Abstract
Pro-inflammatory cytokine and brain-derived neurotrophic factor (BDNF) are modulated in post-traumatic stress disorder (PTSD). This study investigated the effects of ibuprofen (IBU) on enhanced anxiety in a rat model of PTSD induced by a single prolonged stress (SPS) procedure. The effects of IBU on inflammation and BDNF modulation in the hippocampus and the mechanisms underlying for anxiolytic action of IBU were also investigated. Male Sprague-Dawley rats were given IBU (20 or 40 mg/kg, i.p., once daily) for 14 days. Daily IBU (40 mg/kg) administration signifi cantly increased the number and duration of open arm visits in the elevated plus maze (EPM) test, reduced the anxiety index in the EPM test, and increased the time spent in the center of an open fi eld after SPS. IBU administration signifi cantly decreased the expression of pro-inflammatory mediators, such as tumor necrosis factor-α, interleukin-1β, and BDNF, in the hippocampus, as assessed by reverse transcription-polymerase chain reaction analysis and immunohistochemistry. These fi ndings suggest that IBU exerts a therapeutic effect on PTSD that might be at least partially mediated by alleviation of anxiety symptoms due to its anti-inflammatory activity and BDNF expression in the rat brain.
Keywords: Anxiety, Ibuprofen, Inflammation, Post-traumatic stress disorder, Single prolonged stress
INTRODUCTION
Post-traumatic stress disorder (PTSD), an anxiety disorder recently reclassified as a trauma- and stressor-related disorder, can develop in response to real or perceived life-threatening situations [1]. This psychopathological response to traumatic stressors is characterized by intense memories in which patients re-experience the original traumatic experience, the avoidance of trauma-related stimuli, intrusive recollections, the avoidance of associated stimuli, negative cognitions/mood, hyper-arousal, and marked social impairment [2,3]. PTSD is often co-morbid with anxiety and major depression, as well as with substance abuse, sleep disturbances, and marked psychosocial and occupational impairment [4,5].
Although selective serotonin reuptake inhibitors (SSRIs) have proven to be very efficacious in many sufferers, they have adverse effects that limit their utility, including cognitive dysfunction, weight gain, sexual dysfunction, sedation, dependence, and withdrawal. There is a major unmet medical need to find more promising treatments for PTSD [6]. Consequently, much attention has been devoted to the use of new non-steroidal anti-inflammatory drugs (NSAIDs) that are safer for long-term treatment [7].
Ibuprofen (IBU) is an NSAIDs that is used widely to reduce pain, fever, and inflammation. In animal models of stroke, IBU reduces neuronal injury and improves cerebral blood flow and neurological outcome in global ischemia [8], and decreases infarct size in focal ischemia [9]. IBU also suppresses cerebral plaque formation and inflammation in a mouse model of Alzheimer's disease [10]. These observations have led to the hypothesis that IBU may also be effective for improving PTSD-associated psychiatric symptoms, such as anxiety.
While endocrine changes associated with PTSD have been researched extensively, findings during the last decade also suggest that alternations in the immune system are strongly linked with PTSD [11]. Cytokines, most notably interleukin-1α (IL-1β), interleukin-6 (IL-6), and tumor necrosis factor-α (TNF-α), are thought to mediate the inflammatory dysregulation observed in PTSD [12,13]. Recently, circulating IL-1β and TNF-α levels were reported to be chronically elevated in PTSD patients [14].
Brain-derived neurotrophic factor (BDNF) is one of several endogenous proteins that play critical roles in the survival, maintenance, and growth of the brain and peripheral neurons [15]. Evidence suggests that BDNF mediates the pathophysiology of mood disorders, including PTSD [16]. A recent study reported significantly lower plasma BDNF levels in PTSD patients compared with healthy controls [17]. These findings support the idea that novel pharmacological tools targeting inflammatory mediators and BDNF expression might be promising new anti-PTSD drugs.
To evaluate the effects of IBU on inflammation and BDNF modulation, we used a single prolonged stress (SPS) animal model of PTSD that demonstrates three hallmark features of the disorder: hormonal abnormalities, long-lasting traumatic memories, and persistent anxiety [18]. This model also possesses both predictive and construct validity, making it sensitive to effective pharmacologic agents while displaying similarities to human PTSD [19].
This study investigated whether IBU affects SPS-induced anxiety-like behavior, representing the core symptom of PTSD-related abnormalities. Anxiety-like behaviors were assessed using the elevated plus maze (EPM) test and open field test (OFT). The neurobiological mechanism underlying these behaviors was also investigated by evaluating inflammatory activity and BDNF expression in the hippocampus of rats following SPS.
METHODS
Animals and IBU administration
The present study utilized adult male Sprague-Dawley (SD) rats weighing 200~220 g (6 weeks-old, Samtako Animal Company; Seoul, Korea). All rats were housed in a limited access rodent facility with five rats per polycarbonate cage. The room controls were set to maintain the temperature at 22±2℃ and the relative humidity at 55±15%, the cages were lit by artificial light for 12 h each day, and sterilized drinking water and a standard chow diet were supplied ad libitum throughout the experiments. All animal experiments began a minimum of 7 days after the animals arrived, were conducted in accordance with the Guide for the Care and Use of Laboratory Animals from the National Institutes of Health (NIH Publications No. 80-23; revised in 1996), and were approved by the Kyung Hee University Institutional Animal Care and Use Committee. All efforts were made to minimize the number and suffering of animals.
