Abstract
Introduction
The accuracy of echocardiography in evaluating left ventricular (LV) contractility has not been validated in children. The objective of this study was to compare echocardiographic measures of contractility vs. those derived from pressure-volume loop (PVL) analysis in children.
Methods
Patients with relatively normal loading conditions undergoing routine left heart catheterization were prospectively enrolled. PVLs were obtained via conductance catheters. The gold-standard measure of contractility, end-systolic elastance (Ees), was obtained via balloon occlusion of one or both vena cavae. Echocardiograms were performed immediately after PVL analysis under the same anesthetic conditions. Single-beat estimations of echocardiographic Ees were calculated using four different methods. These estimates were calculated using a combination of non-invasive blood pressure readings, ventricular volumes derived from 3D echocardiography, and Doppler time intervals.
Results
Of 24 patients, 18 patients were heart transplant recipients, 6 patients had a small patent ductus arteriosus or small coronary fistula. Mean age was 9.1 ± 5.6 years. The average invasive Ees was 3.04 ± 1.65 mmHg/mL. Invasive Ees correlated best with echocardiographic Ees by method of Tanoue (r = 0.85, p < 0.01) with a mean difference of −0.07 mmHg/mL (95% limits of agreement: −2.0, 1.4 mm Hg/mL).
Conclusion
Echocardiographic estimates of Ees correlate well with gold-standard measures obtained via conductance catheters in children with relatively normal loading conditions. The use of these non-invasive measures in accurately assessing LV contractility appears promising and merits further study in children.
Keywords: Pressure-volume relationship, pediatric, contractility, echocardiography
Introduction
The advanced assessment of left ventricular mechanics in the pediatric population has the potential to provide valuable insights into the natural history and results of medical and surgical interventions in patients with congenital heart disease. However, such an assessment is rarely performed in children due to the invasive nature of studies that are required to carry out pressure-volume loop (PVL) analysis.1 As such, the development of accurate non-invasive indices of myocardial mechanics has long been a goal in pediatric echocardiography.2
Left ventricular end-systolic elastance (Ees) is a load independent measure of myocardial contractility, defined as the slope of the end-systolic pressure-volume relationship.3 The ratio of arterial elastance to Ees (Ea/Ees), is the reference-standard measure of ventriculo-arterial (VA) coupling as it describes the interaction between myocardial performance and vascular function.4 A number of studies have been performed in animals and humans attempting to develop non-invasive estimates of these measures.5–8 Few studies have been performed attempting to independently validate these methods in adults.9 However, it is clear that adult data supporting the accuracy of non-invasive assessments of myocardial mechanics may not be applicable in children.10 As such, before these non-invasive measures can be used in children, they should be validated against the reference-standard.
The goal of this study was to assess the validity of echocardiographic indices of contractility and VA coupling by direct comparison to reference-standard indices derived from PVL analysis in children. We hypothesized that non-invasive estimates of Ees and Ea/Ees would correlate well with invasive Ees and Ea/Ees, respectively.
Methods
Children (<21 years of age) with biventricular circulation undergoing a clinically indicated diagnostic left heart catheterization were recruited prospectively. Exclusion criteria included: 1) medical status for which participation in the study presented more than minimal risk as determined by the attending physician, 2) non-sinus rhythm, 3) patients with right-sided cardiac pathology (tetralogy of Fallot, atrial septal defect, etc.), and 4) significantly abnormal loading conditions (Qp:Qs > 1.5 or left ventricular outflow tract gradient > 15 mmHg) - a significant left to right shunt would adversely affect conductance catheter volume calibration and left ventricular outflow tract obstruction would significantly affect the non-invasive estimation of left ventricular pressure. Therefore, patients with significantly abnormal loading conditions were excluded, keeping the study population relatively homogenous. The protocol was approved by our institutional review board. Informed consent was obtained from the parent or legal guardian of minors or from the participants of age ≥ 18.
Study Catheterization and PVL Analysis Protocol
All patients underwent general anesthesia per institutional protocol. All study data were collected following the patient’s primary diagnostic and interventional procedures. A 4 Fr high fidelity microconductance catheter (CD Leycom®, Netherlands) was placed in the apex of the left ventricle via the femoral approach. The conductance catheter’s micromanometer was calibrated in normal saline for 15 seconds prior to placement. PVLs were volume calibrated using hypertonic saline to account for parallel conductance. Conductance catheter volumes have been shown to correlate well with cardiac MRI volumes, though they do underestimate absolute volumes.11, 12 Cardiac output was determined by thermodilution. Conductance electrodes outside of the ventricle were excluded from analysis. Preload reduction was acheived via balloon occlusion of one or both vena cavae. Ees was then calculated using the iterative regression method.13 Invasive Ea was calculated as end-systolic pressure divided by invasive stroke volume.14 All PVL data were recorded in triplicate over 10 seconds during an expiratory breath hold. Microconductance data was recorded at a sampling rate of 250 Hz. Invasive data was obtained using standard equipment approved for use in human subjects (INCA® intracardiac analyzer; CD Leycom, Netherlands). PVL analysis was performed offline using specialized software (ConductNT® v.3.18; CD Leycom, Netherlands).
