Abstract
Cryptotanshinone (CPT) is a natural compound isolated from traditional Chinese medicine Salvia miltiorrhiza Bunge. In the present study, the regulatory effect and potential mechanisms of CPT on tumor necrosis factor alpha (TNF-α) induced lectin-like receptor for oxidized low density lipoprotein (LOX-1) were investigated. Human umbilical vein endothelial cells (HUVECs) were cultured and the effect of TNF-α on LOX-1 expression at mRNA and protein levels was determined by Real-time PCR and Western blotting respectively. The formation of intracellular ROS was determined with fluorescence probe CM-DCFH2-DA. The endothelial ox-LDL uptake was evaluated with DiI-ox-LDL. The effect of CPT on LOX-1 expression was also evaluated with SD rats. TNF-α induced LOX-1 expression in a dose- and time-dependent manner in endothelial cells. TNF-α induced ROS formation, phosphorylation of NF-κB p65 and ERK, and LOX-1 expression, which were suppressed by rotenone, DPI, NAC, and CPT. NF-κB inhibitor BAY11-7082 and ERK inhibitor PD98059 inhibited TNF-α-induced LOX-1 expression. CPT and NAC suppressed TNF-α-induced LOX-1 expression and phosphorylation of NF-κB p65 and ERK in rat aorta. These data suggested that TNF-α induced LOX-1 expression via ROS activated NF-κB/ERK pathway, which could be inhibited by CPT. This study provides new insights for the anti-atherosclerotic effect of CPT.
INTRODUCTION
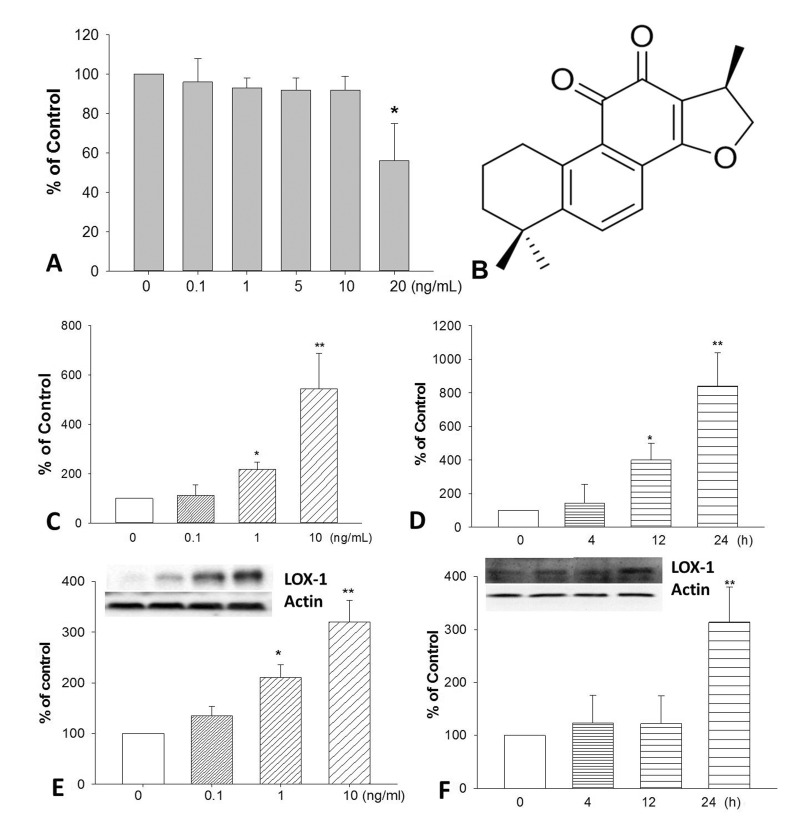
Cryptotanshinone (CPT, Fig. 1B) is a natural diterpene quinone isolated from Salvia miltiorrhiza Bunge, a widely prescribed Chinese herb for cardiovascular diseases treatment. Accumulated studies provide data for the beneficial effect of CPT in cardiovascular diseases. CPT protects the myocardium against ischemia-induced derangements in a hypoxic perfusion and reoxygenation isolated heart model [1]. CPT attenuates isoprenaline-induced cardiac fibrosis in mice associated with upregulation and activation of matrix metalloproteinase-2 [2]. CPT protects against hypoxia-induced mitochondrial apoptosis in H9c2 cells [3]. In human aortic smooth muscle cells (HASMC), CPT inhibits TNF-α-induced matrix metalloproteinase-9 production and HASMC migration via down-regulated NF-κB and AP-1 [4]. In human umbilical vein endothelial cells (HUVECs), CPT inhibits TNF-α-induced endothelin-1, vascular cell adhesion molecule-1 (VCAM-1), and intracellular adhesion molecule-1 (ICAM-1) expression [5,6] and stimulates nitric oxide (NO) production [5]. It also suppresses ox-LDL induced ICAM-1, VCAM-1 expression, endothelial-monocyte adhesion and increased NO level in HUVECs [7].
Fig. 1. The cytotoxicity of TNF-α on HUVECs after 24 h treatment.
(A). The chemical structure of CPT (B). Cells were incubated with TNF-α, the expression of LOX-1 at mRNA (C and D) and protein (E and F) levels was determined by realtime PCR and Western blotting respectively. *p<0.05 and **p<0.01 vs. untreated group.
Lectin-like oxidized low-density lipoprotein receptor (LOX-1) is a type II membrane protein with a typical C-type lectin structure at the extracellular C-terminus expressed in endothelial cells. It has been identified as the main receptor for oxidized low-density lipoprotein (ox-LDL), the main risk factor for atherosclerosis, on endothelial cells [8]. Accumulated data has revealed that LOX-1 plays an important role in atherosclerosis and might provide new therapeutic approaches for the treatment of atherosclerosis and other related disorders [9,10,11]. The expression of LOX-1 has been found to be up-regulated by a series of atherogenic factors, such as homocysteine, lysophophatidylcholine, ox-LDL, angiotensin II, phorbol 12-myristate 13-acetate, hypertension, hyperlipidemia [12].
