Abstract
The large adhesin protein LapA mediates adhesion and biofilm formation by Pseudomonas fluorescens. Although adhesion is thought to involve the long multiple repeats of LapA, very little is known about the molecular mechanism by which this protein mediates attachment. Here we use atomic force microscopy to unravel the biophysical properties driving LapA-mediated adhesion. Single-cell force spectroscopy shows that expression of LapA on the cell surface via biofilm-inducing conditions (i.e., phosphate-rich medium) or deletion of the gene encoding the LapG protease (LapA+ mutant) increases the adhesion strength of P. fluorescens toward hydrophobic and hydrophilic substrates, consistent with the adherent phenotypes observed in these conditions. Substrate chemistry plays an unexpected role in modulating the mechanical response of LapA, with sequential unfolding of the multiple repeats occurring only on hydrophilic substrates. Biofilm induction also leads to shortening of the protein extensions, reflecting stiffening of their conformational properties. Using single-molecule force spectroscopy, we next demonstrate that the adhesin is randomly distributed on the surface of wild-type cells and can be released into the solution. For LapA+ mutant cells, we found that the adhesin massively accumulates on the cell surface without being released and that individual LapA repeats unfold when subjected to force. The remarkable adhesive and mechanical properties of LapA provide a molecular basis for the “multi-purpose” adhesion function of LapA, thereby making P. fluorescens capable of colonizing diverse environments.
Graphical abstract
In nature, most bacteria form surface-associated pluricellular communities known as biofilms.1–4 Biofilm formation involves a series of regulated steps that include initial attachment of the cells to biotic or abiotic surfaces, followed by cell–cell interactions.2,5 The soil bacterium Pseudomonas fluorescens forms biofilms on a wide variety of biotic6–9 and abiotic surfaces.10–13 Besides being found in soils as a root colonizer and biological control agent,12,14 P. fluorescens can also be isolated from water and is sometimes involved in nosocomial diseases.15,16 The P. fluorescens large adhesin LapA is a cell-surface protein required for substrate attachment and biofilm formation.10,17–20 This ~520 kDa protein is composed of an N-terminal region containing the LapG cleavage site, followed by 37 repeats each of ~100 amino acids, and a C-terminal region composed of a Calx-β domain, a von Willebrand factor type A (vWA) domain, six repeats-in-toxins (RTX), and a type 1 secretion system signal.20 LapA at the cell surface is regulated by the LapD-LapG signaling system that allows precise control of cell attachment and subsequent biofilm formation.11,21,22 The LapD-LapG system is controlled by the environmental signal inorganic phosphate (Pi)19 and via regulating the levels of the secondary intracellular messenger, cyclic dimeric GMP (c-di-GMP).11,19,21–24 LapA is exported from the cytoplasm to the cell surface by the ABC transporter encoded by the lapEBC genes.17 At low Pi concentrations, induction of the Pho regulon reduces the intracellular concentration of c-di-GMP via expression of the c-di-GMP-degrading phosphodiesterase RapA.19,22,24 Under conditions of low c-di-GMP, LapD-mediated inhibition of the LapG protease is relieved, and the LapG protease cleaves the N-terminus of LapA.19,22,25,26 The proteolytically processed LapA is then released from the cell surface, preventing cell adhesion.11,17,25 Thus, strains in which the lapG gene has been deleted (designated here as LapA+ mutants) accumulate the LapA adhesin on the cell surface leading to a hyper-adherent biofilm phenotype.11,25 Although the mechanisms regulating LapA at the cell surface have been widely investigated, the molecular interactions driving LapA-mediated adhesion are poorly understood.