Different groups of rats, 6~7 animals per group, were used for drugs treatment and tests. All the experimental animals including control and drug-treatment groups were administration. The standard doses of IBU in the rat and considering the long-term treatment used in the present study was based on previous study [20]. Ibuprofen (20 and 40 mg/kg, body weight; Sigma-Aldrich Chemical Co. St. Louise, MO, USA) and the positive drug fluoxetine (10 mg/kg, FLX, Fluoxetine hydrochloride; Eli Lilly and Company, Basingstoke, Hampshire) were administered by intraperitoneally (i.p.) in a volume of 1 ml/kg for 14 days. All drugs were freshly prepared right before every experiment.
Single prolonged stress
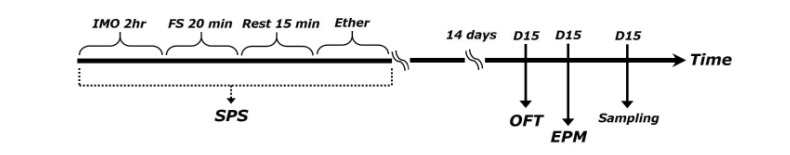
SPS model of PTSD was performed as previously described [21]. Briefly, rats were first immobilized for 2 h on restraint tubes (20 cm height, 7 cm diameter). Following immobilization, rats were immediately subjected to forced swim for 20 min in a plexiglass cylinder (50 cm height, 20 cm diameter) filled to two-thirds with 24℃ fresh water. The animals were dried and allowed to recuperate for 15 min and then exposed to ether vapor until loss of consciousness. The SPS procedure was performed between 10 AM and 4 PM. Following SPS stressors, animals were housed one per cage and left undisturbed for 14 days to allow PTSD-like symptoms to manifest [21]. The following parameters were measured to monitor the effects of the development of psychiatric disorders by SPS model of PTSD: changes of body weight gains and serum corticosterone (CORT) levels. Behavioral testing for anxiety-like behaviors was done 24 h after the end of the undisturbed condition. The entire experimental schedules of SPS and behavioral examinations are shown in Fig. 1. To avoid carryover from one test to another, each behavioral test was performed on separate or interval between each behavioral test. Following behavioral testing and the measurement of body weight, all rats were sacrificed and their brain tissues were collected immediately for use in the study or stored at -70℃ for later use.
Fig. 1. Experimental schedule used for developing SPS-induced anxiety-like behaviors and IBU treatment.
Different groups of rats, with seven animals per group, were used for all experiments.
Elevated plus maze test
Animals exhibiting anxiety-like behaviors typically experience a reduced number of entries and amount of time spent in the open arms of the maze and, thus, an increased amount of time in the closed arms of the maze. This apparatus used in the present study consisted of two open arms (50×10 cm each), two closed arms (50×10×20 cm each) and a central platform (10×10 cm) arranged such that the open arms and closed arms were directly opposite each other. The EPM apparatus was constructed from black Plexiglas and elevated 50 cm above the floor. Exploration of the open arms was encouraged by testing under indirect dim light (2×60 W). At the beginning of each trial, the animals were placed in the center of the maze facing a closed arm, and the following parameters were recorded during the 5-min test period: number of open-arm entries, number of closed-arm entries, time spent in open arms, and time spent in closed arms. Entry into an arm was defined as the placement of four paws within a particular arm. The maze was cleaned with alcohol after each subject had been tested. Behavior in the maze was recorded by a video camera mounted on the ceiling above the center of the maze with the S-MART program (PanLab; Barcelona, Spain). Anxiety reduction was defined as an increase in the number of entries into the open arms relative to total entries into both the open and closed arms, and total arm entries were used as an indicator of changes in locomotor activity. Total arm entries were also used as indicators of changes in locomotor activities of the rats. Anxiety index was calculated as follows:
Anxiety index values range from 0 to 1 where an increase in the index expresses increased anxiety-like behavior [21].
Open field test
Before the completion of EPM test, the animals were subjected to the open field test, normally used to assess emotionality, based on the same conflict situation as in the EMP. The open filed area consisted of an enclosed square arena made of dark opaque Plexiglas (60×60 cm) surrounded by walls (30 cm high). The arena was divided by transverse lines into 20×20 equal squares (total 9 squares). A central light source (60 W) on the ceiling gave invariant illumination in an otherwise dark room. All locomotor activities (animal's movements) was measured by a video camera mounted above the center of the maze. The speed and distance of the movements of the rats were monitored by a computerized video-tracking system using the S-MART program (PanLab Co., Barcelona, Spain). The rat to be tested was transported from the home cage to the recording room in a black box. Each rat was gently placed into the same corner of the arena facing the same direction and allowed to feely explore the arena for 5 min. Every time both hind limbs entered a square, a crossing was recorded. The locomotor activity was evaluated total distance traveled in the container, and exploratory activity was evaluated from measurements of total number of line crossings [22]. The floor surface of each chamber was cleaned thoroughly with 70% ethanol between tests for different subjects.
Corticosterone measurement
For this, the unanesthetized rats were rapidly decapitated, and blood was quickly collected via the abdominal aorta. Rats were randomly divided into five groups consisting of four individuals each, the same as above. The blood samples were centrifuged at 4000 g for 10 min, and serum was collected. The CORT concentration was measured by a competitive enzyme-linked immunosorbent assay (ELISA) using a rabbit polyclonal CORT antibody (Abcam Corticosterone ELISA kit; Cambridge, MA, USA) according to the manufacturer's protocol. Samples (or standard) and conjugate were added to each well, and the plate was incubated for 1 h at room temperature without blocking. After the wells were washed several times with buffers and proper color developed, the optical density was measured at 450 nm using an ELISA reader (MultiRead 400; Authos Co., Vienna, Austria).
