Abstract
Background
Studies based on Western populations have found that body mass index (BMI) is positively related to the risk of esophageal adenocarcinoma but inversely associated with esophageal squamous cell carcinoma (ESCC). Little reliable evidence exists of an association between BMI and ESCCin China, where ESCC incidence is high but BMI is low.
Methods
We evaluated the BMI‐ESCC association in a population‐based prospective study of 29 446 Chinese aged 40–69 with 27 years of follow‐up. China‐specific BMI cut‐offs (underweight < 18.5, healthy ≥ 18.5 to <24, overweight ≥ 24 to <28, and obese ≥ 28) and quartile categories were used to define BMI subgroups. Adjusted hazard ratios (HRs) and confidence intervals (CIs) for death from ESCC by BMI subgroups were calculated using Cox proportional hazards models.
Results
During a median follow‐up duration of 21.2 years (555 439 person‐years), 2436 ESCC deaths were identified. BMI was protective for death from ESCC with an HR of 0.97 (95% CI 0.95–0.99) for each unit increase in BMI. Relative to healthy weight, HRs for BMI were 1.21 (95% CI 1.02–1.43) for the underweight group and 0.87 (95% CI 0.78–0.98) for the overweight. Categorical quartile analyses found people with BMIs in the Q3 and Q4 groups had 16% and 13% reductions in the risk of ESCC, respectively. Gender‐specific analyses found that clear effects were evident in women only.
Conclusions
Higher BMI was associated with a reduced risk of ESCC in aChinese population.
Keywords: Body mass index (BMI), Chinese population, esophageal squamous cell carcinoma (ESCC), overweight, underweight
Background
Esophageal cancer (EC) is the eighth most common cancer worldwide, and the sixth most common cause of death from cancer.1 Around 50% of the worldwide cases occur in China.1 There are two main histological types of EC, namely adenocarcinomas (EAC) and squamous cell carcinomas (ESCC), which have similar presentations but quite different risk factors. Body mass index (BMI), an anthropometric measure of nutritional status, is associated with all‐cause and total cancer mortality, but shows opposite risk patterns for the two main histological types of EC. High BMI is a major risk factor for EAC, with the risk directly increasing with increasing BMI.4, 5, 6, 7 Moreover, an Australian study found that the population attributable fraction for overweight/obese in EAC was as high as 31%.8 In contrast, observational and cohort studies have shown that BMI is inversely associated with ESCC.9, 10 A meta‐analysis that included 10 studies on ESCC indicated that each 5 kg/m2 increase in BMI was associated with 51% and 31% lower risks of ESCC in case‐control and prospective studies, respectively.11 Another systematic review found that an overweight condition or obesity decreased the risk of death by 20% in ESCC patients.12
However, most of the published data have been from Western populations where BMIs are high and EC incidence is low, and the type of EC is predominantly EAC. In contrast to Western populations, Chinese populations have low BMI, high incidence of EC, with predominantly ESCC; however, little reliable epidemiological evidence exists on the association between BMI and ESCC in the Chinese population. Definitions of underweight and obesity in most current studies were based on criteria from Western populations.13 No study has evaluated the association of BMI and ESCC using the more suitable Chinese BMI cut‐offs released by the National Health and Family Planning Commission (NHFPC) of the People's Republic of China in 2013.14
Therefore, we conducted this analysis in a large cohort of approximately 30 000 people in Linxian, China, who were followed for nearly 30 years. With incidence rates exceeding 100 per 100 000 person‐years, the people of Linxian have some of the highest rates of ESCC in the world.15 As a result, more than 2400 ESCC cases were identified in this cohort during the follow‐up period. Thus, this study can address the issue of the long‐term risk of ESCC by BMI in a Chinese population, and provide data relevant to nutrition guidance regarding weight for future efforts to prevent and control EC in China and other Asian countries.
Methods
Study population
The design of the Linxian Nutrition Intervention Trial (NIT) and its extended follow‐up have been previously described.10, 15, 16 In brief, the NIT started in 1985 in Linxian, a rural county in north central China. Residents aged 40–69 with no history of cancer or debilitating disease were eligible and were invited to participate in the trial. A total of 29 584 subjects, 60% of those invited, were randomly assigned to the trial. The current analyses were conducted on 29 446 (99.5%) of 29 584 initial study participants. Subjects were excluded because of missing information on the questionnaire (n = 115) and loss to follow‐up (n = 23).