Here, we use atomic force microscopy (AFM) to unravel the biophysical properties of LapA, i.e., cell-surface density, localization and dynamics, adhesion forces, and molecular elasticity, at the single-cell and single-molecule levels. We compare the behavior of two P. fluorescens strains, a wild-type (WT) strain in which LapA localization and release are controlled by the Pi concentration and a mutant strain (LapA+ mutant) that accumulates LapA at the cell surface.11,25 Using single-cell force spectroscopy (SCFS)27,28 we quantify the adhesion forces between individual bacteria (WT and LapA+) on hydrophobic or hydrophilic substrates and investigate the influence of biofilm-inducing conditions (Pi concentration). We correlate these data with the localization, adhesion, and extension of individual LapA molecules on live cells using single-molecule force spectroscopy with specific antibody probes (SMFS).29,30 The results show that the multimodular nature of LapA endows P. fluorescens with remarkable adhesive and mechanical properties, thereby favoring a model in which the protein functions as a “multi-purpose” adhesive able to attach to a variety of surfaces.12,17
RESULTS AND DISCUSSION
LapA Mediates Bacterial Adhesion to Hydrophilic and Hydrophobic Solid Substrates
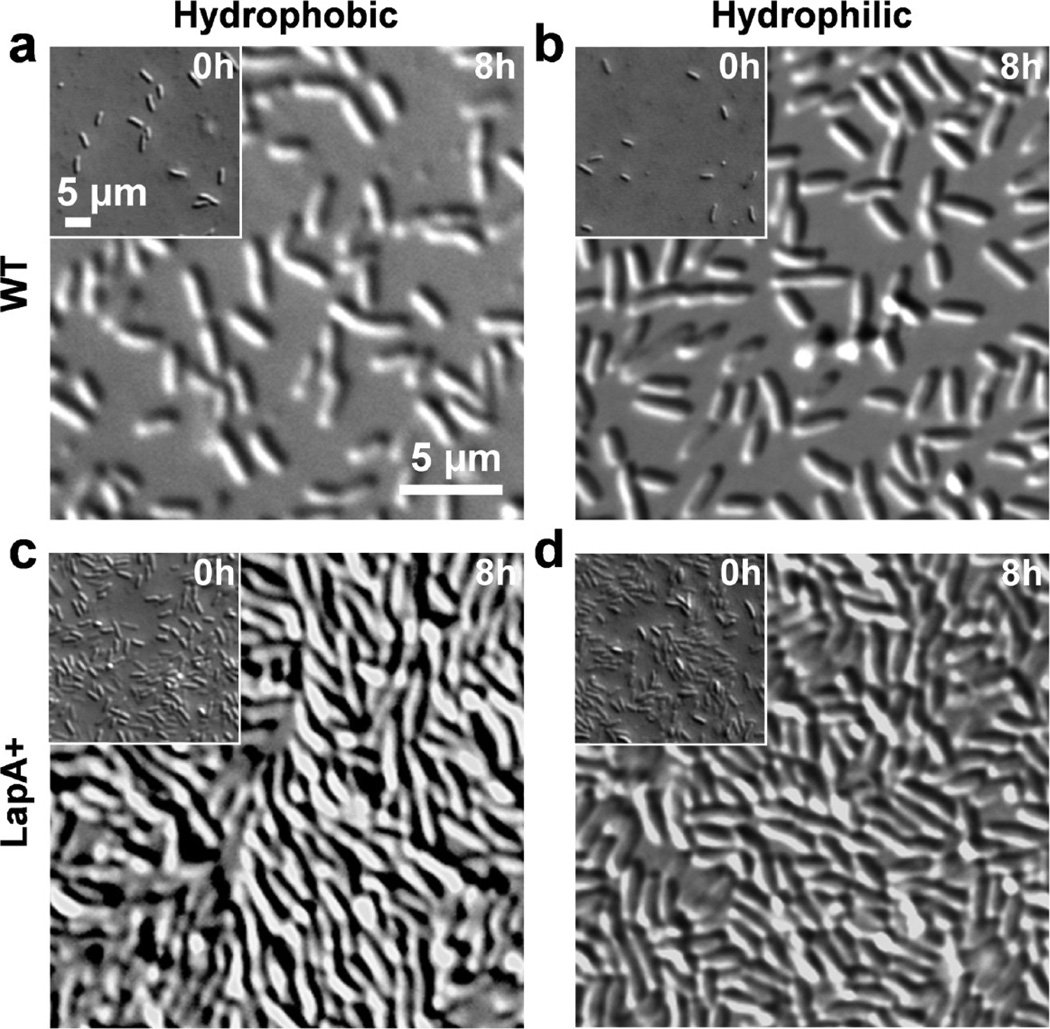
We first investigated the role of LapA in controlling bacterial adhesion to hydrophobic and hydrophilic substrates (Figure 1). We compared the behavior of the WT strain with that of the LapA+ mutant that increases cell-surface LapA as a result of deleting the LapG protease11,25 and with that of the LapA- mutant (Supplementary Figures 1a and 1c), which does not expose LapA as a result of inactivation of LapB, a component of the LapA transporter.19 Adhesion assays demonstrated that biofilm induction promoted WT adhesion since surface coverage on both hydrophobic and hydrophilic substrates increased from ~1% at 0 h to ~25% at 8 h (Figures 1a and 1b). LapA+ mutant cells were more adhesive than WT cells under all conditions tested as they occupied ~40% of the surfaces at 0 h, and ~90% at 8h, forming densely packed monolayers (Figures 1c and 1d). By contrast, no adhesive cells were observed for LapA- mutant cells (Supplementary Figures 1a and 1c, surface coverage <1%). Consistent with earlier studies,12,17–20 these results show that LapA plays a major role in P. fluorescens surface adhesion and that LapA+, with increased cell-surface LapA, leads to hyper-adherent phenotypes, without any apparent preference between hydrophobic and hydrophilic chemistries.
Figure 1.
LapA mediates adhesion of P. fluorescens to hydrophobic and hydrophilic substrates. DIC images showing the microscopic adhesion behavior of the wild-type (WT) strain (a, b) and of the mutant strain (a lapG mutant designated “LapA+”) that accumulates cell-surface LapA (c, d), on hydrophobic (a, c) and hydrophilic (b, d) substrates. Bacterial cells were incubated with the subtratum for 8 h in Pi-rich medium to induce cell adhesion. The insets show the adhesion behavior of noninduced cells (0 h), i.e., cells incubated with the substrates for 8 h in LB. Similar results were obtained in duplicate experiments using independent cultures.
Single-Cell and Single-Molecule Analyses
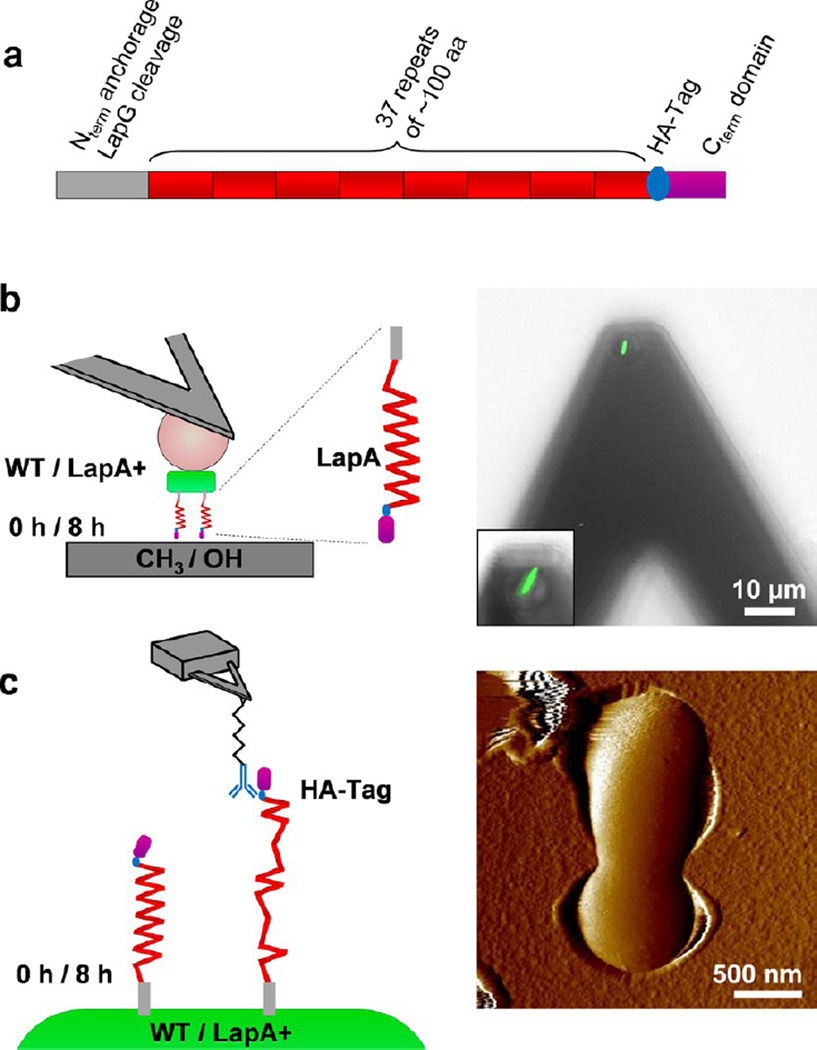
We used two advanced AFM modalities to provide complementary views of the LapA adhesion behavior (Figure 2). On the one hand, the forces engaged in single whole-cell adhesion were quantified using SCFS.31 Single cells were picked up with colloidal probe cantilevers coated with polydopamine (Figure 2b), without altering their viability (Figure 2b, right panel; green color indicates that the cell membrane is intact). Using these cellular probes, we measured force–distance curves between single WT or LapA+ mutant bacteria and hydrophobic or hydrophilic substrates, before and after biofilm induction in Pi-rich medium (Figure 2b). On the other hand, we used SMFS to map and functionally analyze single LapA molecules on the cell surface (Figure 2c).32 LapA proteins containing an HA-tag located directly after the repeats regions were probed with AFM tips functionalized with monoclonal anti-HA antibodies.19 Force–distance curves recorded across single cells immobilized on porous membranes (Figure 2c, right panel) enabled us to measure the localization, adhesion, and mechanics of individual adhesins.
