Abstract
Diet is an important variable in toxicology. There are mixed reports on the impact of soy components on energy utilization, fat deposition, and reproductive parameters. Three generations of CD-1 mice were fed irradiated natural ingredient diets with varying levels of soy (NIH-41, 5K96, or 5008/5001), purified irradiated AIN-93 diet, or the AIN-93 formulation modified with ethanol-washed soy protein concentrate (SPC) or SPC with isoflavones (SPC-IF). NIH-41 was the control for pairwise comparisons. Minimal differences were observed among natural ingredient diet groups. F0 males fed AIN-93, SPC, and SPC-IF diets had elevated glucose levels and lower insulin levels compared with the NIH-41 group. In both sexes of the F1 and F2 generations, the SPC and SPC-IF groups had lower body weight gains than the NIH-41 controls and the AIN-93 group had an increased percent body fat at postnatal day 21. AIN-93 F1 pups had higher baseline glucose than NIH-41 controls, but diet did not significantly affect breeding performance or responses to glucose or uterotrophic challenges. Reduced testes weight and sperm in the AIN-93 group may be related to low thiamine levels. Our observations underline the importance of careful selection, manufacturing procedures, and nutritional characterization of diets used in toxicological studies.
Keywords: soy, isoflavones, natural ingredient diet, purified ingredient diet, thiamine, CD-1 mouse
Introduction
Diet has long been recognized as an important variable that can influence the results of rodent toxicology studies (Brown and Setchell, 2001; Conner and Newberne, 1984; Cooper et al., 2006; Fullerton et al., 1992; Greenman and Fullerton, 1986; Greenman et al., 1987; Heindel and vom Saal, 2008; Kozul et al., 2008; Newberne and Conner, 1986; Rao and Knapka, 1998; Thigpen et al., 2013; Thigpen et al., 2004). In the case of studies of potential endocrine disruptors, and, in particular, agents with potential estrogenic activity, there has been much concern about the possible confounding effects of soy components, particularly estrogenic soy isoflavones (Boettger-Tong et al., 1998; Muhlhauser et al., 2009; Patisaul et al., 2012; Thigpen et al., 2004; Wang et al., 2005). This focus is due to the widespread use of varying levels of soy meal in natural ingredient diets typically used in rodent studies and fluctuations in isoflavone levels in diets of equivalent amounts of soy meal based on weather and other growing conditions (Brown and Setchell, 2001; Heindel and vom Saal, 2008; Thigpen et al., 2007; Thigpen et al., 1999). The interpretation of studies on the impact of diets containing soy is complicated by the presence of many non-isoflavone bioactive components in soy meal that can vary independently of the isoflavones (Kang et al., 2010; Velasquez and Bhathena, 2007). In addition, other dietary factors, including energy content, impact study results (Odum et al., 2001; Odum et al., 2004; Thigpen et al., 2002).
The present study was prompted by consideration of conducting long-term studies with hormonally active agents in a mouse model. We had previously used a soy-free diet in several studies with strong and weak estrogens in Sprague-Dawley rats, including multigenerational and chronic studies (Delclos et al., 2014; National Toxicology Program, 2008a, b, 2010a, b). Similarly, others have used soy-free diets in multigenerational reproductive studies in rats and mice (e.g., Kendig et al., 2012; Sprando et al., 2000); however, some literature suggested that the specific soy- and alfalfa-free diet that we had used might be problematic in studies in the CD-1 mouse (Ruhlen et al., 2008). This diet, designated 5K96, was originally designed to be nutritionally equivalent to the open-formula NIH-31 diet. The soy and alfalfa meal present in NIH-31 were replaced by casein in 5K96 to reduce considerably the estrogenic isoflavones and coumestrol found respectively in soy and alfalfa meals. Ruhlen et al. (2008) reported that CD-1 mice fed the 5K96 diet from one week prior to mating produced offspring that were obese as adults, with males, but not females, also showing increased serum leptin and impaired glucose regulation at PND 90 relative to mice fed soy meal-containing 5008 diet. Weights of the testes, epididymides, and seminal vesicles were decreased in the soy-free diet group, while prostate weight was increased. These effects were hypothesized to be due to the increase in fetal estrogen levels measured in mice fed 5K96 diet relative to those in mice fed the soy-containing diet. Cederroth et al. (2007) reported a similar finding with respect to body weight and fat deposition in CD-1 mice maintained from conception on a soy-free modified natural ingredient diet (Ziegler Phytoestrogen Reduced Rodent Diet I) similar to that used by Ruhlen et al. (2008). Cederroth and Nef (2009a) further reported that the beneficial effects of a high phytoestrogen diet on obesity were confined to postnatal exposure and beneficial effects on glucose tolerance were confined to intrauterine exposure, with the latter effects dependent on the intrauterine position of the pups. In addition, Cederroth et al. (2010) reported opposite effects on the male reproductive tract from Ruhlen et al. (2008), with the high soy diet resulting in reduced sperm counts, reduced seminal vesicle weight, and reduced fertility.
While the studies listed above were major motivators for the study described here, other literature also presents an unclear picture of the effects of dietary soy components on body weight, fat deposition, and reproductive function, as well as on the ability to detect the effects of weak estrogens in rodents. Multiple studies have suggested beneficial effects of phytoestrogens and soy protein on obesity (reviewed in Orgaard and Jensen, 2008; Velasquez and Bhathena, 2007). Soy has been implicated as a protective factor against the development of metabolic syndrome in rodents (Cederroth and Nef, 2009b), and both isoflavones and soy protein, due to its amino acid composition or peptides derived from the protein (Moriyama et al., 2004; Tachibana et al., 2010), have been implicated in this beneficial effect. Similar to the results mentioned above in CD-1 mice (Cederroth et al., 2007; Ruhlen et al., 2008), Badger et al. (2001) reported that Sprague-Dawley rats fed an AIN-93 based diet with a soy protein isolate replacing casein from conception had lower body weights than the animals fed the casein diet. Andreoli et al. (2015) also reported that feeding adult male Wistar rats a low phytoestrogen diet induced obesity and impaired glucose metabolism. Conversely, exposure of Wistar rats to a soy meal-containing diet during development has been reported to result in increased adult body weight in males, but not females (Cao et al., 2015), while developmental exposure of Sprague-Dawley rats to genistein was reported to result in obese adult females, but not males (Strakovsky et al., 2014). Lifetime exposure of Sprague-Dawley rats to 500 ppm, but not 5 or 100 ppm, genistein reduced body weight gain in the F1 and F2 generation females and in the F1 generation males (National Toxicology Program, 2008a). Developmental exposure of Wistar rats to soy protein-containing diet was reported to increase body weight of both male and female rats as adults compared to those fed a casein diet, but glucose intolerance was more pronounced in adult males (Jahan-Mihan et al., 2011). The effects of the dietary protein difference were largely attributed to the dams’ diet during pregnancy (Jahan-Mihan et al., 2012). The data regarding soy diet effects on body weight and obesity are thus somewhat difficult to interpret and may depend on species and strain, the specific soy preparations or components examined, or other diet constituents or properties (e.g. pellet hardness or moisture content) that differ across studies.
The goals of the present study were to assess the suitability of a soy- and alfalfa-free modified natural ingredient diet for use in toxicology studies with CD-1 mice, to explore the utility of modified purified ingredient diets to alleviate the variability inherent in natural ingredient diets, and to better separate the effects that are due to soy isoflavones from those due to soy protein or other soy components. The results affirm marked diet-related differences in basic animal parameters, underline the difficulties in attributing diet effects to specific dietary components, and point to the importance of careful consideration of micronutrient variations when using irradiated purified diets.
Materials and Methods
Animals
Weanling CD-1 mice [Crl:CD1(ICR)], approximately three to four weeks old, were obtained from Charles River Laboratories (Wilmington, MA). The experiment was loaded in two equal sized animal groups spaced two weeks apart. Males born a week earlier than females were obtained to ensure that littermates were not mated. Upon receipt, animals were fed NIH-41 Irr diet and housed in polysulfone cages with hardwood chip bedding (P.J. Murphy, Montville, NJ), wire lids, and microisolator tops. Millipore-filtered tap water was provided in glass bottles with silicone stoppers and stainless steel sipper tubes. Feed and water were provided ad libitum. Animal rooms were maintained at 23° ± 3°C with a relative humidity of 50% ± 20%. Lights were on for 12 hours per day starting at 6 AM.
Within a week of arrival, animals were randomly allocated to one of six experimental diet groups by a stratified randomization procedure by sex based on body weight to give approximately equivalent mean body weights in each diet group. Males and females were randomly paired one-to-one within diet groups when the F0 females were approximately 11–12 weeks of age. Pairs were separated after 10 days. Except for the mating period and the pre-weaning period when dams and pups were co-housed, animals were individually housed to allow for more accurate evaluation of food consumption. Sentinel animals were maintained with NIH-41 Irr diet in each animal room and one animal per room was removed and evaluated for viral and bacterial pathogens at the midpoint and end of the study in accordance with the sentinel animal program at the National Center for Toxicological Research (NCTR). No pathogens were detected in any sentinel animal. All animal procedures were approved by the NCTR Animal Care and Use Committee.
Diets
There were six diet groups and 10 diets in the experiment. Four of the groups had separate growth (G) and maintenance (M) diet formulations, while the remaining two groups had one diet formulation fed through all stages of the experiment. The six diet groups were: 1) NIH-41, an irradiated formulation of NIH-31, 2) 5K96, 3) 5008(G)/5001(M), 4) AIN-93 G and M, 5) SPC G and M, which were modifications of AIN-93 in which casein was replaced with ethanol-washed soy protein concentrate (SPC, Arcon® SJ, product 066408, Archer Daniels Midland, Decatur, IL), and 6) SPC-IF G and M, the SPC diet formulations supplemented with isoflavones (IF, NovaSoy® 650, containing 73.08% isoflavones, product 152650, Archer Daniels Midland). The open formula purified diets AIN-93, SPC, and SPC-IF were obtained from Envigo (Harlan) Teklad Diets (Madison, WI). SPC was used in this study rather than soy protein isolate because a commercially available ethanol-washed, and thus isoflavone-free, soy protein isolate product could not be identified at the time that the study started. NIH-41 open formula, and 5K96 and 5008/5001, which are closed formula diets, were obtained from PMI Nutrition International (LabDiet/TestDiet), St. Louis, MO. Single lots of each diet were used in this study. All of the diets were provided in pelleted form and were irradiated by the manufacturers prior to shipment. Natural ingredient diets from Lab Diet were irradiated with Cobalt 60 (gamma irradiation) at an average dose of 20–25 kGy for approximately one hour (personal communication, Dr. Carrie Schultz, PMI Nutrition International). The other diets were irradiated with a Cobalt 60 source at 20–50 kGy for approximately six hours (personal communication, Dr. Barbara Mickelson, Envigo (Harlan) Teklad). Diets were confirmed to be free of microbiological contaminants. All were stored at 4°C until given to the animals and fresh diet was supplied weekly. Compositions and calculated energy contents of the natural ingredient diets are summarized in Table 1. Compositions and calculated energy contents of the three AIN-93 based diets are shown in Table 2. The AIN-93 based diets had higher energy densities than did the natural ingredient diets, including the natural ingredient growth diet, 5008. F0 animals were assigned as described above to one of the six maintenance diets within one week of arrival at NCTR. One week before mating, the F0 and F1 breeding animals were fed the growth diet in the four diet groups that had growth and maintenance diets. F1 animals were switched to maintenance formulations on PND 21.
Table 1.
Composition and calculated energy density of the natural ingredient-based diets used in this studya
| NIH-41 | 5K96b | 5001 (maintenance)b | 5008 (growth)b |
|---|---|---|---|
| Ground wheat 34.90% | Ground wheat | Ground corn | Ground corn |
| Ground corn 21.00% | Ground corn | Dehulled soybean meal | Dehulled soybean meal |
| Wheat middlings 10.00% | Wheat middlings | Dried beet pulp | Ground wheat |
| Ground whole oats 10.00% | Ground oats | Fish meal | Fish meal |
| Fish meal (60% protein) 9.00% | Fish meal | Ground oats | Wheat middlings |
| Soy oil 2.00% | Casein | Brewers dry yeast | Porcine animal fat with BHA |
| Soybean meal (47.5% protein) 5.00% | Corn gluten meal | Cane molasses | Cane molasses |
| Alfalfa meal (17% protein), 2.00% | Dicalcium phosphate | Alfalfa meal | Brewers dry yeast |
| Corn gluten meal (60% protein) 2.00% | Monocalcium phosphate | Dried whey | Porcine meat meal |
| Dicalcium phosphate 1.50% | Soybean oil | Wheat germ | Wheat germ |
| Brewers dried yeast 1.00% | Brewers dry yeast | Porcine animal fat with butylated hydroxyanisole | Ground oats |
| Premixes 0.60% | Calcium carbonate | Porcine meat meal | Dried beet pulp |
| Ground limestone 0.50% | Salt | Wheat middlings | Alfalfa meal |
| Salt 0.50% | Salt | Calcium carbonate | |
| Dried whey | |||
| Salt | |||
| Crude fiber, %c | |||
| ≤ 5.0 | ≤ 5.0 | ≤ 6.0 | ≤ 4.0 |
| Metabolizable energy, Kcal/g | |||
| 3.11 | 3.15 | 3.02 | 3.31 |
NIH-41 is an open-formula diet (http://www.ors.od.nih.gov/sr/dvr/drs/nutrition/Documents/SpecsDiets/41.pdf) and percentages of the ingredients are included. The other diets are closed formula and thus the ingredients are listed in descending content order. 5K96 is nutritionally similar to the NIH-41 formulation, with soy and alfalfa meals replaced by casein. Both NIH-41 and 5K96 diets support growth and reproduction, so there are no separate G and M formulations. All diets were irradiated by the manufacturer.
Further information on the closed formula natural ingredient diets are available at the manufacturer’s web site: 5K96, http://www.labdiet.com/cs/groups/lolweb/@labdiet/documents/web_content/mdrf/mdi4/~edisp/ducm04_028427.pdf; 5001, http://www.labdiet.com/cs/groups/lolweb/@labdiet/documents/web_content/mdrf/mdi4/~edisp/ducm04_028021.pdf; 5008, http://www.labdiet.com/cs/groups/lolweb/@labdiet/documents/web_content/mdrf/mdi4/~edisp/ducm04_028444.pdf.
Crude fiber limit data for each of the diets as provided by the manufacturer. Fiber levels for the specific lots of the natural ingredient diets used were not determined.
Table 2.
