Abstract
Purpose
To quantify the relationship between aggregated preoperative risk factors and cataract surgery complications, as well as to build a model predicting outcomes on an individual-level—given a constellation of demographic, baseline, preoperative, and intraoperative patient characteristics.
Setting
Stanford Hospital and Clinics between 1994 and 2013.
Design
Retrospective cohort study
Methods
Patients age 40 or older who received cataract surgery between 1994 and 2013. Risk factors, complications, and demographic information were extracted from the Electronic Health Record (EHR), based on International Classification of Diseases, 9th edition (ICD-9) codes, Current Procedural Terminology (CPT) codes, drug prescription information, and text data mining using natural language processing. We used a bootstrapped least absolute shrinkage and selection operator (LASSO) model to identify highly-predictive variables. We built random forest classifiers for each complication to create predictive models.
Results
Our data corroborated existing literature on postoperative complications—including the association of intraoperative complications, complex cataract surgery, black race, and/or prior eye surgery with an increased risk of any postoperative complications. We also found a number of other, less well-described risk factors, including systemic diabetes mellitus, young age (<60 years old), and hyperopia as risk factors for complex cataract surgery and intra- and post-operative complications. Our predictive models based on aggregated outperformed existing published models.
Conclusions
The constellations of risk factors and complications described here can guide new avenues of research and provide specific, personalized risk assessment for a patient considering cataract surgery. The predictive capacity of our models can enable risk stratification of patients, which has utility as a teaching tool as well as informing quality/value-based reimbursements.
Introduction
Cataracts are the most common cause of reversible visual impairment in the United States, affecting over 20 million people, with approximately 3 million surgeries performed annually at a cost of $3.4 billion to Centers for Medicare and Medicaid Services (CMS)—the largest single procedure source of CMS expenditures.[1, 2] Rates of surgery continue to rise; between 1990 and 2010, the incidence of cataract surgery increased between 2.5- and 6.5-fold, varying by region in the United States.[3–5] Although a safe and effective surgery, rare complications add up to substantial numbers due to the sheer volume of cataract surgeries performed annually.[6] These complications can have significant functional and financial impact. Direct clinical costs of managing complications range from $400 to $6000 for an episode of care[7] and indirect costs are even greater.
Previous epidemiologic studies have quantified the incidence of a number of intraoperative and postoperative complications of cataract surgery.[8–11] However, much of the existing literature focuses on the relationship between risk factors and post-operative visual acuity.[12–15] Studies examining the effect of risk factors on complications have tended to look at aggregated outcomes (e.g. any intraoperative complication).[12, 16, 17] Studies exploring specific outcomes have tended to focus either on the relationship between preoperative risk factors and intraoperative complications,[10, 18–25] or on the relationship between intraoperative and postoperative complications.[26–28] Few studies have examined relationships between preoperative risk factors and postoperative complications, or explored the combined effects of a large number of risk factors and complications simultaneously.
The aims of our study are two-fold: 1) to quantify the relationship between aggregated pre-operative risk factors and complications in cataract surgery (as recorded in the electronic health record, or EHR); and 2) to build a model to predict the occurrence of these outcomes on an individual level given a constellation of demographic, baseline, preoperative and intraoperative characteristics for a given patient. We selected cataract surgery as a high volume and well-studied procedure (with accepted risk factors, complications, and results) to validate our models.
A framework to identify risk factors among patient characteristics and their combined effects may guide new avenues of research and help quantify individual risk for a patient considering cataract surgery. We envision a predictive model that can be valuable in case-specific risk stratification—with utility for patient selection, as a teaching tool, and potentially informing risk adjustment for anticipated implementation of value-based reimbursement.
Methods
Data Source and Study Population
The patient population was drawn from the Stanford Translational Research Integrated Database Environment (STRIDE), which contains data from the EHR for 1.8 million patients seen at the Stanford Hospital and Clinics and Stanford Children’s Health Network between 1994 and 2013. Extraction and processing of the datasets was approved by the Stanford Institutional Review Board.
Cohort and Feature Selection