Figure 2.
Single-cell and single-molecule analysis of LapA-mediated adhesion. (a) Primary structure of LapA representing the N-terminal region containing the cleavage site for the LapG protease; long multiple repeats, each consisting of ~100 amino acids, that constitute more than three-quarters of the total length of the protein; the HA tag for single-molecule detection and immunofluorescence; and the C-terminal domain. (b) Single-cell force spectroscopy. Living P. fluorescens bacteria (green) were attached on polydopamine-coated colloidal probes (pink). The adhesion forces between an individual bacterium (WT, Lap+ mutant) and hydrophobic (CH3) or hydrophilic (OH) substrates were measured, before (0 h) and after (8 h) biofilm induction. The right panel is an optical microscope image of a single bacterium attached to the colloidal cantilever probes (inset: higher magnification of another cell), documenting that the cell is properly located and alive (green color). (c) Single-molecule force spectroscopy. AFM tips functionalized with a single monoclonal anti-HA antibody were used to detect HA-tagged LapA molecules on the surface of a single bacterium immobilized on a porous polymer substrate and enabled us to measure the localization, adhesion, and mechanics of individual adhesins. The approach used should result in a single antibody on each tip; however, the antibody is linked to a 6-nm spacer, meaning that an individual antibody can explore ~10 nm, allowing for the possibility of the same adhesin molecule being detected more than once. The right panel shows an AFM image of a single bacterium trapped in a pore (center of the image).
Accumulation of Cell-Surface LapA Strengthens Adhesion to Hydrophobic Substrates
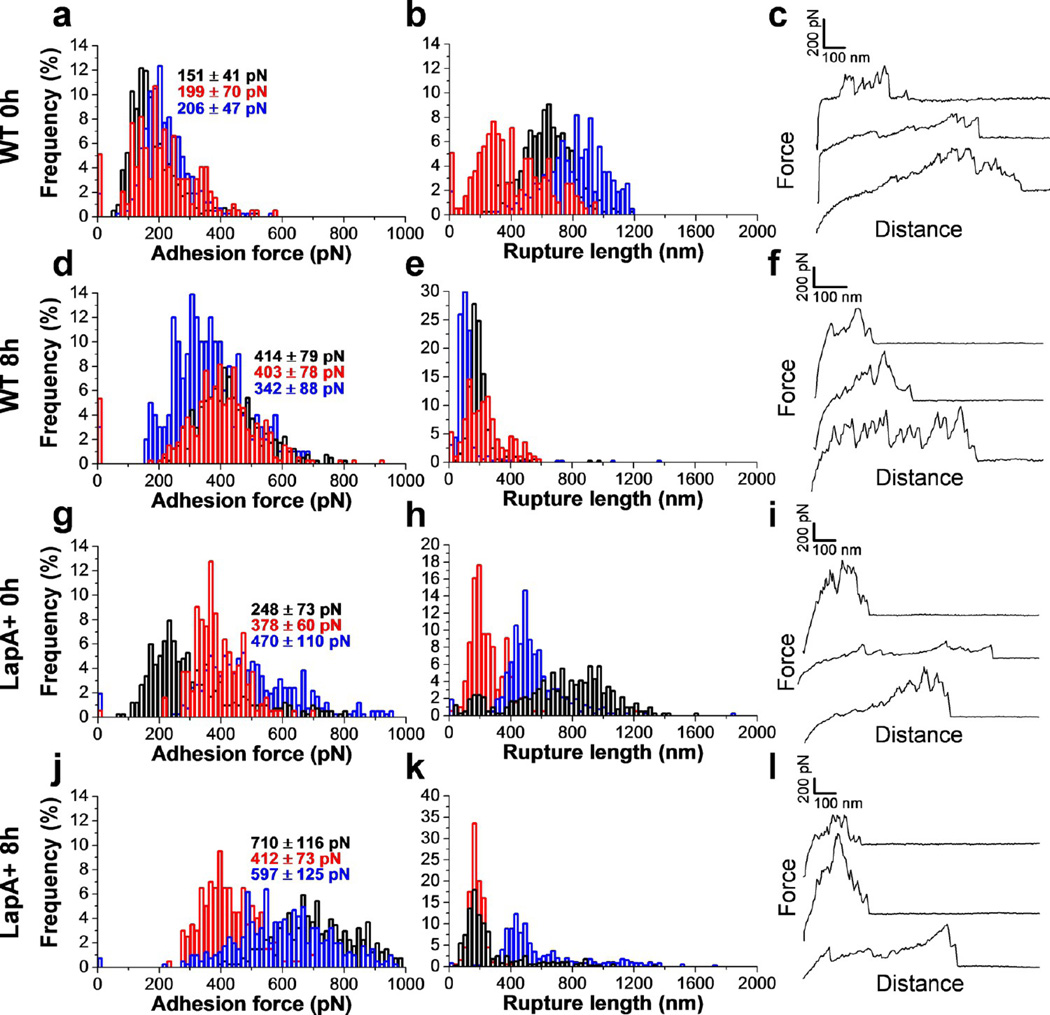
We first investigated the adhesion of single cells toward hydrophobic substrates using SCFS (Figure 3). Figure 3a–f shows the adhesion force and rupture length histograms, together with representative force curves, obtained between WT cells and hydrophobic substrates. The general features of the curves did not substantially change when recording consecutive force curves on different spots of the substrate, indicating that force measurements did not alter the cell-surface properties. Cells from independent cultures generally yielded similar behavior, indicating homogeneity and reproducibility of the cell populations. Before biofilm induction (Figure 3a–c), most force curves (95%) showed multiple force peaks of 150–250 pN magnitude and 100–1200 nm rupture length, which we attribute to LapA adhesive interactions. The measured ruptures, although quite long, were shorter than expected for fully stretched LapA proteins. Indeed, assuming that each amino acid contributes 0.36 nm to the contour length of a fully extended polypeptide chain, and that LapA is made of 5211 amino acids, full extension of the protein should give a length of ~1875 nm. Our findings suggest that most repeats were not unfolded, consistent with the lack of equally spaced, well-defined peaks (sawtooth patterns) in the force signatures (Figure 3c). Upon biofilm induction (Figure 3d–f), similar adhesion frequency was observed (95%), but larger adhesion forces (250–500 pN) and shorter ruptures (50–400 nm) were noted. Hence, surface exposure of LapA in Pi-rich medium not only increases the strength of cell-surface adhesion but also decreases LapA flexibility and extension. We suggest that exposure of the adhesin at high density on the cell surface may lead to stiffer, mechanically more stable conformations. Increase of LapA on the cell surface by biofilm induction in Pi-rich medium was further confirmed by dot blot analysis showing that WT cells present a 6-fold higher level of LapA after induction (Supplementary Figure 2). Finally, we note that the LapA-mutant showed very low adhesion frequency (mean <10%) and adhesion forces (~50 pN) (Supplementary Figure 1a and b), demonstrating that the adhesive force profiles measured on the WT indeed originate from LapA interactions.
