Abstract
This review gives a short overview on the widespread use of nanostructured and nanocomposite materials for disease diagnostics, drug delivery, imaging and biomedical sensing applications. Nanoparticle interaction with a biological matrix/entity is greatly influenced by its morphology, crystal phase, surface chemistry, functionalization, physicochemical and electronic properties of the particle. Various nanoparticle synthesis routes, characteristization, and functionalization methodologies to be used for biomedical applications ranging from drug delivery to molecular probing of underlying mechanisms and concepts are described with several examples (150 references).
Keywords: Nanoparticles, synthesis, therapeutic application, surface functionalization
1. INTRODUCTION
Growing interest in the field of nano-biotechnology research has attracted significant attention, mainly due to the expanding potential of drug delivery approaches, imaging techniques and sensor technology, where nanoparticles are deliberately placed inside the body. Particles of 1 to 100 nm have unique physicochemical characteristics, which makes them advantageous as drug molecule carriers, therapeutic and deep tissue image contrast agents and sensitive probes. In this article, we consider the role of various interdisciplinary domains of biomedical engineering and nanoparticle technology in improving current pharmaceutical design, delivery and imaging processes. In section 2, nanoparticles of biomedical importance, more particularly for multimodal disease diagnostics, imaging and sensing, are given. In this section, nanoparticles and their importance are discussed independently of their synthesis process. In section 3, current nanoparticle synthesis methods, with a specific focus on aerosol routes, are presented. Section 4 focuses on the characterization of the nanoscale phenomenon of these particles and their clinical importance. Section 5 reviews the surface modification of synthesized nanomaterials, which makes them useful for diagnostic purposes by enhancing cellular internalization, acceptance, circulation time in the bio-matrix and minimizing the risk of the toxicological sign.
2. NANOPARTICLES OF BIOMEDICAL IMPORTANCE
Due to their unique physical, chemical and electronic characteristics, nanoscale engineered particles find broad application for energy, environmental, and, more recently, biomedical applications [2–6]. There are some reports that claim the nanoscale form of metallic gold was used for medicinal purposes as early as 2500 B.C. by civilizations in India, China, and Egypt [7, 8]. However, recently there has been a mushrooming of applications in the biomedical arena due to advances in the science of synthesis and characterization. Tunable geometric, optical, and surface properties of organic and inorganic nanomaterials enables engineering for a number of applications, such as drug delivery, controlled release, deep tissue imaging and sensing of cellular behavior. In the following section we discuss the use of various nanoparticles for drug delivery, diagnostics, imaging and sensor applications.
2.1. Drug delivery and diagnostics
Both, inorganic (metal and metal oxide) and organic (lipid, protein, DNA, carbon) nanoparticles can be used to target specific organ sites in the animal body. Understanding of colloidal interface science for nanoparticle–bio-matrix interaction depends primarily on particle size and surface properties. Factors affecting cellular internalization and biocompatibility are discussed in the later section. Nanotechnology-based novel drug delivery approaches address issues associated with the current pharmaceutical approach by enhancing shelf-life and acceptability either by uptake efficacy or patient compliance [9–11]. Nanoparticles can be delivered by various entry routes, including oral administration [12, 13], vaccination [14] and aerosol-based drug delivery [15–17], depending on the therapeutic requirement. The selection of the drug administration approach is based principally on the target localization, drug retention time, physiological barriers and pathobiology of the target disease [18].
Despite the potential risk of spreading infectious diseases, the invasive technique of vaccination is still an important instrument to deliver drugs to animals and humans. For instance, Jung et al., [19] established a methodology for topical vaccination using nanosized liposomes in the hair follicle. Liposomes penetrate deeper into hair follicles than a standard formulation, leading to an increased trans-follicular drug uptake. The uptake of liposome further depends on surface charge [17, 20]. Alternative, non-invasive approaches, such as nasal-based mucosal and oral administration of drugs, has become more popular but widespread use is constrained due to drug insolubility problems [21]. Another noninvasive approach of pulmonary drug delivery based on aerosol science and technology is used for the treatment of respiratory disorders such as asthma, cystic fibrosis, respiratory infection and lung cancer [22–25].
An aerosol based target delivery to the affected pulmonary tissue may improve therapeutic efficiency and minimize unwanted side effects [26–28]. Dames et al., [15] performed computer-aided simulations, along with experimental tests on mice, for the targeted delivery of aerosolized droplets containing iron oxide nanoparticles to the lung. Targeted aerosol delivery may be used to treat localized pulmonary infection with bacterial species and viruses. Chattopadhyay [17] summarized the use of nanoscale liposomes for pulmonary drug delivery, including different atomization techniques, with an emphasis on aerosol particle deposition and absorption on the lung surface. Recent advancement in multifunctional nano therapeutics with a suitable optimally engineered carrier and drug delivery system may revolutionize the clinical impact and practices in the near future.
2.1.1. Nanoparticle/drug carrier fate after administration
The crucial steps of nanoparticle uptake are surface recognition and transport by a physiological cellular membrane. To facilitate the uptake and targeted delivery, nanoparticle/nano-drug carriers can be functionalized with various hydrophobic/hydrophilic molecules. Once nanoparticles are internalized (either active or passive administration) by the cell, diverse phisico-chemical and biological events occurs in blood/cytoplasm. As soon as nanoparticles/nano-based drug carriers come in contact with constituents such as amino acids, bioenergetics molecules and peptides in a biological matrix, it leads to the formation of a protein corona as a result of conditioning by plasma/cytoplasmic glycoproteins and peptides [29].
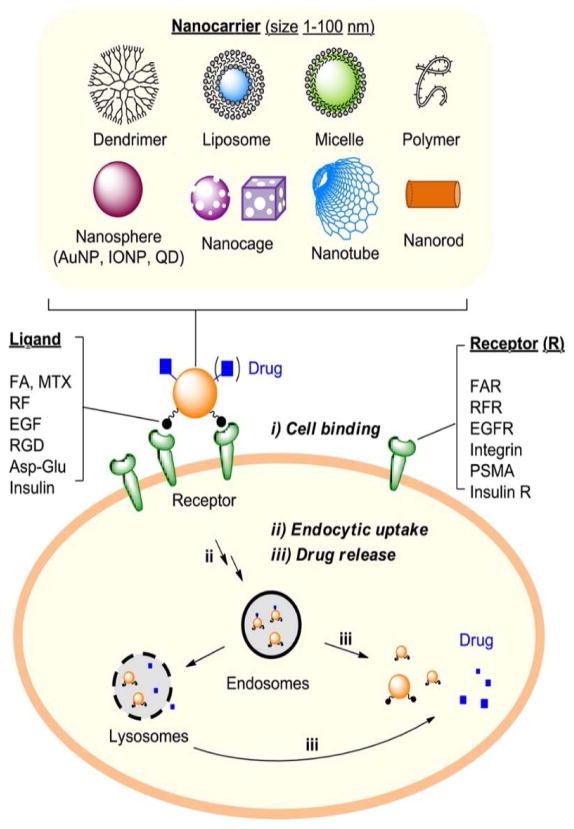
The alteration of nanoparticle surfaces can facilitate the immunological actions and defense mechanism of the cell to release either the drug from the nano-carrier or the individual nanoparticles [29–31]. The release mechanism of nanoparticles/ drugs from nanocarriers includes: various digestive enzymes (hydrolases, phosphatase, esterase, peptidase, lipase, and collagenase), hydrolysis by changing pH, ionic strength, thermolysis, or enzymatic reduction [1]. The released nanoparticles/drug molecules either reach the target site by an enhanced permeation and retention effect (passive targeting) or receptor recognition, binding and uptake (active targeting). A schematic for nanotechnology based cell-targeted delivery of therapeutic agents is illustrated in Figure 1.
Figure 1.
Schematic diagram for cell targeted delivery of therapeutic agents carried by a nanoparticle. The process is comprised of three sequential steps: (i) nanoparticle binding to target cells via multivalent receptor–ligand interactions, (ii) intracellular uptake of the nanoparticle via receptor-mediated endocytosis, and (iii) intracellular drug release or action [1].
Abbrevation: AuNP: Gold nanoparticle;IONP: Iron Oxide nanoparticle; QD: Quantum dots; FA:folic acid; MTX: methotrexate; RF: riboflavin; EGF: epidermal growth factor; RGD: Arg-Gly-Asp; FAR: folate receptor; RFR:riboflavin receptor; EGFR: epidermal growth factor receptor; PSMA: prostate-specific membrane antigen
2.1.2. Imaging
Nanoparticle features, such as multi-functionality of the surface, multivalence, and the ability to carry large payloads have made them the subject of disease diagnostic, drug delivery, probe sensing and imaging research. Engineered particles of 1–100 nm size have emerged as a versatile tool for bio and molecular imaging due to their unique surface phenomenon and tunable absorption and emission properties [32, 33]. The preparation of nanoparticles for bioimaging applications include a variety of steps such as synthesis, coating, and surface functionalization (discussed in the later section of this article). Surface engineering of nanoparticles exhibit highly selective binding, making them useful for fluorescence imaging, magnetic resonance imaging (MRI), positron emission tomography (PET) imaging, and multimodal imaging [34, 35]. The literature is brimming with multi-functional metal (Au, Ag)/metal oxide (Fe3O4), semiconductor nanocrystal (quantum dots), carbon, polymer, and lipid nanoparticles that have been proposed for biomedical imaging, some of which are discussed here.