Immunohistochemistry for proinflammatory markers
For immunohistochemical studies, three rats in each group were deeply anesthetized with sodium pentobarbital (80 mg/kg, by intraperitoneal injection) and perfused through the ascending aorta with normal saline (0.9%), followed by 300 ml (per rat) of 4% paraformaldehyde in 0.1 M phosphate-buffered saline (PBS). The brains were removed in a randomized order, post-fixed overnight, and cryoprotected with 20% sucrose in 0.1 M PBS at 4℃. Coronal sections 30 µm thick were serially cut through the hippocampus using a cryostat (Leica CM1850; Leica Microsystems Ltd., Nussloch, Germany). The sections were obtained according to the rat atlas of Paxinos and Watson (hippocampus; between bregma -2.6 and -3.6) [23]. The sections were immunostained for TNF-α and IL-1β expression using the avidin-biotin-peroxidase complex (ABC) method. The sections were incubated with primary rabbit anti-TNF-α antibody (1:200 dilution; Novus Biochemicals LLC., Littleton, CO, USA) and rabbit anti-IL-1β antibody (1:200 dilution; Santa Cruz Biotechnology Inc., Santa Cruz, CA, USA) in PBST (PBS plus 0.3% Triton X-100) for 48 h at 4℃, respectively. The sections were incubated for 120 min at room temperature with secondary antibody. The secondary antibodies were obtained from Vector Laboratories Co. (Burlingame, CA, USA) and diluted 1:200 in PBST containing 2% normal serum. To visualize immunoreactivity, the sections were incubated for 90 min in ABC reagent (Vectastain Elite ABC kit; Vector Labs. Co., Burlingame, CA, USA), and incubated in a solution containing 3,3'-diaminobenzidine (DAB; Sigma) and 0.01% H2O2 for 1 min. Images were captured using an AxioVision 3.0 imaging system (Carl Zeiss, Inc., Oberkochen, Germany) and processed using Adobe Photoshop (Adobe Systems, Inc., San Jose, CA, USA). The sections were viewed at 200× magnification, and the numbers of cells within 100×100-µm2 grids were counted by observers blinded to the experimental groups. Counting immunopositive cells was performed in at least three different hippocampal sections per rat brain. Stained sections were randomly chosen from equal levels of serial sections along the rostral-caudal axis. The TNF-α- and IL-1β-immunopositive cells were only counted when their densities reached a defined darkness above the background level. Distinct brown spots for TNF-α and IL-1β were observed in the cytoplasms and in the membranes of the cone-shaped cells of the hippocampus. The cells were anatomically localized according to the stereotactic rat brain atlas of Paxinos and Watson [23]. The brightness and contrast between images were not adjusted to exclude any possibility of subjective selection of immunoreactive cells.
Total RNA preparation and reverse transcription-polymerase chain reaction
The expression levels of TNF-α, IL-1β, and BDNF mRNAs were determined by the reverse transcription-polymerase chain reaction (RT-PCR). The brain hippocampus was isolated from three rats per group. The total RNAs were prepared from the brain tissues using a TRIzol® reagent (Invitrogen Co., Carlsbad, CA, USA) according to the supplier's instructions. Complementary DNA was first synthesized from total RNA using a reverse transcriptase (Takara Co., Shiga, Japan). PCR was performed using a PTC-100 programmable thermal controller (MJ Research, Inc., Watertown, MA, USA). All primers were designed using published mRNA sequences of those cytokines and a primer designing software, Primer 3, offered by the Whitehead Institute for Biomedical Research (Cambridge, MA, USA; http://primer3.wi.mit.edu) on the website. The PCR products were separated on 1.2% agarose gels and stained with ethidium bromide. The density of each band was quantified using an image-analyzing system (i-Max™, CoreBio System Co., Seoul, Korea). The expression levels were compared each other by calculating the relative density of target band, such as TNF-α, IL-1β, and BDNF, to that of glyceraldehyde 3-phosphate dehydrogenase (GAPDH).
Statistical analysis
All measurements were performed by an independent investigator blinded to the experimental conditions, and the results are expressed as means±standard error of the mean (SE). Differences within or between normally distributed data were analyzed using an analysis of variance (ANOVA) using SPSS (Version 13.0, SPSS, Inc.; Chicago, IL, USA) followed by Tukey's post hoc test. Statistical significance was set at p<0.05.
RESULTS
Effects of IBU on changes in body weight and serum CORT levels following single prolonged stress
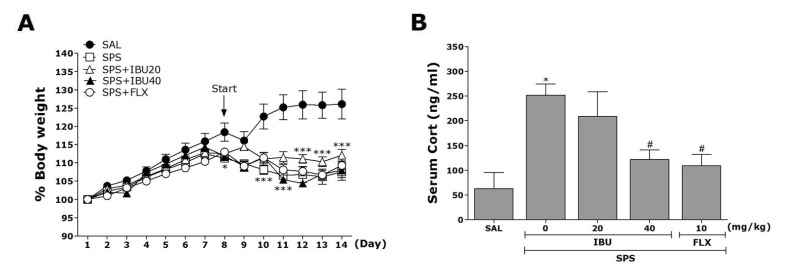
We measured the body weight of each rat in each group for 14 days. In the present study, rats exposed to single prolonged stress began to lose body weight on the first day of the SPS procedure (difference between daily weights and starting weight of the SPS procedure; Fig. 2A). Compared with the control rats (SAL group), subjects in the SPS group showed a significant gradual reduction in body weight gain over 7 days. However, subjects treated with 20 or 40 mg/kg IBU exhibited no significant differences of this reduction in body weight gain compared with the SPS group.
Fig. 2. The effects of IBU administration on body weights gain (A) and serum CORT levels (B) of rats exposed to SPS.
