Abstract
Purpose
The aim of the study was to evaluate two methods of endometrial preparation for frozen-thawed single euploid blastocyst transfer: modified natural and artificial cycle with GnRH-agonist pituitary suppression.
Methods
In this prospective, controlled randomized trial, a total of 236 patients undergoing infertility treatment were randomized in 1:1 ratio; 118 received a frozen-thawed single euploid blastocyst transfer in a modified natural cycle and 118 in an artificial cycle with GnRH-agonist pituitary suppression. In the artificial protocol, GnRH-agonist combined with estradiol valerate was administered. In the natural protocol, only final oocyte maturation was induced using human chorionic gonadotropin administration. The primary end-points were the clinical pregnancy and implantation rates; the secondary end-points were the cost-benefit in terms of drug cost and the number of visits and the woman psychological distress caused by the treatment.
Results
No significant differences were found in clinical pregnancy, implantation, and miscarriage rates between protocols. The number of clinical and ultrasound controls and the number of laboratory dosages and venous samplings were similar in both study groups. No significant differences were found between the groups in the anxiety and depression values before the start of treatment, on the days of progesterone administration, the blastocyst transfer, and pregnancy test.
Conclusions
The findings of this study evidence that in case of frozen-thawed single euploid blastocyst transfer, both protocols are equally effective in terms of clinical outcomes, cost-benefit, and patient compliance. The choice of endometrial preparation protocol should be based on women menstrual and ovulatory characteristics or otherwise on patient need for cycle planning.
Trial registration
www.clinicaltrials.gov with number NCT02378584
Keywords: Blastocyst transfer, Euploid embryos, Vitrification, Endometrial preparation
Introduction
The embryo implantation is a critical step affecting the success rate of in vitro fertilization (IVF) and controlled ovarian stimulation programs. Successful embryo implantation and pregnancy depend on the transfer of a vital embryo into a molecularly receptive endometrium [1–3]. The quality of the embryo is today considered the main determinant of a successful implantation.
The choice of the best embryo for transfer becomes of fundamental importance especially when a single embryo transfer program is adopted to avoid multiple pregnancies. The embryo morphological assessment at cleavage stage is the conventional way to select the embryos to transfer into the uterus. There is today a wide scientific consensus that the microscopic appearance of an embryo is weakly correlated with its viability [4]. Moreover, it has been demonstrated that the standard embryo evaluation strategies do not reveal embryos with the incorrect number of chromosomes [5]. Thus, a variety of noninvasive methods, such as time-lapse imaging for embryo morphokinetic evaluation [6], proteomic evaluation [7], and metabolomic study [8], were proposed to assess the embryo quality.
At the same time, several studies have demonstrated that embryo aneuploidy is the most important reason of implantation failure and miscarriage, enhancing the importance of genetic embryo screening [9, 10]. Embryo chromosome aneuploidy screening by microarray comparative genomic hybridization (aCGH) at blastocyst stage has been demonstrated to be a very powerful method for selecting chromosomally healthy embryos, and its clinical effectiveness expressed in terms of pregnancy, live birth, and miscarriage rates has been highlighted in recently published papers [11, 12].
The blastocyst cryopreservation is an essential component of the preimplantation genetic screening (PGS) programs, especially when they develop on day 6 or 7 of culture and therefore out of implantation window [13–15]. Moreover, a detrimental effect on the clinical results of a fresh embryo transfer on day 6 on an advanced endometrium cannot be completely excluded. Vitrification and warming of euploid biopsied blastocysts has been demonstrated to be a very safe and efficient method for embryo cryopreservation [16, 17].
The ovarian stimulation protocols used for IVF result in high estrogen and progesterone levels that may impair endometrial receptivity [3, 14, 18–23]. In fact, many studies have suggested that frozen-thawed embryo transfer (FTET) gives a better clinical and obstetrical outcomes compared to fresh cycles [2, 3, 13, 15, 24].
To optimize embryo implantation, several protocols for endometrium preparation are available. They include natural (NC), modified natural cycle (modified-NC), or artificial cycle (AC). In natural cycle, the endometrium develops under endogenous hormonal stimulation and FTET is timed after the determination of the spontaneous luteinizing hormone (LH) surge. In modified-NC, the development of the dominant follicle and the endometrium is monitored by ultrasound and the embryo transfer is planned after ovulation induction and progesterone administration. In an AC, the estrogens and progesterone are administered to prepare the endometrium for implantation with or without gonadotropin-releasing hormone (GnRH)-agonist suppression in the luteal phase [1, 25, 26]. The main advantages of the NC and modified-NC are avoidance of multiple medications and improvement of cost benefits, although the timing of ovulation increases scheduling difficulties and cancellation rate. The AC with GnRH-agonist use offers better control over the timing of the cycle and minimizes any risk of premature ovulation but is the most intensive and expensive of the protocols.
The currently available data on morphological selected embryos suggest no difference in implantation, ongoing pregnancy, and live-birth rates, in cryopreserved-thawed cycles comparing natural and artificial protocols. Anyway, most of the studies are retrospective or small in size and not randomized controlled trials [27–30]. A recently published randomized and controlled pilot trial of natural versus hormone replacement therapy cycles in frozen embryo transfer showed similar clinical outcomes between both protocols [31].
However, until now, there are no randomized studies evaluating clinical outcomes from vitrified-thawed single euploid blastocyst transfer in different endometrium preparation protocols. This kind of procedure, in which the bias of an embryo-dependent implantation factor can be theoretically minimized, allows a more precise method to assess which is the best method to prepare endometrium and achieve the best clinical results.
Moreover, many studies have shown that procedures involved in the IVF might be a cause of patient psychological distress [32, 33]. It has been also described that ovarian suppression with the use of GnRH-agonists can cause in some patients symptoms such depression, anxiety [34], and headache [35].
In order to overcome the lack of data, we conducted a controlled randomized study comparing modified-NC and AC with GnRH-agonist pituitary suppression for endometrial preparation, in FTET performed with a single vitrified euploid blastocyst transfer. The aim of the study was to evaluate pregnancy and implantation rates, the patients’ psychological distress, and cost-benefit ratio between the two protocols.
Materials and methods
Study design
The objective of this study was to evaluate two methods of endometrial preparation for frozen-thawed single euploid blastocyst transfer: modified-NC and AC with pituitary suppression by GnRH-agonist started in the luteal phase of previous menstrual cycle.
To address these issues, we designed a randomized control trial started on February 2015 and completed on September 2015. The Institutional Review Board of the European Hospital and the Genoma Laboratory approved the study before initiation. All participants gave written consent after having been informed on all aspects of the study. An independent monitor reviewed all study records. The registration number on www.clinicaltrials.gov was NCT02378584. All the clinical and biological procedures were conducted at the Reproductive Medicine of European Hospital, Rome, Italy, whereas the blastocyst genetic screening was performed at the Genoma Laboratory, Rome, Italy. All procedures were performed according to the Helsinki declaration of 1975 and its further modifications. All women were enrolled in this study only once and it was their first IVF cycle.
The primary end-point was the clinical pregnancy rate defined as a pregnancy which had completed 12 gestational weeks with embryo heart activity. The secondary end-points were the cost-benefit of two protocols, the woman psychological distress induced by the treatment and live birth rate considered as the number of deliveries that resulted in a live born with respect to the number of started cycles. In addition, the implantation rate was defined as the percentage of embryos transferred that develop to the stage of ultrasound-documented fetal heartbeat. The miscarriage rate was considered as the percentage of spontaneous pregnancy loss before the 24-week gestation with respect to the number of transfers performed. Moreover, biochemical pregnancy was defined as evidence of conception based only on biochemical data in the serum without ultrasound evidence of a gestational sac.
Patient population
Two hundred thirty-six patients were included in the study and randomized in two groups according to computer-generated, not cancelled, simple randomization list with allocation assignment 1.1. Therefore, 118 patients were planned to receive a frozen-thawed single euploid blastocyst transfer in a modified-NC whereas an AC with GnRH-agonist suppression was proposed to the other half. Both the patient and the clinicians were informed of the assigned treatment. The randomization took place during the visit to discuss PGS results. All women were enrolled in this study for their first cycle of treatment.
