Abstract
Objective. The eradication rate of Helicobacter pylori (H. pylori) following standard triple therapy has declined over the past few decades. This study has determined whether high dose dual therapy (PPI and amoxicillin) is adequate for eradicating H. pylori in Korea. Methods. This was an open-labeled study of H. pylori infected treatment-naive patients. Subjects received dual therapy for 14 days: ilaprazole 40 mg tablets given twice a day and amoxicillin 750 mg tablets given 4 times a day. At the end of the therapy, the subjects visited the clinic to confirm compliance and monitor for any side effects. Subjects visited again after 4–6 weeks to confirm H. pylori status through a urea breath test. Results. The cure rate of H. pylori was 79.3% (23 of 29) (95% confidence interval: 61.6–90.2) in the intention-to-treat analysis and 82.1% (23 of 28) in the per-protocol analysis. Compliance rates were high (96.6%) and side effects were minimal and tolerable. Conclusion. A high dose of ilaprazole + amoxicillin was ineffective as the first-line therapy for eradicating H. pylori in Korea. Future studies should focus on intragastric pH measurements and assess amoxicillin resistance.
1. Introduction
Helicobacter pylori (H. pylori) has been related to a range of gastrointestinal (GI) diseases, such as peptic ulcers, gastric adenocarcinoma, and chronic gastritis [1]. Peptic ulcers are considered to be an infectious disease, and eradicating the causative microbe is a cure. Many studies have reported that H. pylori is etiologically related to mucosa-associated lymphoid tissue (MALT) lymphoma and gastric cancer [2–4]. Elimination of H. pylori induces the regression of MALT lymphomas and decreases the occurrence of metachronous relapse, following the removal of early gastric cancer by endoscopic resection [5, 6].
Until recently, a proton pump inhibitor (PPI) and the antibiotics clarithromycin and amoxicillin/metronidazole, the so-called standard triple therapy, was used as the first-line eradication therapy for H. pylori infection, worldwide [7–10]. Unfortunately, the efficacy of this standard triple regimen has decreased over the past decades, which is thought to be due in large part to increasing antimicrobial resistance. The declined effectiveness of standard triple therapy was also confirmed in a recent Korean study, using meta-analysis. The overall success rate was 74.6% in an intention-to-treat (ITT) analysis and 82% in a per-protocol (PP) analysis [11]. Indiscriminate antibiotics use for the treatments of upper respiratory infections, urinary tract infections, and nonbacterial diseases, such as the common cold, may cause resistance-associated H. pylori eradication failure.
The high dose dual regimen, as an alternative first-line medication, is comprised of a high dose PPI and amoxicillin to which H. pylori has shown relatively low resistance [12]. Some studies that revealed an increasing effectiveness of a high dose and/or more frequent PPI and amoxicillin administration also reported eradication rates greater than 90% [13, 14]. Despite the advantage of high doses, however, eradication rates vary among studies, and several studies have reported that the CYP2C19 genotype may affect the eradication rate [15, 16]. It is generally thought that high dose PPI therapy is required to reliably increase gastric pH to 6 or above [17, 18]. In addition, data regarding the efficacy, side effects, and patient adherence to the new therapy is scarce in Korea. Therefore, this study was designed to demonstrate whether or not high dose PPI, plus amoxicillin, is efficient as the first-line treatment for H. pylori and elucidate compliance and side effects of this therapy.
2. Subjects and Methods
2.1. Subjects
Eligible subjects were 20–80 years of age. Candidates were consecutive, treatment-naive subjects who underwent upper GI endoscopy with proven H. pylori based on Giemsa stained histological findings, H. pylori IgG serology, or the rapid urease test (CLO test; Kimberly-Clark Co., Draper, UT, USA) performed at Dongguk University Ilsan Hospital, Korea, between January 8, 2015 and May 8, 2015. Subjects who consented were recruited into the study and their demographic information, including age, sex, underlying gastric disease, smoking, and alcohol consumption, was also collected.
Subjects who fulfilled 1 or more of the following criteria were excluded from the study: allergy or contraindication to any study medication; abnormal laboratory findings (total bilirubin or creatinine > 1.5 times the upper normal limit, or aspartate aminotransferase, and alanine aminotransferase or blood urea nitrogen > 2 times the upper normal limit); PPI or antibiotics taking within the preceding 4 weeks; pregnancy or lactating; female of childbearing age not using a medically accepted contraceptive method (menopausal women with more than twelve months of amenorrhea were regarded as nonchild bearing); presence of uncontrolled diabetes mellitus, hypertension, or hepatic disease; alcohol abuse; history of GI malignancy, except gastric adenoma or early gastric cancer resected by an endoscopic procedure within the preceding 5 years; previous stomach or esophageal surgery, such as gastrectomy or partial esophagostomy; and enrollment in another clinical research study within the preceding 30 days.
