Abstract
Background:
Suboptimal utilisation of pharmacotherapy, non-adherence to prescribed treatment, and a lack of monitoring all contribute to poor blood (BP) pressure control in patients with hypertension.
Objective:
The objective of this study was to evaluate the implementation of a pharmacist-led hypertension management service in terms of processes, outcomes, and methodological challenges.
Method:
A prospective, controlled study was undertaken within the Australian primary care setting. Community pharmacists were recruited to one of three study groups: Group A (Control – usual care), Group B (Intervention), or Group C (Short Intervention). Pharmacists in Groups B and C delivered a service comprising screening and monitoring of BP, as well as addressing poor BP control through therapeutic adjustment and adherence strategies. Pharmacists in Group C delivered the shortened version of the service.
Results:
Significant changes to key outcome measures were observed in Group C: reduction in systolic and diastolic BPs at the 3-month visit (P<0.01 and P<0.01, respectively), improvement in medication adherence scores (P=0.01), and a slight improvement in quality of life (EQ-5D-3L Index) scores (P=0.91). There were no significant changes in Group B (the full intervention), and no differences in comparison to Group A (usual care). Pharmacists fed-back that patient recruitment was a key barrier to service implementation, highlighting the methodological implications of screening.
Conclusion:
A collaborative, pharmacist-led hypertension management service can help monitor BP, improve medication adherence, and optimise therapy in a step-wise approach. However, blood pressure screening can effect behaviour change in patients, presenting methodological challenges in the evaluation of services in this context.
Keywords: Hypertension, Community Pharmacy Services, Interprofessional Relations, Medication Adherence, Medication Therapy Management, Methodology, Australia
INTRODUCTION
There is a need for targeted services in chronic disease management, particularly in hypertension where up to 67.5% of people are reported to have inadequate blood pressure (BP) control.1 Clinical inertia, non-adherence by patients, and a lack of monitoring underpin poor BP control.2,3,4 Here, an opportunity exists for a multi-faceted, collaborative approach involving the community pharmacist and GP to optimise hypertension management.5 In the Australian primary care setting, a number of studies have shown that pharmacist-led interventions can rationalise medicines use in patients6,7, improve clinicians’ prescribing of evidence-based therapies in cardiovascular disease7,8,9, and improve patient self-management as well as clinical outcomes.10,11
In regard to the management of hypertension, previous studies have demonstrated the positive impact that pharmacists can have on a range of outcomes. Even dating back to the 1990s, studies have shown that community pharmacist-led services comprising patient education and blood pressure monitoring (including the use home blood pressure readings obtained from monitors that wirelessly transmit information to the pharmacy) can improve patient knowledge about hypertension, significantly decrease mean blood pressure, improve blood pressure control in those not at treatment targets, and rationalise the use of antihypertensive medication.12,13,14,15 A Cochrane review also reports the positive outcomes from pharmacist-led care, with the majority of controlled trials associated with improved blood pressure control.16 Even those studies that have focused on patient self-management (e.g., self-monitoring of BP) have shown that additional support from health professionals may enhance the BP lowering effect of any intervention.17 Pharmacy practice has, of course, evolved in recent times, comprising more specialised roles and an expanded scope of practice. This has been highlighted by the RxACTION trial (i.e., the Rural Alberta Clinical Trial in Optimizing Hypertension).18 which evaluated the impact of enhanced pharmacist care on the management of patients with hypertension; this enhanced care involved pharmacists taking a more active role and independently prescribing antihypertensive medication. The study showed that the enhanced care resulted in significantly larger reductions in both systolic and diastolic blood pressure, with patients twice as likely to reach their recommended blood-pressure targets, compared to usual care. Although this type of expanded care is not yet legislated in Australia, pilot studies have also demonstrated the potential for pharmacist prescribing in hypertension management, suggesting that credentialed pharmacists are able to make appropriate therapeutic decisions.19
In delivering such interventions, it is important to recognise that the management of hypertension is relatively complex, comprising screening and monitoring of blood pressure, addressing adherence barriers, and reviewing pharmacotherapy.20,21 It is these aspects of hypertension management that Australian community pharmacists need to currently focus on, in preparation for future expanded care services.22,23 Therefore, the aim of this paper is to report on the lessons learned from a pilot study of a pharmacist-led intervention (hypertension management service) in Australian primary care. Specifically, the objectives of this paper are to: 1) describe the key outcomes following delivery of the intervention; 2) report pharmacists’ feedback regarding service implementation and any methodological challenges; and 3) discuss the challenges encountered using a program evaluation framework.
METHODS
Study Design - Pilot study of a pharmacist-led intervention
A pharmacist-led intervention was evaluated in a 3-group prospective, controlled study commencing at the beginning of 2013, and concluding in mid-2014: initially, Group A (Control) and Group B (Intervention) were implemented, followed later by Group C (Short Intervention; Figure 1).
Figure 1.

Study design
BP = blood pressure; QoL = Quality of Life; MMAS = Morisky Medication Adherence Scale; GP = General Practitioner. Use of the ©MMAS is protected by US copyright laws. Permission for use is required. A license agreement is available from: Donald E. Morisky, ScD, ScM, MSPH, Professor, Department of Community Health Sciences, UCLA School of Public Health, Los Angeles, CA.
Group C intervention was truncated to a period of 3-months and comprised a convenience sample of pharmacists. This group was introduced later to the study, after the recruitment of patients to Groups A and B had concluded, and was based on the initial experience of Group B pharmacists. Group C represented a variation to the study protocol to maximise patient recruitment and to model the approaches used commonly in clinical practice and in previous hypertension studies.24 However recruitment was not aligned to guideline recommendations.
Setting and Participants
Community pharmacists and their patients (customers) were recruited to the study.
The main study (Groups A and B) was conducted within pharmacies located in two Sydney metropolitan regions that were comparable in terms of socioeconomic characteristics and disease burden (Northern Sydney and Sutherland Medicare Locals, NSW, Australia). Pharmacists were therefore recruited based on their location in these regions and were cluster-randomised to Group A or B to minimise contamination between intervention and control patients.25 Group C was a convenience sample of pharmacists within both Sydney metropolitan and regional sites (Illawarra: n=2, Southern Highlands: n=1) who had not previously offered hypertension services. They were recruited using the snowball technique i.e., the researchers approached one pharmacist who was known to them from participation in previous research studies, and that pharmacist then approached other pharmacists who they thought might be interested in the study.
Pharmacists within each Medicare Local region were initially contacted by telephone (by Project Officer) to gauge their interest in participating. Pharmacists were eligible to participate if their pharmacy had:
at least 2 pharmacists on duty to allow the service to proceed uninterrupted
a designated quiet and relatively private area for patient education/counselling
a portable Omron™ BP monitor (with a range of cuff sizes), which was to be calibrated (by Omron™) both before and during the trial to ensure accuracy
a pharmacist who could attend specialised training to deliver the service
Pharmacists who were interested and eligible to participate were then provided an information sheet and consent form. All were compensated for their time in providing the pharmacy service and for data collection (Group A: AUD40 per patient; Groups B and C: AUD100 per patient).