*p<0.05, ***p<0.001 vs. SAL group; #p<0.05 vs. SPS group.
Serum CORT levels were also measured in each group following the single prolonged stress for 7 days (Fig. 2B). A ELISA revealed that single prolonged stress significantly increased serum CORT concentration by 402.24% compared to saline-treated rats (p<0.05). However, the administration of IBU significantly inhibited the single prolonged stress-induced increase in serum CORT levels (p<0.05). Thus, the single prolonged stress procedure induced anxiety-like symptoms in rats, and this was exploited to develop the PTSD. Inhibition of increase in serum CORT levels by IBU also hardly provide the basis for the inference that SPS induces anxiety-like symptoms.
Effects of IBU on anxiety-like behavior following single prolonged stress
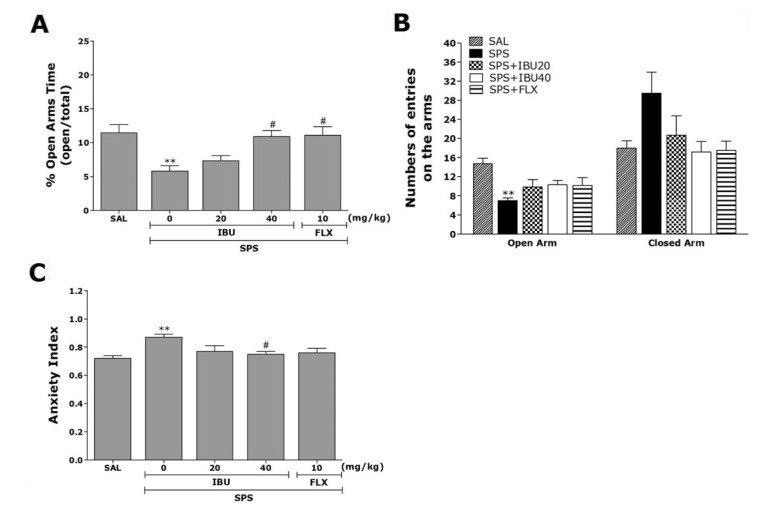
The impact of single prolonged stress on different parameters of anxiety-like behaviors is shown in Fig. 3. Seven days after single prolonged stress were applied rats demonstrated lower exploration behavior and higher anxiety index compared to the SAL group. The effects of IBU administration on anxiety-like behaviors, which were characterized by decreases open arm exploration in the EPM test, were also investigated. Post hoc comparisons revealed a significant decrease in the percentage of time spent in the open arms of the maze by rats exposed to the single prolonged stress for 7 days compared to the saline-treated rats not exposed to stress (p<0.01; Fig. 3A). However, the rats in the SPS+IBU40 group showed a significant restoration of the percentage of time spent in the open arms of the maze, which had previously decreased following single prolonged stress, compared to the SPS group (p<0.05). Similarly, post hoc comparisons revealed a significant decrease in the number of entries in the open arms of the maze by rats exposed to the single prolonged stress for 7 days compared to the SAL group (p<0.01; Fig. 3B). Rats in the SPS+IBU40 group showed a slightly greater entries in the open arms of the maze, as compared with those in the SPS group, although these differences did not reach statistical significance (p=0.416). Because there were no significant differences in the number of entries into the closed arms among the groups, the observed anxiety-like behaviors of the rats exposed to single prolonged stress were likely not attributable to differences in their locomotor activities (Fig. 3B). The administration of IBU prior single prolonged stress did elicit anxiolytic or anxiogenic behavior in the present study. These findings indicated that the increase in the percentage of time spent into the open arms of the maze in the SPS+ IBU40 group was almost comparable to the percentage of time spent of open arm entries in the SPS+FLX group. Overall anxiety index calculated based on the number of visits and duration in open and closed arms was also different in these five groups of rats with lower values in the IBU treated rats (p<0.05; Fig. 3C).
Fig. 3. The effects of IBU administration on the percentage of time spent in open arms (A), the numbers of entries into open and closed arms (B), and the anxiety index (C) in the EPM test following SPS.
**p<0.01 vs. SAL group; #p<0.05 vs. SPS group.
Effects of IBU on spontaneous locomotor activity and exploratory behaviors following single prolonged stress
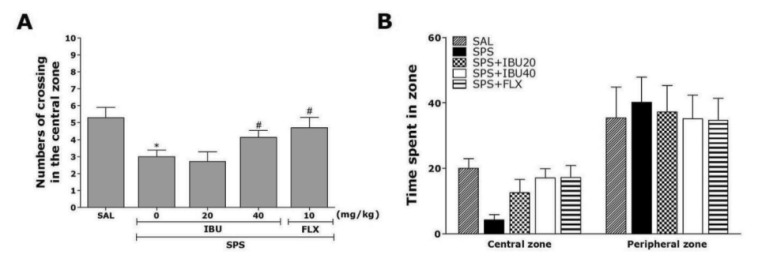
Open-field activity was used to evaluate locomotion and exploratory behavior among the rats exposed to the single prolonged stress for 7 days (Fig. 4). Rats exposed to the single prolonged stress slightly decreased the time spent in central zone with corresponding increase in the time spent in the peripheral zone compared to the SAL group, although these differences did not reach statistical significance (p=0.348). There was also significant decrease the numbers of crossing in the central zone following single prolonged stress (p<0.05). This finding suggests that SPS-treated rats subsequently produce exploration activities that are closely associated with anxiety-like behaviors in the open-field test. However, IBU-treated rats (40 mg/kg) displayed a significant increase in central zone crossings compared to the SPS group (p<0.05), indicating that anxiety-like behaviors in the SPS+IBU40 group was almost comparable to those of the SPS+FLX group.