The conduct of the Linxian NIT was approved by the institutional review boards of the Cancer Institute of Chinese Academy of Medical Sciences (CICAMS) and the United States National Cancer Institute, and written informed consent was obtained from all participants prior to participation. The trial is registered as ClinicalTrials.gov number, NCT00342654.
Baseline examination and the intervention trial
All individuals were interviewed by questionnaire to obtain information on medical history, family history of cancer, diet, and alcohol and tobacco consumption. Smoking was defined as regular cigarette or pipe use for at least six months (including ever and current smokers), and alcohol consumption was defined as any consumption in the previous 12 months (drinkers were defined as persons who reported drinking in a range of categories from “several times per year” to “several times per day”). Family history of cancer was considered positive if cancer was reported in at least one first degree relative, including parents, siblings, or offspring. A brief physical examination was conducted by a village doctor. Body weight and height were measured during the physical examination, and BMI was calculated as weight in kilograms divided by the square of height in meters.
Nine nutrients were included in this trial which employed a one‐half 2 fractional factorial design to evaluate four different combinations of these nine nutrients in eight different intervention groups, including a placebo.4 Participants were randomly assigned to one of the eight intervention groups. During the trial period from March 1, 1986, to May 1, 1991, village health workers visited participants monthly to assess compliance and ascertain vital and disease status. Compliance, measured by the rate of medication use (93%) and biochemical measurements, was excellent.17, 18 Diagnostic materials (case records, pathology slides, and x‐rays) for 85% of the cancer cases in the trial period were available and reviewed by a panel of American and Chinese experts to confirm diagnoses.
Post‐trial follow‐up data collection
In the subsequent 22 years post trial, village health workers or study interviewers continued to contact participants monthly. For new EC diagnoses and deaths, diagnostic materials were collected and EC diagnoses were verified by a panel of American and Chinese experts or by senior Chinese diagnosticians from CICAMS. Throughout the trial and the post trial follow‐up, case ascertainment was considered essentially complete and loss to follow‐up was minimal (<1%). Outcomes of this study were based on data from the full 27.1 years of follow‐up, from March 1, 1986 to March 31, 2013.
Statistical methods
The main outcome of this study was ESCC mortality. Participants were censored at their last known follow‐up date, date of death, or the administrative closure of follow‐up (March 31, 2013), whichever came first. As Asians have relatively low BMIs, the World Health Organization, the International Association for the Study of Obesity, and the International Obesity Task Force all recommended lower BMI cut‐offs to define overweight and obese in Asian populations. Therefore, BMI was divided into different groups according to the “Criteria of Weight for Adults” released by the NHFPC of China in 2013, which defined four groups according to the following BMI (kg/m2) cut‐offs: underweight (<18.5), healthy (≥18.5 to <24), overweight (≥24 to <28), and obese (≥28).14As this study was conducted in a relatively lean population, only a few people were in the overweight and obese groups. To improve statistical power, we also divided BMI into quartiles with the following cut‐offs: Q1 (<20.3), Q2 (≥20.3 to <21.8), Q3 (≥21.8 to <23.3), and Q4 (≥23.3).
We tabulated baseline frequencies and percentages by demographics for participants with different BMI groups and compared risk factors between these groups by Chi‐square or analysis of variance tests. Cox proportional hazards models were used to calculate hazard ratios (HRs) and 95% confidence intervals (CIs) for different BMI groups or a continuous BMI variable, adjusting for age at enrollment, gender, cigarette smoking, alcohol, and family history of cancer. Study participants in the healthy BMI category (18.5 to <24.0) were used as the reference group for the analysis with Chinese BMI categories, while subjects in the lowest BMI quartile (Q1) were used as the reference group for quartile analysis. All P values are two sided and P values less than 0.05 were considered to show associations. Analyses were conducted using SAS version 9.1.3 service pack 4 (SAS Institute Inc., Cary, NC, USA).
