and elongation at 72 °C for 30 s, respectively, and the final extension at 72 °C for 5 min. The PCR products were separated on 2 % agarose gels and visualized by ethidium bromide staining. The intensity of individual bands was semi-quantitatively analyzed using Image/J® software 1.43u (National Institutes of Health, USA), and the relative expression levels of each gene were obtained by normalization using the expression of GAPDH (Table 2).
Table 1.
Primer sequences used for reverse-transcription polymerase chain reaction
| Gene | Primer sequences | Product size (bp) |
|---|---|---|
| MCL-1 | F TTGCTGGAGTAGGAGCTGGT | 197 |
| R TCCTCTTGCCACTTGCTTTT | ||
| BCL-2 | F GCGATGACTTCTCTCGTCGCT | 350 |
| R ACAGCCAGGAGAAATCAAATAG | ||
| BCL-XL | F ACAGCGTATCAGAGCTTTGAGCA | 296 |
| R CGTCAGGAACCATCGGTTGAAG | ||
| BAX | F AAGCGCATTGGAGATGAACT | 183 |
| R GGCCTTGAGCACCAGTTTAC | ||
| BAK | F CTGCCCCTAGAACCTAGCAG | 185 |
| R TTGATGCCACTCTCGAACAG | ||
| GAPDH | F TTCCACGGCACAGTCAAGGC | 151 |
| R CATGGTCGTGAAGACACCAG |
Table 2.
Effects of acteoside supplementation of IVM medium on porcine oocyte maturation in vitro
| Acteoside (μM) | No. of GV oocytes | No. of MII oocytes | Maturation rate (% ± SD)NS |
|---|---|---|---|
| 0 (control) | 193 | 137 | 71.13 ± 5.5 |
| 10 | 193 | 147 | 75.96 ± 3.1 |
| 30 | 192 | 140 | 72.95 ± 3.6 |
| 50 | 192 | 142 | 73.68 ± 4.7 |
Maturation rate: the number of MII/ the number of GV oocytes × 100. Experiments were repeated five times independently
NS not significant
Statistical analysis
All data were analyzed using one way-ANOVA software program (SPSS Statistics Inc., ver. 18.0.0). A P value < 0.05 was considered statistically significant.
Results
Effect of acteoside on nuclear maturation during IVM
Acteoside is known to act in a concentration-dependent manner and displayed apparent activity at 30 μM concentration [42]. To investigate the effect of acteoside on nuclear maturation of porcine parthenogenetic oocytes, acteoside was added to the IVM medium at concentrations of 10, 30, and 50 μM. Supplementation of IVM medium with 10, 30, or 50 μM acteoside did not improve the rate of recovery of MII stage oocytes in comparison with that of the oocytes without acteoside treatment (0 μM). There was also no statistically significant difference in nuclear maturation rate among the groups exposed to the three different concentrations of acteoside (P < 0.05).
Acteoside supplementation during IVM affects the pre-implantation embryo development
After establishing that acteoside supplementation of IVM medium did not alter the rate of nuclear maturation of porcine oocytes, developmental ability of acteoside-treated oocytes subjected to electrical parthenogenetic activation was examined (Table 3). The cleavage rate of embryos did not differ significantly between the control group (89.89 %) and the groups supplemented with 10, 30, and 50 μM acteoside (95.70, 96.04, and 88.38 %). However, acteoside supplementation at 10 μM concentration significantly increased the rate of blastocyst formation compared to that in the control group (40.03 vs. 22.95 %) (P < 0.05).
Table 3.
In vitro development of parthenogenetic embryos after IVM in the presence of acteoside
| Acteoside (μM) | No. of examined oocytes | No. of embryos (% ± SD) | |
|---|---|---|---|
| 2-cell | Blastocyst | ||
| 0 (control) | 118 | 108 (89.89 ± 8.0) | 24 (22.95 ± 12.8a) |
| 10 | 127 | 121 (95.70 ± 5.8) | 48 (40.03 ± 11.5b) |
| 30 | 126 | 121 (96.04 ± 4.4) | 44 (35.76 ± 12.5a, b) |
| 50 | 129 | 115 (88.38 ± 8.4) | 33 (24.72 ± 12.6a) |
Experiments were repeated six times independently
a, bValues with different superscripts are significantly different (P < 0.05)
Effect of acteoside on ROS levels in mature oocytes
The ROS level in treated oocytes was examined to determine whether cytoplasmic competency was improved by acteoside supplementation during IVM. Varying levels of ROS were detected in most oocytes matured in vitro in the absence of acteoside (Fig. 1a). However, ROS was rarely detected in oocytes cultured in IVM medium supplemented with acteoside regardless of the concentration (Fig. 1b–d). The relative rate of ROS generation was also significantly lower in oocytes, which were matured in IVM medium supplemented with acteoside, compared to those matured without acteoside (P < 0.05) (Fig. 1e).