Composition and calculated energy density of AIN-93, SPC, and SPC-IF growth (G) and maintenance (M) formulationsa
| Ingredient | Dietb | |||||
|---|---|---|---|---|---|---|
| AIN-93 G | AIN-93 M | SPC G | SPC M | SPC-IF G | SPC-IF M | |
| Casein | 200 | 140 | 0 | 0 | 0 | 0 |
| Arcon® SJc | 0 | 0 | 260 | 182 | 260 | 182 |
| NovaSoy®650d | 0 | 0 | 0 | 0 | 0.85 | 0.85 |
| L-cystine | 3 | 1.8 | 1.24 | 0.57 | 1.24 | 0.57 |
| L-methionine | 0 | 0 | 2.34 | 1.65 | 2.34 | 1.65 |
| Corn starch | 383.5 | 451.3 | 392.0 | 456.8 | 391.1 | 455.9 |
| Maltodextrin | 132 | 155 | 132 | 155 | 132 | 155 |
| Sucrose | 100 | 100 | 100 | 100 | 100 | 100 |
| Soybean oil | 70 | 40 | 70 | 40 | 70 | 40 |
| Cellulosee | 50 | 50 | 0.6 | 15.42 | 0.6 | 15.42 |
| Fibere | 50 | 50 | 50 | 50 | 50 | 50 |
| Vitamin mixf | 15 | 15 | 15 | 15 | 15 | 15 |
| Choline bitartrate | 2.75 | 2.75 | 2.75 | 2.75 | 2.75 | 2.75 |
| Tert-butylhydroquinone | 0.014 | 0.008 | 0.014 | 0.008 | 0.014 | 0.008 |
| Trace mineral mixf | 6 | 6 | 6 | 6 | 6 | 6 |
| Calcium carbonateg | 15.7 | 15.7 | 4.2 | 4.1 | 4.2 | 4.1 |
| Potassium phosphate, monobasicg | 10.1 | 11.9 | 0 | 0 | 0 | 0 |
| Potassium citrate, monohydrateg | 4.7 | 3.25 | 0 | 4.4 | 0 | 4.4 |
| Sodium chloride | 1.22 | 1.22 | 1.22 | 1.22 | 1.22 | 1.22 |
| Potassium sulfateg | 1.63 | 1.63 | 0 | 0 | 0 | 0 |
| Magnesium oxideg | 2 | 2 | 0.67 | 1.07 | 0.67 | 1.07 |
| Sodium carbonateg | 2.1 | 2.1 | 0 | 0.65 | 0 | 0.65 |
| Ferric citrateg | 0.33 | 0.33 | 0.21 | 0.25 | 0.21 | 0.25 |
| Calcium phosphate, dibasicg | 0 | 0 | 11.8 | 13.15 | 11.8 | 13.15 |
| Metabolizable energy, Kcal/g | 3.7 | 3.6 | 3.8 | 3.6 | 3.8 | 3.6 |
Across all diets, fat and micronutrient levels were matched where practical. Within a set of maintenance or growth diets, protein and sulfur amino acids are equivalent. Vitamin levels were higher than needed to compensate for losses during irradiation.
Ingredient levels in each diet are given as g ingredient/kg diet.
Arcon® SJ, product 066408, NutriSoy® isolated soy protein concentrate (SPC), Archer Daniels Midland Company, IL.
NovaSoy®650, isoflavone isolate (IF), Archer Daniels Midland Company, IL.
Cellulose, a fiber source, was reduced in diets containing Arcon® SJ, which has a fiber content of 19%. All diets contained 5%, or 50 g/kg, fiber.
AIN-93 vitamin mix (Reeves et al., 1993) was increased to 15 g/kg diet from the usual 10 g/kg to account for potential losses during irradiation. The trace mineral mix was as specified in Reeves et al. (1993).
These macrominerals were adjusted by the manufacturer where necessary to adjust for contributions from the SPC product and to support reproduction.
Chemical analyses of diets
Results of analyses of selected dietary components and contaminants assayed after arrival at NCTR are reported in Table 3. Fat was extracted with a Soxhlet apparatus and analyzed by gravimetry; total protein was quantified using a FP28 nitrogen/protein determinator (LECO Corporation, St. Joseph, MI); vitamins A and E were determined by high pressure liquid chromatography (HPLC) with fluorescence detection, and thiamine by the method of Gehring et al. (1995). Organochlorine pesticides, organophosphate pesticides, and polychlorinated biphenyls were determined following a modified FDA Pesticides Analytical Manual (PAM) method by gas chromatography with electron capture detector. Aflatoxins were measured by thermospray mass spectroscopy after derivitization with iodine (Holcomb et al., 1991). Fumonisins were measured with a RIDASCREEN®FAST fumonisin immunoassay kit (R-Biopharm Inc, Marshall, MI) according to the manufacturer’s instructions. Heavy metals (arsenic, cadmium, selenium, and mercury) were analyzed by Applied Research and Development Laboratory, Inc. (Mount Vernon, IL), using standard EPA methods. Daidzein, genistein, and zearalenone levels in feed were determined by LC tandem mass spectrometry following an acid hydrolysis standard addition method. Background levels of bisphenol A (BPA) in feed and cage bedding were determined by LC-MS/MS following liquid-liquid extraction and cleanup using solid phase extraction.
Table 3.
Levels of selected dietary components and contaminants in diets post-irradiationa
| Analyte | Diet | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| NIH-41 | 5K96 | 5008 | 5001 | AIN-93 G | AIN-93 M | SPC G | SPC M | SPC-IF G | SPC-IF M | |
| Fat, % | 5.4 | 6.9 | 8.3 | 6.7 | 8.4 | 4.3 | 7.6 | 4.8 | 8.6 | 5.0 |
| Protein, % | 17.8 | 20.9 | 23.1 | 23.4 | 15.8 | 10.4 | 15.7 | 10.9 | 15.8 | 10.8 |
| Vitamin A, ppm (IU/g) | 3.1 (10.3) | 3.8 (12.7) | 5.5 (18.3) | 3.5 (11.7) | 1.3 (4.3) | 1.4 (4.7) | 1.8 (6.0) | 1.5 (5.0) | 1.2 (4.0) | 1.6 (5.3) |
| Thiamine, ppmb | 15.5 | 19.2 | 14.9 | 13.2 | <LOQ (4.0) | <LOQ (4.7)c | 3.1 (7.1)c | <LOQ (3.9) | 3.8 (7.6)c | <LOQ (3.7) |
| Vitamin E, ppm | 32.0 | 48.0 | 58.8 | 36.0 | 78.0 | 77.7 | 133.4 | 143.2 | 111.3 | 127.3 |
| Genistein, ppm | 23.02 | 0.62 | 103.69 | 86.90 | 0.02 | 0.14 | 1.16 | 0.95 | 189.31 | 121.65 |
| Daidzein, ppm | 18.13 | 0.32 | 100.20 | 50.47 | 0.02 | 0.02 | 0.97 | 0.56 | 114.66 | 91.56 |
| Coumestrol, ppm | 2.04 | < 0.5 | 0.81 | 5.88 | < 0.5 | < 0.5 | < 0.5 | < 0.5 | < 0.5 | < 0.5 |
| Zearalenone, ppm | < 0.5 | < 0.5 | < 0.5 | < 0.5 | < 0.5 | < 0.5 | < 0.5 | < 0.5 | < 0.5 | < 0.5 |
| BPA, ppb | 0.93 | 3.64 | 0.77 | 1.57 | 0.46 | 0.52 | 2.94 | 2.20 | 3.13 | 2.22 |
| Arsenic, ppm | 0.10 | 0.16 | 0.06 | 0.10 | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD |
| Lead, ppm | 0.40 | 0.37 | 0.37 | 0.34 | 0.20 | <LOD | <LOD | 0.29 | <LOD | <LOD |
| Mercury, ppm | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD |
| Cadmium, ppm | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD |
| Selenium, ppm | 0.49 | 0.49 | 0.37 | 0.44 | 0.29 | 0.28 | 0.45 | 0.33 | 0.31 | 0.32 |
| Fumonisins, ppb | 78 | 70 | 96 | 58 | 44 | 50 | 101 | 42 | 99 | 124 |
| Aflatoxins (B1, B2, G1, and G2), ppb | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD |
| Malathion, ppb | 74.6 | 609 | 11.9 | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD |
| PCBs, ppb | <LOD | 59.3 | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD |
LODs (limits of detection) = 0.03 ppm (arsenic), 0.20 ppm lead, 0.08 ppm (mercury), 0.1 ppm (cadmium); LOQs (limits of quantitation) = aflatoxins B1, B2, G1, and G2 ranges from 0.025 – 5 ppb; MDL (method detection limits) = 24 ppb (malathion), 3 ppb (PCBs). For coumestrol and zearalenone, 0.5 ppm was set as the limit of concern and samples were spiked with that amount of these compounds. Estimates are provided for samples that had peaks greater than the spike.
Numbers in parentheses in this row are thiamine values obtained in pre-irradiation diet samples. While the LOQ for the thiamine assay is 5 ppm, estimates were reported for samples that had distinct peaks below this level.
Reanalysis of diets frozen for approximately 3 years after diet irradiation by the manufacturer using AOAC Official Methods 942.23, 953.17, 957.17. (Modified) [Official Methods of Analysis of AOAC International (2005), 18th Ed., AOAC INTERNATIONAL, Gaithersburg, Maryland, USA] gave the following values: AIN-93 M, 0.8 ppm; SPC G, 2.4 ppm; SPC-IF G, 2 ppm.
Body weights, food consumption, and F1 pup allocation
Animals were weighed on arrival and weekly thereafter, except for the period when they were paired for mating. Litter weights were recorded by sex on PND 1 (day of birth = PND 0) and body weights of individual pups were recorded on PND 4, 7, and weekly thereafter. Body weights were also collected on the day on which vaginal opening and preputial separation were observed, on the day of glucose challenge, and at removal for necropsy. Food consumption was measured weekly except for the mating period. Food consumption data are approximate, since waste was not accounted for.
For each diet group, the first 20 litters born that met the minimal litter criterion of at least three male and three female pups were continued on the study beyond weaning. Litters with more than 10 pups were randomly culled to 10 on PND 4 with a distribution of five males and five females when possible. At PND 21, one pup per sex per litter was euthanized and the carcass scanned by dual x-ray absorptiometry (DXA). Two other pups per sex per litter were randomly selected to continue on the study after weaning at PND 21. One retained pup of each sex was mated with a non-littermate at 11 – 12 weeks of age to produce F2 litters (breeding or BR arm), and the other pup of each sex underwent glucose challenge and necropsy (GN arm) as described below. F2 litters were treated similarly to the F1 litters, except that there was no minimum litter size requirement and all pups retained after the culling of the litter on PND 4 were euthanized at PND 21.
In some cases, pups from any litters produced above the 20 above or that did not match the litter size criterion were used in the uterotrophic assay (see below) or as sentinels.
Uterotrophic assay
On PND 17, 180 F1 female pups (30 per diet group; maximum two per litter) were weaned and housed up to six per cage within diet groups so that no animals in any given cage were littermates. Starting on PND 17, daily subcutaneous injections of 0, 0.1, 1, 10, or 100 μg/kg body weight (bw)/day ethinyl estradiol (EE2; Sigma product # E4876-10G, lot # 028K1411) in corn oil (Sigma, product # C8267, lot # MKBG0329V) were administered for three consecutive days, with animals in a given cage all receiving the same EE2 dose. On the morning of PND 20, the mice were euthanized and body weights recorded. The uterus was removed as prescribed in OECD Test Guideline 440 (OECD, 2007) and the wet (i.e., uterus and luminal fluid) and blotted (i.e., uterus after expression and blotting of luminal fluid) uterine weights were recorded.
Markers of sexual development and vaginal cytology
Anogenital distance was measured using an ocular micrometer (Delclos et al., 2014) at PND 21 on all F1 pups kept in the study after the PND 21 sacrifice. The anogenital distance measured at weaning has been reported to reflect the intrauterine androgen environment in mice (Vandenbergh and Huggett, 1995; Hotchkiss and Vandenbergh, 2005). Preputial separation and vaginal opening were monitored in F1 pups from PND 22 until occurrence. Vaginal smears were taken on the day of vaginal opening and daily thereafter for 20 consecutive days to determine the time of first estrus.
Glucose challenge
On PND 90 ± 3, one F1 animal per sex per litter (GN arm) had food removed from the cage at 6 AM. Starting at 1 PM, the animals were weighed and a zero-time blood sample collected by a tail vein prick made with a 3 mm lancet. Each animal received an intraperitoneal injection of 2.0 g/kg bw of glucose in 0.9% saline, and blood drops were collected from the tail vein at 15, 30, 45, 60, and 120 minutes after dosing for measurement of glucose levels using a glucometer (Accu-chek Aviva, Roche Diagnostics, Indianapolis, IN). After the final glucose measurement, animals were returned to their cages and continued on the study until necropsy on PND 105 ± 5.
DXA
On PND 21 (F1 and F2 males and females) and PND 105 (F0 male and F1 male and female breeders), animals were sacrificed and analyzed on the same day using a DXA PIXImus densitometer (Lunar GE Medical Systems, Madison, WI). The instrument was calibrated daily using the manufacturer’s phantom mouse. The whole carcasses were scanned in the ventral position, with the limbs held splayed and the tail wrapped around the left side of the body. The head of each mouse was excluded from analysis by placing it outside the X-ray field and/or using the software’s exclusion area.
Necropsy, sperm analyses, and testicular histopathology
F0 breeder males and females were sacrificed after the 10 day mating period or after weaning of litters, respectively. Animals were anesthetized with gaseous carbon dioxide and blood was collected by cardiac puncture into a serum separator tube. The blood was allowed to clot and centrifuged at 1000 × g for 10 minutes. Serum was utilized for the following clinical chemistry measurements: cholesterol, triglycerides, glucose, insulin, leptin, and adiponectin. Clinical chemistry analyses were conducted on an Alfa Wassermann ALERA (West Caldwell, New Jersey), as previously described (Delclos et al., 2014). Leptin concentration was measured using a mouse leptin ELISA kit (Millipore, St. Charles, MO). Plates were read on an ELx800 Universal Microplate Reader (Bio-Tek, Winooski, VT). Insulin was assayed using a “Coat-a-Count” radioimmunoassay (RIA) (Siemens, Los Angeles, CA) and adiponectin was assayed with a mouse adiponectin RIA kit (Millipore, St. Charles, MO). The insulin and adiponectin tubes were then counted on a Wizard2 (PerkinElmer, Shelton, CT) gamma counter. An aliquot of the male serum was also used for isoflavone analyses (Twaddle et al., 2002). For males, the terminal body weight was recorded and the carcass was scanned by DXA for body composition analysis. For females, the uterus was removed and placed in 10% ammonium sulfide for enumeration of implantation sites. The uteri of F0 dams that did not deliver litters or delivered litters with less than 3 pups per sex were also evaluated for implantation sites and non-viable fetuses.
For F1 animals in the GN arm, food, but not water, was removed at 6 AM on the morning of scheduled necropsy on PND 105 ± 5. After a mean fast of 7.6 ± 0.8 (SD) h, animals were anesthetized with gaseous CO2, blood was collected by cardiac puncture, and serum prepared as described above. The serum was used for the following assays: total protein, albumin, urea nitrogen, creatinine, alanine aminotransferase, gamma glutamyl transpeptidase, sorbitol dehydrogenase, aspartate aminotransferase, alkaline phosphatase, total bile acids, glucose, cholesterol, triglycerides, insulin, leptin, and adiponectin. The following organs were removed and weighed: adrenals, brain, prostate (ventral and dorsolateral after fixation), epididymides, kidneys, liver, ovaries, seminal vesicles with coagulating gland, testes, epididymal, ovarian and parametrial (combined), and retroperitoneal fat pads, thyroid (after fixation), and uterus. Paired organs were weighed together, except for testes and epididymides, which were weighed separately because of the sperm evaluations described below. A vaginal smear was taken at the time of necropsy to determine the stage of the estrous cycle; in all diet groups, except the NIH-41 group, there was one animal with a poor smear from which the estrous cycle stage could not be determined. The cycle stage at necropsy was not taken into account in the analyses of the clinical chemistry or organ weights. With the exception of the testes, which were processed for microscopic evaluation, all weighed tissues were fixed in 10% neutral buffered formalin and held as wet tissue. The right testes was removed, fixed in modified Davidson’s fixative, embedded in infiltrating media (Formula R, Leica, Buffalo Grove, IL). Five μm sections were stained with Periodic Acid Schiff’s stain for histological evaluation. The left testis was frozen on dry ice and stored at −80°C for later use in determining testicular spermatid head counts. The left epididymis was dissected away from the testis, weighed, and processed for determination of sperm motility and morphology and total sperm counts as previously described (Delclos et al., 2014).