Super-paramagnetic nanoparticles (SPIO) are a major class of nanoscale materials with the potential to revolutionize the current noninvasive deep tissue magnetic resonance imaging (MRI) through their application as contrast agents capable of providing high resolution anatomical images [36]. Nanoscale SPIO have increased surface-to-volume ratios resulting in pronounced surface effects, such as noncollinear spins, spin canting, and spin-glass-like behavior, which can significantly enhance their magnetization [37]. Iron oxide nanoparticles catalyzed the growth of heterostructures complexes with near-infrared fluorescent single-walled carbon nanotubes utilized as multimodal imaging agents [38]. A recent compressive review by Sharifi et al., [39] summarizes the SPIO application for in vitro and in vivo noninvasive imaging. Nanoparticles having surface plasmon resonance (SPR,) properties have been harnessed extensively for bioimaging. SPR is a phenomenon where free electrons oscillate at the interface of a metal and surrounding medium in resonance with applied external electromagnetic field [40]. SPR and strong surface-enhanced Raman scattering characteristics of noble metal nanostructures, such as gold spheres, rods, shells, and cages can be easily tuned by changing the size, shape and surface properties, which has great interest for imaging and photothermal therapy [6, 41–43]. In addition to SPIO and gold nanostructures, several other nanoparticles such as conjugated polymer nanoparticles [44], quantum dots of various materials such as graphene [45], magnetic nanoparticles [46], semiconductor core-shell nanoparticles [47], nucleic acid functionalized and aptamers [48, 49] are used for imaging purposes. Challenges such as long-term storage stability and toxicological impact of nanostructure enabled novel imaging is still in its early phase of clinical translation.
2.1.3. Sensor
The development of sensors that respond rapidly, reversibly, and specifically to the concentration of a biologically important analyte is a vital and active area of research in both academia and industry. These sensors have application in a broad spectrum of areas including clinical evaluation, on-line bioprocess control, and manufacturing quality assurance [50]. Recent advances in the ability to engineer nanomaterials, including precise control over size, morphology, and composition, have greatly improved the analytical capacity of these sensors. Sensor detection mechanisms include electrochemical, thermal, gravimetric, and optical. The focus of this section will be on advances in electrochemical systems, where aerosol-based routes for material synthesis show great promise. Chemiresistor gas sensors have been studied extensively since their introduction in 1962 to detect gasses through the change in electrical resistance of a semiconducting material as a function of the surrounding atmosphere [51, 52]. Metal oxide semiconductors are already a pervasive tool in some industries where the monitoring of gasses or volatiles are important, including environmental monitoring, domestic safety, and industrial processing. The morphology and composition of the semiconductor material plays an integral role in the receptor function (ability of the oxide surface to interact with the target gas), transducer function (ability to convert the chemical signal into an electrical signal), and utility factor (accessibility of inner oxide grains to the target gas), all of which dictate sensor performance [53, 54]. More recently, aerosol-based nanostructured materials have been designed for use in this emerging field. The aerosol assisted chemical vapor deposition (AACVD) technique has been used to produce Au-and Pt-nanoparticle-functionalized tungsten oxide nanoneedles and Au-nanoparticle-functionalized WO3 nanoneedles for ethanol gas sensing [55, 56]. Flame spray pyrolysis (FSP) was used to produce acetone gas sensors through the single-step deposition of pure and SiO2-doped WO3 nanoparticle layers on Al2O3 substrates [57–59], which showed good agreement with PTR-MS results when tested on human breath [60]. Spray pyrolysis was used to prepare CuO-loaded SnO2 hollow spheres for real-time diagnosis of halitosis [61]. Also, nanotechnology-based techniques can be used to decorate films [62] to enhance the sensitivity and selectivity of materials synthesized through alternative routes. Materials being explored for non-enzymatic amperometric sensors include metals, metal oxides, complexes, and carbon nanomaterials [63]. Kim et al. explored the role of graphene and reviewed its application as a glucose biosensor [64]. More recently, the same group synthesized silver-graphene-titanium dioxide nanocomposites used as a glucose biosensor [65]. Upon a review of the literature, the synthesis of metal oxide nanostructures for application in amperometric glucose sensors is limited but offers great potential, specifically as nonenzymatic sensors become more prevalent.
3. SYNTHESIS OF NANOPARTICLES
Precise control over nanoparticle morphology, chemical composition, and crystal phases enables the engineering of their properites, such as solubility, payload, optical, electronic, mechanical, chemical, magnetic and surface chemistry, a specific biomedical use. The modification of these nanoparticle properties is required to enhance their performance, sensitivity, selectivity and biocompatibility. Numerous methods have been developed in the past two decades to synthesize nanoparticle with controlled characteristics. Table 1 summarizes the major synthesis routes for nanoparticles/nanocomposites, which are discussed in detail here.
Table 1.
Nanoparticles/nanocomposite synthesis approach
| Features | Aerosol | Biological | Chemical | Physical |
|---|---|---|---|---|
| Size and Size Distribution | <100 nm | <100 nm | <100 nm | 15–100 nm |
| Advantages |
|
|
|
|
| Disadvantages |
|
|
|
Broad PSD and poly shape |
3.1. Aerosol route for nanoparticle synthesis
An aerosol is a suspension of solid or liquid particles in a gas [66]. Aerosols may be inadvertently released which can have negative impacts on human health and the environment or can be engineered to be useful for specific applications. Engineered aerosol may be put to several use such as energy, environmental and therapeutic applications, whereas, inadvertently produced aerosols are pollutant particles generated as a result of various combustion process as well as tiny biological organism such as harmful viruses, bacteria also known as bio-aerosol [67]. In recent decades, administration of medicine and diagnosis of health conditions through aerosol-based (pulmonary) delivery has become an increasingly important field due to effective aerosol doses, rapid onset drug action, and targeted and systematic delivery [68]. Here, we will discuss various aerosol routes to synthesise nanoparticles and nanocarriers used for payloading the drug/biomolecules, bioimaging and sensor applications. Aerosol-based methods for nanomaterial synthesis are advantageous in their scalability, simplicity, and flexibility for the deposition of tailored nanomaterials [69]. These industrially relevant processes allow direct deposition of nanoparticles as thin (granular, smooth, columnar) films [70] or collection as powdered particles [71], which can then be suspended in a desired matrix for the use as sensor technology and pharmaceutical drugs, respectively. Aerosol synthesis of nanoparticles is accomplished by various techniques such as flame, furnace, chemical vapor deposition, atomization and electrospray [72]. The basic steps in all the gas/vapor phase nanoparticle synthesis are nucleation and condensation of gases and vapor molecules of the precursor, followed by cluster formation and coagulation leading to the formation of primary particles which may further grow into aggregates by sintering, agglomeration and aggregation [72–74]. Various inorganic and organic base nanoparticles including, but not limited to, magnetite, silica, graphene, lipid and titanium dioxide, are used widely as MRI contrast agents, coatings, drug carriers, sensors and coloring agents, as discussed in the previous section, has been reported to be synthesized by flame [75–79], furnace [80], aerosol chemical vapor deposition [81, 82] and electrospray [17, 20, 83, 84]. A typical process of aerosol based nanoparticles and nanostructure synthesis of nanoparticle can be seen in Figure 2. Aerosol routes for nanomaterial synthesis is becoming increasingly important for biomedical engineering because a) pristine nanoparticles and composites can be synthesized without any trace contamination, b) multicomponent materials with desired chemical composition, c) better process and product in terms of strictly controlled physicochemical and crystal characteristics d) passivation of the surface e) monodispersity, and d) large scale and continue synthesis..
Figure 2.
A typical steps for an aerosol synthesis of nanoparticles and application (a) Schematic of aerosol-based routes for material synthesis. The two major synthesis routes are vapor-to-particle formation and droplet-to-particle formation. In droplet-to-particle formation, a precursor solution is atomized to form small droplets, which evaporate until the solute precipitates and homogenous reaction takes place on the surface of the droplet to form a particle. In vapor-to-particle formation, the precursor takes the form of a vapor, which can undergo either homogenous gas-phase reaction to form molecules of the desired material that then nucleate and grow through coagulation or sintering or heterogeneous reaction on the substrate surface to form thin films of the desired material. (b) Schematic of aerosol-based routes for drug delivery. Traditional drug delivery routes are through the respiratory system; however, more recent advances are focused on delivery directly to the brain, through the blood-brain barrier and axonal transport.