This clinical trial was approved by the DUIH Institutional Review Board (2014.121) and is registered at Clinical Trials.gov (NCT02401477). All subjects were provided with the study medication after written informed consent had been obtained.
2.2. Study Design
2.2.1. Eradication Regimen
This was an open-labeled study and both the physicians and the subjects were not blinded to the study medication. Subjects received eradication therapy consisting of 2 drugs. The newly introduced ilaprazole (IL-Yang Pharmaceutical Co., Ltd., Seoul, Korea) as 40 mg tablets were taken twice a day, 1 hour before breakfast and dinner, approximately 12-hour interval, and 750 mg amoxicillin tablets were taken 4 times a day, 30 min after meals and before sleep. A 5- to 6-hour interval was recommended as the interval of administration. The total daily doses were 80 mg ilaprazole and 3,000 mg amoxicillin for 14 days. These drugs were offered in customized blister packs to assure that subjects kept to the study regimen.
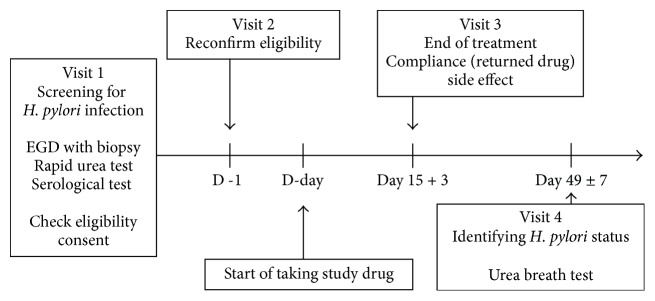
Subjects were requested to revisit the clinic after 14 days from the start of taking the study medication to determine compliance and any side effects. Subjects were instructed to return empty blister packs and all unused medications. Compliance was estimated using the remaining pills; taking less than 80% of all prescribed drugs was classified as poor compliance. Side effects were recorded as experienced symptoms after ingesting the drugs. PPIs and amoxicillin can cause unpleasant symptoms including nausea, vomiting, diarrhea, and abdominal pain. Side effects were assessed using a standard questionnaire, and any symptom that caused subjects to discontinue taking the medication was considered severe. Blood samples were taken to identify any systemic side effects, including drug-related liver function test abnormalities (Figure 1).
Figure 1.
Flow chart of study methodology. EGD: esophagogastroduodenoscopy.
2.2.2. Eradication Assessment
All subjects returned 4 to 6 weeks after treatment to assess eradication of the infection through a urea breath test (UBT). PPI use was prohibited during the 2-week period prior to assessment of therapeutic efficacy. Breath samples were obtained before and 15 min after oral administration of 100 mg urea labeled with 13C (UBIT; Korea Otsuka Pharmaceutical Co., Seoul, Korea), after an overnight fast. All samples were analyzed by infrared spectrophotometry (UBiT-IR300; Otsuka Electronics Co., Ltd., Osaka, Japan). The difference between the baseline 15-minute values was expressed as delta (δ) over baseline (DOB, δ‰), and DOB > 2.5‰ was considered positive [19]. We determined the eradication rate using an ITT analysis to estimate the practical efficacy and a PP analysis to estimate the biological efficacy. A success rate > 80% in the ITT analysis and > 90% in the PP analysis was defined as a suitable first-line eradication therapy [10, 20]. Entry of subjects into the study was designed to be ceased if it became clear that these rates could not be achieved to limit exposure of subjects to unnecessary risks according to the study termination criteria of 6 or more failures within 50 subjects or a success rate of less than 80% [21].
2.3. Statistical Analyses
Analyses were performed using SPSS for Windows ver. 20.0 software package (SPSS Inc., Chicago, IL, USA). Categorical variables were presented as number (percentage) and continuous variables as mean ± standard deviation. The eradication rate was calculated as a percentage of all enrolled subjects with a 95% confidence interval (CI). Categorical variables were compared among groups through the chi-square or Fisher's exact test as appropriate, and continuous variables were compared using Student's t-test. A P value of less than 0.05 was considered significant. All statistical methods were reviewed by the biostatistician (Chi Yeon Lim).
