Abstract
Background
Lung cancer is a major public health issue in most countries, including China. The expression of RelB is associated with poor prognosis in diverse cancers. However, whether RelB expression could be an indicator of poor prognosis in non‐small cell lung cancer (NSCLC) is still unclear.
Methods
The expression of RelB in NSCLC tumor tissue and adjacent non‐neoplastic tissues were examined by immunohistochemistry. Chi‐square or two‐tailed Fisher's exact tests were used to analyze possible associations between qualitative clinicopathological variables and RelB expression. Kaplan–Meier analysis and a Cox regression model were employed to determine independent prognostic factors.
Results
The expression of RelB was increased in tumor tissue compared with adjacent non‐neoplastic tissue in NSCLC patients. High RelB expression was significantly correlated with degree of differentiation (P = 0.023), depth of tumor invasion (P < 0.001), lymph node metastasis (P = 0.017), distant metastases (P = 0.004), and tumor node metastasis stage (P < 0.001) in patients with NSCLC. NSCLC patients with high RelB expression had significantly shorter overall survival than those with low RelB expression (P < 0.001). Our results indicate that high RelB expression is an independent prognostic factor for patients with NSCLC (P < 0.001).
Conclusions
High RelB expression could provide a basis for judgment of prognosis in patients with NSCLC.
Keywords: NF‐κB, non‐small cell lung cancer, prognosis, RelB
Introduction
Lung cancer is the leading cause of cancer‐related death worldwide.1 According to the American Cancer Society, the five‐year survival rate of stage I non‐small cell lung cancer (NSCLC) is 49%, while in stage IV it is only 1%. In China, lung cancer is one of the most common cancers and the leading cause of cancer death in both urban and rural areas.2 NSCLC accounts for approximately 85% of lung cancer diagnoses. Adenocarcinoma and squamous cell carcinoma are the two main types of NSCLC. Current therapeutic approaches include surgery, chemotherapy, radiation therapy, and targeted therapy; however these treatments do not cure the disease.1, 2 Therefore, it is important to identify useful molecular makers to assist early diagnosis and accurate prediction of prognosis in patients with NSCLC.
The mammalian nuclear transcription factor kappa B (NF‐κB) family consists of five members, including: p105/p50 (NF‐κB1), p100/p52 (NF‐κB2), p65 (RelA), RelB, and c‐Rel.3 These subunits form diverse homo‐dimers and hetero‐dimers that regulate transcription of their respective target genes. Upon stimulation with different receptors, NF‐κB subunits can be activated by classical and alternative NF‐κB pathways.4, 5 NF‐κB subunits participate in various biological processes and play critical roles in cell growth and survival, and immune and inflammatory responses.4 Aberrant NF‐κB activities are involved in many inflammatory diseases, leukemia, and solid tumors.5, 6, 7
In the alternative NF‐κB signaling pathway, the central players are RelB and p100/p52.4 The signaling pathway is activated by IκB kinase α (IKK)α‐dependent processing of the inhibitory NF‐κB2/p100 protein to p52, resulting in transcriptionally activated RelB/p52 hetero‐dimers.5 The role of RelB has been studied in hematopoietic malignancies and solid tumors. In breast cancer cells, RelB and aryl hydrocarbon receptor (AhR) play a critical role in the regulation of interleukin (IL)‐8 and the anti‐apoptotic response.8 In Hodgkin lymphoma (HL), HL cells require RelB for sustaining viability.9 In multiple myeloma (MM), RelB is frequently activated in MM patient samples, and is a crucial positive regulator for MM cell survival.10 In mesenchymal glioma, RelB is an oncogenic driver of mesenchymal glioma tumor growth and invasion.11 However, the role of RelB in NSCLC has not been thoroughly examined.
In this study, we found that RelB was present at different expression levels in adenocarcinoma and squamous cell carcinoma. In addition, the NSCLC patients with high RelB expression had significantly shorter overall survival (OS) than those with low RelB expression. High RelB expression could be considered an independent prognostic factor for lower OS in patients with NSCLC.