Statistics
NIH-41, the standard institutional diet at NCTR, was considered the reference control diet for pairwise comparisons among the six diet groups. In addition, exploratory analyses using AIN-93 as the reference control diet for comparisons were conducted within the three diet groups based on the AIN-93 diet (AIN-93, SPC, and SPC-IF). Results of the latter analyses are discussed in the text and summarized in Supplemental Table 1. Analyses were performed separately for females and males and for the BR and GN study arms. Adjustment for multiple comparisons of diet groups to the reference control diet was performed using Dunnett’s method for analysis of variance (ANOVA), analysis of covariance (ANOCOVA), the log-rank test, logistic regression, and Poisson regression; pairwise comparisons of diets to the reference control diet were performed within contrasts. Holm’s method of adjustment was used for Fisher’s exact test. For analyses of repeated time point measures, within-group correlations were modeled using a heterogeneous first-order autoregressive (ARH(1)) correlation structure; for correlation between litter mates, correlation was modeled using a compound symmetric correlation structure. All statistical tests were conducted as two-sided at the 0.05 significance level.
Body weights, food consumption, and metabolic efficiency (g of body weight gained per g of food consumed in a given time period) were analyzed using a repeated measures mixed model ANOVA with terms for diet, week, and interaction. Food consumption outliers were defined as daily average greater than 80% of an animal’s body weight at the end of the week; outliers for average weekly percent metabolic efficiency were identified using Grubb’s test.
Mating success, defined as the proportion of females that littered to number mated, was analyzed using Fisher’s exact test. Pup counts at birth (number alive, number of males, number of females, and number of unsexed) were analyzed using Poisson regression. Litter size was defined as the total number of pups (male, female, and unsexed) born alive. Sex proportions within litters were analyzed using logistic regression. Unsexed pups were assigned as male for the analysis reported, but assignment of the unsexed pups as female did not change the results. For litter weight data, analysis was performed using ANOVA. For mean animal weights, ANOCOVA was performed adjusted for litter size. Numbers of implants and resorptions were analyzed using ANOVA; the number of resorptions was calculated as the number of implants minus the sum of the number of pups born alive and dead. For anogenital distance (AGD), repeated measures mixed model ANOVA was performed for the mean of three measurements of AGD and for anogenital distance index (AGI), defined as the mean of AGD divided by the cube root of body weight.
Age and body weight at vaginal opening for females and at preputial separation for males were analyzed using ANOVA. Daily vaginal swabs were reported as estrus (E), proestrus (P), or diestrus (D), in addition to some reported as indeterminate (E/D, P/E, and D/P). For the analysis, E/D and P/E were categorized as E and D/P as D. Time to first estrus was defined as the first occurrence of an estrus smear during data collection up to 21 days following vaginal opening. Log rank analysis was performed for time to first estrus, with animals considered censored if dead or moribund, or if estrus was unobserved. The distribution of D, E, and P animals at necropsy for each diet compared to the reference control was analyzed using Fisher’s Exact test with Monte Carlo estimation.
Glucose levels at baseline and at 15, 30, 45, 60, and 120 min after the injection of glucose were analyzed using a repeated measures mixed model ANOCOVA with terms for diet group, time, interaction, and covariate baseline glucose level. Area under the curve (AUC) was also calculated using the trapezoidal rule. One male animal was excluded due to an accidental early death.
For clinical chemistries, ANOVA was performed using a nonparametric method with midranks and an unstructured covariance. Measurements beyond detection limits were defined as one-half the lower limit or equal to the upper limit of quantification; samples with insufficient volume for analysis were considered missing.
ANOVA was performed for DXA scan of percent fat of tissue using arcsine square root transformation. In addition, ANOVA was performed for bone mineral density, bone mineral content, bone area, tissue area, and total tissue mass. Organ weights were analyzed using ANOVA for absolute weights. ANOCOVA was performed with covariate brain or terminal body weight in separate analyses. Ovaries with grossly observable cysts were excluded from the ovary weight analysis; in addition, the ovarian weights of one animal in the NIH-41 diet group and one animal in the AIN-93 diet group were excluded because the ovaries had been weighed together with the oviduct. Fisher’s Exact test was performed to compare diet groups to NIH-41 control for the number of these exclusions. For seminiferous tubule degeneration of the testes in the study diet groups compared to the NIH-41 control, Fisher’s Exact test was used for incidence, and Shirley’s method, modified by Williams, was performed for severity scores; tests were conducted as one-sided and no adjustment was made for multiple comparisons.
Results
Diet analyses and serum isoflavones
The results of analyses of diets for the estrogenic substances, macronutrients, and vitamins in the single lots of each irradiated diet used in the study are shown in Table 3. The estrogenic compounds assayed (genistein, daidzein, coumestrol, zearalenone, and BPA) were specific for this study and the other analyses are standard analyses conducted at NCTR for all diets. As expected, the natural ingredient diets contained varying levels of soy-derived isoflavones in the order 5008/5001 > NIH-41> 5K96. Minimal soy isoflavones were reported in the AIN-93 G/M and SPC G/M diets, while SPC-IF had the highest measured isoflavone content of all diets used. Assessment of the serum of F0 breeder males confirmed that only animals fed 5001, SPC-IF, or NIH-41 had measurable levels of circulating isoflavones (Table 4). Coumestrol levels were highest in 5001 and NIH-41. Levels of other measured contaminants varied across diets, but were within tolerances established by the National Toxicology Program (National Toxicology Program, 2011) with the exception of malathion levels in 5K96, which exceeded the 0.5 ppm tolerance by approximately 20%. Low ppb levels of BPA were also found in all diets tested for the present study.
Table 4.
Total isoflavone levels (μM) in serum of F0 adult male micea
| Diet | ||||||
|---|---|---|---|---|---|---|
| NIH-41 | 5K96 | 5001 | AIN-93 | SPC | SPC-IF | |
| n | 17 | 17 | 17 | 17 | 17 | 17 |
| Genistein | 0.07 ± 0.03 | < LOD | 0.56 ± 0.44 | < LOD | < LOD | 0.42 ± 0.20 |
| Daidzein | 0.09 ± 0.06 | < LOD | 0.55 ± 0.29 | < LOD | < LOD | 0.45 ± 0.23 |
| Equol | 0.92 ± 0.36 | < LOD | 3.40 ± 1.75 | < LOD | < LOD | 3.36 ± 2.39 |
Mean ± SD. LOD = 0.03 μM (genistein), 0.02 μM (daidzein), and 0.1 μM (equol). Values presented are total (aglycone plus conjugates) isoflavones measured after enzymatic deconjugation (Twaddle et al., 2002).
Vitamin A and B1 (thiamine) were higher in the natural ingredient diets than in the AIN-93-based diets, while vitamin E levels were higher in the AIN-93-based diets, particularly in the SPC and SPC-IF diets. All vitamins, except thiamine, exceeded NRC recommended levels in all diets (National Research Council, 1995). As shown in Table 3, several of the AIN-93-based diets (AIN-93 G and M, SPC M, SPC-IF M) had thiamine levels that were not detectable by the standard method used for diet thiamine analysis at this institution. After all of the study data had been collected and analyzed, samples of the purified diets were returned to the manufacturer for reanalysis, which confirmed low levels of thiamine in the diets (Table 3, footnote), although the length of time that had elapsed between diet preparation and analysis precludes the exact determination of the thiamine level at the time the diet was fed to the animals.
Body weights, food consumption, and metabolic efficiency
For F0 females, pairwise comparisons to NIH-41 indicated that the only significant difference for the females was at 6 weeks with the mean weight of the 5K96 group higher (6%) than that of the NIH-41 group (Supplemental Figure 1). There were no significant differences between any diet and NIH-41 at any week in pairwise comparisons for F0 males (Supplemental Figure 1).
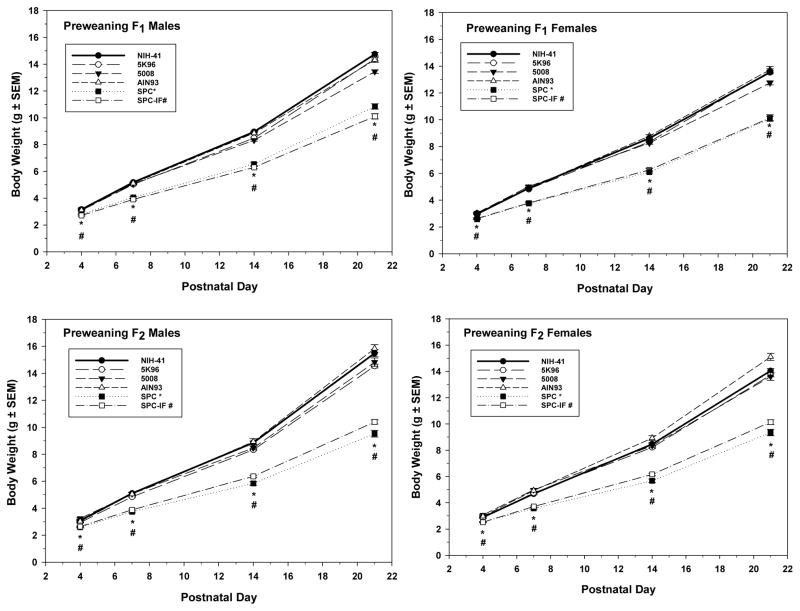
Mean male pup PND 1 weights, adjusted for litter size, were 5% lower in the SPC-IF group relative to the reference NIH-41 group in both the F1 (p = 0.041) and F2 (p = 0.030) generations (Supplemental Table 2). In both generations, body weights of both sexes in the SPC and SPC-IF groups were significantly less than those in the NIH-41 group at PND 4, 7, 14, and 21 (Figure 1). Although no formal statistical comparison was conducted, these weight differences were similar for SPC and SPC-IF groups relative to the other natural ingredient diets. The mean body weights in animals fed SPC or SPC-IF ranged from 11 – 15% lower than those fed NIH-41 at PND 4 and 25 – 31% lower at PND 21 (Figure 1). For the AIN-93-related diet subset, there was no difference in PND 1 weights among the AIN-93, SPC, and SPC-IF groups. At PND 4 and after, both sexes in both the SPC and SPC-IF groups generally had significantly lower mean body weights than the AIN-93 group (Supplemental Table 1).
Figure 1.
Pre-wean pup body weights of F1 (top row) and F2 (bottom row) males (left column) and females (right column) measured at PNDs 4, 7, 14, and 21. Mean weights ± S.E.M. are shown. Inset indicates the line type and symbol associated with each diet and the symbols (*, SPC; #, SPC-IF) used to indicate a significant difference from the NIH-41 diet group in the same week. During this time period, the growth diets were fed in those groups that had both G and M formulations.
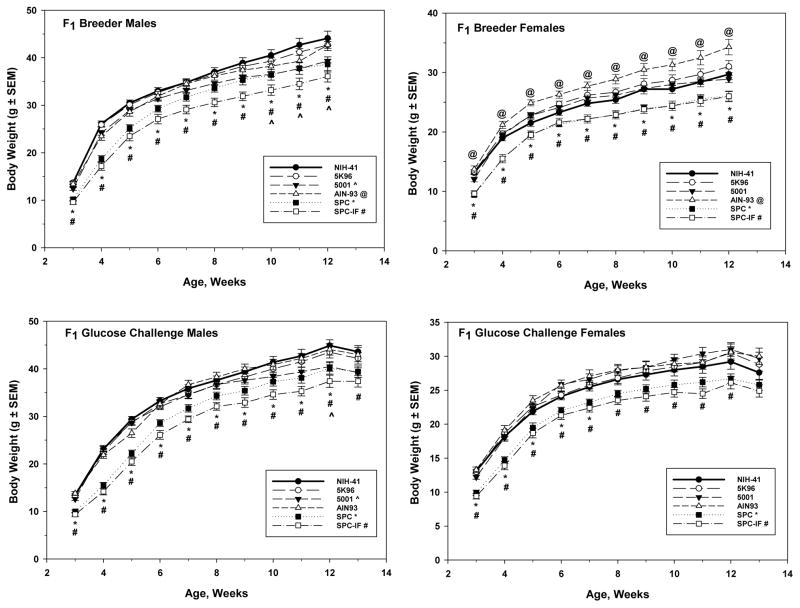
After weaning, the animals that were assigned to the BR and GN arms were analyzed separately, with similar results in both study arms. As with the pre-weaning animals, the body weights of the SPC and SPC-IF groups were lower than those of the NIH-41 reference animals during the post-weaning period of both sexes (Figure 2). The animals in the SPC-IF group had the lightest body weights, ranging from approximately 30% lighter than the NIH-41 group in the week after weaning to approximately 10 – 18% at week 12 (BR arm) or 13 (GN arm). For both pre- and post-wean animals, the analysis of the modified AIN-93 diet subsets showed similar significant differences for SPC and SPC-IF groups versus the AIN-93 control group, with the SPC and SPC-IF animals having lower body weights than the AIN-93 group (Supplemental Table 1). Males in the 5001 group had mean body weights that were 10 – 12% lighter than the reference NIH-41 group animals at weeks 10, 11, and 12 for the BR arm (Figure 2, top left) and at week 12 in the GN arm (Figure 2, bottom left). For the F1 BR arm, the AIN-93 group females had a higher body weight (11 – 15%) than NIH-41 animals during the post-weaning period (Figure 2, top right).
Figure 2.
Post-wean F1 male (left column) and female (right column) weekly body weights for the breeder (top row) and glucose challenge (bottom row) study arms. For the breeder animals, weights recorded from the time of weaning to the time of pairing for mating are shown. For the glucose challenge animals, weights recorded from the time of weaning through the glucose challenge study are shown. All data are mean weights ± S.E.M. Inset indicates the line type and symbol associated with each diet and the symbols (^, 5001; @, AIN-93; *, SPC; #, SPC-IF) used to indicate a significant difference from the NIH-41 diet group in the same week.