3.2. Other approaches
In addition to aerosol approaches, chemical synthesis of noble metal nanoparticles, particularly gold nanostructures [85–87], core-shell structures [88], quantum dots [89] and luminescent particles [90] are used for deep tissue bio-imaging and thermal therapy. In spite of several advantages, including simple approaches for surface and morphological tuning, functionalization, and monodispersity, the major concerns of chemical synthesis techqniues are stability (high agglomeration rate), undesired chemical coating (due to a part of synthesis procedure) and batch synthesis, which either limits their use or requires addition work to make them biocompatible [91]. Similarly, physical routes, such as the grinding and heating of precursor salts to make nanoparticles, are a more popular industrial large scale synthesis pathway to be used for engineering and environmental application [92–94]. A wide particle size distribution, low rate of solubility, and mixed-phase crystalline nature limits the wider application of nanoparticles synthesized by physical approaches. In the last decade, green synthesis of nanoparticles has become prevalent as the origin and coating of natural source material is believed to enhance biocompatibility [95]. A literature review of the field revealed that microbial (yeast, fungi, bacteria) strains, plant extract and animal tissue lysate have been used to synthesize gold [96, 97], silver [98], iron oxide [99], phosphorous [100], ZnO [95], TiO2 [101] and MgO [102], could be used for biomedical applications. However, large-scale production and control over various physical and chemical characteristics is still challenging.
4. MORPHOLOGICAL AND PHYSICOCHEMICAL CHARACTERIZATION
Nanoparticle properties and behavior in biological media depend on the shape, size, surface charge, and chemical and crystal phase of existence. The goal is to develop drug or drug delivery vehicles with the ability to efficiently cross physiological barriers and reach to the desired locations for disease containment and control. Different nanoscale morphologies allow precise delivery of not only small-molecule drugs but also the delivery of nucleic acids and proteins that reduce systemic side effects [4, 103]. Surface of nanoparticles could be meso-porous, have tiny pores of 2–50 nm over the surface or non-porous, have plane surfaces that theoretically contain no pores [104]. As a versatile material, silica nanoparticles, with both meso and non-porous surface properties, are used in nanomedicine because of their proven biocompatibility [105]. Meso-porous silica is used widely for delivery of active drug molecules based on physical or chemical adsorption. The small pores greatly enhance the capacity of drug loading [106]. In contrast, non-porous particles deliver molecules through encapsulation or conjugation. The interaction with the carrier molecules and silica nanoparticles is weak, although it can be engineered and tuned according to the cellular environment. Similarly, metal nanoparticle gold nanostructures (sphere, rod, shell, cage, cube, star) used extensively for photo-thermal therapy for selective killing of cancer cure due to its tunable SPR behavior [107], which can be controlled by the particle morphology [42, 43]. The SPR peaks of gold nanostructures can be tuned from the visible to the near-infrared region by controlling the shape and structure [43]. In addition to inorganic nanoparticles, carbon-based nanomaterials, such as graphene and its derivatives, peptides, and lipid nanoparticles are extensively used for drug delivery applications [5, 11, 108].
Nanoparticle behavior (toxic or compatible) in the biological matrix depends not only on the type of nanoparticles, but also on the size, shape, crystal phase, surface charge, chemical composition, and agglomeration state [20, 84, 109–111]. Jiang et al., [110] studied reactive oxygen species (ROS) generation capacity of TiO2 nanoparticles, independent of size range (4–195 nm) and crystal phase (anatase, rutile, amorphous, and mixed phase). The highest ROS activity per unit area was observed for 30 nm TiO2 particles, whereas, the trend for crystal phase was amorphous followed by anatase, anatase-rutile mixture and rutile. Similar observations of crystal phase, chemical composition and size were also reported by other research groups [112–114]. To minimize the toxicity of nanoparticles, either biocompatible base materials or surface modification by several surface coating agents must be used and are discussed in the following section of this article. Therefore, nanomaterial synthesis and independent characteristics play a vital role to determine the biomedical application. Some techniques are developed to characterize nanoparticles in the biological matrix as well as in native or aerosol form. Table 2 summarizes the various characterization techniques developed so far and their importance.
Table 2.
Technical approach for characterization of nanoparticles
| Nanoparticle properties | Microscopy and related techniques | Centrifugation and filtration techniques | Spectroscopic and related techniques | Techniques Other |
|---|---|---|---|---|
| Aggregation | STEM, TEM, SEM, AFM, STM | ANUC | XRD, SANS | Zeta potential |
| Chemical composition | AEM, CFM | NMR, XPS, Auger AES, AAS, ICP-MS/OES, XRD, EBSD | ||
| Mass concentration | AEM, CFM | Gravimetry, thermal analysis | ||
| Particle number concentration | Particle counter, CPC | |||
| Shape | STEM, TEM, SEM, AFM, STM | UC | ||
| Size | STEM, TEM, SEM, AFM, STM | DMA | ||
| Size distribution | STEM, TEM, SEM, AFM, STM | CFF, UC, CFUF | SPMS. SAXS | UCPC, SMPS |
| Dissolution | Dialysis, CFUF | Voltammetry, | ||
| Speciation Structure | STEM, TEM, SEM, AFM, STM | XAFS, XRD, ANS | ||
| Surface area (& porosity) | BET | |||
| Surface charge | Zeta Potential, in-flight DMA/SMPS | |||
| Surface Chemistry | AEM, CFM | FTIR, Raman Spectroscopy |
Abbreviations- STEM: scanning transmission electron microscope; TEM: transmission electron microscopy; SEM: scanning electron microscope; AFM: Atomic Force Microscope; STM: Scanning Tunneling Microscope; AEM: analytical electron microscopy; CFM: chemical force microscopy; ANUC: analytical ultracentrifuge; UC: ultracentrifuge; CFF: cross-flow filtration; CFUF: Cross-flow ultrafiltration; XRD: X-ray diffraction; SANS: small-angle neutron scattering; NMR: nuclear magnetic resonance; XPS: X-ray photoelectron spectroscopy; AES: Auger electron spectroscopy; AAS: atomic absorption spectroscopy; ICP-MS/OES: inductively coupled plasma mass / optical emission spectrometry; EBSD: Electron backscatter diffraction; SPMS: Solid Phase Molecular Spectroscopy; SAXS: Small angle x-ray scattering spectroscopy; XAFS: X-ray absorption fine structure; CPC: Condensation particle counter; DMA: differential mobility analyzer; UCPC: ultrafine condensation particle counter; SMPS: Scanning mobility particle sizer; BET: Brunauer–Emmett–Teller, NMs: Nanomaterials; DMA: Differential Mobility Analyzer; SMPS: Scanning Mobility Particle Sizer; FTIR: Fourior Transform Infra-red
5. FUNCTIONALIZATION AND SURFACE MODIFICATION OF NANOPARTICLES
The active surface of nanoparticles and nanocomposites needs to be carefully designed before being applied to biomedical applications. The altering of surfaces can enable the use of material for: a) targeted drug delivery, b) selective binding to desired epitope, c) controlled drug release, d) facilitation of cellular internalization, e) biodistribution f) enhances circulation in bio-fluid, and g) colloidal aggregation stability. Nanoparticles synthesized by aerosol routes are used as pharmacological drug or drug carriers in an emulsifying/colloidal solution. The stability of colloidal suspensions depends on the equilibrium between attractive and repulsive forces by various weak interactions among molecules [115–118]. The theoretical description of these two forces on nanoparticle surfaces can be understood by the Derjaguin-Landau-Verwey-Overbeek theory [109, 119–121]. To engineer nanoparticle surfaces for a desired application, various biocompatible stabilizing agents are used (Figure 2). They are categorized primarily as a) monomeric stabilizers, eg., Thiol group, b) inorganic materials, eg., Silica, gold, and c) organic polymer such as PEG, PVA, DNA molecules, peptides, polysaccharides [117, 122, 123]. Details of nanoparticle stabilizers in colloidal suspension and surface modifiers, based on their properties and corresponding biological applications, are outlined in Table 3. The principle of surface functionalization is mimicking cellular surface compatibility. In spite of functionalization, the cellular reach out of nanomaterials further depends on active morphology, surface zeta potential, surface energy, oxidation state, pH of the dispersion medium, surface and number concentration, aggregation kinetics, deposition rate and exposure duration [122, 124–128]. Instead of direct functionalization, other strategies such as core-shell nanoparticle synthesis or use of polymeric matrix can be applied, however, they limit the potential application and drug release rate.
Table 3.