Fig. 1.
Acteoside decreased the level of ROS in porcine oocytes matured in vitro with acteoside supplementation. The relative ROS production was detected in oocytes treated with a 0 μM (control), b 10 μM, c 30 μM, and d 50 μM acteoside by fluorescence microscopy after 44 h IVM (60 oocytes per group). e The relative intensity of fluorescence was measured using NIS-Elements BRsoftware 3.00. Different superscripts above each bar represent significant difference (P < 0.05). The data shown represent the mean ± standard error of results from four independent experiments
Intracellular localization of mitochondria in oocytes after IVM in the presence of acteoside
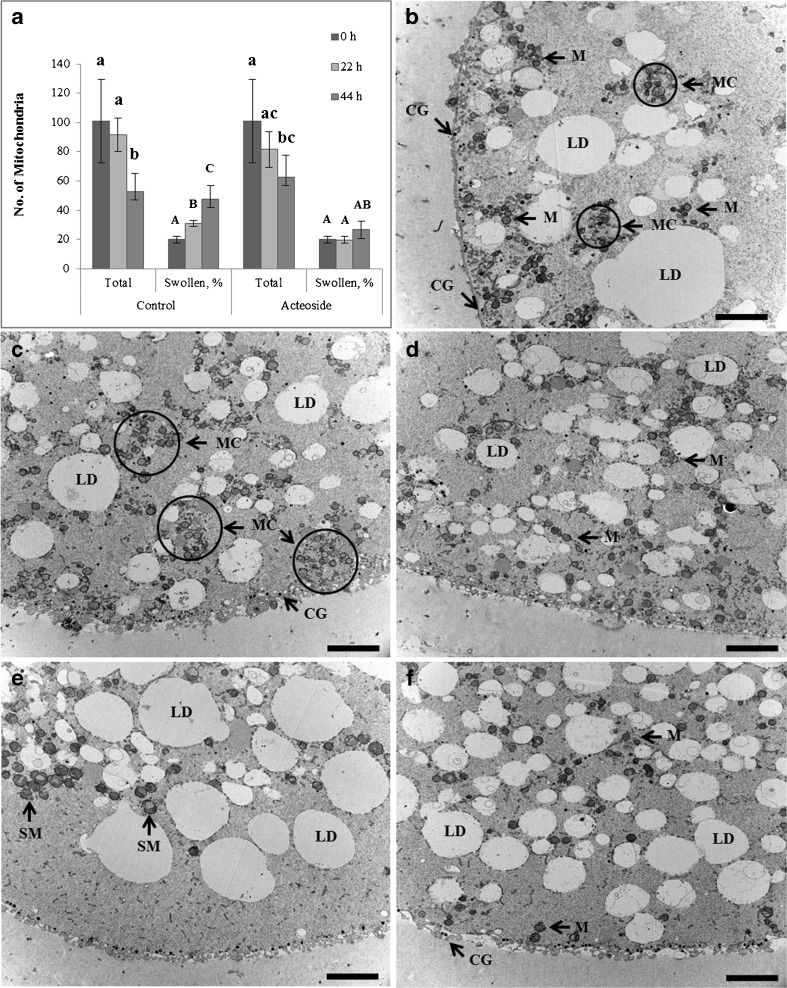
To estimate cytoplasmic maturation level of mature oocytes after acteoside treatment, the intracellular localization of mitochondria was analyzed by labeling with a mildly thiol-reactive chloromethyl moiety. The total number of active mitochondria and the number of swollen mitochondria were counted in oocytes matured in the presence of 10 μM acteoside at 0, 22, and 44 h during IVM (Fig. 2a). The total number of mitochondria was not affected by acteoside treatment, but decreased over time both with and without acteoside treatment (Fig. 2a). However, acteoside treatment delayed the decrease in the total number of mitochondria. The number of swollen mitochondria was significantly higher in mature oocytes (22 and 44 h) without acteoside compared to immature oocytes (0 h), but was not different in oocytes with acteoside (P < 0.05) (Fig. 2a). In oocytes matured without acteoside treatment, active mitochondria were mainly localized in the peripheral regions of oocytes (Supplement Fig 1a). In oocytes matured in the presence of acteoside, mitochondria were distributed evenly within the oocytes (Supplement Fig 1b).
Fig. 2.