Results
Baseline characteristics of the study participants are presented according to the four BMI categories in Table 1. The majority of the participants (76.3%) were in the healthy BMI group, followed by those who were overweight (16.4%), while only a few were underweight (5.3%) or obese (2.0%). Underweight participants were generally older, reported relatively less alcohol use, and were more likely to have ESCC. The overweight and obese groups were predominantly female, and only ≈7% were diagnosed with ESCC during follow‐up. All of the baseline characteristics differed (P < 0.05) among the four BMI groups, except for family history of cancer.
Table 1.
Baseline characteristics of subjects from nutrition intervention trial according to BMI
| Characteristics | <18.5 | Body mass index (kg/m2) | P value* | ||
|---|---|---|---|---|---|
| >=18.5, <24 | >=24, <28 | > = 28 | |||
| Number of participants | 1555 | 22471 | 4839 | 581 | |
| Age at baseline, mean (SD), years | 55.96 (8.55) | 52.31 (8.90) | 51.74 (8.73) | 52.86 (8.57) | <0.001 |
| Gender | |||||
| Male, N (%)† | 507 (32.6) | 10915 (48.6) | 1598 (33.0) | 94 (16.2) | <0.001 |
| Female, N (%)† | 1048 (67.4) | 11556 (51.4) | 3241 (67.0) | 487 (83.8) | |
| Ever regularly smoke tobacco: yes, N (%)† | 389 (25.0) | 7448 (33.1) | 937 (19.4) | 56 (9.6) | <0.001 |
| Any alcohol in previous 12 months: yes, N (%)† | 259 (16.7) | 5516 (24.5) | 1036 (21.4) | 93 (16.0) | <0.001 |
| Family history of cancer: yes, N (%)† | 524 (33.7) | 7704 (34.3) | 1699 (35.1) | 206 (35.5) | 0.606 |
| Esophageal cancer cases, N (%)† | 155 (10.0) | 1909 (8.5) | 333 (6.9) | 39 (6.7) | <.001 |
| Male cases, N (%)† | 49 (9.7) | 1026 (9.4) | 118 (7.4) | 3 (3.2) | 0.012 |
| Female cases, N (%)† | 106 (10.1) | 883 (7.6) | 215 (6.6) | 36 (7.4) | 0.003 |
| BMI, mean (SD) | 17.59 (0.82) | 21.35 (1.38) | 25.33 (1.03) | 29.73 (2.21) | <0.001 |
P values come from Chi‐square test, except for age at baseline and body mass index (BMI) which were calculated by analysis of variance.
Percent was calculated by the target variable divided by the number of participants in each BMI category. SD, standard deviation.
During a median follow‐up time of 21.2 years (555 439 person‐years), 2436 ESCC deaths were documented in this cohort. BMI played an important role in ESCC mortality, with a HR of 0.97 (95% CI 0.95–0.99), indicating a 3% reduction of risk of ESCC for each one unit increase in BMI. To further investigate the effect of different levels of body mass on ESCC mortality, BMI was stratified into four quartiles for subgroup analysis. The association of BMI with ESCC mortality remained after multivariate adjustment for important risk factors, including baseline age, gender, cigarette smoking, alcohol consumption, and cancer family history. Relative to the healthy weight BMI group, the multivariate‐adjusted HRs by BMI were 1.21 (95% CI 1.02–1.43) for the underweight group, 0.87 (95% CI 0.78–0.98) for the overweight group, and 0.91 (95% CI 0.66–1.25) for the obese group. Relative to the Q1 group, the multivariate‐adjusted HRs by BMI were 0.95 (95% CI 0.85–1.05) for Q2, 0.84 (95% CI 0.75–0.94) for Q3, and 0.87 (95% CI 0.77–0.97) for Q4. A 5% reduction in risk of ESCC was found for each quartile increase in BMI (HR 0.95, 95% CI 0.91–0.98).