Inclusion criteria for participation in the study were as follows: maternal age <42 years, regular menstrual cycle, normal intrauterine cavity on pretreatment assessment, the presence of at least one vitrified euploid blastocyst obtained after intracytoplasmic sperm injection (ICSI) followed by preimplantation genetic diagnosis by aCGH, and a consent to undergo a frozen-thawed single transfer in a modified-NC or after hormonal endometrium preparation. Exclusion criteria were as follows: ovulation disorders, body mass index >29 kg/m2, endometriosis grade ≥III according to the American Fertility Society criteria, and the use of testicular sperm for ICSI.
IVF-ICSI treatment and embryo culture
Controlled ovarian stimulation was performed using recombinant follicle stimulating hormone (FSH) (Gonal F, Merck Serono, London, UK) and GnRH-agonist in a long suppression protocol or GnRH antagonist flexible protocol according to ovarian reserve and anti-Mullerian hormone (AMH) values. When at least three follicles reached 19 mm in diameter, human chorionic gonadotropin (hCG) (Gonasi, 10.000 IU, IBSA, Lodi, Italy) was administered by intramuscular injection. Oocytes were retrieved 36–38 h later by ultrasound-guided transvaginal follicular puncture.
The denudation procedure was completed between 38 and 40 h after hCG administration, and the oocytes were treated by ICSI immediately thereafter. Particular attention was paid to the removal of all adhering cumulus and coronal cells with the aim to avoid maternal DNA contamination during the amplification steps. ICSI was performed 38–40 h after hCG administration, using previously described techniques and instrumentation [36].
Fertilization was considered successful when two clearly distinct pronuclei and two polar bodies were presented 16–18 h after ICSI, as described elsewhere [36]. Embryo culture was carried out in cleavage medium under mineral oil (Sage In-Vitro Fertilization, Inc., Trumbull, CT,USA) up to day 3 and then followed by blastocyst medium (Sage In-Vitro Fertilization, Inc., Trumbull, CT, USA) up to day 5, 6, or 7 at 37 °C and under 5 % O2 and 6 % CO2. Embryo culture was performed in Embryoscope or in a mini-incubator (SANYO), where all embryos from each patient were kept separately from other couples throughout the whole culture duration.
Biopsy procedure and cell preparation
On day 3, when the embryos reached the 6–8-cell stage, a noncontact 1.48 diode laser was used to create a circular 6–9-diameter opening in the zona pellucida, in order to allow the biopsy of 5–10 herniated trophectoderm (TE) cells on day 5, 6, or 7, depending on the speed of blastocyst development. On the day of biopsy, TE cells were gently aspirated into the biopsy pipette (biopsy pipette; Cook Ireland Ltd., Limerick, Ireland) followed, if necessary, by a laser-assisted removal from the body of the blastocyst. The obtained cells were washed in sterile phosphate-buffered saline solution (PBS) and then placed in microcentrifuge tubes containing 2 mL of PBS, spinned down for a few seconds, and sent to GENOMA Laboratory for analysis.
TE cells were lysed and genomic DNA was amplified using the SurePlex DNA Amplification System (BlueGnome, Cambridge, UK), according to the manufacturer’s instructions. Whole Genome Amplification (WGA) products were fluorescently labeled and competitively hybridized to 24sure V3 arrays (BlueGnome, Cambridge, UK) with a matched control in an array CGH experiment format. A laser scanner was used to excite the hybridized fluorophores and read and store the resulting images of the hybridization. Scanned images were then analyzed and quantified by algorithm-fixed settings in BlueFuse Multi Software (BlueGnome, Cambridge, UK), a software package that performed the steps of grid placement, quantification, normalization, and postprocessing automatically. The whole procedure was completed within 12–24 h.
Blastocyst vitrification and warming
Vitrification procedure was used to cryopreserve all blastocysts, both euploid ones for later use and aneuploid ones whose storage is dictated by the Italian law which forbids any embryo destruction. Vitrification was carried out with the use of the Kuwayama protocol with Cryotop support as previously described [16]. Briefly, blastocysts were placed in equilibration solution (Kitazato Vitrification Kit, BioPharma, Shizuoka, Japan) containing 7.5 % ethylene glycol and 7.5 % dimethyl sulfoxide for 15 min at room temperature, then moved to a vitrification solution composed of 15 % ethylene glycol, 15 % dimethyl sulfoxide, and 0.5 mol/L sucrose for 30 to 60 s. The blastocysts were singly loaded onto the polypropylene strip of the Cryotop in a volume of <0.1 μL, quickly plunged into liquid nitrogen, capped with a protective cover, and stored in a liquid N2 storage tank at −196 °C.
Warming was carried out still with the use of the Kuwayama protocol previously described [16]. In brief, warming was performed by placing the Cryotop in a thawing solution (Kitazato Warming Kit, BioPharma, Shizouka, Japan) of 1 mol/L sucrose for 45 to 60 s at 37 °C. Blastocysts then were transferred to a dilution solution of 0.5 mol/L sucrose for 3 min, followed by washing with medium without sucrose for 5 min. The survived blastocysts were incubated for 4 h before their transfer to the uterus.
Blastocyst grading
Blastocyst stages were graded before freezing by using the system of Gardner and Schoolcraft [37]. Blastocysts were given a number based on the degree of expansion and hatching status: (1) early blastocyst: the blastocoel accounts for less than one half of the volume of the embryo; (2) blastocyst: the blastocoel occupies more than one half of the volume of the embryo; (3) full blastocyst: the blastocoel fills the embryo completely; (4) expanded blastocyst: the blastocoel is now larger than the early embryo, and the zona pellucida has begun to thin; (5) hatching blastocyst: TE cells have begun to herniate through the zona pellucida; and (6) hatched blastocyst: the blastocyst has completely escaped from the zona pellucida.
For fully developed blastocysts (grades 3–6), a second scoring step was performed under an inverted microscope to assess the inner cell mass (ICM) and the TE. For the ICM, the following descriptions are used: (A) tightly packed with many cells; (B) loosely grouped with several cells; and (C) very few cells. For the TE, the following grading is used: (A) many cells forming a cohesive epithelium; (B) few cells forming a loose epithelium; and (C) very few large cells. After thawing, blastocysts were subjectively graded for degree of blastocoel reexpansion, degree of cell reorganization, and degree of cell survival by two embryologists.
On the basis of this evaluation, blastocysts showing the degree of blastocoel expansion ≥4, grade A of both ICM and TE, were considered of high quality; blastocysts showing the degree of blastocoel expansion ≥3, grade A ICM and grade B TE or vice versa, were considered of good quality; blastocysts showing the degree of blastocoel expansion ≥3 and at least a grade C ICM or TE were considered of poor quality.
Artificial protocol
GnRH-agonist (buserelin acetate; Suprefact®; Hoechst, Marion Roussel, Milan, Italy) was started at the dose of 0.2 mg twice daily on day 21 of the previous menstrual cycle. When serum estradiol concentrations were <40 pg/mL and progesterone <1.5 ng/mL and no ovarian cystic structures were observed by transvaginal ultrasound, increasing doses of oral estradiol valerate (Progynova, Bayer, New Zealand limited, Auckland) were given. In general, patients started estradiol at a dose of 2 mg twice a day. This dose was increased every 3–5 days up to a maximum dose of 2 mg three times a day. Serial serum estradiol measurements and transvaginal ultrasound evaluation of the endometrium were monitored. After adequate endometrial proliferation (>7 mm), serum estradiol (>200 pg) and progesterone concentration (<1.5 ng/mL) were documented, GnRH-agonist treatment was stopped, and treatment with intramuscular progesterone (Prontogest, IBSA, Lodi, Italy), 50 mg/day, was initiated. In cases of pregnancy, estradiol and progesterone were continued until the 12th gestational week. All the cycles in which endometrial thickness was <7 mm and progesterone was >1.5 ng/mL were cancelled.
Modified-natural cycle
All patients assessed on cycle day 3 the FSH, LH, estradiol, and progesterone levels in order to check if they corresponded to the early follicular phase. Subsequently, serum estradiol and LH levels and transvaginal ultrasound evaluation of the endometrium were performed serially according to the physician’s decision starting from day 8 of the cycle.