Echocardiographic Acquisition and Analysis Protocol
Echocardiograms were performed immediately after PVL analysis under the same anesthetic conditions using a Phillips IE33 system (Andover, MA). Echocardiograms were sent uncompressed and at native frame rates to the encrypted server for analysis. All measurements were made off-line by a single blinded reviewer (SC) and averaged over three beats. Ventricular volumes and ejection fraction used in the calculation of Ees were derived from 3D echocardiography (3DE) (QLAB v. 9.0, Phillips, Andover, MA). ECG-gated 3DE volumes were acquired during expiratory breath-hold over four beats and the sub-volumes were stitched together. The average frame rate of the 3DE volumes was 29.7 ± 5.1 frames/sec with an average heart rate during acquisition of 86.8 ± 17.2 bpm.
Single-beat estimations of echocardiographic Ees (Eessb) were calculated using four different methods, which have been previously validated in adult patients. Methods 1 (Eessb1)5, 2 (Eessb2)6, and 3 (Eessb3)7 use echocardiographic ventricular volumes, Doppler time intervals, and blood pressure cuff measurements to estimate Ees. In addition, Eessb2 and Eessb3 require an estimation of ventricular end-diastolic pressure. Method 4 (Eessb4)8 is a simpler method that requires only echocardiographic ventricular volumes and blood pressure cuff measurements to estimate Ees. Please see the Appendix for details on the methods to calculate these Eessb estimates.
Echocardiographic Ea was calculated as (0.9*systolic blood pressure)/(3DE stroke volume). A second set of calculations of Ees and Ea was made using 2D echocardiography by calculating volumes using the 5/6 area length method. Non-invasive blood pressures (systolic, diastolic, and mean) were obtained supine at the time of echocardiography by automated sphygmomanometer and averaged over three measurements. Intra- and inter-observer variability of Eessb was performed on 50% of studies by observers blinded to the original measurements.
Statistics
The agreement between invasive Ees and echocardiographic Eessb was expressed as percent error of invasive Ees (Eessb−Ees)/Ees with 95% limits of agreement (± 1.96*standard deviation). One sample t-tests were used to determine if the percent error of the mean was statistically significantly different from zero to assess if the non-invasive measure systematically over- or under-estimated the invasive measure. Differental bias (ex. increased error in estimation as the absolute value of the measure increases) in the accuracy of Eessb estimation vs. invasive Ees was tested using linear regression. This procedure was repeated for invasive Ea vs. echocardiographic Ea and for invasive Ea/Ees vs. echocardiographic Ea/Ees. Pearson’s correlation was performed to evaluate for a linear relationship between invasive and echocardiographic measures. Intra- and inter-observer variability of Eessb was reported using intraclass correlation coefficients assessing absolute agreement and by calculating the absolute value of the percent error of the mean (observation 2 − observation1)/((observation2 + observation 1)/2). A p-value < 0.05 was considered statistically significant. All statistics were performed using IBM® SPSS® Statistics software v. 22.
Results
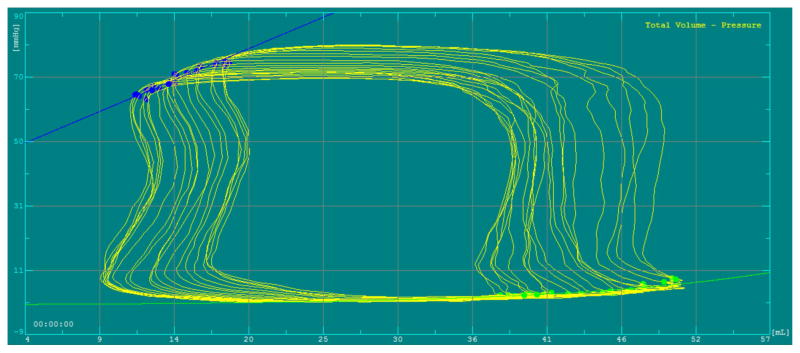
Twenty-four patients were enrolled; 18 patients were status post heart transplant, 5 patients had a trivial or small patent ductus arteriosus, and one had a small coronary fistula. All patent ductus arteriosus and coronary fistula patients were successfully intervened upon. No transplant patients had evidence of coronary artery disease. Demographic, clinical, and catheterization data from these patients are presented in Table 1. A representative PVL during preload reduction and the resulting end-systolic pressure-volume relationship is shown in Figure 1.
Table 1.
Patient Demographics and Invasive Data
| Age (years) | 9.6 ± 5.8 |
| Female, n (%) | 12 (50%) |
| Height (cm) | 126 (58.1) |
| Weight (kg) | 32.9 (36.4) |
| BSA (m2) | 0.96 (0.85) |
| Systolic blood pressure (mm Hg) | 88 ± 9 |
| Diastolic blood pressure (mm Hg) | 47 ± 7 |
| Baseline heart rate (bpm) | 86 ± 18 |
| O2 Saturation (%) | 99 (2.8) |
| EDP (mm Hg) | 10.6 ± 3.3 |
| Cardiac index (L/min/m2) | 3.5 ± 1.2 |
| MvO2 (%) | 75 ± 5 |
| Rp (Wood units) | 1.8 ± 0.7 |
| Rs (Wood units) | 19.2 ± 6.0 |
| Qp:Qs | 1.03 ± 0.21 |
| Ees (mm Hg/mL) | 2.9 ± 1.6 |
| Ea (mm Hg/mL) | 2.2 ± 0.9 |
| Ea/Ees | 0.88 ± 0.35 |
Results reported as mean ± standard deviation for parametric data and median (interquartile range) for non-parametric data. BSA = body surface area. EDP = end-diastolic pressure. MvO2 = mixed venous oxygen saturation. Rp = pulmonary vascular resistance. Rs = systemic vascular resistance. Qp:Qs = ratio of pulmonary to systemic blood flow.