Previous studies showed that TNF-α induced LOX-1 expression in endothelial cells [13], macrophages [14], and vascular smooth muscle cells [15] while its underlying mechanisms remain to be clear. 15-deoxy-Delta(12,14)-prostaglandin J(2), pioglitazone and troglitazone [16], mulberry leaf aqueous fractions [17], Leonurus sibiricus herb extract [18], H(2) (a therapeutic antioxidant) [19], and docosahexaenoic acid [20] were reported to inhibit TNF-α-induced LOX-1 expression in endothelial cells and THP-1 cells. In this study, we discussed the mechanism of TNF-α-induced LOX-1 expression and the inhibitory effect of CPT in HUVECs.
METHODS
Materials
CPT (>95%) was purchased from SICHUAN WEIKEQI BIOLOGICAL TECHNOLOGY CO., LTD (Chengdu, China). TNF-α and Trizol were purchased from Sangon Biotech (Shanghai, China). 5-(6)-carboxy-2',7'-dichlorodihydrofluorescein diacetate (CM-DCFH2-DA) was purchased from Sigma (St. Louis, MO, USA). Antibodies for phospho-ERK (p-ERK), phospho-NF-κB p65 (p-p65) and actin antibodies were purchased from Cell Signaling Technology (Beverly, USA). Anti-LOX-1 antibody was obtained from R&D Systems (Minneapolis, USA). Diphenyleneiodonium chloride (DPI), rotenone (Rot), PD98059, BAY11-7082, and N-acetyl-L-cysteine (NAC) were purchased from Beyotime (Haimen, China). Primers and other materials for Real-Time PCR were purchase from Sangon Biotech (Shanghai, China) and TaKaRa Bio Group (Japan). BCA protein kit was purchased from Thermo Fisher (Rockford, USA). DiI-ox-LDL was purchased from Yiyuan Biotechnology (Guangzhou, China).
Cell culture
HUVECs were purchased from Xiangya Cell Bank (Changsha, China). Cells were cultured in RPMI-1640 medium supplemented with 10% (v/v) fetal calf serum, penicillin (100 U/mL) and streptomycin (100 µg/mL) at 37℃ under a humidified atmosphere containing 5% CO2 and 95% air.
Animals
Male SD rats (200±20 g) were purchased from Experimental Animal Center of Daping Hospital at the Third Military Medical University. All animals were housed under standard environmental conditions and allowed free access to tap water and food. Great care was taken to minimize the number of animals and their suffering which was in accordance with the legislation of experimental animal administration of Zunyi Medical College.
MTT assay
The cytotoxic effect of TNF-α on HUVECs was determined by assessment of cell viability using the MTT assay as previously report with minor modifications [21]. Confluent HUVECs monolayers in 96-well microplates were exposed to different concentrations (0~20 ng/mL) of TNF-α for 24 h and then added 100 µL of MTT solution (0.5 mg/mL) to each well. After 4 h of incubation at 37℃, the supernatant was removed and 150 µl of dimethyl sulfoxide (DMSO) was added to each well to solubilize the formazan crystals. The absorbance was measured at 490 nm using a Microplate Reader. The cellular viability was calculated by comparison with untreated cells, which was set to 100% viability.
Measurement of intracellular ROS production
The intracellular ROS production was determined with the fluorescence probe CM-DCFH2-DA [22]. HUVECs were cultured in 12-well plates and treated with TNF-α (10 ng/mL) for 24 h. The cells were incubated with CM-DCFH2-DA (final concentration 10 µM) in the dark at 37℃ for 30 min. After washing twice with PBS, the cellular fluorescence was observed with a fluorescent microscopy. H2O2 (50 µM) was used as a positive control. To explore the effect of CPT, Rot, DPI, and NAC on TNF-α induced ROS formation, cells were pretreated with CPT (0.25, 0.5, 1.0 µM), Rot (10 µM), DPI, (10 µM), NAC (1 mM) respectively for 2 h before TNF-α challenge.
DiI-ox-LDL uptake assay
The method for DiI-ox-LDL uptake assay was following our previous study with minor revisions [23]. Cells were cultured in 96-well plate and treated with TNF-α (10 ng/mL) for 24 h. After incubated with 25 µg/mL DiI-ox-LDL for another 4 h at 37℃ in the dark, the cells were gently washed with PBS for three times and the uptake of ox-LDL was measured on a fluorescent microscopy. To explore the effect of CPT, Rot, DPI, and NAC on TNF-α induced ox-LDL uptake, cells were pretreated with CPT (0.25, 0.5, 1.0 µM), Rot (10 µM), DPI, (10 µM), NAC (1 mM) respectively for 2 h before TNF-α challenge.
RT-PCR analysis
The mRNA expression of LOX-1 was determined with RT-PCR. After cells were incubated with TNF-α (0~10 ng/mL) for 0~24 h, the total RNA was extracted with TRIzol Reagent. Two µg total RNA was reverse-transcribed into cDNA using Super-Script TMIII First-Stand Synthesis System Kit (Toyobo, Japan). RT-PCR was performed using SYBR Green PCR reagents (Applied Biosystems). The specific primers for LOX-1: 5'-CTC GGG CTC ATT TAA CTG GGA-3' (forward), 5'-AGG AAA TTG CTT GCT GGA TGA' (reverse); For Actin: 5'-AGA AGG CTG GGG CTC ATT TG-3' (forward), 5'AGG GGC CAT CCA CAG TCT TC-3' (reverse). The levels of LOX-1 expression were determined by normalizing to actin expression.