Figure 3.
Single-cell analysis shows that accumulation of cell-surface LapA strengthens adhesion to hydrophobic substrates. (a, d, g, j) Adhesion force histograms, (b, e, h, k) rupture length histograms, and (c, f, i, l) representative retraction force profiles, obtained by recording multiple force–distance curves between either WT cells (a–f) or LapA+ mutant cells (g–l) and a hydrophobic substratum, before (a–c, g–i) and after (d–f, j–l) biofilm induction in Pi-rich medium. Black, red, and blue colors represent results from three cells from independent cultures (n > 200 force–distance curves for each cell).
We then examined whether accumulation of LapA at the cell surface in the lapG mutant background (LapA+ strain) influences the cell biophysical properties. As seen in Figure 3g–i, the LapA+ mutant showed force profiles that clearly differed from the WT. Before induction (Figure 3g–i), larger adhesion forces (200–600 pN) were observed, compared to the WT, suggesting that more LapA adhesins were surface-exposed. This increase in adhesion forces was more pronounced after induction (250–1000 pN; Figure 3j–l), providing direct evidence that loss of the LapG protease enhances cell adhesion properties due to accumulation of LapA on the cell surface, as previously reported.11,12,17,19,25 Again, much shorter ruptures were observed on induced cells (50–600 nm vs 50–1200 nm on noninduced mutant cells), supporting the view that a high-level of LapA molecules stiffens their mechanical behavior. While the structural reason for this stiffening is unclear, it could be due to the triggering of adhesin–adhesin aggregation when the protein density is high. For instance, the ChsA adhesin from Streptococcus gordonii, which shares homologous sequences with the LapA repeats, has been shown to form surface fibrils that confer adhesive properties.33
Substrate Chemistry Influences LapA Mechanics
LapA has been shown to mediate adhesion of P. fluorescens to a wide variety of substrates.10,12,17,34 In line with these reports, we found that bacteria adhere similarly to hydrophobic and hydrophilic substrates (Figure 1). These observations suggested that the molecular interactions and properties of LapA are not substrate-dependent. Surprisingly, our single-cell experiments revealed that this is not the case. Figure 4 shows the force data collected for the interaction between WT or LapA+ mutant cells and hydrophilic substrates. Before induction (Figure 4a–c), a moderate fraction of the force curves (66–80% vs 95% on hydrophobic substrates) showed adhesion force peaks, meaning that a smaller number of LapA molecules interacted with the surface for the WT cells (~100% for LapA+). We speculate that when LapA is poorly exposed on the cell surface (low Pi), it has a stronger tendency to bind to hydrophobic surfaces owing to its hydrophobic residues. After induction, the adhesion frequency strongly increased (up to 95–100%), and the adhesion events were larger (200–600 pN) and shorter (50–500 nm for most events), suggesting again that high-level LapA expression increased both the adhesion and the macromolecular stiffness of the cell surface (Figure 4d–f).
Figure 4.
Single-cell adhesion forces depend on substratum chemistry. (a, d, g, j) Adhesion force histograms, (b, e, h, k) rupture length histograms, and (c, f, i, l) representative retraction force profiles, obtained by recording multiple force–distance curves between either WT cells (a–f) or LapA+ mutant cells (g–l) on a hydrophilic substratum, before (a–c, g–i) and after (d–f, j–l) biofilm induction in Pi-rich medium. Unlike on hydrophobic substrates, a substantial fraction of the force profiles could be described by the worm-like-chain model (red lines), using a persistence length lp of 0.4 nm: F(x) = kBT/lp [0.25(1 − x/Lc)−2 + x/Lc − 0.25], where Lc is the contour length of the molecule, kB is the Boltzmann constant, and T the absolute temperature. On average, ~12 data points per peak were fitted. As shown in panel l (inset), enlarged views of the fits show that they do not perfectly fit the data, which could reflect the complexity of the cellular systems investigated. Black, red, and blue colors represent results from three cells from independent cultures (n > 200 force–distance curves for each cell).
Notably, a fraction of the curves obtained before (7% for WT, 28% for LapA+; Figure 4c and i) and after (25% for WT, 21% for LapA+; Figure 4f and l) induction showed sawtooth patterns with well-defined, equally spaced force peaks of 300–400 pN magnitude and 400–1800 nm rupture lengths. We believe these signatures reflect the sequential unfolding of the LapA repeats because (i) the measured forces are in the range of unfolding forces reported for β-folds domains, such as Ig and fibronectin domains in titin35 and larger than the forces needed to unfold α-helical domains;36 (ii) force peaks were reasonably well-described by the worm-like-chain model (WLC, Figure 4c, f, i, and l, red lines), suggesting they reflect the unfolding of secondary structures; and (iii) these signatures were never observed on LapA- mutant cells (Supplementary Figure 1c and d). Strikingly, the peak-to-peak distance, or change in contour length ΔLc, that we measured, 57 ± 10 nm (from n > 500 peaks), was much larger than that expected for the unfolding of a single LapA repeat. Since an amino acid contributes 0.36 nm to the contour length of a fully extended polypeptide, a single repeat of ~100 amino acids is expected to give a protein extension of ~36 nm. Considering the error on the measurement, the uncertainty on the exact repeat size, and the fact that the folded repeats have a finite size (most likely >5 nm), our 57 nm value strongly suggests that the repeats are unraveled not individually but by pairs, as observed earlier for the SiiE adhesin from Salmonella enterica.37 Although the structural reason behind this mechanical response is unclear, a reasonable explanation is that post-translational modifications or interactions of β sheets stabilize the interacting repeats two-by-two, and therefore, we hypothesize the basic mechanical unit of LapA would consist of two adjacent repeats. These mechanical properties could be important for the biological function of LapA, namely, the modular nature of the protein may strengthen cell adhesion by increasing both the lifetime and “energy” (work of adhesion) of the protein–substrate bond.
A pertinent question is why were unfolding signatures never found on hydrophobic substrates? We hypothesize that the LapA binding mechanism is substrate-dependent. While the adhesin may preferentially bind hydrophilic surfaces through its C-terminal domain, enabling sequential unfolding of its multidomains upon separation, it would bind hydrophobic surfaces primarily through its full length structure, particularly its repeat domains, which are known to contain hydrophobic amino acids.20 To test this hypothesis, we analyzed the behavior of a ΔCterm LapA+ mutant, which accumulates LapA at the cell surface and is thus similar to the LapA+ mutant, but in which the C-terminal domain of the adhesin is missing (Figure 2a, Supplementary Figure 1e–h). After 8 h of induction, we observed a drastic reduction of adhesion frequency on hydrophilic substrates compared to the LapA+ mutant (Figure 4j–l), as well as much smaller adhesion forces and rupture lengths (Supplementary Figure 1g and h). Also, less than 1% (vs 21%) of the curves showed unfolding profiles, indicating that the multiple repeats can only be unfolded when they are pulled through the C-terminal domain. These single-cell data correlated with a very low adhesion density at the microscale (Supplementary Figure 1g, inset). By contrast, truncation of the adhesin had only moderate effect on hydrophobic substrates, i.e., large adhesions (100–300 pN) with moderate extensions (50–600 nm) being observed in many of the curves (Supplementary Figure 1e and f). Yet the adhesion frequency varied from one cell to another, and the adhesion density was lower (20%) than that of the LapA+ mutant (Figure 1c), suggesting that the C-terminal domain may also play a role on hydrophobic substrates (Supplementary Figure 1e, inset). These results strongly support our model in which LapA mediates adhesion to hydrophilic and hydrophobic substrates via its C-terminal and multirepeat regions, respectively.