Acteoside altered the distribution of cellular organelles of porcine oocytes during IVM. Oocytes were cultured for 0 (immature), 22 (maturing), and 44 h (mature) in IVM medium with or without 10 μM acteoside (15 oocytes from each group were used for TEM). a The total number of mitochondria and the percentage of abnormally swollen mitochondria at 0, 22, and 44 h maturation, b immature oocyte (at 0 h and no acteoside), c maturing oocyte (at 22 h and without acteoside), d maturing oocyte (at 22 h and with 10 μM acteoside), e matured oocyte (at 44 h and without acteoside), and f matured oocyte (at 44 h and with 10 μM acteoside). CG cortical granules, LD lipid droplets, M mitochondria, MC mitochondrial clusters, SM swollen mitochondria. Bar = 5 μm. Different superscripts above each bar represent significant difference (P < 0.05). The data shown represent the mean ± standard error
Acteoside supplementation induces rearrangement of organelles in mature oocytes
To check whether the ultrastructure of oocytes was affected by acteoside, transmission electron microscopy (TEM) was performed on immature (0 h of maturation; hm), maturing (22 hm), and mature oocytes (44 hm). Immature oocytes at 0 hm were characterized by peripherally located mitochondrial clusters (MC) along with cortical granules (CG) and conspicuously large cytoplasmic lipid droplets (LD) (Fig. 2b). In control maturing oocytes at 22 hm, MC, CG, and LD heterogeneous in their size were observed in the sub-oolemmal cortex (Fig. 2c). However, mitochondria and CG were evenly distributed within the oocyte, and the size of LD was decreased in acteoside-treated oocytes at 22 hm (Fig. 2d). Finally, the organelle rearrangement in fully matured oocytes was observed at 44 hm. In oocytes fully matured for 44 h in the presence of acteoside, mitochondrial distribution in the ooplasm became more even, and a delimited region with well-developed smooth endoplasmic reticulum was observed with lipid droplets that were relatively homogenous and smaller (Fig. 2f) compared to those in the control oocytes (Fig. 2e). CG localized to the marginal region under the oolemma in both the control and acteoside-treated groups (Fig. 2e, f). In addition, hooded swollen mitochondria (SM) were arranged in large groups and associated with lipid droplets at the periphery in control matured oocytes.
Apoptosis-related gene expression in blastocysts after IVM with acteoside
Blastocysts derived from parthenogenetically activated oocytes matured in the presence of acteoside were examined by RT-PCR, to investigate the expression patterns of apoptosis-related genes (Fig. 3a). The relative mRNA levels of MCL-1, BCL-2, and BCL-XL (antiapoptotic genes) were significantly higher in 10 μM acteoside-treated blastocysts than non-treated blastocysts (P < 0.05). However, BAX and BAK (pro-apoptotic genes) were expressed at significantly higher levels in non-treated blastocysts than in 10 μM acteoside-treated blastocysts (P < 0.05) (Fig. 3b).
Fig. 3.
Expression of anti- and pro-apoptotic mRNAs was affected by acteoside in parthenogenetic blastocysts. a The result of RT-PCR was visualized by ethidium bromide staining after agarose gel electrophoresis. b The relative expression levels of genes were semi-quantitatively analyzed using Image/J® software 1.43u, and normalized to the expression of GAPDH. Control: parthenogenetic blastocysts produced from oocytes cultured in IVM medium without acteoside supplementation. Acteoside: parthenogenetic blastocysts produced from oocytes cultured in IVM medium with 10 μM acteoside. Different superscripts above each bar represent significant difference (P < 0.05). The data shown represent the mean ± standard error of three independent experiments
Discussion
Oocytes from animals and humans have been used widely to study embryo development, and to develop methods for producing healthy embryos in vitro [43]. For in vitro embryo production, IVM of oocytes is the first crucial determinant step in the successful sequential embryonic development. In humans, IVM has been especially used for women who need urgent oocyte preservation prior to anticancer treatment or who are in need of the conventional IVF. However, there are many differences between in vivo and in vitro environments that differently affect oocyte maturation. For instance, the quality of oocytes matured in vitro is significantly lower than that of oocytes matured in vivo [44]. Despite many efforts to delineate the mechanism of IVM, much of the IVM mechanism and the factors affecting IVM remain unknown.
In this study, acteoside, which has been known to have an effective antioxidative activity, was investigated in the experimental model of porcine oocytes to determine whether supplementation of the IVM medium with acteoside enhances the efficiency of oocyte maturation in vitro. Acteoside supplementation of IVM medium had no significant effect on IVM efficiency. However, the rate of blastocyst formation was increased, and it improved embryonic developmental competence in parthenogenetic embryos. The data indicated that acteoside supplementation of the oocyte maturation medium has a positive effect on in vitro development of parthenogenetic embryos. This finding implies that IVM is a critical initial event for the further successful development of parthenogenetic embryos.