The recipients of the service were patients (i.e., the customers of each community pharmacist) who required management of their hypertension. The specific inclusion criteria were:
aged ≥18 years of age
able to provide written informed consent to participate in study
able to return for all follow-up visits (as per Figure 1) and were accessible by telephone
had a diagnosis of essential hypertension (new diagnosis or established diagnosis) which was not controlled and meeting criteria for therapy as defined by current Australian guidelines.12
Patients in any of the following categories were excluded from study participation: history of recent myocardial infarction, unstable congestive heart failure, chronic kidney disease (CKD), or poorly controlled asthma; pregnant female; enrolled in any other trial.
The patients were initially identified from dispensed medication histories (dispensing program). As each patient presented to the pharmacy for refill prescriptions, the pharmacist invited them to have their blood pressure (BP) measured and to participate in the study (if eligible). The pharmacists performed an initial screen for poorly controlled hypertension by measuring the patients BP in the pharmacy; for confirmation, all patients in Groups A and B underwent repeated BP measurements (1-week apart) prior to study enrolment, per guideline recommendations.20 In Group C, the same intervention was delivered but there was only 1 BP screening step (i.e., baseline BP was not re-checked after 1-week) (Figure 1). Patients received a voucher (AUD15 for use in the pharmacy) after the final follow-up visit.
Intervention
Group A patients received usual care upon presentation to the pharmacist for refill prescriptions; i.e., supply of their medication and any medication counselling (Figure 1). Pharmacists in Group B and Group C were specially trained9 to deliver a pharmacist-led service, based on the Health Collaboration Model (HCM).26 The HCM describes five key barriers to treatment adherence that are amenable to change (i.e., regimen knowledge barriers; recall barriers; motivational barriers; side effect barriers; and access barriers) and which can be purposefully targeted through pharmacist intervention via monitoring, identification of barriers, engagement of patients, regular follow-up, and provision of support to patients and clinicians alike.
The intervention was delivered via face-to-face visits (follow-up) in the pharmacy interspersed with brief (5 minute) telephone follow-up by the pharmacist (Figure 1). In Group C the intervention was shortened (i.e., final face-to-face visit was conducted at 3-months). Each face-to-face visit involved BP measurement, as well as adherence and quality of life assessments (and/or therapeutic adjustment in Groups B and C) as follows:
screening and monitoring patients to identify patients with poor BP control (Figure 1)
applying a systematic approach to identify the potential cause(s) of poor control by reviewing patients’ medication management including adherence (Figure 2)
assessment and review of medicines for hypertension (agent, dosage, regimen)
addressing and providing support for any adherence issues and/or making therapeutic adjustment recommendations to the patient’s GP to optimise medicines use
monitoring patients at regular intervals to ensure ongoing adherence to medications and assessing BP control.9
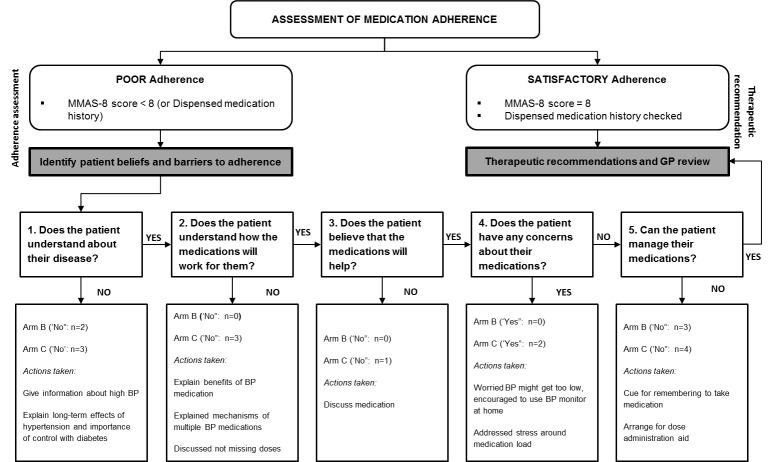
Figure 2.
Flowchart of adherence program based on the Health Collaboration Model (HCM) (undertaken for 14 patients)
All pharmacists were trained in accurate BP measurement (using Omron™ BP monitors), per the National Heart Foundation’s measurement protocol.20 The service was delivered with the cooperation of, and referral to, the local GP as appropriate; each pharmacist contacted their local GPs to advise them of the study and to agree on processes for communication and discussion regarding patient management (i.e., issues identified, actions taken, therapeutic recommendations). The researchers provided each pharmacy with document templates to help efficiently communicate important information to the GPs. This collaboration between the pharmacist and the GP in managing the patient’s hypertension was based on the principles of shared care, ensuring that the care provided was: patient-centred; effectively communicated and coordinated; supported by both the GP and the pharmacist; and delivered according to best practice guidelines.27
Process and outcome measures
Purpose-designed data collection forms were provided to pharmacists to document service provision and relevant outcome measures. Process measures included the number of pharmacists and patients recruited to the study. Pharmacists’ interventions (adherence interventions and therapeutic recommendations) were initially categorised by the pharmacists themselves (using the flowchart of adherence program, Figure 2) during the documentation process; the nature of these interventions was verified by the Project Officer and one of the researchers, in-line with the categories used by the Pharmaceutical Society of Australia.28 The key clinical outcome measures were: BP control (change in systolic BP) and self-reported medication adherence (using the Morisky medication adherence scale (MMAS-8).29,30,31 Quality of life (QoL)(EQ-5D-3L).32,33,34, was the primary humanistic outcome. Comparisons in outcomes were made between the treatment groups at baseline, 3 months and 12 months (Groups A and B only for the latter).
Sample size
For the main study (Groups A and B), the target sample size was based on an anticipated decrease in systolic BP of 5 to 10mmHg35,36 as the primary outcome. Using 80% power (1-sided alpha of 0.05), and allowing for a patient drop-out rate of 20%, the calculated sample size was 15 (for a 5mmHg BP reduction) to 50 patients per group (for a 10mmHg BP reduction), i.e., minimum of 5 pharmacists recruiting 3 to 10 patients each. To account for drop-out of pharmacists, additional pharmacists were recruited. For Group C, a purposive sample of 8 pharmacists (from 6 pharmacies) was recruited to deliver the short intervention.
Data analysis
SPSS37 Version 19 was used to analyse the data pertaining to process and outcome measures, and included Wilcoxon signed-rank test (pre/post mean BPs/MMAS-8 scores), Kruskal-Wallis ANOVA (continuous variables, e.g., age), and Chi-square test for independent proportions; significance level was set at 0.05. EQ-5D-3L values, pertaining to measures of QoL, were calculated using the New Zealand VAS value set.38
End-of-study evaluation
At the conclusion of the study, all pharmacists across the three study groups were invited to participate in a face-to-face interview to elicit qualitative feedback on their experience of service implementation. These interviews were facilitated by members of the research team who were experienced in qualitative interview techniques, and who were not in direct contact with the pharmacists during the service implementation. A semi-structured interview guide, modelled on that used by the researchers in previous studies and guided by the study objectives, was used to guide the questioning and discussion. Pharmacists’ responses were digitally (audio) recorded to allow for verbatim transcription. The transcripts were first analysed by an independent contracted researcher (not associated with the present study) using manual inductive coding to generate themes.39 Subsequently, the research team reviewed the transcripts to check the reliability of the analysis and ensure consensus was attained regarding the themes (theme verification).