Fig. 4. The effects of IBU administration on locomotion and exploratory behavior in the open-field test following SPS.
Change of numbers of crossing in the central zone (A) and the time spent in central zone and peripheral zone (B). *p<0.05 vs. SAL group; #p<0.05 vs. SPS group.
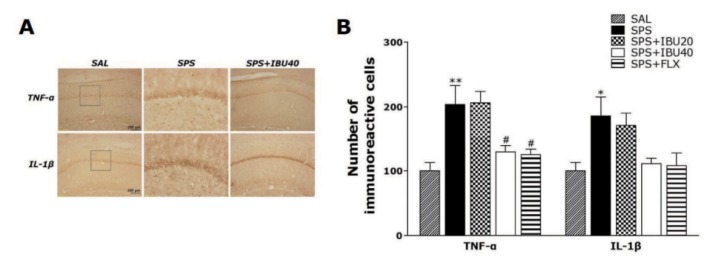
Effects of IBU on immunohistochemical changes of inflammatory mediators in the hippocampus following single prolonged stress
Following the behavioral tasks, brain tissue samples from the rats were analyzed using immunohistochemistry to investigate the effect of administering IBU on the expression of the proinflammatory markers activated by SPS-induced inflammation in the rat brains (Fig. 5). Distinct yellow stains for TNF-α and IL-1β were observed in the cytoplasm and membranes of the coneshaped cells of the hippocampus. The immunoreactivity analysis showed the deepest yellow staining in the rat hippocampus. The brain immunohistochemistry of the SPS group showed a significant increase in TNF-α expression in the hippocampus of this group compared with that in the hippocampus of the SAL group (p<0.01; Fig. 5B). TNF-α immunoreactive cells decreased significantly in the hippocampal regions in the SPS+IBU40 group as compared with the SPS group (p<0.05). Similarly, brain sections taken from the SPS group showed significantly increased IL-1β expression in the hippocampus compared with those taken from the SAL group (p<0.05). Despite the appearance of IL-1β-immunoreactive cells in the hippocampal regions in the SPS+IBU40 group, no significant difference was observed when this group was compared with the SPS group is this regard (p=0.254). Increases in pro-inflammatory mediator such as TNF-α-immunoreactive cells by SPS-induced inflammation were significantly inhibited by IBU and the restoration was similar to that observed in the SPS+FLX group.
Fig. 5. The effects of IBU administration on the mean number of TNF-α- and IL-1β-stained hippocampal areas after behavioral tests.
Representative photographs (A) and the relative percentage values (B). Sections were cut coronally at 30 µm. Scale bar indicates 50 µm. *p<0.05 and **p<0.01 vs. SAL group; #p<0.05 vs. SPS group.
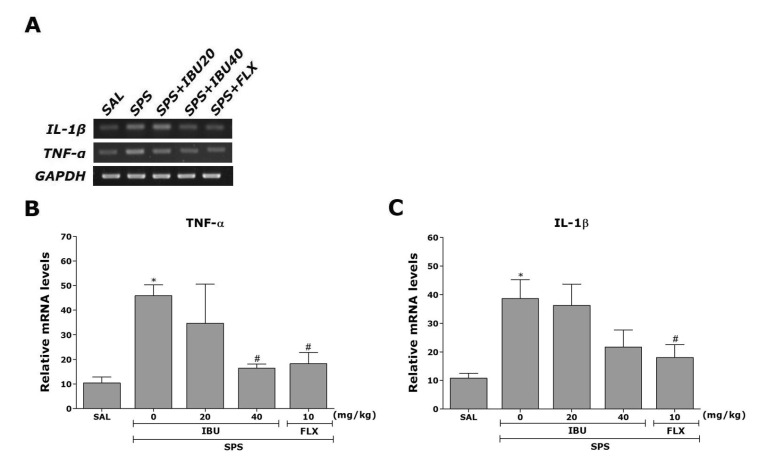
Effects of IBU on TNF-α, IL-1β and BDNF mRNAs in the hippocampus following single prolonged stress
The effects of IBU administration on SPS-induced expression of TNF-α and IL-1β mRNAs in the rat hippocampus was investigated using RT-PCR analysis (Fig. 6). Hippocampal expression of TNF-α and IL-1β mRNAs in the SPS group were significantly increased compared with that in the SAL group (p<0.05). The increased TNF-α mRNA expression in the SPS group was significantly restored in the SPS+IBU40 group (p<0.05). The restored levels of these inflammatory mediators (e.g., TNF-α and IL-1β) were similar to those observed in the SPS+FLX group.
Fig. 6. The effects of IBU on expression of TNF-α and IL-1β mRNAs in rats with SPS-induced hippocampal impairment.
PCR bands on an agarose gel (A) and their relative intensities. The expression of TNF-α (B) and IL-1β (C) mRNAs were normalized to that of glyceraldehyde 3-phosphate dehydrogenase (GAPDH) mRNA as an internal control. *p<0.05 vs. SAL group; #p<0.05 vs. SPS group.
