Abstract
This study was performed to investigate whether the spinal cholinergic and serotonergic analgesic systems mediate the relieving effect of electroacupuncture (EA) on oxaliplatin-induced neuropathic cold allodynia in rats. The cold allodynia induced by an oxaliplatin injection (6 mg/kg, i.p.) was evaluated by immersing the rat's tail into cold water (4℃) and measuring the withdrawal latency. EA stimulation (2 Hz, 0.3-ms pulse duration, 0.2~0.3 mA) at the acupoint ST36, GV3, or LI11 all showed a significant anti-allodynic effect, which was stronger at ST36. The analgesic effect of EA at ST36 was blocked by intraperitoneal injection of muscarinic acetylcholine receptor antagonist (atropine, 1 mg/kg), but not by nicotinic (mecamylamine, 2 mg/kg) receptor antagonist. Furthermore, intrathecal administration of M2 (methoctramine, 10 µg) and M3 (4-DAMP, 10 µg) receptor antagonist, but not M1 (pirenzepine, 10 µg) receptor antagonist, blocked the effect. Also, spinal administration of 5-HT3 (MDL-72222, 12 µg) receptor antagonist, but not 5-HT1A (NAN-190, 15 µg) or 5-HT2A (ketanserin, 30 µg) receptor antagonist, prevented the anti-allodynic effect of EA. These results suggest that EA may have a signifi cant analgesic action against oxaliplatin-induced neuropathic pain, which is mediated by spinal cholinergic (M2, M3) and serotonergic (5-HT3) receptors.
Keywords: Acetylcholine, Cold allodynia, Electroacupuncture, Oxaliplatin, Serotonin
INTRODUCTION
Oxaliplatin is a third-generation platinum-based chemotherapeutic agent, which when coupled with 5-fluorouracil and leucovorin (FOLFOX) is one of the most effective chemotherapy regimens for colorectal cancer [1]. Oxaliplatin plays a major role in FOLFOX treatment, and it is widely used as it does not cause nephrotoxicity and ototoxicity compared to other platinum-based drugs such as cisplatin and carboplatin [2,3,4]. However, side effects including paraesthesia and dysesthesia of the hands and feet, aggravated by cold stimulation, limit its clinical usage [4,5]. About 90% of oxaliplatin-treated patients rapidly develop significant pain without motor dysfunction during or shortly after a single infusion peaking at the first 24~48 h [6]. Since no optimum drug without side effects is available to date, an effort to develop an efficient therapeutic method is critically needed.
Acupuncture has been proven to be clinically efficacious in various types of pain, and electroacupuncture (EA) is a procedure that combines acupuncture with electric current stimulation. The analgesic effect of acupuncture or EA on different types of pain has been reported in humans and rodents [7,8]. Studies performed in animals have shown that EA significantly relieves behavioral signs such as hyperalgesia and allodynia in peripheral nerve injury-induced neuropathic pain models [9,10,11]. Clinical trials have also demonstrated the effect of acupuncture on chemotherapy-induced peripheral neuropathy (CIPN) [12,13]. For several decades, many research groups have concentrated their efforts on clarifying the anti-allodynic mechanism of EA. Not only spinal opioidergic receptors but also non-opioidergic receptors (e.g. noradrenergic, serotonergic, cholinergic, GABAergic) have been reported to mediate the analgesic effect by activating the central descending pain inhibitory system. Our previous studies, using peripheral nerve-injured rats, have demonstrated that each of these endogenous analgesic systems take part in the effect of EA [9,11,14,15].
In a study conducted in our laboratory, it has been demonstrated that EA significantly diminishes cold allodynia induced by a single oxaliplatin injection via the opioidergic pathway, but not via the noradrenergic pathway [16]. The analgesic effects of EA and morphine involved opioidergic receptors; however, their analgesic actions were not identical. The pain relieving effect of morphine was stronger in the first 30 minutes, but it diminished rapidly compared to that of EA. Thus, we supposed that other non-opioid analgesic systems such as cholinergic and serotonergic as well as opioidergic systems may play an important role in the relieving effect of EA on oxaliplatin-induced neuropathic pain, as previously published articles that used a different pain model also demonstrate [9,14].
Cholinergic and serotonergic systems are known to be involved in the mechanism of pain attenuation [17], and articles reporting that EA attenuates pain via spinal cholinergic and serotonergic receptors have been published [15,18]. Cholinergic activities are classified into two main receptor types which are muscarinic and nicotinic receptors. Both these receptors are located in the dorsal horn of the spinal cord where nociceptive processing occurs [19] and both muscarinic and nicotinic receptors have several subtypes, of which analgesic mechanisms have not yet been clearly identified yet [20,21]. Serotonin is known to have spinal anti-nociceptive effects, which are dependent on the receptor subtypes [22,23]. Among the several subtypes of serotonin receptors, 5-HT1, 5-HT2 and 5-HT3 receptors are known to be commonly implicated in the spinal pain processing [20,22,24,25].
In this article, we examined the effect of EA on oxaliplatin-induced cold allodynia in rats and determined which cholinergic and serotonergic receptor subtypes mediate the anti-allodynic effect of EA.
METHODS
Experimental animals
Young adult male Sprague-Dawley rats (210~250 g, 7-week-old, Daehan Biolink, Chungbuk, Korea) were housed in cages (3~4 rats per cage) with water and food available ad libitum. The room was maintained with a 12 h-light/dark cycle (light cycle; 08:00~20:00, dark cycle; 20:00~08:00) and room temperature kept at 23±2℃. All animals were acclimated to their environment for 1 week prior to any experiments. All procedures involving animals were approved by the Institutional Animal Care and Use Committee of Kyung Hee University (KHUASP(SE)-15 -088) and were conducted in accordance with the guidelines of the International Association for the Study of Pain [26].
Oxaliplatin injection
As described previously [3,27], oxaliplatin (Sigma, St Louis, MO, USA) was dissolved in a 5% glucose (Sigma, USA) solution at a concentration of 2 mg/ml and was intraperitoneally (i.p.) administered at 6 mg/kg.