We utilized a retrospective cohort study design. The cohort was defined to include all patients who had a Current Procedural Terminology (CPT) code for cataract surgery (either 66982 or 66984) and who were at least 40 years old at the time of surgery. To account for the fact that many patients may be referred to Stanford to receive cataract surgery and have this surgery as their only encounter in our database, we limited our cohort to patients who had at least one year of records prior to their surgery. This step helps ensure we capture as many pre-operative risk factors as possible. Since most patients would return to the provider who performed their surgery in the case of any postoperative complications, we did not impose any such restriction on the follow-up records after cataract surgery.
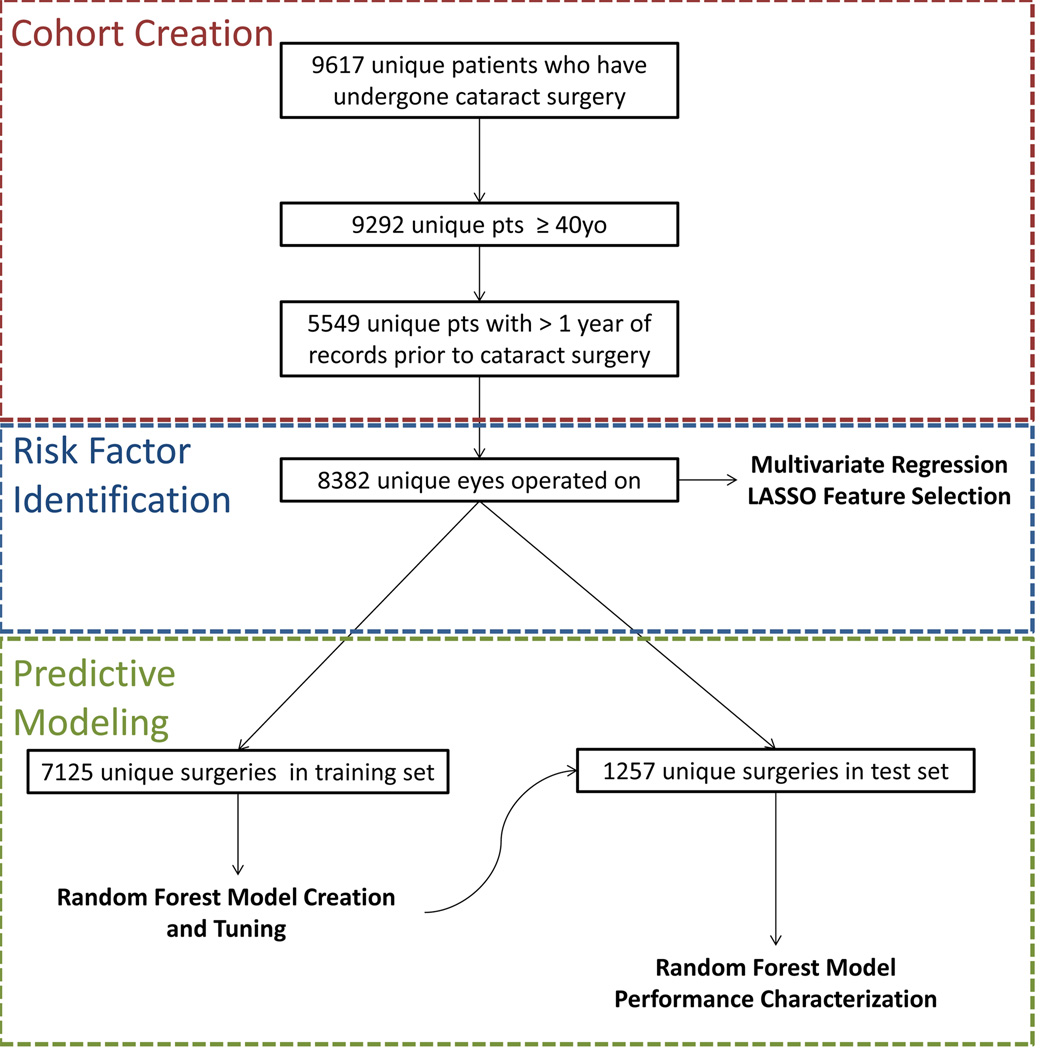
In total, there were 5,549 unique patients accounting for 8,382 unique surgeries, since patients could each have up to two surgeries—one surgery per eye occurring at least 30 days apart (Figure 1). Potential risk factors and complications were determined by an expert clinician (Table 1). These characteristics, as well as key demographic information and the Charlson comorbditiy score to serve as a proxy for the patient’s overall health[29], were extracted from the EHR using International Classification of Diseases, 9th edition (ICD-9) codes, CPT codes, drug prescription information, and text data mining via natural language processing methods (described previously).[30] A full list of all codes used is provided in Appendix Tables S1 and S2.
Figure 1.
Schematic outline of the methodology for this paper. We first created a cohort consisting of 5,549 unique patients who had undergone cataract surgery and had sufficient records. We then used the 8.382 distinct surgeries to identify the relationship between risk factors and complications using multivariate regression and LASSO feature selection. Finally, we split the 8.382 surgeries into a training set consisting of 85% of the data and a test set of 15% of the data. Using the training set, we created and tuned random forest predictive models and then ran them on the individuals in the test set to characterize the performance of our predictive models.
Table 1.
Prevalence of risk factors and complications in study population.
| Preoperative Risk Factors | N | (%)1 | Complication | N | (%)* |
|---|---|---|---|---|---|
| Age (by half-decade) | Intraoperative Complications | ||||
| 40–45 years | Complex surgery3 | 768 | 9 | ||
| 46–50 years | Posterior capsular tear | 35 | 0 | ||
| 51–55 years | Dropped nucleus or retained lens material | 76 | 1 | ||
| 56–60 years | Unplanned anterior vitrectomy | 128 | 2 | ||
| 61–65 years | Intraoperative Floppy Iris Syndrome (IFIS) or iris damage |
49 | 1 | ||
| 66–70 years | Suprachoroidal Hemorrhage | 1 | 0 | ||
| 71–75 years | Any Intraoperative Complication4 | 214 | 3 | ||
| 76–80 years | |||||
| 81–85 years | Short-term Postoperative Complications (within 90 days) | ||||
| ≥86 years | Endophthalmitis | 82 | 1 | ||
| Gender | Glaucoma or ocular hypertension | 175 | 2 | ||
| Male | 3464 | (41) | Pars plana vitrectomy/lensectomy | 107 | 1 |
| Female | 4918 | (59) | Retinal tear/detachment | 107 | 1 |
| Race/Ethnicity | Corneal edema | 81 | 1 | ||
| Caucasian | 5715 | (68) | Wound leak | 39 | 0 |
| Asian | 948 | (11) | Intraocular lens subluxation | 13 | 0 |
| Hispanic | 298 | (4) | |||
| Black | 245 | (3) | Long-term Postoperative Complications (within 1 year) | ||
| Other/Unknown | 1176 | (14) | Posterior capsule opacification/YAG capsulotomy | 406 | 5 |
| Myopia | 936 | 11 | Cystoid macular edema | 160 | 2 |
| Hyperopia | 362 | 4 | Post-cataract refractive surgery | 277 | 3 |
| Diabetes mellitus | 2750 | 33 | Intraocular lens exchange or repositioning, or secondary intraocular lens |
123 | 2 |
| Age-related macular degeneration |
844 | 10 | |||
| Glaucoma | 2003 | 24 | Aggregated Postoperative Complications | ||
| History of uveitis (or posterior synechiae) |
244 | 3 | Any lens-related complication5 | 896 | 11 |
| History of eye trauma | 172 | 2 | Any postoperative complication6 | 692 | 8 |
| History of retinal tear/detachment |
1158 | 14 | Any posterior segment complications7 | 237 | 3 |
| History of steroid use (systemic or ocular) |
563 | 7 | Any anterior segment complication8 | 233 | 3 |
| History of alpha-blocker use |
436 | 5 | Any intraocular lens malfunction9 | 116 | 1 |
| Fuch's endothelial dystrophy |
91 | 1 | |||
| Prior refractive surgery | 389 | 5 | |||
| Prior vitrectomy | 486 | 6 | |||
| Other prior intraocular surgery |
367 | 4 | |||
| Intravitreal injection | 248 | 3 | |||
| Pseudoexfoliation | 995 | 12 | |||
| Corneal scarring or significant corneal arcus |
381 | 5 | |||
| Corneal Edema | 80 | 1 | |||
| Nystagmus | 192 | 2 | |||
| Lens Subluxation | 85 | 1 | |||
| Surgery on both eyes2 | 2829 | 51 |
Out of 5549 unique individuals
Not considered as a risk factor per se, but included in the model to adjust for cumulative increase in risk from bilateral surgery
Though not by definition a complication, we included complex cataract surgery (Current Procedural Terminology, or CPT, code 66982) in the model, as compared to routine cataract surgery (CPT code 66984).