Figure 1.
Representative PVL during preload reduction. The end-systolic pressure-volume relationship is represented by the blue line.
3D Echocardiographic Agreement with Invasive Measures – Ees
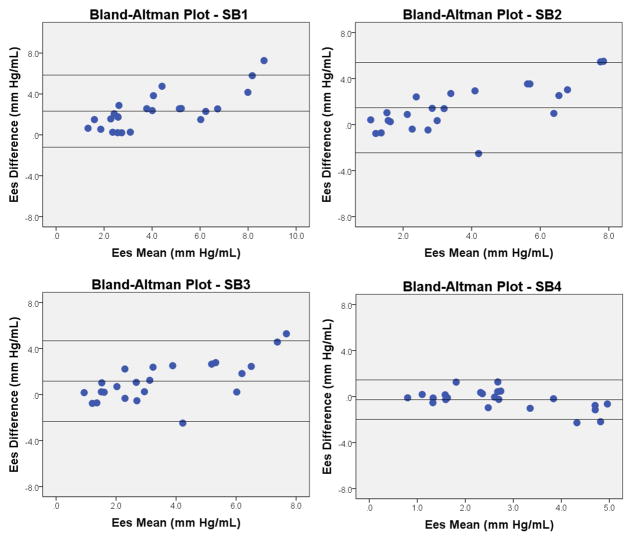
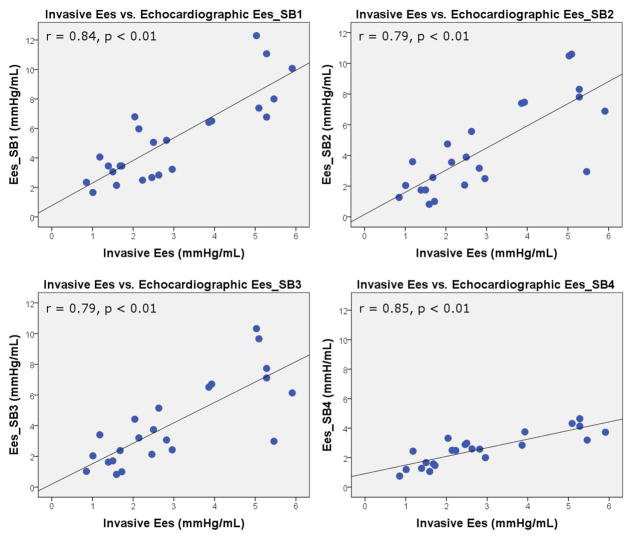
Descriptive echocardiographic estimates of 3DE Ees are reported in Table 2. Correlations and agreement between invasive and echocardiographic Ees are reported in Table 3. Bland-Altman plots displaying agreement between invasive and echocardiographic estimation of Ees are shown in Figure 2. Eessb1, Eessb2, and Eessb3 all systematically overestimated invasive Ees. Only Eessb4 showed good agreement with invasive Ees. There was positive differential bias when estimating Ees (i.e. error increased as Ees increased) using Eessb1 (R2 = 0.58, p < 0.01), Eessb2 (R2 = 0.52, p < 0.01), and Eessb3 (R2 = 0.44, p < 0.01). There was negative differential bias when using Eessb4 (R2 = 0.34, p < 0.01). Scatterplots and correlations between invasive and echocardiographic estimates of Ees are displayed in Figure 3. In general, correlations between invasive and all echocardiographic Eessb estimates were strong. Results of observer variability analysis for Eessb methods and their components can be found in Table 4.
Table 2.
Echocardiographic Estimations of Ees and Ea/Ees
| Ees Method | Echocardiographic Ees | Echocardiographic Ea/Ees |
|---|---|---|
| Eessb1 (mm Hg/mL) | 5.3 ± 2.9 | 0.59 ± 0.16 |
| Eessb2 (mm Hg/mL) | 4.3 ± 3.1 | 0.85 ± 0.41 |
| Eessb3 (mm Hg/mL) | 4.0 ± 2.8 | 0.90 ± 0.43 |
| Eessb4 (mm Hg/mL) | 2.5 ± 1.1 | 1.17 ± 0.40 |
Measures reported as mean ± standard deviation. Ea = arterial elastance. Ees = end-systolic elastance.
Table 3.
Correlations and agreement between invasive and 3D echocardiographic Ees
| Echocardiographic vs. Invasive Ees | ||
|---|---|---|
| 3DE Echocardiographic Method | Correlation coefficient | % error of invasive Ees (95% LoA) |
| SB1 | 0.84* | 91% (−1.2, 5.8 mm Hg/mL)† |
| SB2 | 0.79* | 51% (−2.5, 5.4 mm Hg/mL)† |
| SB3 | 0.79* | 42% (−2.3, 4.7 mm Hg/mL)† |
| SB4 | 0.85* | −0.7% (−2.0, 1.4 mm Hg/mL) |
p-value < 0.05.
% error is statistically significantly different from zero, p < 0.05.