Criteria for hCG administration included the following: mean diameter of dominant follicle of at least 17 mm, the endometrial thickness >7 mm, serum estradiol >200 pg, serum progesterone <1.5 ng/mL, and absence of a spontaneous LH surge. Final oocyte maturation was induced using 10.000 UI of hCG (Gonasi, 10.000 IU, IBSA, Lodi, Italy). The spontaneous LH surge was defined as LH concentration rise by 180 % [38] above the latest available value. In these cases, hCG was not administrated and the cycles were canceled. Intramuscular administration of progesterone at dose of 50 mg/day (Prontogest, IBSA, Lodi, Italy) was started in all patients 2 days after hCG. The blastocyst transfer was performed on day 6 of progesterone treatment.
Embryo transfer technique
All embryo transfers were performed under ultrasound control. For this scope, patients were asked to fill their bladder to provide an acoustic window for uterus visualization. The catheter tip (Wallace, Smiths Medical, Dublin, Ireland) was placed 1.0–2.0 cm below the apex of the uterus cavity. Care was taken to avoid contact of the transfer catheter with the uterine fundus in order to prevent uterine contractions.
Psychological assessment
All the women participating in the study were asked to complete a psychological questionnaire on four time points as follows: before the start of treatment (T1), on the day of progesterone administration (T2), on the day of the blastocyst transfer (T3), and on the day of pregnancy test (T4). We adopted the Hospital Anxiety and Depression Scale (HADS) [39, 40], a widely used self-report tool developed for measurement of anxiety and depression in clinical patients [40]. It consists of two scales (range 0–21) of seven items, which are scored on a four-point-like scale from 0 to 3. Higher scores are associated with more symptoms. Cutoff scores for possible and probable depressive and anxiety disorder are 7/8 and 10/11, respectively. The Italian version of the HADS has been clinically validated [39].
Sample size and statistical analysis
Considering an expected difference of 20 % from the estimated clinical pregnancy rate (60 %) between the two groups suggested by previous report [41] with a two-sided 5 % significance level and a power of 80 %, a sample size of 100 patients per group was necessary. One hundred eighteen patients per group were recruited to compensate for possible missing data or dropouts. Quantitative variables were compared between the two groups by unpaired Student’s t test. Chi square test was used to compare categorical variables. HADS questionnaire values at T0 and at T4 were compared separately between the two groups, by paired Student’s t test. Statistical analyses were performed with Stata software (StataCorp. 2013. Stata Statistical Software: Release 13. College Station, TX, USA: StataCorp LP). The significance level was set at p < 0.05.
Results
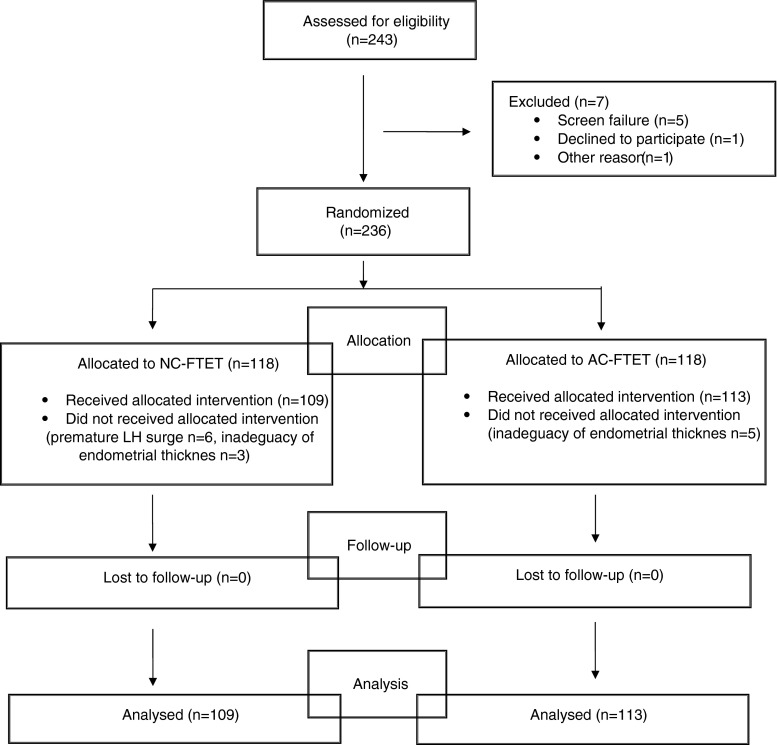
A breakdown of 236 enrolled patients is shown in Fig. 1. Of these, 109 underwent a single euploid blastocyst transfer in the modified-NC and 113 in the AC. The demographic and clinical patient characteristics are presented in Table 1. There were no differences in the age, BMI, AMH, and infertility history between the two groups. No allergic reaction and complication following the hormonal drug administration were encountered.
Fig. 1.
Disposition of enrolled patients
Table 1.
Demographic and clinical characteristics of women undergoing different endometrium preparation for frozen-thawed euploid blastocyst transfer
| Modified-NC (n = 109) | AC (n = 113) | ||
|---|---|---|---|
| Mean ± SD (range) | Mean ± SD (range) | p value | |
| Age (years) | 35.2 ± 3.6 (25.0–42.0) | 35.5 ± 3.8 (26.0–42.0) | 0.481 |
| BMI (kg/m2) | 22.1 ± 3.1 (17.1–38.1) | 22.1 ± 3.8 (17.1–38.9) | 0.436 |
| AMH (pmol/L) | 22.6 ± 8.7 (12.0–33.4) | 25.6 ± 13.8 (10.9–37.1) | 0.621 |
| Primary IVF indication (%): | |||
| Female factor | 28 (25.7 %) | 31 (27.4 %) | 0.868 |
| Male factor | 36 (33.0 %) | 27 (23.9 %) | 0.173 |
| Couple factor | 28 (25.7 %) | 41 (36.3 %) | 0.314 |
| Unexplained | 17 (15.6 %) | 14 (12.4 %) | 0.620 |
| PGS indication (%): | |||
| Advanced maternal age | 15 (13.8 %) | 21 (18.6 %) | 0.428 |
| Recurrent implantation failure | 15 (13.8 %) | 9 (8.0 %) | 0.240 |
| Recurrent pregnancy loss | 8 (7.3 %) | 7 (6.2 %) | 0.942 |
| Embryo chromosomal evaluation | 53 (48.6 %) | 58 (51.3 %) | 0.788 |
| Multiple factor | 18 (16.5 %) | 18 (15.9 %) | 0.906 |
The characteristics of FTET cycles are summarized in Table 2. The maximum endometrial thickness and peak estradiol levels were similar between the two groups. The ovulation induction in women undergoing a modified-NC was performed on mean cycle day 12.6 ± 2.3 and mean follicular diameter of 17.8 ± 1.0 at LH level under 15 mIU/mL in all cases. In the AC protocol, the mean duration of estradiol treatment until the pregnancy test was 28.1 ± 2.2. The mean duration of clinical observation until the day of progesterone initiation was comparable in both study groups. Moreover, no significant differences were observed in the number of clinical or ultrasound controls and the number of laboratory dosages. However, the number of venous samplings was significantly lower in the modified-NC group (p < 0.008) (Table 2).
Table 2.
Comparison of frozen-thawed euploid blastocyst transfer cycles in both study groups
| Modified-NC (n = 109) | AC (n = 113) | ||
|---|---|---|---|
| Mean ± SD (range) | Mean ± SD (range) | p value | |
| Endometrium (mm) | 9.4 ± 1.4 (6–13) | 9.,6 ± 1.3 (6–15) | 0.509 |
| Peak E2 level (pg/mL) | 339.6 ± 146.08 (162–1026) | 36.0 ± 135.2 (146–808) | 0.099 |
| Peak LH level (mIU/mL) | 7.7 ± 2.5 (2.1–14.2) | NA | NA |
| Follicle diameter on HCG trigger (mm) | 17.8 ± 1.0 (16–20) | NA | NA |
| Cycle day on HCG trigger (n) | 12.6 ± 2.3 (9–16) | NA | NA |
| Days of estradiol treatment until pregnancy test (n) | NA | 28.1 ± 2.2 (24–38) | NA |
| Cycle day on progesterone starting (n) | 13.6 ± 2.3 (9–16) | 14.1 ± 2.6 (10–17) | 0.265 |
| Clinical or ultrasound controls (n) | 3.8 ± 1.1 (2–6) | 4.2 ± 0.87 (2–6) | 0.128 |
| Venous sampling (n) | 1.5 ± 0.5 (1–2) | 3.1 ± 1.0 (1–6) | 0.008 |
| Laboratory dosages (n) | 3.5 ± 1.5 (2–6) | 3.7 ± 1.8 (2–8) | 0.432 |
The dose and the cost of the drugs administered during the endometrial preparation for FTET is presented in Table 3.The estimated mean cost for AC and modified-NC was 64.0 ± 1.6 and 59.88 ± 0.0 euro, respectively, and no statistically significant difference was documented between both study groups.