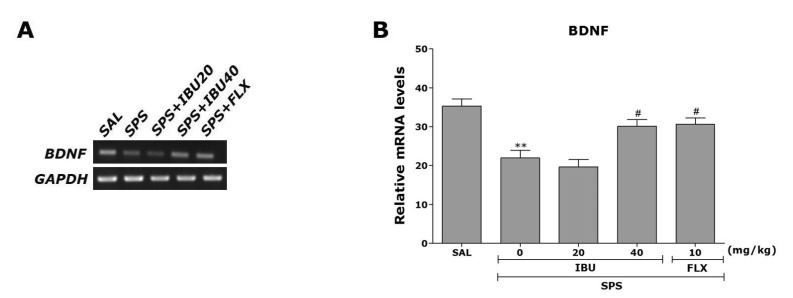
Also, to investigate the effect of IBU administration on SPS-induced expression of BDNF mRNA, which is molecules crucial for anxiety-like behavior, we conducted RT-PCR analysis following single prolonged stress (Fig. 7). Hippocampal expression of BDNF mRNA in the SPS group was significantly decreased compared with that in the SAL group (p<0.01). The decreased expression of BDNF mRNA in the SPS group was significantly restored in the SPS+IBU40 group (p<0.05). This also indicated that the expression of BDNF mRNA in the hippocampus in rats receiving 40 mg/kg IBU administration was closely associated with that in rats receiving 10 mg/kg FLX administration.
Fig. 7. The effects of IBU administration on expression of BDNF mRNA in rats subjected to SPS for 14 consecutive days.
PCR bands on an agarose gel (A) and their relative intensities (B). **p<0.01 vs. SAL group; #p<0.05 vs. SPS group.
DISCUSSION
The results of this study revealed that SPS induced PTSD-like symptoms in rats as evidenced by the EPM test and OFT results. The behavioral effects were likely based on the modulation of inflammatory activity and BDNF expression in the hippocampus in the brains of PTSD animals, which is the primary projection area that underlies the development of anxiety. We found that the administration of IBU before inducing SPS significantly increased the number and duration of open-arm visits in the EPM test, reduced the anxiety index, and increased the time spent at the center in the OFT. IBU appears to act as an anxiolytic, which could lead to the development of novel therapeutics for treating psychiatric diseases in humans.
We used SPS as traumatic stress because it is a reliable model of PTSD, characterized by high face validity and core etiological factors of this disease, such as reduced sensorimotor gating of the startle response, anxiety-like behaviors, and enhanced negative feedback of the hypothalamic-pituitary-adrenal (HPA) axis [21]. Our slightly modified model of SPS clearly increased anxiety-like behaviors in stressed animals compared with the unstressed controls, and consistent with previous findings [21], these animals dealt with stressful situations more successfully.
After SPS, we observed a gradual decrease in body weight gain and an increase in serum CORT levels compared with immediately before testing, indicating that SPS was sufficiently stressful [24,25]. Many studies have shown that, consistent with PTSD models, SPS increases the serum CORT concentrations of rats [24,25]. This forced maintenance of high CORT levels likely supports anxiety-like symptoms; a similar mechanism may underlie the progression or exacerbation of PTSD in humans [21]. Therefore, IBU had anxiolytic activity, suggesting that this therapy inhibits the HPA axis-associated psychological dysfunction by decreasing serum CORT levels, thereby normalizing behavioral and neurochemical responses.
Our behavioral investigations revealed the anxiolytic-like effects of IBU in an animal model of anxiety. Administration of a higher dose of IBU (40 mg/kg) before SPS significantly reduced anxiety-like behaviors in the EPM test [26], as indicated by more entries and exploratory behaviors, and less avoidance of the open arms, yielding lower anxiety indices, i.e., in the EPM test, animals spent much more time in the open arms. The administration of IBU after SPS also significantly reduced anxiety-like behaviors, as indicated by an increase in the total number of line crossings in the OFT, reflecting less activation of the central nervous system (CNS) by novelty and fear [27]. These changes in anxiety-like behaviors appear to have been specific and not the result of impaired locomotor activity, as the track length in the EPM test and OFT was similar for all groups. Accordingly, the greater total number of line crossing observed in the OFT among IBU-treated rats was similar to the behavioral effects observed in the EPM test, likely due to anxiolytic activity. These results suggest that IBU decreased trauma-induced potentiation of vulnerability to the novel stress of the EPM. Therefore, because behaviors in the EPM test and the OFT are related to the HPA axis, these results suggest that inhibition caused by IBU attenuates HPA activity.
The role of inflammation in pathological conditions such as cardiovascular disease, diabetes mellitus, metabolic syndrome, and neurological disease has been established [28]. Some studies have reported higher levels of IL-1β, IL-6, and TNF-α in the plasma or elevated levels of IL-6 in the cerebrospinal fluid of PTSD patients compared with controls [29]. Our results demonstrated that SPS-induced neuroinflammation caused significant increases in the pro-inflammatory mediators in the hippocampus and produced anxiety-like behaviors.
BDNF has also been implicated in the etiology of anxiety and the mechanisms underlying anxiolytic treatments [30]. Clinical investigations have demonstrated that stress-related psychiatric disorders, including anxiety, are associated with reductions in brain BDNF levels [26]. For example, stress following chronic social defeat and SPS results in a significant decrease in BDNF expression in the hippocampus [31]. Consistent with these findings, we found that SPS decreases the expression of BDNF mRNA in the rat hippocampus, as well as decreasing anxiety-like behaviors. IBU restored hippocampal BDNF mRNA levels, suggesting that modulation of BDNF signaling plays a role in the anxiolytic actions of IBU [30].
In summary, this study demonstrated that the administration of IBU is associated with anxiolytic-like effects in the EPM test and OFT, possibly via modification of the neuroinflammation caused by BDNF expression in the hippocampus. IBU may be a useful alternative medicine for treating psychiatric diseases, including PTSD.
ACKNOWLEDGEMENTS
This research was supported by a Grant from the National Research Foundation of Korea funded by the Korean government (MEST) (2013R1A1A2063051).
Footnotes
Author contributions: B.L. concepted and designed. B.L. and B.S. carried out the experiments. B.L. and B.S. acquired data. B.L. and D.H.H. analysed and interpreted data. B.L. drafted the article. B.L. performed statistical analysis. M.Y. I. S. and H. L. supervised the study.