Methods
Patients and clinicopathological features
The Clinical Research Ethics Committee of the First Affiliated Hospital of Soochow University, China, approved the use of patient samples. The study included all patients with lung cancer who were diagnosed, treated, and followed at the First Affiliated Hospital of Soochow University from 2009 to 2014. All patients accepted treatment according to the following principles. Stage I: surgical treatment is preferred. Stage II: surgical treatment is preferred; chemotherapy (a cisplatin‐based doublet chemotherapeutic regimen) was administered. Stage III: resectable stage III includes T3N1, T4N0–1, and a single N2 lymph node smaller than 3 cm. Surgical treatment is preferred; chemotherapy and sequential radiotherapy were administered. Stage IV: treatment includes isolation of brain metastases, adrenal metastases, or contralateral lung nodules. Surgical treatment was performed, followed by whole brain stereotactic ablative radiotherapy (SABR) or local therapy for adrenal lesions.
Inclusion criteria for the study were: (i) completely surgically resected and pathologically confirmed primary NSCLC; (ii) complete medical records; and (iii) available and well‐preserved paraffin‐embedded blocks. Exclusion criteria were: (i) radiotherapy and chemotherapy treatment before resection; (ii) staging data not available; and (iii) incomplete follow‐up data. In total, 280 patients who underwent complete resection were recruited into our study. Detailed follow‐up was available for 222 patients and 115 patients were analyzed for RelB expression. Clinicopathological information was obtained from medical records and pathology reports. Tumor diagnosis and histological classification were based on a new multidisciplinary classification of lung cancer proposed by the International Association for the Study of Lung Cancer, the American Thoracic Society, and the European Respiratory Society.12 Tumor node metastasis (TNM) pathological staging was classified according to the seventh edition staging system of the American Joint Committee on Cancer.13 Clinicopathological information is detailed in Table 1.
Table 1.
Relationship between RelB expression and clinicopathological characteristics
| Characteristics | RelB expression | P value | |
|---|---|---|---|
| High | Low | ||
| N = 60 (52%) | N = 55 (48%) | ||
| Gender | 0.731 | ||
| Male | 27 (54.0) | 23 (46.0) | |
| Female | 33 (50.8) | 32 (49.2) | |
| Age | 0.596 | ||
| Mean ± SD | 60.04 ± 10.25 | 60.19 ± 10.34 | |
| Range | 34–83 | 39–87 | |
| Smoking | 0.733 | ||
| Yes | 20 (50.0) | 20 (50.0) | |
| No | 40 (53.3) | 35 (46.7) | |
| Histology | 0.899 | ||
| ADC | 43 (51.8) | 40 (48.2) | |
| SCC | 17 (53.1) | 15 (46.9) | |
| Degree of differentiation | 0.023* | ||
| Low | 23 (67.6) | 11 (32.4) | |
| Middle | 34 (47.2) | 38 (52.8) | |
| High | 3 (33.3) | 6 (66.7) | |
| Depth of tumor invasion | <0.001* | ||
| T1 | 10 (29.4) | 24 (70.6) | |
| T2 | 28 (53.9) | 24 (46.1) | |
| T3 | 13 (72.2) | 5 (27.8) | |
| T4 | 9 (81.8) | 2 (18.2) | |
| Lymph node metastasis | 0.017* | ||
| N0 | 20 (41.7) | 28 (58.3) | |
| N1 | 30 (54.5) | 25 (45.6) | |
| N2 | 10 (83.3) | 2 (16.7) | |
| Distant metastases | 0.004* | ||
| No | 47 (47.0) | 53 (53.0) | |
| Yes | 13 (86.7) | 2 (13.3) | |
| TNM stage | <0.001* | ||
| I | 9 (27.3) | 24 (72.7) | |
| II | 17 (41.5) | 24 (58.5) | |
| III | 21 (80.8) | 5 (19.2) | |
| IV | 13 (86.7) | 2 (13.3) | |
The difference had statistical significance. ADC, adenocarcinoma; NSCLC, non‐small cell lung cancer; SD, standard deviation; SCC, squamous cell carcinoma; TNM, tumor node metastasis.