Western blotting assay
After TNF-α treatment, the protein expressions of LOX-1, phosphorylation of NF-κB p65, ERK in cells and aorta tissues were determined by Western blotting. 50 µg proteins were subjected to 10% SDS-PAGE and transferred onto PVDF membrane. After blocking with 5% non-fat milk in TBST at room temperature for 1 h, the membranes were washed with TBST for three times and then incubated with specific primary antibodies at 4℃ for overnight. After washing with TBST, the membranes were incubated with horseradish-peroxidase conjugated secondary antibodies at room temperature for 2 h. Actin was used as the reference protein. The protein-antibody complexes were detected by ECL Advanced Western Blot detection Kit. To explore the effect of CPT, Rot, DPI, NAC, PD98059, and BAY11-7802 on TNF-α induced LOX-1, ERK, NF-κB p65 expression, HUVECs were pretreated with CPT (0.25, 0.5, 1.0 µM), Rot (10 µM), DPI (10 µM), NAC (1 mM), PD98059 (50 µM), and BAY11-7802 (50 µM) respectively for 2 h before TNF-α challenge.
Animal experiment
The male SD rats were randomly assigned into four groups. After overnight fasting, they were intraperitoneal injection of TNF-α (20 ng/kg) with or without 2 h pretreatment of CPT (50 mg/kg) or NAC (150 mg/kg) by intragastric administration. The rats were sacrificed after 24 h TNF-α challenge and the aorta was isolated. The protein expression of LOX-1, phosphorylation of NF-κB p65 and ERK were determined by Western blotting.
Statistical analysis
Data were expressed as the means±SD from at least three separate experiments. The differences between groups were analyzed using SPSS 11.5 and the statistical analysis was performed by analysis of variance (one-way ANOVA). p<0.05 is considered statistically significant.
RESULTS
TNF-α induced LOX-1 mRNA and protein expression in HUVECs
Firstly, we determined the cytotoxic effect of TNF-α on HUVECs by MTT assay. Results (Fig. 1A) showed that TNF-α at concentration of 20 ng/mL could significantly inhibit endothelial cell proliferation after 24 h incubation. To investigate the effects of TNF-α on LOX-1 expression, HUVECs were treated with various concentrations of TNF-α (0.1, 1, 10 ng/mL) and treated for different time (0, 4, 12, 24 h). TNF-α remarkably induced LOX-1 mRNA expression in a time- and concentration-dependent manner in HUVECs (Fig. 1C and 1D). Furthermore, TNF-α also increased LOX-1 protein expression in a concentration-dependent manner (Fig. 1E and 1F).
CPT inhibited TNF-α-induced ROS formation in HUVECs
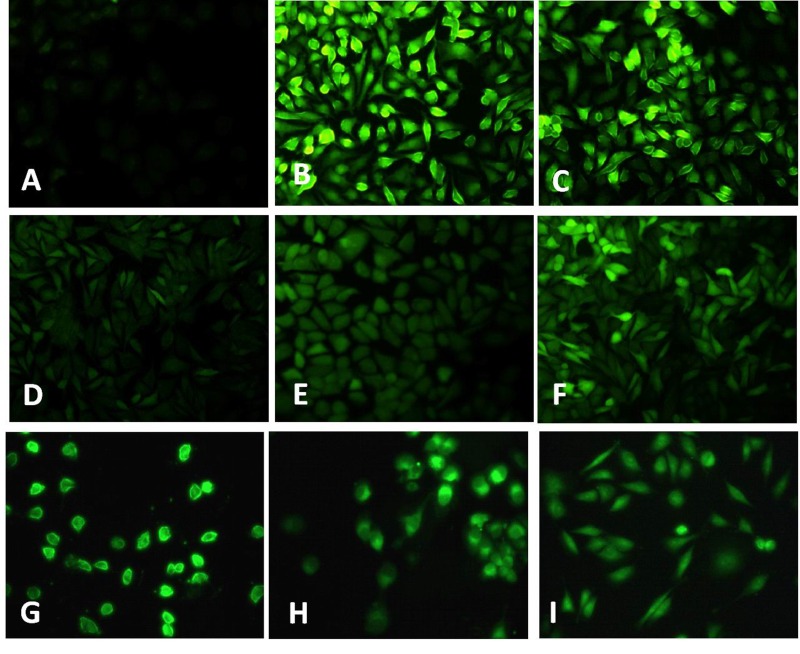
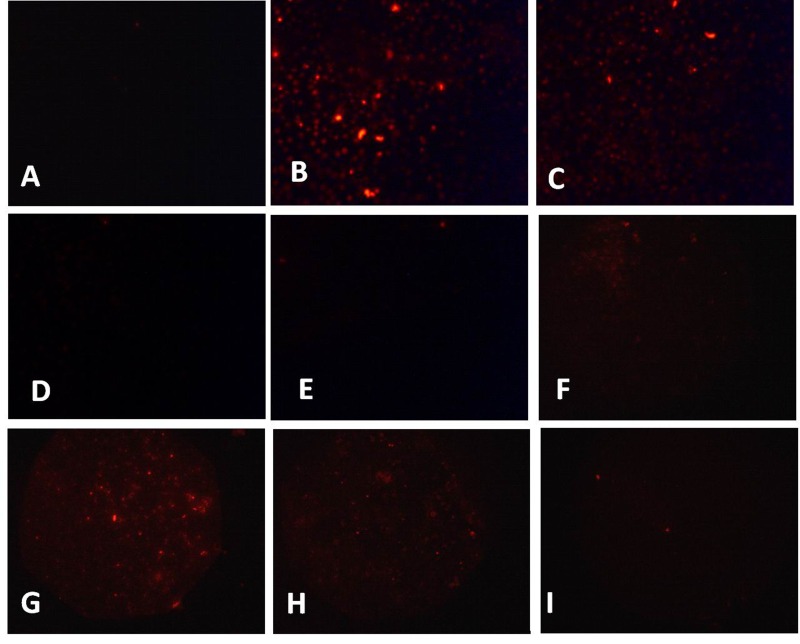
TNF-α has been implicated as an important ROS inducer in endothelial cells [24]. Here, we investigated the effect of TNF-α on endothelial ROS production. Results showed that TNF-α triggers significant increase in ROS formation as indicated by the intense green fluorescence in TNF-α treated group (Fig. 2B), which could be inhibited by DPI (inhibitor of NADPH oxidase) (Fig. 2D), rotenone (inhibitor for mitochondria electron transport chain) (Fig. 2E), and NAC (Fig. 2F). Furthermore, CPT also significantly inhibits TNF-α induced ROS formation in HUVECs (Fig. 2G~I).