EA stimulation
To determine the optimal acupoint for the treatment of oxaliplatin-induced neuropathic pain, EA was administered at three different acupoints after a single injection of oxaliplatin: Quchi (LI11), Zusanli (ST36), and Yaoyangguan (GV3). After baseline cold sensitivity was measured, rats were randomly divided into three groups and EA was administered. LI11 is located at the depression medial to the origin of extensor carpi radialis, at the lateral end of the cubital crease [28]. ST36 is located in the tibialis anterior muscle, 5 mm lateral and distal to the anterior tubercle of the tibia [14]. GV3 acupoint is located between the spinous processes of the fourth and fifth lumbar vertebrae [28]. EA stimulation at ST36 is known to produce analgesic effects on various types of pain, including neuropathic pain, through activation of the endogenous analgesic systems.
EA stimulation at acupoints was carried out by connecting the output terminals of the electrical stimulator (Nihon Kohden, Japan) to the two acupuncture needles. Constant rectangular current pulses (2 Hz, 0.3-ms pulse duration) were applied for 20 min (Fig. 1a). To exclude the stress effect that might be induced by EA stimulation itself, the intensity was adjusted according to the muscle twitch threshold (0.2~0.3 mA).
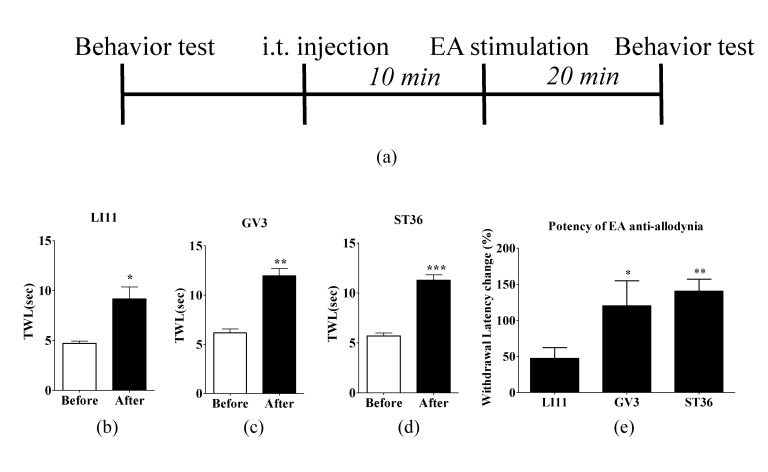
Fig. 1. Experimental schedule and acupoint specific effect of EA on oxaliplatin-induced cold allodynia.
(A) Behavioral tests for cold allodynia were performed before and 20 min after EA treatment in the three groups: (B) LI11 (n=8), (C) GV3 (n=6), and (D) ST36 (n=10). The effect of EA at LI11, GV3 and ST36 significantly increased the TWL. (E) Potency of EA against cold allodynia was significantly greater at GV3 and ST36 comparing with LI11. Data are presented as mean±S.E.M.; ***p<0.001, **p<0.01, *p<0.05; by paired t-test.
Drug treatment
To investigate the role of cholinergic receptors in the analgesic effect of EA in oxaliplatin-administered rats, three different agents were intraperitoneally administered with EA treatment: Normal Saline (NS), atropine (non-selective muscarinic acetylcholine receptor antagonist, 1 mg/kg, Sigma), and mecamylamine (non-selective nicotinic acetylcholine receptor antagonist, 2 mg/kg, Sigma).
Furthermore, to clarify which muscarinic acetylcholine receptor subtypes mediate the analgesic effect of EA, pirenzepine (M1 receptor antagonist, 10 µg), methoctramine (M2 receptor antagonist, 10 µg) and 4-DAMP (M3 receptor antagonist, 10 µg) were injected intrathecally (i.t.). In addition, to observe the effect of various serotonergic receptor subtypes in the analgesic mechanism of EA, NAN-190 (5-HT1A receptor antagonist, 15 µg), ketanserin (5-HT2A receptor antagonist, 30 µg), and MDL-72222 (5-HT3 receptor antagonist, 12 µg) were administered (i.t.). Pirenzepine, methoctramine, and 4-DAMP were dissolved in 50 µl of NS. NAN-190, ketanserin, and MDL-72222 were dissolved in 50 µl of 20% DMSO. All chemicals were obtained from Sigma Chemical Co., USA. To administer the antagonists intrathecally with direct lumbar puncture, rats were anaesthetized with isofluorane and placed on a board in such a way that the spine is curved at the level of the L3~L5 vertebrae. A 1-ml syringe was used to deliver the drugs directly to the spinal cord. Tail or paw flicking was considered as a demonstration of the correct placement of the needle. This method is known to show no motor dysfunction- or stress-induced changes in pain scores [29,30]. The doses of each antagonist was selected based on previously published studies [9,14,25,31,32,33], and the antagonist itself at the concentration used in this study did not affect the behavior after intrathecal administration (Supplementary figure 2).
Behavioral test
To determine whether cold allodynia was induced, cold immersion test was carried out as described previously [9,32,33]. Each animal was gently immobilized in a plastic holder and its tail was drooped for proper application of cold water stimuli. The rats were adapted to the holder for 2 days before starting behavioural tests. The tail was immersed in 4℃ water, and then the tail withdrawal latency (TWL) was measured with a cutoff time of 15 seconds. Before the cold allodynia assessment, a beaker containing the water was put in an ice filled box. We measured the temperature of the water before each assessment to make sure that the temperature is well maintained at 4℃. The cold immersion test was repeated five times at 5 min intervals. The average latency was taken as a measure for the severity of cold allodynia; a shorter TWL was interpreted as more severe allodynia. Because a previous study [16] demonstrated that a significant cold allodynia sign is induced at day three and lasted up to day seven after a single oxaliplatin (6 mg/kg, i.p.) injection, all our tests were conducted at day three after the oxaliplatin injection.
Statistical analysis
All data are presented as mean±S.E.M. Paired t-test was used for statistical analysis. In all cases, p<0.05 was considered to be statistically significant.