ROS are produced in vivo under physiological conditions and stimulate cell growth and proliferation. However, excessive ROS induce oxidative stress and cellular injury (e.g., damage to DNA, lipid membranes, and proteins) [19]. Unlike the situation in vivo, ROS concentration has been reported to be higher in oocytes cultured in vitro compared to the in vivo ROS levels. ROS negatively affect oocyte quality, and limit the in vitro fertilization and embryonic developmental competence [45]. For example, excessive ROS cause peroxidation of the cell membrane lipids during oocyte maturation and embryonic development [18, 46]. Previous studies reported that the supplementation of IVM medium with antioxidants rescued oocytes and embryos from cellular injury by reducing ROS [47, 48]. Parthenogenetic embryos have widely used in scientific researches and medical sciences related to ART as somatic cell nuclear transfer to clone animals and to generate transgenic animals. Moreover, pluripotent stem cells have been tried to generate from parthenogenetic embryos to avoid ethical problem. In the present study, acteoside was used to treat immature porcine oocytes during IVM prior to parthenogenesis. Acteoside enhanced parthenogenetic embryo developmental competence by decreasing intracellular ROS levels. It is inferred that acteoside acted as a powerful antioxidant by scavenging ROS, which in turn improved the maturation of oocytes in vitro.
Mitochondria contain their own genomic DNA (mtDNA), which is maternally inherited [37]. These organelles play various essential roles in cellular functions by supplying energy, regulating apoptosis, and Ca2+ homeostasis [37, 49]. Mitochondria are an important determinant of the developmental competence of oocytes and embryos owing to their central role in cellular metabolism [50]. However, little is known about the mitochondrial morphology and activity in oocytes regarding ROS. During meiotic maturation, re-localization of mitochondria and lipid droplets occurs in oocytes. This redistribution, which is important for oocyte maturation and subsequent embryo development, might be in response to energy demands of oocytes during a given stage of development [49]. In this study, mitochondria in oocytes were observed and counted at random areas as representative samples. Mitochondria were found to be irregularly distributed and accumulated in the peripheral regions in oocytes during maturation. However, acteoside supplementation induced homogenous distribution of mitochondria with reduction of ROS level in oocytes matured in vitro. Moreover, the number of abnormal, swollen mitochondria decreased with the addition of acteoside to IVM medium. This implies that maintaining the number of normal mitochondria and re-localization of mitochondria from the periphery to a more uniform distribution positively affect the cytoplasmic maturation of oocytes and support subsequent embryonic development by more efficiently supplying energy essential for the formation of blastocysts. Similarly, it had been reported that the re-organization of mitochondria is correlated with increased ATP content in bovine oocytes during IVM [51]. In addition, it is possible that clusters of swollen mitochondria, which were observed in oocytes matured in vitro without acteoside supplementation, are likely to cause cytoplasmic damage by oxidative stress [37, 52, 53].
Apoptosis is the process of programmed cell death triggered by extrinsic and intrinsic stimuli/responses, such as the perforin/granzyme pathway, that leads to cellular changes like cell blebbing, cell shrinking, DNA fragmentation, chromatin condensation, and consequent phagocytosis [54]. Apoptosis is strongly involved in both oocyte maturation and pre-implantation embryo development. The expression of apoptosis-related genes such as p53, BAD, BCL-2, and other BCL family genes, is known to regulate apoptosis during pre-implantation embryo development in murine and human systems [55–57]. In the present study, acteoside treatment during IVM decreased the expression of BAK and BAX in parthenogenetic blastocysts. On the other hand, the expression of antiapoptotic genes MCL-1, BCL-2, and BCL-XL was increased. This result implied that acteoside would be related to apoptosis in oocytes during early embryo development.
In conclusion, the acteoside supplement as an antioxidant in IVM medium supports pre-implantation developmental competence of parthenogenetic embryos by reducing ROS, improving oocyte cytoplasmic conditions, increasing expression of antiapoptotic genes, and decreasing expression of pro-apoptotic genes. Therefore, acteoside, an antioxidant is a supplement that has the potential to improve oocyte quality during IVM and subsequent embryo development competence in vitro. This finding would contribute to the understanding of the involvement of mitochondria and ROS during the maturation of oocytes, which in turn will help improve the efficacy of IVM to achieve successful IVF.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Active mitochondrial distribution in porcine oocytes matured in vitro with a 0 μM (control) or b 10 μM acteoside. Oocytes were stained with MitoTracker Red FM. Bar = 20 μm (30 oocytes per groups were used, and three independent experiments were performed) (GIF 115 kb)
Acknowledgments
This study was supported by a grant from the Next-Generation BioGreen 21 program (Grant No. PJ011359) funded by the Rural Development Administration, Republic of Korea. We are thankful to Dr. John Hammond in USDA-ARS for his scientific comments and writing support on the manuscript.
Footnotes
Capsule Acteoside supplementation in IVM medium improves the oocyte quality and subsequent development of pre-implantation embryos that would eventually contribute to produce embryos with high embryonic development competence.