Fig. 2. TNF-α induced ROS formation in HUVECs.
Cells were treated with TNF-α (10 ng/mL) (B) and H2O2 (50 µM) (C) for 24 h, the ROS formation was determined with CM-DCFH2-DA. After 2 h pretreated with DPI (10 µM) (D), rotenone (10 µM) (E), NAC (1 mM) (F), CPT (0.25 µM) (G), CPT (0.5 µM) (H), CPT (1 µM) (I), the ROS gene ration was measured. A. Without TNF-α treatment.
DPI, rotenone, and NAC suppressed TNF-α-induced LOX-1 expression in HUVECs
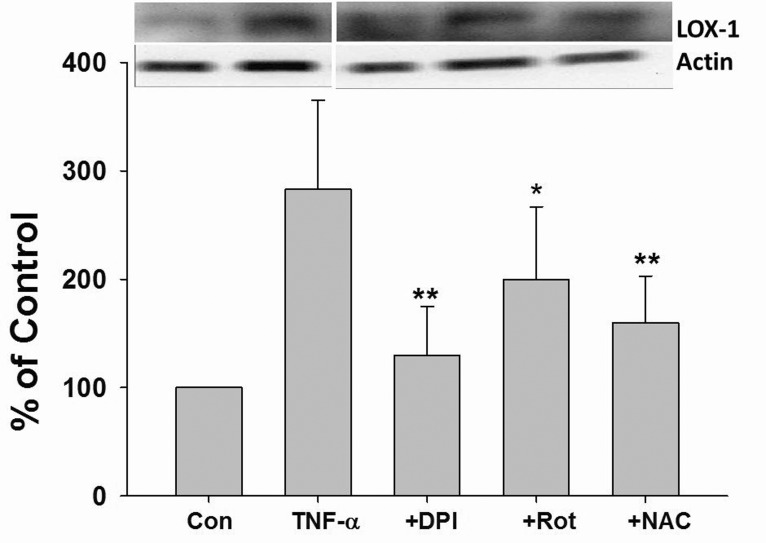
The role of ROS in TNF-α induced LOX-1 expression was investigated. As shown in Fig. 3, TNF-α dramatically induced LOX-1 protein expression in HUVECs. Furthermore, TNF-α induced LOX-1 protein expression was significantly suppressed by DPI, Rot, and NAC pretreatment (Fig. 3).
Fig. 3. ROS mediated TNF-α-induced LOX-1 expression in HUVECs.
Cells were incubated with TNF-α (10 ng/mL) for 24 h with or without 2 h pretreatment with DPI (10 µM), Rot (10 µM), NAC (1 mM), the LOX-1 protein expression was determined by Western blotting. Rot, rotenone; *p<0.05 and **p<0.01 vs. TNF-α treated group.
CPT, PD98059, and BAY11-7802 inhibited TNF-α-induced LOX-1 expression in HUVECs
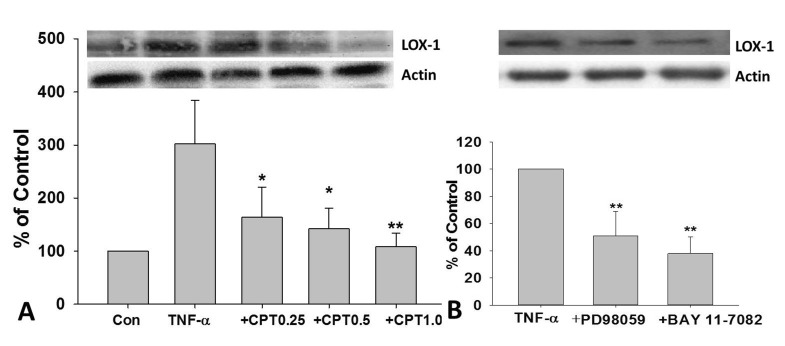
As shown in Fig. 4A, TNF-α-induced LOX-1 expression in HUVECs was dramatically inhibited by CPT pretreatment. Furthermore, a dose-dependent manner was observed. PD98059, an inhibitor for ERK, and BAY11-7802, an inhibitor for NF-κB, also dramatically reversed TNF-α-induced LOX-1 protein expression in HUVECs (Fig. 4B).
Fig. 4. CPT reversed TNF-α-induced LOX-1 expression in HUVECs.
Cells were incubated with TNF-α (10 ng/mL) for 24 h with or without CPT pretreatment for 1 h, the protein expression of LOX-1 were determined (A). Cells were pretreated with PD98059 (50 µM) or BAY11-7802(50 µM) for 1 h and then incubated with TNF-α (10 ng/mL) for 24 h, the protein expression of LOX-1 was determined (B). CPT, cryptotanshinone; *p<0.05 and **p<0.01 vs. TNF-α treated group.
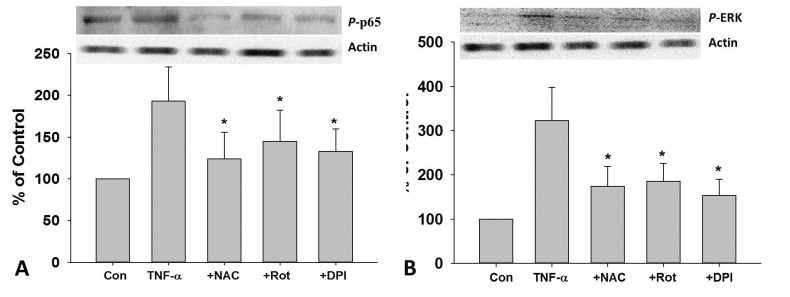
DPI, rotenone, and NAC inhibited TNF-α-induced NF-κB p65 and ERK phosphorylation in HUVECs
As shown in Fig. 5A, TNF-α caused increase in the phosphorylation of NF-κB p65, which could be inhibited by DPI, Rot and NAC pretreatment. Similarly, DPI, Rot and NAC pretreatment also dramatically inhibit TNF-α induced increase in the phosphorylation of ERK in HUVECs (Fig. 5B).