Nanoparticles surface modifying agent/(bio) molecules used for stabilization and biocompatibility
| Surface modifying agent | Properties | Advantages/applications | References |
|---|---|---|---|
| Polyethylene Glycol (PEG) | Hydrophilic, water-soluble, | Increase the biocompatibility, internalization efficiency, dispersions and circulation times | [127, 129–131] |
| Polyvinylpyrrolidone (PVP) | Water-soluble polymer made from the monomer N-vinylpyrrolidone | Enhances the blood circulation time and stabilizes the colloidal solution, used as binder | [132] |
| Polyvinyl Alcohol (PVA) | Hydrophilic linear polymer that forms copolymers | Biocompatible, PVA coating onto the particle surface prevents their agglomeration, giving rise to monodisperse particles | |
| Chitosan | An alkaline, non-toxic, hydrophilic | Biocompatible and biodegradable | [133–135] |
| Alginate | Electrolytic polysaccharide with carboxyl groups | Stability and functionalization | [136–139] |
| Cellulose | Hydrophilic polymer | Entrap hydrophilic drugs, impart stealth character to nanocarriers, controlled drug release, and increase half-life | [126, 138, 140] |
| Gelatin | Gelling agent, hydrophilic | Emulsifier, biocompatible, natural polymer | [3, 141] |
| Polypeptides | Selectivity for hydrophilicity and hydrophobicity | Increase nanoparticle binding ability to the ligand and cellular internalization, target drug delivery | [125, 142, 143] |
| Fatty acids | Carboxylic acid with a long aliphatic tail | Colloidal stability, terminal functionalization using carboxyl groups | [144, 145] |
| Dextran | α-linked D-glucopyranosyl repeating units | Enhances the blood circulation time, stabilizes the colloidal solution | [146, 147] |
| Silica | Oxide of silicon, Commonly found in nature | Prevent aggregation, improve chemical stability, biocompatible | [148] |
| Nucleic acid | Easy to absorb on the nanoparticle surface | Surface functionalization, Gene delivery, regulation of gene expression | [149] |
| Thiol | Functional group of amino acid | Surface functionalization, target drug delivery | [2, 150] |
6. FUTURE OUTLOOK
Advancements in the field of nanotechnology and the increasing control over nanoparticle structure and composition has unmasked the potential of the use of nanoparticles for drug delivery, imaging and sensing. Smart nanomaterials, seen as the future of nanomedicine of are further advancing the role of nanotechnology in improving human health. These smart nanomaterials are “stimuli responsive” allowing their functionality to be controlled by internal or external stimuli. These stimuli maybe light magnetic field, pH, or heat. These smart materials find tremendous application in targeted drug delivery, controlled release drug delivery, medical implants, tissue scaffolding and wound dressing.
Growing use of nanomaterials and their application directly to human systems has tremendous benefits but has also raised concerns over the unknown detrimental effects of these materials. Toxicity of nanomaterials has been a growing area of research and concern. Possible routes for drug delivery are also possible routes for entry of toxic nanomaterials into the human body. For example, inhalation based drug delivery is very promising and has been used extensively for direct delivery of drugs to the blood stream due to its non-invasive nature but the same route is seen as the most susceptible to toxic nanoparticles. The delivery of drugs via the olfactory system is being seen as an extremely attractive approach to direct delivery to the brain bypassing the blood brain barrier. However, this has also brought forward the issue of nanoparticle toxicity via the olfactory system which has far reaching toxicological implications. Thus, while research advances are made on nanoparticle for biomedical applications, their potential inadvertent impact on human health need also to be considered by the same research.
7. CONCLUSIONS
Nanomaterials are being extensively researched for use as therapeutic agents and other nanomedicine applications. Engineered organic and inorganic nanoparticles are currently being used mainly for deep tissue non-invasive imaging using superparamagnetic nanoparticles and quantum dots as image contrast agents. Aerosol-based pulmonary drug delivery for quick measurable response, principally by atomization is being used and explored for several applications. Photo-thermal therapy for cancer treatment using gold nanostructured materials is being explored. Porous silica nanoparticles as a drug carrier, lipid nanoparticles for targeted gene and drug delivery and aerosol assisted thin film structure for sensor applications are other methodologies being researched. Both the synthesis route and surface functionalization play an important role in nanoparticle biocompatibility. However, long-term stability, toxicological impact, site-specific internalization and metabolism of nanotechnology-based drug/drug carrying vehicles is still an open question and more extensive research is needed.
Footnotes
CONFLICT OF INTEREST
The authors declare that they have no conflict of interest.
References
- 1.Wong PT, Choi SK. Mechanisms of Drug Release in Nanotherapeutic Delivery Systems. Chemical Reviews. 2015;115:3388–3432. doi: 10.1021/cr5004634. [DOI] [PubMed] [Google Scholar]
- 2.Aznar E, Climent E, Mondragon L, Sancenón F, Martínez-Máñez R. Functionalized Mesoporous Materials with Gate-Like Scaffoldings for Controlled Delivery. Polymers in Regenerative Medicine: Biomedical Applications from Nano-to Macro-Structures. 2015:337–366. [Google Scholar]
- 3.Che E, Gao Y, Wan L, Zhang Y, Han N, Bai J, Li J, Sha Z, Wang S. Paclitaxel/gelatin coated magnetic mesoporous silica nanoparticles: Preparation and antitumor efficacy in vivo. Microporous and Mesoporous Materials. 2015;204:226–234. [Google Scholar]
- 4.Hughes GA. Nanostructure-mediated drug delivery. Nanomedicine: Nanotechnology, Biology and Medicine. 2005;1:22–30. doi: 10.1016/j.nano.2004.11.009. [DOI] [PubMed] [Google Scholar]
- 5.Chung C, Kim YK, Shin D, Ryoo S-R, Hong BH, Min DH. Biomedical Applications of Graphene and Graphene Oxide. Accounts of Chemical Research. 2013;46:2211–2224. doi: 10.1021/ar300159f. [DOI] [PubMed] [Google Scholar]
- 6.Dykman L, Khlebtsov N. Gold nanoparticles in biomedical applications: recent advances and perspectives. Chemical Society Reviews. 2012;41:2256–2282. doi: 10.1039/c1cs15166e. [DOI] [PubMed] [Google Scholar]
- 7.Chaudhary A. Ayurvedic Bhasma: nanomedicine of ancient India—its global contemporary perspective. Journal of biomedical nanotechnology. 2011;7:68–69. doi: 10.1166/jbn.2011.1205. [DOI] [PubMed] [Google Scholar]
- 8.Pal D, Sahu CK, Haldar A. Bhasma: the ancient Indian nanomedicine. Journal of advanced pharmaceutical technology & research. 2014;5:4–12. doi: 10.4103/2231-4040.126980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.De Villiers MM, Aramwit P, Kwon GS. Nanotechnology in drug delivery. Springer Science & Business Media; 2008. p. 662. [Google Scholar]
- 10.Farokhzad OC, Langer R. Impact of nanotechnology on drug delivery. ACS Nano. 2009;3:16–20. doi: 10.1021/nn900002m. [DOI] [PubMed] [Google Scholar]
- 11.Sahoo SK, Labhasetwar V. Nanotech approaches to drug delivery and imaging. Drug discovery today. 2003;8:1112–1120. doi: 10.1016/s1359-6446(03)02903-9. [DOI] [PubMed] [Google Scholar]
- 12.Gelperina S, Kisich K, Iseman MD, Heifets L. The potential advantages of nanoparticle drug delivery systems in chemotherapy of tuberculosis. American journal of respiratory and critical care medicine. 2005;172:1487–1490. doi: 10.1164/rccm.200504-613PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pridgen EM, Alexis F, Farokhzad OC. Polymeric nanoparticle drug delivery technologies for oral delivery applications. Expert opinion on drug delivery. 2015:1–15. doi: 10.1517/17425247.2015.1018175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Smith JD, Morton LD, Ulery BD. Nanoparticles as synthetic vaccines. Current opinion in biotechnology. 2015;34:217–224. doi: 10.1016/j.copbio.2015.03.014. [DOI] [PubMed] [Google Scholar]