Tissue specimens and immunohistochemistry (IHC)
RelB expression was analyzed by immunohistochemistry (IHC) in four micrometer‐thick, formalin‐fixed, paraffin‐embedded (FFPE) sections. Tissue IHC was performed using a standard peroxidase‐based staining method. Tissue sections were incubated in a dry oven at 60°C for one hour, dewaxed in xylene for 3 × 10 minutes, and rehydrated with graded ethanol in 100%, 100%, 95%, 90%, 80%, and 70% ethanol for five minutes each. Antigen retrieval was then performed by pretreatment of the slides in 0.01 M citrate buffer (pH 6.0) using a microwave oven. Subsequently, the sections were treated with 3% hydrogen peroxide (H2O2) for 10 minutes in order to block endogenous peroxidase. The sections were washed with 1 × phosphate buffered saline (PBS; pH 7.4) and were incubated with rabbit anti‐RelB antibody (dilution 1:200; Santa Cruz Biotechnology Inc., Santa Cruz, CA, USA) overnight at 4°C. The sections were then washed with 1 × PBS and incubated with biotinylated goat anti‐rabbit immunoglobulin G. For each sample, the omission of primary antibody was used as a negative control. Finally, 3, 3‐diaminobenzine (DAB) was used to visualize the immunoreactive products. The results were evaluated by System Microscope IX71 (Olympus America Inc., Center Valley, PA, USA).
Evaluation of IHC
Two experienced pathologists analyzed the IHC results. RelB expression was graded on a scale of 0–3 according to the cells’ staining intensity and positive rate. Cytoplasmic staining with or without nuclear staining was considered positive for RelB expression. RelB expression was scored as follows: 0, no staining or <10% of tumor cells expressing RelB; 1, weak staining in >10% of tumor cells or moderate staining in 10–70% of tumor cells expressing RelB; 2, moderate staining in >70% of tumor cells or strong staining in 10–70% of tumor cells expressing RelB; and 3, strong staining in >70% of tumor cells expressing RelB. All scores were divided into two groups: low (0–1) and high RelB expression (2–3) in NSCLC samples.14
Survival and correlation analysis
Overall survival was defined from the first surgery until death and was analyzed in March 2015. The follow‐up duration ranged from 2 to 66 months (median follow‐up 29 months). The causes of death were mainly cancer relapse. The Kaplan–Meier method applying the log‐rank test was used to estimate the differences in OS between high and low RelB expression cases in patients with NSCLC. Univariate and multivariate Cox proportional hazards regression analyses were carried out to identify factors that had a significant impact on OS. Significant variables (P < 0.05) in univariate Cox proportional hazards regression analysis were entered into multivariate analysis.
Statistical analysis
Clinical characteristics of the subjects are summarized as mean ± standard deviations for continuous variables and number (%) for categorical variables. Chi‐square or two‐tailed Fisher's exact tests were used to analyze possible associations between qualitative clinicopathological variables and RelB expression. SPSS statistical software version 16.0 (SPSS Inc., Chicago, IL, USA) was used for all statistical analyses. P < 0.05 was considered statistically significant.
Results
RelB expression increased in non‐small cell lung cancer (NSCLC) formalin‐fixed, paraffin‐embedded samples
RelB expression was hardly detected in adjacent non‐neoplastic tissue (Fig 1a–c); however, RelB expression could be detected in adenocarcinoma by IHC, although a clear heterogeneity of RelB expression was observed among different samples of adenocarcinoma patients. Overall, low RelB expression was detected in 40/83 (48.2%) samples, with high RelB expression in 43/83 (51.8%) samples. While RelB expression was detected in the cytoplastic fraction of all adenocarcinoma cells, the nuclear expression of RelB was detected only in parts of samples (Fig 1d–f).
Figure 1.