As the proportion of female participants differed significantly among the four BMI groups by Chinese categories, and almost all of the females in Linxian were non‐smokers, we also calculated gender‐specific HRs for BMI and ESCC mortality. The risk of ESCC for low BMI in the total population was also observed in females alone (HR 1.28, 95% CI 1.04–1.56), but not in males alone (HR 1.13, 95% CI 0.85–1.50). No associations between BMI and risk were seen in the overweight or obese groups, but the number of subjects in the obese category was low (94 men, 487 women) and the number of ESCC deaths few (3 men, 36 women; Tables 1, 2). In the quartile analysis, high BMI showed a protective effect in women with a risk reduction of 7% for each quartile increase in BMI. The HRs by BMIi n women were 0.85 (95% CI 0.73–0.99) for Q2, 0.74 (95% CI 0.63–0.87) for Q3, and 0.82 (95% CI 0.71–0.95) for Q4.
Table 2.
Cumulative HRs and 95% CIs for ESCC by gender
| Risk factor | Esophageal squamous cell carcinoma | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Total | Male | Female | |||||||
| HRs‡ | 95% CI | HRs‡ | 95% CI | HRs‡ | 95% CI | ||||
| Gender | 0.86 | 0.76 | 0.97 | – | – | – | – | – | – |
| Age at baseline | 1.07 | 1.06 | 1.07 | 1.07 | 1.06 | 1.08 | 1.06 | 1.05 | 1.07 |
| Ever regularly smoke tobacco: Yes | 1.36 | 1.20 | 1.55 | 1.37 | 1.20 | 1.55 | 1.16 | 0.37 | 3.60 |
| Any alcohol in previous 12 months: Yes | 0.85 | 0.76 | 0.94 | 0.88 | 0.78 | 0.99 | 0.77 | 0.62 | 0.95 |
| Family history of cancer: Yes | 1.40 | 1.30 | 1.52 | 1.29 | 1.15 | 1.45 | 1.52 | 1.36 | 1.70 |
| BMI (continuous variable, kg/m2)† | 0.97 | 0.95 | 0.99 | 0.97 | 0.94 | 1.00* | 0.97 | 0.95 | 0.99 |
| Underweight, <18.5 | 1.21 | 1.02 | 1.43 | 1.13 | 0.85 | 1.50 | 1.28 | 1.04 | 1.56 |
| Healthy, 18.5–24 | 1.00 | – | – | 1.00 | – | – | 1.00 | – | – |
| Overweight, 24–28 | 0.87 | 0.78 | 0.98 | 0.83 | 0.69 | 1.01 | 0.90 | 0.78 | 1.05 |
| Obese, >= 28 | 0.91 | 0.66 | 1.25 | 0.36 | 0.12 | 1.11 | 1.04 | 0.75 | 1.46 |
| BMI (categorical variable, Q1‐Q4§) | 0.95 | 0.91 | 0.98 | 0.97 | 0.91 | 1.02 | 0.93 | 0.89 | 0.98 |
| Q1 < 20.3 | 1.00 | – | – | 1.00 | – | – | 1.00 | – | – |
| Q2 20.3–21.8 | 0.95 | 0.85 | 1.05 | 1.05 | 0.90 | 1.23 | 0.85 | 0.73 | 0.99 |
| Q3 21.8–23.3 | 0.84 | 0.75 | 0.94 | 0.95 | 0.80 | 1.11 | 0.74 | 0.63 | 0.87 |
| Q4 >= 23.3 | 0.87 | 0.77 | 0.97 | 0.92 | 0.77 | 1.11 | 0.82 | 0.71 | 0.95 |
P = 0.052.
Body mass index (BMI) was calculated as weight in kilograms divided by the square of height in meters.
Multivariate‐adjusted hazard ratios (HRs) were calculated using study participants with a BMI of 18.5 to 24 as the reference group, adjusted for age, gender, cigarette smoking, alcohol consumption, and family history of cancer.
BMI was divided into quartiles, and the HRs were calculated as the effect on the risk of esophageal squamous cell carcinoma (ESCC) for each 25% increase of BMI. CI, confidence interval.