Fig. 5. Role of ROS in TNF-α-induced phosphorylation of NF-κB p65 and ERK in HUVECs.
After 2 h pretreated with DPI (10 MM), Rot (10 µM), NAC (1 mM), cells were treat ed with TNF-α (10 ng/mL) for 24 h, the protein expression of p-p65 (A) and p-ERK (B) was measured by Western blotting. Rot, rotenone; *p<0.05 vs. TNF-α treated group.
CPT inhibited TNF-α-induced phosphorylation of NF-κB p65 and ERK in HUVECs
TNF-α-induced phosphorylation of NF-κB p65 and ERK in endothelial cells could be reversed by BAY11-7802 and PD98059. Furthermore, CPT pretreatment significantly suppress TNF-α-induced phosphorylation of NF-κB p65 and ERK in a dose-dependent manner (Fig. 6).
Fig. 6. CPT inhibited TNF-α-induced phosphorylation of NF-κB p65 and ERK in HUVECs.
After 1 h pretreated with CPT (0–1 MM), PD98059(50 µM), or BAY11-7802 (50 µM), cells were treated with TNF-α (10 ng/mL) for 24 h, the protein expression of p-p65 (A) and p-ERK (B) was measured by Western blotting. CPT, cryptotanshinone; *p<0.05 and **p<0.01 vs. TNF-α treated group.
CPT inhibited TNF-α-induced cellular uptake of DiIox-LDL in HUVECs
LOX-1 has been identified as the main receptor for ox-LDL in endothelial cells and mediated ox-LDL endocytosis [8]. We test the DiI-ox-LDL uptake after TNF-α treatment. As shown in Fig. 7A, the resting endothelial cells exhibit sporadic red fluorescence suggesting the endocytosis of little DiI-ox-LDL. However, TNF-α and H2O2 treated cells demonstrated intensive red fluorescence suggesting the large amount of DiI-ox-LDL uptake (Fig. 7B, C). Furthermore, TNF-α-induced intensive red fluorescence could be markedly decreased by DPI, Rot, and NAC pretreatment (Fig. 7D~F). Furthermore, CPT treatment also dramatically decreased the red fluorescence (Fig. 7G~I).
Fig. 7. CPT inhibited TNF-α-induced DiI-ox-LDL uptake in HUVECs.
Cells were treated with TNF-α (10 ng/mL) (B) and H2O2 (50 µM) (C) for 24 h, then the cells were incubated with DiI-ox-LDL and the uptake of DiI- ox-LDL was measured with a fluorescent microscopy. After 2 h pretreated with DPI (10 µM) (D), rotenone (10 µM) (E), NAC (1 mM) (F), CPT (0.25 µM) (G), CPT (0.5 µM) (H), or CPT (1 µM) (I), the uptake of DiI-ox-LDL was measured. A. Without TNF-α treatment.
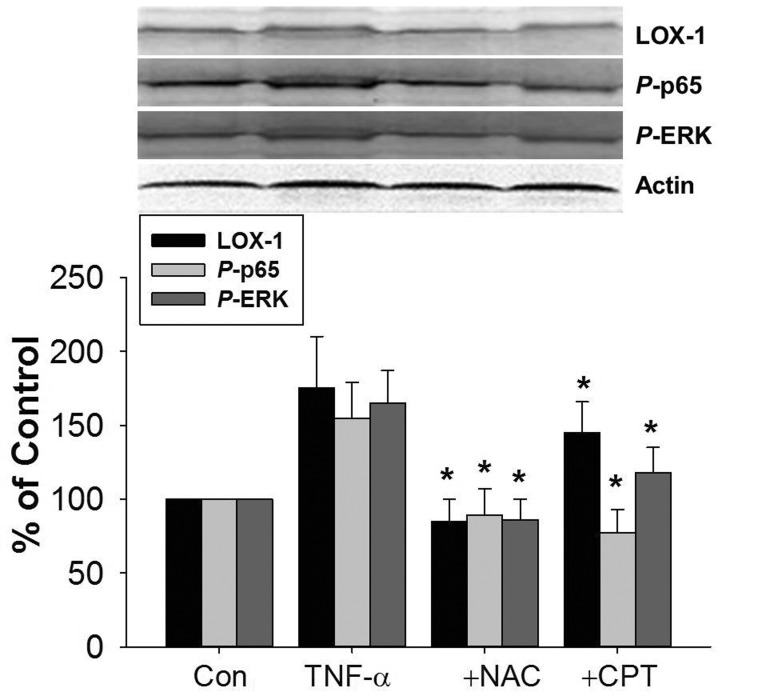
CPT inhibited TNF-α-induced LOX-1, phosphorylation of NF-κB p65 and ERK in rat aorta
The effect of CPT on TNF-α-induced LOX-1 expression was also investigated in vivo. As shown in Fig. 8, TNF-α injection induced LOX-1 protein expression in rat aorta tissues. The protein expression of phosphorylated NF-κB p65 and ERK was also increased. CPT pretreatment inhibited TNF-α-induced protein expression of LOX-1, p-p65, and p-ERK. Furthermore, the increased protein expression of LOX-1, p-p65, and p-ERK were also reversed by NAC pretreatment.
Fig. 8. CPT inhibited TNF-α-induced LOX-1 expression in rat aorta tissues.
Male SD mice were pretreated with CPT (50 mg/kg) or NAC (150 mg/kg) for 2 h by ig and then administrated with TNF-α (20 ng/mL) by intraperitoneal injection for 24 h. The aorta tissues were isolated and the protein expression of LOX-1, p-p65, and p-ERK was determined by Western blotting. CPT, cryptotanshinone; *p<0.05 vs. TNF-α treated group.