- 15.Dames P, Gleich B, Flemmer A, Hajek K, Seidl N, Wiekhorst F, Eberbeck D, Bittmann I, Bergemann C, Weyh T. Targeted delivery of magnetic aerosol droplets to the lung. Nature nanotechnology. 2007;2:495–499. doi: 10.1038/nnano.2007.217. [DOI] [PubMed] [Google Scholar]
- 16.Beck-Broichsitter M, Merkel OM, Kissel T. Controlled pulmonary drug and gene delivery using polymeric nano-carriers. Journal of Controlled Release. 2012;161:214–224. doi: 10.1016/j.jconrel.2011.12.004. [DOI] [PubMed] [Google Scholar]
- 17.Chattopadhyay S. Aerosol generation using nanometer liposome suspensions for pulmonary drug delivery applications. Journal of liposome research. 2013;23:255–267. doi: 10.3109/08982104.2013.802332. [DOI] [PubMed] [Google Scholar]
- 18.Labhasetwar V. Nanotechnology for drug and gene therapy: the importance of understanding molecular mechanisms of delivery. Current Opinion in Biotechnology. 2005;16:674–680. doi: 10.1016/j.copbio.2005.10.009. [DOI] [PubMed] [Google Scholar]
- 19.Jung S, Patzelt A, Otberg N, Thiede G, Sterry W, Lademann J. Strategy of topical vaccination with nanoparticles. Journal of biomedical optics. 2009;14:021001–021007. doi: 10.1117/1.3080714. [DOI] [PubMed] [Google Scholar]
- 20.Chadha TS, Chattopadhyay S, Venkataraman C, Biswas P. Study of the charge distribution on liposome particles aerosolized by air-jet atomization. Journal of aerosol medicine and pulmonary drug delivery. 2012;25:355–364. doi: 10.1089/jamp.2011.0967. [DOI] [PubMed] [Google Scholar]
- 21.Csaba N, Garcia-Fuentes M, Alonso MJ. Nanoparticles for nasal vaccination. Advanced Drug Delivery Reviews. 2009;61:140–157. doi: 10.1016/j.addr.2008.09.005. [DOI] [PubMed] [Google Scholar]
- 22.O’Riordan TG. Aerosol delivery devices and obstructive airway disease. 2005;2:197–203. doi: 10.1586/17434440.2.2.197. [DOI] [PubMed] [Google Scholar]
- 23.Dinwiddie R. Anti-inflammatory therapy in cystic fibrosis. Journal of cystic fibrosis: official journal of the European Cystic Fibrosis Society. 2005;4:45–48. doi: 10.1016/j.jcf.2005.05.010. [DOI] [PubMed] [Google Scholar]
- 24.Hagerman JK, Hancock KE, Klepser ME. Aerosolised antibiotics: a critical appraisal of their use. 2006;3:71–86. doi: 10.1517/17425247.3.1.71. [DOI] [PubMed] [Google Scholar]
- 25.Rao R, Markovic S, Anderson P. Aerosol therapy for malignancy involving the lungs. Current cancer drug targets. 2003;3:239–250. doi: 10.2174/1568009033481895. [DOI] [PubMed] [Google Scholar]
- 26.Bennett WD, Brown JS, Zeman KL, Hu S-C, Scheuch G, Sommerer K. Targeting delivery of aerosols to different lung regions. Journal of aerosol medicine. 2002;15:179–188. doi: 10.1089/089426802320282301. [DOI] [PubMed] [Google Scholar]
- 27.Card JW, Zeldin DC, Bonner JC, Nestmann ER. Pulmonary applications and toxicity of engineered nanoparticles. American Journal of Physiology-Lung Cellular and Molecular Physiology. 2008;295:L400–L411. doi: 10.1152/ajplung.00041.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dolovich MB, Dhand R. Aerosol drug delivery: developments in device design and clinical use. The Lancet. 2011;377:1032–1045. doi: 10.1016/S0140-6736(10)60926-9. [DOI] [PubMed] [Google Scholar]
- 29.Lundqvist M, Stigler J, Elia G, Lynch I, Cedervall T, Dawson KA. Nanoparticle size and surface properties determine the protein corona with possible implications for biological impacts. Proceedings of the National Academy of Sciences. 2008;105:14265–14270. doi: 10.1073/pnas.0805135105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tenzer S, Docter D, Kuharev J, Musyanovych A, Fetz V, Hecht R, Schlenk F, Fischer D, Kiouptsi K, Reinhardt C. Rapid formation of plasma protein corona critically affects nanoparticle pathophysiology. Nature nanotechnology. 2013;8:772–781. doi: 10.1038/nnano.2013.181. [DOI] [PubMed] [Google Scholar]
- 31.Monopoli MP, Walczyk D, Campbell A, Elia G, Lynch I, Baldelli Bombelli F, Dawson KA. Physical– chemical aspects of protein corona: relevance to in vitro and in vivo biological impacts of nanoparticles. Journal of the American Chemical Society. 2011;133:2525–2534. doi: 10.1021/ja107583h. [DOI] [PubMed] [Google Scholar]
- 32.Nune SK, Gunda P, Thallapally PK, Lin Y-Y, Laird Forrest M, Berkland CJ. Nanoparticles for biomedical imaging. Expert opinion on drug delivery. 2009;6:1175–1194. doi: 10.1517/17425240903229031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Choi HS, Frangioni JV. Nanoparticles for biomedical imaging: fundamentals of clinical translation. Molecular imaging. 2010;9:291–310. [PMC free article] [PubMed] [Google Scholar]
- 34.Erathodiyil N, Ying JY. Functionalization of inorganic nanoparticles for bioimaging applications. Accounts of Chemical Research. 2011;44:925–935. doi: 10.1021/ar2000327. [DOI] [PubMed] [Google Scholar]
- 35.Nam J, Won N, Bang J, Jin H, Park J, Jung S, Jung S, Park Y, Kim S. Surface engineering of inorganic nanoparticles for imaging and therapy. Advanced Drug Delivery Reviews. 2013;65:622–648. doi: 10.1016/j.addr.2012.08.015. [DOI] [PubMed] [Google Scholar]
- 36.Jin R, Lin B, Li D, Ai H. Superparamagnetic iron oxide nanoparticles for MR imaging and therapy: design considerations and clinical applications. Current opinion in pharmacology. 2014;18:18–27. doi: 10.1016/j.coph.2014.08.002. [DOI] [PubMed] [Google Scholar]
- 37.Sun C, Lee JSH, Zhang M. Magnetic nanoparticles in MR imaging and drug delivery. Advanced Drug Delivery Reviews. 2008;60:1252–1265. doi: 10.1016/j.addr.2008.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Choi JH, Nguyen FT, Barone PW, Heller DA, Moll AE, Patel D, Boppart SA, Strano MS. Multimodal biomedical imaging with asymmetric single-walled carbon nanotube/iron oxide nanoparticle complexes. Nano Letters. 2007;7:861–867. doi: 10.1021/nl062306v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sharifi S, Seyednejad H, Laurent S, Atyabi F, Saei AA, Mahmoudi M. Superparamagnetic iron oxide nanoparticles for in vivo molecular and cellular imaging. Contrast media & molecular imaging. 2015 doi: 10.1002/cmmi.1638. [DOI] [PubMed] [Google Scholar]
- 40.Jain PK, Huang X, El-Sayed IH, El-Sayed MA. Noble metals on the nanoscale: optical and photothermal properties and some applications in imaging, sensing, biology, and medicine. Accounts of Chemical Research. 2008;41:1578–1586. doi: 10.1021/ar7002804. [DOI] [PubMed] [Google Scholar]
- 41.Bao C, Conde J, Polo E, del Pino P, Moros M, Baptista PV, Grazu V, Cui D, de la Fuente JM. A promising road with challenges: where are gold nanoparticles in translational research? Nanomedicine. 2014;9:2353–2370. doi: 10.2217/nnm.14.155. [DOI] [PubMed] [Google Scholar]
- 42.Hu M, Chen J, Li Z-Y, Au L, Hartland GV, Li X, Marquez M, Xia Y. Gold nanostructures: engineering their plasmonic properties for biomedical applications. Chemical Society Reviews. 2006;35:1084–1094. doi: 10.1039/b517615h. [DOI] [PubMed] [Google Scholar]
- 43.Cobley CM, Chen J, Cho EC, Wang LV, Xia Y. Gold nanostructures: a class of multifunctional materials for biomedical applications. Chemical Society Reviews. 2011;40:44–56. doi: 10.1039/b821763g. [DOI] [PubMed] [Google Scholar]
- 44.Kim S, Lim C-K, Na J, Lee Y-D, Kim K, Choi K, Leary JF, Kwon IC. Conjugated polymer nanoparticles for biomedical in vivo imaging. Chemical Communications. 2010;46:1617–1619. doi: 10.1039/b923309a. [DOI] [PubMed] [Google Scholar]
- 45.Zhu S, Zhang J, Tang S, Qiao C, Wang L, Wang H, Liu X, Li B, Li Y, Yu W. surface chemistry routes to modulate the photoluminescence of graphene quantum dots: from fluorescence mechanism to up-conversion bioimaging applications. Advanced Functional Materials. 2012;22:4732–4740. [Google Scholar]
- 46.Selvan S, Patra PK, Ang CY, Ying JY. Synthesis of silica-coated semiconductor and magnetic quantum dots and their use in the imaging of live cells. Angewandte Chemie. 2007;119:2500–2504. doi: 10.1002/anie.200604245. [DOI] [PubMed] [Google Scholar]