3DE = 3D echocardiography. Ees = end-systolic elastance. LoA = limits of agreement. SB = single beat method.
Figure 2.
Bland Altman Plots: Invasive Ees vs. Eessb. Ees = end-systolic elastance. SB = single beat method.
Figure 3.
Scatterplots between invasive and echocardiographic estimates of Ees. Ees = end-systolic elastance. SB = single beat method.
Table 4.
Observer Variability
| Measure | Intraobserver ICC | Intraobserver % error of the mean | Interobserver ICC | Interobserver % error of the mean |
|---|---|---|---|---|
| Eessb1 | 0.93 | 8% | 0.87 | 12% |
| Eessb2 | 0.85 | 13% | 0.82 | 19% |
| Eessb3 | 0.87 | 13% | 0.84 | 15% |
| Eessb4 | 0.98 | 6% | 0.92 | 10% |
| EDV | 0.99 | 4% | 0.98 | 12% |
| ESV | 0.99 | 4% | 0.98 | 10% |
| PEP | 0.84 | 10% | 0.73 | 21% |
| ET | 0.94 | 3% | 0.88 | 3% |
EDV = end-diastolic volume. Ees = end-systolic elastance. ESV = end-systolic volume. ET = ejection time. PEP = pre-ejection period. SB = single beat method.
3D Echocardiographic Agreement with Invasive Measures – Ea
Mean echocardiographic Ea was 3.0 ± 1.3 mm Hg/mL. Correlation between invasive and echocardiographic Ea was r = 0.94, p < 0.01. Echocardiographic Ea systematically overestimated invasive Ea by 33.4% (95% limits of agreement −0.32, 1.81 mm Hg/mL), p < 0.01 due to positive differential bias. That is, as Ea increased, the difference between invasive and 3DE increased (r = 0.84, p < 0.01).
3D Echocardiographic Agreement with Invasive Measures – Ea/Ees
Descriptive echocardiographic estimates of 3DE Ea/Ees are reported in Table 2. Correlations and agreement between invasive and echocardiographic Ees and Ea/Ees are reported in Table 5.
Table 5.
Correlations and agreement between invasive and 3D echocardiographic Ea/Ees
| Echocardiographic vs. Invasive Ea/Ees | ||
|---|---|---|
| 3DE Echo Method | Correlation coefficient | % error of invasive Ea/Ees (95% LoA) |
| SB1 | 0.60* | −21% (−0.89, 0.39)† |
| SB2 | −0.27 | 9% (−0.88, 0.87) |
| SB3 | 0.32 | 14% (−0.80, 0.89) |
| SB4 | 0.60* | 46% (−0.37, 0.95)† |
p-value < 0.05.
% error is statistically significantly different from zero, p < 0.05.
3DE = 3D echocardiography. Ea = arterial elastance. Ees = end-systolic elastance. LoA = limits of agreement. SB = single beat method.
Agreement with Invasive Measures – Ventricular Volumes, Ejection Fraction, and End-systolic Pressure
In order to assess for sources of disagreement between invasive and non-invasive Ees, we evaluated the agreement between invasive and non-invasive ventricular volumes, ejection fraction, and end-systolic pressure. Results can be found in Appendix Table 1. There were better correlations between invasive vs. non-invasive ventricular volumes than between invasive vs. non-invasive ejection fraction and end-systolic pressure. Non-invasive measures tended to underestimate ventricular volumes and ejection fraction when compared to invasive analysis.
2D Echocardiographic Agreement with Invasive Measures – Ees, Ea, and Ea/Ees
Correlations and agreement between invasive and 2D echocardiographic Ees and Ea/Ees are reported in Appendix Table 2. Correlation between invasive and 2D echocardiographic Ea was r = 0.90, p < 0.01. 2D echocardiographic Ea systematically overestimated invasive Ea by 21.3% (95% limits of agreement −0.70, 1.75 mm Hg/mL), p < 0.01. In general, Ees, Ea, and Ea/Ees estimates by 2D echocardiography were comparable to estimates obtained by 3D echocardiography.
Discussion
To our knowledge, this is the first study to comprehensively evaluate the correlation and agreement of echocardiographic vs. invasive measures of contractility and systolic pump function using gold-standard methods for PVL acquisition in children. The main findings of this study are that all four methods of 3DE estimation of Ees show strong correlation with PVL-derived Ees, however, only 3DE Eessb4 showed good agreement with invasive Ees.
The purpose of measuring non-invasive 3DE Eessb is to to detect abnormal contractility in children. Our results beg the question: do the 3DE Eessb methods with good correlation but poor agreement with invasive Ees hold the potential to accurately assess contractility in this population? It seems clear, with good correlation, Eessb will be able to classify children as having normal or abnormal contractility regardless of absolute value. However, due to poor agreement, normal values established using invasive methods will not be applicable to non-invasive methods. Therefore, new normative values will need to be established using these 3DE methods.