DISCUSSION
TNF-α, an inflammatory cytokine, plays a pivotal role in the disruption of endothelial cells both in vivo and in vitro [25]. LOX-1, the main receptor for ox-LDL on endothelial cells, plays an important role in cardiovascular diseases [9,26]. Consistent with previous reports [13,16,27], our results demonstrated that TNF-α upregulated LOX-1 expression at both mRNA and protein levels in HUVECs. Furthermore, the aorta expression of LOX-1 is also enhanced by TNF-α insult. These data further support our previous results that LOX-1 contributes to TNF-α induced endothelial dysfunction [28].
ROS are important mediators of cell injury and dysfunction in endothelial cells [24]. Previous data showed that ROS act as a key mediator for ox-LDL-induced LOX-1 expression in endothelial cells and smooth muscle cells [29,30]. In present study, TNF-α treatment caused significant intracellular ROS formation, which was inhibited by DPI and Rot suggesting that the ROS were mainly derived from NADPH oxidase and mitochondria. The inhibitory effect of DPI, Rot, and NAC suggested that ROS mediated TNF-α induced LOX-1 expression in endothelial cells. Previous results showed that ox-LDL, 15-lipoxygenase-modified LDL, and glucose induced LOX-1 expression in endothelial cell through p38MAPK, ERK1/2, and NF-κB [31,32,33]. Our data showed that TNF-α induced phosphorylation of ERK and NF-κB p65, which was reversed by DPI, Rot, and NAC in HUVECs and by NAC in aorta. Furthermore, TNF-α-induced LOX-1 expression was inhibited by PD98059 and BAY11-7802. Collectively, these results suggested that TNF-α-induced LOX-1 expression was mediated by ROS through activation of NF-κB and ERK. CPT was found to induce ROS production in cancer cells [34,35]. Interestingly, CPT suppressed TNF-α-induced ROS formation in endothelial cells. Furthermore, TNF-α-induced activation of NF-κB and ERK was reversed by CPT in a dose-dependent manner. Thus, inhibitory effect of CPT on TNF-α-induced LOX-1 expression was attributed, at least in part, to suppressing the activation of NF-κB and ERK by inhibiting ROS formation.
Up-regulated LOX-1 expression mediated uptake of DiI-ox- LDL in endothelial cells [13], vascular smooth muscle cells [36], human monocyte-derived macrophage [37]. Here, we found that TNF-α treatment resulted in increased ox-LDL uptake in HUVECs, which could be reversed by DPI, Rot, and CPT. In view of the key role of ox-LDL in the pathogenesis of atherosclerosis, this provide new clue for TNF-α in atherogenesis and the antiatherosclerosis mechanism of CPT. Particularly, a latest study showed that CPT inhibited ox-LDL-induced LOX-1 expression, monocytes adhesion to HUVECs, generation of superoxide radicals and NF-κB activation in HUVECs. It also attenuated atherosclerosis in ApoE KO mice [38]. Thus, our results provided further evidence and potential mechanisms for the antiatherosclerotic effect of CPT.
In summary, our results showed that TNF-α treatment caused intracellular ROS formation in endothelial cells mediated by mitochondria and NADPH oxidase. ROS induced phosphorylation of ERK and NF-κB p65, which result in LOX-1 expression. Elevated LOX-1 expression mediated ox-LDL endocytosis. CPT inhibited TNF-α-induced LOX-1 expression by suppressing ROS formation in HUVECs and rat aorta, which provides new insight into its beneficial effect in atherosclerosis.
ACKNOWLEDGMENTS
This study was supported by the National Natural Science Foundation of China (No. 81160048), the Science and Technology Development Fund of Macau Special Administrative Region (021/2012/A1).
Footnotes
CONFLICTS OF INTEREST: We declare that none of the authors has any kind of conflict of interest related to the present work.
Author contributions: XL.R., WW.Z., and WP.L. performed the cell-based assay experiments and the animal experiment. XL.R. drafted the manuscript. JS.S. and XP.C. supervised and coordinated the study and revised the manuscript.
References
- 1.Yagi A, Fujimoto K, Tanonaka K, Hirai K, Takeo S. Possible active components of tan-shen (Salvia miltiorrhiza) for protection of the myocardium against ischemia-induced derangements. Planta Med. 1989;55:51–54. doi: 10.1055/s-2006-961824. [DOI] [PubMed] [Google Scholar]
- 2.Ma S, Yang D, Wang K, Tang B, Li D, Yang Y. Cryptotanshinone attenuates isoprenaline-induced cardiac fibrosis in mice associated with upregulation and activation of matrix metalloproteinase-2. Mol Med Rep. 2012;6:145–150. doi: 10.3892/mmr.2012.866. [DOI] [PubMed] [Google Scholar]
- 3.Jin HJ, Xie XL, Ye JM, Li CG. TanshinoneIIA and cryptotanshinone protect against hypoxia-induced mitochondrial apoptosis in H9c2 cells. PLoS One. 2013;8:e51720. doi: 10.1371/journal.pone.0051720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Suh SJ, Jin UH, Choi HJ, Chang HW, Son JK, Lee SH, Jeon SJ, Son KH, Chang YC, Lee YC, Kim CH. Cryptotanshinone from Salvia miltiorrhiza BUNGE has an inhibitory effect on TNF-alpha-induced matrix metalloproteinase-9 production and HASMC migration via down-regulated NF-kappaB and AP-1. Biochem Pharmacol. 2006;72:1680–1689. doi: 10.1016/j.bcp.2006.08.013. [DOI] [PubMed] [Google Scholar]