Table 3.
Cost comparison in different protocols for endometrium preparation for frozen-thawed euploid blastocyst transfer cycles in both study groups
| Modified-NC (n = 109) | AC (n = 113) | p value | |||
|---|---|---|---|---|---|
| Total dose | Cost | Total dose | Cost | ||
| GnRH-analog | NA | NA | 4.2 ± 0.8 mg (3.8–4.5) | 19.3 ± 0.0 euro | NA |
| hCG | 10.000 ± 0.0 UI | 30.0 ± 0.0 euro | NA | NA | NA |
| Progynova | NA | NA | 164 ± 22.1 mg (140–204) | 14.9 ± 1.6 euro (11.9–17.8) | NA |
| Prontogest | 800 ± 0.0 mg | 29.8 ± 0.0 euro | 800 ± 0.0 mg | 29.8 ± 0.0 euro | NA |
| Mean total cost | 59.8 ± 0.0 euro | 64.0 ± 1.6 | 0.438 | ||
The blastocyst survival rate was 100 %, and only one embryo was transferred per cycle. Table 4 compares the characteristics of transferred blastocysts reporting no considerable differences in their quality and biopsy day among the groups.
Table 4.
Biological outcomes of frozen-thawed euploid blastocyst transfer cycles in the study groups
| Modified-NC (n = 109) | AC (n = 113) | p value | |
|---|---|---|---|
| Blastocyst biopsy on day 5 (%) | 47 (43.1 %) | 48 (42.5 %) | 0.923 |
| Blastocyst biopsy on day 6 (%) | 57 (52.3 %) | 60 (53.1 %) | 0.904 |
| Blastocyst biopsy on day 7 (%) | 5 (4.6 %) | 5 (4.4 %) | 0.953 |
| High blastocyst quality (%) | 55 (50.4 %) | 48 (42.5 %) | 0.290 |
| Good blastocyst quality (%) | 39 (35.8 %) | 53 (46.9 %) | 0.122 |
| Poor blastocyst quality (%) | 15 (13.8 %) | 12 (10.6 %) | 0.609 |
The overall pregnancy, clinical pregnancy, and implantation rates in modified-NC (62.3, 54.1, and 54.1 %, respectively) were almost overlapping with respect to the same parameters in AC (61.9, 50.4, and 50.4 %, respectively). The biochemical pregnancy and miscarriage rates (8.2 and 5.5 %, respectively) in modified-NC resulted to be comparable to those observed in AC (11.5 and 7.0 %, respectively). The live-birth rate in modified-NC of 45.8 % was similar to that observed in AC (41.5 %). No statistical significance was documented between the groups (Table 5).
Table 5.
Clinical outcome following frozen-thawed euploid blastocyst transfer in the study groups
| Modified-NC (n = 109) | AC (n = 113) | p value | |
|---|---|---|---|
| Overall pregnancy rate (%) | 68 (62.3 %) | 70 (61.9 %) | 0.946 |
| Biochemical pregnancy rate (%) | 9 (8.2 %) | 13 (11.5 %) | 0.558 |
| Clinical pregnancy rate (%) | 59 (54.1 %) | 57 (50.4 %) | 0.677 |
| Miscarriage (%) | 6 (5.5 %) | 8 (7.0 %) | 0.836 |
| Implantation rate (%) | 59 (54.1 %) | 57 (50.4 %) | 0.677 |
| Live-birth rate (%) | 50 (45.8 %) | 47 (41.5 %) | 0.612 |
The results of psychological tests showed no statistical differences between the groups at T1, T2, T3, and T4 for both anxiety and depression values (Table 6). The T4 anxiety values in both groups showed a statistically significant increment compared to the baseline point-time screening. However, the mean increment in both groups was similar (5.2 ± 1.9 for modified-NC and 5.5 ± 2.1 for AC protocol).
Table 6.
HADS questionnaire results in the study groups
| Anxiety scale | Depression scale | |||||
|---|---|---|---|---|---|---|
| Modified-NC (n = 109) | AC (n = 113) | p value | Modified-NC (n = 109) | AC (n = 113) | p value | |
| T1 | 7.1 ± 1.4 | 7.3 ± 1.5 | 0.231 | 5.3 ± 1.8 | 4.9 ± 1.5 | 0.321 |
| T2 | 7.6 ± 1.8 | 7.8 ± 1.7 | 0.093 | 5.8 ± 1.2 | 6.1 ± 1.3 | 0.564 |
| T3 | 9.5 ± 1.2 | 9.8 ± 1.3 | 0.452 | 5.8 ± 1.3 | 5.6 ± 1.5 | 0.325 |
| T4 | 12.3 ± 2,1 | 12.8 ± 2.6 | 0.569 | 6.1 ± 1.1 | 6.8 ± 1.6 | 0.658 |
Discussion
Implantation is the most critical step for the success of assisted reproduction techniques and depends on endometrial receptivity, embryo quality, and appropriate synchrony between embryo and endometrium. The literature review suggests that ovarian stimulation, followed by high estrogen levels, may lead to the embryo-endometrium asynchrony and progesterone premature elevation with consequent endometrial advancement [42]. There is also evidence that different genes and implantation factors highly expressed during the implantation window in NC tend to be downregulated at higher estrogen concentrations [43–45].
The negative effect of ovarian stimulation can be expressed by decreased clinical results and, as recently suggested, by higher adverse obstetrics and perinatal outcomes [3, 42]. In fact, the study by Shapiro et al. [2] showed that the clinical pregnancy rate transferring embryos in fresh cycle was approximately two thirds of the rate obtained in FTET. The difference in implantation rate of 29.3 % in favor of cryopreservation may estimate the proportion of fresh transfer failure that may be attributed to the endometrial impairment due to the multiple follicular growths [2]. Implantation pattern in cycles with ovarian stimulation has shown greater implantation rates of blastocysts obtained on day 5 when compared with those formed on day 6, but not in cycles without gonadotrophin administration [14, 46]. In the same time, there is evidence that day 6 blastocysts have a better implantation rate in FTET [13–15]. Therefore, growing application of genetic embryo screening increases the need for blastocyst cryopreservation while awaiting the results and transfer after euploidity confirmation [42].
These data taken together suggest that embryo cryopreservation, until now considered only a way to improve the cumulative chance of conception per oocyte retrieval in case of embryo surplus [25], may become a routine IVF approach. Several studies have demonstrated significant differences between the natural and stimulated cycle, suggesting that FTET is able to give a better ongoing pregnancy rate, live birth, and obstetrical outcomes [47–49]. Moreover, some authors declare that cohort cryopreservation might convey an a priori greater chance of success [24]. At the same time, the development of vitrification and improved long-term in vitro culture systems lead to the better survival rate of frozen-thawed embryos, limiting cellular damage and enabling treated embryos to maintain the reproductive potential, comparable to fresh ones [43, 50, 51].
For many couples, the possibility to transfer chromosomally healthy blastocyst may be unrepeatable and every effort should be done to optimize the treatment results. In the present study, 57.2 % blastocysts were obtained and subjected to biopsy on day 6 or 7, after in vitro fertilization requiring cryopreservation as a mandatory approach. However, based on the literature data presented above, we adopted a strategy of cohort blastocyst vitrification, independently on the day of biopsy. In addition, the survival rate of euploid blastocyst was of 100 %, confirming the literature data regarding the safety and efficiency of vitrification.
Cycle regimens used for endometrial preparation in FTET are commonly classified into three groups: NC with or without ovulation induction using hCG [1, 25, 26]; AC in which the endometrium is artificially prepared using estrogens, with or without use of GnRH-agonist [1, 25, 26]; and stimulated cycles, in which follicular development is supported by follicle-stimulating drugs [43].