Includes all intraoperative complications except Complicated Surgery
Includes posterior capsule opacification/YAG capsulotomy, intraocular lens (IOL) dislocation, IOL repair, secondary IOL placement, and post-cataract refractive surgery
Includes all postoperative complications listed above (short-term and long-term)
Includes endophthalmitis (or vitreous tap with intravitreal antibiotics in the immediate postoperative period), retinal tear/detachment, pars plana vitrectomy/lensectomy, and cystoid macular edema
Includes corneal edema, wound leak/dehiscence, and glaucoma or ocular hypertension
Includes IOL dislocation, IOL repair, and secondary IOL placement
To reduce the possibility of misidentifying a historical event or preexisting condition as a surgical complication, we excluded instances where a complication was identified in the medical record preoperatively. That is, a complication was “counted” only if it was incident—first noted during or after the surgery.
Complications were classified as: 1) Intraoperative, 2) Short-term postoperative, or 3) Long-term postoperative. Intraoperative complications included those most likely to occur during cataract surgery, and we included instances mentioned within 5 days of the date of the surgery (to account for notes or codes that may have been inputted in the immediate post-operative period). Short-term postoperative complications were defined as occurring within 90 days of the cataract surgery, and long-term postoperative complications defined as occurring within 365 days of surgery. In addition to exploring individual complications, we also aggregated related outcomes (e.g. all lens-related complications or all complications occurring in the posterior segment of the eye) to improve our statistical power and identify trends among associated conditions.
Statistical Analyses
In order to identify and quantify relationships across a spectrum of risk factors and complications, we built multiple multivariate logistic regression models. We created a separate model for each complication, including the same variables in each model (risk factors plus key demographic information)—except in cases where a particular complication could also be a risk factor (e.g. glaucoma/ocular hypertension was not counted as a complication if it was present preoperatively). Intraoperative complications were included as risk factors for postoperative complications (e.g. posterior capsule tear as a risk factor for endophthalmitis). To account for the increased exposure to risk among patients who have had two distinct surgeries (up to twice the cumulative risk for intra- or post-operative complications as compared to a patient with unilateral surgery), we controlled for one surgery versus two distinct surgeries as a binary variable in our models.
To assess and quantify the importance of each variable, we calculated odds ratios (ORs) and 95% confidence intervals (CIs) for each variable/outcome pair. We also calculated p-values quantifying the significance of the relationship between each variable/outcome pair. Unlike other methods examining the relationships of a very large number of m variables on n outcomes using m × n univariate regressions (e.g. in genomic analysis), we only performed n—where n = 20 outcomes—unique multivariate regressions. Thus, we did not feel that it was necessary to use a method, like the Bonferroni correction, to account for multiple comparisons.
To identify the most important variables, we used a bootstrapped least absolute shrinkage and selection operator (LASSO) model, which utilizes a penalized logistic regression in order to shrink some of the regression coefficients to zero, eventually performing variable selection.[31] The tuning parameter (λ) was optimized through 10-fold cross-validation and the subset of variables selected in the model was recorded. We repeated this process through 100 bootstrapped iterations. Those variables that were present in ≥90% of the bootstrapped LASSO models were then input back into a multivariate logistic regression model and ORs, 95% CIs and p-values were calculated as above. Results were displayed graphically through bubble plots of statistically significant values (p<0.05), with axes organized through hierarchical clustering to show similarity between groups of risk factors and complications.
To create a predictive model, we used the random forest classification method.[31] We left out 15% of the study population to serve as a test set and used the other 85% to train and tune our model. Since there was a great deal of class imbalance, even for our most prevalent outcomes (>15:1 ratio between unaffected and affected individuals), we downsampled the majority class for each complication in the training set, such that there was a 1:1 ratio of unaffected to affected individuals. The test set data was not downsampled and was a representative sample of our population used to determine the model’s performance. In order to achieve the best predictive performance, we used 5-fold cross validation of the training set to determine the optimal number of trees and the optimal p-value cutoff for determining whether to call an individual “positive” or “negative” for a given predicted outcome. Then, we built a model for each individual outcome. After tuning the characteristics of the model, it was run on the held out test set. The predictions of the model were then compared to the known results from our test set. Relevant statistics describing the outcomes are reported, including sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV) and the area-under-the-curve (AUC) describing the receiver operating curve (ROC). All statistical analyses were conducted in R version 3.0.2.[32]
Results
Patient Population and Demographics
Demographic characteristics of our population are shown in Table 1. Our population was predominantly female (59%, vs. 41% males), consistent with other large studies examining cataract surgery.[3, 33] The mean age at the time of surgery was 74.4 years (SD=10.9); this age distribution is also similar to other studies.[3, 8] Our population was predominantly white (68%), with Asians making up the next largest racial/ethnic group (11%).
Quantifying Risk Factors
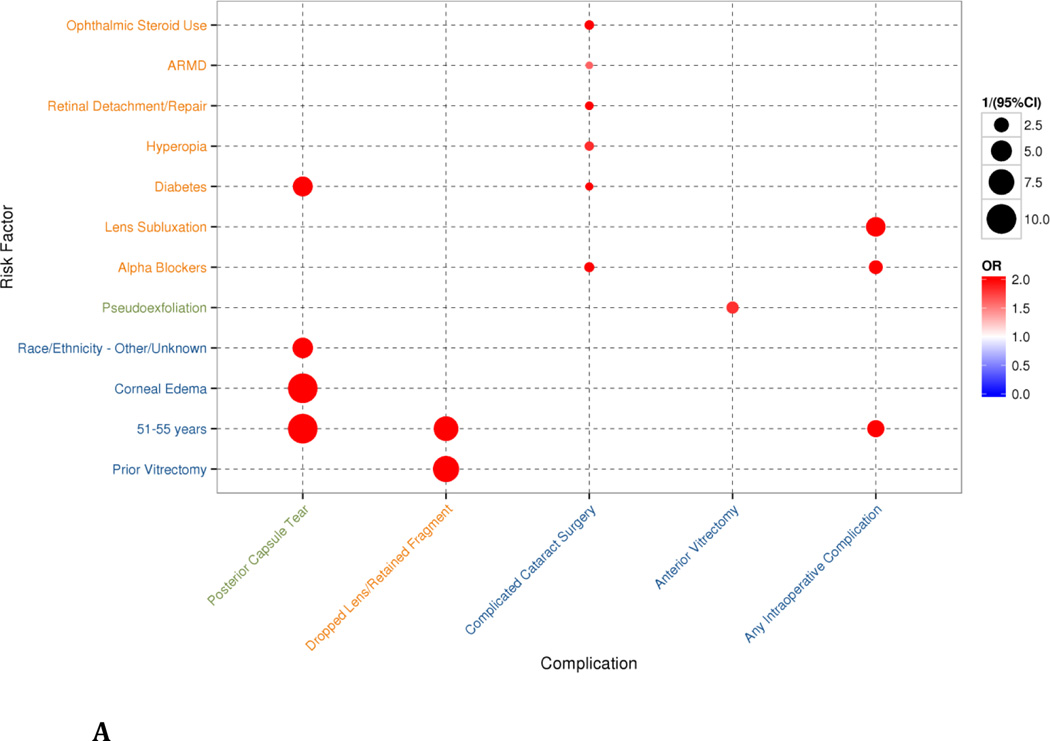
When evaluating intraoperative complications, we found, as expected, that the feature-selecting LASSO model (Figures 2A and 2B) produced a more parsimonious subset of the risk factors found in the full multivariate regression model (Supplementary Figures S1A and S1B). That is, while all of the risk factors from the LASSO model were also found in the multivariate regression, the regression model also included many more features, which were excluded by the LASSO model. In most instances, the LASSO model de-emphasized potentially protective factors. Similarly, risk factors that weakly increased risk of complications in the multivariate model were dropped out or deemphasized during feature-selection in the LASSO model (e.g., glaucoma was found to be a weak risk factor for any intraoperative complication in the multivariate model, but was not selected in the LASSO model). Being on alpha-adrenergic antagonists, having a pre-existing diagnosis of diabetes and being relatively young (between 51–55 years) were all risk factors significantly associated with intraoperative complications.