Since all Eessb methods showed good correlation with invasive Ees, determining the most robust method for clinical use will rely upon other characteristics of these methods. For example, compared to Eessb2 and Eessb3, Eessb1 and Eessb4 appear to have better observer reliability and correlate with invasive Ea/Ees when assessing VA coupling by echocardiography. Therefore, Eessb1 and Eessb4 appear to hold the most promise. While Eessb4 is simple to calculate and shows good agreement with invasive Ees, it makes the assumption that the volume intercept of the end-systolic pressure-volume relationship is 0. It may also be quite susceptible to changes in loading conditions due to it only relying on two load-sensitive components – systolic blood pressure and end-systolic volume. Eessb1 may be more load insensitive due to its reliance on relatively insensitive Doppler time intervals. However, its complexity makes it more difficult to calculate. The number of factors in the formula also add “noise” that increases its observer variability. In addition, assumptions in the calculation do not hold in certain disease processes, such as ischemic cardiomyopathy.15 To determine the ideal method for estimating 3DE Eessb, future studies should assess these methods’ ability to predict patient outcomes and their accuracy during altered loading/inotropic states in order to make a more accurate assessment of their utility.
While the correlation between Eessb and invasive Ees was good for all methods, SB methods 1, 2, and 3 demonstrated significant systematic overestimation of Ees. This is likely related to the intrinsic nature of performing these measurements in children. These three methods were developed in adults and utilize time intervals, such as pre-ejection period. In children, whose heart rates are significantly higher than adults, these time intervals become quite short and likely contribute to the overestimation of Ees. In addition, as contractility improves the pre-ejection period shortens, likely leading to the positive differential bias in increasing overestimation of Eessb1-3 with higher invasive Ees. Moreover, due to the poor measurement resolution of short Doppler time intervals, these measurements have high observer variability.16 In contrast, the only method with no time interval incorporated into the equation, Eessb4, showed good agreement with invasive Ees. Another source of error in Eessb methods 2 and 3 is the need to estimate left ventricular end-diastolic pressure. While we have shown good correlation between multiple methods of non-invasive Eessb estimation and PVL-derived Ees in children with relatively normal loading conditions, the development of more accurate methods to estimate Eessb in children may be prudent.
A number of studies purport the accuracy of invasive single-beat estimation of Ees.7, 17–19 However, each study uses a different method to calculate Eessb, leaving clinicians and researchers little guidance on the most robust method. Similar patterns are found when these methods are translated non-invasively.5, 6, 8 Studies attempting to independently validate non-invasively derived Eessb are rare. Yotti et al assessed the correlation between Eessb1 and Eessb4 vs. Ees derived from PVL analysis in adults.9 They found poor correlation between Eessb4 and invasive Ees and no correlation between Eessb1 and invasive Ees, findings that are different from the current study. Disparate results between these two studies may be due to a number of reasons. First, their population was quite heterogeneous in their diagnoses and loading conditions. These formulae were developed in animals and adult humans with relatively normal loading conditions. Abnormal loading conditions are known to produce inaccuracies in the estimation of Eessb, which likely contributed to the poor correlation between Eessb and invasive Ees in the previous study.15, 20 Second, ventricular volumes were assessed using the 2D biplane Simpson’s methods, which has shown to be less accurate and have greater observer variability compared to 3DE.21 Finally, the time and method of blood pressure measurement was not reported in the study, leading to concerns about more sources of error.
We found only a modest correlation between invasive and 3DE Ea/Ees. This was likely due to the fact that there were small, but compounded, sources of error in the measurements needed to estimate 3DE Ea/Ees, such as the error seen in estimating end-systolic pressure using blood-pressure cuff. This is consistent with previous studies.22 Some groups have estimated Eessb using arterial tonometry to estimate end-systolic pressure more accurately.23 This method merits further study in children. In addition, measurement of ventricular volumes and EF for Eessb estimation may be more accurately measured using cardiac magnetic resonance imaging; however, such methodology does not lend itself to validation using simultaneous conductance derived PVL analysis.
Clinical Implications
The validation of the non-invasive assessment of Ees and Ea/Ees has the potential to provide important insights into disease progression and response to treatment in patients with congenital heart disease – many of who spend their lifetime at risk for heart failure. With a constant preload, Ea/Ees is directly related to ejection fraction.14 Therefore, we can use Ea and Ees to assist in management decisions. For example, in a patient with dilated cardiomyopathy and reduced ejection fraction, if the Ea is elevated and the Ees is in a relatively normal range, but cannot compensate for the high Ea enough to result in a normal ejection fraction, it would seem reasonable to treat with medications designed to decrease afterload. Alternatively, if the patient had an Ea in the low or normal range and a low Ees, it would seem clear that this patient would benefit from inotropic support to improve ejection fraction.
Ea and Ees have been shown to be associated with mortality, B-type natriuretic peptide, and exercise performance in adults with cardiovascular disease.24–28 In addition, they can be used to elucidate the mechanism of improvement in heart failure symptoms after therapy.29–31 This is important in pediatrics because children with heart failure have not shown the same response to heart failure therapy as adults.32, 33 Investigating Ea and Ees may allow us to gain insight into the pathophysiology behind the lack of efficacy of standard heart failure therapies in children.
Limitations
The study population was relatively small; our results may deserve validation in a larger cohort. The majority of our patients were status post heart transplantation, and therefore cannot be considered to have absolutely normal cardiac function or loading conditions. We did not perform repeated measures after a change in loading conditions or inotropic states to avoid further complexity in the PVL catheterization procedure. To be applicable to the broader congenital heart disease population, 3DE Eessb methods should next be validated under differing loading conditions, inotropic states, heart rates, and ventricular sizes, masses, and morphologies. Prior to clinical use, normative values need to be established and the clinical utility of these measures need to be validated by assessing their relationship to patient outcomes.