Three retrospective papers have shown equivalent ongoing pregnancy and live birth rates between NC or modified-NC and AC with GnRH-agonist [28, 30, 52]. Other three papers have shown that GnRH-agonist administration is irrelevant for endometrial preparation in AC [29, 53, 54], counteracting a randomized controlled trial that evidenced an improved live-birth rate in AC with GnRH-agonist hormone [25, 47]. Levron et al. [55] showed that natural endometrium preparation yields better outcome compared with AC cycle, as demonstrated by higher implantation and clinical pregnancy rate. Some studies have found a higher pregnancy loss in women undergoing a hormone replacement therapy for FTET compared with natural cycle [27, 56, 57] suggesting that excessive estrogen environment or suboptimal ratio between estrogen and progesterone may compromise pregnancy development [27].
However, there are very few studies evaluating the endometrium preparation protocol for transfer of vitrified-thawed blastocyst, in which implantation occurs only during a brief period of the secretory phase of the menstrual cycle, usually referred as “implantation window.” The only one retrospective cohort analysis supported that blastocyst transfer in AC has a higher live-birth rate when compared to NC [1]. Another retrospective study documented that the implantation, clinical, and ongoing pregnancy rates were significantly higher in NC with spontaneous LH peak with respect to the hormonally manipulated cycle, although comparable to NC with the use of hCG for ovulation triggering [43]. Unfortunately, no studies reported any data regarding the transfer of single and euploid frozen-thawed blastocyst.
The results from our prospective controlled randomized trial documented that overall pregnancy rate in women undergoing FTET in modified-NC (62.3 %) was similar to those noted in patients receiving hormonal stimulation for endometrial growth (61.9 %). Moreover, the clinical pregnancy, implantation, and live-birth rates showed similar results. The biochemical pregnancy and miscarriage rates observed in modified-NC showed a trend to be lower than in AC. Substantially, no statistical significant differences were observed between the two groups, showing that both protocols are equally effective in FTET. This finding evidences that in case of a single euploid FTET at blastocyst stage, the choice of endometrial preparation protocol should be based only on women menstrual and ovulatory characteristics or otherwise on patient need for cycle planning.
The administration of estrogen and progesterone does not guarantee complete pituitary suppression, and a dominant follicle growth may occur. The follicle spontaneous luteinization may expose the endometrium to progesterone influence earlier leading to incorrect timing of transfer. Therefore, in order to optimize the clinical outcome, the study population undergoing AC-FTET blastocyst transfer received a GnRH-agonist from day 21 of the previous menstrual cycle.
Endometrial thickness and its ultrasound pattern evaluation provide an effective approach to assess endometrial development and predict endometrial receptivity. Some studies have documented that the endometrial thickness was not associated with implantation and clinical pregnancy rates on the day of fresh and frozen embryo transfer [58]. However, the study by Zhao et al. [59] suggested that pregnancy was unlikely to occur when the endometrial thickness was <6 mm. In our randomized clinical trial, the endometrium thickness lower than 7 mm was a cycle cancellation criterion.
Mechanical endometrial injury in the cycle preceding IVF has been proposed to improve implantation, but a recent retrospective cohort study demonstrated that endometrial disruption does not improve implantation in patients who failed the transfer of euploid blastocyst [60].
Pregnancy rates in NC-FTET are closely dependent on the timely identification of ovulation and calculation of the likely subsequent period of optimal endometrial receptivity [61, 62] in which the transfer should be performed. Modified NC-FTET overcomes the disadvantages of LH monitoring of ovulation triggering by hCG as soon as the dominant follicle is of sufficient size to surrogate to the endogenous LH surge for final oocyte maturation [63]. However, NC-FTET carries the risk of unexpected ovulation and difficulty in ensuring timely thawing and transfer of the embryo. In the study by Fatemi et al. [64], an LH surge at the moment of hCG administration was not a cancellation criterion. The authors reported that women who received hCG despite of the LH rise had a very low pregnancy rate (4.3 %). These results might be explained by the fact that the hCG and LH share the same receptor, and their simultaneous presence could affect the pregnancy development. Actually, there is no consensus regarding premature LH surge at the moment of hCG injection [26, 65], although based on Fatemi et al.’s experience [64], we considered reasonable the exclusion from the study patients with a spontaneous LH peak who did not receive the hCG.
It was suggested that the timing of blastocyst formation is not linked to or affected by chromosomal abnormalities and embryos reaching the expanded blastocyst stage on day 6 or 7 have a similar risk of being aneuploid as faster growing ones [66, 67]. At the same time, it was evidenced that lower-quality euploid embryos yield the same ongoing implantation rate compared with blastocysts of good morphological quality [68]. A side reflection deriving from this study regards the comparability of clinical results from transfer of blastocysts with different characteristics in both endometrial preparation protocols. In our experience, slower growing and/or poor-quality blastocysts retain a clinically important chance of implantation and should be considered for biopsy and subsequently for embryo transfer, if euploid.
There are only few published studies comparing patient preference or cost-benefit related to the different methods of monitoring for FTET. Weissman et al. [69] extrapolated the number of visits per cycle to calculate cost-efficiency and patient preference. It was demonstrated that the number of clinical visits in the natural cycle with hCG triggering was significantly lower compared with spontaneous ovulation [64, 69]. However, there are no available data regarding the same question in the AC. In our study, no statistical difference was observed in the number of medical visits as well as hormonal dosages between both protocols. However, the number of venous samplings resulted to be higher in the AC cycle, but based on the results obtained from psychological tests performed in different time points of clinical preparation for FTET, this fact does not seem to influence the patient compliance. Moreover, the estimated drug cost for both study groups, despite different pharmaceuticals used, was almost identical.
The role of stress in relation to assisted reproductive technologies, such as IVF, has long been studied [70, 71]. Stress during fertility treatment is thought to be related to the diagnosis of infertility [72], the medical procedures, the awaiting of a positive outcome [73], the physiological effects of gonadotropin stimulation [74], and the biopsy procedure and genetic testing when PGS is performed [75]. Stress and anxiety are usually measured at different time points during IVF treatment. The oocyte retrieval and embryo transfer stages are the most stressful events for most women, independently from sociodemographic and biomedical reasons; women with more than a moderate level of financial burden were relatively more stable, as could be the case of PGS couples [70].
The open question is whether stress or anxiety has an impact on success of fertility treatment and whether interventions to decrease stress are useful. Data coming from the literature are conflicting and contradictory [32, 33]. Even if the number of venous samplings was significantly different between the protocols, the number of clinical controls performed was comparable. This can probably explain why no statistical differences were observed in the results obtained from psychological tests performed in different time points of clinical preparation for FTET.
We suppose that the patient knowledge of high implantation rate of an euploid blastocyst, the high survival rates of vitrified blastocyst, and carrying out a single embryo transfer avoiding the risk (<4 %) of multiple pregnancies are fundamental in the controlling of the emotional distress. Anxiety outcomes were elevated at the same degree, just on the day of pregnancy test in both the protocols, as expected. These data taken together with the drug charge evidence a comparability of both protocols in term of cost-benefit and patient compliance.
On the basis of our prospective randomized trial, there is an emerging evidence that modified-NC and AC with GnRH-agonist pituitary suppression for single vitrified warmed euploid blastocyst transfer ensure the same clinical results, patient compliance, and socioeconomic cost. Therefore, the choice of the protocol for FTET at blastocyst stage should be based on women menstrual and ovulatory characteristics or otherwise on patient need for cycle planning.
Compliance with ethical standards
The Institutional Review Board of the European Hospital and the Genoma Laboratory approved the study before initiation. All participants gave written consent after having been informed on all aspects of the study. All procedures were performed according to the Helsinki declaration of 1975 and its further modifications.
Footnotes
Capsule Frozen-thawed single euploid blastocyst transfer can be successfully performed in a modified-natural cycle and after hormonal endometrial preparation. The protocol choice depends on a women’s ovulatory characteristics or need for cycle planning.