3. Results
3.1. Subject Characteristics
A total of 29 subjects were enrolled (17 male and 12 female; mean age, 47.9 ± 2 years). The H. pylori infection state was proven using rapid urease testing in 21 patients (72.4%) and using histology in 8 subjects (27.6%). Underlying gastric diseases diagnosed by endoscopy included gastritis (n = 27, 93.1%), gastric ulcer (n = 1, 4.3%), and duodenal ulcer (n = 1, 4.3%). Eight patients (27.6%) were current smokers, and 20 patients (69.0%) were social or heavy drinkers. All factors in the eradication success and failure groups are presented in Table 1. No differences were found in these factors between groups.
Table 1.
Baseline characteristics of the study population.
| Characteristics | Eradication success∗
n = 23 |
Eradication failure n = 6 |
P value |
|---|---|---|---|
| Gender, n (%) | 0.20 | ||
| Male | 15 (65.2) | 2 (33.3) | |
| Female | 8 (34.8) | 4 (66.7) | |
| Age, years, mean ± SD | 47.5 ± 10.6 | 49.3 ± 7.1 | 0.63 |
| Underlying gastric disease, n (%) | |||
| Gastritis | 21 (91.3) | 6 (100.0) | 1.00 |
| Gastric ulcer | 1 (4.3) | 0 (0.0) | 1.00 |
| Duodenal ulcer | 1 (4.3) | 0 (0.0) | 1.00 |
| Smoking, n (%) | |||
| Current smoker | 7 (30.4) | 1 (16.7) | 0.65 |
| Ex-smoker | 5 (21.7) | 0 (0) | 0.55 |
| Never smoker | 11 (47.9) | 5 (83.3) | 0.18 |
| Alcohol, n (%) | |||
| Nonalcohol drinker | 6 (26.1) | 3 (50.0) | 0.55 |
| Social drinker† | 14 (60.9) | 2 (33.3) | 0.25 |
| Heavy drinker‡ | 3 (13.0) | 1 (16.7) | 0.36 |
| Compliance, n (%) | 0.20 | ||
| Good | 23 (100) | 5 (83.3) | |
| Bad§ | 0 (0) | 1 (16.7) | |
| Side effect, n (%) | 3 (13.0) | 0 (0) | 1.00 |
∗ Helicobacter pylori eradication rate was 79.3% based on the intention-to-treat analysis. †Drinking fewer than 3 bottles/week, ‡drinking more than 3 bottles/week, and §taking less than 80% of all study drugs. The P value was derived from Student's t-test for age variable, and the P values for the other variables were derived from the chi-square or Fisher's exact test. SD: standard deviation.
3.2. Eradication Rates
The success rate of H. pylori eradication was 23 of 29 or 79.3% (95% CI: 61.6–90.2) in the ITT analysis and 23 of 28 or 82.1% (95% CI: 64.4–93.2) in the PP analysis.
3.3. Compliance and Drug-Induced Side Effects
Compliance was found to be 96.6% (28/29). Only 1 patient did not take over 80% of the study drugs due to reasons unrelated to the medication or study itself. Side effects were reported by 3 subjects (10.3%): 2 cases of diarrhea and 1 of epigastric discomfort. These symptoms were mostly mild, and no subjects ceased treatment due to side effects. According to the blood test results, one patient exhibited a mild drug-related elevation of transaminases; however, these levels normalized 2 weeks later (Table 2).
Table 2.
Side effects of high dose ilaprazole plus amoxicillin therapy.
| Side effects | Number of subjects (%) n = 29 |
|---|---|
| Nausea | 0 (0.0%) |
| Diarrhea | 2 (6.9%) |
| Vomiting | 0 (0.0%) |
| Abdominal pain | 0 (0.0%) |
| Metallic taste | 0 (0.0%) |
| Epigastric soreness | 1 (3.4%) |
| Drug related LFT abnormality | 1 (3.4%) |
LFT: liver function test.
4. Discussion
A large number of previous studies have found abundant evidence that H. pylori is etiologically related to GI diseases, including peptic ulcers, MALT lymphoma, and gastric cancer. The present recommendation is that an H. pylori infection should be eradicated with antibiotics in the above-mentioned diseases [22, 23].
Standard triple therapy based on PPI is the first-line treatment regimen, most commonly used worldwide. The eradication success rate using this therapy however has declined over the past decade and fails to reach even 80% in most studies in the central and southern European countries of Spain, France, Italy, and Turkey [24, 25]. These results are thought to be largely due to clarithromycin-resistant strains of H. pylori [26, 27], and, as such, the development of several alternative first-line therapeutic options is required. The most recent Maastricht IV/Florence Consensus Report recommended an alternative first-line therapy consisting of a bismuth-containing and/or nonbismuth quadruple therapy or sequential treatment to replace standard triple therapy in areas with a high resistance rate to clarithromycin (resistance rate over 15–20%) [28]. In addition to the above-mentioned method, high dose dual PPI plus amoxicillin or alternative antibiotics, such as rifabutin, furazolidone, and levofloxacin, has also been suggested as an alternative first-line therapy [29–32].