Figure 1 shows the cumulative event rate curves for ESCC mortality by Chinese BMI categories. Compared with healthy weight subjects, higher ESCC mortality was observed in the underweight group (total population: 10.0% vs. 8.5%, P = 0.04; men: 9.7% vs. 9.4%, P = 0.84; women: 10.1% vs. 7.6%, P = 0.004), while the opposite (lower mortality) was observed in the overweight group (total population: 6.9% vs. 8.5%, P < 0.001; men: 7.4% vs. 9.4%, P = 0.009; women: 6.6% vs.7.6%, P = 0.05). However, results in the obese group were not consistent by gender: lower mortality was seen in men (3.2% vs. 9.4%, P = 0.039), but not in women (7.4% vs. 7.6%, P = 0.84).
Figure 1.
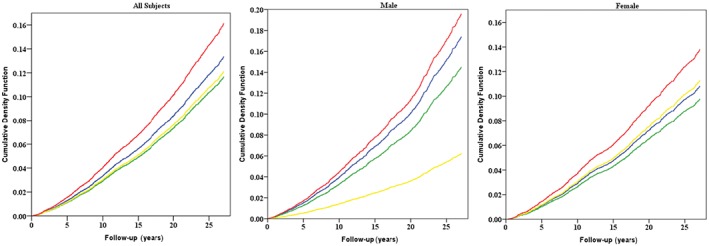
Effects of baseline body mass index (BMI) on esophageal squamous cell carcinoma mortality for all subjects, male, and female, as shown by cumulative event rates (cumulative density function, as %) from Kaplan–Meier estimates. Red lines represent underweight participants (BMI < 18.5), blue lines represent healthy participants (BMI >= 18.5, <24), green lines represent overweight participants (BMI >= 24, <28), and yellow lines represent obese participants (BMI >= 28).
Discussion
This study reports results on the association between BMI and long‐term mortality from ESCC in a cohort with over 27 years of follow‐up in a relatively lean population. Using Chinese criteria for BMI cut‐offs, we found a linear relation between higher BMI and lower risk of death from ESCC, with a 3% reduction in risk for every one unit increase in BMI. Further, relative to healthy weight subjects, ESCC mortality was reduced 13% in the overweight group but increased 21% in the underweight group. Gender‐specific analyses found generally similar effect point estimates, but a clear association was only observed in the female underweight group. BMI quartile analysis supported these results. Each quartile increase of BMI showed a 5% reduction in risk of ESCC in the total population and a 7% reduction in women, but not in men. Categorical quartile analyses showed strong and consistent reductions in the risk for higher quartiles, but only in women. Because women in Linxian have little or no exposure to tobacco or alcohol, the evidence for women is more robust as to the effect of BMI on the risk of ESCC.
The increased ESCC mortality risk we observed with a low BMI is also supported by results from other studies. A large Chinese male cohort study similarly found a strong inverse association between BMI and EC death, with each 5 kg/m2 higher BMI associated with 25% lower EC mortality.11 A Norwegian cohort study found that low BMI was associated with an increased ESCC risk, while high BMI was protective.19 Although our study showed that increased BMI was protective against ESCC death overall, when we examined ESCC mortality by BMI groups, BMI was only associated with ESCC death in the overweight group. As our study was conducted in a lean population, the small size of the obese group may explain our inability to observe an effect in this group. The quartile BMI analysis supplemented the results of the Chinese BMI categories and provided results that were generally stronger and clearer. We found that people with BMIs in the Q3 and Q4 groups had 16% and 13% reductions in the risk of ESCC, respectively, and that these effects were limited to women only. As this study was conducted in a poor nutrient population, the lean population studied may have lower nutritional status. Low serum levels of essential micronutrients, such as vitamin E and selenium, have been associated with an increased risk of ESCC, which may explain these results.20, 21 Moreover, data from other epidemiological and clinical studies also provided convincing evidence to support our results. A meta‐analysis of 2.88 million individuals found that an overweight condition was associated with lower all‐cause mortality.2 A European study focused on abdominal obesity found that BMI and waist circumference were both inversely related to ESCC.22 As BMI presented quite different risk patterns for the two main types of EC, the physiologic mechanism of its protective effect on ESCC and the harmful effect on EAC need to be further investigated by future basic research. Together, the inverse association between BMI and ESCC in our study was probably real, although not necessarily causal. Because there is a long latency in the carcinogenesis process, latency models are needed to distinguish between real results as opposed to results of reversal causality for associations between low BMI and risk of ESCC. We excluded ESCCcases within three years of the baseline and performed a lag analysis, and results showed similar effects for the different BMI groups on the risk of ESCC (data not shown).