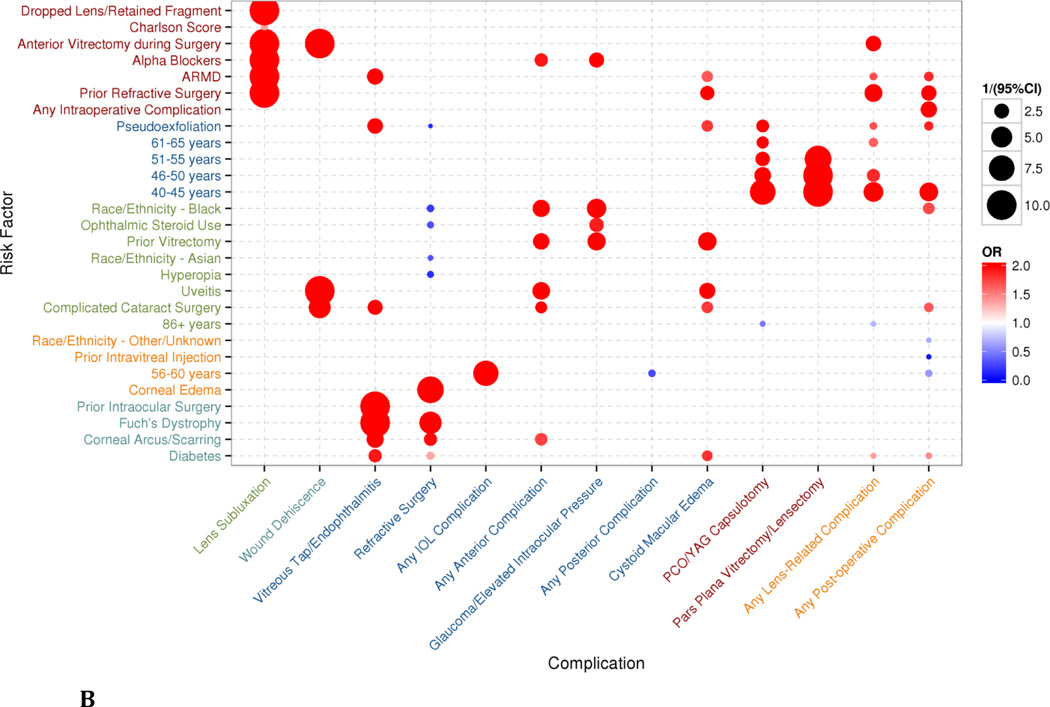
Figure 2.
Bubbles represent statistically-significant (p<0.05) results of multivariate regression of variables identified by a bootstrapped least absolute shrinkage and selection operator (LASSO) model. The size of the bubble corresponds to the inverse of the 95% confidence interval (CI)—that is, 1/(95%CI)—such that results with a larger variance are shown as smaller bubbles and results with a smaller variance are shown as larger bubbles. The color of the bubbles is related to the odds ratio (OR), with blue indicating an OR <1 and red indicating an OR>1 (the maximum value of the OR was capped at 2 in the graphical representation). Axes are organized through complete linkage hierarchical clustering to show similarity between groups of risk factors and complications. A) Intraoperative complications from multivariate regression on risk factors selected through bootstrapped LASSO model, clustered in 3 groups for each axis. B) Postoperative complications from multivariate regression on risk factors selected through bootstrapped LASSO model, clustered in 5 groups for each axis.
The LASSO model for postoperative complications behaved similarly to the model for intraoperative complications, with risk-reducing and weakly-associated features from the full multivariate regression being de-emphasized or dropped.
The greater number of evaluated postoperative complications allowed additional patterns to emerge. Younger age (<60 years old), prior anterior vitrectomy or refractive surgery, history of age-related macular degeneration (ARMD), and complex cataract surgery were all risk factors associated with postoperative complications.
The calculated odds ratios, 95% CIs, and p-values for intraoperative and postoperative the LASSO regressions are in Appendix Table S3.
Predictive Modeling
The results of the random forest predictive models for intraoperative and postoperative complications and evaluated on the test set data are presented in Table 2. In general, the predictive models tended to have very high negative predictive value (NPV >0.95) with moderate sensitivity (>67%) and moderate AUC (>0.65). Predictive models for relatively prevalent complications (like posterior capsule opacification/YAG capsulotomy) and predictive models for aggregated outcomes (like any posterior segment complication or any anterior segment complication) tended to have better reliability than models for other individual complications. However, several less-prevalent complications also had good predictive value—e.g., intraocular lens subluxation (sensitivity 100%, AUC 0.79) and endophthalmitis (sensitivity 75%, AUC 0.70).