RESULTS
Acupoint specific effect of EA on oxaliplatin-induced cold allodynia in rats
To determine the optimal acupoint of EA for oxaliplat-induced cold allodynia, rats were randomly divided into three groups: LI11, GV3, and ST36. The anti-allodynic effects of EA at different acupoints (LI11, GV3, or ST36) are shown in Fig. 1. TWL of all groups was significantly increased after EA stimulation (LI11: p<0.05, Fig. 1B; GV3: p<0.01, Fig. 1C; ST36: p<0.001, Fig. 1D). Comparison of the withdrawal latency change (%) after EA administration at LI11, GV3, and ST36 showed that the potency of EA anti-allodynia was significantly greater in GV3 and ST36 groups than in LI11 group (Fig. 1E). The significant analgesic effect of EA at both ST36 and GV3 lasted until one hour after the administration (Supplementary figure 1).
Effects of non-selective muscarinic and nicotinic acetylcholine receptor antagonists on EA-induced anti-allodynia
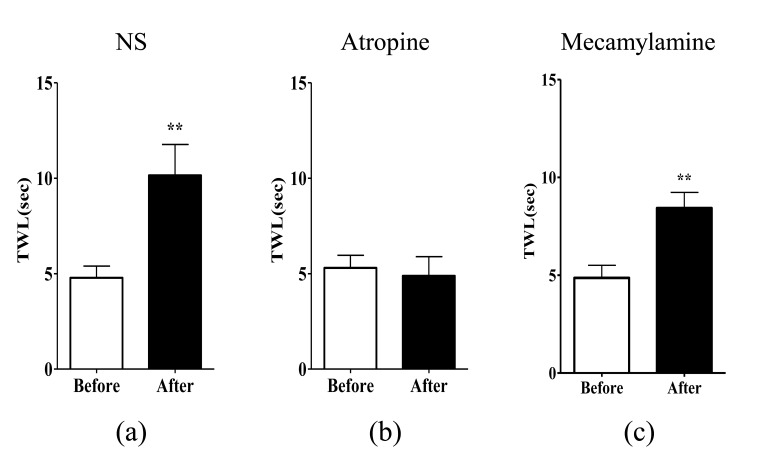
To assess which acetylcholine receptors mediate the suppressive effect of EA at ST36 on cold allodynia, atropine (non-selective muscarinic receptor antagonist) or mecamylamine (non-selective nicotinic receptor antagonist) was administered intraperitoneally to oxaliplatin-injected rats. NS was injected as control. In the NS group, there was a significant increase in TWL (p<0.01, Fig. 2A), which demonstrates the analgesic effect of EA on oxaliplatin-induced cold allodynia in rats. An injection of atropine (1 mg/kg, i.p.) markedly blocked the analgesic effect of EA (p>0.05, Fig. 2B), whereas an injection of mecamylamine (2 mg/kg, i.p.) did not alter the anti-allodynic effect of EA (p<0.01, Fig. 2C). Neither atropine nor mecamylamine itself changed the TWL after intraperitoneal administration (data not shown). This indicates that muscarinic acetylcholine receptors, but not nicotinic acetylcholine receptors, are involved in the relieving effect of EA on oxaliplatin-induced cold allodynia in rats.
Fig. 2. Effects of non-selective muscarinic and nicotinic acetylcholine receptor antagonists on EA-induced anti-allodynia.
Rats with cold allodynia induced by a single injection of oxaliplatin were randomly divided into three groups. The behavioral tests for cold allodynia were performed before an intraperitoneal injection of antagonists and after EA treatment: (A) NS (n=5), (B) atropine (n=5), and (C) mecamylamine (n=7). The analgesic effect of EA at ST36 was blocked by intraperitoneal injection of atropine (non-selective muscarinic receptor antagonist, 1 mg/kg), but not by intraperitoneal injection of mecamylamine (non-selective nicotinic receptor antagonist, 2 mg/kg). Data are presented as mean±S.E.M.; **p<0.01; by paired t-test.
Effects of spinal muscarinic acetylcholine receptor subtype antagonists on EA-induced anti-allodynia
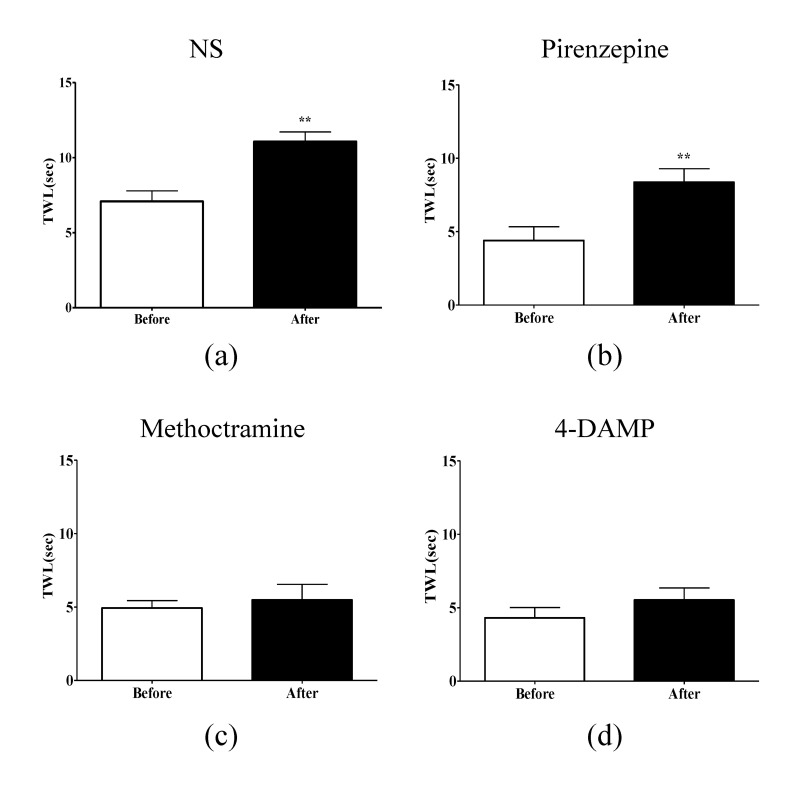
To determine which muscarinic receptor subtypes in the spinal cord play an important role in mediation of the analgesic action of EA, selective muscarinic acetylcholine receptor antagonists: pirenzepine (M1 receptor antagonist), methoctramine (M2 receptor antagonist), and 4-DAMP (M3 receptor antagonist) were injected intrathecally 20 min before treatment with EA (Fig. 3). The injection of NS or pirenzepine did not block the relieving effects of EA on cold allodynia (p<0.01 Fig. 3A, B), whereas injection of methoctramine and 4-DAMP completely blocked the analgesic effect of EA (p>0.05, Fig. 3C, D). These data suggest that spinal M2 and M3 acetylcholine receptor subtypes, but not M1 receptor subtype, are involved in the analgesic effect of EA on cold allodynia evoked by oxaliplatin in rats.