- 5.Zhou Z, Wang SQ, Liu Y, Miao AD. Cryptotanshinone inhibits endothelin-1 expression and stimulates nitric oxide production in human vascular endothelial cells. Biochim Biophys Acta. 2006;1760:1–9. doi: 10.1016/j.bbagen.2005.09.009. [DOI] [PubMed] [Google Scholar]
- 6.Jin YC, Kim CW, Kim YM, Nizamutdinova IT, Ha YM, Kim HJ, Seo HG, Son KH, Jeon SJ, Kang SS, Kim YS, Kam SC, Lee JH, Chang KC. Cryptotanshinone, a lipophilic compound of Salvia miltiorrriza root, inhibits TNF-alpha-induced expression of adhesion molecules in HUVEC and attenuates rat myocardial ischemia/reperfusion injury in vivo. Eur J Pharmacol. 2009;614:91–97. doi: 10.1016/j.ejphar.2009.04.038. [DOI] [PubMed] [Google Scholar]
- 7.Ang KP, Tan HK, Selvaraja M, Kadir AA, Somchit MN, Akim AM, Zakaria ZA, Ahmad Z. Cryptotanshinone attenuates in vitro oxLDL-induced pre-lesional atherosclerotic events. Planta Med. 2011;77:1782–1787. doi: 10.1055/s-0030-1271119. [DOI] [PubMed] [Google Scholar]
- 8.Sawamura T, Kume N, Aoyama T, Moriwaki H, Hoshikawa H, Aiba Y, Tanaka T, Miwa S, Katsura Y, Kita T, Masaki T. An endothelial receptor for oxidized low-density lipoprotein. Nature. 1997;386:73–77. doi: 10.1038/386073a0. [DOI] [PubMed] [Google Scholar]
- 9.Chen XP, Zhang TT, Du GH. Lectin-like oxidized low-density lipoprotein receptor-1, a new promising target for the therapy of atherosclerosis? Cardiovasc Drug Rev. 2007;25:146–161. doi: 10.1111/j.1527-3466.2007.00009.x. [DOI] [PubMed] [Google Scholar]
- 10.Renier G. Lectin-like oxidized low-density lipoprotein receptor-1 (LOX-1), a relevant target for diabetic vasculopathy? Cardiovasc Hematol Disord Drug Targets. 2008;8:203–211. doi: 10.2174/187152908785849107. [DOI] [PubMed] [Google Scholar]
- 11.Ulrich-Merzenich G, Zeitler H. The lectin-like oxidized low-density lipoprotein receptor-1 as therapeutic target for atherosclerosis, inflammatory conditions and longevity. Expert Opin Ther Targets. 2013;17:905–919. doi: 10.1517/14728222.2013.805748. [DOI] [PubMed] [Google Scholar]
- 12.Chen XP, DU GH. Lectin-like oxidized low-density lipoprotein receptor-1: protein, ligands, expression and pathophysiological significance. Chin Med J (Engl) 2007;120:421–426. [PubMed] [Google Scholar]
- 13.Kume N, Murase T, Moriwaki H, Aoyama T, Sawamura T, Masaki T, Kita T. Inducible expression of lectin-like oxidized LDL receptor-1 in vascular endothelial cells. Circ Res. 1998;83:322–327. doi: 10.1161/01.res.83.3.322. [DOI] [PubMed] [Google Scholar]
- 14.Moriwaki H, Kume N, Kataoka H, Murase T, Nishi E, Sawamura T, Masaki T, Kita T. Expression of lectin-like oxidized low density lipoprotein receptor-1 in human and murine macrophages: upregulated expression by TNF-alpha. FEBS Lett. 1998;440:29–32. doi: 10.1016/s0014-5793(98)01414-8. [DOI] [PubMed] [Google Scholar]
- 15.Kume N, Moriwaki H, Kataoka H, Minami M, Murase T, Sawamura T, Masaki T, Kita T. Inducible expression of LOX-1, a novel receptor for oxidized LDL, in macrophages and vascular smooth muscle cells. Ann N Y Acad Sci. 2000;902:323–327. doi: 10.1111/j.1749-6632.2000.tb06332.x. [DOI] [PubMed] [Google Scholar]
- 16.Chiba Y, Ogita T, Ando K, Fujita T. PPARgamma ligands inhibit TNF-alpha-induced LOX-1 expression in cultured endothelial cells. Biochem Biophys Res Commun. 2001;286:541–546. doi: 10.1006/bbrc.2001.5361. [DOI] [PubMed] [Google Scholar]
- 17.Shibata Y, Kume N, Arai H, Hayashida K, Inui-Hayashida A, Minami M, Mukai E, Toyohara M, Harauma A, Murayama T, Kita T, Hara S, Kamei K, Yokode M. Mulberry leaf aqueous fractions inhibit TNF-alpha-induced nuclear factor kappaB (NF-kappaB) activation and lectin-like oxidized LDL receptor-1 (LOX-1) expression in vascular endothelial cells. Atherosclerosis. 2007;193:20–27. doi: 10.1016/j.atherosclerosis.2006.08.011. [DOI] [PubMed] [Google Scholar]
- 18.Lee MJ, Lee HS, Park SD, Moon HI, Park WH. Leonurus sibiricus herb extract suppresses oxidative stress and ameliorates hypercholesterolemia in C57BL/6 mice and TNF-alpha induced expression of adhesion molecules and lectin-like oxidized LDL receptor-1 in human umbilical vein endothelial cells. Biosci Biotechnol Biochem. 2010;74:279–284. doi: 10.1271/bbb.90582. [DOI] [PubMed] [Google Scholar]
- 19.Song G, Tian H, Liu J, Zhang H, Sun X, Qin S. H2 inhibits TNF-α-induced lectin-like oxidized LDL receptor-1 expression by inhibiting nuclear factor κB activation in endothelial cells. Biotechnol Lett. 2011;33:1715–1722. doi: 10.1007/s10529-011-0630-8. [DOI] [PubMed] [Google Scholar]
- 20.Yamagata K, Tusruta C, Ohtuski A, Tagami M. Docosahexaenoic acid decreases TNF-α-induced lectin-like oxidized low-density lipoprotein receptor-1 expression in THP-1 cells. Prostaglandins Leukot Essent Fatty Acids. 2014;90:125–132. doi: 10.1016/j.plefa.2013.12.011. [DOI] [PubMed] [Google Scholar]