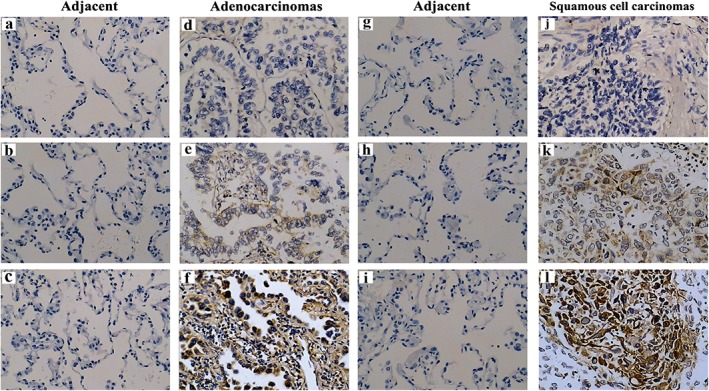
RelB expression in non‐small cell lung cancer tissues and adjacent non‐neoplastic tissue based on immunohistochemistry (magnification ×200). (a–c) Negative RelB expression in adjacent non‐neoplastic adenocarcinoma tissues; (d–f) negative, low, and high RelB expression in adenocarcinoma tissues, respectively; (g–i) negative RelB expression in adjacent non‐neoplastic squamous cell carcinoma tissues; (j–l) negative, low, and high RelB expression in squamous cell carcinoma tissues, respectively.
Similarly, heterogeneity of RelB expression was also observed in squamous cell carcinoma. RelB expression was hardly detected in adjacent non‐neoplastic tissue (Fig 1g–i). Low and high RelB expression was detected in 15/32 (46.9%) and in 17/32 (53.1%) squamous cell carcinoma samples, respectively (Fig 1j–l). The frequency of high RelB expression was slightly higher in squamous cell carcinoma 17/32 (53.1%) than in adenocarcinoma 43/83 (51.8%); however no statistical significance was observed (P = 0.89). This data suggests that RelB expression was absent in adjacent non‐neoplastic tissue in adenocarcinoma and squamous cell carcinoma. RelB expression was increased in either adenocarcinoma or squamous cell carcinoma, although different expression levels were observed.
Correlation between RelB expression and clinicopathological features of NSCLC patients
The relationship between RelB expression and the clinicopathological characteristics of NSCLC patients is summarized in Table 1. The RelB expression level was not correlated with gender, age, smoking status, or pathology of NSCLC patients. A high level of RelB expression was more frequently observed in low‐differentiation than in high‐differentiation samples. In low, middle, and high‐differentiation samples, the frequencies of high RelB expression were 67.6%, 47.2%, and 33.3%, respectively, indicating that the RelB expression level was negatively correlated with tumor differentiation status (P = 0.023). Among T1, T2, T3, and T4 stage samples, the frequencies of high RelB expression were 29.4%, 53.9%, 72.2%, and 81.8%, respectively, indicating that RelB expression was positively correlated with tumor progression (P < 0.001). In N0, N1, and N2 stage samples, the frequencies of high RelB expression were 41.7%, 54.5%, and 83.3%, indicating that RelB expression was significantly correlated with lymph node metastasis (P < 0.017). High RelB expression occurred more frequently in patients with distant metastasis (86.7%) than in patients without (47.0%), indicating that RelB expression was significantly correlated with distant metastasis (P = 0.004).
High RelB expression was more frequently observed in advanced clinical stage than in early stage samples. Among stage I, II, III, and IV samples, the percentages of high RelB expression were 27.3%, 41.5%, 80.8%, and 86.7%, respectively, suggesting that RelB expression was positively significantly correlated with TNM stage (P < 0.001).
Survival analysis and prognostic significance of high RelB expression in NSCLC patients
The relationship between RelB expression and OS in 115 NSCLC patients was analyzed by Kaplan–Meier using the log‐rank test (Fig 2). Patients with high RelB expression had significantly shorter OS than those with low RelB expression (χ2 = 32.993, P < 0.001; Fig 2). OS was not significantly correlated with age, gender, smoking status, or histology according to univariate Cox regression analysis (Table 2). However, short OS was correlated with low differentiation (hazard ratio [HR] 2.608, P = 0.004), advanced T (HR 2.196, P = 0.015), lymph node metastasis (HR 2.386, P = 0.014), distant metastasis (HR 2.726, P = 0.009), advanced TNM stage (HR 3.148, P < 0.001), and high RelB expression (HR 6.942, P < 0.001; Table 2). Moreover, RelB expression in adenocarcinoma and squamous cell carcinoma samples (HR 6.983, P < 0.001), along with the differentiation level of cancer (HR 2.522, P = 0.008) was an independent prognostic indicator of OS in NSCLC patients.