Discussion
Identifying Known Associations
A number of previous studies have looked at the effect of risk factors on visual outcomes following cataract surgery.[12–15] Risk factors commonly associated with worse visual outcomes include: age-related macular degeneration (ARMD), diabetic retinopathy, corneal opacity/pathology, older age, female sex, previous vitrectomy, previous retinal detachment surgery, alpha-blockers, complex surgery, and intraoperative complications.[12–15] We found similar trends in our LASSO models, including ARMD, corneal pathology, alpha-blocker medications, and complex surgery each as recurring risk factors for a number of complications. For example, we find an increased risk of intraoperative complications associated with preoperative use of alpha-blockers such as tamsulosin—a relationship which has reported extensively in the literature.[34]
Our methods allowed us to examine complications and patient/surgical characteristics (potential risk factors) individually and in aggregate, confirming recognized associations as well as identifying possible new ones. Many existing studies of predictors for cataract surgery complications have evaluated risk factors for aggregated complications. [16, 21, 26, 34, 35] Our findings were consistent with the literature describing risk factors for any postoperative complication, including: complex cataract surgery, any intraoperative complication, black race and previous eye surgery (including keratorefractive surgery). [17, 26]
Research on individual complications is more limited, with studies tending to concentrate on intraoperative complications, such as posterior capsule tear[13, 24, 25, 36–38] and intraoperative floppy iris syndrome (IFIS).[39–43] Postoperative endophthalmitis and retinal tear/detachment are also well-studied[16, 28, 44–49]—and our analysis also found complex cataract surgery to be associated with higher risk for endophthalmitis—however, other post-operative complications have been less extensively described in the literature.[17, 35, 50–53]
Novel Associations
In addition to confirming known associations, we also found a number of novel risk factors. Prior studies have demonstrated that diabetic retinopathy increases the risk of poor visual acuity outcomes and surgical complications.[14–16, 25] However, our study indicates a significant association between systemic diabetes mellitus itself (irrespective of diabetic retinopathy) and a number of complications, including posterior capsule tear, endophthalmitis, and cystoid macular edema. Additionally, while young age (<60 years old) has previously been linked to endophthalmitis[28] and retinal detachment/tear,[27, 48] our work found it as a risk factor for many other intra- and post-operative complications.
We also found other factors which appeared to increase the risk for several intra- and post-operative complications and are clinically plausible. Previous vitrectomy appeared to increase the risk for a number of complications (including cystoid macular edema and dropped nucleus or retained lens fragments), and hyperopia appeared to increase risk of complex cataract surgery. Steroids have long been known to increase the risk for glaucoma[54]—a finding which was confirmed in our data—but our study suggests that alpha-antagonist drugs may also increase the risk for glaucoma/ocular hypertension after cataract surgery.
Results using these methods can be used to guide future research and studies to explore the nature and physiological underpinnings behind observed associations. Additionally, awareness of the potential effects of these risk factors can help guide clinical practice. Knowing that a patient has one or more of these risk factors may make a surgeon more cognizant of potential intraoperative complications. Similarly, patients with one or more associated risk factors might be given more specific warnings or precautions, and/or be followed more closely postoperatively to help prevent complications. Availability of large national empiric clinical data (such as the predominantly EHR-based American Academy of Ophthalmology Intelligent Research in Sight, or IRIS, data registry[55]) will provide the opportunity to apply these methods with greater statistical power—to more accurately determine risk and identify clinical associations.
Predictive Modeling for Cataract Surgery
The very high NPV of our predictive models suggests potential utility for preoperative screening. Our models overall outperformed existing published models, [26] with our AUCs ranging from approximately 0.63–0.84. One such model, which relies on labor-intensive questionnaires, achieved an AUC of 0.627 for predicting any postoperative complication, compared to our AUC of 0.68 for the same outcome using a model independent of human input.[26] Other studies have developed systems to predict visual function/acuity after cataract with similar performance to our complication-specific models.[56–58] Complication risk can thus be reliably predicted from preoperative data alone, and results used for patient selection, counseling, and risk adjustment.
Predictive tools are already being used in other disciplines to help patients make informed decisions about treatments based on their own personal data and risk factors (for example, an online risk calculator to help patients decide between Coronary Artery Bypass Graft surgery and Percutaneous Coronary Intervention).[59] And, in ophthalmology, a prognosis calculator is available for uveal melanoma.[60] Although cataract surgery is routine and safe, it remains a significant event for patients, who may value personalized information. Thus, one potentially valuable outcome of this work may be the development of a predictive tool for assessing individualized risk of complications from cataract surgery. Additionally, these models could be used in teaching hospital settings to evaluate risk in a structured way, selecting appropriate teaching cases and assisting in training of resident surgeons.[61–63] Also, these models may be useful for evidence-based risk-adjustment of surgeon quality metrics for value-based payments (e.g., Medicare value-based payment modifier beginning in 2015).[16, 63, 64] In this setting, false positives may be better tolerated than false negatives for an acceptable quality benchmarking system.
Not only valuable at the aggregate level, a growing body of literature suggests that site-specific models may be more accurate than generalized clinical guidelines in predicting outcomes.[65–67] Rather than treating differences in demographics, patient comorbidities, and institutional characteristics as confounders to be controlled for a given population, it may be more effective to incorporate these factors into models—improving predictive value for that specific population. The methods used in our study are portable and can be used to develop local predictive models in other systems.
Limitations
Readers should consider some limitations when interpreting our findings. Since our results were derived from a single clinical site, our study population may not be representative of the general population. While the text-analysis techniques used in this study have been shown to be quite accurate in detecting negated terms (97% accuracy), drug mentions (93% accuracy) and disease conditions (86% accuracy),[68–71] it is possible that some conditions or procedures were not adequately captured in our analysis (IFIS, for example, is almost certainly underreported in our sample since there it lacks specific codes and short acronyms are difficult to detect and classify through text-analysis). Furthermore, in addition to free text analysis, we also used ICD-9 and CPT codes—which have inherent limitations in accuracy. Finally, we stress that predictive accuracy for identifying associations should not be interpreted as evidence of causality.
Conclusions
We have constructed a series of models with good reliability for predicting cataract surgery complications, and identified several novel clinical associations. Although our results are derived from a single clinical site, the methods may be disseminated for broader application (including site-specific local analysis) and guide new avenues for research. The potential to directly integrate these predictive tools into EHRs may enable personalized medicine and decision-making at the point of care[72]—for patient counseling and as a teaching tool—as well as informed risk adjustment for value-based payment programs.
Supplementary Material
Bubbles represent statistically-significant (p<0.05) results of multivariate regression. The size of the bubble corresponds to the inverse of the 95% confidence interval (CI)—that is, 1/[95%CI]—such that results with a larger variance are shown as smaller bubbles and results with a smaller variance are shown as larger bubbles. The color of the bubbles is related to the odds ratio (OR), with blue indicating an OR <1 and red indicating an OR>1 (the maximum value of the OR was capped at 2 in the graphical representation). Axes are organized through complete linkage hierarchical clustering to show similarity between groups of risk factors and complications. A) Intraoperative complications from multivariate regression on all risk factors, clustered in 3 groups for each axis. B) Postoperative complications from multivariate regressions on all risk factors, clustered in 5 groups for each axis.
What Was Known
While cataract surgery is generally a very safe surgery, severe complications do exist. Given the large volume of cataract surgeries each year, even relatively infrequent complications occur in significant numbers
Pre-existing conditions are known to predispose patients to intraoperative and postoperative complications. Risk factors commonly associated with generally poor surgical outcomes include: age-related macular degeneration (ARMD), diabetic retinopathy, corneal opacity/pathology, older age, female sex, previous vitrectomy, previous retinal detachment surgery, alpha-blockers, complex surgery, and intraoperative complications.