- 21.Chen X, Zhong Z, Xu Z, Chen L, Wang Y. No protective effect of curcumin on hydrogen peroxide-induced cytotoxicity in HepG2 cells. Pharmacol Rep. 2011;63:724–732. doi: 10.1016/s1734-1140(11)70584-9. [DOI] [PubMed] [Google Scholar]
- 22.Chen X, Zhong Z, Xu Z, Chen L, Wang Y. 2',7'-Dichlorodihydr ofluorescein as a fluorescent probe for reactive oxygen species measurement: Forty years of application and controversy. Free Radic Res. 2010;44:587–604. doi: 10.3109/10715761003709802. [DOI] [PubMed] [Google Scholar]
- 23.Zhang T, Huang Z, Dai Y, Chen X, Zhu P, Du G. The expression of recombinant human LOX-1 and identifying its mimic ligands by fluorescence polarization-based high throughput screening. J Biotechnol. 2006;125:492–502. doi: 10.1016/j.jbiotec.2006.03.040. [DOI] [PubMed] [Google Scholar]
- 24.Chen X, Andresen BT, Hill M, Zhang J, Booth F, Zhang C. Role of reactive oxygen species in tumor necrosis factor-alpha induced endothelial dysfunction. Curr Hypertens Rev. 2008;4:245–255. doi: 10.2174/157340208786241336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang H, Park Y, Wu J, Chen Xp, Lee S, Yang J, Dellsperger KC, Zhang C. Role of TNF-alpha in vascular dysfunction. Clin Sci (Lond) 2009;116:219–230. doi: 10.1042/CS20080196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dunn S, Vohra RS, Murphy JE, Homer-Vanniasinkam S, Walker JH, Ponnambalam S. The lectin-like oxidized low-density-lipoprotein receptor: a pro-inflammatory factor in vascular disease. Biochem J. 2008;409:349–355. doi: 10.1042/BJ20071196. [DOI] [PubMed] [Google Scholar]
- 27.Liang M, Zhang P, Fu J. Up-regulation of LOX-1 expression by TNF-alpha promotes trans-endothelial migration of MDA- MB-231 breast cancer cells. Cancer Lett. 2007;258:31–37. doi: 10.1016/j.canlet.2007.08.003. [DOI] [PubMed] [Google Scholar]
- 28.Chen X, Zhang H, McAfee S, Zhang C. The reciprocal relationship between adiponectin and LOX-1 in the regulation of endothelial dysfunction in ApoE knockout mice. Am J Physiol Heart Circ Physiol. 2010;299:H605–H612. doi: 10.1152/ajpheart.01096.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nagase M, Ando K, Nagase T, Kaname S, Sawamura T, Fujita T. Redox-sensitive regulation of lox-1 gene expression in vascular endothelium. Biochem Biophys Res Commun. 2001;281:720–725. doi: 10.1006/bbrc.2001.4374. [DOI] [PubMed] [Google Scholar]
- 30.Sun Y, Chen X. Ox-LDL-induced LOX-1 expression in vascular smooth muscle cells: role of reactive oxygen species. Fundam Clin Pharmacol. 2011;25:572–579. doi: 10.1111/j.1472-8206.2010.00885.x. [DOI] [PubMed] [Google Scholar]
- 31.Pirillo A, Reduzzi A, Ferri N, Kuhn H, Corsini A, Catapano AL. Upregulation of lectin-like oxidized low-density lipoprotein receptor-1 (LOX-1) by 15-lipoxygenase-modified LDL in endothelial cells. Atherosclerosis. 2011;214:331–337. doi: 10.1016/j.atherosclerosis.2010.11.006. [DOI] [PubMed] [Google Scholar]
- 32.Li L, Sawamura T, Renier G. Glucose enhances endothelial LOX-1 expression: role for LOX-1 in glucose-induced human monocyte adhesion to endothelium. Diabetes. 2003;52:1843–1850. doi: 10.2337/diabetes.52.7.1843. [DOI] [PubMed] [Google Scholar]
- 33.Fu R, Yan T, Wang Q, Guo Q, Yao H, Wu X, Li Y. Suppression of endothelial cell adhesion by XJP-1, a new phenolic compound derived from banana peel. Vascul Pharmacol. 2012;57:105–112. doi: 10.1016/j.vph.2012.05.006. [DOI] [PubMed] [Google Scholar]
- 34.Lee WY, Liu KW, Yeung JH. Reactive oxygen species-mediated kinase activation by dihydrotanshinone in tanshinones-induced apoptosis in HepG2 cells. Cancer Lett. 2009;285:46–57. doi: 10.1016/j.canlet.2009.04.040. [DOI] [PubMed] [Google Scholar]
- 35.Chen W, Liu L, Luo Y, Odaka Y, Awate S, Zhou H, Shen T, Zheng S, Lu Y, Huang S. Cryptotanshinone activates p38/JNK and inhibits Erk1/2 leading to caspase-independent cell death in tumor cells. Cancer Prev Res (Phila) 2012;5:778–787. doi: 10.1158/1940-6207.CAPR-11-0551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mukai E, Kume N, Hayashida K, Minami M, Yamada Y, Seino Y, Kita T. Heparin-binding EGF-like growth factor induces expression of lectin-like oxidized LDL receptor-1 in vascular smooth muscle cells. Atherosclerosis. 2004;176:289–296. doi: 10.1016/j.atherosclerosis.2004.03.028. [DOI] [PubMed] [Google Scholar]
- 37.Li L, Sawamura T, Renier G. Glucose enhances human macrophage LOX-1 expression: role for LOX-1 in glucose-induced macrophage foam cell formation. Circ Res. 2004;94:892–901. doi: 10.1161/01.RES.0000124920.09738.26. [DOI] [PubMed] [Google Scholar]
- 38.Liu Z, Xu S, Huang X, Wang J, Gao S, Li H, Zhou C, Ye J, Chen S, Jin ZG, Liu P. Cryptotanshinone, an orally bioactive herbal compound from Danshen, attenuates atherosclerosis in apolipoprotein E-deficient mice: role of lectin-like oxidized LDL receptor-1 (LOX-1) Br J Pharmacol. 2015 doi: 10.1111/bph.13068. 10.1111/bph.13068 [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]