Fig. 3. Effects of spinal muscarinic acetylcholine receptor subtype antagonists on EA-induced anti-allodynia.
The behavioral tests for cold allodynia were performed before the intrathecal injection of receptor antagonists and after EA treatment in four groups: (A) NS (n=4), (B) pirenzepine (n=6), (C) methoctramine (n=8), and (D) 4-DAMP (n=7). Methoctramine (M2 receptor antagonist), and 4-DAMP (M3 receptor antagonist) blocked the analgesic effect of EA, whereas, NS and pirenzepine (M1 receptor antagonist) did not block the analgesic effect of EA. Data are presented as mean±S.E.M.; **p<0.01; by paired t-test.
Effect of spinal serotonergic receptor subtype antagonists on EA-induced anti-allodynia
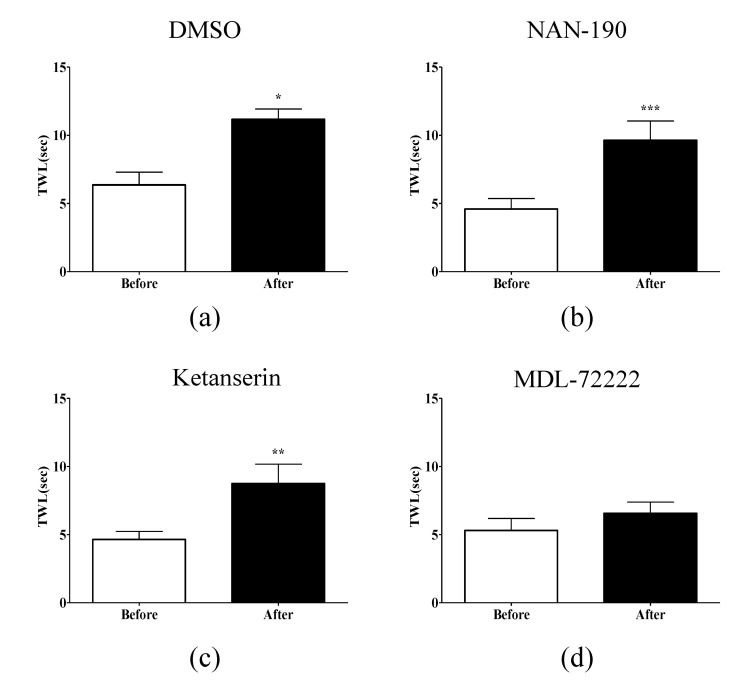
To clarify which serotonin receptor subtypes are involved in the analgesic effect of EA on oxaliplatin-induced cold allodynia, NAN-190 (5-HT1A receptor antagonist), ketanserin (5-HT2A receptor antagonist), and MDL-72222 (5-HT3 receptor antagonist) were administered intrathecally 20 min before treatment with EA. DMSO was injected in the control group. In the DMSO group, there was a significant increase in TWL (p<0.05, Fig. 4A), showing the analgesic effect of EA on oxaliplatin-induced cold allodynia. NAN-190 and ketanserin did not block the analgesic effect of EA (Fig. 4B, C), whereas MDL-72222 significantly blocked the analgesic effect of EA (Fig. 4D). These results indicate that spinal 5-HT3 receptors are involved in the analgesic effect of EA on oxaliplatin-induced cold allodynia in rats, whereas 5-HT1A and 5-HT2A receptors are not involved in the analgesic effect of EA.
Fig. 4. Effect of spinal serotonergic receptor subtypes antagonist on EA-induced anti-allodynia.
The behavioral tests for cold allodynia were performed before the intrathecal injection of antagonists and after EA treatment in four groups: (A) DMSO (n=4), (B) NAN-190 (n=6), (C) ketanserin (n=8), and (D) MDL-72222 (n=6). MDL-72222 (5-HT3 receptor antagonist) completely blocked the analgesic effect of EA; however, DMSO, NAN-190 (5-HT1A receptor antagonist), and ketanserin (5-HT2A receptor antagonist) did not alter the anti-allodynic effect of EA. Data are presented as mean±SEM.; ***p<0.001, **p<0.01, *p<0.05; by paired t-test.
DISCUSSION
Peripheral neuropathy triggered or aggravated in cold conditions after administration of oxaliplatin is an adverse side effect that limits the widespread use of the substance. Anticonvulsants, serotonin-norepinephrine reuptake inhibitors, and topical lidocaine are recommended as first line treatment options, but it has been suggested that they cause side effects such as sedation, dizziness, and cardiac complications [34,35]. Thus, there is a need for better therapeutic options. EA is commonly used in oriental medicine to treat various diseases [8,36]. Recently, it has been reported to be a safe and effective treatment method for CIPN [12,13], and a study from our laboratory has demonstrated the effect of EA in a rat model of oxaliplatin-induced neuropathic pain [16].
This study, firstly, presents experimental evidence suggesting that the analgesic effect of EA is acupoint-specific. Low frequency stimulation with EA (2 Hz, 0.3-ms pulse duration, 0.2~0.3 mA) at the acupoint (ST36 or GV3) proximal to the tail showed a greater anti-allodynic effect, than EA administered at the distal acupoint (LI11). Although EA administered at LI 11, ST36 or GV3 acupoint all had a significant analgesic effect, ST36 was used in our experiments, as the potency of EA anti-allodynia was greater in ST36 group. Also, 2 Hz was chosen, as low frequency was more effective than high frequency in our previous studies (i.e. 2 Hz and 100 Hz, respectively) [9,37].