CONFLICTS OF INTEREST: The authors declare no conflicts of interest.
References
- 1.Zhang LM, Zhou WW, Ji YJ, Li Y, Zhao N, Chen HX, Xue R, Mei XG, Zhang YZ, Wang HL, Li YF. Anxiolytic effects of ketamine in animal models of posttraumatic stress disorder. Psychopharmacology (Berl) 2015;232:663–672. doi: 10.1007/s00213-014-3697-9. [DOI] [PubMed] [Google Scholar]
- 2.Brunello N, Davidson JR, Deahl M, Kessler RC, Mendlewicz J, Racagni G, Shalev AY, Zohar J. Posttraumatic stress disorder: diagnosis and epidemiology, comorbidity and social consequences, biology and treatment. Neuropsychobiology. 2001;43:150–162. doi: 10.1159/000054884. [DOI] [PubMed] [Google Scholar]
- 3.Nemeroff CB, Bremner JD, Foa EB, Mayberg HS, North CS, Stein MB. Posttraumatic stress disorder: a state-of-the-science review. J Psychiatr Res. 2006;40:1–21. doi: 10.1016/j.jpsychires.2005.07.005. [DOI] [PubMed] [Google Scholar]
- 4.Ji MH, Jia M, Zhang MQ, Liu WX, Xie ZC, Wang ZY, Yang JJ. Dexmedetomidine alleviates anxiety-like behaviors and cognitive impairments in a rat model of post-traumatic stress disorder. Prog Neuropsychopharmacol Biol Psychiatry. 2014;54:284–288. doi: 10.1016/j.pnpbp.2014.06.013. [DOI] [PubMed] [Google Scholar]
- 5.Rau V, DeCola JP, Fanselow MS. Stress-induced enhancement of fear learning: an animal model of posttraumatic stress disorder. Neurosci Biobehav Rev. 2005;29:1207–1223. doi: 10.1016/j.neubiorev.2005.04.010. [DOI] [PubMed] [Google Scholar]
- 6.Liberzon I, López JF, Flagel SB, Vázquez DM, Young EA. Differential regulation of hippocampal glucocorticoid receptors mRNA and fast feedback: relevance to post-traumatic stress disorder. J Neuroendocrinol. 1999;11:11–17. doi: 10.1046/j.1365-2826.1999.00288.x. [DOI] [PubMed] [Google Scholar]
- 7.Nie H, Peng Z, Lao N, Wang H, Chen Y, Fang Z, Hou W, Gao F, Li X, Xiong L, Tan Q. Rosmarinic acid ameliorates PTSD-like symptoms in a rat model and promotes cell proliferation in the hippocampus. Prog Neuropsychopharmacol Biol Psychiatry. 2014;51:16–22. doi: 10.1016/j.pnpbp.2014.01.002. [DOI] [PubMed] [Google Scholar]
- 8.de Souza CO, Kurauti MA, de Fatima Silva F, de Morais H, Borba-Murad GR, de Andrade FG, de Souza HM. Effects of celecoxib and ibuprofen on metabolic disorders induced by Walker-256 tumor in rats. Mol Cell Biochem. 2015;399:237–246. doi: 10.1007/s11010-014-2250-9. [DOI] [PubMed] [Google Scholar]
- 9.Antezana DF, Clatterbuck RE, Alkayed NJ, Murphy SJ, Anderson LG, Frazier J, Hurn PD, Traystman RJ, Tamargo RJ. High-dose ibuprofen for reduction of striatal infarcts during middle cerebral artery occlusion in rats. J Neurosurg. 2003;98:860–866. doi: 10.3171/jns.2003.98.4.0860. [DOI] [PubMed] [Google Scholar]
- 10.Zara S, De Colli M, Rapino M, Pacella S, Nasuti C, Sozio P, Di Stefano A, Cataldi A. Ibuprofen and lipoic acid conjugate neuroprotective activity is mediated by Ngb/Akt intracellular signaling pathway in Alzheimer's disease rat model. Gerontology. 2013;59:250–260. doi: 10.1159/000346445. [DOI] [PubMed] [Google Scholar]
- 11.Pace TW, Heim CM. A short review on the psychoneuroimmunology of posttraumatic stress disorder: from risk factors to medical comorbidities. Brain Behav Immun. 2011;25:6–13. doi: 10.1016/j.bbi.2010.10.003. [DOI] [PubMed] [Google Scholar]
- 12.Hoge EA, Brandstetter K, Moshier S, Pollack MH, Wong KK, Simon NM. Broad spectrum of cytokine abnormalities in panic disorder and posttraumatic stress disorder. Depress Anxiety. 2009;26:447–455. doi: 10.1002/da.20564. [DOI] [PubMed] [Google Scholar]
- 13.Jones KA, Thomsen C. The role of the innate immune system in psychiatric disorders. Mol Cell Neurosci. 2013;53:52–62. doi: 10.1016/j.mcn.2012.10.002. [DOI] [PubMed] [Google Scholar]
- 14.von Känel R, Hepp U, Kraemer B, Traber R, Keel M, Mica L, Schnyder U. Evidence for low-grade systemic proinflammatory activity in patients with posttraumatic stress disorder. J Psychiatr Res. 2007;41:744–752. doi: 10.1016/j.jpsychires.2006.06.009. [DOI] [PubMed] [Google Scholar]
- 15.Hoffman JR, Ostfeld I, Stout JR, Harris RC, Kaplan Z, Cohen H. β-Alanine supplemented diets enhance behavioral resilience to stress exposure in an animal model of PTSD. Amino Acids. 2015;47:1247–1257. doi: 10.1007/s00726-015-1952-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tyagi E, Agrawal R, Zhuang Y, Abad C, Waschek JA, Gomez-Pinilla F. Vulnerability imposed by diet and brain trauma for anxiety-like phenotype: implications for post-traumatic stress disorders. PLoS One. 2013;8:e57945. doi: 10.1371/journal.pone.0057945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dotta-Panichi RM, Bins HD, Tramontina JF, Ceresér KM, Aguiar BW, Paz AC, Taborda JG. Serum concentrations of brain-derived neurotrophic factor and mental disorders in imprisoned women. Rev Bras Psiquiatr. 2015;37:113–120. doi: 10.1590/1516-4446-2014-1421. [DOI] [PubMed] [Google Scholar]