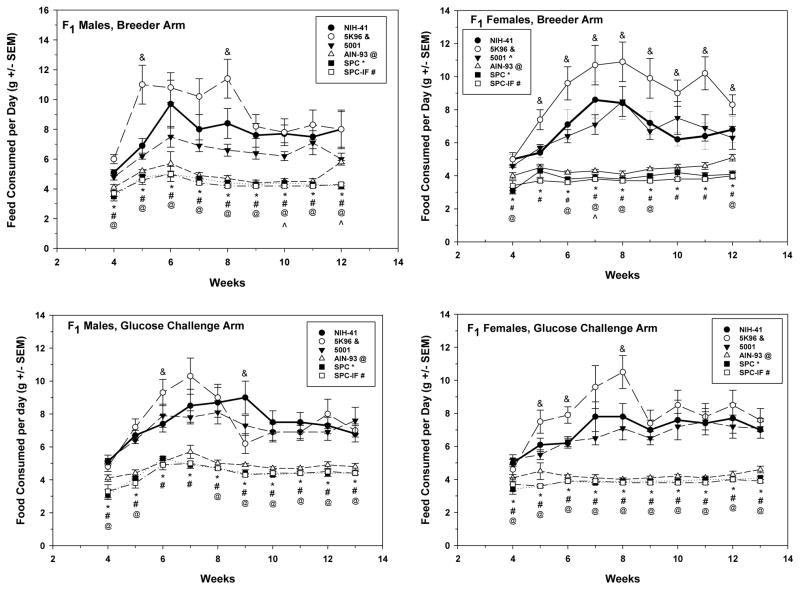
Food consumption was measured only for the F1 post-weaning animals and similar results were seen in both the BR and GN study arms. Food consumption was lower than that in the NIH-41 group for the AIN-93, SPC, and SPC-IF groups in both sexes (Figure 3). Consumption in the 5K96 group was higher (24 – 59%) in females in all weeks after week 4 in the BR arm (Figure 3, top right) and in weeks 5, 6, and 8 in the GN arm (Figure 3, bottom right). Males did not show a consistent difference in food consumption for 5K96 versus the NIH-41 control, with higher consumption in week 6 and lower consumption in week 9 in the GN arm (Figure 3, bottom left) and higher consumption in weeks 5 and 8 in the BR arm (Figure 3, top left). For the AIN-93-related diet subset, females and males in both the SPC and SPC-IF groups in both the GN and BR study arm had significantly lower food consumption than the AIN-93 group over the course of the study, although the numerical differences were less than those between these diets and NIH-41 (Figure 3 and Supplemental Table 1). Food consumption data were also plotted as calories consumed, since the diets differed in energy density, with similar results to those of grams consumed (Supplemental Figure 2).
Figure 3.
Post-wean F1 male (left column) and female (right column) mean daily food consumption per week for the breeder (top row) and glucose challenge (bottom row) study arms. For the breeder animals, food consumption data from the week after weaning to the time of pairing for mating are shown. For the glucose challenge animals, food consumption data from the week after weaning through the glucose challenge study are shown. All data are mean weights ± S.E.M. Inset indicates the line type and symbol associated with each diet and the symbols (&, 5K96; ^, 5001; @, AIN-93*, SPC; #, SPC-IF) used to indicate a significant difference from the NIH-41 diet group in the same week.
Metabolic efficiency, while variable over the course of the study, was generally numerically higher for AIN-93, SPC, and SPC-IF relative to NIH-41, and was higher in all significant comparisons (Supplemental Figure 3). For the AIN-93-related diet subset, significant differences in metabolic efficiencies were observed in a few specific weeks that varied by sex and study arm (Supplemental Table 1).
F0 and F1 mating and litter summaries
Mating success of the F0 and F1 generations and basic litter parameters are shown in Supplemental Table 2. There were no statistically significant differences in the proportion of mated pairs producing litters, implant sites, resorptions, litter size, or pup sex ratio in either generation. The percentage of mated pairs producing litters ranged from 83 – 97% in all diet groups in both generations, except in the F1 AIN-93 group, where it was 70%; however, this decrease was not significantly different from the NIH-41 diet control.
Markers of sexual development and estrous cycle
A summary of the markers of pubertal development measured in both study arms is shown in Table 5. Statistically significant effects on the timing of vaginal opening were confined to the SPC diet group in the BR arm (1.9 day delay). The difference of 0.9 days in the GN study arm was in the same direction, but it was not statistically significant. The body weight at vaginal opening was significantly lower in the SPC and SPC-IF diet groups, consistent with the generally lighter body weights of the animals in these groups reported above. In both study arms, the time of first estrus was delayed relative to that in the NIH-41 diet group in the 5001, SPC, and SPC-IF groups. The time of first estrus was significantly less than that in the NIH-41 group in the AIN-93 group in the BR arm, but not in the GN arm, where the median time of first estrus was numerically later than that in the NIH-41 group. All smears in the breeding group for 21 days were read regardless of the time that the first estrus smear was detected and evaluated for cycle parameters; however, the majority of mice did not show regular cycles within this time period, consistent with previous reports that regular cycles in mice can take weeks to become established after vaginal opening and first vaginal estrus are observed (Nelson et al., 1990). The results of these analyses, which indicated limited statistical differences across diets, are not shown. The distributions of D/E/P animals at necropsy were as follows: NIH-41, 8/12/0; 5K96, 9/9/1; 5001, 13/4/2; AIN-93, 9/8/2; SPC, 12/5/2; SPC-IF, 10/7/2. These proportions did not differ significantly in comparisons of diets to the reference control.
Table 5.
| Endpoint | Diet | |||||
|---|---|---|---|---|---|---|
| NIH-41 | 5K96 | 5001 | AIN-93 | SPC | SPC-IF | |
| Males, breeder arm | ||||||
| n | 20 | 20 | 20 | 19 | 19 | 20 |
| Day of PPS, PND | 29.4 ± 0.4 | 29.0 ± 0.3 | 29.6 ± 0.6 | 28.0 ± 0.4 | 29.3 ± 0.6 | 29.8 ± 0.5 |
| Weight at PPS, g | 26.0 ± 0.6 | 25.7 ± 0.7 | 24.5 ± 0.6 | 22.4 ± 0.8* | 18.2 ± 0.9* | 17.6 ± 0.8* |
| Males, glucose challenge arm | ||||||
| n | 20 | 20 | 19 | 18 | 20 | 19 |
| Day of PPS, PND | 28.1 ± 0.3 | 27.8 ± 0.3 | 28.2 ± 0.2 | 28.1 ± 0.3 | 27.8 ± 0.4 | 29.5 ± 0.3* |
| Weight at PPS, g | 24.0 ± 0.6 | 23.4 ± 0.6 | 23.5 ± 0.6 | 22.9 ± 0.6 | 16.0 ± 0.6* | 16.3 ± 0.6* |
| Females, breeder arm | ||||||
| n | 19 | 19 | 19 | 20 | 20 | 20 |
| Day of VO, PND | 25.0 ± 0.4 | 25.2 ± 0.5 | 25.8 ± 0.5 | 25.0 ± 0.3 | 26.9 ± 0.4* | 25.0 ± 0.3 |
| Weight at VO, g | 16.6 ± 0.5 | 17.0 ±0.5 | 17.1 ± 0.4 | 17.9 ± 0.5 | 14.0 ± 0.4* | 12.3 ± 0.5* |
| Time to first estrus, Median PND (95% CI) | 32 (NA) | 33 (32 – 33) | 34 (34 – 36)* | 30 (29 – 30)* | 36 (NA)* | 33 (33 – 34)* |
| Females, glucose challenge arm | ||||||
| n | 20 | 20 | 20 | 20 | 20 | 20 |
| Day of VO, PND | 25.5 ± 0.4 | 25.5 ± 0.4 | 25.5 ± 0.4 | 24.7 ± 0.3 | 26.4 ± 0.4 | 24.7 ± 0.3 |
| Weight at VO, g | 17.9 ± 0.5 | 17.9 ± 0.5 | 17.1 ± 0.4 | 17.5 ± 0.5 | 14.0 ± 0.6* | 12.2 ± 0.5* |
| Time to first estrus, Median PND (95% CI) | 37 (35 – NA) | 34 (33 – 36) | 42 (42 – NA)* | 40 (34 – NA) | 42 (40 – 44)* | 42 (38 – 43)* |
NA, 95% confidence interval (CI) could not be calculated due to right censoring.
Mean counts ± S.E.M., except time to first estrus.
Shaded cell indicates statistically significant difference from NIH-41 diet group ;
p < 0.05.
AGD and AGDI were measured in all retained pups at PND 21. There were no AGD differences among diet groups that were not explained by the body weight reductions in the SPC and SPC-IF groups, as indicated by the lack of statistically significant differences for the AGDI (Supplemental Table 3).
Uterotrophic assay
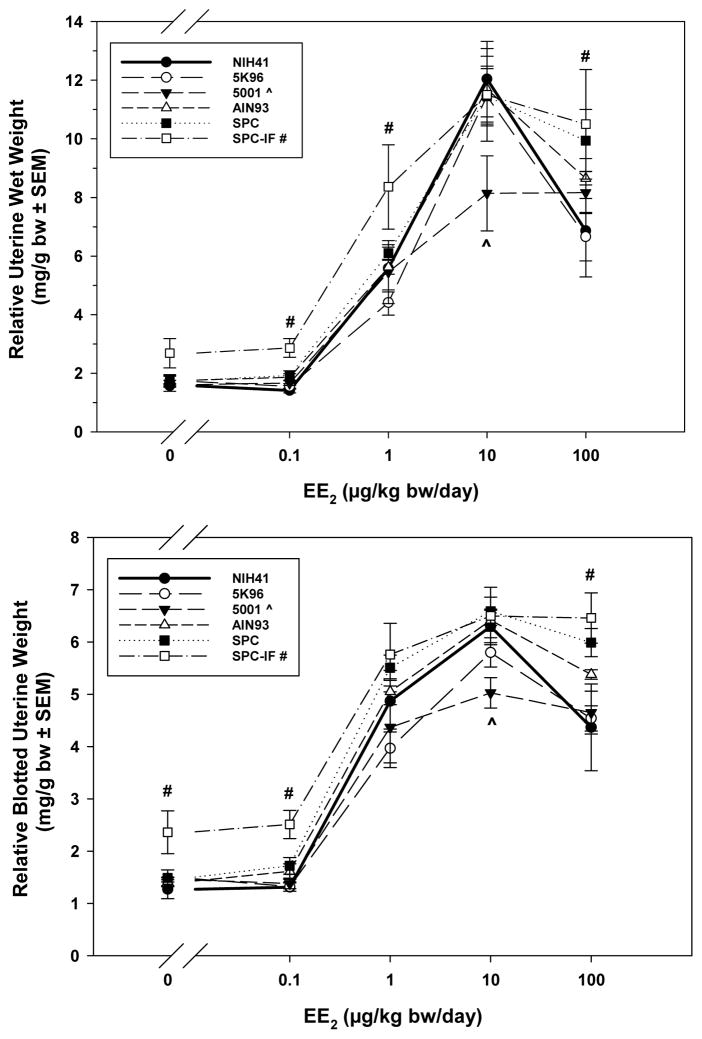
Data from the uterotrophic assay were analyzed based on both absolute and body-weight adjusted uterine wet and blotted weights. Body weight-adjusted results are discussed because of the significant effect of the SPC and SPC-IF diets on body weight. Both wet and blotted uterine weight relative to body weight (Figure 4, top and bottom, respectively) showed an increase over the corn oil control at 1 μg EE2/kg bw/day and above. For the SPC-IF group, the wet uterine weight was significantly higher than the NIH-41 control at 0.1, 1, and 100 μg EE2/kg bw/day; similarly, the blotted uterine weights were significantly higher than the NIH-41 control at 0, 0.1, and 100 μg EE2/kg bw/day. Both wet and blotted uterine weights were lower in 5001 than that in the control diet group at 10 μg EE2/kg bw/day.
Figure 4.
Uterine weights relative to body weights for PND 20 F1 females treated with three consecutive i.p. doses of EE2 (0, 0.1, 1, 10, or 100 μg/kg bw/day) from PND 17. Mean weights ± S.E.M. are shown. Inset indicates the line type and symbol associated with each diet and the symbols (^, 5001; #, SPC-IF) used to indicate a significant difference from the NIH-41 diet group at the same dose of EE2.
Glucose tolerance
The baseline glucose level for the AIN-93 diet was 30% higher compared to the NIH-41 diet for males; however, there were no significant differences in post-baseline glucose levels, adjusted for baseline, between any of the diet groups compared to the NIH-41 diet for either females or males (Supplemental Figure 4). In addition, there were no differences across diets in the area-under-the-blood-glucose*time curve (Supplemental Table 4).
Clinical chemistry
Selected clinical chemistry measurements are shown in Figure 5; terminal blood was collected from unfasted F0 animals, while F1 animals were fasted for approximately 7 hours.
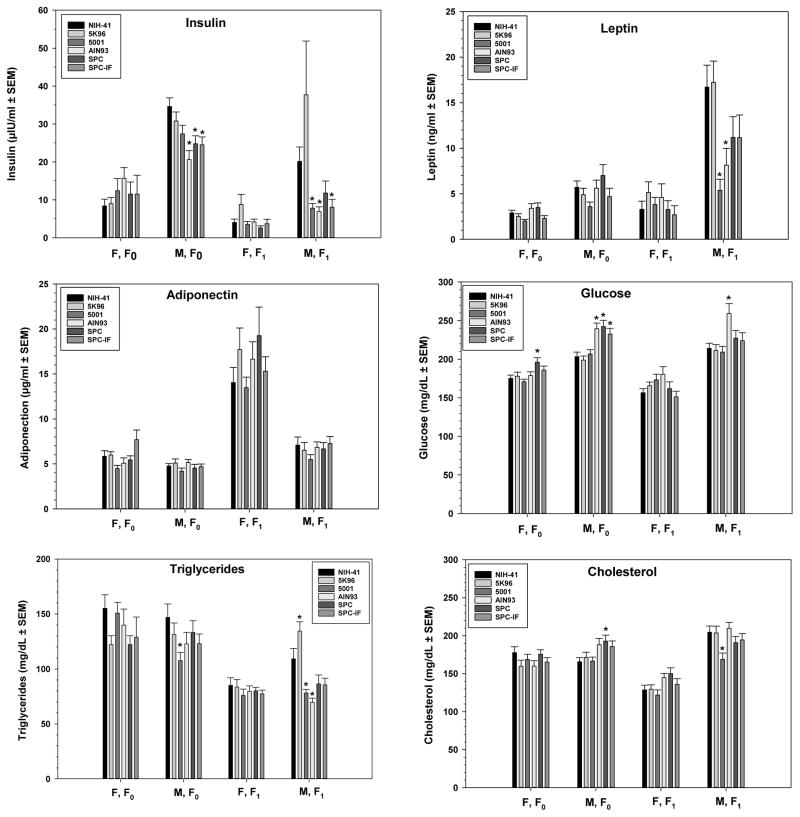
Figure 5.
Serum clinical chemistry measurements made in both F0 and F1 adult males and females. Serum samples were collected from unfasted F0 females and males and fasted (approximately 7 hours) F1 females and males at necropsy. Mean ± S.E.M. are shown. There were 20 animals for all endpoints except for the following groups of females: 5001, n =19 for insulin; SPC, n = 19 for leptin, n = 18 for insulin; SPC-IF, n = 19 for leptin and insulin. Inset indicates the bar type associated with each diet. Asterisks (*) indicate a significant difference from the NIH-41 diet group within the same generation and sex.