For many years, our laboratory has conducted various experiments to elucidate the analgesic mechanism of EA in different neuropathic pain rat models, and involvement of opioidergic and non-opiodergic receptors (e.g. noradrenergic, serotonergic, cholinergic, GABAergic) has been reported [9,11,14,37]. However, little is known about the mechanism of EA in CIPN. Previously, we have demonstrated that opioidergic receptors, but not adrenergic receptors, mediate the analgesic effect of EA on oxaliplatin-induced neuropathic pain in rats [16]. But the involvement of other non-opioid receptors located at the spinal cord has not yet been clarified. Therefore, in this study, we have focused on the spinal cholinergic and serotonergic systems.
Our results demonstrate that muscarinic acetylcholine receptors, but not nicotinic acetylcholine receptors, are involved in the anti-allodynic effect of EA. Specifically, spinal M2 and M3 acetylcholine receptors, but not M1 acetylcholine receptors, were found to mediate the effect of EA. Also, spinal 5-HT3 receptors, but not 5-HT1A or 5-HT2A receptors, were shown to mediate the analgesic action of EA against oxaliplatin-induced cold allodynia in rats. These results suggest that the analgesic effect of EA is mediated by M2, M3 acetylcholine receptors and 5-HT3 receptors in the spinal cord. The analgesic effect of EA at GV3 was also blocked by M2, M3, or 5-HT3 receptor antagonist (Supplementary figure 3), suggesting that EA administered at GV3 and ST36 have a same analgesic pathway at the spinal level. Based on our previous results, we suppose that EA action on oxaliplatin-induced neuropathic pain is similar to that of EA action on nerve injury induced neuropathic pain as they are both mediated by muscarinic acetylcholine and serotonergic receptors present at the spinal level. However, subtle differences also exist between the results on nerve-injury and oxaliplatin model: on nerve-injury model, M1 receptor antagonist blocked the anti-allodynic effect [14], whereas on oxaliplatin model M2 or M3 receptor antagonist blocked the effect. Also, 5-HT3 receptor antagonist was shown to prevent the analgesic effect of EA on both pain model, whereas on nerve-injury model, 5-HT1 receptor antagonist also blocked the effect of EA [9].
Both muscarinic and nicotinic acetylcholine receptors are located in the spinal dorsal horn, but muscarinic receptors have been reported to be more important in the regulation of pain. Five classes of muscarinic receptors have been identified (i.e., M1-5) but the subtypes implicated in spinal nociceptive transmission and modulation are mainly M1, M2 and M3 [20,21]. Our results do not concur with previously published studies which suggested that M1 mediates the analgesic effects of EA in nerve-injured rats [14]. However, an article has reported that as M2 and M3 binding sites, but not M1 binding sites, are localized in the superficial laminae of the dorsal horn where nociceptive Aδ and C fibers terminate, either or both of these muscarinic receptor subtypes may modulate antinociception [38]. Serotonin has been reported to produce receptor specific and dosage specific anti-allodynic effects. They are known to be mediated by the descending pain inhibitory system including the periaqueductal gray, 7nucleus raphe magnus, and spinal serotonergic receptors. Of the seven serotonin receptor subtypes (5-HT1-7) reported, 5-HT1, 5-HT2, and 5-HT3 receptors have so far been the most commonly implicated in spinal anti-allodynic processes [20,22,25]. In a previous study conducted in rats with nerve injury-induced neuropathic pain, 5-HT1 and 5-HT3 receptors, but not 5-HT2 receptors, were shown to be involved in the effects of EA [9]. However, in this study, 5-HT3 receptors, but not 5-HT1 or 5-HT2 receptors was found to mediate the effect of EA. These discrepancies in results might be due to the difference in the type of pain model used (nerve injury vs. oxaliplatin).
Furthermore, results from our former and present experiments demonstrate that opioidergic, cholinergic, serotonergic antagonists, all completely abolished the analgesic effect of EA on oxaliplatin induced neuropathic pain in rats. Although we cannot explain all the details of the process, hitherto complex interrelationships between opioid, cholinergic, and serotonergic systems at the spinal or supraspinal level have been suggested. For example, microinjection of morphine, which effect is most likely mediated via opioid receptors, into the periaqueductal grey has been found to evoke the release of serotonin from the spinal cord [39,40]. Also, other article reported that systemic morphine causes spinal acetylcholine release in animals and humans, suggesting that spinal endogenous acetylcholine plays an important role in mediating the analgesic effect of systemic morphine [41,42]. Thus, based on these results, we can suppose that opioidergic, cholinergic, and serotonergic system are related in the antiallodynic effect of EA on oxaliplatin-induced neuropathic pain in rats.
Bee venom acupuncture (BVA) and EA both stimulate peripheral nerves. However, they differ in stimulation means, as BVA stimulate chemically, and EA electrically. Moreover, BVA has been suggested to produce analgesic effect by activating mostly Aδ fibers and capsaicin-insensitive primary afferents [43], whereas EA effects are known to be produced by stimulation of Aβ, Aδ, and C fibers [36]. BVA is reported to produce its analgesic effect via spinal adrenergic, nicotinic, and serotonergic receptors, but not via muscarinic and opioidergic receptors in rats with oxaliplatin-induced cold allodynia [32,33,43,44]. Meanwhile, our previous results [16] indicate that the relieving effect of EA on oxaliplatin-induced cold allodynia does not involve the activation of noradrenergic and nicotinic receptors, but the activation of opioid, muscarinic and serotonin receptors. Taken together, these findings suggest that EA and BVA could have somewhat different mechanisms of action in the anti-allodynic effects on oxaliplatin-induced neuropathic pain.
This study clearly demonstrates a key role of the cholinergic and serotonergic inhibitory systems in the relieving effects of EA on oxaliplatin-induced neuropathic cold allodynia. It also provides basic evidence for the use of EA as an alternative therapeutic option in the management of oxaliplatin-induced peripheral neuropathy, and presents the possibility of combined use of EA and well-known analgesics, such as morphine, serotonin agonists, serotonin selective reuptake inhibitors, cholinesterase inhibitors, and antidepressants, to decrease their side effects and increase their analgesic effect. For example, the combination of EA stimulation with low doses of intrathecal neostigmine (cholinesterase inhibitor) yielded a synergistic antiallodynic effect without apparent side effects in a rat model of neuropathic pain [45].