References
- 1.Hill MJ, Miller A, Frattarelli JL. A GnRH agonist and exogenous hormone stimulation protocol has a higher live-birth rate than a natural endogenous hormone protocol for frozen-thawed blastocyst-stage embryo transfer cycles: an analysis of 1391 cycles. Fertil Steril. 2010;93:416–422. doi: 10.1016/j.fertnstert.2008.11.027. [DOI] [PubMed] [Google Scholar]
- 2.Shapiro BS, Daneshmand ST, Garner FC, Aguirre M, Hudson C, Thomas S. Evidence of impaired endometrial receptivity after ovarian stimulation for in vitro fertilization: a prospective randomized trial comparing fresh and frozen-thawed embryo transfer in normal responders. Fertil Steril. 2011;96:344–348. doi: 10.1016/j.fertnstert.2011.05.050. [DOI] [PubMed] [Google Scholar]
- 3.Weinerman R, Mainigi M. Why we should frozen instead of fresh embryos: the translational rationale. Fertil Steril. 2014;102:10–18. doi: 10.1016/j.fertnstert.2014.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Abeyta M, Behr B. Morphological assessment of embryo viability. Semin Reprod Med. 2014;32(2):114–126. doi: 10.1055/s-0033-1363553. [DOI] [PubMed] [Google Scholar]
- 5.Munné S, Lee A, Rosenwaks Z, Grifo J, Cohen J. Diagnosis of major chromosome aneuploidies in human preimplantation embryos. Hum Reprod. 1993;8(12):2185–2191. doi: 10.1093/oxfordjournals.humrep.a138001. [DOI] [PubMed] [Google Scholar]
- 6.Desai N, Ploskonka S, Goodman LR, Austin C, Goldberg J, Falcone T. Analysis of embryo morphokinetics, multinucleation and cleavage anomalies using continuous time-lapse monitoring in blastocyst transfer cycles. Reprod Biol Endocrinol. 2014;20:12–54. doi: 10.1186/1477-7827-12-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Katz Jaffe MG, McReynolds S. Embryology in the era of proteomics. Fertil Steril. 2013;15(4):1073–7. doi: 10.1016/j.fertnstert.2012.12.038. [DOI] [PubMed] [Google Scholar]
- 8.Uyar A, Seli E. Metabolomic assessment of embryo viability. Semin Reprod Med. 2014;32(2):141–152. doi: 10.1055/s-0033-1363556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dahdouh EM, Balayla J, García-Velasco JA. Impact of blastocyst biopsy and comprehensive chromosome screening technology on preimplantation genetic screening: a systematic review of randomized controlled trials. Reprod Biomed Online. 2015;30(3):281–289. doi: 10.1016/j.rbmo.2014.11.015. [DOI] [PubMed] [Google Scholar]
- 10.Sahin L, Bozkurt M, Sahin H, Gürel A, Yumru AE. Is preimplantation genetic diagnosis the ideal embryo selection method in aneuploidy screening? Kaohsiung J Med Sci. 2014;30(10):491–498. doi: 10.1016/j.kjms.2014.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee E, Illingworth P, Wilton L, Chambers GM. The clinical effectiveness of preimplantation genetic diagnosis for aneuploidy in all 24 chromosomes (PGD-A): systematic review. Hum Reprod. 2015;30(2):473–483. doi: 10.1093/humrep/deu303. [DOI] [PubMed] [Google Scholar]
- 12.Hellani A, Abu-Amero K, Azouri J, El-Akoum S. Successful pregnancies after application of array-comparative genomic hybridization in PGS-aneuploidy screening. Reprod Biomed Online. 2008;17(6):841–847. doi: 10.1016/S1472-6483(10)60413-0. [DOI] [PubMed] [Google Scholar]
- 13.Murata Y, Oku H, Morimoto Y, Tokuda M, Murata T, Sugihara K, Nagata F, Nakaoka Y, Fukuda A. Freeze-thaw programmes rescue the implantation of day 6 blastocyts. Reprod Biomed Online. 2005;1:428–433. doi: 10.1016/S1472-6483(10)61134-0. [DOI] [PubMed] [Google Scholar]
- 14.Shapiro BS, Daneshmand ST, Garner FC, Aguirre M, Thomas S. Large blastocyst diameter, early blastulation, and low preovulatory serum progesterone are dominant predictors of clinical pregnancy in fresh autologous cycles. Fertil Steril. 2008;90:302–309. doi: 10.1016/j.fertnstert.2007.06.062. [DOI] [PubMed] [Google Scholar]
- 15.Shapiro BS, Daneshmand ST, Restrepo H, Garner FC, Aguirre M, Hudson C. Matched-cohort comparison of single-embryo transfers in fresh and frozen-thawed embryo transfer cycles. Fertil Steril. 2013;99:389–392. doi: 10.1016/j.fertnstert.2012.09.044. [DOI] [PubMed] [Google Scholar]
- 16.Kuwayama M. Highly efficient vitrification for cryopreservation of human oocytes and embryos: the Cryotop method. Theriogenology. 2007;67(1):73–80. doi: 10.1016/j.theriogenology.2006.09.014. [DOI] [PubMed] [Google Scholar]
- 17.Lopes AS, Frederickx V, Van Kerkhoven G, Campo R, Puttemans P, Gordts S. Survival, re-expansion and cell survival of human blastocysts following vitrification and warming using two vitrification systems. J Assist Reprod Genet. 2015;32(1):83–90. doi: 10.1007/s10815-014-0373-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Check JH, Choe JK, Katsoff D, Summers-Chase D, Wilson C. Controlled ovarian hyperstimulation adversely affects implantation after in vitro fertilization-embryo transfer. J Assist Reprod Genet. 1999;16:416–420. doi: 10.1023/A:1020565408018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Haouzi D, Assou S, Mahmoud K, Tondeur S, Reme T, Hedon B, De Vos J, Hamamah S. Gene expression profile of human endometrial receptivity: comparison between natural and stimulated cycles for the same patients. Hum Reprod. 2009;24:1436–1445. doi: 10.1093/humrep/dep039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Horcajadas JA, Dıaz-Gimeno P, Pellicer A, Simon C. Uterine receptivity and the ramifications of ovarian stimulation on endometrial function. Semin Reprod Med. 2007;25:454–460. doi: 10.1055/s-2007-991043. [DOI] [PubMed] [Google Scholar]
- 21.Kolibianakis E, Bourgain C, Albano C, Osmanagaoglu K, Smitz J, Van Steirteghem A, et al. Effect of ovarian stimulation with recombinant follicle-stimulating hormone, gonadotropin releasing hormone antagonists, and human chorionic gonadotropin on endometrial maturation on the day of oocyte pick-up. Fertil Steril. 2002;78:1025–1029. doi: 10.1016/S0015-0282(02)03323-X. [DOI] [PubMed] [Google Scholar]
- 22.Nikas G, Develioglu OH, Toner JP, Jones HW., Jr Endometrial pinopodes indicate a shift in the window of receptivity in IVF cycles. Hum Reprod. 1999;14:787–792. doi: 10.1093/humrep/14.3.787. [DOI] [PubMed] [Google Scholar]
- 23.Papanikolaou EG, Bourgain C, Kolibianakis E, Tournaye H, Devroey P. Steroid receptor expression in late follicular phase endometrium in GnRH antagonist IVF cycles is already altered, indicating initiation of early luteal phase transformation in the absence of secretory changes. Hum Reprod. 2005;20:1541–1547. doi: 10.1093/humrep/deh793. [DOI] [PubMed] [Google Scholar]
- 24.Roque M, Lattes K, Serra S, Sola I, Geber S, Carreras R, et al. Fresh embryo transfer versus frozen embryo transfer in in vitro fertilization cycles: a systematic review and meta-analysis. Fertil Steril. 2013;99:156–162. doi: 10.1016/j.fertnstert.2012.09.003. [DOI] [PubMed] [Google Scholar]
- 25.Ghobara T, Vandekerckhove P. Cycle regimens for frozen-thawed embryo transfer. Cochrane Database Syst Rev. 2008;1:CD003414. doi: 10.1002/14651858.CD003414.pub2. [DOI] [PubMed] [Google Scholar]