Accordingly, our single-cell experiments show that biofilm induction (Pi-rich medium), LapA cell-surface overexpression (LapA+ mutant), and substrate chemistry play important roles in controlling LapA adhesion and mechanics and in turn cell adhesion. We have shown that (i) without biofilm induction, WT cells feature adhesive force profiles with moderate adhesions and rather long extensions; these are more frequent on hydrophobic substrates than on hydrophilic ones, suggesting a role of hydrophobicity in cell adhesion; (ii) LapA+ mutant cells show increased adhesion forces, consistent with the accumulation of LapA molecules on the cell surface; (iii) biofilm induction of the two strains leads to increased adhesion forces, attributed to surface expression of LapA in Pi-rich medium, and causes shorter protein extensions, suggesting that the conformational properties of LapA are stiffer; and (iv) substrate chemistry modulates the mechanical response of LapA, sequential unfolding of the repeats being observed only on hydrophilic substrates.
Single-Molecule Imaging Demonstrates the Accumulation and Release of LapA on the Cell Surface
In WT cells, LapA is localized to the cell surface and is either retained at the surface or proteolytically processed by LapG to be released into the solution. By contrast, in LapA+ mutant cells, LapA is localized to the cell surface but is never cleaved from the cell surface, due to loss of LapG protease activity, leading to an accumulation of the adhesin on the surface.11,25 Consistent with these findings, fluorescence imaging using anti-HA antibodies and FITC-conjugated secondary antibodies revealed that the surface of WT cells was poorly labeled both prior and after induction (Figure 5a and b), while the surface of LapA+ mutant cells was massively labeled (Figure 5c and d). On close inspection, however, both strains showed local spots, about 200 nm in size, suggesting that LapA may accumulate or aggregate at specific regions on the cell surface. This finding correlates with a recent super-resolution fluorescence imaging study showing that LapA forms ~200-nm patches on the cell surface.18 We note that biofilm induction had little effect on the fluorescence contrast, presumably due to a lack of resolution and sensitivity.
Figure 5.
Fluorescence imaging of the LapA cell wall distribution. Fluorescent and false-colored merged (phase and fluorescence; insets) images of P. fluorescens cells expressing LapA-HA and labeled with anti-HA antibodies and FITC-conjugated secondary antibodies: comparison of WT cells (a, b) and LapA+ mutant cells (c, d), before (a, c) and after (b, d) biofilm induction. Similar results were obtained in duplicate experiments using independent cultures.
Unlike fluorescence microscopy, SMFS enables us to map the distribution of single constituents on the outermost cell surface and to quantify their adhesion forces and biophysical properties. We mapped the cell surface using SMFS with AFM tips functionalized with specific antibodies (Figure 2c), with the aim to answer the following questions: what are the organization and biophysical properties of single LapA molecules on the cell surface, how do these characteristics change in the hyper-adherent biofilm strain, and how do they correlate with SCFS data? To address these questions, Figure 6a–f shows the adhesion force map, the adhesion force histogram with representative force curves, and the rupture length histogram recorded between the antibody tip and the surface of WT cells. Before induction (Figure 6a–c), a moderate proportion (11%) of force curves showed single adhesion force peaks of 50–100 pN (77 ± 21 pN) magnitude and 100–200 nm length. Because these force profiles are very similar to those obtained for other antibody–adhesin interactions using the same protocol32,38 and were not observed on the LapA- mutant (Supplementary Figure 3a–c), we attribute them to the weak interaction between the anti-HA antibody tip and the HA-epitope tag in the C-terminal domain of LapA. The result indicates that even without biofilm induction, P. fluorescens expresses LapA on its surface at a substantial surface density, corresponding to ~450 sites/µm2. Note that this value may be an overestimation if the same molecule or cluster of molecules is detected more than once. We note that the upper portions of the maps systematically showed poor detection. During imaging, the tip scans the surface line-by-line horizontally, starting from the bottom. The loss of detection may therefore reflect inactivation of the antibody probe by loosely bound LapA, consistent with the fact that this adhesin is released into the supernatant.11,25
Figure 6.
Single-molecule imaging demonstrates the accumulation and release of cell-surface LapA. (a, d, g, j) Adhesion force maps (500 nm × 500 nm; scale bars = 100 nm; z range = 300 pN; bright pixels correspond to the detection of single adhesins) recorded in Pi-rich medium between an AFM tip bearing an anti-HA antibody and the surface of WT cells (a–f) or LapA+ mutant cells (g–l), before (a–c, g–i) and after (d–f, j–l) biofilm induction. Insets: second maps from independent experiments. (b, e, h, k) Corresponding adhesion force histograms (n = 1024) and (c, f, i, l) corresponding histograms of rupture distances (n = 1024) together with representative force curves. Red lines on unfolding force curves correspond to WLC fits. Panel l (inset) shows an enlarged view of the fits.
We found that biofilm induction strongly alters the adhesin distribution. Figure 6d–f shows that cells grown in Pi-rich medium showed little LapA detection (1%). Presumably, this striking behavior is due to the cellular regulatory mechanism of LapA exposure on that cell surface. Once localized to the cell surface by the LapBCE ABC transporter, a portion of LapA is released into the medium by LapG protease cleavage, even under conditions of biofilm induction.11,19 Thus, while biofilm induction increases LapA exposure, a sufficient portion of the adhesin is released to block the antibody tip. Strongly supporting this view, a few LapA molecules were always detected at the beginning of an experiment, i.e., in the lower part of the maps, thus when the probes are still functional and not binding released LapA.