In conclusion, the findings of the present study demonstrate that EA administration at acupoint proximal to the site of the measurement significantly attenuates the cold allodynia induced by a single injection of oxaliplatin in rats compared to EA administered at the distal acupoint. This anti-allodynic effect of EA is shown to be mediated by spinal M2 and M3 acetylcholine receptors, and 5-HT3 serotonergic receptor. Thus, based on these results, we propose that EA treatment can be a potential therapeutic option for oxaliplatin-induced neuropathic pain.
ACKNOWLEDGEMENTS
This work was supported by a grant of the National Research Foundation of Korea (NRF) Grant funded by the Korea Government (NRF-2013R1A1A1012403).
Footnotes
CONFLICTS OF INTEREST: The authors declare no conflicts of interest.
Author contributions: J.H.L., D.G. and S.K.K. conceived and designed the study. J.H.L., D.G., W.K. and G.L. performed the experiments. J.H.L., D.G., H.B., F.S.Q. and S.K.K. analyzed and interpreted the data. J.H.L., D.G., W.K., H.B. and S.K.K. wrote the manuscript.
SUPPLEMENTARY MATERIALS
Supplementary data including three figures can be found with this article online at http://pdf.medrang.co.kr/paper/pdf/Kjpp/Kjpp020-04-10-s001.pdf.
The duration of the anti-allo dynic effect of EA at GV3 and ST36.
Assessment of anti-allodynic effect of EA were performed 20 min, 1, and 2 hours after EA treatment in two groups: (a) GV3 (n=6), (b) ST36 (n=5). The effect of EA at GV3, ST36 lasted until one hour after EA stimulation. Data are presented as mean±S.E.M.; ***p<0.001, **p<0.01, *p<0.05; by paired t-test.
Effects of muscarinic acetylcholine and serotonergic receptor antagonists.
(a, f) CON refers to non-oxaliplatin treated group. Behavioral tests to measure the cold allodynia were performed before and after the intrathecal injection of muscarinic acetylcholine and serotonergic receptor antagonists in four groups: (b) NS (n=4), (c) pirenzepine (n=9), (d) methoctramine (n=7), and (e) 4-DAMP (n=6). (g) DMSO (n=4), (h) NAN-190 (n=6), (i) ketanserin (n=6), and (j) MDL-72222 (n=6). None of the antagonists affected the pain behavior. Data are presented as mean mean±S.E.M.
Effects of spinal muscarinic acetylcholine and serotonergic receptor antagonist on EA (GV3) induced anti-allodynia.
(a, d) NS and DMSO were used as control for muscarinic acetylcholine and serotonergic receptor antagonists, respectively. The behavioral tests for cold allodynia were performed before the intrathecal injection of receptor antagonists and after EA treatment in three groups: (b) methoctramine (n=6), (c) 4-DAMP (n=6), and (e) MDL-72222 (n=6). Data are presented as mean±S.E.M.; **p<0.01; by paired t-test.
References
- 1.Goldberg RM, Sargent DJ, Morton RF, Fuchs CS, Ramanathan RK, Williamson SK, Findlay BP, Pitot HC, Alberts SR. A randomized controlled trial of fluorouracil plus leucovorin, irinotecan, and oxaliplatin combinations in patients with previously untreated metastatic colorectal cancer. J Clin Oncol. 2004;22:23–30. doi: 10.1200/JCO.2004.09.046. [DOI] [PubMed] [Google Scholar]
- 2.Arany I, Safirstein RL. Cisplatin nephrotoxicity. Semin Nephrol. 2003;23:460–464. doi: 10.1016/s0270-9295(03)00089-5. [DOI] [PubMed] [Google Scholar]
- 3.Desoize B, Madoulet C. Particular aspects of platinum compounds used at present in cancer treatment. Crit Rev Oncol Hematol. 2002;42:317–325. doi: 10.1016/s1040-8428(01)00219-0. [DOI] [PubMed] [Google Scholar]
- 4.Pasetto LM, D'Andrea MR, Rossi E, Monfardini S. Oxaliplatinrelated neurotoxicity: how and why? Crit Rev Oncol Hematol. 2006;59:159–168. doi: 10.1016/j.critrevonc.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 5.Gamelin E, Gamelin L, Bossi L, Quasthoff S. Clinical aspects and molecular basis of oxaliplatin neurotoxicity: current management and development of preventive measures. Semin Oncol. 2002;29(5 Suppl 15):21–33. doi: 10.1053/sonc.2002.35525. [DOI] [PubMed] [Google Scholar]
- 6.Lehky TJ, Leonard GD, Wilson RH, Grem JL, Floeter MK. Oxaliplatin-induced neurotoxicity: acute hyperexcitability and chronic neuropathy. Muscle Nerve. 2004;29:387–392. doi: 10.1002/mus.10559. [DOI] [PubMed] [Google Scholar]
- 7.Murotani T, Ishizuka T, Nakazawa H, Wang X, Mori K, Sasaki K, Ishida T, Yamatodani A. Possible involvement of histamine, dopamine, and noradrenalin in the periaqueductal gray in electroacupuncture pain relief. Brain Res. 2010;1306:62–68. doi: 10.1016/j.brainres.2009.09.117. [DOI] [PubMed] [Google Scholar]
- 8.Deluze C, Bosia L, Zirbs A, Chantraine A, Vischer TL. Electroacupuncture in fibromyalgia: results of a controlled trial. BMJ. 1992;305:1249–1252. doi: 10.1136/bmj.305.6864.1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kim SK, Park JH, Bae SJ, Kim JH, Hwang BG, Min BI, Park DS, Na HS. Effects of electroacupuncture on cold allodynia in a rat model of neuropathic pain: mediation by spinal adrenergic and serotonergic receptors. Exp Neurol. 2005;195:430–436. doi: 10.1016/j.expneurol.2005.06.018. [DOI] [PubMed] [Google Scholar]
- 10.Kim SK, Moon HJ, Park JH, Lee G, Shin MK, Hong MC, Bae H, Jin YH, Min BI. The maintenance of individual differences in the sensitivity of acute and neuropathic pain behaviors to electroacupuncture in rats. Brain Res Bull. 2007;74:357–360. doi: 10.1016/j.brainresbull.2007.07.006. [DOI] [PubMed] [Google Scholar]