The present study evaluated effectiveness of high dose ilaprazole plus amoxicillin for H. pylori eradication. Ilaprazole is a new benzimidazole compound, so called third generation PPI, synthesized in Korea. In terms of pharmacokinetics, ilaprazole showed more prolonged plasma half-life time (8.1–10.1 hr) than prior PPIs (0.5–2 hr) [33] and it has been well known that ilaprazole is minimally metabolized by CYP2C19, main metabolic enzyme for first- and second-generation PPIs, so that lower variability of the medicinal effect was found among these PPI takers and there were no differences in mean intragastric pH for 24 hr, the percentage of time of a pH > 4, and healing rate of duodenal ulcer among the different phenotypes of CYP2C19 [34, 35]. In the present study, the eradication rate of H. pylori also had no difference among the different CYP2C19 phenotypes. To date, ilaprazole has several clinical data in spite of relatively short time of postmarket drug usage as compared with other PPIs. In the recent two studies, only studies that compared the gastric acid suppression of ilaprazole with that of other PPIs in healthy volunteers in China and Korea, ilaprazole exhibited significantly greater and prolonged suppression of gastric acid than omeprazole (ilaprazole 10, 20 mg versus omeprazole 20 mg) and esomeprazole (ilaprazole 20 mg, 40 mg versus esomeprazole 40 mg) [34, 36]. In the study for the efficacy of ilaprazole in duodenal ulcer, four randomized control trials displayed that ilaprazole was as effective as omeprazole [37].
The high dose dual therapy regimen in this study is based on two observations. Firstly, primary resistance to amoxicillin is usually low worldwide, and secondary amoxicillin resistance is also rare. The amoxicillin resistance rate of H. pylori is 0.5–2.2% but is as high as 17.8% in Africa [12, 28]. The bactericidal effect of amoxicillin against H. pylori is thought to be time-dependent, thus making more frequent doses better than less frequent doses [38, 39]. Secondly, H. pylori is thought to move into a nonreplicative but viable state when the intragastric pH is less than 6 but higher than 3. The bacteria move into the replicative state when the pH increases over 6 and in turn become susceptible to amoxicillin [40–42].
A study in Japan, using 10 mg rabeprazole and 500 mg amoxicillin, 4 times a day for 2 weeks, reported a cure rate of 90.9% (95% CI: 81.2–96.6) in the ITT analysis and 93.8% (95% CI: 84.8–98.3) in the PP analysis [43]. In addition, a study in China reported that eradication was achieved in 89.8% (95% CI: 82.1–87.6) of 117 subjects in the ITT analysis and 93.0% (95% CI: 86.4–99.6) in the PP analysis, using 20 mg rabeprazole twice and 1 g amoxicillin 4 times a day, for 2 weeks [44]. In this study, the eradication rate was 79.3% (23/29) in the ITT analysis and 82.1% (23/28) in the PP analysis. We administered 40 mg ilaprazole, which is 4 times the recommended dose for a duodenal ulcer [45]; yet the results did not meet our expectations. In a USA based study, using 40 mg esomeprazole plus 750 mg amoxicillin 3 times a day for 2 weeks, reported an ITT eradication rate of 72.2% (95% CI: 56–84) and 74.2% in the PP analysis (95% CI: 56–87) [46]. Another study in Korea using 30 mg lansoprazole plus 750 mg amoxicillin 3 times a day for 2 weeks showed that cure rates of based on ITT and PP analyses were 67.3% (95% CI: 58.3–76.3) and 74% (95% CI: 65.5–82.4), respectively [47]. The treatment success rate in the present study did not satisfy the requirements for first-line therapy according to the “report card” format with scales of A ≥ 95%, B = 90–94%, C = 85–89%, D = 81–84%, and F ≤ 80% from the ITT analyses and A = 95–100%, B = 90–94%, C = 86–89%, and F ≤ 85% from the PP analyses. The effectiveness grades based on the ITT and PP analyses were “F” and “unacceptable” [48]. Unfortunately, we found no higher eradication rate, even compared with the overall rate from a meta-analysis of standard triple therapy in Korea. This study set a goal of enrolling 54 subjects based on the sample size calculation prior to the study. We stopped enrolling additional subjects after the interim analysis for ethical reasons and to limit exposing the subjects to unnecessary risks according to the stop rule (6 or more failures within 50 subjects or an eradication rate of less than 80%).