A program evaluation framework (Table 1), adapted from Bauman and Nutbeam40, was then used to map the components of the study and challenges encountered during implementation of the intervention. This organised framework describes a process for evaluating intervention programs in healthcare, and maps out key steps including: an assessment of the current problem, consultation with stakeholders and the community, measurement and monitoring of intervention components, assessment of short-term impact, and assessment of the longer term outcomes. This type of logic model allows researchers to identify any barriers to the implementation and/or long-term sustainability of any intervention. In this study, post-service delivery, qualitative feedback from the pharmacists, a review of the study outcomes, and reflections from the investigators informed the framework inputs.
Table 1.
Program evaluation framework for the pilot study of a pharmacist-led hypertension management service
| Aspect of Evaluation | Pre-planning phase | Planning phase | Implementation phase | Impact and Outcomes |
|---|---|---|---|---|
| Recommended activities and actions | • Identify policies and resources • Ascertain community and epidemiological need • Engage relevant community / stakeholders |
• Develop implementation plan • Describe outcome measures • Describe specific strategies |
• Prepare timeline • Develop program components |
• Assess short-term and medium long impact of program |
| Activities completed as part of this pilot study | • Hypertension management guidelines identified (i.e., National Heart Foundation) (20) • Health Collaboration Model (HCM) (26) utilised • Liaison with representative GP organisations (i.e., Medicare Locals) consulted • Consultation with advisory group (comprising GPs, pharmacists) |
• Study protocol developed in accordance with hypertension management guidelines and HCM (Figures 1 and 2) • Outcome measures defined, including process, clinical, and humanistic measures • Participant feedback to be canvassed via semi-structured qualitative interviews • Pharmacist training program developed (9) |
• Timeline for pilot study prepared (Figure 1) • Pharmacist training program delivered (9) • Service resources amassed (e.g., BP monitors) and/or developed (e.g., data recording forms) • Pharmacists supported by Project Officer and investigators with respect to provision of resources, access to information, and assistance with promotion of intervention |
• Process measures - # pharmacists participating in study - # patients recruited - uptake of therapeutic adjustment recommendations • Clinical outcomes - change in systolic BP - change in medication adherence (MMAS score) • Humanistic outcomes - change in QoL •Participant feedback - pharmacist feedback (qualitative interview) - patient feedback (qualitative interview) |
| Challenges encountered in study | • Absence of need assessment to identify areas of need / service gaps; over-estimating potential impact of service • Limitation on pharmacists’ scope of practice - inability to independently manage patients or prescribe medication due to current Australian practice regulations • Not well established relationships with local GPs (limited inter-professional collaboration) |
• Not conceptualising the initial ‘screening’ step of the study (the initial BP checking step, prior to patient enrolment) as a key part of the intervention • Underestimating the impact of the specific characteristics of hypertension (i.e., asymptomatic, variable), and it's responsiveness of changes in patient behaviour |
• Complexity and comprehensiveness of study documents impacting on pharmacists ability to recruit patients and record outcome measures • Pharmacy support staff (e.g., pharmacy assistants) not included in training – unable to assist in patient recruitment |
• Small sample size impacting on outcome measures • Challenges encountered in pre-planning, planning, and implementation phases reflected in outcome measures • Lack of feedback from GPs |
GP = general practitioner; HCM = Health Collaboration Model; BP = blood pressure; MMAS= Morisky Medication Adherence Scale; QoL = quality of life; # = number of participants
RESULTS
Process measures
Of the 25 pharmacists initially enrolled in the study (8-9 pharmacists per group), 15 successfully recruited patients: 37.5% of pharmacists in Group A; 66.7% of Group B pharmacists; and 75.0% of Group C pharmacists (Figure 1). As expected, Group A pharmacists spent significantly less time during the first patient visit compared to Group B or Group C (P<0.05; Table 1). In delivering the service, interventions addressing medication adherence issues were recorded for 14 patients (Figure 2).
Participant characteristics
Thirty eight patients participated in the study (Table 1) after excluding those lost to follow-up (i.e., unable to be contacted), discontinued, or with incomplete or invalid data (Figure 1). Patients in Group C were significantly younger (P=0.03) than those in Groups A or B (Table 2). Only 6 patients had hypertension without other comorbidities. Given the sample size, an analysis of study outcomes taking into account specific participant characteristics was beyond the scope of this pilot study.
Table 2.
Group characteristics at time of recruitment
| Group characteristics (n): | Group A (Control) (n=11) | Group B (Intervention) (n=10) | Group C (Short Pilot) (n=17) |
|---|---|---|---|
| Age (mean ± SD) in years | 71.6 ±16.1 | 69.7 ± 13.2 | 59.1 ±12.4 |
| Male n | 6 | 3 | 6 |
| Retired n | 8 | 8 | 9 |
| Smoking status n Current Never Previous |
1 8 2 |
1 5 4 |
2 9 6 |
| Number of days per week they exercise >30 minutes (mean ± SD) | 3.6 ±2.0 | 3.9 ±2.6 | 3.7 ±2.8 |
| Number of standard drinks of alcohol consumed per day (mean ± SD) | 0.8 ±1.1 | 0.8 ±0.8 | 0.96 ±1.06 |
| Medical conditions n Diabetes Heart disease (angina, heart failure) High cholesterol Previous stroke Asthma COPD Depression Anxiety Other |
1 1 3 1 1 1 1 2 2 |
2 3 6 0 1 0 1 2 3 |
7 0 7 1 3 1 4 3 5 |
| Years since hypertension diagnosis (mean ± SD) | 13.0 ±13.7 | 18.3 ±17.0 | 9.3 ±6.7 |
SBP= systolic blood pressure; DBP = diastolic blood pressure; SD = standard deviation
Outcome measure: blood pressure control
At the screening assessment (i.e., initial BP check prior to patient enrolment) there was no difference in mean systolic BP (SBP; P=0.45) or diastolic BP (DBP; P=0.72) readings between Group A and Group B. Group C did not undertake this screening step.
At the time of patient recruitment into the study (Baseline Visit), there was no significant difference in SBP or DBP between Group A and Group B (P=0.15 and P=0.79, respectively). However, SBP and DBP were significantly higher in Group C compared to Group B (P=0.02 and P=0.01, respectively) (Table 3).
Table 3.