The LapA+ mutant featured a very different behavior (Figure 6g–l). Before induction, 24% of the curves were adhesive, corresponding to a maximum surface density of ~1000 sites/µm2 (Figure 6g–i), although this value may be an overestimation if the same molecule or cluster of molecules is detected more than once. This increased surface density is consistent with the accumulation and firm anchorage of LapA on the surface of mutant cells lacking the LapG protease11,25 and correlates with our enhanced single-cell adhesion forces and fluorescence images shown above. Interestingly, the adhesins seemed to form clusters of about 200 nm, thus correlating with the patchy organization observed in fluorescence. Such aggregates may form at high LapA density and enhance the adhesion properties of the cells. In addition, several (2%) force curves featured sawtooth patterns that were very similar to those measured with whole cells on hydrophilic substrates and well-fitted with the WLC model (Figure 6i, red lines). Upon induction, the above features were much more pronounced (Figure 6j–l). First, the LapA+ mutant showed massive LapA detection, i.e., 87% corresponding to 3600 sites/µm2. Note that the actual density is probably lower because we cannot exclude that, at high coverage, the antibody tip will detect the same adhesin multiple times. Also, clusters may be present but not detected because of this multiple detection issue or because they are too large (>500 nm, the image size). Nevertheless, this high level of detection is in marked contrast with the poor detection on the WT, supporting the view that WT cells release LapA into the solution. Second, a much larger proportion (10%) of sawtooth profiles were seen, revealing that they indeed originate from the unfolding of LapA repeats. The changes in contour length, ΔLc = 51 ± 8 nm (from n > 500 peaks), were in the same range as those measured on whole cells, supporting strongly our interpretation that applying mechanical force on LapA through its C-terminal domain induces the unfolding of two repeats at a time.
Toward a Molecular View of LapA-Mediated Adhesion
Knowledge of the molecular interactions and biophysical properties of Lap proteins is critical to our understanding of the molecular basis of biofilm formation in a number of environmentally or medically important bacteria. Our singlecell and single-molecule experiments provide novel insights into the molecular mechanisms driving LapA-mediated adhesion in P. fluorescens. Our main finding is that LapA exhibits remarkable adhesive and mechanical properties that are function-related, as they provide a molecular basis for the ability of P. fluorescens to colonize diverse environments. The results indicate that while LapA binds hydrophobic surfaces through its full-length structure, particularly its hydrophobic multiple repeats (Figure 7a), it attaches to hydrophilic surfaces through its C-terminal domain, enabling sequential unfolding of its multi-repeats upon separation (Figure 7b). Before induction, LapA proteins are distributed randomly across the surface of WT cells and are readily desorbed from the cell surface (SMFS), consistent with the well-known LapA processing that can result in loss of this adhesin.11,25 LapA contributes to adhesion of whole single cells to solid surfaces, via adhesive force profiles with moderate adhesions and rather long extensions (SCFS). The probability of binding is higher on hydrophobic substrates, suggesting a role of hydrophobicity in cell adhesion, most likely through the repeats. Biofilm induction leads to increased cell adhesion forces, attributed to increased surface expression of LapA, and to less extended adhesins. That the conformational properties of LapA are stiffer could be due to the triggering of adhesin–adhesin associations (aggregation) at high protein density. Interestingly, studies of bacterial attachment in the 1930s and 1940s postulated a two-step process whereby bacteria first attached weakly to a surface (so-called “reversible” attachment), followed by subsequent tighter or “irreversible” attachment.39–41 Perhaps this transition from weak-to-strong attachment requires an accumulation of such surface adhesins at the interface and their subsequent increase in conformational stiffness, which is associated with an increased adhesive force. Deletion of the lapG gene leads to a massive increase in LapA surface density (SMFS), particularly after biofilm induction, and strengthens adhesion forces (SCFS) and microscopic adhesion (optical imaging) to solid substrates, providing a molecular basis for the hyper-adherent phenotypes.11,25 Substrate chemistry influences the mechanical response of LapA since sequential unfolding of the LapA repeats is observed only on hydrophilic substrates. These data suggest that while LapA mediates adhesion on a wide range of surfaces, the mechanism whereby attachment is mediated might differ from surface to surface or, alternatively, exploit different parts of the molecule. Furthermore, unfolding signatures observed on these hydrophilic substrata strongly suggest that two adjacent repeats are unfolded simultaneously, and thus we propose that the basic mechanical unit of LapA consists of two repeats. Thus, the multiple, environment-dependent mechanical properties of LapA provide a molecular basis for the ability of P. fluorescens to attach to a wide variety of substrates.
Figure 7.
Toward a dynamic and molecular view of LapA-mediated adhesion. (a, b) Plots of the maximum adhesion forces versus rupture distances measured by SCFS between WT bacteria and hydrophobic (a) or hydrophilic (b) substrata, before (0 h, black symbols) or after biofilm induction (8 h, red symbols). Data points from 3 different cells are shown: before induction, n = 1015 and 580 for hydrophobic and hydrophilic substrata, respectively (the adhesion frequency was lower in the latter case); after induction, n = 973 and 1001, respectively. The cartoons emphasize the key role of biofilm induction and substrate chemistry in tuning the biophysical properties (adhesion, extension, and unfolding) of LapA, providing a molecular basis for its “multi-purpose” adhesion function (see text for details).
METHODS
Microorganisms and Growth Conditions
The following P. fluorescens strains were used in these studies: Wild type (WT) strain (SMC4798) containing three HA epitope tags after the 4093rd amino acid residue of LapA;19 LapA+ mutant (SMC5207, ΔlapG), which overexpresses cell-surface, HA-tagged LapA due to loss of the LapG protease;11 LapA- mutant (SMC5164, lapB::pMQ89) derived from the WT strain and also expressing HA-tagged LapA, but the adhesin is not secreted due to loss of function of the LapBCE transporter;19 and ΔCterm LapA+ mutant (SMC6074, ΔlapG ΔCterm, see Supporting Information for details), a derivative of SMC5207 containing a clean deletion of amino acids Thr4018–Asn5151 of LapA. Strains were cultivated overnight in lysogeny broth (LB) at 30 °C and shaken at 200 rpm. Gentamycin (30 µg mL−1, Sigma) was added for LapA-cultures. For biofilm induction, overnight cultures were diluted 1:75 in Pi-rich medium (K10T) and incubated 8 h at 30 °C and 200 rpm.42 While LapA does play a role in early biofilm formation, there is a period of “induction” required for bacteria from the overnight cultures (which become limited for nutrients) to begin to grow and produce cell-surface LapA after being reintroduced into fresh medium. Extended incubation also allows larger amount of LapA to accumulate on the cell surface, thus facilitating some of the experiments presented here. Previous studies19,42 have shown that this microbe continues to make robust biofilms through this 8 h period.
Substrate Preparation
For preparing hydrophobic and hydrophilic substrates, glass coverslips coated with a thin layer of gold were immersed overnight in solutions of 1 mM 1-dodecanethiol (Sigma) or 1 mM of 11-mercapto-1-undecanol (Sigma), then rinsed with ethanol, and dried under N2.
Atomic Force Microscopy
Single-cell force spectroscopy (SCFS) and single-molecule force spectroscopy (SMFS) analyses were performed at RT (20 °C) in Pi-rich medium using Bioscope Catalyst and Nanoscope VIII Multimode AFMs (Bruker AXS Corporation). We used triangular shaped tipless cantilevers (NP-O10, Microlevers, Bruker Corporation) for SCFS and oxide-sharpened microfabricated Si3Ni4 cantilevers (Microlevers, Bruker Corporation) for SMFS. The nominal spring constant of the cantilever was determined by the thermal noise method. Detailed information about cell probe, tip, and sample preparation as well as recording conditions can be found in Supporting Information.