Compliance of the proposed eradication regimen in the present study was high and side effects were mild and minimal. No subjects stopped taking the prescribed drugs due to side effects, and no significant difference was found in the prevalence of side effects between the eradication success and failure groups. Therefore, these factors apparently did not influence the low eradication rate in this study. Smoking has previously been considered a significant factor related to treatment failure of dual therapy [49]. Smoking lowers intragastric pH by raising acid secretion and decreasing pancreatic and duodenal bicarbonate secretion [50–52]. Although our study population was small, we found no association between smoking and eradication rate. We also hypothesized that drinking could affect drug compliance, but drinking status did not affect the eradication rate. Consequently, compliance, side effects, smoking, and drinking states were not associated with the eradication rate in this study.
This study had several limitations. We did not measure 24 hr intragastric pH during the study, so we were unable to ascertain whether intragastric pH was maintained over 6 and for how long. However, we did calculate the dose of ilaprazole to be equivalent to the lansoprazole dose that has been shown to maintain the intragastric pH over 6.
In conclusion, high dose ilaprazole twice daily and amoxicillin 4 times daily was ineffective as a first-line therapy to eradicate H. pylori in Korea. The best reported results have been obtained by administering a PPI and amoxicillin 4 times a day [13]. It seems prudent in the future to start with 4 times a day PPI and amoxicillin administration or alter the timing of PPI administration based upon the intragastric pH measurements and to test for amoxicillin resistance.
Acknowledgments
The present study was supported by IL-Yang Pharmaceutical Co., Ltd.
Competing Interests
The authors declare that there is no conflict of interests regarding the publication of this paper.
References
- 1.Suerbaum S., Michetti P. Helicobacter pylori infection. The New England Journal of Medicine. 2002;347(15):1175–1186. doi: 10.1056/nejmra020542. [DOI] [PubMed] [Google Scholar]
- 2.Forman D., Newell D., Fullerton F., et al. Association between infection with Helicobacter pylori and risk of gastric cancer: evidence from a prospective investigation. British Medical Journal. 1991;6788(302):1302–1305. doi: 10.1136/bmj.302.6788.1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nomura A., Stemmermann G. N., Chyou P.-H., Kato I., Perez-Perez G. I., Blaser M. J. Helicobacter pylori infection and gastric carcinoma among Japanese Americans in Hawaii. The New England Journal of Medicine. 1991;325(16):1132–1136. doi: 10.1056/nejm199110173251604. [DOI] [PubMed] [Google Scholar]
- 4.Parsonnet J., Friedman G. D., Vandersteen D. P., et al. Helicobacter pylori infection and the risk of gastric carcinoma. New England Journal of Medicine. 1991;325(16):1127–1131. doi: 10.1056/NEJM199110173251603. [DOI] [PubMed] [Google Scholar]
- 5.Bayerdörffer E., Rudolph B., Neubauer A., et al. Regression of primary gastric lymphoma of mucosa-associated lymphoid tissue type after cure of Helicobacter pylori infection. The Lancet. 1995;345(8965):1591–1594. doi: 10.1016/s0140-6736(95)90113-2. [DOI] [PubMed] [Google Scholar]
- 6.Uemura N., Mukai T., Okamoto S., et al. Effect of Helicobacter pylori eradication on subsequent development of cancer after endoscopic resection of early gastric cancer. Cancer Epidemiology Biomarkers and Prevention. 1997;6(8):639–642. [PubMed] [Google Scholar]
- 7.Lee J. W., Kim N., Kim J. M., et al. Prevalence of primary and secondary antimicrobial resistance of helicobacter pylori in Korea from 2003 through 2012. Helicobacter. 2013;18(3):206–214. doi: 10.1111/hel.12031. [DOI] [PubMed] [Google Scholar]