The current study used a large Chinese cohort, with 27 years of follow‐up, to assess the risk of ESCC mortality by different BMI categories at baseline. BMI information was based on actual measurements as opposed to self reports, and was collected prospectively before the onset of the disease, which strengthens the evidence found in support of a causal association between BMI and EC risk. Accurate ESCC diagnoses by a panel of expert diagnosticians minimized misdiagnosis of EAC or gastric cardia cancer with ESCC. Moreover, this study used both the China‐specific BMI criteria and a more robust analysis of BMI categorized in quartiles, so that our results will provide evidence relevant for nutrition guidance regarding weight in the prevention of EC among Chinese populations.
This study had some limitations that must be addressed. First, weight and height were collected only once (at baseline), although participants'weight (and other exposures) might change during a follow‐up period that extends 27 years. With rapid economic development, there have been large changes in dietary patterns in China during recent decades. Consequently, lack of multiple measurements limits our ability to determine the stability of BMI in study participants.Second, although use of the new Chinese criteria for BMI categorization provides important information for cancer control in Asian populations, our use of different categories means that our results cannot be directly compared with those of Western countries. Third, unknown geographical factors in Linxian and other etiological factors, such as microorganisms, which may affect EC mortality were not available and, thus, could not be evaluated in this study.
Conclusion
Esophageal squamous cell carcinoma is characterized by late presentation and poor survival. Better knowledge about modifiable risk factors, such as BMI, offer the potential dual benefits of decreasing ESCC incidence and mortality. According to our study, both the China‐specific BMI categories and the quartile BMI analyses showed strong and consistent reductions in the risk of ESCC with higher BMI, but only in women. Appropriate lifestyle choices to maintain a healthy weight may facilitate the prevention and control of ECs in high‐risk regions, such as China.
Disclosure
No authors reportany conflict of interest.
Acknowledgments
This study was funded by a US National Cancer Institute contract (HHSN261201200034C) to the Cancer Hospital/Institute, Chinese Academy of Medical Sciences and a basic research grant (2013JKB01) to the Cancer Hospital/Institute, Chinese Academy of Medical Sciences.
References
- 1. Ferlay J, Soerjomataram I, Ervik M, et al GLOBOCAN 2012 v1.0, Cancer Incidence and Mortality Worldwide: IARC CancerBase No. 11 [Internet]. Lyon, France: International Agency for Research on Cancer; 2013. [Cited 25 Dec 2015.] Available from URL: http://globocan.iarc.fr
- 2. Flegal KM, Kit BK, Orpana H, Graubard BI. Association of all‐cause mortality with overweight and obesity using standard body mass index categories: A systematic review and meta‐analysis. JAMA 2013; 309: 71–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Gu D, He J, Duan X et al Body weight and mortality among men and women in China. JAMA 2006; 295: 776–783. [DOI] [PubMed] [Google Scholar]
- 4. Cook MB, Freedman ND, Gamborg M, Sorensen TI, Baker JL. Childhood body mass index in relation to future risk of oesophageal adenocarcinoma. Br J Cancer 2015; 112: 601–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hoyo C, Cook MB, Kamangar F et al Body mass index in relation to oesophageal and oesophagogastric junction adenocarcinomas: A pooled analysis from the International BEACON Consortium. Int J Epidemiol 2012; 41: 1706–1718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Löfdahl HE, Lane A, Lu Y et al Increased population prevalence of reflux and obesity in the United Kingdom compared with Sweden: A potential explanation for the difference in incidence of esophageal adenocarcinoma. Eur J Gastroenterol Hepatol 2011; 23: 128–132. [DOI] [PubMed] [Google Scholar]