Supplementary Material
Acknowledgments
Work at the Université catholique de Louvain was supported by the National Fund for Scientific Research (FNRS), the Université catholique de Louvain (Fonds Spéciaux de Recherche), the Federal Office for Scientific, Technical and Cultural Affairs (Interuniversity Poles of Attraction Programme), and the Research Department of the Communauté française de Belgique (Concerted Research Action). Y.F.D. is Research Director of the FNRS. This work was also supported by the National Science Foundation through grant MCB 9984521 to G.A.O. We thank H. Sondermann for helpful discussions.
Footnotes
ASSOCIATED CONTENT
Supporting Information
This material is available free of charge via the Internet at http://pubs.acs.org.
The authors declare no competing financial interest.
REFERENCES
- 1.Costerton JW, Lewandowski Z, Caldwell DE, Korber DR, Lappin-Scott HM. Microbial biofilms. Annu. Rev. Microbiol. 1995;49:711–745. doi: 10.1146/annurev.mi.49.100195.003431. [DOI] [PubMed] [Google Scholar]
- 2.Costerton JW, Stewart PS, Greenberg EP. Bacterial biofilms: a common cause of persistent infections. Science. 1999;284:1318–1322. doi: 10.1126/science.284.5418.1318. [DOI] [PubMed] [Google Scholar]
- 3.Davey ME, O′Toole GA. Microbial biofilms: from ecology to molecular genetics. Microbiol. Mol. Biol. Rev. 2000;64:847–867. doi: 10.1128/mmbr.64.4.847-867.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kolter R, Greenberg EP. Microbial sciences: The superficial life of microbes. Nature. 2006;441:300–302. doi: 10.1038/441300a. [DOI] [PubMed] [Google Scholar]
- 5.O′Toole G, Kaplan HB, Kolter R. Biofilm formation as microbial development. Annu. Rev. Microbiol. 2000;54:49–79. doi: 10.1146/annurev.micro.54.1.49. [DOI] [PubMed] [Google Scholar]
- 6.Dekkers LC, Bloemendaal CJ, de Weger LA, Wijffelman CA, Spaink HP, Lugtenberg BJ. A two-component system plays an important role in the root-colonizing ability of Pseudomonas fluorescens strain WCS365. Mol. Plant-Microbe Interact. 1998;11:45–56. doi: 10.1094/MPMI.1998.11.1.45. [DOI] [PubMed] [Google Scholar]
- 7.Dekkers LC, Phoelich CC, van der Fits L, Lugtenberg BJ. A site-specific recombinase is required for competitive root colonization by Pseudomonas fluorescens WCS365. Proc. Natl. Acad. Sci. U.S.A. 1998;95:7051–7056. doi: 10.1073/pnas.95.12.7051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dekkers LC, van der Bij AJ, Mulders IH, Phoelich CC, Wentwoord RA, Glandorf DC, Wijffelman CA, Lugtenberg BJ. Role of the O-antigen of lipopolysaccharide, and possible roles of growth rate and of NADH:ubiquinone oxidoreductase (nuo) in competitive tomato root-tip colonization by Pseudomonas fluorescens WCS365. Mol. Plant-Microbe Interact. 1998;11:763–771. doi: 10.1094/MPMI.1998.11.8.763. [DOI] [PubMed] [Google Scholar]
- 9.Simons M, van der Bij AJ, Brand I, de Weger LA, Wijffelman CA, Lugtenberg BJ. Gnotobiotic system for studying rhizosphere colonization by plant growth-promoting Pseudomonas bacteria. Mol. Plant-Microbe Interact. 1996;9:600–607. doi: 10.1094/mpmi-9-0600. [DOI] [PubMed] [Google Scholar]
- 10.Duque E, de la Torre J, Bernal P, Molina-Henares MA, Alaminos M, Espinosa-Urgel M, Roca A, Fernandez M, de Bentzmann S, Ramos JL. Identification of reciprocal adhesion genes in pathogenic and non-pathogenic Pseudomonas. Environ. Microbiol. 2013;15:36–48. doi: 10.1111/j.1462-2920.2012.02732.x. [DOI] [PubMed] [Google Scholar]
- 11.Newell PD, Boyd CD, Sondermann H, O′Toole GA. A c-di-GMP effector system controls cell adhesion by inside-out signaling and surface protein cleavage. PLoS Biol. 2011;9:e1000587. doi: 10.1371/journal.pbio.1000587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.O’Toole GA, Kolter R. Initiation of biofilm formation in Pseudomonas fluorescens WCS365 proceeds via multiple, convergent signalling pathways: a genetic analysis. Mol. Microbiol. 1998;28:449–461. doi: 10.1046/j.1365-2958.1998.00797.x. [DOI] [PubMed] [Google Scholar]
- 13.Spiers AJ, Rainey PB. The Pseudomonas fluorescens SBW25 wrinkly spreader biofilm requires attachment factor, cellulose fibre and LPS interactions to maintain strength and integrity. Microbiology. 2005;151:2829–2839. doi: 10.1099/mic.0.27984-0. [DOI] [PubMed] [Google Scholar]
- 14.Haas D, Defago G. Biological control of soil-borne pathogens by fluorescent pseudomonads. Nat. Rev. Microbiol. 2005;3:307–319. doi: 10.1038/nrmicro1129. [DOI] [PubMed] [Google Scholar]
- 15.Picot L, Abdelmoula SM, Merieau A, Leroux P, Cazin L, Orange N, Feuilloley MG. Pseudomonas fluorescens as a potential pathogen: adherence to nerve cells. Microbes Infect. 2001;3:985–995. doi: 10.1016/s1286-4579(01)01462-9. [DOI] [PubMed] [Google Scholar]
- 16.Picot L, Mezghani-Abdelmoula S, Chevalier S, Merieau A, Lesouhaitier O, Guerillon J, Cazin L, Orange N, Feuilloley MG. Regulation of the cytotoxic effects of Pseudomonas fluorescens by growth temperature. Res. Microbiol. 2004;155:39–46. doi: 10.1016/j.resmic.2003.09.014. [DOI] [PubMed] [Google Scholar]
- 17.Hinsa SM, Espinosa-Urgel M, Ramos JL, O′Toole GA. Transition from reversible to irreversible attachment during biofilm formation by Pseudomonas fluorescens WCS365 requires an ABC transporter and a large secreted protein. Mol. Microbiol. 2003;49:905–918. doi: 10.1046/j.1365-2958.2003.03615.x. [DOI] [PubMed] [Google Scholar]
- 18.Ivanov IE, Boyd CD, Newell PD, Schwartz ME, Turnbull L, Johnson MS, Whitchurch CB, O′Toole GA, Camesano TA. Atomic force and super-resolution microscopy support a role for LapA as a cell-surface biofilm adhesin of Pseudomonas fluorescens. Res. Microbiol. 2012;163:685–691. doi: 10.1016/j.resmic.2012.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Monds RD, Newell PD, Gross RH, O′Toole GA. Phosphate-dependent modulation of c-di-GMP levels regulates Pseudomonas fluorescens Pf0–1 biofilm formation by controlling secretion of the adhesin LapA. Mol. Microbiol. 2007;63:656–679. doi: 10.1111/j.1365-2958.2006.05539.x. [DOI] [PubMed] [Google Scholar]