- 18.Yamamoto S, Morinobu S, Fuchikami M, Kurata A, Kozuru T, Yamawaki S. Effects of single prolonged stress and D-cycloserine on contextual fear extinction and hippocampal NMDA receptor expression in a rat model of PTSD. Neuropsychopharmacology. 2008;33:2108–2116. doi: 10.1038/sj.npp.1301605. [DOI] [PubMed] [Google Scholar]
- 19.Serova LI, Laukova M, Alaluf LG, Pucillo L, Sabban EL. Intranasal neuropeptide Y reverses anxiety and depressive-like behavior impaired by single prolonged stress PTSD model. Eur Neuropsychopharmacol. 2014;24:142–147. doi: 10.1016/j.euroneuro.2013.11.007. [DOI] [PubMed] [Google Scholar]
- 20.Wixey JA, Reinebrant HE, Buller KM. Post-insult ibuprofen treatment attenuates damage to the serotonergic system after hypoxia-ischemia in the immature rat brain. J Neuropathol Exp Neurol. 2012;71:1137–1148. doi: 10.1097/NEN.0b013e318277d4c7. [DOI] [PubMed] [Google Scholar]
- 21.Serova LI, Tillinger A, Alaluf LG, Laukova M, Keegan K, Sabban EL. Single intranasal neuropeptide Y infusion attenuates development of PTSD-like symptoms to traumatic stress in rats. Neuroscience. 2013;236:298–312. doi: 10.1016/j.neuroscience.2013.01.040. [DOI] [PubMed] [Google Scholar]
- 22.Sudakov SK, Nazarova GA, Alekseeva EV, Bashkatova VG. Estimation of the level of anxiety in rats: differences in results of open-field test, elevated plus-maze test, and Vogel's conflict test. Bull Exp Biol Med. 2013;155:295–297. doi: 10.1007/s10517-013-2136-y. [DOI] [PubMed] [Google Scholar]
- 23.Paxinos G, Watson C. The rat brain in stereotaxic coordinates. 3th ed. New York: Academic Press; 1986. pp. 54–85. [Google Scholar]
- 24.Patki G, Li L, Allam F, Solanki N, Dao AT, Alkadhi K, Salim S. Moderate treadmill exercise rescues anxiety and depression-like behavior as well as memory impairment in a rat model of posttraumatic stress disorder. Physiol Behav. 2014;130:47–53. doi: 10.1016/j.physbeh.2014.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Juven-Wetzler A, Cohen H, Kaplan Z, Kohen A, Porat O, Zohar J. Immediate ketamine treatment does not prevent posttraumatic stress responses in an animal model for PTSD. Eur Neuropsychopharmacol. 2014;24:469–479. doi: 10.1016/j.euroneuro.2013.08.007. [DOI] [PubMed] [Google Scholar]
- 26.Shang L, Xu TL, Li F, Su J, Li WG. Temporal dynamics of anxiety phenotypes in a dental pulp injury model. Mol Pain. 2015;11:40–57. doi: 10.1186/s12990-015-0040-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee B, Sur B, Yeom M, Shim I, Lee H, Hahm DH. L-tetrahydropalmatine ameliorates development of anxiety and depression-related symptoms induced by single prolonged stress in rats. Biomol Ther (Seoul) 2014;22:213–222. doi: 10.4062/biomolther.2014.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Acosta SA, Diamond DM, Wolfe S, Tajiri N, Shinozuka K, Ishikawa H, Hernandez DG, Sanberg PR, Kaneko Y, Borlongan CV. Influence of post-traumatic stress disorder on neuroinflammation and cell proliferation in a rat model of traumatic brain injury. PLoS One. 2013;8:e81585. doi: 10.1371/journal.pone.0081585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Peng Z, Wang H, Zhang R, Chen Y, Xue F, Nie H, Chen Y, Wu D, Wang Y, Wang H, Tan Q. Gastrodin ameliorates anxiety-like behaviors and inhibits IL-1beta level and p38 MAPK phosphorylation of hippocampus in the rat model of posttraumatic stress disorder. Physiol Res. 2013;62:537–545. doi: 10.33549/physiolres.932507. [DOI] [PubMed] [Google Scholar]
- 30.Solanki N, Alkadhi I, Atrooz F, Patki G, Salim S. Grape powder prevents cognitive, behavioral, and biochemical impairments in a rat model of posttraumatic stress disorder. Nutr Res. 2015;35:65–75. doi: 10.1016/j.nutres.2014.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Takei S, Morinobu S, Yamamoto S, Fuchikami M, Matsumoto T, Yamawaki S. Enhanced hippocampal BDNF/TrkB signaling in response to fear conditioning in an animal model of posttraumatic stress disorder. J Psychiatr Res. 2011;45:460–468. doi: 10.1016/j.jpsychires.2010.08.009. [DOI] [PubMed] [Google Scholar]