Process measures and key outcomes reported across the three study groups
| Outcome | Group A (Control) (n=11) | Group B (Intervention) (n=10) | Group C (Short Pilot) (n=17) |
|---|---|---|---|
| DURATION OF PHARMACIST CONSULTATIONS | |||
| Time (minutes): Screening (mean ± SD) | 16.1 ± 2.7 | 14.1 ± 8.2 | Not applicable |
| Time (minutes): Visit – Baseline (mean ± SD) | 17.5 ± 2.9 | 24.3 ± 15.5 | 22.5 ± 6.0 |
| Time (minutes): Visit – 1 month (mean ± SD) | Not applicable | 13.5 ± 8.1 | 11.6 ± 3.8 |
| Time (minutes): Visit – 3 months (mean ± SD) | 18.8 ± 2.5 | 12.8 ± 3.7 | 11.3 ± 4.3 |
| Time (minutes): Visit – 12 months (mean ± SD) | 17.5 ± 2.7 | 14.4 ± 3.2 | Not applicable |
| BLOOD PRESSURE (BP) | |||
| BP (mmHg): Screening (mean ± SD) | SBP: 147 ± 9 DBP: 85 ± 12 |
SBP: 153 ± 12 DBP: 85 ± 11 |
Not applicable |
| BP (mmHg): Visit – Baseline (mean ± SD) | SBP: 139 ± 8 DBP: 82 ± 9 |
SBP: 145 ± 19 DBP: 81 ± 12 |
SBP: 157 ± 14 DBP: 92 ± 10 |
| BP (mmHg): Visit – 1 month (mean ± SD) | Not applicable | SBP: 139 ± 18 DBP: 76 ± 13 |
SBP: 141 ± 9 DBP: 86 ± 11 |
| BP (mmHg): Visit – 3 months (mean ± SD) | SBP: 132 ± 9 DBP: 79 ± 8 |
SBP: 137 ± 18 DBP: 74 ± 12 |
SBP: 132 ± 10 DBP: 79 ± 8 |
| Change from Baseline to Visit – 3 months (Wilcoxon) |
SBP: P=0.34 DBP: P=0.08 |
SBP: P=0.14 DBP: P=0.04 |
SBP: P<0.01 DBP: P<0.01 |
| BP (mmHg): Visit – 12 months (mean ± SD) | SBP: 125 ± 9 DBP: 75± 12 |
SBP: 132 ± 14 DBP: 73 ± 12 |
Not applicable |
| Change from Visit – 3 months to Visit – 12 months (Wilcoxon) |
SBP: P=0.23 DBP: P=0.69 |
SBP: P=0.68 DBP: P=0.87 |
Not applicable |
| MEDICATION ADHERENCE | |||
| MMAS***
Baseline
n (%) Low adherence (<6) Medium adherence (6 to <8) High adherence (=8) |
0 (0%) 2 (18%) 9 (82%) |
2 (20%) 1 (10%) 7 (70% |
7 (42%) 5 (29%) 5 (29%) |
| Visit – 3 months
n (%) Low adherence (<6) Medium adherence (6 to <8) High adherence (=8) |
0 (0%) 1 (9%) 10 (91%) |
1 (10%) 3 (30%) 6 (60%) |
1 (6%) 7 (44%) 8 (50%) |
| Change from Baseline to Visit – 3 months (Pearson Chi-Square) | P=0.62 | P=0.19 | P=0.01 |
| Visit – 12 months
n (%) Low adherence (<6) Medium adherence (6 to <8) High adherence (=8) |
1 (9%) 2 (18%) 8 (73%) |
0 4 (45%) 5 (55%) |
Not applicable |
| Change from Visit –3 months to Visit –12 months (Pearson Chi-Square) | P=0.81 | P=0.68 | Not applicable |
| Median MMAS score: Visit – 1 month | 7.82 ± 0.41 | 7.10 ± 1.73 | 6.06 ± 1.71 |
| Median MMAS score: Visit – 3 months | 7.82 ± 0.60 | 7.20 ± 1.32 | 7.25 ± 0.93 |
| Change from Baseline to Visit – 3 months (Wilcoxon) | P=1.00 | P=1.00 | P=0.004 |
| Median MMAS score: Visit –12 months | 7.55 ± 0.93 | 7.33 ± 0.87 | Not applicable |
| Change from Visit – 3 months to Visit – 12 months (Wilcoxon) | P=0.46 | P=0.71 | Not applicable |
| QUALITY OF LIFE (QoL) | |||
| EQ-5D-3L – INDEX values | |||
| Median value: Baseline (25th and 75th percentiles) | 1.00 (0.68 - 1.00) | 0.70 (0.61 – 1.00) | 0.71 (0.62 – 1.00) |
| Median value: Visit – 3 months (25th and 75th percentiles) | 1.00 (0.71 – 1.00) | 0.71 (0.54 – 1.00) | 0.67 (0.59 – 1.00) |
| Change from Baseline to Visit – 3 months (Wilcoxon) | P=0.66 | P=0.79 | P=0.91 |
| Median value: Visit – 12 months (25th and 75th percentiles) | 1.00 (0.63 – 1.00) | 0.78 (0.46 – 1.00) | Not applicable |
| Change from Visit – 3 months to Visit – 12 months (Wilcoxon) | P=0.58 | P=1.00 | Not applicable |
| EQ VAS values | |||
| Median value: Baseline (25th and 75th percentiles) | 80.0 (75.0 – 90.0) | 70.0 (63.8 – 87.5) | 80.0 (55.0 – 87.5) |
| Median score: Visit – 3 months (25th and 75th percentiles) | 86.0 (80.0 – 93.0) | 80.0 (67.5 – 85.0) | 74.0 (60.0 – 84.3) |
| Change from Baseline to Visit – 3 months (Wilcoxon) | P=0.15 | P=0.80 | P=0.75 |
| Median score: Visit – 12 months (25th and 75th percentiles) | 92.5 (80.0 – 98.5) | 80.0 (55.0 – 90.0) | Not applicable |
| Change from Visit – 3 months to Visit – 12 months (Wilcoxon) | P=0.05 | P=1.00 | Not applicable |
SBP= systolic blood pressure; DBP = diastolic blood pressure; VAS = visual analogue scale; MMAS = Morisky Medication Adherence Scale; SD = Standard Deviation;
Use of the ©MMAS is protected by US copyright laws. Permission for use is required. A license agreement is available from: Donald E. Morisky, ScD, ScM, MSPH, Professor, Department of Community Health Sciences, UCLA School of Public Health, 650 Charles E. Young Drive South, Los Angeles, CA 90095-17
At the 3 month Visit any changes in BP (compared to the readings at Baseline Visit) were assessed in each group; there were no significant changes to BP within Group A patients (SBP: P=0.34, DBP: P=0.08). Within Group B a change in DBP was noted (P=0.04), but not in SBP (P=0.14). Within Group C, a significant change was noted in both SBP and DBP (P<0.01 and P=<0.01, respectively) (Table 3). Overall, between-group comparisons showed that there were no significant differences in the 3-month SBP (P>0.5) or DBP (P>0.5) between any of the three groups.