- 20.Yousef F, Espinosa-Urgel M. In silico analysis of large microbial surface proteins. Res. Microbiol. 2007;158:545–550. doi: 10.1016/j.resmic.2007.04.006. [DOI] [PubMed] [Google Scholar]
- 21.Navarro MV, Newell PD, Krasteva PV, Chatterjee D, Madden DR, O′Toole GA, Sondermann H. Structural basis for c-di-GMP-mediated inside-out signaling controlling periplasmic proteolysis. PLoS Biol. 2011;9:e1000588. doi: 10.1371/journal.pbio.1000588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Newell PD, Monds RD, O′Toole GA. LapD is a bis-(3′,5′)-cyclic dimeric GMP-binding protein that regulates surface attachment by Pseudomonas fluorescens Pf0–1. Proc. Natl. Acad. Sci. U.S.A. 2009;106:3461–3466. doi: 10.1073/pnas.0808933106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Boyd CD, O′Toole GA. Second messenger regulation of biofilm formation: breakthroughs in understanding c-di-GMP effector systems. Annu. Rev. Cell. Dev. Biol. 2012;28:439–462. doi: 10.1146/annurev-cellbio-101011-155705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Romling U, Galperin MY, Gomelsky M. Cyclic di-GMP: the first 25 years of a universal bacterial second messenger. Microbiol. Mol. Biol. Rev. 2013;77:1–52. doi: 10.1128/MMBR.00043-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Boyd CD, Chatterjee D, Sondermann H, O′Toole GA. LapG, required for modulating biofilm formation by Pseudomonas fluorescens Pf0–1, is a calcium-dependent protease. J. Bacteriol. 2012;194:4406–4414. doi: 10.1128/JB.00642-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hinsa SM, O′Toole GA. Biofilm formation by Pseudomonas fluorescens WCS365: a role for LapD. Microbiology. 2006;152:1375–1383. doi: 10.1099/mic.0.28696-0. [DOI] [PubMed] [Google Scholar]
- 27.Helenius J, Heisenberg CP, Gaub HE, Muller DJ. Single-cell force spectroscopy. J. Cell Sci. 2008;121:1785–1791. doi: 10.1242/jcs.030999. [DOI] [PubMed] [Google Scholar]
- 28.Müller DJ, Helenius J, Alsteens D, Dufrêne YF. Force probing surfaces of living cells to molecular resolution. Nat. Chem. Biol. 2009;5:383–390. doi: 10.1038/nchembio.181. [DOI] [PubMed] [Google Scholar]
- 29.Dupres V, Menozzi FD, Locht C, Clare BH, Abbott NL, Cuenot S, Bompard C, Raze D, Dufrene YF. Nanoscale mapping and functional analysis of individual adhesins on living bacteria. Nat. Methods. 2005;2:515–520. doi: 10.1038/nmeth769. [DOI] [PubMed] [Google Scholar]
- 30.Hinterdorfer P, Dufrene YF. Detection and localization of single molecular recognition events using atomic force microscopy. Nat. Methods. 2006;3:347–355. doi: 10.1038/nmeth871. [DOI] [PubMed] [Google Scholar]
- 31.Beaussart A, El-Kirat-Chatel S, Herman P, Alsteens D, Mahillon J, Hols P, Dufrene YF. Single-cell force spectroscopy of probiotic bacteria. Biophys. J. 2013;104:1886–1892. doi: 10.1016/j.bpj.2013.03.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Alsteens D, Garcia MC, Lipke PN, Dufrene YF. Force-induced formation and propagation of adhesion nanodomains in living fungal cells. Proc. Natl. Acad. Sci. U.S.A. 2010;107:20744–20749. doi: 10.1073/pnas.1013893107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McNab R, Forbes H, Handley PS, Loach DM, Tannock GW, Jenkinson HF. Cell wall-anchored CshA polypeptide (259 kDas) in Streptococcus gordonii forms surface fibrils that confer hydrophobic and adhesive properties. J. Bacteriol. 1999;181:3087–3095. doi: 10.1128/jb.181.10.3087-3095.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fuqua C. Passing the baton between laps: adhesion and cohesion in Pseudomonas putida biofilms. Mol. Microbiol. 2010;77:533–536. doi: 10.1111/j.1365-2958.2010.07250.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rief M, Gautel M, Oesterhelt F, Fernandez JM, Gaub HE. Reversible unfolding of individual titin immunoglobulin domains by AFM. Science. 1997;276:1109–1112. doi: 10.1126/science.276.5315.1109. [DOI] [PubMed] [Google Scholar]
- 36.Rief M, Pascual J, Saraste M, Gaub HE. Single molecule force spectroscopy of spectrin repeats: low unfolding forces in helix bundles. J. Mol. Biol. 1999;286:553–561. doi: 10.1006/jmbi.1998.2466. [DOI] [PubMed] [Google Scholar]
- 37.Griessl MH, Schmid B, Kassler K, Braunsmann C, Ritter R, Barlag B, Stierhof YD, Sturm KU, Danzer C, Wagner C, Schaffer TE, Sticht H, Hensel M, Muller YA. Structural insight into the giant Ca(2)(+)-binding adhesin SiiE: implications for the adhesion of Salmonella enterica to polarized epithelial cells. Structure. 2013;21:741–752. doi: 10.1016/j.str.2013.02.020. [DOI] [PubMed] [Google Scholar]
- 38.Beaussart A, Alsteens D, El-Kirat-Chatel S, Lipke PN, Kucharikova S, Van Dijck P, Dufrene YF. Single-molecule imaging and functional analysis of Als adhesins and mannans during Candida albicans morphogenesis. ACS Nano. 2012;6:10950–10964. doi: 10.1021/nn304505s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Henrici AT. Studies of freshwater bacteria: I. A direct microscopic technique. J. Bacteriol. 1933;25:277–287. doi: 10.1128/jb.25.3.277-287.1933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zobell CE. The influence of solid surface upon the physiological activities of bacteria in seawater. J. Bacteriol. 1937;33:86. [Google Scholar]
- 41.Zobell CE. The effect of solid surfaces upon bacterial activity. J. Bacteriol. 1943;46:39–56. doi: 10.1128/jb.46.1.39-56.1943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Monds RD, Newell PD, Schwartzman JA, O′Toole GA. Conservation of the Pho regulon in Pseudomonas fluorescens Pf0–1. Appl. Environ. Microbiol. 2006;72:1910–1924. doi: 10.1128/AEM.72.3.1910-1924.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.