What This Paper Adds
In addition to confirming existing risk factor-complication relationships, we also identify novel associations, such as a significant association between systemic diabetes mellitus itself (irrespective of diabetic retinopathy) and a number of complications, including posterior capsule tear, endophthalmitis, and cystoid macular edema.
We have also created models for predicting cataract surgery complications on an individual level, which outperform existing published models.
Acknowledgments
We would like to thank Paea Lependu and Tanya Podchiyska for their help on retrieving data from the STRIDE database. We would also like to thank Yen Low for her help with visualizing the results and Kenneth Jung for providing guidance and support for the predictive modeling.
Financial Support: This work was supported in part by NIH grant U54 HG004028 for the National Center for Biomedical Ontology, NLM grant R01 LM011369, and NIGMS grant R01 GM101430. We also acknowledge support from the Medical Scholars Research Program at Stanford School of Medicine.
Footnotes
Conflict of Interest: No conflicting relationship exists for any author
References
- 1.Group EDPR. Causes and prevalence of visual impairment among adults in the United States. Archives of ophthalmology. 2004;122(4):477. doi: 10.1001/archopht.122.4.477. [DOI] [PubMed] [Google Scholar]
- 2.Group EDPR. Prevalence of cataract and pseudophakia/aphakia among adults in the United States. Archives of Ophthalmology. 2004;122(4):487. doi: 10.1001/archopht.122.4.487. [DOI] [PubMed] [Google Scholar]
- 3.Gollogly HE, et al. Increasing incidence of cataract surgery: population-based study. Journal of Cataract & Refractive Surgery. 2013;39(9):1383–1389. doi: 10.1016/j.jcrs.2013.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Klein BE, et al. Changing Incidence of Lens Extraction over 20 Years: The Beaver Dam Eye Study. Ophthalmology. 2014;121(1):5–9. doi: 10.1016/j.ophtha.2013.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Javitt JC, et al. Geographic variation in utilization of cataract surgery. Medical care. 1995:90–105. doi: 10.1097/00005650-199501000-00008. [DOI] [PubMed] [Google Scholar]
- 6.Clark A, et al. Whole population trends in complications of cataract surgery over 22 years in Western Australia. Ophthalmology. 2011;118(6):1055–1061. doi: 10.1016/j.ophtha.2010.11.001. [DOI] [PubMed] [Google Scholar]
- 7.Busbee BG, et al. Incremental cost-effectiveness of initial cataract surgery. Ophthalmology. 2002;109(3):606–612. doi: 10.1016/s0161-6420(01)00971-x. [DOI] [PubMed] [Google Scholar]
- 8.Behndig A, et al. One million cataract surgeries: Swedish National Cataract Register 1992–2009. J Cataract Refract Surg. 2011;37(8):1539–1545. doi: 10.1016/j.jcrs.2011.05.021. [DOI] [PubMed] [Google Scholar]
- 9.Fong CS, et al. Long-term outcomes of phacoemulsification cataract surgery performed by trainees and consultants in an Australian cohort. Clin Experiment Ophthalmol. 2012;40(6):597–603. doi: 10.1111/j.1442-9071.2012.02759.x. [DOI] [PubMed] [Google Scholar]
- 10.Woodfield AS, et al. Intraoperative phacoemulsification complication rates of second- and third-year ophthalmology residents a 5-year comparison. Ophthalmology. 2011;118(5):954–958. doi: 10.1016/j.ophtha.2010.08.047. [DOI] [PubMed] [Google Scholar]
- 11.Chatziralli IP, et al. First postoperative day review after uneventful phacoemulsification cataract surgery: Is it necessary? BMC research notes. 2012;5(1):333. doi: 10.1186/1756-0500-5-333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lai FH, et al. Clinical Outcomes of Cataract Surgery in Very Elderly Adults. Journal of the American Geriatrics Society. 2014;62(1):165–170. doi: 10.1111/jgs.12590. [DOI] [PubMed] [Google Scholar]
- 13.Lundström M, et al. The Changing Pattern of Cataract Surgery Indications: A 5-Year Study of 2 Cataract Surgery Databases. Ophthalmology. 2014 doi: 10.1016/j.ophtha.2014.07.047. [DOI] [PubMed] [Google Scholar]
- 14.Lundström M, et al. Visual outcome of cataract surgery; Study from the European Registry of Quality Outcomes for Cataract and Refractive Surgery. Journal of Cataract & Refractive Surgery. 2013;39(5):673–679. doi: 10.1016/j.jcrs.2012.11.026. [DOI] [PubMed] [Google Scholar]
- 15.Sparrow J, et al. The Cataract National Dataset electronic multi-centre audit of 55 567 operations: risk indicators for monocular visual acuity outcomes. Eye. 2012;26(6):821–826. doi: 10.1038/eye.2012.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stein JD, et al. Severe adverse events after cataract surgery among medicare beneficiaries. Ophthalmology. 2011;118(9):1716–1723. doi: 10.1016/j.ophtha.2011.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Greenberg PB, et al. Prevalence and predictors of ocular complications associated with cataract surgery in United States veterans. Ophthalmology. 2011;118(3):507–514. doi: 10.1016/j.ophtha.2010.07.023. [DOI] [PubMed] [Google Scholar]
- 18.Briszi A, et al. Complication rate and risk factors for intraoperative complications in resident-performed phacoemulsification surgery. Graefes Arch Clin Exp Ophthalmol. 2012;250(9):1315–1320. doi: 10.1007/s00417-012-2003-y. [DOI] [PubMed] [Google Scholar]
- 19.Hyams M, et al. Intraoperative complications of phacoemulsification in eyes with and without pseudoexfoliation. J Cataract Refract Surg. 2005;31(5):1002–1005. doi: 10.1016/j.jcrs.2004.09.051. [DOI] [PubMed] [Google Scholar]
- 20.Clark A, et al. Long-term trends and outcomes of anterior vitrectomy in Western Australia. Acta Ophthalmol. 2014 doi: 10.1111/aos.12453. [DOI] [PubMed] [Google Scholar]
- 21.Rutar T, Porco TC, Naseri A. Risk factors for intraoperative complications in resident-performed phacoemulsification surgery. Ophthalmology. 2009;116(3):431–436. doi: 10.1016/j.ophtha.2008.10.028. [DOI] [PubMed] [Google Scholar]
- 22.Blomquist PH, et al. Risk factors for vitreous complications in resident-performed phacoemulsification surgery. J Cataract Refract Surg. 2012;38(2):208–214. doi: 10.1016/j.jcrs.2011.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Drolsum L, Haaskjold E, Sandvig K. Phacoemulsification in eyes with pseudoexfoliation. Journal of Cataract & Refractive Surgery. 1998;24(6):787–792. doi: 10.1016/s0886-3350(98)80132-6. [DOI] [PubMed] [Google Scholar]