At the follow up visit (12 months), no changes in SBP or DBP were noted (compared to 3 month visit) within either Group A (P=0.23 and P=0.69, respectively) or Group B (P=0.68 and P=0.87, respectively). Overall, there were no significant differences between Group A and Group B in regard to the 12-month SBP (P>0.5) or DBP (P>0.5).
In terms of the absolute mean differences in SBPs recorded within each group from the time of the baseline measurement to the final visit (12 months for Groups A and B; 3 months for Group C), the largest change was observed within Group C (decrease of 25mmHg; P<0.01), followed by Groups A (22mmHg; P<0.01) and B Group (21mmHg; P<0.01)
Outcome measure: medication adherence
In terms of median adherence (MMAS-8) scores there were no significant differences between the 3 study groups at any time-point (P>0.05). When patients were categorised according to their MMAS-8 scores there were no significant differences in the proportion of patients assessed as having low, medium or high medication adherence based between the three study groups at any time-point, although a slightly higher proportion of patients in Group C were assessed as having ‘low adherence’ at baseline (Table 3).
Post-intervention, changes in the median adherence (MMAS-8) scores were observed in Groups B and C: 10.0% of Group B patients (n=10) and 41.2% of Group C patients (n=17) reported an improvement of 2 points or more (minimal detectable change41) in their MMAS-8 score (i.e., from baseline to 3 month visit). No Group A patients recorded an improvement of 2 points or more. At the 3 month visit, a significant improvement in the median MMAS-8 score was only observed in Group C (P=0.01). At the 12 month follow-up visit, there was no significant change in median MMAS-8 scores (compared to the 3 month Visit), in either Group A or Group B (P=0.81 and P=0.68, respectively). Overall, there were no significant differences MMAS-8 scores between groups (P>0.5) at the 3-month or 12-month (Group A versus Group B) visits.
Outcome measure: quality of life
At baseline, all patients had good QoL with no significant differences between groups. No changes were observed in EQ-5D-3L scores (Table 3).
Outcome measure: pharmacist interventions
Among the 14 patients for whom ‘adherence’ interventions were recorded, the most common issue identified was patients’ difficulties in managing their medications (50.0% of patients), followed by patients’ poor understanding about their disease (35.7%) (Figure 2). A lack of belief that the medication would be helpful was an issue in only 1 patient. The vast majority of adherence-based interventions comprised education and patient counselling; for the patients unable to manage their medications, interventions included strategies to act as ‘cues’ for remembering to take doses and dose administration aids.
Therapeutic adjustment accounted for approximately 40% of all pharmacist interventions in both Groups B and C at the baseline visit. Recommendations for dosage changes were more commonly reported as part of initial visit: adding antihypertensive agents and changing to alternative antihypertensives were more often recommended in subsequent visits. In Group A only 5 changes were identified in the patients’ antihypertensive medication regimens over the 12-month study period, compared to 20 in Group B and 18 in Group C.
Pharmacist feedback on service implementation
Most pharmacists identified patient recruitment as the greatest difficulty encountered in implementation. Some pharmacists printed study leaflets/posters and set-up information tables to help promote the service and recruit more patients. Those who struggled to recruit patients cited the following reasons during the feedback:
“It’s difficult to actually get someone to sign on the dotted line and it’s not necessarily because they were unwilling; I had people that were willing but didn’t meet the criteria. The criteria was pretty tough”
“…a lot of people simply didn’t come back for the second one regardless of phoning them or were just not willing to”
“I took their blood pressure and it was really high …. they don’t want to answer a questionnaire or they don’t want to come back in three months.”
“…. patients were not prepared to commit to an ongoing process as required for the study”
In Groups A and B, pharmacists reported that, in a number of cases, the patient’s BP improved from the initial screening assessment to the baseline Week 1 Visit (re-check of BP), which excluded them from further participation. Group C, the shorter intervention (without BP re-check after the initial screening) added later, avoided this issue. Pharmacists speculated on possible reasons for their observations:
“Each time I’ve tested their blood pressure since [screening] it’s been fine. Some of them have had changes in medications and they’ve increased or they’ve added another medication in like a calcium blocker. A lot of them it was just about compliance at the beginning”
“…With each one of them, compliance must have been an issue because each person came back apart from two of them, completely controlled the next week”.
A couple of pharmacists commented on how time-pressures had made it difficult to recruit since they were ‘not [always] the person - face-to-face - that [the patients] spoke to’ during that visit to the pharmacy, where other pharmacists and/or assistants may have been the primary contacts. This highlights the need for pharmacists to be able to commit sufficient time and effort to the chronic disease management program.
Overview of the challenges in intervention implementation
Synthesis of the study outcomes, participant feedback, and investigators’ reflections at the conclusion of the study, identified some key issues impacting on the actual implementation phase and program outcomes within the program evaluation framework (Table 1). Activities and actions in the ‘pre-planning’ and ‘planning’ phases of the evaluation framework were identified as needing particular attention. First, the pharmacists’ restricted scope of practice, and an absence of well established relationships between some pharmacists and GPs, limited their ability to facilitate specific therapeutic interventions. Second, neither the pharmacists nor the investigators had conceptualised the initial screening step of the study (the initial BP checking step to identify poor BP control, prior to patient enrolment) as an intervention in its own right, and had underestimated the responsiveness of BP to changes in patient behaviour. Subsequently, pharmacists’ ability to recruit patients was affected (in following best practice guidelines for confirming uncontrolled BP), impacting on the overall sample size and power to identify significant differences in outcome measures between groups. Indeed, Group C was implemented within a modified study protocol to address this issue and maximize patient recruitment.
DISCUSSION
Overall, this study has piloted a complex pharmacy-based intervention to help optimise hypertension management in those with poor BP control, and has additionally highlighted some methodological challenges. In terms of key outcomes, changes were observed in Group C (i.e., shorter intervention), specifically a reduction in BP at the 3-month visit, a significant improvement in medication adherence scores, and a trend to improvement in quality of life scores. In comparison, there were no significant changes in outcomes in Group B (full intervention), and no differences compared to Group A (usual care). Although the latter observations were perhaps disappointing, other studies exploring pharmacist-led interventions in chronic disease management (e.g., diabetes) have also shown no significant differences in outcomes between control and intervention groups.42
Possible explanations for these observations include the critical difference between study groups, i.e., the initial BP screening step prior to service delivery. Group B required an initial BP ‘screening’ step, followed by a re-check of the BP 1-week later, consistent with guidelines.20 In contrast, Group C, patients were directly recruited after the initial BP assessment. The absence of this re-check step may explain key study observations. Pharmacists in Groups A and B expressed difficulties in recruiting patients; patients who were initially assessed as having elevated BP were 1-week later often re-assessed as being within normal range, precluding their enrolment. While this, at face value, is simply an expression of the study inclusion/exclusion criteria, several pharmacists identified that the initial BP check prompted many patients into action (e.g., resuming their prescribed medications and/or consulting with GPs).