In F0 males, the AIN-93, SPC, and SPC-IF diet groups had significantly elevated glucose levels (18, 19, and 14%, respectively) and depressed insulin levels (40, 28, and 29%, respectively) relative to those in the NIH-41 diet. Cholesterol was elevated by 16% in the SPC group and triglycerides depressed by 26% in the 5001 group relative to levels in the NIH-41 diet group. For females, the only statistically significant difference in the clinical chemistry parameters measured was a 12% increase in glucose in the SPC group relative to levels in the NIH-41 animals.
For F1 animals, significant differences between the NIH-41 control diet and the other diets for the analytes shown in Figure 5 were confined to males. In AIN-93 males, glucose was elevated 21%, while insulin, leptin, and triglycerides were decreased by 66%, 51%, and 36%, respectively. The insulin levels in SPC-IF were decreased by 60%, but glucose, leptin, and triglycerides were not significantly different from the control diet level. None of these parameters differed in the SPC diet versus the NIH-41 diet. In the 5001 group, cholesterol, triglycerides, leptin, and insulin were all significantly lower than NIH-41 levels by 18%, 28%, 68%, and 61%, respectively. Triglycerides were increased by 23% in the 5K96 diet compared to NIH-41 diet. There were few statistically significant changes in the other serum components measured (Supplemental Tables 5 and 6 for males and females, respectively). All three of the AIN-93-related diet groups had reduced alanine aminotransferase levels relative to the NIH-41 control in both sexes.
Organ weights
Selected organ weights recorded at necropsy are shown for males and females in Tables 6 and 7, respectively. Other recorded organ weights are listed in Supplemental Tables 7 (males) and 8 (females). The percentage differences mentioned in this paragraph are based on the least squares means generated by the statistical model. In males, brain, kidney, testes, and thyroid gland showed significant differences across diets. In females, significant diet effects were confined to the kidney and thyroid gland. Male brain weight, corrected for body weight, in the SPC-IF group was 12% higher than that in the NIH-41 group. Kidney weights were lower than those in the NIH-41 group in the AIN-93, SPC, and SPC-IF groups in both males (9%, 19%, and 20%, respectively) and females (11%, 21%, and 26%, respectively). In males, thyroid weights were significantly lower than those in the NIH-41 group in the AIN-93, SPC, and SPC-IF groups (29%, 33%, and 30%, respectively). In females, only the SPC-IF diet group had a significantly different thyroid to body weight ratio (35% lower). Mean thyroid weights were numerically lower than the NIH-41 control mean in the 5001, AIN-93, and SPC diet groups, but these differences were not statistically significant. Testes weight in the AIN-93 group was significantly reduced compared to the mean weight in the NIH-41group (21% less). Summaries of the significant results from the analysis of organ weight adjusted for body weight in the AIN-93-related diet subset are shown in Supplemental Table 1. In both sexes, kidney weights were lower in the SPC and SPC-IF diet groups compared to the AIN-93 group, while liver weight was lower in both sexes in the SPC group and in females in the SPC-IF group. Testes weights were significantly higher in the SPC and SPC-IF groups than in the AIN-93 group.
Table 6.
Adult F1 Male Organ Weights (Absolute, Relative to Brain Weight, and Relative to Terminal Body Weight)a
| Organ | Diet | |||||
|---|---|---|---|---|---|---|
| NIH-41 | 5K96 | 5001 | AIN-93 | SPC | SPC-IF | |
| Body Weight (g) | 45.80 ± 1.382 | 44.16 ± 1.267 (19) | 41.18 ± 1.122 | 43.03 ± 1.441 | 41.06 ± 1.224* | 38.50 ± 1.395** |
| Brain | ||||||
| Absolute (g) | 0.542 ± 0.006 | 0.525 ± 0.007 | 0.525 ± 0.004 | 0.531 ± 0.005 | 0.520 ± 0.004* | 0.507 ± 0.007*** |
| Rel to Body (mg/g) | 12.04 ± 0.375 | 12.05 ± 0.383 | 12.87 ± 0.282 | 12.57 ± 0.404 | 12.88 ± 0.383 | 13.46 ± 0.435** |
| Retroperitoneal Fat Pad | ||||||
| Absolute (g) | 0.635 ± 0.062 | 0.641 ± 0.032 | 0.414 ± 0.041* | 0.520 ± 0.083 | 0.501 ± 0.062 | 0.447 ± 0.052 |
| Rel. to Brain (g/g) | 1.169 ± 0.114 | 1.228 ± 0.067 | 0.785 ± 0.076 | 0.979 ± 0.157 | 0.961 ± 0.118 | 0.881 ± 0.105 |
| Rel. to Body (mg/g) | 13.45 ± 1.052 | 14.47 ± 0.607 | 9.781 ± 0.829 | 11.38 ± 1.481 | 11.66 ± 1.226 | 11.08 ± 0.919 |
| Kidney | ||||||
| Absolute (g) | 0.684 ± 0.022 (19) | 0.665 ± 0.020 | 0.677 ± 0.023 | 0.606 ± 0.014* | 0.521 ± 0.017*** | 0.490 ± 0.017*** |
| Rel. to Brain (g/g) | 1.266 ± 0.041 | 1.276 ± 0.048 | 1.289 ± 0.040 | 1.142 ± 0.026* | 1.002 ± 0.033*** | 0.971 ± 0.037*** |
| Rel. to Body (mg/g) | 15.21 ± 0.509 | 15.10 ± 0.302 | 16.45 ± 0.362 | 14.28 ± 0.433* | 12.74 ± 0.326*** | 12.87 ± 0.388*** |
| Liver | ||||||
| Absolute (g) | 1.890 ± 0.089 | 2.044 ± 0.113 | 1.762 ± 0.049 (19) | 1.896 ± 0.128 | 1.597 ± 0.076 | 1.493 ± 0.071* |
| Rel. to Brain (g/g) | 3.484 ± 0.160 | 3.917 ± 0.230 | 3.363 ± 0.086 | 3.571 ± 0.235 | 3.065 ± 0.139 | 2.951 ± 0.150 |
| Rel. to Body (mg/g) | 41.03 ± 1.001 | 45.77 ± 1.332 | 42.83 ± 0.658 | 43.43 ± 1.591 | 38.67 ± 0.990 | 38.71 ± 0.913 |
| Testes | ||||||
| Absolute (g) | 0.256 ± 0.006 | 0.241 ± 0.008 | 0.269 ± 0.010 | 0.198 ± 0.015*** | 0.236 ± 0.006 | 0.243 ± 0.007 |
| Rel. to Brain (g/g) | 0.472 ± 0.009 | 0.461 ± 0.016 | 0.512 ± 0.018 | 0.372 ± 0.028*** | 0.454 ± 0.011 | 0.480 ± 0.014 |
| Rel. to Body (mg/g) | 2.834 ± 0.085 | 2.712 ± 0.126 | 3.303 ± 0.114 | 2.312 ± 0.194*** | 2.911 ± 0.099 | 3.218 ± 0.161 |
| Thyroid Gland | ||||||
| Absolute (g) | 0.006 ± 0.0 | 0.006 ± 0.0 (17) | 0.005 ± 0.001 | 0.004 ± 0.0** | 0.004 ± 0.0** | 0.004 ± 0.0** |
| Rel. to Brain (g/g) | 0.011 ± 0.001 | 0.011 ± 0.001 | 0.010 ± 0.001 | 0.008 ± 0.0** | 0.008 ± 0.0** | 0.008 ± 0.0* |
| Rel. to Body (mg/g) | 0.135 ± 0.011 | 0.137 ± 0.009 | 0.129 ± 0.015 | 0.101 ± 0.006** | 0.100 ± 0.007** | 0.111 ± 0.007** |
Mean counts ± S.E.M., n = 20 except were indicated by numbers in parentheses.
Shaded cell indicates statistically significant difference from NIH-41 diet group;
p < 0.05,
p < 0.01,
p < 0.001.
Table 7.
Adult F1 Female Organ Weights (Absolute, Relative to Brain Weight, and Relative to Terminal Body Weight)a
| Organ | Diet | |||||
|---|---|---|---|---|---|---|
| NIH-41 | 5K96 | 5001 | AIN-93 | SPC | SPC-IF | |
| Body Weight (g) | 29.37 ± 0.95 | 29.60 ± 0.87 | 31.17 ± 1.06 | 30.15 ± 1.20 | 26.94 ± 0.80 | 26.24 ± 0.81 |
| Kidney | ||||||
| Absolute (g) | 0.418 ± 0.011 | 0.443 ± 0.010 | 0.452 ± 0.013 | 0.376 ± 0.010* | 0.315 ± 0.009*** | 0.292 ± 0.008*** |
| Rel. to Brain (g/g) | 0.783 ± 0.019 | 0.821 ± 0.018 | 0.849 ± 0.025 | 0.711 ± 0.016* | 0.603 ± 0.019*** | 0.569 ± 0.017*** |
| Rel. to Body (mg/g) | 14.36 ± 0.358 | 15.12 ± 0.440 | 14.65 ± 0.409 | 12.67 ± 0.374** | 11.80 ± 0.373*** | 11.19 ± 0.253*** |
| Liver | ||||||
| Absolute (g) | 1.215 ± 0.049 | 1.266 ± 0.051 | 1.326 ± 0.047 | 1.314 ± 0.072 | 1.053 ± 0.044 | 1.050 ± 0.029 |
| Rel. to Brain (g/g) | 2.282 ± 0.097 | 2.350 ± 0.099 | 2.485 ± 0.080 | 2.491 ± 0.140 | 2.012 ± 0.081 | 2.051 ± 0.067 |
| Rel. to Body (mg/g) | 41.40 ± 1.076 | 42.81 ± 1.296 | 42.65 ± 0.784 | 43.25 ± 0.795 | 39.02 ± 1.029 | 40.29 ± 0.922 |
| Ovary | ||||||
| Absolute (g) | 0.016 ± 0.001 (18)b | 0.018 ± 0.001 (11) | 0.016 ± 0.001 (14) | 0.017 ± 0.002 (8) | 0.013 ± 0.001 (8) | 0.015 ± 0.001 (16) |
| Rel. to Brain (g/g) | 0.030 ± 0.001 | 0.034 ± 0.002 | 0.030 ± 0.002 | 0.032 ± 0.004 | 0.024 ± 0.001 | 0.028 ± 0.002 |
| Rel. to Body (mg/g) | 0.549 ± 0.033 | 0.617 ± 0.044 | 0.520 ± 0.038 | 0.542 ± 0.083 | 0.463 ± 0.023 | 0.554 ± 0.033 |
| Thyroid Gland | ||||||
| Absolute (g) | 0.006 ± 0.0 | 0.006 ± 0.001 | 0.005 ± 0.0 | 0.005 ± 0.001 | 0.004 ± 0.0 | 0.004 ± 0.0* |
| Rel. to Brain (g/g) | 0.011 ± 0.001 | 0.010 ± 0.001 | 0.009 ± 0.001 | 0.009 ± 0.001 | 0.008 ± 0.001 | 0.007 ± 0.0* |
| Rel. to Body (mg/g) | 0.196 ± 0.015 | 0.195 ± 0.021 | 0.156 ± 0.012 | 0.160 ± 0.026 | 0.166 ± 0.014 | 0.142 ± 0.008* |
Mean counts ± S.E.M., n = 20 except were indicated by numbers in parentheses.
Shaded cell indicates statistically significant difference from NIH-41 diet group;
p < 0.05,
p < 0.01,
p < 0.001.
Only weights of ovaries without macrocysts were used in the analysis; in addition, the ovarian weights of one animal in the NIH-41 diet group and one animal in the AIN-93 diet group were excluded because the ovaries had been weighed together with the oviduct. The original sample size for ovary weight was 20 per diet group, except for the 5001 diet group where sample size was 19.
While there were no diet-related differences in ovary weights, grossly observable cysts led to the exclusion of many ovaries from weighing at the time of necropsy, and the percentage of animals affected was significantly different across diets (Table 7). The 5K96, AIN-93, and SPC diet groups had 9 of 20 (45%), 11 of 20 (55%), and 12 of 20 (60%), respectively, excluded for ovarian cysts compared to 1 of 20 (5%) in the NIH-41 group. All of these low isoflavone diet groups differed significantly from the NIH-41 control (p = 0.025, 0.005, and 0.002, for 5K96, AIN-93, and SPC, respectively). Histological examination of the ovaries confirmed an association between the gross cysts and bursal cysts (data not shown).
DXA scans
DXA scans were conducted on the carcasses of F0 males, F1 and F2 PND 21 weans, and F1 males and females from the BR arm. The results for percent body fat are shown in Table 8. PND 21 females and males in the AIN-93 diet group had significantly increased percent body fat in both the F1 (13–14% higher) and F2 (28–30% higher) generations compared to the NIH-41 diet group. There were no statistically significant differences between any of the diets and the NIH-41 control in percent fat in F0 adult males or F1 adult males or females, except for F1 adult females fed SPC-IF. The DXA software also calculated bone area, bone mineral content, bone mineral density, total area, and total tissue mass (Supplemental Tables 9 and 10 for males and females, respectively).
Table 8.
DXA evaluation of percent fat in weanling and adult male and female micea
| Fat % | ||||||
|---|---|---|---|---|---|---|
| NIH-41 | 5K96 | 5001 | AIN-93 | SPC | SPC-IF | |
| Males | ||||||
| F0breeders (PND 105) | 15.29 ± 0.66 (30) | 15.20 ± 0.66 (30) | 13.47 ± 0.54 (30) | 16.81 ± 0.87 (30) | 17.16 ± 0.86 (29) | 15.11 ± 0.68 (30) |
| F1 (PND 21) | 11.59 ± 0.29 (20) | 11.18 ± 0.31 (20) | 10.84 ± 0.24 (20) | 13.25 ± 0.43** (20) | 11.74 ± 0.28 (20) | 11.71 ± 0.30 (20) |
| F1 breeders (PND 105) | 22.97 ± 1.27 (20) | 22.12 ± 1.07 (20) | 19.08 ± 0.54 (20) | 25.36 ± 1.14 (20) | 24.18 ± 1.32 (19) | 19.70 ± 1.18 (19) |
| F2 weanlings (PND 21) | 11.86 ± 0.28 (16) | 12.16 ± 0.25 (17) | 11.99 ± 0.25 (14) | 15.25 ± 0.52*** (12) | 11.89 ± 0.45 (13) | 11.94 ± 0.23 (16) |
| Females | ||||||
| F1 weanlings (PND 21) | 11.37 ± 0.29 (20) | 11.15 ± 0.27 (20) | 10.61 ± 0.22 (20) | 12.80 ± 0.31** (20) | 12.16 ± 0.30 (20) | 11.32 ± 0.23 (19) |
| F1 breeders (PND 105) | 18.96 ± 0.94 (16) | 19.58 ± 1.25 (17) | 16.38 ± 0.84 (14) | 22.92 ± 1.35 (12) | 16.26 ± 0.82 (13) | 15.21 ± 0.82* (16) |
| F2 weanlings (PND 21) | 11.85 ± 0.33 (16) | 11.68 ± 0.23 (17) | 11.95 ± 0.26 (14) | 15.36 ± 0.61*** (12) | 12.21 ± 0.38 (13) | 11.61 ± 0.30 (16) |
Mean ± S.E.M., n is given in parentheses in each cell.
Shaded cell indicates statistically significant difference from NIH-41 diet group ;
p < 0.05,
p < 0.01,
p < 0.001.