Figure 2.
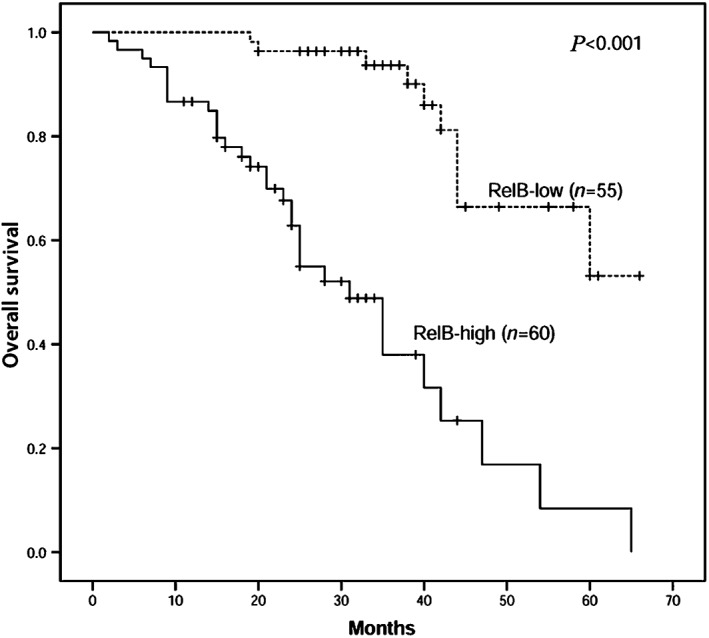
Correlation of RelB expression with overall survival in 115 non‐small cell lung cancer patients. The high RelB expression group has significantly shorter overall survival (P < 0.001) than the low RelB expression group.
Table 2.
Univariate and multivariate analyses of OS in 115 patients with NSCLC
| Characteristics | Univariate analysis | Multivariate analysis | ||
|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | |
| Age (>60 vs. ≤ 60) | 1.505 (0.804–2.818) | 0.201 | — | — |
| Gender (male vs. female) | 1.552 (0.832–2.894) | 0.167 | — | — |
| Smoking (yes vs. no) | 1.206 (0.637–2.285) | 0.565 | — | — |
| Histology (ADC vs. SCC) | 0.796 (0.410–1.543) | 0.498 | — | — |
| Differentiation (low vs. middle, high) | 2.608 (1.517–5.674) | 0.004* | 2.522 (1.276–4.985) | 0.008* |
| T stage (T3, T4 vs. T1, T2) | 2.196 (1.167–4.132) | 0.015* | — | — |
| N stage (N1 N2 vs. N0) | 2.386 (1.194–4.768) | 0.014* | — | — |
| M stage (M1 vs. M0) | 2.726 (1.287–5.773) | 0.009* | — | — |
| TNM stage (III IV vs. I II) | 3.148 (1.689–5.869) | <0.001* | — | — |
| RelB expression (high vs. low) | 6.942 (3.256–14.799) | <0.001* | 6.983 (3.237–15.064) | <0.001* |
The difference had statistical significance. ADC, adenocarcinoma; CI, confidence interval; HR, hazard ratio; NSCLC, non‐small cell lung cancer; OS, overall survival; SCC, squamous cell carcinoma; TNM, tumor node metastasis.
Discussion
In this study, we found that RelB expression was present at different expression levels in adenocarcinoma and squamous cell carcinoma. RelB expression was mainly detected in the cytoplasm of tumor cells, which was significantly correlated with degree of differentiation, depth of tumor invasion, lymph node metastasis, distant metastases, and TNM stage in patients with NSCLC. In addition, NSCLC patients with high RelB expression had significantly shorter OS than those with low RelB expression. Importantly, high RelB expression could be considered an independent prognostic factor for lower OS in patients with NSCLC.