- 47.Law WC, Yong KT, Roy I, Ding H, Hu R, Zhao W, Prasad PN. Aqueous-phase synthesis of highly luminescent CdTe/ZnTe core/shell quantum dots optimized for targeted bioimaging. Small. 2009;5:1302–1310. doi: 10.1002/smll.200801555. [DOI] [PubMed] [Google Scholar]
- 48.Kong RM, Zhang XB, Chen Z, Tan W. Aptamer-assembled nanomaterials for biosensing and biomedical applications. Small. 2011;7:2428–2436. doi: 10.1002/smll.201100250. [DOI] [PubMed] [Google Scholar]
- 49.Hu R, Zhang X-B, Kong R-M, Zhao X-H, Jiang J, Tan W. Nucleic acid-functionalized nanomaterials for bioimaging applications. Journal of Materials Chemistry. 2011;21:16323–16334. [Google Scholar]
- 50.Meadows D. Recent developments with biosensing technology and applications in the pharmaceutical industry. Advanced drug delivery reviews. 1996;21:179–189. [Google Scholar]
- 51.Taguchi N. A Metal Oxide Gas Sensor. Jpn Patent. 1962:45–38200. [Google Scholar]
- 52.Seiyama T, Kato A, Fujiishi K, Nagatani M. A New detector for gaseous components using semiconductive thin films. Analytical Chemistry. 1962;34:1502–1503. [Google Scholar]
- 53.Korotcenkov G. Metal oxides for solid-state gas sensors: What determines our choice? Materials Science and Engineering: B. 2007;139:1–23. [Google Scholar]
- 54.Zhan Z, Wang W-N, Zhu L, An W-J, Biswas P. Flame aerosol reactor synthesis of nanostructured SnO2 thin films: High gas-sensing properties by control of morphology. Sensors and Actuators B: Chemical. 2010;150:609–615. [Google Scholar]
- 55.Vallejos S, Stoycheva T, Umek P, Navio C, Snyders R, Bittencourt C, Llobet E, Blackman C, Moniz S, Correig X. Au nanoparticle-functionalised WO3 nanoneedles and their application in high sensitivity gas sensor devices. Chemical Communications. 2011;47:565–567. doi: 10.1039/c0cc02398a. [DOI] [PubMed] [Google Scholar]
- 56.Vallejos S, Umek P, Stoycheva T, Annanouch F, Llobet E, Correig X, De Marco P, Bittencourt C, Blackman C. Single-step deposition of au-and pt-nanoparticle-functionalized tungsten oxide nanoneedles synthesized via aerosol-assisted cvd, and used for fabrication of selective gas microsensor arrays. Advanced Functional Materials. 2013;23:1313–1322. [Google Scholar]
- 57.Righettoni M, Tricoli A, Pratsinis SE. Thermally stable, silica-doped ε-WO3 for sensing of acetone in the human breath. Chemistry of Materials. 2010;22:3152–3157. [Google Scholar]
- 58.Righettoni M, Tricoli A, Pratsinis SE. Sensors, 2010. IEEE; 2010. Si:WO3 sensors for noninvasive diabetes diagnosis by breath analysis; pp. 1491–1495. [Google Scholar]
- 59.Righettoni M, Tricoli A. Toward portable breath acetone analysis for diabetes detection. Journal of Breath Research. 2011;5:037109. doi: 10.1088/1752-7155/5/3/037109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Righettoni M, Tricoli A, Gass S, Schmid A, Amann A, Pratsinis SE. Breath acetone monitoring by portable Si: WO3 gas sensors. Analytica chimica acta. 2012;738:69–75. doi: 10.1016/j.aca.2012.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Choi K-I, Kim H-J, Kang YC, Lee J-H. Ultraselective and ultrasensitive detection of H2S in highly humid atmosphere using CuO-loaded SnO2 hollow spheres for real-time diagnosis of halitosis. Sensors and Actuators B: Chemical. 2014;194:371–376. [Google Scholar]
- 62.Cui S, Guo X, Ren R, Zhou G, Chen J. Decoration of vertical graphene with aerosol nanoparticles for gas sensing. Journal of Physics D: Applied Physics. 2015;48:314008. [Google Scholar]
- 63.Si P, Huang Y, Wang T, Ma J. Nanomaterials for electrochemical non-enzymatic glucose biosensors. RSC Advances. 2013;3:3487–3502. [Google Scholar]
- 64.Kim SK, Chang H, Choi J-W, Huang J, Jang HD. Aerosol processing of graphene and its application to oil absorbent and glucose biosensor. KONA Powder and Particle Journal. 2014;31:111–125. [Google Scholar]
- 65.Jang HD, Kim SK, Chang H, Jo EH, Roh KM, Choi J-H, Choi J-W. Synthesis of 3D silver-graphene-titanium dioxide composite via aerosol spray pyrolysis for sensitive glucose biosensor. Aerosol Science and Technology. 2015;49:538–546. [Google Scholar]
- 66.Hinds WC. Aerosol technology: properties, behavior, and measurement of airborne particles. New York: Wiley-Interscience; 1982. p. 442. [Google Scholar]
- 67.AAQRL. [Accessed on July 30, 2015];Aerosol and Air Quality Reesearch laboratory. http://aerosols.eece.wustl.edu/
- 68.Hickey AJ. Pharmaceutical inhalation aerosol technology. CRC Press; 2003. p. 288. [Google Scholar]
- 69.Tricoli A, Graf M, Mayer F, Kuühne S, Hierlemann A, Pratsinis SE. Micropatterning Layers by Flame Aerosol Deposition-Annealing. Advanced Materials. 2008;20:3005–3010. [Google Scholar]
- 70.An W-J, Thimsen E, Biswas P. Aerosol-chemical vapor deposition method for synthesis of nanostructured metal oxide thin films with controlled morphology. The Journal of Physical Chemistry Letters. 2009;1:249–253. [Google Scholar]
- 71.Wang Z-M, Yang G, Biswas P, Bresser W, Boolchand P. Processing of iron-doped titania powders in flame aerosol reactors. Powder technology. 2001;114:197–204. [Google Scholar]
- 72.Kruis FE, Fissan H, Peled A. Synthesis of nanoparticles in the gas phase for electronic, optical and magnetic applications—a review. Journal of Aerosol Science. 1998;29:511–535. [Google Scholar]
- 73.Fang J, Leavey A, Biswas P. Controlled studies on aerosol formation during biomass pyrolysis in a flat flame reactor. Fuel. 2014;116:350–357. [Google Scholar]
- 74.Hahn H. Gas phase synthesis of nanocrystalline materials. Nanostructured Materials. 1997;9:3–12. [Google Scholar]
- 75.Basak S, Rane KS, Biswas P. Hydrazine-assisted, low-temperature aerosol pyrolysis method to synthesize γ-Fe2O3. Chemistry of Materials. 2008;20:4906–4914. [Google Scholar]
- 76.Li C, Ma C, Wang F, Xi Z, Wang Z, Deng Y, He N. Preparation and biomedical applications of core–shell silica/magnetic nanoparticle composites. Journal of nanoscience and nanotechnology. 2012;12:2964–2972. doi: 10.1166/jnn.2012.6428. [DOI] [PubMed] [Google Scholar]
- 77.Deng Y-H, Wang C-C, Hu J-H, Yang W-L, Fu S-K. Investigation of formation of silica-coated magnetite nanoparticles via sol–gel approach. Colloids and Surfaces A: Physicochemical and Engineering Aspects. 2005;262:87–93. [Google Scholar]
- 78.Jiang J, Chen D-R, Biswas P. Synthesis of nanoparticles in a flame aerosol reactor with independent and strict control of their size, crystal phase and morphology. Nanotechnology. 2007;18:285603. [Google Scholar]
- 79.Tiwari V, Jiang J, Sethi V, Biswas P. One-step synthesis of noble metal–titanium dioxide nanocomposites in a flame aerosol reactor. Applied Catalysis A: General. 2008;345:241–246. [Google Scholar]
- 80.Wang W-N, Jiang Y, Biswas P. Evaporation-induced crumpling of graphene oxide nanosheets in aerosolized droplets: confinement force relationship. The Journal of Physical Chemistry Letters. 2012;3:3228–3233. doi: 10.1021/jz3015869. [DOI] [PubMed] [Google Scholar]
- 81.Chadha TS, Park J, An WJ, Biswas P. Gold nanocage coupled single crystal TiO2 nanostructures for near-infrared water photolysis. Journal of Nanoparticle Research. 2014;16:1–9. [Google Scholar]
- 82.Tricoli A, Righettoni M, Teleki A. Semiconductor gas sensors: dry synthesis and application. Angewandte Chemie International Edition. 2010;49:7632–7659. doi: 10.1002/anie.200903801. [DOI] [PubMed] [Google Scholar]
- 83.Basak S, Chen D-R, Biswas P. Electrospray of ionic precursor solutions to synthesize iron oxide nanoparticles: modified scaling law. Chemical engineering science. 2007;62:1263–1268. [Google Scholar]
- 84.Chattopadhyay S, Modesto-Lopez LB, Venkataraman C, Biswas P. Size distribution and morphology of liposome aerosols generated by two methodologies. Aerosol Science and Technology. 2010;44:972–982. [Google Scholar]
- 85.Sun Y, Xia Y. Shape-controlled synthesis of gold and silver nanoparticles. Science. 2002;298:2176–2179. doi: 10.1126/science.1077229. [DOI] [PubMed] [Google Scholar]