- 24.Berler DK. Intraoperative complications during cataract surgery in the very old. Transactions of the American Ophthalmological Society. 2000;98:127. [PMC free article] [PubMed] [Google Scholar]
- 25.Narendran N, et al. The Cataract National Dataset electronic multicentre audit of 55 567 operations: risk stratification for posterior capsule rupture and vitreous loss. Eye. 2008;23(1):31–37. doi: 10.1038/sj.eye.6703049. [DOI] [PubMed] [Google Scholar]
- 26.Gonzalez N, et al. Factors affecting cataract surgery complications and their effect on the postoperative outcome. Can J Ophthalmol. 2014;49(1):72–79. doi: 10.1016/j.jcjo.2013.09.013. [DOI] [PubMed] [Google Scholar]
- 27.Haug SJ, Bhisitkul RB. Risk factors for retinal detachment following cataract surgery. Curr Opin Ophthalmol. 2012;23(1):7–11. doi: 10.1097/ICU.0b013e32834cd653. [DOI] [PubMed] [Google Scholar]
- 28.Hatch WV, et al. Risk factors for acute endophthalmitis after cataract surgery: a population-based study. Ophthalmology. 2009;116(3):425–430. doi: 10.1016/j.ophtha.2008.09.039. [DOI] [PubMed] [Google Scholar]
- 29.Charlson ME, et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. Journal of chronic diseases. 1987;40(5):373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 30.Cole TS, et al. Profiling risk factors for chronic uveitis in juvenile idiopathic arthritis: a new model for EHR-based research. Pediatric Rheumatology. 2013;11(1):45. doi: 10.1186/1546-0096-11-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hastie T, et al. The elements of statistical learning. Vol. 2. Springer; 2009. [Google Scholar]
- 32.Team RC. R: A Language and Environment for Statistical Computing. Vienna, Austria: Foundation for Statistical Computing; 2014. [Google Scholar]
- 33.Jaycock P, et al. The Cataract National Dataset electronic multi-centre audit of 55 567 operations: updating benchmark standards of care in the United Kingdom and internationally. Eye. 2007;23(1):38–49. doi: 10.1038/sj.eye.6703015. [DOI] [PubMed] [Google Scholar]
- 34.Haridas A, et al. Intraoperative floppy iris syndrome (IFIS) in patients receiving tamsulosin or doxazosin—a UK-based comparison of incidence and complication rates. Graefe's Archive for Clinical and Experimental Ophthalmology. 2013;251(6):1541–1545. doi: 10.1007/s00417-013-2260-4. [DOI] [PubMed] [Google Scholar]
- 35.Bell CM, et al. Association between tamsulosin and serious ophthalmic adverse events in older men following cataract surgery. JAMA. 2009;301(19):1991–1996. doi: 10.1001/jama.2009.683. [DOI] [PubMed] [Google Scholar]
- 36.Ti SE, et al. A 5-year audit of cataract surgery outcomes after posterior capsule rupture and risk factors affecting visual acuity. Am J Ophthalmol. 2014;157(1):180–185. e1. doi: 10.1016/j.ajo.2013.08.022. [DOI] [PubMed] [Google Scholar]
- 37.Lundstrom M, et al. Decreasing rate of capsule complications in cataract surgery: eight-year study of incidence, risk factors, and data validity by the Swedish National Cataract Register. J Cataract Refract Surg. 2011;37(10):1762–1767. doi: 10.1016/j.jcrs.2011.05.022. [DOI] [PubMed] [Google Scholar]
- 38.Hashemi H, et al. Incidence of and risk factors for vitreous loss in resident-performed phacoemulsification surgery. J Cataract Refract Surg. 2013;39(9):1377–1382. doi: 10.1016/j.jcrs.2013.03.028. [DOI] [PubMed] [Google Scholar]
- 39.Artzen D, et al. Capsule complication during cataract surgery: Case-control study of preoperative and intraoperative risk factors: Swedish Capsule Rupture Study Group report 2. J Cataract Refract Surg. 2009;35(10):1688–1693. doi: 10.1016/j.jcrs.2009.05.026. [DOI] [PubMed] [Google Scholar]
- 40.Casuccio A, et al. Pharmacologic pupil dilation as a predictive test for the risk for intraoperative floppy-iris syndrome. Journal of Cataract & Refractive Surgery. 2011;37(8):1447–1454. doi: 10.1016/j.jcrs.2011.02.030. [DOI] [PubMed] [Google Scholar]
- 41.Vollman DE, et al. Intraoperative floppy iris and prevalence of intraoperative complications: results from ophthalmic surgery outcomes database. Am J Ophthalmol. 2014;157(6):1130–1135. e1. doi: 10.1016/j.ajo.2014.02.052. [DOI] [PubMed] [Google Scholar]
- 42.Oshika T, et al. Incidence of Intraoperative Floppy Iris Syndrome in Patients on Either Systemic or Topical α1-Adrenoceptor Antagonist. American journal of ophthalmology. 2007;143(1):150–151. doi: 10.1016/j.ajo.2006.07.046. [DOI] [PubMed] [Google Scholar]
- 43.Chang DF, Campbell JR. Intraoperative floppy iris syndrome associated with tamsulosin. Journal of Cataract & Refractive Surgery. 2005;31(4):664–673. doi: 10.1016/j.jcrs.2005.02.027. [DOI] [PubMed] [Google Scholar]
- 44.Asencio MA, et al. A case-control study of post-operative endophthalmitis diagnosed at a Spanish hospital over a 13-year-period. Epidemiol Infect. 2014:1–6. doi: 10.1017/S095026881400034X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Combey de Lambert A, et al. Baseline factors predictive of visual prognosis in acute postoperative bacterial endophthalmitis in patients undergoing cataract surgery. JAMA Ophthalmol. 2013;131(9):1159–1166. doi: 10.1001/jamaophthalmol.2013.4242. [DOI] [PubMed] [Google Scholar]
- 46.Keay L, et al. Postcataract surgery endophthalmitis in the United States: analysis of the complete 2003 to 2004 Medicare database of cataract surgeries. Ophthalmology. 2012;119(5):914–922. doi: 10.1016/j.ophtha.2011.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cao H, et al. Risk factors for acute endophthalmitis following cataract surgery: a systematic review and meta-analysis. PLoS One. 2013;8(8):e71731. doi: 10.1371/journal.pone.0071731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tuft SJ, Minassian D, Sullivan P. Risk factors for retinal detachment after cataract surgery: a case-control study. Ophthalmology. 2006;113(4):650–656. doi: 10.1016/j.ophtha.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 49.West ES, et al. The incidence of endophthalmitis after cataract surgery among the US Medicare population increased between 1994 and 2001. Ophthalmology. 2005;112(8):1388–1394. doi: 10.1016/j.ophtha.2005.02.028. [DOI] [PubMed] [Google Scholar]