- 7. O'Doherty MG, Freedman ND, Hollenbeck AR, Schatzkin A, Abnet CC. A prospective cohort study of obesity and risk of oesophageal and gastric adenocarcinoma in the NIH‐AARP Diet and Health Study. Gut 2012; 61: 1261–1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kendall BJ, Wilson LF, Olsen CM et al Cancers in Australia in 2010 attributable to overweight and obesity. Aust N Z J Public Health 2015; 39: 452–457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lahmann PH, Pandeya N, Webb PM, Green AC, Whiteman DC, Australian Cancer Study . Body mass index, long‐term weight change, and esophageal squamous cell carcinoma: Is the inverse association modified by smoking status? Cancer 2012; 118: 1901–1909. [DOI] [PubMed] [Google Scholar]
- 10. Tran GD, Sun XD, Abnet CC et al Prospective study of risk factors for esophageal and gastric cancers in the Linxian general population trial cohort in China. Int J Cancer 2005; 113: 456–463. [DOI] [PubMed] [Google Scholar]
- 11. Smith M, Zhou M, Whitlock G et al Esophageal cancer and body mass index: Results from a prospective study of 220 000 men in China and a meta‐analysis of published studies. Int J Cancer 2008; 122: 1604–1610. [DOI] [PubMed] [Google Scholar]
- 12. Fahey PP, Mallitt KA, Astell‐Burt T, Stone G, Whiteman DC. Impact of pre‐diagnosis behavior on risk of death from esophageal cancer: A systematic review and meta‐analysis. Cancer Causes Control 2015; 26: 1365–1373. [DOI] [PubMed] [Google Scholar]
- 13. Clinical guidelines on the identification, evaluation, and treatment of overweight and obesity in adults: Executive summary. Expert panel on the identification, evaluation, and treatment of overweight in adults. Am J Clin Nutr 1998; 68: 899–917. [DOI] [PubMed] [Google Scholar]
- 14. China NHaFPCotPsRo . Criteria of weight for adults. File No WS/T 428–2013 2013.
- 15. Qiao YL, Dawsey SM, Kamangar F et al Total and cancer mortality after supplementation with vitamins and minerals: Follow‐up of the Linxian General Population Nutrition Intervention Trial. (Published erratum appears in J Natl Cancer Inst 2010; 102: 140) J Natl Cancer Inst 2009; 101: 507–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Li JY, Taylor PR, Li B et al Nutrition intervention trials in Linxian, China: Multiple vitamin/mineral supplementation, cancer incidence, and disease‐specific mortality among adults with esophageal dysplasia. J Natl Cancer Inst 1993; 85: 1492–1498. [DOI] [PubMed] [Google Scholar]
- 17. Li B, Taylor PR, Li JY et al Linxian nutrition intervention trials. Design, methods, participant characteristics, and compliance. Ann Epidemiol 1993; 3: 577–585. [DOI] [PubMed] [Google Scholar]
- 18. Blot WJ, Li JY, Taylor PR, Guo W, Dawsey SM, Li B. The Linxian trials: Mortality rates by vitamin‐mineral intervention group. Am J Clin Nutr 1995; 62 (6 Suppl): 1424S–1426. [DOI] [PubMed] [Google Scholar]
- 19. Engeland A, Tretli S, Bjorge T. Height and body mass index in relation to esophageal cancer; 23‐year follow‐up of two million Norwegian men and women. Cancer Causes Control 2004; 15: 837–843. [DOI] [PubMed] [Google Scholar]
- 20. Mark SD, Qiao YL, Dawsey SM et al Prospective study of serum selenium levels and incident esophageal and gastric cancers. J Natl Cancer Inst 2000; 92: 1753–1763. [DOI] [PubMed] [Google Scholar]
- 21. Taylor PR, Qiao YL, Abnet CC et al Prospective study of serum vitamin E levels and esophageal and gastric cancers. J Natl Cancer Inst 2003; 95: 1414–1416. [DOI] [PubMed] [Google Scholar]
- 22. Steffen A, Schulze MB, Pischon T et al Anthropometry and esophageal cancer risk in the European prospective investigation into cancer and nutrition. Cancer Epidemiol Biomarkers Prev 2009; 18: 2079–2089. [DOI] [PubMed] [Google Scholar]