Sperm evaluation and testicular histopathology
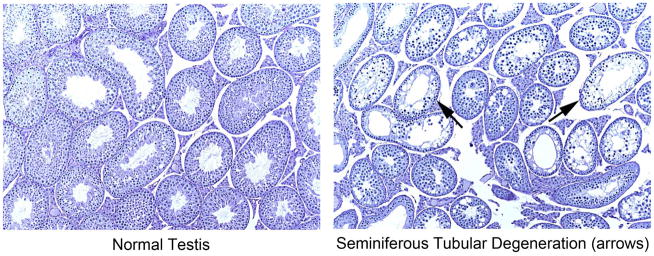
No significant differences in F1 caudal sperm motility or morphology were found between any diet group and the NIH-41 group (data not shown). Testicular spermatid head counts were significantly lower in the AIN-93 group than in the NIH-41 group, but caudal epididymal sperm counts did not differ among diet groups (Table 9). Analysis of the AIN-93-related diet subset indicated that both SPC and SPC-IF groups had significantly higher mean testicular spermatid head counts than the AIN-93 group (Supplemental Table 1). Data from the microscopic evaluation of the testes are summarized in Table 10 and Figure 6. In the AIN-93 diet group, there was a 65% incidence of seminiferous tubule degeneration characterized by degenerate and necrotic spermatogenic cells with reduced numbers of germinal epithelial cells and with multinucleated giant cells. In some areas, the germ cells were markedly depleted and only flattened Sertoli cells remained. Sixty-two percent (8 or 13) of the affected animals had a marked (grade 4, the highest grade) lesion severity. Low incidences (5 – 10%) of minimal severity (grade 1) lesions were seen in the NIH-41, 5K96, 5001, and SPC diet groups and were considered background.
Table 9.
Caudal sperm and testicular spermatid head counts (millions per gram tissue)a
| Endpoint | Diet | |||||
|---|---|---|---|---|---|---|
| NIH-41 | 5K96 | 5001 | AIN-93 | SPC | SPC-IF | |
| n | 20 | 19 | 20 | 20 | 20 | 20 |
| Cauda epididymis sperm count | 897.4 ± 84.6 | 811.5 ± 86.1 | 732.9 ± 54.0 | 798.7 ± 62.8 | 868.6 ± 80.8 | 914.9 ± 63.7 |
| Testicular spermatid head count | 105.8 ± 5.8 | 96.9 ± 6.6 | 103.6 ± 6.2 | 77.5 ± 9.6* | 108.5 ± 6.3 | 117.4 ± 6.0 |
Mean counts ± S.E.M.
Shaded cell indicates statistically significant difference from NIH-41 diet group;
p < 0.05.
Table 10.
Incidences of seminiferous tubule degeneration in the testes of F1 adult CD-1 mice fed the various study diets
| Lesion | Diet | |||||
|---|---|---|---|---|---|---|
| NIH-41 | 5K96 | 5001 | AIN-93 | SPC | SPC-IF | |
| Testes, Semiferous tubule degeneration | ||||||
| Incidencea | 1/20 | 1/19 | 2/20 | 13/20 | 1/20 | 0/20 |
| Severity profileb | 1/0/0/0 | 1/0/0/0 | 2/0/0/0 | 2/2/1/8 | 1/0/0/0 | 0/0/0/0 |
Incidence is given as the number of affected animals/total animals examined.
Severity profile is given as the number of affected animals in the group showing minimal/mild/moderate/marked lesions.
Figure 6.
Micrographs of normal (left panel) and affected (right panel) testes of F1 adult animals in the AIN-93 diet group, ×10. The affected testes shown were scored as having grade 4 (marked) seminiferous tubule degeneration.
Discussion
The primary purpose of this study was to assess the suitability of a soy- and alfalfa-free modified natural ingredient diet for use in toxicology studies with CD-1 mice. The study was initiated in light of reports of both low and high soy diets interfering with evaluations of estrogenic agents or directly inducing adverse effects (Cederroth et al., 2007; Cederroth et al., 2010; Ruhlen et al., 2008). Uncertainty with regard to the soy components that are responsible for various soy diet-related effects prompted us to investigate the purified ingredient diet AIN-93 and modifications of that diet with casein, the sole protein source in that diet, replaced with an ethanol washed soy protein concentrate with or without added isoflavones. As indicated previously, the soy- and alfalfa-free modified natural ingredient diet 5K96 was tested because it has been the diet used for several rat studies of chemicals with estrogenic activity at this institution (Delclos et al., 2014; Delclos et al., 2009; National Toxicology Program, 2008a, b, 2010a, b) and because it was reported to induce a neonatal estrogenization-like metabolic syndrome in CD-1 mice (Ruhlen et al., 2008). NIH-41, an irradiated version of NIH-31, was tested because it is the formulation on which 5K96 is based, with soy and alfalfa meals replaced by casein, so it is nutritionally equivalent to NIH-41, but has a lower soy content than diets to which soy-free diets have been compared in previous mouse studies (e.g., Cederroth et al., 2007; Cederroth et al., 2008; Cederroth et al., 2010; Ruhlen et al., 2008). 5008 and 5001, a G/M combination of diets, were used because they are the comparison diets used by Ruhlen et al. (2008) and they have a variable, but relatively high, phytoestrogen content (Brown and Setchell, 2001; Thigpen et al., 2004). Under the conditions of this study, where feeding of a single lot of 5K96 was started shortly after weaning of the F0 generation and continued throughout the study, we did not observe significant differences between the 5K96 group and the NIH-41 group with regard to body weight, fat pad weights, serum leptin, adiponectin, insulin, triglycerides, total cholesterol or glucose, response to glucose or estrogen challenges, sperm parameters in F1 animals, or reproduction over two generations. Although no formal statistical comparisons were conducted comparing the 5K96 diet group to the 5008/5001 diet group, no marked differences were apparent in the means of these endpoints between these groups. It should be noted that the specific lots of 5008 and 5001 used in this study had lower chemically measured isoflavone levels (Table 3) than have been reported previously (Brown and Setchell, 2001; Thigpen et al., 2004). The level of phytoestrogens in the diet could influence the observed effects. For example, Lephart et al. (2004) reported lower post-weaning body weights and serum leptin and insulin levels in Long Evans rat pups exposed to a soy-containing diet with 600 ppm isoflavones from conception compared to rats exposed similarly to a low phytoestrogen diet. Our results show no major differences among the natural ingredient diets tested and suggest that all should be suitable for multigenerational toxicology studies in mice.
Advantages and disadvantages of using natural ingredient diets versus purified diets have been recognized and discussed (Barnard et al., 2009; Rao and Knapka, 1998). Among the potential advantages of purified diets are the minimization of contaminants and reduction of batch-to-batch variability. Conversely, natural ingredient diets have higher micronutrient levels than purified diets (Oller et al., 1989; Rao and Knapka, 1998) and marginal micronutrient levels may contribute to occasional reports of breeding difficulties on the purified diets (US Food and Drug Administration, 2007). There is a lack of data supporting the suitability of the purified ingredient diets for chronic studies (Duffy et al., 2002; Lewis et al., 2003). The AIN-93 G and M purified diets used in the present study, which contain casein as their sole protein source, are widely used phytoestrogen-free diets that can be modified to accommodate dietary component additions. The ethanol-washed soy protein concentrate used in the SPC diet, Arcon® SJ, consisted of soy protein with a relatively low content of isoflavones or other ethanol-extractable low-molecular-weight phytochemicals. A soy isoflavones concentrate, NovaSoy® 650, was added to the SPC-IF diet to supply isoflavones. The most marked and consistent effect observed in the study was the significantly lower body weights of animals of both sexes after birth in the F1 and F2 generations in both the SPC and SPC-IF diet groups, suggesting that the soy protein was the prime driver of this effect. F0 animals fed these diets only during the post-weaning period did not show significant differences in body weights from the natural ingredient diets or from the AIN-93 group, indicating that the diet consumed by the dam during gestation and lactation influenced the pup body weights. The importance of the diet consumed by the dam on pup body weight throughout life has been reported in other studies of soy- versus casein-containing diets, although inconsistent effects have been found, with the soy-consuming F1 CD-1 mice having lower body weights (Cederroth et al., 2007; Ruhlen et al., 2008) and Wistar rats having higher body weights (Jahan-Mihan et al., 2011; Jahan-Mihan et al., 2012). A study in Sprague-Dawley rats reported no differences in adult body weight between rats fed purified diets with either casein or soy protein isolate as the protein sources (Badger et al., 2001).
There were no notable organ weight differences seen in the F1 animals among the natural ingredient diets, but in males, the kidney and thyroid weights, adjusted for body weight, were lower than the NIH-41 organ weights in all three AIN-93-related diets. The same was true for kidneys in females, but in females, only the thyroid weight of the SPC-IF diet group was lower than that in the NIH-41 group. Within the AIN-93-related diet subset, the SPC and SPC-IF females had lower kidney and liver weights than the AIN-93 females and the SPC-IF females also had a significantly lower retroperitoneal fat pad weight. For males, the SPC and SPC-IF groups had lower kidney weights than the AIN-93 group, liver weight was decreased in the SPC group and adrenal and brain weights were lower in the SPC-IF group. Testis weight differences are discussed below. Soy protein has been reported to have beneficial effects on kidney function (Ogborn et al., 1998; Ogborn et al., 2010), and soy protein has been reported to lower liver weight, as well as reduce serum and liver cholesterol and triglycerides (Ascencio et al., 2004; Nagata et al., 1981; Torre-Villalvazo et al., 2008; Wanezaki et al., 2015), although the latter effects were not observed in the present study. While there were no significant diet effects on ovary weights, there was a higher incidence of gross ovarian cysts in the three low isoflavone diets compared to the NIH-41 diet. Patisaul et al. (2014) recently reported an increased incidence of cystic follicles in Wistar rats fed a soy meal containing diet.
An unexpected aspect of this study that limits the ability to compare it with other published studies using AIN-93 diets and modifications thereof was the low thiamine level reported in the AIN-93, SPC, and SPC-IF diets (Table 3). Despite these low thiamine levels, there was no apparent effect on growth or general health of the animals in any of the diet groups, and gestation and lactation were supported by all diets. In fact, throughout the course of the study, none of the classical signs of thiamine deficiency in rodents, including poor growth or neurological symptoms, were noted in any of the diet groups reported to have low thiamine. The U.S. National Research Council (NRC) has estimated a thiamine requirement for mice of 5 mg thiamine hydrochloride/kg diet (National Research Council, 1995), although the similar estimate for rats (4 mg thiamine hydrochloride/kg diet) has been questioned in that approximately 5 to 7-fold lower levels were able to maintain adequate growth in rats (Rains et al., 1997; Shibata and Fukuwatari, 2013). In both rodents and humans, the thiamine requirement has long been known to vary according to the carbohydrate content of the diet, with higher thiamine levels required in high carbohydrate diets (Elmadfa et al., 2001; Reinhold et al., 1944; Wainio, 1942). Generally, natural ingredient diets have higher levels of thiamine than the NRC estimated requirement, while purified diets have been reported to have lower and variable levels (Oller et al., 1989; Rao and Knapka, 1998). Vitamin A levels were also lower in the AIN-93-related diets, although all were higher than the NRC estimated mouse requirement of 0.72 mg/kg (2.4 IU/g) diet. Vitamin E levels were higher in the AIN-93-related diets, particularly in the SPC and SPC-IF diets. As discussed below, the low levels of thiamine in the diet may have impacted significantly the results of the current study.
The reason for the low thiamine levels in the AIN-93-related diets in the present study is not clear, although irradiation seems to have been a factor (Table 3). It is known that heat and irradiation can reduce thiamine and vitamin A levels, and this is taken into account when formulating diets that will be autoclaved or irradiated. Irradiation has been reported to have relatively modest effects in a natural ingredient diet matrix (Caulfield et al., 2008), while thiamine was reported to be unstable on storage at room temperature in the purified AIN-76 diet relative to a natural ingredient diet (Fullerton et al., 1982). In the current study, the vitamin mix added to the AIN-93-based diets should have been sufficient to account for loss due to irradiation, and the diets were stored refrigerated to maintain nutrient levels. However, specifics of pellet preparation were likely a contributing factor as, in an attempt to normalize hardness across all purified diets, some had higher moisture content than others. Factors that contribute to thiamine loss in irradiated purified diets include a combination of moisture content, irradiation dose, and form of thiamine (personal communication, Dr. B. Mickelson, Envigo (Harlan) Teklad). The longer irradiation time and higher dose range used by the manufacturer of the purified diets (see Materials and Methods) may have contributed to the lower thiamine levels in these diets. In any case, although the thiamine levels fed to the animals in the study were below 5 ppm, they were clearly adequate in all cases to support growth, gestation, and lactation.
Endpoints to evaluate spermatogenesis (testes weight, testicular spermatid head count, caudal epididymal sperm count, motility, and morphology, and histopathology) were included based on the reports of soy/phytoestrogen detrimental effects on spermatogenesis in CD-1 mice (Cederroth et al., 2010). Although our data did not support such a link, abnormal spermatogenesis was noted in animals fed AIN-93, and its low thiamine may have contributed to this observed effect. Testicular degeneration had been reported decades ago in rats subjected to severe thiamine deficiency (Morris and Dubnik, 1947), and spermatogenesis defects have been reported in thiamine transporter knockout mice in the absence of the more severe thiamine deficiency manifestations (Fleming et al., 2003; Oishi et al., 2004). An interesting aspect of the present study is that defects in spermatogenesis were observed only in the AIN-93 diet group, which had non-detectable levels of thiamine reported in both the G and M formulations, while the SPC and SPC-IF groups, which had detectable thiamine levels in the M, but not G, formulation, did not show effects on testicular weights, testicular spermatid head counts, or histology. This could indicate that an early developmental subclinical thiamine deficiency may be critical to the noted effect on spermatogenesis and/or that there is an interaction between the subclinical thiamine deficiency and the lack of soy protein. Furthermore, although consistent effects on testes were noted, there was no observed deficit in epididymal sperm in the AIN-93 diet group. This could indicate a delayed effect on testicular spermatogenesis, similar to the delayed effect on spermatogenesis reported in aromatase knockout mice (Robertson, 2002). Further targeted experiments in mice fed low thiamine diets would be needed to address these questions more definitively. The issue of thiamine deficiency affecting reproductive toxicity assessments in rodents is unlikely to be an issue when natural ingredient diets are used, due to their high thiamine content; however, this may be a potential issue for rodent studies when purified diets, and particularly sterilized purified diets, are used. It must also be noted that, while the low measured thiamine levels are possibly involved in the observed effect, other micronutrients that were lower in the AIN-93-related diets may have also played a role. For example, as noted previously, vitamin A levels were lower in these diets than in the natural ingredient diets and vitamin A also plays a critical role in the development of the testis and the maintenance of spermatogenesis (Chihara et al., 2013; Hogarth and Griswold, 2010).