Keun Jung Kim and Ju Lan Chun contributed equally to this work.
References
- 1.Wei D, Zhang C, Xie J, Song X, Yin B, Liu Q, et al. Supplementation with low concentrations of melatonin improves nuclear maturation of human oocytes in vitro. J Assist Reprod Genet. 2013;30(7):933–8. doi: 10.1007/s10815-013-0021-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Machtinger R, Combelles CM, Missmer SA, Correia KF, Williams P, Hauser R, et al. Bisphenol-A and human oocyte maturation in vitro. Hum Reprod. 2013;28(10):2735–45. doi: 10.1093/humrep/det312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gomez E, Tarin JJ, Pellicer A. Oocyte maturation in humans: the role of gonadotropins and growth factors. Fertil Steril. 1993;60(1):40–6. doi: 10.1016/S0015-0282(16)56033-6. [DOI] [PubMed] [Google Scholar]
- 4.Alak BM, Coskun S, Friedman CI, Kennard EA, Kim MH, Seifer DB. Activin A stimulates meiotic maturation of human oocytes and modulates granulosa cell steroidogenesis in vitro. Fertil Steril. 1998;70(6):1126–30. doi: 10.1016/S0015-0282(98)00386-0. [DOI] [PubMed] [Google Scholar]
- 5.Krisher RL. The effect of oocyte quality on development. J Anim Sci. 2004;82(E-Suppl):E14–23. doi: 10.2527/2004.8213_supplE14x. [DOI] [PubMed] [Google Scholar]
- 6.Jimenez-Macedo AR, Izquierdo D, Urdaneta A, Anguita B, Paramio MT. Effect of roscovitine on nuclear maturation, MPF and MAP kinase activity and embryo development of prepubertal goat oocytes. Theriogenology. 2006;65(9):1769–82. doi: 10.1016/j.theriogenology.2005.10.012. [DOI] [PubMed] [Google Scholar]
- 7.Cheng WM, Sun XL, An L, Zhu SE, Li XH, Li Y, et al. Effect of different parthenogenetic activation methods on the developmental competence of in vitro matured porcine oocytes. Anim Biotechnol. 2007;18(2):131–41. doi: 10.1080/10495390601096148. [DOI] [PubMed] [Google Scholar]
- 8.Chappaz E, Albornoz MS, Campos D, Che L, Palin MF, Murphy BD, et al. Adiponectin enhances in vitro development of swine embryos. Domest Anim Endocrinol. 2008;35(2):198–207. doi: 10.1016/j.domaniend.2008.05.007. [DOI] [PubMed] [Google Scholar]
- 9.Kim HS, Jeong YI, Lee JY, Jeong YW, Hossein MS, Hyun HS, et al. Effects of recombinant relaxin on in vitro maturation of porcine oocytes. J Vet Med Sci. 2010;72(3):333–7. doi: 10.1292/jvms.09-0411. [DOI] [PubMed] [Google Scholar]
- 10.Yuan Y, Krisher RL. Effect of ammonium during in vitro maturation on oocyte nuclear maturation and subsequent embryonic development in pigs. Anim Reprod Sci. 2010;117(3–4):302–7. doi: 10.1016/j.anireprosci.2009.05.012. [DOI] [PubMed] [Google Scholar]
- 11.Kim JS, Song BS, Lee SR, Yoon SB, Huh JW, Kim SU, et al. Supplementation with estradiol-17beta improves porcine oocyte maturation and subsequent embryo development. Fertil Steril. 2011;95(8):2582–4. doi: 10.1016/j.fertnstert.2011.03.013. [DOI] [PubMed] [Google Scholar]
- 12.Tareq KM, Akter QS, Khandoker MA, Tsujii H. Selenium and vitamin E improve the in vitro maturation, fertilization and culture to blastocyst of porcine oocytes. J Reprod Dev. 2012;58(6):621–8. doi: 10.1262/jrd.2012-064. [DOI] [PubMed] [Google Scholar]
- 13.Jeong YW, Hossein MS, Bhandari DP, Kim YW, Kim JH, Park SW, et al. Effects of insulin-transferrin-selenium in defined and porcine follicular fluid supplemented IVM media on porcine IVF and SCNT embryo production. Anim Reprod Sci. 2008;106(1–2):13–24. doi: 10.1016/j.anireprosci.2007.03.021. [DOI] [PubMed] [Google Scholar]
- 14.Devasagayam TP, Tilak JC, Boloor KK, Sane KS, Ghaskadbi SS, Lele RD. Free radicals and antioxidants in human health: current status and future prospects. J Assoc Phys India. 2004;52:794–804. [PubMed] [Google Scholar]
- 15.Ribarov SR, Benov LC. Relationship between the hemolytic action of heavy metals and lipid peroxidation. Biochim Biophys Acta. 1981;640(3):721–6. doi: 10.1016/0005-2736(81)90102-4. [DOI] [PubMed] [Google Scholar]