- 26.Groenewoud ER, Cantineau AE, Kollen BJ, Macklon NS, Cohlen BJ. What is the optimal means of preparing the endometrium in frozen-thawed embryo transfer cycles? A systematic review and meta-analysis. Hum Reprod Update. 2013;19(5):458–470. doi: 10.1093/humupd/dmt030. [DOI] [PubMed] [Google Scholar]
- 27.Candido T, Alsbjerg B, Martikainen H, Humaidan P. Pregnancy lost after frozen-embryo transfer-a comparison of three protocols. Fertil Steril. 2012;98:1165–1169. doi: 10.1016/j.fertnstert.2012.07.1058. [DOI] [PubMed] [Google Scholar]
- 28.Gelbaya TA, Nardo LG, Hunter HR, Fitgerald CT, Horne G, Pease EEH, Brison DR, Lieberman BA. Cryopreserved-thawed embryo transfer in natural or down-regulated hormonally controlled cycles: a retrospective study. Fertil Steril. 2006;85:603–609. doi: 10.1016/j.fertnstert.2005.09.015. [DOI] [PubMed] [Google Scholar]
- 29.Prato LD, Borini A, Cattoli M, Bonu MA, Sciajno R, Flamigni C. Endometrial preparation for frozen-thawed embryo transfer with or without pretreatment with gonadotropin-releasing hormone agonist. Fertil Steril. 2002;77:956–960. doi: 10.1016/S0015-0282(02)02960-6. [DOI] [PubMed] [Google Scholar]
- 30.Spandorfer SD, Fasouliotis SJ, Cimmino C, Shpizner M, Veeck L, Rosenwaks Z. Blastocyst frozen embryo transfer (FET): comparison of outcome with replacement in natural or programmed/medicated cycle. Fertil Steril. 2004;82:s154. doi: 10.1016/j.fertnstert.2004.07.393. [DOI] [Google Scholar]
- 31.Mounce G, McVeigh E, Turner K, Child TJ. Randomized, controlled pilot trial of natural versus hormone replacement therapy cycles in frozen embryo replacement in vitro fertilization. Fertil Steril. 2015;104:915–920. doi: 10.1016/j.fertnstert.2015.07.1131. [DOI] [PubMed] [Google Scholar]
- 32.An Y, Sun Z, Li L, Zhang Y, Ji H. Relationship between psychological stress and reproductive outcome in women undergoing in vitro fertilization treatment: psychological and neurohormonal assessment. J Assis Reprod Genet. 2013;30(1):35–41. doi: 10.1007/s10815-012-9904-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pasch LA, Gregorich SE, Katz PK, Millstein SG, Nachtigall RD, Bleil ME, Adler NE. Psychological distress and in vitro fertilization outcome. Fertil Steril. 2012;98(2):459–464. doi: 10.1016/j.fertnstert.2012.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Eyal S, Weizman A, Toren P, Dor Y, Mester R, Rehavi M. Chronic GnRH agonist administration down-regulates platelet serotonin transporter in women undergoing assisted reproductive treatment. Psychopharmacol (Berl) 1996;125(2):141–145. doi: 10.1007/BF02249413. [DOI] [PubMed] [Google Scholar]
- 35.Amir BY, Yaacov B, Guy B, Gad P, Itzhak W, Gal I. Headaches in women undergoing in vitro fertilization and embryo-transfer treatment. Headache. 2005;45(3):215–219. doi: 10.1111/j.1526-4610.2005.05047.x. [DOI] [PubMed] [Google Scholar]
- 36.Greco E, Litwicka K, Ferrero S, Baroni E, Sapienza F, Rienzi L. GnRH antagonists in ovarian stimulation for ICSI with oocyte restriction: a matched, controlled study. Reprod Biomed Online. 2008;14(5):572–578. doi: 10.1016/S1472-6483(10)61048-6. [DOI] [PubMed] [Google Scholar]
- 37.Gardner DK, Schoolcraft WB. In vitro culture of human blastocysts. In: Jansen R, Mortimer D, editors. Toward reproductive certainty: fertility and genetics beyond. London: Parthenon Publishing; 1999. pp. 378–388. [Google Scholar]
- 38.Testart J, Frydman R, Feinstein MC, Thebault A, Roger M, Scholler R. Interpretation of plasma luteinizing hormone assay for the collection of mature oocytes from women: definition of a luteinizing hormone-surge-initiating rise. Fertil Steril. 1981;36:50–54. doi: 10.1016/S0015-0282(16)45617-7. [DOI] [PubMed] [Google Scholar]
- 39.Iani L, Lauriola M, Costantini M. A confirmatory bifactor analysis of the Hospital Anxiety and Depression Scale in an Italian community sample. Health Qual Life Outcomes. 2014;5:12–84. doi: 10.1186/1477-7525-12-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand. 1983;67(6):361–370. doi: 10.1111/j.1600-0447.1983.tb09716.x. [DOI] [PubMed] [Google Scholar]
- 41.Taylor TH, Patrick JL, Gitlin SA, Crain JL, Wilson JM, Griffin DK. Blastocyst euploidy and implantation rates in a young (<35 years) and old (≥35 years) presumed fertile and infertile patient population. Fertil Steril. 2014;102(5):1318–1323. doi: 10.1016/j.fertnstert.2014.07.1207. [DOI] [PubMed] [Google Scholar]
- 42.Shapiro BS, Daneshmand ST, Garner FC, Aguirre M, Hudson C. Clinical rationale for cryopreservation of entire embryo cohorts in lieu of fresh transfer. Fertil Steril. 2014;102(1):3–9. doi: 10.1016/j.fertnstert.2014.04.018. [DOI] [PubMed] [Google Scholar]
- 43.Chang EM, Han JE, Kim YS, Lyu SW, Lee WS, Yoon TK. Use of the natural cycle and vitrification thawed blastocyst transfer results in better in-vitro fertilization outcomes: cycle regimens of vitrification thawed blastocyst transfer. J Assist Reprod Genet. 2011;28(4):369–374. doi: 10.1007/s10815-010-9530-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Creus M, Ordi J, Fábregues F, Casamitjana R, Carmona F, Cardesa A, Vanrell JA, Balasch J. The effect of different hormone therapies on integrin expression and pinopode formation in the human endometrium: a controlled study. Hum Reprod. 2003;18(4):683–693. doi: 10.1093/humrep/deg177. [DOI] [PubMed] [Google Scholar]
- 45.Morozov V, Ruman J, Kenigsberg D, Moodie G, Brenner S. Natural cycle cryo-thaw transfer may improve pregnancy outcome. J Assist Reprod Genet. 2007;24(4):119–123. doi: 10.1007/s10815-006-9100-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Richter KS, Shipley SK, McVearry I, Tucker MJ, Widra EA. Cryopreserved embryo transfers suggest that endometrial receptivity may contribute to reduced success rates of later developing embryos. Fertil Steril. 2006;86(4):862–866. doi: 10.1016/j.fertnstert.2006.02.114. [DOI] [PubMed] [Google Scholar]
- 47.El-Toukhy T, Taylor A, Khalaf Y, Al-Darazi K, Rowell P, Seed P, Braude P. Pituitary suppression in ultrasound-monitored frozen embryo replacement cycles. A randomized study. Hum Reprod. 2004;19:874–879. doi: 10.1093/humrep/deh183. [DOI] [PubMed] [Google Scholar]
- 48.Karlström PO, Bergh T, Forsberg AS, Sandkvist U, Wikland M. Prognostic factors for the success rate of embryo freezing. Hum Reprod. 1997;12(6):1263–1266. doi: 10.1093/humrep/12.6.1263. [DOI] [PubMed] [Google Scholar]
- 49.Toner JP, Veeck LL, Acosta AA, Muasher SJ. Predictive value of pregnancy during original in vitro fertilization cycle on implantation and pregnancy in subsequent cryothaw cycles. Fertil Steril. 1991;56(3):505. doi: 10.1016/S0015-0282(16)54549-X. [DOI] [PubMed] [Google Scholar]
- 50.Hong SW, Sepilian V, Chung HM, Kim TJ. Cryopreserved human blastocysts after vitrification result in excellent implantation and clinical pregnancy rates. Fertil Steril. 2009;92(6):2062–2064. doi: 10.1016/j.fertnstert.2009.06.008. [DOI] [PubMed] [Google Scholar]