- 8.An B., Moon B. S., Kim H., et al. Antibiotic resistance in Helicobacter pylori strains and its effect on H. pylori eradication rates in a single center in Korea. Annals of Laboratory Medicine. 2013;33(6):415–419. doi: 10.3343/alm.2013.33.6.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yoon K.-H., Park S. W. O., Lee S. W. O., Kim B. J. I., Kim J. G. Y. Clarithromycin-based standard triple therapy can still be effective for Helicobacter pylori eradication in some parts of the Korea. Journal of Korean Medical Science. 2014;29(9):1240–1246. doi: 10.3346/jkms.2014.29.9.1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Malfertheiner P., Megraud F., O'Morain C. A., et al. Management of Helicobacter pylori infection—the Maastricht IV/Florence consensus report. Gut. 2012;61(5):646–664. doi: 10.1136/gutjnl-2012-302084. [DOI] [PubMed] [Google Scholar]
- 11.Gong E. J. E., Yun S.-C., Jung H.-Y., et al. Meta-analysis of first-line triple therapy for Helicobacter pylori eradication in Korea: is it time to change? Journal of Korean Medical Science. 2014;29(5):704–713. doi: 10.3346/jkms.2014.29.5.704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.de Francesco V., Giorgio F., Hassan C., et al. Worldwide H. pylori antibiotic resistance: a systematic review. Journal of Gastrointestinal and Liver Diseases. 2010;19(4):409–414. [PubMed] [Google Scholar]
- 13.Furuta T., Graham D. Y. Pharmacologic aspects of eradication therapy for Helicobacter pylori infection. Gastroenterology Clinics of North America. 2010;39(3):465–480. doi: 10.1016/j.gtc.2010.08.007. [DOI] [PubMed] [Google Scholar]
- 14.Yang J.-C., Lin C.-J., Wang H.-L., et al. High-dose dual therapy is superior to standard first-line or rescue therapy for Helicobacter pylori infection. Clinical Gastroenterology and Hepatology. 2015;13(5):895–905.e5. doi: 10.1016/j.cgh.2014.10.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Furuta T., Shirai N., Takashima M., et al. Effect of genotypic differences in CYP2C19 on cure rates for Helicobacter pylori infection by triple therapy with a proton pump inhibitor, amoxicillin, and clarithromycin. Clinical Pharmacology and Therapeutics. 2001;69(3):158–168. doi: 10.1067/mcp.2001.113959. [DOI] [PubMed] [Google Scholar]
- 16.Furuta T., Sagehashi Y., Shirai N., et al. Influence of CYP2C19 polymorphism and Helicobacter pylori genotype determined from gastric tissue samples on response to triple therapy for H pylori infection. Clinical Gastroenterology and Hepatology. 2005;3(6):564–573. doi: 10.1016/s1542-3565(04)00779-7. [DOI] [PubMed] [Google Scholar]
- 17.Graham D. Y., Shiotani A. New concepts of resistance in the treatment of Helicobacter pylori infections. Nature Clinical Practice Gastroenterology and Hepatology. 2008;5(6):321–331. doi: 10.1038/ncpgasthep1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Marcus E. A., Inatomi N., Nagami G. T., Sachs G., Scott D. R. The effects of varying acidity on Helicobacter pylori growth and the bactericidal efficacy of ampicillin. Alimentary Pharmacology and Therapeutics. 2012;36(10):972–979. doi: 10.1111/apt.12059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ohara S., Kato M., Asaka M., Toyota T. Studies of 13C-urea breath test for diagnosis of Helicobacter pylori infection in Japan. Journal of Gastroenterology. 1998;33(1):6–13. doi: 10.1007/pl00009968. [DOI] [PubMed] [Google Scholar]
- 20.Fock K. M., Katelaris P., Sugano K., et al. Second Asia-Pacific Consensus Guidelines for Helicobacter pylori infection. Journal of Gastroenterology and Hepatology. 2009;24(10):1587–1600. doi: 10.1111/j.1440-1746.2009.05982.x. [DOI] [PubMed] [Google Scholar]
- 21.Graham D. Y. Efficient identification and evaluation of effective Helicobacter pylori therapies. Clinical Gastroenterology and Hepatology. 2009;7(2):145–148. doi: 10.1016/j.cgh.2008.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Marcus E. A., Sachs G., Wen Y., Scott D. R. Phosphorylation-dependent and Phosphorylation-independent Regulation of Helicobacter pylori Acid Acclimation by the ArsRS Two-component System. Helicobacter. 2016;21(1):69–81. doi: 10.1111/hel.12235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sugano K., Tack J., Kuipers E. J., et al. Kyoto global consensus report on Helicobacter pylori gastritis. Gut. 2015;64:1353–1367. doi: 10.1136/gutjnl-2015-309252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Graham D. Y., Fischbach L. Helicobacter pylori treatment in the era of increasing antibiotic resistance. Gut. 2010;59(8):1143–1153. doi: 10.1136/gut.2009.192757. [DOI] [PubMed] [Google Scholar]