- 16.Evans MD, Dizdaroglu M, Cooke MS. Oxidative DNA damage and disease: induction, repair and significance. Mutat Res. 2004;567(1):1–61. doi: 10.1016/j.mrrev.2003.11.001. [DOI] [PubMed] [Google Scholar]
- 17.Guerin P, El Mouatassim S, Menezo Y. Oxidative stress and protection against reactive oxygen species in the pre-implantation embryo and its surroundings. Hum Reprod Update. 2001;7(2):175–89. doi: 10.1093/humupd/7.2.175. [DOI] [PubMed] [Google Scholar]
- 18.Nasr-Esfahani MH, Aitken JR, Johnson MH. Hydrogen peroxide levels in mouse oocytes and early cleavage stage embryos developed in vitro or in vivo. Development. 1990;109(2):501–7. doi: 10.1242/dev.109.2.501. [DOI] [PubMed] [Google Scholar]
- 19.Agarwal A, Aponte-Mellado A, Premkumar BJ, Shaman A, Gupta S. The effects of oxidative stress on female reproduction: a review. Reprod Biol Endocrinol. 2012;10:49. doi: 10.1186/1477-7827-10-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Watanabe H, Okawara S, Bhuiyan M, Fukui Y. Effect of lycopene on cytoplasmic maturation of porcine oocytes in vitro. Reprod Domest Anim = Zuchthygiene. 2010;45(5):838–45. doi: 10.1111/j.1439-0531.2009.01365.x. [DOI] [PubMed] [Google Scholar]
- 21.Kang JT, Koo OJ, Kwon DK, Park HJ, Jang G, Kang SK, et al. Effects of melatonin on in vitro maturation of porcine oocyte and expression of melatonin receptor RNA in cumulus and granulosa cells. J Pineal Res. 2009;46(1):22–8. doi: 10.1111/j.1600-079X.2008.00602.x. [DOI] [PubMed] [Google Scholar]
- 22.Tao Y, Chen H, Tian NN, Huo DT, Li G, Zhang YH, et al. Effects of L-ascorbic acid, alpha-tocopherol and co-culture on in vitro developmental potential of porcine cumulus cells free oocytes. Reprod Domest Anim = Zuchthygiene. 2010;45(1):19–25. doi: 10.1111/j.1439-0531.2008.01129.x. [DOI] [PubMed] [Google Scholar]
- 23.Lee K, Wang C, Chaille JM, Machaty Z. Effect of resveratrol on the development of porcine embryos produced in vitro. J Reprod Dev. 2010;56(3):330–5. doi: 10.1262/jrd.09-174K. [DOI] [PubMed] [Google Scholar]
- 24.Murai M, Tamayama Y, Nishibe S. Phenylethanoids in the herb of Plantago lanceolata and inhibitory effect on arachidonic acid-induced mouse ear edema. Planta Med. 1995;61(5):479–80. doi: 10.1055/s-2006-958143. [DOI] [PubMed] [Google Scholar]
- 25.Xiong Q, Kadota S, Tani T, Namba T. Antioxidative effects of phenylethanoids from Cistanche deserticola. Biol Pharm Bull. 1996;19(12):1580–5. doi: 10.1248/bpb.19.1580. [DOI] [PubMed] [Google Scholar]
- 26.Kyriakopoulou I, Magiatis P, Skaltsounis AL, Aligiannis N, Harvala C. Samioside, a new phenylethanoid glycoside with free-radical scavenging and antimicrobial activities from Phlomis samia. J Nat Prod. 2001;64(8):1095–7. doi: 10.1021/np010128+. [DOI] [PubMed] [Google Scholar]
- 27.Saimaru H, Orihara Y. Biosynthesis of acteoside in cultured cells of Olea europaea. J Nat Med. 2010;64(2):139–45. doi: 10.1007/s11418-009-0383-z. [DOI] [PubMed] [Google Scholar]
- 28.Afanas’ev IB. Superoxide and nitric oxide in pathological conditions associated with iron overload: the effects of antioxidants and chelators. Curr Med Chem. 2005;12(23):2731–9. doi: 10.2174/092986705774462941. [DOI] [PubMed] [Google Scholar]
- 29.Brevini TA, Vassena R, Francisci C, Gandolfi F. Role of adenosine triphosphate, active mitochondria, and microtubules in the acquisition of developmental competence of parthenogenetically activated pig oocytes. Biol Reprod. 2005;72(5):1218–23. doi: 10.1095/biolreprod.104.038141. [DOI] [PubMed] [Google Scholar]
- 30.Liu L, Hammar K, Smith PJ, Inoue S, Keefe DL. Mitochondrial modulation of calcium signaling at the initiation of development. Cell Calcium. 2001;30(6):423–33. doi: 10.1054/ceca.2001.0251. [DOI] [PubMed] [Google Scholar]