NF‐κB was originally characterized as a molecule that could bind to the enhancer regions of immunoglobulin light chain genes.15 In resting cells, NF‐κB heterodimers were inactive and were sequestered in the cytoplasm by the binding of various inhibitory molecules, known as inhibitors of κB (IκB).3, 16 When triggered by different stimulators, NF‐κB is activated via classical or alternative NF‐κB pathways.4, 5
RelB is one member of the alternative NF‐κB pathways. The RelB gene locates on chromosome 19q13.32, and encodes messenger ribonucleic acid from 11 exons, producing a protein of 579 amino acids.17 The RelB protein is very labile and normally sequestered in the cytoplasm by p100. RelB regulates important biological functions, such as lymphoid organ development, circadian rhythm, inflammatory response, and the xenobiotic detoxifying pathway.3, 18, 19, 20, 21
Deregulated alternative NF‐κB activity is an important aspect of many hematological malignancies and solid tumors.22 In prostate cancer, RelB enhances prostate cancer cell growth and inhibits the radiosensitivity of cancer cells.23, 24 In breast tumors, RelB promotes cellular survival and confers more highly invasive phenotypes.25 In MM, Francoise et al. found that 40% of newly diagnosed MM patients have constitutive RelB DNA‐binding activity in CD138+ tumor cells.10 In chronic lymphocytic leukemia (CLL) cells, enhanced RelB activity increases the sensitivity of CLL cells to the proteasome inhibitor, bortezomib.26 Baicalin, as a potential therapeutic candidate for hepatocellular carcinoma, regulates RelB/p52 activation.27 Thus, according to previous studies, RelB has a tumor‐supportive role in diverse cancers. However, little is known about the role of RelB in NSCLC. Dimitrakopoulos et al. reported that RelB may play a role in the development and progression of NSCLC.28 In this study, we found that RelB expression was present at different expression levels in adenocarcinoma and squamous cell carcinoma. Moreover, we found that RelB expression was predominant in the cytoplasm of tumor cells, consistent with previous reports.11, 23
To further clarify the role of RelB in the development and progression of NSCLC, the relationship between RelB expression and clinicopathological features was analyzed in NSCLC patients. High RelB expression was significantly associated with low differentiation, deep tumor invasion, positive lymph node metastasis, distant metastases, and advanced clinical stage. These results suggest that high RelB expression could play a role in NSCLC tumor progression and metastasis (data not shown), in line with a previous report indicating that RelB was correlated with tumor stage in NSCLC patients.28
High RelB expression has been shown to serve as an independent poor prognostic factor, such as in mesenchymal glioma.10, 11 However, whether RelB expression could be an independent poor prognostic factor in NSCLC patients was unknown. In this study, for the first time, we demonstrated that the RelB protein expression in NSCLC samples was inversely correlated with OS, as the patients with higher RelB protein expression experienced shorter survival duration (χ2 = 32.993, P < 0.001). Moreover, according to multivariate analyses, the high expression of RelB protein was a significant predictor of poor prognosis in NSCLC patients (HR 6.983, P < 0.001).
In conclusion, based on IHC assay combined with clinicopathological characteristic analyses, our study indicated a strong clinical and prognostic significance of high RelB expression in NSCLC patients. Particularly, high RelB expression could be a strong prognostic marker for NSCLC in Chinese patients.
Disclosure
No authors report any conflicts of interest.
Acknowledgments
The authors would like to thank the patients for their participation and cooperation. This work was supported by the National Natural Science Foundation of China (No. 81172433) and the Project of Jiangsu Provincial College graduate research and innovation program (No. KYLX_1268).