Second, the impact of the initial BP measurement step on patients’ behaviours was also suggested by differences in mean BP readings between the study groups at baseline; the mean BPs reported at the re-check steps were slightly lower than initial readings, even in patients who were ultimately recruited to Group B. In Group C, the baseline BP readings were significantly higher than either Group A or B. While this may represent regression to the mean, our contextual information (pharmacists’ observations) suggests that the initial BP measurement step itself played a greater role than just procedural confirmation of hypertension prior to study enrolment, acting as an important intervention itself. Indeed, such so-called screening has been shown to effect behaviour change.43
Third, the changes in outcomes observed in Group C may have been due to the absence of the BP re-check step, thereby preventing patients from initiating behaviour changes prior to study enrolment. The net effect of this was higher mean BPs at baseline (and greater potential for demonstrating reduction in BP), and an increased opportunity to record patient-centred interventions as part of pharmacist recommendations (e.g., behaviour changes in relation to medication adherence, lifestyle measures) in Group C. The findings support this, given that adherence-based interventions were more common in Group C than in Group B. This highlights the potential impact of screening processes on prompting behaviour change in patients, at least in the short-term.
These observations highlight the methodological challenges in evaluating interventions for hypertension management. Good clinical practice mandates that a diagnosis of hypertension is confirmed through repeated BP testing at designated intervals20; in applying these screening procedures, the diagnostic process may become a component of the intervention itself, thereby confounding study findings. At the first level the screening may have such a profound effect on potential participants that recruitment into studies becomes more difficult; secondarily, the impact of the screening and the components of the purpose-designed intervention may become conflated. In this study two intervention study groups were included to differentiate the potential impact of the screening step on process measures and patient outcomes, addressing this very issue.
Additional methodological considerations relate to the ‘planning’ around study implementation, and more carefully exploring the local context. The current restrictions on Australian pharmacists’ scope of practice, suboptimal selection of geographical regions (i.e., with regard to access to health services), and lack of strong interprofessional relationships between some pharmacists and GPs, have limited the potential benefits afforded by this service model, compared to findings from other studies.44 These challenges aside, other potential study limitations must be acknowledged. This study was conducted in specific regions within Australia and may not be generalisable to other settings. The difficulties in recruiting patients may have under-powered the study to detect significant changes in patient outcomes, and any changes observed in BP (Group C) may have been due to natural variations (regression to the mean), rather than the intervention. Furthermore, this study did not evaluate the characteristics of the participating pharmacists or pharmacies, and how these may have influenced any of the outcomes. A future larger scale study could address these limitations to provide more robust evidence for the impact of such an intervention.
Overall, the within-group comparisons for the two interventions groups in this study have hinted at the potential benefits of a pharmacist-led hypertension management service beyond the initial measurement of BP and immediate improvement in adherence, including optimisation of therapy through therapeutic adjustment and persistence of adherence, to support GPs and patients alike. Although the methodological challenges and sample size limitations preclude a robust analysis of the key study outcomes (particularly in terms of between-group comparisons), the results from this pilot study show that pharmacists have engaged in processes that can help monitor appropriate clinical parameters and identify appropriate strategies to optimise hypertension management in a step-wise approach, including generating recommendations for adjustment of therapeutic regimens that are acceptable to GPs. Previous studies have demonstrated the impact that pharmacists can have in improving hypertension management within community pharmacies45 or home-based monitoring services46, as well as in improving the quality of prescribing.47 Furthermore, this study specifically highlights what the critical components of such a service are, and how these can also present methodological challenges in the evaluation of services in this context.
CONCLUSIONS
A collaborative, pharmacist-led hypertension management service can help monitor BP, improve medication adherence, and optimise therapy in a step-wise approach. This study highlights the contribution of BP screening to effecting behaviour change in patients, particularly through adherence to medication. However, this can also present methodological challenges in the development and evaluation of services in this context, both in terms of recruiting patients as well as in assessing patient outcomes.
ACKNOWLEDGEMENTS
The authors gratefully acknowledge the assistance and support of Pradnya Naik Panvelkar (Project Officer) in the conduct of this study. Components of the study were guided by an Advisory Group specifically convened for this purpose.
Footnotes
CONFLICT OF INTEREST
The authors declare that they have no conflicts of interest.
Funding: This study received funding from the National Heart Foundation (Australia).
Contributor Information
Beata Bajorek., Academic Pharmacist and Associate Professor. Graduate School of Health – Pharmacy, University of Technology Sydney. Broadway, NSW (Australia). beata.bajorek@uts.edu.au.
Kate S. Lemay, Woolcock Institute of Medical Research, University of Sydney. Glebe, NSW (Australia). kate.lemay@sydney.edu.au
Parker Magin., Academic General Practitioner and Conjoint Professor, Discipline of General Practice, University of Newcastle. Callaghan, NSW (Australia). parker.magin@newcastle.edu.au.
Christopher Roberts., Associate Professor in Primary Care and Medical Education. Sydney Medical School – Northern, Hornsby Ku-ring-Gai Hospital. Hornsby, NSW (Australia). christopher.roberts@sydney.edu.au.
Ines Krass, Professor of Pharmacy Practice. Faculty of Pharmacy, University of Sydney. Sydney, NSW (Australia). ines.krass@sydney.edu.au.