- 31.Brookes PS, Levonen AL, Shiva S, Sarti P, Darley-Usmar VM. Mitochondria: regulators of signal transduction by reactive oxygen and nitrogen species. Free Radic Biol Med. 2002;33(6):755–64. doi: 10.1016/S0891-5849(02)00901-2. [DOI] [PubMed] [Google Scholar]
- 32.Hao ZD, Liu S, Wu Y, Wan PC, Cui MS, Chen H, et al. Abnormal changes in mitochondria, lipid droplets, ATP and glutathione content, and Ca(2+) release after electro-activation contribute to poor developmental competence of porcine oocyte during in vitro ageing. Reprod Fertil Dev. 2009;21(2):323–32. doi: 10.1071/RD08157. [DOI] [PubMed] [Google Scholar]
- 33.Torner H, Brussow KP, Alm H, Ratky J, Pohland R, Tuchscherer A, et al. Mitochondrial aggregation patterns and activity in porcine oocytes and apoptosis in surrounding cumulus cells depends on the stage of pre-ovulatory maturation. Theriogenology. 2004;61(9):1675–89. doi: 10.1016/j.theriogenology.2003.09.013. [DOI] [PubMed] [Google Scholar]
- 34.Dumollard R, Duchen M, Carroll J. The role of mitochondrial function in the oocyte and embryo. Curr Top Dev Biol. 2007;77:21–49. doi: 10.1016/S0070-2153(06)77002-8. [DOI] [PubMed] [Google Scholar]
- 35.Jansen RP, Burton GJ. Mitochondrial dysfunction in reproduction. Mitochondrion. 2004;4(5–6):577–600. doi: 10.1016/j.mito.2004.07.038. [DOI] [PubMed] [Google Scholar]
- 36.Sun QY, Wu GM, Lai L, Park KW, Cabot R, Cheong HT, et al. Translocation of active mitochondria during pig oocyte maturation, fertilization and early embryo development in vitro. Reproduction. 2001;122(1):155–63. doi: 10.1530/rep.0.1220155. [DOI] [PubMed] [Google Scholar]
- 37.Van Blerkom J. Mitochondria in human oogenesis and preimplantation embryogenesis: engines of metabolism, ionic regulation and developmental competence. Reproduction. 2004;128(3):269–80. doi: 10.1530/rep.1.00240. [DOI] [PubMed] [Google Scholar]
- 38.Katayama M, Zhong Z, Lai L, Sutovsky P, Prather RS, Schatten H. Mitochondrial distribution and microtubule organization in fertilized and cloned porcine embryos: implications for developmental potential. Dev Biol. 2006;299(1):206–20. doi: 10.1016/j.ydbio.2006.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dell’Aquila ME, Ambruosi B, De Santis T, Cho YS. Mitochondrial distribution and activity in human mature oocytes: gonadotropin-releasing hormone agonist versus antagonist for pituitary down-regulation. Fertil Steril. 2009;91(1):249–55. doi: 10.1016/j.fertnstert.2007.10.042. [DOI] [PubMed] [Google Scholar]
- 40.Liu S, Li Y, Gao X, Yan JH, Chen ZJ. Changes in the distribution of mitochondria before and after in vitro maturation of human oocytes and the effect of in vitro maturation on mitochondria distribution. Fertil Steril. 2010;93(5):1550–5. doi: 10.1016/j.fertnstert.2009.03.050. [DOI] [PubMed] [Google Scholar]
- 41.Dunning KR, Cashman K, Russell DL, Thompson JG, Norman RJ, Robker RL. Beta-oxidation is essential for mouse oocyte developmental competence and early embryo development. Biol Reprod. 2010;83(6):909–18. doi: 10.1095/biolreprod.110.084145. [DOI] [PubMed] [Google Scholar]
- 42.Lee KW, Kim HJ, Lee YS, Park HJ, Choi JW, Ha J, et al. Acteoside inhibits human promyelocytic HL-60 leukemia cell proliferation via inducing cell cycle arrest at G0/G1 phase and differentiation into monocyte. Carcinogenesis. 2007;28(9):1928–36. doi: 10.1093/carcin/bgm126. [DOI] [PubMed] [Google Scholar]
- 43.Coticchio G, Dal-Canto M, Guglielmo MC, Mignini-Renzini M, Fadini R. Human oocyte maturation in vitro. Int J Dev Biol. 2012;56(10–12):909–18. doi: 10.1387/ijdb.120135gv. [DOI] [PubMed] [Google Scholar]
- 44.Kim DH, Ko DS, Lee HC, Lee HJ, Park WI, Kim SS, et al. Comparison of maturation, fertilization, development, and gene expression of mouse oocytes grown in vitro and in vivo. J Assist Reprod Genet. 2004;21(7):233–40. doi: 10.1023/B:JARG.0000042008.83699.cc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dennery PA. Effects of oxidative stress on embryonic development. Birth Defects Res C Embryo Today. 2007;81(3):155–62. doi: 10.1002/bdrc.20098. [DOI] [PubMed] [Google Scholar]