Conclusion
Non-invasive estimates of Eessb derived from 3DE accurately represents invasive Ees derived from PVL analysis in children with normal loading conditions. The use of these non-invasive estimates of Ees in accurately assessing LV contractility appears promising and merits further study in children.
Highlights.
The objective of this study was to compare echocardiographic measures of contractility vs. those derived from pressure-volume loop (PVL) analysis in children.
Non-invasive estimations of end-systolic elastance correlate well with invasive gold-standard methods in children with biventricular circulation and relatively normal loading conditions.
The use of these non-invasive estimates of Ees in accurately assessing LV contractility appears promising and merits further study in children.
Acknowledgments
Funding Sources
This study was funded by the American Society of Echocardiography Foundation and the Mend a Heart Foundation. Dr. Chowdhury was supported by NIH/NHLBI grant T32 HL07710.
Abbreviations
- Ea
arterial elastance
- Ees
end-systolic elastance
- PVL
pressure-volume loop
- VA
ventriculo-arterial
Appendix – Methods used to estimate 3DE Eessb
Method 1 (Eessb1) by Chen et al:5
Where Pd = diastolic blood pressure, Ps = systolic blood pressure, SV = stroke volume, and where EF = ejection fraction, Pes = end-systolic pressure estimated as 0.9 * Ps, and ENDavg is an empirical estimation of normalized population-average elastance at the onset of ejection fitted by a 7-degree polynomial to the ratio of pre-ejection time to total systolic ejection time measured by spectral Doppler.5
Method 2 (Eessb2) by Kim et al:6
Where EDP = end-diastolic pressure – estimated as 10 mmHg in this cohort, ET = ejection time as defined by the duration of systolic aortic flow by spectral Doppler, PEP = pre-ejection period defined as the time interval between the beginning of the QRS and the start of aortic outflow, and α = 1.171 * EF + 0.222.
Method 3 (Eessb3) by Shishido et al is similar to that of Kim et al, except for the use of a bivariate model to predict α:7
Where α = 0.210 +1.348 * EF + 0.682 * PEP/(PEP + ET).
Method 4 (Eessb4) by Tanuoue et al:8
Where ESV = end-systolic volume.
Appendix Table 1.
Invasive vs. Non-invasive – 3DE Ventricular Volume, Ejection Fraction, and End-systolic Pressure
| Measure | Invasive mean | Non- invasive mean | Correlation coefficient with invasive measure | % error of invasive measure (95% LoA) |
|---|---|---|---|---|
| EDV (mL) | 69 ± 28 | 64 ± 29 | 0.94* | −7% (−24, 16)† |
| ESV (mL) | 32 ± 17 | 29 ± 17 | 0.89* | −7% (−18, 12) |
| EF (%) | 60 ± 8 | 57 ± 17 | 0.73* | −4% (−14, 8)† |
| ESP (mm Hg) | 80 ± 11 | 79 ± 8 | 0.79* | −0.8% (−13, 13) |
p-value < 0.05.
% error is statistically significantly different from zero, p < 0.05.
3DE = 3D echocardiography. EDV = end-diastolic volume. EF = ejection fraction. ESP = end-systolic pressure calculated as 0.9 * systolic pressure from blood pressure cuff. ESV = end-systolic volume by LoA = limits of agreement.
Appendix Table 2.
Correlations and agreement between invasive and 2D echocardiographic Ees and Ea/Ees
| Echocardiographic vs. Invasive Ees | Echocardiographic vs. Invasive Ea/Ees | |||
|---|---|---|---|---|
| 2DE Echo Method | Correlation coefficient | % error of invasive Ees (95% LoA) | Correlation coefficient | % error of invasive Ea/Ees (95% LoA) |
| SB1 | 0.84* | 58% (−1.1, 5.9 mm Hg/mL)† | 0.49* | −46% (−0.94, 0.28)† |
| SB2 | 0.85* | 31% (−1.8, 3.9 mm Hg/mL)† | 0.28 | −3% (−0.86, 0.81) |
| SB3 | 0.86* | 24% (−1.7, 3.3 mm Hg/mL)† | 0.35 | 3% (−0.82, 0.88) |
| SB4 | 0.74* | −6.2% (−2.1, 2.1 mm Hg/mL) | 0.52* | 24% (−0.59, 1.07)† |
p-value < 0.05.
% error is statistically significantly different from zero, p < 0.05.
2DE = 2D echocardiography. Ea = arterial elastance. Ees = end-systolic elastance. LoA = limits of agreement. SB = single beat method.