- 86.Brust M, Walker M, Bethell D, Schiffrin DJ, Whyman R. Synthesis of thiol-derivatised gold nanoparticles in a two-phase liquid–liquid system. Journal of the Chemical Society, Chemical Communications. 1994:801–802. [Google Scholar]
- 87.Gu H, Zheng R, Zhang X, Xu B. Facile one-pot synthesis of bifunctional heterodimers of nanoparticles: a conjugate of quantum dot and magnetic nanoparticles. Journal of the American Chemical Society. 2004;126:5664–5665. doi: 10.1021/ja0496423. [DOI] [PubMed] [Google Scholar]
- 88.Du H, Hamilton PD, Reilly MA, d’Avignon A, Biswas P, Ravi N. A facile synthesis of highly water-soluble, core–shell organo-silica nanoparticles with controllable size via sol–gel process. Journal of Colloid and Interface Science. 2009;340:202–208. doi: 10.1016/j.jcis.2009.08.032. [DOI] [PubMed] [Google Scholar]
- 89.Yi DK, Selvan ST, Lee SS, Papaefthymiou GC, Kundaliya D, Ying JY. Silica-coated nanocomposites of magnetic nanoparticles and quantum dots. Journal of the American Chemical Society. 2005;127:4990–4991. doi: 10.1021/ja0428863. [DOI] [PubMed] [Google Scholar]
- 90.Selvan ST, Bullen C, Ashokkumar M, Mulvaney P. Synthesis of Tunable, highly luminescent QD-glasses through sol-gel processing. Advanced Materials. 2001;13:985–988. [Google Scholar]
- 91.Daniel M-C, Astruc D. Gold nanoparticles: assembly, supramolecular chemistry, quantum-size-related properties, and applications toward biology, catalysis, and nanotechnology. Chemical reviews. 2004;104:293–346. doi: 10.1021/cr030698+. [DOI] [PubMed] [Google Scholar]
- 92.Eliezer S, Eliaz N, Grossman E, Fisher D, Gouzman I, Henis Z, Pecker S, Horovitz Y, Fraenkel M, Maman S. Synthesis of nanoparticles with femtosecond laser pulses. Physical Review B. 2004;69:144119. [Google Scholar]
- 93.Deguchi S, Mukai S-a, Tsudome M, Horikoshi K. Facile Generation of fullerene nanoparticles by hand-grinding. Advanced Materials. 2006;18:729–732. [Google Scholar]
- 94.Lu J, Ng KM, Yang S. Efficient, one-step mechanochemical process for the synthesis of ZnO nanoparticles. Industrial & engineering chemistry research. 2008;47:1095–1101. [Google Scholar]
- 95.Raliya R, Rathore I, Tarafdar JC. Development of microbial nanofactory for zinc, magnesium, and titanium nanoparticles production using soil fungi. Journal of Bionanoscience. 2013;7:590–596. [Google Scholar]
- 96.Raliya R, Biswas P. Environmentally benign bio-inspired synthesis of Au nanoparticles, their self-assembly and agglomeration. RSC Advances. 2015;5:42081–42087. [Google Scholar]
- 97.Raliya R, Tarafdar J. Biosynthesis of gold nanoparticles using rhizoctonia bataticola TFR-6. Advanced Science, Engineering and Medicine. 2013;5:1073–1076. [Google Scholar]
- 98.Raliya R, Tarafdar J. Novel approach for silver nanoparticle synthesis using Aspergillus terreus CZR-1: mechanism perspective. Journal of Bionanoscience. 2012;6:12–16. [Google Scholar]
- 99.Raliya JCTaR. Rapid, low-cost, and ecofriendly approach for iron nanoparticle synthesis using Aspergillus oryzae TFR9. Journal of Nanoparticles. 2013;2013:141274. [Google Scholar]
- 100.Tarafdar J, Raliya R, Rathore I. Microbial synthesis of phosphorous nanoparticle from tri-calcium phosphate using Aspergillus tubingensis TFR-5. Journal of Bionanoscience. 2012;6:84–89. [Google Scholar]
- 101.Tarafdar A, Raliya R, Wang W-N, Biswas P, Tarafdar J. Green synthesis of TiO2 nanoparticle using Aspergillus tubingensis. Advanced Science, Engineering and Medicine. 2013;5:943–949. [Google Scholar]
- 102.Raliya R, Tarafdar JC, Choudhary K, Mal P, Raturi A, Gautam R, Singh SK. Synthesis of MgO Nanoparticles Using Aspergillus Tubingensis TFR-3. Journal of Bionanoscience. 2014;8:34–38. [Google Scholar]
- 103.Tang L, Cheng J. Nonporous silica nanoparticles for nanomedicine application. Nano today. 2013;8:290–312. doi: 10.1016/j.nantod.2013.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Callejas-Fernández J, Estelrich J, Quesada-Pérez M, Forcada J. Soft Nanoparticles for Biomedical Applications. Royal Society of Chemistry. 2014 [Google Scholar]
- 105.Slowing II, Vivero-Escoto JL, Wu C-W, Lin VS-Y. Mesoporous silica nanoparticles as controlled release drug delivery and gene transfection carriers. Advanced drug delivery reviews. 2008;60:1278–1288. doi: 10.1016/j.addr.2008.03.012. [DOI] [PubMed] [Google Scholar]
- 106.Tzur-Balter A, Shatsberg Z, Beckerman M, Segal E, Artzi N. Mechanism of erosion of nanostructured porous silicon drug carriers in neoplastic tissues. Nature Communication. 2015;6 doi: 10.1038/ncomms7208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Chen J, Wiley B, Li ZY, Campbell D, Saeki F, Cang H, Au L, Lee J, Li X, Xia Y. Gold nanocages: engineering their structure for biomedical applications. Advanced Materials. 2005;17:2255–2261. [Google Scholar]
- 108.Zhang S. Fabrication of novel biomaterials through molecular self-assembly. Nature biotechnology. 2003;21:1171–1178. doi: 10.1038/nbt874. [DOI] [PubMed] [Google Scholar]
- 109.Jiang J, Oberdörster G, Biswas P. Characterization of size, surface charge, and agglomeration state of nanoparticle dispersions for toxicological studies. Journal of Nanoparticle Research. 2009;11:77–89. [Google Scholar]
- 110.Jiang J, Oberdörster G, Elder A, Gelein R, Mercer P, Biswas P. Does nanoparticle activity depend upon size and crystal phase? Nanotoxicology. 2008;2:33–42. doi: 10.1080/17435390701882478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Suttiponparnit K, Jiang J, Sahu M, Suvachittanont S, Charinpanitkul T, Biswas P. Role of surface area, primary particle size, and crystal phase on titanium dioxide nanoparticle dispersion properties. Nanoscale Res Lett. 2011;6:27. doi: 10.1007/s11671-010-9772-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Auffan M, Rose J, Bottero J-Y, Lowry GV, Jolivet J-P, Wiesner MR. Towards a definition of inorganic nanoparticles from an environmental, health and safety perspective. Nature nanotechnology. 2009;4:634–641. doi: 10.1038/nnano.2009.242. [DOI] [PubMed] [Google Scholar]
- 113.Jin C, Tang Y, Yang FG, Li XL, Xu S, Fan XY, Huang YY, Yang YJ. Cellular toxicity of TiO2 nanoparticles in anatase and rutile crystal phase. Biological trace element research. 2011;141:3–15. doi: 10.1007/s12011-010-8707-0. [DOI] [PubMed] [Google Scholar]
- 114.Hinton TM, Grusche F, Acharya D, Shukla R, Bansal V, Waddington LJ, Monaghan P, Muir BW. Bicontinuous cubic phase nanoparticle lipid chemistry affects toxicity in cultured cells. Toxicology Research. 2014;3:11–22. [Google Scholar]
- 115.Kreyling WG, Biswas P, Messing ME, Gibson N, Geiser M, Wenk A, Sahu M, Deppert K, Cydzik I, Wigge C. Generation and characterization of stable, highly concentrated titanium dioxide nanoparticle aerosols for rodent inhalation studies. Journal of Nanoparticle Research. 2011;13:511–524. [Google Scholar]
- 116.Gupta AK, Gupta M. Synthesis and surface engineering of iron oxide nanoparticles for biomedical applications. Biomaterials. 2005;26:3995–4021. doi: 10.1016/j.biomaterials.2004.10.012. [DOI] [PubMed] [Google Scholar]
- 117.Mout R, Moyano DF, Rana S, Rotello VM. Surface functionalization of nanoparticles for nanomedicine. Chemical Society Reviews. 2012;41:2539–2544. doi: 10.1039/c2cs15294k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Villanueva A, Canete M, Roca AG, Calero M, Veintemillas-Verdaguer S, Serna CJ, del Puerto Morales M, Miranda R. The influence of surface functionalization on the enhanced internalization of magnetic nanoparticles in cancer cells. Nanotechnology. 2009;20:115103. doi: 10.1088/0957-4484/20/11/115103. [DOI] [PubMed] [Google Scholar]
- 119.Keller AA, Wang H, Zhou D, Lenihan HS, Cherr G, Cardinale BJ, Miller R, Ji Z. Stability and aggregation of metal oxide nanoparticles in natural aqueous matrices. Environmental science & technology. 2010;44:1962–1967. doi: 10.1021/es902987d. [DOI] [PubMed] [Google Scholar]
- 120.Zhou J, Ralston J, Sedev R, Beattie DA. Functionalized gold nanoparticles: synthesis, structure and colloid stability. Journal of Colloid and Interface Science. 2009;331:251–262. doi: 10.1016/j.jcis.2008.12.002. [DOI] [PubMed] [Google Scholar]