- 46.Noda Y, Matsumoto H, Umaoka Y, Tatsumi K, Kishi J, Mori T. Involvement of superoxide radicals in the mouse two-cell block. Mol Reprod Dev. 1991;28(4):356–60. doi: 10.1002/mrd.1080280408. [DOI] [PubMed] [Google Scholar]
- 47.Kang JT, Kwon DK, Park SJ, Kim SJ, Moon JH, Koo OJ, et al. Quercetin improves the in vitro development of porcine oocytes by decreasing reactive oxygen species levels. J Vet Sci. 2013;14(1):15–20. doi: 10.4142/jvs.2013.14.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kwak SS, Cheong SA, Jeon Y, Lee E, Choi KC, Jeung EB, et al. The effects of resveratrol on porcine oocyte in vitro maturation and subsequent embryonic development after parthenogenetic activation and in vitro fertilization. Theriogenology. 2012;78(1):86–101. doi: 10.1016/j.theriogenology.2012.01.024. [DOI] [PubMed] [Google Scholar]
- 49.Ramalho-Santos J, Varum S, Amaral S, Mota PC, Sousa AP, Amaral A. Mitochondrial functionality in reproduction: from gonads and gametes to embryos and embryonic stem cells. Hum Reprod Update. 2009;15(5):553–72. doi: 10.1093/humupd/dmp016. [DOI] [PubMed] [Google Scholar]
- 50.Romek M, Gajda B, Rolka M, Smorag Z. Mitochondrial activity and morphology in developing porcine oocytes and pre-implantation non-cultured and cultured embryos. Reprod Domest Anim = Zuchthygiene. 2011;46(3):471–80. doi: 10.1111/j.1439-0531.2010.01691.x. [DOI] [PubMed] [Google Scholar]
- 51.Stojkovic M, Machado SA, Stojkovic P, Zakhartchenko V, Hutzler P, Goncalves PB, et al. Mitochondrial distribution and adenosine triphosphate content of bovine oocytes before and after in vitro maturation: correlation with morphological criteria and developmental capacity after in vitro fertilization and culture. Biol Reprod. 2001;64(3):904–9. doi: 10.1095/biolreprod64.3.904. [DOI] [PubMed] [Google Scholar]
- 52.Zhang W, Liu Y, An Z, Huang D, Qi Y, Zhang Y. Mediating effect of ROS on mtDNA damage and low ATP content induced by arsenic trioxide in mouse oocytes. Toxicol in Vitro. 2011;25(4):979–84. doi: 10.1016/j.tiv.2011.03.009. [DOI] [PubMed] [Google Scholar]
- 53.Menezo Y, Dale B, Cohen M. DNA damage and repair in human oocytes and embryos: a review. Zygote. 2010;18(4):357–65. doi: 10.1017/S0967199410000286. [DOI] [PubMed] [Google Scholar]
- 54.Elmore S. Apoptosis: a review of programmed cell death. Toxicol Pathol. 2007;35(4):495–516. doi: 10.1080/01926230701320337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Boise LH, Gonzalez-Garcia M, Postema CE, Ding L, Lindsten T, Turka LA, et al. bcl-x, a bcl-2-related gene that functions as a dominant regulator of apoptotic cell death. Cell. 1993;74(4):597–608. doi: 10.1016/0092-8674(93)90508-N. [DOI] [PubMed] [Google Scholar]
- 56.Jurisicova A, Latham KE, Casper RF, Casper RF, Varmuza SL. Expression and regulation of genes associated with cell death during murine preimplantation embryo development. Mol Reprod Dev. 1998;51(3):243–53. doi: 10.1002/(SICI)1098-2795(199811)51:3<243::AID-MRD3>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 57.Metcalfe AD, Hunter HR, Bloor DJ, Lieberman BA, Picton HM, Leese HJ, et al. Expression of 11 members of the BCL-2 family of apoptosis regulatory molecules during human preimplantation embryo development and fragmentation. Mol Reprod Dev. 2004;68(1):35–50. doi: 10.1002/mrd.20055. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Active mitochondrial distribution in porcine oocytes matured in vitro with a 0 μM (control) or b 10 μM acteoside. Oocytes were stained with MitoTracker Red FM. Bar = 20 μm (30 oocytes per groups were used, and three independent experiments were performed) (GIF 115 kb)