- 51.Youssry M, Ozmen B, Zohni K, Diedrich K, Al-Hasani S. Current aspects of blastocyst cryopreservation. Reprod Biomed Online. 2008;16(2):311–320. doi: 10.1016/S1472-6483(10)60591-3. [DOI] [PubMed] [Google Scholar]
- 52.Al-Shawaf T, Yang D, Al-Magid Y, Seaton A, Iketubosin F, Craft I. Infertility: ultrasonic monitoring during replacement of frozen/thawed embryos in natural and hormone replacement cycles. Hum Reprod. 1993;8:2068–2074. doi: 10.1093/oxfordjournals.humrep.a137983. [DOI] [PubMed] [Google Scholar]
- 53.Queenan JT, Ramey JW, Seltman HJ, Eure L, Veeck LL, Muasher SJ. Transfer of cryopreserved-thawed pre-embryos in a cycle using exogenous steroids without prior gonadotropin-releasing hormone agonist suppression yields favourable pregnancy results. Hum Reprod. 1997;12:1176–1180. doi: 10.1093/humrep/12.6.1176. [DOI] [PubMed] [Google Scholar]
- 54.Simon A, Hurwitz A, Zetner BS, Bdolah Y, Laufer N. Transfer of frozen-thawed embryos in artificially prepared cycles with and without prior gonadotropin-releasing hormone agonist suppression: a prospective randomized study. Hum Reprod. 1998;13:2712–2717. doi: 10.1093/humrep/13.10.2712. [DOI] [PubMed] [Google Scholar]
- 55.Levron J, Yerushalmi GM, Brengauz M, Gat I, Katorza E. Comparison between two protocols for thawed embryo transfer: natural cycle versus exogenous hormone replacement. Gynecol Endocrinol. 2014;30(7):494–497. doi: 10.3109/09513590.2014.900032. [DOI] [PubMed] [Google Scholar]
- 56.Alama P, Melo MAB, Garcia G, Garrido N, Meseguer M, Remohi J. Higher ongoing pregnancy rates in lastocyst transfer of frozen-thawed embryos in natural cycles. Fertil Steril. 2007;8:s161. doi: 10.1016/j.fertnstert.2007.07.559. [DOI] [Google Scholar]
- 57.Givens CR, Markun LC, Ryan IP, Chenette PE, Herbert CM, Schriock ED. Outcomes of natural cycles versus programmed cycles for 1677 frozen-thawed embryo transfers. Reprod Biomed Online. 2009;19:380–384. doi: 10.1016/S1472-6483(10)60172-1. [DOI] [PubMed] [Google Scholar]
- 58.Gingold, J.A., Lee, J.A., Rodriguez-Purata, J., Whitehouse, M.C., Sandler, B., Grunfeld, L., Mukherjee, T., Copperman, A.B. Endometrial pattern, but not endometrial thickness, affects implantation rates in euploid embryo transfers. Fertil Steril. 2015;104(3):620–8. [DOI] [PMC free article] [PubMed]
- 59.Zhao J, Zhang Q, Li Y. The effect of endometrial thickness and pattern measured by ultrasonography on pregnancy outcomes during IVF-ET cycles. Reprod Biol Endocrinol. 2012;10:100. doi: 10.1186/1477-7827-10-100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Werner MD, Forman EJ, Hong KH, Franasiak JM, Bergh PA, Scott RT. Endometrial disruption does not improve implantation in patients who have failed the transfer of euploid blastocysts. J Assist Reprod Genet. 2015;32(4):557–562. doi: 10.1007/s10815-015-0435-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Harper MJ. The implantation window. Baillieres Clin Obstet Gynaecol. 1992;6(2):351–371. doi: 10.1016/S0950-3552(05)80092-6. [DOI] [PubMed] [Google Scholar]
- 62.Tabibzadeh S. Molecular control of the implantation window. Hum Reprod Update. 1998;4(5):465–471. doi: 10.1093/humupd/4.5.465. [DOI] [PubMed] [Google Scholar]
- 63.Andersen AG, Als-Nielsen B, Hornnes PJ, Franch Andersen L. Time interval from human chorionic gonadotrophin (HCG) injection to follicular rupture. Hum Reprod. 1995;10(12):3202–3205. doi: 10.1093/oxfordjournals.humrep.a135888. [DOI] [PubMed] [Google Scholar]
- 64.Fatemi HM, Kyrou D, Bourgain C, Van den Abeel E, Griesunger G, Devroey P. Cryopreserved-thawed human embryo transfer: spontaneous natural cycle is superior to human chorionic gonadotropin-induced natural cycle. Fertil Steril. 2010;94(6):2054–2058. doi: 10.1016/j.fertnstert.2009.11.036. [DOI] [PubMed] [Google Scholar]
- 65.Griesinger G, Weig M, Schroer A, Diedrich K, Kolibianakis EM. Mid-cycle serum levels of endogenous LH are not associated with the likelihood of pregnancy in artificial frozen-thawed embryo transfer cycles without pituitary suppression. Hum Reprod. 2007;22(10):2589–2593. doi: 10.1093/humrep/dem207. [DOI] [PubMed] [Google Scholar]
- 66.Alfarawati S, Fragouli E, Colls P, Stevens J, Gutiérrez-Mateo C, Schoolcraft WB, Katz-Jaffe MG, Wells D. The relationship between blastocyst morphology, chromosomal abnormality, and embryo gender. Fertil Steril. 2011;95(2):520–524. doi: 10.1016/j.fertnstert.2010.04.003. [DOI] [PubMed] [Google Scholar]
- 67.Kroener L, Ambartsumyan G, Briton-Jones C, Dumesic D, Surrey M, Munné S, Hill D. The effect of timing of embryonic progression on chromosomal abnormality. Fertil Steril. 2012;98(4):876–880. doi: 10.1016/j.fertnstert.2012.06.014. [DOI] [PubMed] [Google Scholar]
- 68.Forman EJ, Upham KM, Cheng M, Zhao T, Hong KH, Treff NR, Scott RT., Jr Comprehensive chromosome screening alters traditional morphology-based embryo selection: a prospective study of 100 consecutive cycles of planned fresh euploid blastocyst transfer. Fertil Steril. 2013;100(3):718–724. doi: 10.1016/j.fertnstert.2013.04.043. [DOI] [PubMed] [Google Scholar]
- 69.Weissman A, Horowitz E, Ravhon A, Steinfeld Z, Mutzafi R, Golan A, Levran D. Spontaneous ovulation versus HCG triggering for timing natural-cycle frozen-thawed embryo transfer: a randomized study. Reprod Biomed Online. 2011;23(4):484–489. doi: 10.1016/j.rbmo.2011.06.004. [DOI] [PubMed] [Google Scholar]
- 70.Mahajan NN, Turnbull DA, Davies MJ, Jindal UN, Briggs NE, Taplin JE. Changes in affect and state anxiety across an in vitro fertilization/intracytoplasmic sperm injection cycle. Fertil Steril. 2010;93(2):517–526. doi: 10.1016/j.fertnstert.2008.12.054. [DOI] [PubMed] [Google Scholar]
- 71.Verhaak CM, Smeenk JM, Evers AW, Kremer JA, Kraaimaat FW, Braat DD. Women's emotional adjustment to IVF: a systematic review of 25 years of research. Hum Reprod Update. 2007;13(1):27–36. doi: 10.1093/humupd/dml040. [DOI] [PubMed] [Google Scholar]
- 72.Cousineau TM, Domar AD. Psychological impact of infertility. Best Pract Res Clin Obstet Gynaecol. 2007;21(2):293–308. doi: 10.1016/j.bpobgyn.2006.12.003. [DOI] [PubMed] [Google Scholar]
- 73.Turner K, Reynolds-May MF, Zitek EM, Tisdale RL, Carlisle AB, Westphal LM. Stress and anxiety scores in first and repeat IVF cycles: a pilot study. PLoS One. 2013;23:8(5). doi: 10.1371/journal.pone.0063743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Stenbæk DS, Toftager M, Hjordt LV, Jensen PS, Holst KK, Bryndorf T, Holland T, Bogstad J, Pinborg A, Hornnes P, Frokjaer VG. Mental distress and personality in women undergoing GnRH agonist versus GnRH antagonist protocols for assisted reproductive technology. Hum Reprod. 2015;30(1):103–110. doi: 10.1093/humrep/deu294. [DOI] [PubMed] [Google Scholar]
- 75.Karatas JC, Strong KA, Barlow-Stewart K, McMahon C, Meiser B, Roberts C. Psychological impact of preimplantation genetic diagnosis: a review of the literature. Reprod Biomed Online. 2010;20(1):83–91. doi: 10.1016/j.rbmo.2009.10.005. [DOI] [PubMed] [Google Scholar]