- 25.Cattoir V., Nectoux J., Lascols C., et al. Update on fluoroquinolone resistance in Helicobacter pylori: new mutations leading to resistance and first description of a gyrA polymorphism associated with hypersusceptibility. International Journal of Antimicrobial Agents. 2007;29(4):389–396. doi: 10.1016/j.ijantimicag.2006.11.007. [DOI] [PubMed] [Google Scholar]
- 26.Horiki N., Omata F., Uemura M., et al. Annual change of primary resistance to clarithromycin among helicobacter pylori isolates from 1996 through 2008 in Japan. Helicobacter. 2009;14(5):438–442. doi: 10.1111/j.1523-5378.2009.00714.x. [DOI] [PubMed] [Google Scholar]
- 27.Mégraud F. H pylori antibiotic resistance: prevalence, importance, and advances in testing. Gut. 2004;53(9):1374–1384. doi: 10.1136/gut.2003.022111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yang J.-C., Lu C.-W., Lin C.-J. Treatment of Helicobacter pylori infection: current status and future concepts. World Journal of Gastroenterology. 2014;20(18):5283–5293. doi: 10.3748/wjg.v20.i18.5283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Borody T. J., Pang G., Wettstein A. R., et al. Efficacy and safety of rifabutin-containing “rescue therapy” for resistant Helicobacter pylori infection. Alimentary Pharmacology and Therapeutics. 2006;23(4):481–488. doi: 10.1111/j.1365-2036.2006.02793.x. [DOI] [PubMed] [Google Scholar]
- 30.Isakov V., Domareva I., Koudryavtseva L., Maev I., Ganskaya Z. Furazolidone-based triple ‘rescue therapy’ vs. quadruple ‘rescue therapy’ for the eradication of Helicobacter pylori resistant to metronidazole. Alimentary Pharmacology and Therapeutics. 2002;16(7):1277–1282. doi: 10.1046/j.1365-2036.2002.01299.x. [DOI] [PubMed] [Google Scholar]
- 31.Miehlke S., Krasz S., Schneider-Brachert W., et al. Randomized trial on 14 versus 7 days of esomeprazole, moxifloxacin, and amoxicillin for second-line or rescue treatment of Helicobacter pylori infection. Helicobacter. 2011;16(6):420–426. doi: 10.1111/j.1523-5378.2011.00867.x. [DOI] [PubMed] [Google Scholar]
- 32.Gisbert J. P., Gisbert J. L., Marcos S., Moreno-Otero R., Pajares J. M. Third-line rescue therapy with levofloxacin is more effective than rifabutin rescue regimen after two Helicobacter pylori treatment failures. Alimentary Pharmacology and Therapeutics. 2006;24(10):1469–1474. doi: 10.1111/j.1365-2036.2006.03149.x. [DOI] [PubMed] [Google Scholar]
- 33.De Bortoli N., Martinucci I., Giacchino M., et al. The pharmacokinetics of ilaprazole for gastro-esophageal reflux treatment. Expert Opinion on Drug Metabolism and Toxicology. 2013;9(10):1361–1369. doi: 10.1517/17425255.2013.813018. [DOI] [PubMed] [Google Scholar]
- 34.Du Y. Q., Guo W. Y., Zou D. W., et al. Acid inhibition effect of ilaprazole on Helicobacter pylori-negative healthy volunteers: an open randomized cross-over study. Journal of Digestive Diseases. 2012;13(2):113–119. doi: 10.1111/j.1751-2980.2011.00557.x. [DOI] [PubMed] [Google Scholar]
- 35.Wang L., Zhou L., Hu H., Lin S., Xia J. Ilaprazole for the treatment of duodenal ulcer: a randomized, double-blind and controlled phase III trial. Current Medical Research and Opinion. 2012;28(1):101–109. doi: 10.1185/03007995.2011.639353. [DOI] [PubMed] [Google Scholar]
- 36.Shin J. S., Lee J. Y., Cho K. H., et al. The pharmacokinetics, pharmacodynamics and safety of oral doses of ilaprazole 10, 20 and 40 mg and esomeprazole 40 mg in healthy subjects: a randomised, open-label crossover study. Alimentary Pharmacology and Therapeutics. 2014;40(5):548–561. doi: 10.1111/apt.12860. [DOI] [PubMed] [Google Scholar]
- 37.Bohidar N. P., Krishna K., Panda B. K., Patel C. Ilaprazole: is this a superior proton pump inhibitor for duodenal ulcer? Tropical Gastroenterology. 2013;34(2):95–98. doi: 10.7869/tg.2012.105. [DOI] [PubMed] [Google Scholar]