- 50.Law SK, et al. Clinical Cystoid Macular Edema After Cataract Surgery in Glaucoma Patients. Journal of Glaucoma. 2009:1. doi: 10.1097/IJG.0b013e3181a98b97. [DOI] [PubMed] [Google Scholar]
- 51.Henderson BA, et al. Clinical pseudophakic cystoid macular edema. Risk factors for development and duration after treatment. J Cataract Refract Surg. 2007;33(9):1550–1558. doi: 10.1016/j.jcrs.2007.05.013. [DOI] [PubMed] [Google Scholar]
- 52.Hsiao CH, et al. Wound Dehiscence as a Cataract Surgery-Associated Postoperative Complication in Patients Previously Treated With Alpha-1 Blocker Tamsulosin-A Population-Based Study in Taiwan. Am J Ophthalmol. 2014 doi: 10.1016/j.ajo.2014.08.022. [DOI] [PubMed] [Google Scholar]
- 53.Kim J, et al. Increased intraocular pressure on the first postoperative day following resident-performed cataract surgery. Eye. 2011;25(7):929–936. doi: 10.1038/eye.2011.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Spaeth GL, Rodrigues MM, Weinreb S. Steroid-induced glaucoma: A. Persistent elevation of intraocular pressure B. Histopathological aspects. Transactions of the American Ophthalmological Society. 1977;75:353. [PMC free article] [PubMed] [Google Scholar]
- 55.Ophthalmology, A.A.o. Intelligent Research in Sight (IRIS) Registry. 2014 Available from: http://www.aao.org/iris-registry/index.cfm. [Google Scholar]
- 56.Perea-Milla E, et al. Development and validation of clinical scores for visual outcomes after cataract surgery. Ophthalmology. 2011;118(1):9–16. e3. doi: 10.1016/j.ophtha.2010.04.009. [DOI] [PubMed] [Google Scholar]
- 57.Vryghem JC, et al. Predicting cataract surgery results using a macular function test. Journal of Cataract & Refractive Surgery. 2004;30(11):2349–2353. doi: 10.1016/j.jcrs.2004.05.006. [DOI] [PubMed] [Google Scholar]
- 58.Bertschinger DR, Janin YA, Dosso A. Comparison of adapted Vryghem macular function test and Lotmar-light interferometer in predicting visual acuity after cataract surgery. Acta ophthalmologica. 2008;86(3):307–313. doi: 10.1111/j.1600-0420.2007.01043.x. [DOI] [PubMed] [Google Scholar]
- 59.Newby K. 2014. Coronary Heart Disease Procedure Calculator. Available from: med.stanford.edu/hsr/cabg-pci. [Google Scholar]
- 60.Damato BE, Taktak Azzam. Uveal Melanoma TNM Staging and Survivorship. 2011 Available from: http://clinengnhs.liv.ac.uk:8080/azzam_ws_1/webmeleye_index.htm. [Google Scholar]
- 61.Osborne S, et al. Validation of two scoring systems for the prediction of posterior capsule rupture during phacoemulsification surgery. British journal of ophthalmology. 2006;90(3):333–336. doi: 10.1136/bjo.2005.080754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Osborne SA, et al. The use of a pre-operative scoring system for the prediction of phacoemulsification case difficulty and the selection of appropriate cases to be performed by trainees. BMC ophthalmology. 2006;6(1):38. doi: 10.1186/1471-2415-6-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Muhtaseb M, Kalhoro A, Ionides A. A system for preoperative stratification of cataract patients according to risk of intraoperative complications: a prospective analysis of 1441 cases. British journal of ophthalmology. 2004;88(10):1242–1246. doi: 10.1136/bjo.2004.046003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Services, C.f.M.M. Value-Based Payment Modifier. Medicare FFS Physician Feedback Program/Value-Based Payment Modifier. 2014 Available from: http://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/PhysicianFeedbackProgram/ValueBasedPaymentModifier.html.
- 65.Celi LA, et al. A Database-driven Decision Support System: Customized Mortality Prediction. J Pers Med. 2012;2(4):138–148. doi: 10.3390/jpm2040138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Celi LA, et al. A Clinical Database-Driven Approach to Decision Support: Predicting Mortality Among Patients with Acute Kidney Injury. J Healthc Eng. 2011;2(1):97–110. doi: 10.1260/2040-2295.2.1.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Mayaud L, et al. Dynamic data during hypotensive episode improves mortality predictions among patients with sepsis and hypotension. Crit Care Med. 2013;41(4):954–962. doi: 10.1097/CCM.0b013e3182772adb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.LePendu P, et al. Annotation Analysis for Testing Drug Safety Signals using Unstructured Clinical Notes. J. Biomedical Semantics. 2012;3(S-1):S5. doi: 10.1186/2041-1480-3-S1-S5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.LePendu P, et al. Pharmacovigilance using clinical notes. Clinical pharmacology & therapeutics. 2013;93(6):547–555. doi: 10.1038/clpt.2013.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Leeper NJ, et al. Practice-based evidence: profiling the safety of cilostazol by text-mining of clinical notes. PloS one. 2013;8(5):e63499. doi: 10.1371/journal.pone.0063499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Jonquet C, Shah NH, Musen MA. The open biomedical annotator. Summit on translational bioinformatics. 2009;2009:56. [PMC free article] [PubMed] [Google Scholar]
- 72.Longhurst CA, Harrington RA, Shah NH. A ‘green button’ for using aggregate patient data at the point of care. Health Affairs. 2014;33(7):1229–1235. doi: 10.1377/hlthaff.2014.0099. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Bubbles represent statistically-significant (p<0.05) results of multivariate regression. The size of the bubble corresponds to the inverse of the 95% confidence interval (CI)—that is, 1/[95%CI]—such that results with a larger variance are shown as smaller bubbles and results with a smaller variance are shown as larger bubbles. The color of the bubbles is related to the odds ratio (OR), with blue indicating an OR <1 and red indicating an OR>1 (the maximum value of the OR was capped at 2 in the graphical representation). Axes are organized through complete linkage hierarchical clustering to show similarity between groups of risk factors and complications. A) Intraoperative complications from multivariate regression on all risk factors, clustered in 3 groups for each axis. B) Postoperative complications from multivariate regressions on all risk factors, clustered in 5 groups for each axis.