Footnotes
Disclosures
None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Sagawa K. Cardiac contraction and the pressure-volume relationship. New York: Oxford University Press; 1988. [Google Scholar]
- 2.Pellikka PA, Douglas PS, Miller JG, Abraham TP, Baumann R, Buxton DB, et al. American Society of Echocardiography Cardiovascular Technology and Research Summit: a roadmap for 2020. J Am Soc Echocardiogr. 2013;26:325–38. doi: 10.1016/j.echo.2013.02.003. [DOI] [PubMed] [Google Scholar]
- 3.Sagawa K, Suga H, Shoukas AA, Bakalar KM. End-systolic pressure/volume ratio: a new index of ventricular contractility. Am J Cardiol. 1977;40:748–53. doi: 10.1016/0002-9149(77)90192-8. [DOI] [PubMed] [Google Scholar]
- 4.Fox JM, Maurer MS. Ventriculovascular coupling in systolic and diastolic heart failure. Curr Heart Fail Rep. 2005;2:204–11. doi: 10.1007/BF02696651. [DOI] [PubMed] [Google Scholar]
- 5.Chen CH, Fetics B, Nevo E, Rochitte CE, Chiou KR, Ding PA, et al. Noninvasive single-beat determination of left ventricular end-systolic elastance in humans. J Am Coll Cardiol. 2001;38:2028–34. doi: 10.1016/s0735-1097(01)01651-5. [DOI] [PubMed] [Google Scholar]
- 6.Kim YJ, Jones M, Greenberg NL, Popovic ZB, Sitges M, Bauer F, et al. Evaluation of left ventricular contractile function using noninvasively determined single-beat end-systolic elastance in mitral regurgitation: experimental validation and clinical application. J Am Soc Echocardiogr. 2007;20:1086–92. doi: 10.1016/j.echo.2007.02.009. [DOI] [PubMed] [Google Scholar]
- 7.Shishido T, Hayashi K, Shigemi K, Sato T, Sugimachi M, Sunagawa K. Single-beat estimation of end-systolic elastance using bilinearly approximated time-varying elastance curve. Circulation. 2000;102:1983–9. doi: 10.1161/01.cir.102.16.1983. [DOI] [PubMed] [Google Scholar]
- 8.Tanoue Y, Sese A, Ueno Y, Joh K, Hijii T. Bidirectional Glenn procedure improves the mechanical efficiency of a total cavopulmonary connection in high-risk fontan candidates. Circulation. 2001;103:2176–80. doi: 10.1161/01.cir.103.17.2176. [DOI] [PubMed] [Google Scholar]
- 9.Yotti R, Bermejo J, Benito Y, Sanz-Ruiz R, Ripoll C, Martinez-Legazpi P, et al. Validation of noninvasive indices of global systolic function in patients with normal and abnormal loading conditions: a simultaneous echocardiography pressure-volume catheterization study. Circ Cardiovasc Imaging. 2014;7:164–72. doi: 10.1161/CIRCIMAGING.113.000722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dragulescu A, Mertens L, Friedberg MK. Interpretation of left ventricular diastolic dysfunction in children with cardiomyopathy by echocardiography: problems and limitations. Circ Cardiovasc Imaging. 2013;6:254–61. doi: 10.1161/CIRCIMAGING.112.000175. [DOI] [PubMed] [Google Scholar]
- 11.Winter EM, Grauss RW, Atsma DE, Hogers B, Poelmann RE, van der Geest RJ, et al. Left ventricular function in the post-infarct failing mouse heart by magnetic resonance imaging and conductance catheter: a comparative analysis. Acta Physiol. 2008;194:111–22. doi: 10.1111/j.1748-1716.2008.01863.x. [DOI] [PubMed] [Google Scholar]
- 12.Nielsen JM, Kristiansen SB, Ringgaard S, Nielsen TT, Flyvbjerg A, Redington AN, et al. Left ventricular volume measurement in mice by conductance catheter: evaluation and optimization of calibration. Am J Physiol Heart Circ Physiol. 2007;293:H534–40. doi: 10.1152/ajpheart.01268.2006. [DOI] [PubMed] [Google Scholar]
- 13.Kass DA, Midei M, Graves W, Brinker JA, Maughan WL. Use of a conductance (volume) catheter and transient inferior vena caval occlusion for rapid determination of pressure-volume relationships in man. Cathet Cardiovasc Diagn. 1988;15:192–202. doi: 10.1002/ccd.1810150314. [DOI] [PubMed] [Google Scholar]
- 14.Sunagawa K, Maughan WL, Burkhoff D, Sagawa K. Left ventricular interaction with arterial load studied in isolated canine ventricle. Am J Physiol. 1983;245:H773–80. doi: 10.1152/ajpheart.1983.245.5.H773. [DOI] [PubMed] [Google Scholar]
- 15.Jegger D, Mallik AS, Nasratullah M, Jeanrenaud X, da Silva R, Tevaearai H, et al. The effect of a myocardial infarction on the normalized time-varying elastance curve. J Appl Physiol (1985) 2007;102:1123–9. doi: 10.1152/japplphysiol.00976.2006. [DOI] [PubMed] [Google Scholar]
- 16.Colan SD, Shirali G, Margossian R, Gallagher D, Altmann K, Canter C, et al. The ventricular volume variability study of the Pediatric Heart Network: study design and impact of beat averaging and variable type on the reproducibility of echocardiographic measurements in children with chronic dilated cardiomyopathy. J Am Soc Echocardiogr. 2012;25:842–854. e6. doi: 10.1016/j.echo.2012.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Senzaki H, Chen CH, Kass DA. Single-beat estimation of end-systolic pressure-volume relation in humans. A new method with the potential for noninvasive application. Circulation. 1996;94:2497–506. doi: 10.1161/01.cir.94.10.2497. [DOI] [PubMed] [Google Scholar]