- 11.Park JH, Han JB, Kim SK, Park JH, Go DH, Sun B, Min BI. Spinal GABA receptors mediate the suppressive effect of electroacupuncture on cold allodynia in rats. Brain Res. 2010;1322:24–29. doi: 10.1016/j.brainres.2010.02.001. [DOI] [PubMed] [Google Scholar]
- 12.Bao T, Zhang R, Badros A, Lao L. Acupuncture treatment for bortezomib-induced peripheral neuropathy: a case report. Pain Res Treat. 2011;2011:920807. doi: 10.1155/2011/920807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Donald GK, Tobin I, Stringer J. Evaluation of acupuncture in the management of chemotherapy-induced peripheral neuropathy. Acupunct Med. 2011;29:230–233. doi: 10.1136/acupmed.2011.010025. [DOI] [PubMed] [Google Scholar]
- 14.Park JH, Kim SK, Kim HN, Sun B, Koo S, Choi SM, Bae H, Min BI. Spinal cholinergic mechanism of the relieving effects of electroacupuncture on cold and warm allodynia in a rat model of neuropathic pain. J Physiol Sci. 2009;59:291–298. doi: 10.1007/s12576-009-0035-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim W, Kim SK, Min BI. Mechanisms of electroacupuncture-induced analgesia on neuropathic pain in animal model. Evid Based Complement Alternat Med. 2013;2013:436913. doi: 10.1155/2013/436913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Moon HJ, Lim BS, Lee DI, Ye MS, Lee G, Min BI, Bae H, Na HS, Kim SK. Effects of electroacupuncture on oxaliplatin-induced neuropathic cold hypersensitivity in rats. J Physiol Sci. 2014;64:151–156. doi: 10.1007/s12576-013-0297-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bartolini A, Di Cesare Mannelli L, Ghelardini C. Analgesic and antineuropathic drugs acting through central cholinergic mechanisms. Recent Pat CNS Drug Discov. 2011;6:119–140. doi: 10.2174/157488911795933901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang JY, Meng FY, Chen SP, Gao YH, Liu JL. Analysis on interrelation between electroacupuncture-induced cumulative analgesic effect and hypothalamic cholinergic activities in chronic neuropathic pain rats. Chin J Integr Med. 2012;18:699–707. doi: 10.1007/s11655-012-1059-1. [DOI] [PubMed] [Google Scholar]
- 19.Eisenach JC. Muscarinic-mediated analgesia. Life Sci. 1999;64:549–554. doi: 10.1016/s0024-3205(98)00600-6. [DOI] [PubMed] [Google Scholar]
- 20.Millan MJ. Descending control of pain. Prog Neurobiol. 2002;66:355–474. doi: 10.1016/s0301-0082(02)00009-6. [DOI] [PubMed] [Google Scholar]
- 21.Takeda D, Nakatsuka T, Gu JG, Yoshida M. The activation of nicotinic acetylcholine receptors enhances the inhibitory synaptic transmission in the deep dorsal horn neurons of the adult rat spinal cord. Mol Pain. 2007;3:26. doi: 10.1186/1744-8069-3-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bardin L. The complex role of serotonin and 5-HT receptors in chronic pain. Behav Pharmacol. 2011;22:390–404. doi: 10.1097/FBP.0b013e328349aae4. [DOI] [PubMed] [Google Scholar]
- 23.Xie DJ, Uta D, Feng PY, Wakita M, Shin MC, Furue H, Yoshimura M. Identification of 5-HT receptor subtypes enhancing inhibitory transmission in the rat spinal dorsal horn in vitro. Mol Pain. 2012;8:58. doi: 10.1186/1744-8069-8-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jeong HJ, Mitchell VA, Vaughan CW. Role of 5-HT1 receptor subtypes in the modulation of pain and synaptic transmission in rat spinal superficial dorsal horn. Br J Pharmacol. 2012;165:1956–1965. doi: 10.1111/j.1476-5381.2011.01685.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Radhakrishnan R, King EW, Dickman JK, Herold CA, Johnston NF, Spurgin ML, Sluka KA. Spinal 5-HT2 and 5-HT3 receptors mediate low, but not high, frequency TENS-induced antihyperalgesia in rats. Pain. 2003;105:205–213. doi: 10.1016/s0304-3959(03)00207-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zimmermann M. Ethical guidelines for investigations of experimental pain in conscious animals. Pain. 1983;16:109–110. doi: 10.1016/0304-3959(83)90201-4. [DOI] [PubMed] [Google Scholar]
- 27.Ling B, Coudoré F, Decalonne L, Eschalier A, Authier N. Comparative antiallodynic activity of morphine, pregabalin and lidocaine in a rat model of neuropathic pain produced by one oxaliplatin injection. Neuropharmacology. 2008;55:724–728. doi: 10.1016/j.neuropharm.2008.06.007. [DOI] [PubMed] [Google Scholar]
- 28.Yin CS, Jeong HS, Park HJ, Baik Y, Yoon MH, Choi CB, Koh HG. A proposed transpositional acupoint system in a mouse and rat model. Res Vet Sci. 2008;84:159–165. doi: 10.1016/j.rvsc.2007.04.004. [DOI] [PubMed] [Google Scholar]
- 29.Mestre C, Pélissier T, Fialip J, Wilcox G, Eschalier A. A method to perform direct transcutaneous intrathecal injection in rats. J Pharmacol Toxicol Methods. 1994;32:197–200. doi: 10.1016/1056-8719(94)90087-6. [DOI] [PubMed] [Google Scholar]
- 30.De la Calle JL, Paíno CL. A procedure for direct lumbar puncture in rats. Brain Res Bull. 2002;59:245–250. doi: 10.1016/s0361-9230(02)00866-3. [DOI] [PubMed] [Google Scholar]
- 31.Hagiwara S, Iwasaka H, Takeshima N, Noguchi T. Mechanisms of analgesic action of pulsed radiofrequency on adjuvant-induced pain in the rat: roles of descending adrenergic and serotonergic systems. Eur J Pain. 2009;13:249–252. doi: 10.1016/j.ejpain.2008.04.013. [DOI] [PubMed] [Google Scholar]