- 121.Stebounova LV, Guio E, Grassian VH. Silver nanoparticles in simulated biological media: a study of aggregation, sedimentation, and dissolution. Journal of Nanoparticle Research. 2011;13:233–244. [Google Scholar]
- 122.Moghadam BY, Hou W-C, Corredor C, Westerhoff P, Posner JD. Role of nanoparticle surface functionality in the disruption of model cell membranes. Langmuir. 2012;28:16318–16326. doi: 10.1021/la302654s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Kettler K, Veltman K, van de Meent D, van Wezel A, Hendriks AJ. Cellular uptake of nanoparticles as determined by particle properties, experimental conditions, and cell type. Environmental Toxicology and Chemistry. 2014;33:481–492. doi: 10.1002/etc.2470. [DOI] [PubMed] [Google Scholar]
- 124.Laurent S, Forge D, Port M, Roch A, Robic C, Vander Elst L, Muller RN. Magnetic iron oxide nanoparticles: synthesis, stabilization, vectorization, physicochemical characterizations, and biological applications. Chemical reviews. 2008;108:2064–2110. doi: 10.1021/cr068445e. [DOI] [PubMed] [Google Scholar]
- 125.Sarkar K, Banerjee SL, Kundu PP, Madras G, Chatterjee K. Biofunctionalized surface-modified silver nanoparticles for gene delivery. Journal of Materials Chemistry B. 2015;3:5266–5276. doi: 10.1039/c5tb00614g. [DOI] [PubMed] [Google Scholar]
- 126.Numata Y, Mazzarino L, Borsali R. A slow-release system of bacterial cellulose gel and nanoparticles for hydrophobic active ingredients. International journal of pharmaceutics. 2015;486:217–225. doi: 10.1016/j.ijpharm.2015.03.068. [DOI] [PubMed] [Google Scholar]
- 127.Otsuka H, Nagasaki Y, Kataoka K. PEGylated nanoparticles for biological and pharmaceutical applications. Advanced drug delivery reviews. 2012;64:246–255. doi: 10.1016/s0169-409x(02)00226-0. [DOI] [PubMed] [Google Scholar]
- 128.Pelaz B, del Pino P, Maffre P, Hartmann R, Gallego M, Rivera-Fernández S, de la Fuente JM, Nienhaus GU, Parak WJ. Surface Functionalization of Nanoparticles with Polyethylene Glycol: Effects on Protein Adsorption and Cellular Uptake. ACS Nano. 2015;9:6996–7008. doi: 10.1021/acsnano.5b01326. [DOI] [PubMed] [Google Scholar]
- 129.Gupta AK, Curtis AS. Surface modified superparamagnetic nanoparticles for drug delivery: interaction studies with human fibroblasts in culture. Journal of Materials Science: Materials in Medicine. 2004;15:493–496. doi: 10.1023/b:jmsm.0000021126.32934.20. [DOI] [PubMed] [Google Scholar]
- 130.Gao Y, Lim J, Teoh S-H, Xu C. Emerging translational research on magnetic nanoparticles for regenerative medicine. Chemical Society Reviews. 2015 doi: 10.1039/C4CS00322E. [DOI] [PubMed] [Google Scholar]
- 131.Gref R, Domb A, Quellec P, Blunk T, Müller R, Verbavatz J, Langer R. The controlled intravenous delivery of drugs using PEG-coated sterically stabilized nanospheres. Advanced drug delivery reviews. 2012;64:316–326. doi: 10.1016/0169-409X(95)00026-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Labala S, Mandapalli PK, Kurumaddali A, Venuganti VVK. Layer-by-layer polymer coated gold nanoparticles for topical delivery of imatinib mesylate to treat melanoma. Molecular pharmaceutics. 2015;12:878–888. doi: 10.1021/mp5007163. [DOI] [PubMed] [Google Scholar]
- 133.Feng J-J, Zhao G, Xu J-J, Chen H-Y. Direct electrochemistry and electrocatalysis of heme proteins immobilized on gold nanoparticles stabilized by chitosan. Analytical biochemistry. 2005;342:280–286. doi: 10.1016/j.ab.2005.04.040. [DOI] [PubMed] [Google Scholar]
- 134.Sarkar S, Guibal E, Quignard F, SenGupta A. Polymer-supported metals and metal oxide nanoparticles: synthesis, characterization, and applications. Journal of Nanoparticle Research. 2012;14:1–24. [Google Scholar]
- 135.Unsoy G, Yalcin S, Khodadust R, Gunduz G, Gunduz U. Synthesis optimization and characterization of chitosan-coated iron oxide nanoparticles produced for biomedical applications. Journal of Nanoparticle Research. 2012;14:1–13. [Google Scholar]
- 136.Ma HL, Xu YF, Qi XR, Maitani Y, Nagai T. Superparamagnetic iron oxide nanoparticles stabilized by alginate: pharmacokinetics, tissue distribution, and applications in detecting liver cancers. International journal of pharmaceutics. 2008;354:217–226. doi: 10.1016/j.ijpharm.2007.11.036. [DOI] [PubMed] [Google Scholar]
- 137.Liu Z, Jiao Y, Wang Y, Zhou C, Zhang Z. Polysaccharides-based nanoparticles as drug delivery systems. Advanced drug delivery reviews. 2008;60:1650–1662. doi: 10.1016/j.addr.2008.09.001. [DOI] [PubMed] [Google Scholar]
- 138.Rescignano N, Fortunati E, Armentano I, Hernandez R, Mijangos C, Pasquino R, Kenny JM. Use of alginate, chitosan and cellulose nanocrystals as emulsion stabilizers in the synthesis of biodegradable polymeric nanoparticles. Journal of colloid and interface science. 2015;445:31–39. doi: 10.1016/j.jcis.2014.12.032. [DOI] [PubMed] [Google Scholar]
- 139.Castelló J, Gallardo M, Busquets MA, Estelrich J. Chitosan (or alginate)-coated iron oxide nanoparticles: A comparative study. Colloids and Surfaces A: Physicochemical and Engineering Aspects. 2015;468:151–158. [Google Scholar]
- 140.Mallick N, Asfer M, Anwar M, Kumar A, Samim M, Talegaonkar S, Ahmad FJ. Rhodamine-loaded, cross-linked, carboxymethyl cellulose sodium-coated super-paramagnetic iron oxide nanoparticles: Development and in vitro localization study for magnetic drug-targeting applications. Colloids and Surfaces A: Physicochemical and Engineering Aspects. 2015;481:51–62. [Google Scholar]
- 141.Foox M, Zilberman M. Drug delivery from gelatin-based systems. Expert opinion on drug delivery. 2015;12:1547–1563. doi: 10.1517/17425247.2015.1037272. [DOI] [PubMed] [Google Scholar]
- 142.Ling D, Lee N, Hyeon T. Chemical synthesis and assembly of uniformly sized iron oxide nanoparticles for medical applications. Accounts of Chemical Research. 2015;48:1276–1285. doi: 10.1021/acs.accounts.5b00038. [DOI] [PubMed] [Google Scholar]
- 143.Chen Q, Wang H, Liu H, Wen S, Peng C, Shen M, Zhang G, Shi X. Multifunctional dendrimer-entrapped gold nanoparticles modified with RGD peptide for targeted computed tomography/magnetic resonance dual-modal imaging of tumors. Analytical Chemistry. 2015;87:3949–3956. doi: 10.1021/acs.analchem.5b00135. [DOI] [PubMed] [Google Scholar]
- 144.Sahoo Y, Pizem H, Fried T, Golodnitsky D, Burstein L, Sukenik CN, Markovich G. Alkyl phosphonate/phosphate coating on magnetite nanoparticles: a comparison with fatty acids. Langmuir. 2001;17:7907–7911. [Google Scholar]
- 145.Liao W, Ma Y, Chen A, Yang Y. Preparation of fatty acids coated Fe3O4 nanoparticles for adsorption and determination of benzo (a) pyrene in environmental water samples. Chemical Engineering Journal. 2015;271:232–239. [Google Scholar]
- 146.Easo SL, Mohanan P. In vitro hematological and in vivo immunotoxicity assessment of dextran stabilized iron oxide nanoparticles. Colloids and Surfaces B: Biointerfaces. 2015;134:122–130. doi: 10.1016/j.colsurfb.2015.06.046. [DOI] [PubMed] [Google Scholar]
- 147.Hauser AK, Mathias R, Anderson KW, Hilt JZ. The effects of synthesis method on the physical and chemical properties of dextran coated iron oxide nanoparticles. Materials Chemistry and Physics. 2015;160:177–186. doi: 10.1016/j.matchemphys.2015.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Liu J-N, Bu W-B, Shi J-L. Silica coated upconversion nanoparticles: A versatile platform for the development of efficient theranostics. Accounts of cChemical Research. 2015;48:1797–1805. doi: 10.1021/acs.accounts.5b00078. [DOI] [PubMed] [Google Scholar]
- 149.Mirkin CA, Rosi NL, Thaxton CS, Giljohann DA. Nucleic acid functionalized nanoparticles for therapeutic applications. 8,999,947. US Patent. issued April 7, 2015.
- 150.Karakoti AS, Shukla R, Shanker R, Singh S. Surface functionalization of quantum dots for biological applications. Advances in Colloid and Interface Science. 2015;215:28–45. doi: 10.1016/j.cis.2014.11.004. [DOI] [PubMed] [Google Scholar]