A significant question is whether or not subclinical nutritional deficiencies might contribute to defects in spermatogenesis or to an individual’s susceptibility to reproductive toxicants. This issue seems to have received relatively little attention, although it has been reported that the effect of bisphenol A on the number and motility of sperm in mice is enhanced under conditions of vitamin A deficiency (Aikawa et al., 2004; Nakahashi et al., 2001). Thiamine deficiencies severe enough to produce beriberi are rare in the modern developed world and severe thiamine deficiency associated with severe neurological effects (Wernicke’s encephalopathy) is largely confined to alcoholic adults or other specific conditions or medical procedures (Francini-Pesenti et al., 2009; Frank, 2015; Lallas and Desai, 2014; Stroh et al., 2014; World Health Organization, 1999). Low thiamine levels in diabetics have been noted, and it has been hypothesized that consumption of high energy high carbohydrate diets may lead to low, although subclinical, thiamine levels (Kerns et al., 2015; Lonsdale, 2015; Page et al., 2011; Rosner et al., 2014). In any case, the data reported here and by Oishi et al. (2004) in mouse models suggest that defects in spermatogenesis produced by low thiamine appear before other overt signs of deficiency. The mechanism and relevance of this observation to humans may be worthy of further study.
As noted in the Introduction, multiple studies and reviews over the years have pointed out the potential influence of diet on the outcomes of animal toxicology studies. Despite this, many investigators continue to provide little information on the diet utilized in their studies and likely select diets based largely on familiarity or convenience. The results of this study underline the importance of diet selection and formulation, and thorough nutrient characterization and reporting of the diets tested to enhance interpretability of study results and provide sufficient information to investigators attempting to replicate or build on others’ results.
Supplementary Material
Highlights.
CD-1 mice fed irradiated natural or purified ingredient diets were evaluated over three generations
Groups fed natural ingredient diets were similar, but differed significantly from the groups fed purified diets
Unanticipated low thiamine levels in the AIN-93 diet may have contributed to the decreased spermatogenesis in this group
Diet choice in toxicology requires careful consideration and interaction of nutrients with toxicants warrants attention
Acknowledgments
We are grateful for the outstanding efforts of the NCTR Animal Care, Microbiology, Pathology, and Veterinary Services staffs, as well as Ms. Kathy Carroll of the Office of Scientific Coordination, in the planning, conduct, and reporting of this study. We also thank Ms. Susan Berry of the chemistry group in the Division of Biochemical Toxicology for assistance in the preparation of the manuscript, Dr. Kelly Davis of TPA for assistance in the histological examination of ovaries, and Ms. Kristie Voris-Acuff of TPA for assistance in the clinical chemistry analyses. This study was funded by the NTP under an Interagency Agreement between FDA and NIEHS (FDA IAG # 224-12-0003/NIEHS IAG # AES12013). The opinions expressed in this paper do not necessarily reflect those of the U.S. Food and Drug Administration.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aikawa H, Koyama S, Matsuda M, Nakahashi K, Akazome Y, Mori T. Relief effect of vitamin A on the decreased motility of sperm and the increased incidence of malformed sperm in mice exposed neonatally to bisphenol A. Cell Tissue Res. 2004;315:119–124. doi: 10.1007/s00441-003-0806-1. [DOI] [PubMed] [Google Scholar]
- Andreoli MF, Stoker C, Rossetti MF, Alzamendi A, Castrogiovanni D, Luque EH, Ramos JG. Withdrawal of dietary phytoestrogens in adult male rats affects hypothalamic regulation of food intake, induces obesity and alters glucose metabolism. Mol Cell Endocrinol. 2015;401:111–119. doi: 10.1016/j.mce.2014.12.002. [DOI] [PubMed] [Google Scholar]
- Ascencio C, Torres N, Isoard-Acosta F, Gomez-Perez FJ, Hernandez-Pando R, Tovar AR. Soy protein affects serum insulin and hepatic SREBP-1 mRNA and reduces fatty liver in rats. J Nutr. 2004;134:522–529. doi: 10.1093/jn/134.3.522. [DOI] [PubMed] [Google Scholar]
- Badger TM, Ronis MJ, Hakkak R. Developmental effects and health aspects of soy protein isolate, casein, and whey in male and female rats. Intl J Toxicol. 2001;20:165–174. doi: 10.1080/109158101317097755. [DOI] [PubMed] [Google Scholar]
- Barnard DE, Lewis SM, Teter BB, Thigpen JE. Open- and Closed-Formula Laboratory Animal Diets and Their Importance to Research. JAALAS. 2009;48:709–713. [PMC free article] [PubMed] [Google Scholar]
- Boettger-Tong H, Murthy L, Chiappetta C, Kirkland JL, Goodwin B, Adlercreutz H, Stancel GM, Makela S. A case of a laboratory animal feed with high estrogenic activity and its impact on in vivo responses to exogenously administered estrogens. Environ Health Perspect. 1998;106:369–373. doi: 10.1289/ehp.98106369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown NM, Setchell KDR. Animal models impacted by phytoestrogens in commercial chow: implications for pathways induced by hormones. Lab Invest. 2001;81:735–747. doi: 10.1038/labinvest.3780282. [DOI] [PubMed] [Google Scholar]
- Cao J, Echelberger R, Liu M, Sluzas E, McCaffrey K, Buckley B, Patisaul HB. Soy but Not Bisphenol A (BPA) or the Phytoestrogen Genistin Alters Developmental Weight Gain and Food Intake in Pregnant Rats and Their Offspring. Reprod Toxicol. 2015 doi: 10.1016/j.reprotox.2015.07.077. in press, http://dx.doi.org/10.1016/j.reprotox.2015.07.077. [DOI] [PMC free article] [PubMed]
- Caulfield CD, Cassidy JP, Kelly JP. Effects of gamma irradiation and pasteurization on the nutritive composition of commercially available animal diets. JAALAS. 2008;47:61–66. [PMC free article] [PubMed] [Google Scholar]
- Cederroth CR, Nef S. Fetal programming of adult glucose homeostasis in mice. PloS One. 2009a;4:e7281. doi: 10.1371/journal.pone.0007281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cederroth CR, Nef S. Soy, phytoestrogens and metabolism: A review. Molecular and cellular endocrinology. 2009b;304:30–42. doi: 10.1016/j.mce.2009.02.027. [DOI] [PubMed] [Google Scholar]
- Cederroth CR, Vinciguerra M, Gjinovci A, Kuhne F, Klein M, Cederroth M, Caille D, Suter M, Neumann D, James RW, Doerge DR, Wallimann T, Meda P, Foti M, Rohner-Jeanrenaud F, Vassalli JD, Nef S. Dietary phytoestrogens activate AMP-activated protein kinase with improvement in lipid and glucose metabolism. Diabetes. 2008;57:1176–1185. doi: 10.2337/db07-0630. [DOI] [PubMed] [Google Scholar]
- Cederroth CR, Vinciguerra M, Kuhne F, Madani R, Doerge DR, Visser TJ, Foti M, Rohner-Jeanrenaud F, Vassalli JD, Nef S. A phytoestrogen-rich diet increases energy expenditure and decreases adiposity in mice. Environ Health Perspect. 2007;115:1467–1473. doi: 10.1289/ehp.10413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cederroth CR, Zimmermann C, Beny JL, Schaad O, Combepine C, Descombes P, Doerge DR, Pralong FP, Vassalli JD, Nef S. Potential detrimental effects of a phytoestrogen-rich diet on male fertility in mice. Mol Cell Endocrinol. 2010;321:152–160. doi: 10.1016/j.mce.2010.02.011. [DOI] [PubMed] [Google Scholar]
- Chihara M, Otsuka S, Ihcii O, Kon Y. Vitamin A deprivation affects the progression of the spermatogenic wave and initial formation of the blood-testis barrier, resulting in irreversible testicular degeneration in mice. J Reprod Dev. 2013;59:525–535. doi: 10.1262/jrd.2013-058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conner MW, Newberne PM. Drug-nutrient interactions and their implications for safety evaluations. Fund Appl Toxicol. 1984;4:S341–356. doi: 10.1016/0272-0590(84)90262-8. [DOI] [PubMed] [Google Scholar]
- Cooper S, Latendresse JR, Doerge DR, Twaddle NC, Fu X, Delclos KB. Dietary modulation of p-nonylphenol-induced polycystic kidneys in male Sprague-Dawley rats. Toxicol Sci. 2006;91:631–642. doi: 10.1093/toxsci/kfj171. [DOI] [PubMed] [Google Scholar]
- Delclos KB, Camacho L, Lewis SM, Vanlandingham MM, Latendresse JR, Olson GR, Davis KJ, Patton RE, Gamboa da Costa G, Woodling KA, Bryant MS, Chidambaram M, Trbojevich R, Juliar BE, Felton RP, Thorn BT. Toxicity evaluation of bisphenol A administered by gavage to Sprague Dawley rats from gestation day 6 through postnatal day 90. Toxicol Sci. 2014;139:174–197. doi: 10.1093/toxsci/kfu022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delclos KB, Weis CC, Bucci TJ, Olson G, Mellick P, Sadovova N, Latendresse JR, Thorn B, Newbold RR. Overlapping but distinct effects of genistein and ethinyl estradiol (EE2) in female Sprague-Dawley rats in multigenerational reproductive and chronic toxicity studies. Reprod Toxicol. 2009;27:117–132. doi: 10.1016/j.reprotox.2008.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duffy PH, Lewis SM, Mayhugh MA, McCracken A, Thorn BT, Reeves PG, Blakely SA, Casciano DA, Feuers RJ. Effect of the AIN-93M purified diet and dietary restriction on survival in Sprague-Dawley rats: implications for chronic studies. J Nutr. 2002;132:101–107. doi: 10.1093/jn/132.1.101. [DOI] [PubMed] [Google Scholar]
- Elmadfa I, Majchrzak D, Rust P, Genser D. The thiamine status of adult humans depends on carbohydrate intake. Int J Vitam Nutr Res. 2001;71:217–221. doi: 10.1024/0300-9831.71.4.217. [DOI] [PubMed] [Google Scholar]
- Fleming JC, Tartaglini E, Kawatsuji R, Yao D, Fujiwara Y, Bednarski JJ, Fleming MD, Neufeld EJ. Male infertility and thiamine-dependent erythroid hypoplasia in mice lacking thiamine transporter Slc19a2. Mol Genetics Metabol. 2003;80:234–241. doi: 10.1016/s1096-7192(03)00141-0. [DOI] [PubMed] [Google Scholar]
- Francini-Pesenti F, Brocadello F, Manara R, Santelli L, Laroni A, Caregaro L. Wernicke’s syndrome during parenteral feeding: not an unusual complication. Nutrition. 2009;25:142–146. doi: 10.1016/j.nut.2008.08.003. [DOI] [PubMed] [Google Scholar]
- Frank LL. Thiamin in Clinical Practice. J Parenteral Enteral Nutr. 2015;39:503–520. doi: 10.1177/0148607114565245. [DOI] [PubMed] [Google Scholar]
- Fullerton FR, Greenman DL, Bucci TJ. Effect of diet type on incidence of spontaneous and 2-acetylaminofluorene-induced liver and bladder tumors in BALB/c mice fed AIN-76A versus NIH-07 diet. Fund Appl Toxicol. 1992;18:193–199. doi: 10.1016/0272-0590(92)90046-k. [DOI] [PubMed] [Google Scholar]
- Fullerton FR, Greenman DL, Kendall DC. Effect of storage conditions on nutritional qualities of semipurified (AIN-76) and natural ingredient (NIH-07) diets. J Nutr. 1982;112:567–573. doi: 10.1093/jn/112.3.567. [DOI] [PubMed] [Google Scholar]
- Gehring TA, Cooper WM, Holder CL, Thompson HC., Jr Liquid chromatographic determination of thiamine in rodent feed by postcolumn derivatization and fluorescence detection. JAOAC Intl. 1995;78:307–309. [PubMed] [Google Scholar]
- Greenman DL, Fullerton FR. Comparison of histological responses of BALB/c and B6C3F1 female mice to estradiol when fed purified or natural-ingredient diets. J Toxicol Environ Health. 1986;19:531–540. doi: 10.1080/15287398609530950. [DOI] [PubMed] [Google Scholar]
- Greenman DL, Fullerton FR, Suber R, Farmer J. Effects of purified (AIN-76A) and natural-ingredient (NIH-07) diets on responses of BALB/c and B6C3F1 female mice to estradiol. J Toxicol Environ Health. 1987;22:351–362. doi: 10.1080/15287398709531077. [DOI] [PubMed] [Google Scholar]
- Heindel JJ, vom Saal FS. Meeting report: batch-to-batch variability in estrogenic activity in commercial animal diets--importance and approaches for laboratory animal research. Environ Health Perspect. 2008;116:389–393. doi: 10.1289/ehp.10524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogarth CA, Griswold MD. The key role of vitamin A in spermatogenesis. J Clin Invest. 2010;120:956–962. doi: 10.1172/JCI41303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holcomb M, Korfmacher WA, Thompson HC., Jr Characterization of iodide derivatives of aflatoxin B1 and G1 by thermospray mass spectrometry. J Anal Toxicol. 1991;15:289–292. doi: 10.1093/jat/15.6.289. [DOI] [PubMed] [Google Scholar]
- Hotchkiss AK, Vandenbergh JG. The anogenital distance index of mice (Mus musculus domesticus): an analysis. Contemp Top Lab Anim Sci. 2005;44:46–48. [PubMed] [Google Scholar]
- Jahan-Mihan A, Smith CE, Hamedani A, Anderson GH. Soy protein-based compared with casein-based diets fed during pregnancy and lactation increase food intake and characteristics of metabolic syndrome less in female than male rat offspring. Nutr Res. 2011;31:644–651. doi: 10.1016/j.nutres.2011.08.003. [DOI] [PubMed] [Google Scholar]
- Jahan-Mihan A, Szeto IM, Luhovyy BL, Huot PS, Anderson GH. Soya protein- and casein-based nutritionally complete diets fed during gestation and lactation differ in effects on characteristics of the metabolic syndrome in male offspring of Wistar rats. Brit J Nutr. 2012;107:284–294. doi: 10.1017/S0007114511002686. [DOI] [PubMed] [Google Scholar]
- Kang J, Badger TM, Ronis MJ, Wu X. Non-isoflavone phytochemicals in soy and their health effects. J Ag Food Chem. 2010;58:8119–8133. doi: 10.1021/jf100901b. [DOI] [PubMed] [Google Scholar]
- Kendig EL, Buesing DR, Christie SM, Cookman CJ, Gear RB, Hugo ER, Kasper SN, Kendziorski JA, Ungi KR, Williams K, Belcher SM. Estrogen-like disruptive effects of dietary exposure to bisphenol A or 17alpha-ethinyl estradiol in CD1 mice. Int J Toxicol. 2012;31:537–550. doi: 10.1177/1091581812463254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerns JC, Arundel C, Chawla LS. Thiamin deficiency in people with obesity. Adv Nutr. 2015;6:147–153. doi: 10.3945/an.114.007526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozul CD, Nomikos AP, Hampton TH, Warnke LA, Gosse JA, Davey JC, Thorpe JE, Jackson BP, Ihnat MA, Hamilton JW. Laboratory diet profoundly alters gene expression and confounds genomic analysis in mouse liver and lung. Chem-Biol Interact. 2008;173:129–140. doi: 10.1016/j.cbi.2008.02.008. [DOI] [PubMed] [Google Scholar]
- Lallas M, Desai J. Wernicke encephalopathy in children and adolescents. World J Pediatr. 2014;10:293–298. doi: 10.1007/s12519-014-0506-9. [DOI] [PubMed] [Google Scholar]
- Lephart ED, Porter JP, Lund TD, Bu L, Setchell KD, Ramoz G, Crowley WR. Dietary isoflavones alter regulatory behaviors, metabolic hormones and neuroendocrine function in Long-Evans male rats. Nutr Metabol. 2004:1–16. doi: 10.1186/1743-7075-1-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis SM, Johnson ZJ, Mayhugh MA, Duffy PH. Nutrient intake and growth characteristics of male Sprague-Dawley rats fed AIN-93M purified diet or NIH-31 natural-ingredient diet in a chronic two-year study. Aging Clin Exp Res. 2003;15:460–468. doi: 10.1007/BF03327368. [DOI] [PubMed] [Google Scholar]
- Lonsdale D. Thiamine and magnesium deficiencies: keys to disease. Medical Hypotheses. 2015;84:129–134. doi: 10.1016/j.mehy.2014.12.004. [DOI] [PubMed] [Google Scholar]
- Moriyama T, Kishimoto K, Nagai K, Urade R, Ogawa T, Utsumi S, Mauryama N, Maebuchi M. Soybean beta-conglycinin diet suppresses serum triglyceride levels in normal and genetically obese mice by induction of beta-oxidation, downregulation of fatty acid synthase, and inhibition of triglyceride absorption. Biosci Biotechnol, Biochem. 2004;68:352–359. doi: 10.1271/bbb.68.352. [DOI] [PubMed] [Google Scholar]
- Morris HP, Dubnik CS. Thiamine Deficiency and Thiamine Requirements in C3h Mice. JNCI. 1947;8:127–137. [PubMed] [Google Scholar]
- Muhlhauser A, Susiarjo M, Rubio C, Griswold J, Gorence G, Hassold T, Hunt PA. Bisphenol A effects on the growing mouse oocyte are influenced by diet. Biol Reprod. 2009;80:1066–1071. doi: 10.1095/biolreprod.108.074815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagata Y, Tanaka K, Sugano M. Serum and liver cholesterol levels of rats and mice fed soy-bean protein or casein. J Nutr Sci Vitaminol (Tokyo) 1981;27:583–593. doi: 10.3177/jnsv.27.583. [DOI] [PubMed] [Google Scholar]
- Nakahashi K, Matsuda M, Mori T. Vitamin A Insufficiency Accelerates the Decrease in the Number of Sperm Induced by an Environmental Disruptor, Bisphenol A, in Neonatal Mice. Zoological Science. 2001;18:819–821. [Google Scholar]
- National Research Council. Nutrient Requirements of Laboratory Animals, Fourth Revised Edition, 1995. National Academy Press; Washington, D.C: 1995. [PubMed] [Google Scholar]
- National Toxicology Program. Multigenerational reproductive study of genistein (Cas No. 446-72-0) in Sprague-Dawley rats (feed study) Natl Toxicol Prog Tech Rep Ser. 2008a:1–266. [PubMed] [Google Scholar]
- National Toxicology Program. Toxicology and carcinogenesis studies of genistein (Cas No. 446-72-0) in Sprague-Dawley rats (feed study) Natl Toxicol Prog Tech Rep Ser. 2008b:1–240. [PubMed] [Google Scholar]
- National Toxicology Program. Multigenerational reproductive toxicology study of ethinyl estradiol (CAS No. 57-63-6) in Sprague-Dawley rats. Natl Toxicol Prog Tech Rep Ser. 2010a:1–312. [PubMed] [Google Scholar]
- National Toxicology Program. Toxicology and carcinogenesis study of ethinyl estradiol (CAS No. 57-63-6) in Sprague-Dawley rats (feed study) Natl Toxicol Prog Tech Rep Ser. 2010b:1–210. [PubMed] [Google Scholar]
- National Toxicology Program. Specifications for the conduct of studies to evaluate the toxic and carcinogenic potential of chemical, biological and physical agents in laboratory animals for the National Toxicology Program (NTP) 2011:1–223. https://ntp.niehs.nih.gov/ntp/test_info/finalntp_toxcarspecsjan2011.pdf.