- 32.Lee JH, Li DX, Yoon H, Go D, Quan FS, Min BI, Kim SK. Serotonergic mechanism of the relieving effect of bee venom acupuncture on oxaliplatin-induced neuropathic cold allodynia in rats. BMC Complement Altern Med. 2014;14:471. doi: 10.1186/1472-6882-14-471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yoon H, Kim MJ, Yoon I, Li DX, Bae H, Kim SK. Nicotinic acetylcholine receptors mediate the suppressive effect of an injection of diluted bee venom into the GV3 acupoint on oxaliplatin-induced neuropathic cold allodynia in rats. Biol Pharm Bull. 2015;38:710–714. doi: 10.1248/bpb.b14-00797. [DOI] [PubMed] [Google Scholar]
- 34.Ormseth MJ, Scholz BA, Boomershine CS. Duloxetine in the management of diabetic peripheral neuropathic pain. Patient Prefer Adherence. 2011;5:343–356. doi: 10.2147/PPA.S16358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Serpell MG Neuropathic pain study group. Gabapentin in neuropathic pain syndromes: a randomised, double-blind, placebo-controlled trial. Pain. 2002;99:557–566. doi: 10.1016/S0304-3959(02)00255-5. [DOI] [PubMed] [Google Scholar]
- 36.Lin JG, Lo MW, Wen YR, Hsieh CL, Tsai SK, Sun WZ. The effect of high and low frequency electroacupuncture in pain after lower abdominal surgery. Pain. 2002;99:509–514. doi: 10.1016/S0304-3959(02)00261-0. [DOI] [PubMed] [Google Scholar]
- 37.Kim JH, Min BI, Na HS, Park DS. Relieving effects of electroacupuncture on mechanical allodynia in neuropathic pain model of inferior caudal trunk injury in rat: mediation by spinal opioid receptors. Brain Res. 2004;998:230–236. doi: 10.1016/j.brainres.2003.11.045. [DOI] [PubMed] [Google Scholar]
- 38.Höglund AU, Baghdoyan HA. M2, M3 and M4, but not M1, muscarinic receptor subtypes are present in rat spinal cord. J Pharmacol Exp Ther. 1997;281:470–477. [PubMed] [Google Scholar]
- 39.Reimann W, Schlütz H, Selve N. The antinociceptive effects of morphine, desipramine, and serotonin and their combinations after intrathecal injection in the rat. Anesth Analg. 1999;88:141–145. doi: 10.1097/00000539-199901000-00026. [DOI] [PubMed] [Google Scholar]
- 40.Yaksh TL, Tyce GM. Microinjection of morphine into the periaqueductal gray evokes the release of serotonin from spinal cord. Brain Res. 1979;171:176–181. doi: 10.1016/0006-8993(79)90747-9. [DOI] [PubMed] [Google Scholar]
- 41.Dirksen R, Nijhuis GM. The relevance of cholinergic transmission at the spinal level to opiate effectiveness. Eur J Pharmacol. 1983;91:215–221. doi: 10.1016/0014-2999(83)90467-3. [DOI] [PubMed] [Google Scholar]
- 42.Chen SR, Pan HL. Spinal endogenous acetylcholine contributes to the analgesic effect of systemic morphine in rats. Anesthesiology. 2001;95:525–530. doi: 10.1097/00000542-200108000-00039. [DOI] [PubMed] [Google Scholar]
- 43.Lim BS, Moon HJ, Li DX, Gil M, Min JK, Lee G, Bae H, Kim SK, Min BI. Effect of bee venom acupuncture on oxaliplatin-induced cold allodynia in rats. Evid Based Complement Alternat Med. 2013;2013:369324. doi: 10.1155/2013/369324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Roh DH, Kwon YB, Kim HW, Ham TW, Yoon SY, Kang SY, Han HJ, Lee HJ, Beitz AJ, Lee JH. Acupoint stimulation with diluted bee venom (apipuncture) alleviates thermal hyperalgesia in a rodent neuropathic pain model: involvement of spinal alpha 2-adrenoceptors. J Pain. 2004;5:297–303. doi: 10.1016/j.jpain.2004.05.003. [DOI] [PubMed] [Google Scholar]
- 45.Kim HN, Park JH, Kim SK, Sun B, Koo S, Choi SM, Bae H, Min BI. Electroacupuncture potentiates the antiallodynic effect of intrathecal neostigmine in a rat model of neuropathic pain. J Physiol Sci. 2008;58:357–360. doi: 10.2170/physiolsci.SC008308. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The duration of the anti-allo dynic effect of EA at GV3 and ST36.
Assessment of anti-allodynic effect of EA were performed 20 min, 1, and 2 hours after EA treatment in two groups: (a) GV3 (n=6), (b) ST36 (n=5). The effect of EA at GV3, ST36 lasted until one hour after EA stimulation. Data are presented as mean±S.E.M.; ***p<0.001, **p<0.01, *p<0.05; by paired t-test.
Effects of muscarinic acetylcholine and serotonergic receptor antagonists.
(a, f) CON refers to non-oxaliplatin treated group. Behavioral tests to measure the cold allodynia were performed before and after the intrathecal injection of muscarinic acetylcholine and serotonergic receptor antagonists in four groups: (b) NS (n=4), (c) pirenzepine (n=9), (d) methoctramine (n=7), and (e) 4-DAMP (n=6). (g) DMSO (n=4), (h) NAN-190 (n=6), (i) ketanserin (n=6), and (j) MDL-72222 (n=6). None of the antagonists affected the pain behavior. Data are presented as mean mean±S.E.M.
Effects of spinal muscarinic acetylcholine and serotonergic receptor antagonist on EA (GV3) induced anti-allodynia.
(a, d) NS and DMSO were used as control for muscarinic acetylcholine and serotonergic receptor antagonists, respectively. The behavioral tests for cold allodynia were performed before the intrathecal injection of receptor antagonists and after EA treatment in three groups: (b) methoctramine (n=6), (c) 4-DAMP (n=6), and (e) MDL-72222 (n=6). Data are presented as mean±S.E.M.; **p<0.01; by paired t-test.