- 38.Berry V., Jennings K., Woodnutt G. Bactericidal and morphological effects of amoxicillin on Helicobacter pylori. Antimicrobial Agents and Chemotherapy. 1995;39(8):1859–1861. doi: 10.1128/AAC.39.8.1859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Megraud F., Trimoulet P., Lamouliatte H., Boyanova L. Bactericidal effect of amoxicillin on Helicobacter pylori in an in vitro model using epithelial cells. Antimicrobial Agents and Chemotherapy. 1991;35(5):869–872. doi: 10.1128/AAC.35.5.869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gotoh A., Akamatsu T., Shimizu T., et al. Additive effect of pronase on the efficacy of eradication therapy against Helicobacter pylori. Helicobacter. 2002;7(3):183–191. doi: 10.1046/j.1523-5378.2002.00079.x. [DOI] [PubMed] [Google Scholar]
- 41.Scott D. R., Marcus E. A., Wen Y., Oh J., Sachs G. Gene expression in vivo shows that Helicobacter pylori colonizes an acidic niche on the gastric surface. Proceedings of the National Academy of Sciences of the United States of America. 2007;104(17):7235–7240. doi: 10.1073/pnas.0702300104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Scott D., Weeks D., Melchers K., Sachs G. The life and death of Helicobacter pylori. Gut. 1998;43(1):S56–S60. doi: 10.1136/gut.43.2008.S56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shirai N., Sugimoto M., Kodaira C., et al. Dual therapy with high doses of rabeprazole and amoxicillin versus triple therapy with rabeprazole, amoxicillin, and metronidazole as a rescue regimen for Helicobacter pylori infection after the standard triple therapy. European Journal of Clinical Pharmacology. 2007;63(8):743–749. doi: 10.1007/s00228-007-0302-8. [DOI] [PubMed] [Google Scholar]
- 44.Ren L., Lu H., Li H. Y., et al. New dual therapy for primary treatment of Helicobacter pylori infection: a prospective randomized study in Shanghai, China. Journal of Digestive Diseases. 2014;15(11):622–627. doi: 10.1111/1751-2980.12186. [DOI] [PubMed] [Google Scholar]
- 45.Ji X.-Q., Du J.-F., Chen G., Chen G., Yu B. Efficacy of ilaprazole in the treatment of duodenal ulcers: a meta-analysis. World Journal of Gastroenterology. 2014;20(17):5119–5123. doi: 10.3748/wjg.v20.i17.5119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Graham D. Y., Javed S. U., Keihanian S., Abudayyeh S., Opekun A. R. Dual proton pump inhibitor plus amoxicillin as an empiric anti-H. pylori therapy: studies from the United States. Journal of Gastroenterology. 2010;45(8):816–820. doi: 10.1007/s00535-010-0220-x. [DOI] [PubMed] [Google Scholar]
- 47.Kim S. Y., Jung S. W., Kim J. H., et al. Effectiveness of three times daily lansoprazole/amoxicillin dual therapy for Helicobacter pylori infection in Korea. British Journal of Clinical Pharmacology. 2012;73(1):140–143. doi: 10.1111/j.1365-2125.2011.04048.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Graham D. Y., Lu H., Yamaoka Y. A report card to grade Helicobacter pylori therapy. Helicobacter. 2007;12(4):275–278. doi: 10.1111/j.1523-5378.2007.00518.x. [DOI] [PubMed] [Google Scholar]
- 49.Labenz J., Leverkus F., Börsch G. Omeprazole plus amoxicillin for cure of helicobacter pylori infection factors influencing the treatment success. Scandinavian Journal of Gastroenterology. 1994;29(12):1070–1075. doi: 10.3109/00365529409094890. [DOI] [PubMed] [Google Scholar]
- 50.Bynum T. E., Solomon T. E., Johnson L. R., Jacobson E. D. Inhibition of pancreatic secretion in man by cigarette smoking. Gut. 1972;13(5):361–365. doi: 10.1136/gut.13.5.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Shiotani A., Graham D. Y. Pathogenesis and therapy of gastric and duodenal ulcer disease. Medical Clinics of North America. 2002;86(6):1447–1466. doi: 10.1016/S0025-7125(02)00083-4. [DOI] [PubMed] [Google Scholar]
- 52.Hogan D. L., Ainsworth M. A., Isenberg J. I. Gastroduodenal bicarbonate secretion. Alimentary Pharmacology and Therapeutics. 1994;8(5):475–488. doi: 10.1111/j.1365-2036.1994.tb00319.x. [DOI] [PubMed] [Google Scholar]