Carol L. Armour., Professor of Pharmacology and Executive Director Woolcock Institute of Medical Research, University of Sydney and Sydney Local Health District, Woolcock Institute of Medical Research, University of Sydney. Glebe, NSW (Australia). carol.armour@sydney.edu.au
References
- 1.Chow CK, Teo KK, Rangarajan S, Islam S, Gupta R, Avezum A, Bahonar A, Chifamba J, Dagenais G, Diaz R, Kazmi K, Lanas F, Wei L, Lopez-Jaramillo P, Fanghong L, Ismail NH, Puoane T, Rosengren A, Szuba A, Temizhan A, Wielgosz A, Yusuf R, Yusufali A, McKee M, Liu L, Mony P, Yusuf S PURE (Prospective Urban Rural Epidemiology) Study investigators. PRevalence, awareness, treatment, and control of hypertension in rural and urban communities in high-, middle-, and low-income countries. JAMA. 2013;310(9):959–968. doi: 10.1001/jama.2013.184182. doi: 10.1001/jama.2013.184182. [DOI] [PubMed] [Google Scholar]
- 2.Ho PM, Magid DJ, Shetterly SM, Olson KL, Peterson PN, Masoudi FA, Rumsfeld JS. Importance of therapy intensification and medication nonadherence for blood pressure control in patients with coronary disease. Arch Intern Med. 2008;168(3):271–276. doi: 10.1001/archinternmed.2007.72. doi: 10.1001/archinternmed.2007.72. [DOI] [PubMed] [Google Scholar]
- 3.Janus ED, Bunker SJ, Kilkkinen A, Mc Namara K, Philpot B, Tideman P, Tirimacco R, Laatikainen TK, Heistaro S, Dunbar JA. Prevalence, detection and drug treatment of hypertension in a rural Australian population: the Greater Green Triangle risk factor study 2004-2006. Intern Med J. 2008;38(12):879–886. doi: 10.1111/j.1445-5994.2007.01583.x. doi: 10.1111/j.1445-5994.2007.01583.x. [DOI] [PubMed] [Google Scholar]
- 4.Lebeau JP, Cadwallader JS, Aubin-Auger I, Mercier A, Pasquet T, Rusch E, Hendrickx K, Vermeire E. The concept and definition of therapeutic inertia in hypertension in primary care: a qualitative systematic review. BMC Fam Pract. 2014;15:723. doi: 10.1186/1471-2296-15-130. doi: 10.1186/1471-2296-15-130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Peterson G, Jackson S, Hughes J, Fitzmaurice K, Murphy L. Public perceptions of the role of Australian pharmacists in cardiovascular disease. J Clin Pharm Ther. 2010;35(6):671–677. doi: 10.1111/j.1365-2710.2009.01139.x. doi: 10.1111/j.1365-2710.2009.01139.x. [DOI] [PubMed] [Google Scholar]
- 6.Castelino RL, Bajorek BV, Chen TF. Targeting suboptimal prescribing in the elderly: a review of the impact of pharmacy services. Ann Pharmacother. 2009;43(6):1096–1106. doi: 10.1345/aph.1L700. doi: 10.1345/aph.1L700. [DOI] [PubMed] [Google Scholar]
- 7.Castelino RL, Bajorek BV, Chen TF. Retrospective evaluation of home medicines review by pharmacists in older australian patients using the medication appropriateness index. Ann Pharmacother. 2010;44(12):1922–1929. doi: 10.1345/aph.1P373. doi: 10.1345/aph.1P373. [DOI] [PubMed] [Google Scholar]
- 8.Castelino RL, Chen TF, Guddattu V, Bajorek BV. Use of evidence-based therapy for the prevention of cardiovascular events among older people. Eval Health Prof. 2010;33(3):276–301. doi: 10.1177/0163278710374854. doi: 10.1177/0163278710374854. [DOI] [PubMed] [Google Scholar]
- 9.Bajorek B, Lemay K, Magin P, Roberts C, Krass I, Armour C. Preparing pharmacists to deliver a targeted service in hypertension management: evaluation of an interprofessional training program. BMC Med Educ. 2015;15:723. doi: 10.1186/s12909-015-0434-y. doi: 10.1186/s12909-015-0434-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Krass I, Hebing R, Mitchell B, Hughes J, Peterson G, Song YJ, Stewart K, Armour CL. Diabetes management in an Australian primary care population. J Clin Pharm Ther. 2011;36(6):664–672. doi: 10.1111/j.1365-2710.2010.01221.x. doi: 10.1111/j.1365-2710.2010.01221.x. [DOI] [PubMed] [Google Scholar]
- 11.Armour C1, Bosnic-Anticevich S, Brillant M, Burton D, Emmerton L, Krass I, Saini B, Smith L, Stewart K. Pharmacy Asthma Care Program (PACP) improves outcomes for patients in the community. Thorax. 2007 Jun;62(6):496–502. doi: 10.1136/thx.2006.064709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nemerovski C, Young M, Mariani N, Bugdalski-Stutrud C, Moser L. Project ImPACT: Hypertension Outcomes of a Pharmacist-Provided Hypertension Service. Innovations in Pharmacy. 2013;4(3):126. [Google Scholar]
- 13.Libby EA, Laub JJ. Economic and clinical impact of a pharmacy-based antihypertensive replacement program in primary care. Am J Health Syst Pharm. 1997;54(18):2079–2083. doi: 10.1093/ajhp/54.18.2079. [DOI] [PubMed] [Google Scholar]
- 14.Erickson SR, Slaughter R, Halapy H. Pharmacists’ ability to influence outcomes of hypertension therapy. Pharmacotherapy. 1997;17(1):140–147. [PubMed] [Google Scholar]
- 15.Carter BL, Malone DC, Billups SJ, Valuck RJ, Barnette DJ, Sintek CD, Ellis S, Covey D, Mason B, Jue S, Carmichael J, Guthrie K, Dombrowski R, Geraets DR, Amato M Impact of Managed Pharmaceutical care on resource utilization and Outcomes in Veterans affairs medical centers. Interpreting the findings of the IMPROVE study. Am J Health Syst Pharm. 2001 Jul 15;58(14):1330–1337. doi: 10.1093/ajhp/58.14.1330. [DOI] [PubMed] [Google Scholar]
- 16.Glynn LG, Murphy AW, Smith SM, Schroeder K, Fahey T. Interventions used to improve control of blood pressure in patients with hypertension. Cochrane Database Syst Rev. 2010;(3):723. doi: 10.1002/14651858.CD005182.pub4. doi: 10.1002/14651858.CD005182.pub4. [DOI] [PubMed] [Google Scholar]
- 17.Uhlig K, Patel K, Ip S, Kitsios GD, Balk EM. Self-measured blood pressure monitoring in the management of hypertension: a systematic review and meta-analysis. Ann Intern Med. 2013;159(3):185–194. doi: 10.7326/0003-4819-159-3-201308060-00008. doi: 10.7326/0003-4819-159-3-201308060-00008. [DOI] [PubMed] [Google Scholar]
- 18.Tsuyuki RT, Houle SK, Charrois TL, Kolber MR, Rosenthal MM, Lewanczuk R, Campbell NR, Cooney D, McAlister FA Rx ACTION Investigators. Randomized Trial of the Effect of Pharmacist Prescribing on Improving Blood Pressure in the Community: The Alberta Clinical Trial in Optimizing Hypertension (RxACTION) Circulation. 2015;132(2):93–100. doi: 10.1161/CIRCULATIONAHA.115.015464. doi: 10.1161/CIRCULATIONAHA.115.015464. [DOI] [PubMed] [Google Scholar]
- 19.Krass I, Bajorek B Pharmacists’ therapeutic recommendations in the management of hypertension in primary care. National Medicines Symposium, Sydney (Australia) June. 2012 [Google Scholar]
- 20.National Heart Foundation of Australia (National Blood Pressure and Vascular Disease Advisory Committee) [xccessed November 1 2014];Guide to Management of Hypertension 2008 (Updated December 2010) ISBN 978-1-921226-85-4. www.heartfoundation.org.au .
- 21.Aslani P KI, Bajorek B, Thistlewaite J, Tofler G National Heart Foundation of Australia. on behalf of the Heart Foundation Pharmaceutical Roundtable. [accessed August 4 2014];Improving adherence in cardiovascular care. A toolkit for health professionals. http://wwwheartfoundationorgau/SiteCollectionDocuments/Improving-adherence-in-cardiovascular-care-toolkit.pdf .
- 22.Health Workforce Australia. [accessed June 29 2015];Health Professionals Prescribing Pathway project. Available from: https://www.hwa.gov.au/work-programs/workforce-innovation-and-reform/health-professionals-prescribing-pathway-project .