- Nelson JF, Karelus K, Felicio LS, Johnson TE. Genetic influences on the timing of puberty in mice. Biol Reprod. 1990;42:649–655. doi: 10.1095/biolreprod42.4.649. [DOI] [PubMed] [Google Scholar]
- Newberne PM, Conner MW. Nutrient influences on toxicity and carcinogenicity. Fed Proc. 1986;45:149–154. [PubMed] [Google Scholar]
- Odum J, Tinwell H, Jones K, Van Miller JP, Joiner RL, Tobin G, Kawasaki H, Deghenghi R, Ashby J. Effect of rodent diets on the sexual development of the rat. Toxicol Sci. 2001;61:115–127. doi: 10.1093/toxsci/61.1.115. [DOI] [PubMed] [Google Scholar]
- Odum J, Tinwell H, Tobin G, Ashby J. Cumulative Dietary Energy Intake Determines the Onset of Puberty in Female Rats. Environ Health Perspect. 2004;112:1472–1480. doi: 10.1289/ehp.7039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogborn MR, Bankovic-Calic N, Shoesmith C, Buist R, Peeling J. Soy protein modification of rat polycystic kidney disease. Am J Physiol. 1998;274:F541–549. doi: 10.1152/ajprenal.1998.274.3.F541. [DOI] [PubMed] [Google Scholar]
- Ogborn MR, Nitschmann E, Bankovic-Calic N, Weiler HA, Aukema HM. Dietary soy protein benefit in experimental kidney disease is preserved after isoflavone depletion of diet. Exp Biol Med. 2010;235:1315–1320. doi: 10.1258/ebm.2010.010059. [DOI] [PubMed] [Google Scholar]
- Oishi K, Barchi M, Au AC, Gelb BD, Diaz GA. Male infertility due to germ cell apoptosis in mice lacking the thiamin carrier, Tht1. A new insight into the critical role of thiamin in spermatogenesis. Dev Biol. 2004;266:299–309. doi: 10.1016/j.ydbio.2003.10.026. [DOI] [PubMed] [Google Scholar]
- Oller WL, Kendall DC, Greenman DL. Variability of selected nutrients and contaminants monitored in rodent diets: a 6-year study. J Toxicol Environ Health. 1989;27:47–56. doi: 10.1080/15287398909531277. [DOI] [PubMed] [Google Scholar]
- Orgaard A, Jensen L. The effects of soy isoflavones on obesity. Exp Biol Med. 2008;233:1066–1080. doi: 10.3181/0712-MR-347. [DOI] [PubMed] [Google Scholar]
- Page GL, Laight D, Cummings MH. Thiamine deficiency in diabetes mellitus and the impact of thiamine replacement on glucose metabolism and vascular disease. Intl J Clin Pract. 2011;65:684–690. doi: 10.1111/j.1742-1241.2011.02680.x. [DOI] [PubMed] [Google Scholar]
- Patisaul HB, Mabrey N, Adewale HB, Sullivan AW. Soy but not bisphenol A (BPA) induces hallmarks of polycystic ovary syndrome (PCOS) and related metabolic co-morbidities in rats. Reprod Toxicol. 2014;49C:209–218. doi: 10.1016/j.reprotox.2014.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patisaul HB, Sullivan AW, Radford ME, Walker DM, Adewale HB, Winnik B, Coughlin JL, Buckley B, Gore AC. Anxiogenic effects of developmental bisphenol A exposure are associated with gene expression changes in the juvenile rat amygdala and mitigated by soy. PloS One. 2012;7:e43890. doi: 10.1371/journal.pone.0043890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rains TM, Emmert JL, Baker DH, Shay NF. Minimum thiamin requirement of weanling Sprague-Dawley outbred rats. J Nutr. 1997;127:167–170. doi: 10.1093/jn/127.1.167. [DOI] [PubMed] [Google Scholar]
- Rao GN, Knapka JJ. Animal diets in safety evaluation studies. In: Ioannides C, editor. Nutrition and Chemical Toxicity. John Wiley and Sons; Chichester, England: 1998. pp. 345–374. [Google Scholar]
- Reeves PG, Nielson FH, Fahey GC., Jr AIN-93 purified diets for laboratory rodents: final report of the American Institute of Nutrition ad hoc writing committee on the reformulation of the AIN-76A rodent diet. J Nutr. 1993;123:1939–1951. doi: 10.1093/jn/123.11.1939. [DOI] [PubMed] [Google Scholar]
- Reinhold JG, Nicholson JTL, Elsom KO, Chornock C. The utilization of thiamine in the human subject: The effect of high intake of carbohydrate or of fat. J Nutr. 1944;28:51–62. [Google Scholar]
- Robertson KM. The Phenotype of the Aromatase Knockout Mouse Reveals Dietary Phytoestrogens Impact Significantly on Testis Function. Endocrinol. 2002;143:2913–2921. doi: 10.1210/endo.143.8.8957. [DOI] [PubMed] [Google Scholar]
- Rosner EA, Strezlecki KD, Clark JA, Lieh-Lai M. Low Thiamine Levels in Children With Type 1 Diabetes and Diabetic Ketoacidosis: A Pilot Study. Pediatr Critical Care Med. 2014;16:114–118. doi: 10.1097/PCC.0000000000000302. [DOI] [PubMed] [Google Scholar]
- Ruhlen RL, Howdeshell KL, Mao J, Taylor JA, Bronson FH, Newbold RR, Welshons WV, vom Saal FS. Low phytoestrogen levels in feed increase fetal serum estradiol resulting in the “fetal estrogenization syndrome” and obesity in CD-1 mice. Environ Health Perspect. 2008;116:322–328. doi: 10.1289/ehp.10448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibata K, Fukuwatari T. The body vitamin B1 levels of rats fed a diet containing the minimum requirement of vitamin B1 is reduced by exercise. J Nutr Sci Vitaminol. 2013;59:87–92. doi: 10.3177/jnsv.59.87. [DOI] [PubMed] [Google Scholar]
- Sprando RL, Collins TFX, Black TN, Olejnik N, Rorie JI, Scott M, Wiesenfeld P, Babu US, O’Donnell M. The effect of maternal exposure to flaxseed on spermatogenesis in F1 generation rats. Food Chem Toxicol. 2000;38:325–334. doi: 10.1016/s0278-6915(99)00165-9. [DOI] [PubMed] [Google Scholar]
- Strakovsky RS, Lezmi S, Flaws JA, Schantz SL, Pan YX, Helferich WG. Genistein exposure during the early postnatal period favors the development of obesity in female, but not male rats. Toxicol Sci. 2014;138:161–174. doi: 10.1093/toxsci/kft331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stroh C, Meyer F, Manger T. Beriberi, a severe complication after metabolic surgery - review of the literature. Obesity Facts. 2014;7:246–252. doi: 10.1159/000366012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tachibana N, Iwaoka Y, Hirotsuka M, Horio F, Kohno M. Beta-conglycinin lowers very-low-density lipoprotein-triglyceride levels by increasing adiponectin and insulin sensitivity in rats. Biosci, Biotech, Biochem. 2010;74:1250–1255. doi: 10.1271/bbb.100088. [DOI] [PubMed] [Google Scholar]
- Thigpen JE, Haseman JK, Saunders H, Locklear J, Caviness G, Grant M, Forsythe D. Dietary factors affecting uterine weights of immature CD-1 mice used in uterotrophic bioassays. Cancer Detect Prev. 2002;26:381–393. doi: 10.1016/s0361-090x(02)00122-8. [DOI] [PubMed] [Google Scholar]
- Thigpen JE, Setchell KD, Padilla-Banks E, Haseman JK, Saunders HE, Caviness GF, Kissling GE, Grant MG, Forsythe DB. Variations in phytoestrogen content between different mill dates of the same diet produces significant differences in the time of vaginal opening in CD-1 mice and F344 rats but not in CD Sprague-Dawley rats. Environ Health Perspect. 2007;115:1717–1726. doi: 10.1289/ehp.10165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thigpen JE, Setchell KDR, Ahlmark KB, Locklear J, Spahr T, Caviness GF, Goelz MF, Haseman JK, Newbold RR, Forsythe DB. Phytoestrogen content of purified, open- and closed formula laboratory animal diets. Lab Anim Sci. 1999;49:530–536. [PubMed] [Google Scholar]
- Thigpen JE, Setchell KDR, Kissling GE, Locklear J, Caviness GF, Whteside T, Belcher SM, Brown NM, Collins BJ, Lih FB, Tomer KB, Padilla-Banks E, Camacho L, Adsit FG, Grant M. The estrogenic content of rodent diets, bedding, cages, and water bottles and its effect on bisphenol A studies. JAALAS. 2013;52:130–141. [PMC free article] [PubMed] [Google Scholar]
- Thigpen JE, Setchell KDR, Saunders HE, Haseman JK, Grant MG, Forsythe DB. Selecting the appropriate rodent diet for endocrine disruptor research and testing studies. ILAR J. 2004;45:401–416. doi: 10.1093/ilar.45.4.401. [DOI] [PubMed] [Google Scholar]
- Torre-Villalvazo I, Tovar AR, Ramos-Barragan VE, Cerbon-Cervantes MA, Torres N. Soy protein ameliorates metabolic abnormalities in liver and adipose tissue of rats fed a high fat diet. J Nutr. 2008;138:462–468. doi: 10.1093/jn/138.3.462. [DOI] [PubMed] [Google Scholar]
- Twaddle NC, Churchwell MI, Doerge DR. High-throughput quantification of soy isoflavones in human and rodent blood using liquid chromatography with electrospray mass spectrometry and tandem mass spectrometry detection. J Chromatogr B. 2002;777:139–145. doi: 10.1016/s1570-0232(02)00275-1. [DOI] [PubMed] [Google Scholar]
- US Food and Drug Administration. Diets for toxicity studies. 2007. Toxicological principles for the safety assessment of food ingredients: Redbook 2000 Section IV B5. [Google Scholar]
- Vandenbergh JG, Huggett CL. The anogenital distance index, a predictor of the intrauterine position effects on reproduction in female house mice. Lab Anim Sci. 1995;45:567–573. [PubMed] [Google Scholar]
- Velasquez MT, Bhathena SJ. Role of dietary soy protein in obesity. Int J Med Sci. 2007;4:72–82. doi: 10.7150/ijms.4.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wainio WW. The thiamine requirement of the albino rat as influenced by the substitution of protein for carbohydrate in the diet. J Nutr. 1942;24:317–329. [Google Scholar]
- Wanezaki S, Tachibana N, Nagata M, Saito S, Nagao K, Yanagita T, Kohno M. Soy beta-conglycinin improves obesity-induced metabolic abnormalities in a rat model of nonalcoholic fatty liver disease. Obesity Res Clin Pract. 2015;9:168–174. doi: 10.1016/j.orcp.2014.03.005. [DOI] [PubMed] [Google Scholar]
- Wang H, Tranguch S, Xie H, Hanley G, Das SK, Dey SK. Variation in commercial rodent diets induces disparate molecular and physiological changes in the mouse uterus. Proc Natl Acad Sci USA. 2005;102:9960–9965. doi: 10.1073/pnas.0501632102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization. Thiamine deficiency and its prevention and control in major emergencies. 1999. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.