- 18.Takeuchi M, Igarashi Y, Tomimoto S, Odake M, Hayashi T, Tsukamoto T, et al. Single-beat estimation of the slope of the end-systolic pressure-volume relation in the human left ventricle. Circulation. 1991;83:202–12. doi: 10.1161/01.cir.83.1.202. [DOI] [PubMed] [Google Scholar]
- 19.Kameyama T, Asanoi H, Ishizaka S, Sasayama S. Ventricular load optimization by unloading therapy in patients with heart failure. J Am Coll Cardiol. 1991;17:199–207. doi: 10.1016/0735-1097(91)90728-r. [DOI] [PubMed] [Google Scholar]
- 20.Kjorstad KE, Korvald C, Myrmel T. Pressure-volume-based single-beat estimations cannot predict left ventricular contractility in vivo. Am J Physiol Heart Circ Physiol. 2002;282:H1739–50. doi: 10.1152/ajpheart.00638.2001. [DOI] [PubMed] [Google Scholar]
- 21.Dorosz JL, Lezotte DC, Weitzenkamp DA, Allen LA, Salcedo EE. Performance of 3-dimensional echocardiography in measuring left ventricular volumes and ejection fraction: a systematic review and meta-analysis. J Am Coll Cardiol. 2012;59:1799–808. doi: 10.1016/j.jacc.2012.01.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Suwa M, Hirota Y, Kino M, Saito T, Yoneda Y, Kawamura K. Noninvasive estimation of left ventricular end-systolic pressure. J Cardiol. 1987;17:845–51. [PubMed] [Google Scholar]
- 23.Gayat E, Mor-Avi V, Weinert L, Yodwut C, Lang RM. Noninvasive quantification of left ventricular elastance and ventricular-arterial coupling using three-dimensional echocardiography and arterial tonometry. Am J Physiol Heart Circ Physiol. 2011;301:H1916–23. doi: 10.1152/ajpheart.00760.2011. [DOI] [PubMed] [Google Scholar]
- 24.Antonini-Canterin F, Enache R, Popescu BA, Popescu AC, Ginghina C, Leiballi E, et al. Prognostic value of ventricular-arterial coupling and B-type natriuretic peptide in patients after myocardial infarction: a five-year follow-up study. J Am Soc Echocardiogr. 2009;22:1239–45. doi: 10.1016/j.echo.2009.08.009. [DOI] [PubMed] [Google Scholar]
- 25.Bombardini T, Costantino MF, Sicari R, Ciampi Q, Pratali L, Picano E. End-systolic elastance and ventricular-arterial coupling reserve predict cardiac events in patients with negative stress echocardiography. Biomed Res Int. 2013;2013:235194. doi: 10.1155/2013/235194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ky B, French B, May Khan A, Plappert T, Wang A, Chirinos JA, et al. Ventricular-arterial coupling, remodeling, and prognosis in chronic heart failure. J Am Coll Cardiol. 2013;62:1165–72. doi: 10.1016/j.jacc.2013.03.085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shim CY, Park S, Choi EY, Hong GR, Choi D, Jang Y, et al. The relationship between ventricular-vascular uncoupling during exercise and impaired left ventricular longitudinal functional reserve in hypertensive patients. J Am Soc Hypertens. 2013;7:198–205. doi: 10.1016/j.jash.2013.01.005. [DOI] [PubMed] [Google Scholar]
- 28.Argulian E, Agarwal V, Makani H, Herzog E, Chaudhry FA. Association of exercise tolerance with effective arterial elastance obtained noninvasively in patients with exertional dyspnea. J Am Soc Echocardiogr. 2014;27:675–9. doi: 10.1016/j.echo.2014.01.023. [DOI] [PubMed] [Google Scholar]
- 29.Bozkurt B, Bolos M, Deswal A, Ather S, Chan W, Mann DL, et al. New insights into mechanisms of action of carvedilol treatment in chronic heart failure patients--a matter of time for contractility. J Card Fail. 2012;18:183–93. doi: 10.1016/j.cardfail.2011.11.004. [DOI] [PubMed] [Google Scholar]
- 30.Maurer MS, Sackner-Bernstein JD, El-Khoury Rumbarger L, Yushak M, King DL, Burkhoff D. Mechanisms underlying improvements in ejection fraction with carvedilol in heart failure. Circ Heart Fail. 2009;2:189–96. doi: 10.1161/CIRCHEARTFAILURE.108.806240. [DOI] [PubMed] [Google Scholar]
- 31.Berthelot E, Bihry N, Brault-Melin O, Assayag P, Cohen-Solal A, Chemla D, et al. Changes in ventricular-arterial coupling during decongestive therapy in acute heart failure. Eur J Clin Invest. 2014;44:982–8. doi: 10.1111/eci.12332. [DOI] [PubMed] [Google Scholar]
- 32.Shaddy RE, Boucek MM, Hsu DT, Boucek RJ, Canter CE, Mahony L, et al. Carvedilol for children and adolescents with heart failure: a randomized controlled trial. JAMA. 2007;298:1171–9. doi: 10.1001/jama.298.10.1171. [DOI] [PubMed] [Google Scholar]
- 33.Hsu DT, Zak V, Mahony L, Sleeper LA, Atz AM, Levine JC, et al. Enalapril in infants with single ventricle: results of a multicenter randomized trial. Circulation. 2010;122:333–40. doi: 10.1161/CIRCULATIONAHA.109.927988. [DOI] [PMC free article] [PubMed] [Google Scholar]