- 23.Hoti K, Hughes J, Sunderland B. An expanded prescribing role for pharmacists - an Australian perspective. Australas Med J. 2011;4(4):236–242. doi: 10.4066/AMJ.2011.694. doi: 10.4066/AMJ.2011.694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lau R, Stewart K, McNamara KP, Jackson SL, Hughes JD, Peterson GM, Bortoletto DA, McDowell J, Bailey MJ, Hsueh A, George J. Evaluation of a community pharmacy-based intervention for improving patient adherence to antihypertensives: a randomised controlled trial. BMC Health Serv Res. 2010;10:723. doi: 10.1186/1472-6963-10-34. doi: 10.1186/1472-6963-10-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Torgerson D. Contamination in trials: is cluster randomisation the answer? BMJ. 2001. 322(7282):355–357. doi: 10.1136/bmj.322.7282.355. doi: 10.1136/bmj.322.7282.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Svarstad B, Bultman D Remington: The Science and Practice of Pharmacy. The patient: Behavioral determinants. Baltimore: Lippincott Williams & Wilkins; 2000. [Google Scholar]
- 27.Australian Government - Cancer Australia. [cccessed March 31 2016];Principles of shared care (shared follow-up care) 2013 Available at: https://canceraustralia.gov.au/publications-and-resources/cancer-australia-publications/shared-care-principles-shared-care .
- 28.Pharmaceutical Society of Australia. Standard and guidelines for pharmacists performing clinical interventions. Deakin West: ACT (Australia); 2011. [Google Scholar]
- 29.Morisky D, Ang A, Krousel-Wood M, Ward H. Predictive validity of a medication adherence measure in an outpatient setting. J Clin Hypertens (Greenwich) 2008;10(5):348–354. doi: 10.1111/j.1751-7176.2008.07572.x. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 30.Krousel-Wood M, Islam T, Webber L, Re R, Morisky D, Muntner P. New medication adherence scale versus pharmacy fill rates in seniors with hypertension. Am J Manag Care. 2009;15(1):59–66. [PMC free article] [PubMed] [Google Scholar]
- 31.Morisky D, DiMatteo M. Improving the measurement of self-reported medication nonadherence: Final response. J Clin Epidemiol. 2011;64(3):255–257. doi: 10.1016/j.jclinepi.2010.09.002. doi: 10.1016/j.jclinepi.2010.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dolan P. Modelling valuations for EuroQol health states. Medical Care. 1997;35(11):1095–1108. doi: 10.1097/00005650-199711000-00002. [DOI] [PubMed] [Google Scholar]
- 33.EuroQol Group. EuroQol-a new facility for the measurement of health-related quality of life. Health Policy. 1990;16(3):199–208. doi: 10.1016/0168-8510(90)90421-9. [DOI] [PubMed] [Google Scholar]
- 34.Brooks R. EuroQol: the current state of play. Health Policy. 1996;37(1):53–72. doi: 10.1016/0168-8510(96)00822-6. [DOI] [PubMed] [Google Scholar]
- 35.Machado M, Bajcar J, Guzzo GC, Einarson TR. Sensitivity of patient outcomes to pharmacist interventions. Part II: Systematic reviews and meta-analysis in hypertension management. Ann Pharmacother. 2007;41(11):1770–1181. doi: 10.1345/aph.1K311. [DOI] [PubMed] [Google Scholar]
- 36.McLean DL, McAlister FA, Johnson JA, King KM, Makowsky MJ, Jones CA, Tsuyuki RT SC RIP-HTN Investigators. A randomized trial of the effect of community pharmacist and nurse care on improving blood pressure management in patients with diabetes mellitus: Study of cardiovascular risk intervention by pharmacists–hypertension (SCRIP-HTN) Arch Intern Med. 2008;168(21):2355–2361. doi: 10.1001/archinte.168.21.2355. doi: 10.1001/archinte.168.21.2355. [DOI] [PubMed] [Google Scholar]
- 37.IBM Corp. Released 2010. IBM SPSS Statistics for Windows, Version 19.0. Armonk, NY: IBM Corp; [Google Scholar]
- 38.Szende A OM, Devlin N EQ-5D value sets: Inventory craug. EuroQol Group Monographs. Springer; 2006. [Google Scholar]
- 39.Thomas D. A general inductive approach for analyzing qualitative evaluation data. Am J Eval. 2006;27(2):237–246. doi: 10.1177/1098214005283748. [Google Scholar]
- 40.Bauman A, Nutbeam D. Planning and evaluating population interventions to reduce noncommunicable disease risk - reconciling complexity and scientific rigour? Public Health Res Pract. 2014. 25(1) doi: 10.17061/phrp2511402. doi: 10.17061/phrp2511402. [DOI] [PubMed] [Google Scholar]
- 41.Muntner P, Joyce C, Holt E, He J, Morisky D, Webber LS, Krousel-Wood M. Defining the minimal detectable change in scores on the 8-item Morisky Medication Adherence Scale. Ann Pharmacother. 2011;45(5):569–575. doi: 10.1345/aph.1P677. doi: 10.1345/aph.1P677. [DOI] [PubMed] [Google Scholar]
- 42.Guirguis LM, Johnson JA, Farris KB, Tsuyuki RT, Toth EL. A pilot study to evaluate the impact of pharmacists as certified diabetes educators on the clinical and humanistic outcomes of people with diabetes. Can J Diab Care. 2001;25(4):266–276. [Google Scholar]
- 43.Deutekom M, Vansenne F, McCaffery K, Essink-Bot M-L, Stronks K, Bossuyt P. The effects of screening on health behaviour: a summary of the results of randomized controlled trials. J Public Health (Oxf) 2011;33(1):71–79. doi: 10.1093/pubmed/fdq050. doi: 10.1093/pubmed/fdq050. [DOI] [PubMed] [Google Scholar]
- 44.Tsuyuki RT, Houle SK, Charrois TL, Kolber MR, Rosenthal MM, Lewanczuk R, Campbell NR, Cooney D, McAlister FA Rx ACTION Investigators. A randomized trial of the effect of pharmacist prescribing on improving blood pressure in the community: the Alberta clinical trial in optimizing hypertension (RxACTION) Circulation. 2015;132(2):93–100. doi: 10.1161/CIRCULATIONAHA.115.015464. doi: 10.1161/CIRCULATIONAHA.115.015464. [DOI] [PubMed] [Google Scholar]
- 45.Chabot I, Moisan J, Gregoire JP, Milot A. Pharmacist intervention program for control of hypertension. Ann Pharmacother. 2003;37(9):1186–1193. doi: 10.1345/aph.1C267. [DOI] [PubMed] [Google Scholar]
- 46.Zillich AJ, Sutherland JM, Kumbera PA, Carter BL. Hypertension outcomes through blood pressure monitoring and evaluation by pharmacists (HOME study) J Gen Intern Med. 2005 Dec;20(12):1091–1096. doi: 10.1111/j.1525-1497.2005.0226.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.McGhan WF, Stimmel GL, Hall TG, Gilman TM. A comparison of pharmacists and physicians on the quality of prescribing for ambulatory hypertensive patients. Med Care. 1983;21(4):435–444. doi: 10.1097/00005650-198304000-00006. [DOI] [PubMed] [Google Scholar]