References
- 1. Torre LA, Bray F, Siegel RL, Ferlay J, Lortet‐Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin 2015; 65: 87–108. [DOI] [PubMed] [Google Scholar]
- 2. Chen WQ, Zheng RS, Zhang SW, Zeng HM, Zou XN. The incidences and mortalities of major cancers in China, 2010. Chin J Cancer 2014; 33: 402–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Vallabhapurapu S, Karin M. Regulation and function of NF‐kappaB transcription factors in the immune system. Annu Rev Immunol 2009; 27: 693–733. [DOI] [PubMed] [Google Scholar]
- 4. Hayden MS, Ghosh S. NF‐κB, the first quarter‐century: Remarkable progress and outstanding questions. Genes Dev 2012; 26: 203–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Perkins ND. The diverse and complex roles of NF‐κB subunits in cancer. Nat Rev Cancer 2012; 12: 121–32. [DOI] [PubMed] [Google Scholar]
- 6. DiDonato JA, Mercurio F, Karin M. NF‐κB and the link between inflammation and cancer. Immunol Rev 2012; 246: 379–400. [DOI] [PubMed] [Google Scholar]
- 7. Vallabhapurapu SD, Pagolu KR, Vallabhapurapu S. Regulation of the alternative NF‐κb pathway and its role in cancer. J Cancer Sci Ther 2013; 5: 1–3. [Google Scholar]
- 8. Vogel CF, Li W, Wu D et al. Interaction of aryl hydrocarbon receptor and NF‐κB subunit RelB in breast cancer is associated with interleukin‐8 overexpression. Arch Biochem Biophys 2011; 512: 78–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ranuncolo SM, Pittaluga S, Evbuomwan MO, Jaffe ES, Lewis BA. Hodgkin lymphoma requires stabilized NIK and constitutive RelB expression for survival. Blood 2012; 120: 3756–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Demchenko YN, Glebov OK, Zinqone A, Keats JJ, Bergsagel PL, Kuehl WM. Classical and/or alternative NF‐kappaB pathway activation in multiple myeloma. Blood 2010; 115: 3541–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lee DW, Ramakrishnan D, Valenta J, Parney IF, Bayless KJ, Sitcheran R. The NF‐κB RelB protein is an oncogenic driver of mesenchymal glioma. PLoS ONE 2013; 8 (2): e57489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Travis WD, Brambilla E, Noguchi M et al. International Association for The Study of Lung Cancer/American Thoracic Society/European Respiratory Society International Multidisciplinary Classification of Lung Adenocarcinoma. J Thorac Oncol 2011; 6: 244–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Goldstraw P, Crowley J, Chansky K et al. The IASLC Lung Cancer Staging Project: Proposals for the revision of the TNM stage groupings in the forthcoming (seventh) edition of the TNM classification of malignant tumors. J Thorac Oncol 2007; 2: 706–14. [DOI] [PubMed] [Google Scholar]
- 14. Taylor CR. Quantifiable internal reference standards for immunohistochemistry: The measurement of quantity by weight. Appl Immunohistochem Mol Morphol 2006; 14: 253–9. [DOI] [PubMed] [Google Scholar]
- 15. Sen R, Baltimore D. Inducibility of kappa immunoglobulin enhancer‐binding protein Nf‐kappa B by a posttranslational mechanism. Cell 1986; 47: 921–8. [DOI] [PubMed] [Google Scholar]
- 16. Guo F, Weih D, Meier E, Weih F. Constitutive alternative NF‐kappaB signaling promotes marginal zone B‐cell development but disrupts the marginal sinus and induces HEV‐like structures in the spleen. Blood 2007; 110: 2381–9. [DOI] [PubMed] [Google Scholar]
- 17. Millet P, McCall C, Yoza B. RelB: An outlier in leukocyte biology. J Leukoc Biol 2013; 94: 941–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Guo F, Tänzer S, Busslinger M, Weih F. Lack of nuclear factor‐ kappaB2/p100 causes a RelB‐dependent block in early B lymphopoiesis. Blood 2008; 112: 551–9. [DOI] [PubMed] [Google Scholar]
- 19. Bellet MM, Zocchi L, Sassone‐Corsi P. The RelB subunit of NFkB acts as a negative regulator of circadian gene expression. Cell Cycle 2012; 11: 3304–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. McMillan DH, Baglole CJ, Thatcher TH, Maggirwar S, Sime PJ, Phipps RP. Lung‐targeted overexpression of the NF‐κB member RelB inhibits cigarette smoke‐induced inflammation. Am J Pathol 2011; 179: 125–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Sheridan JA, Zago M, Nair P. Decreased expression of the NF‐κB family member RelB in lung fibroblasts from smokers with and without COPD potentiates cigarette smoke‐induced COX‐2 expression. Respir Res 2015; 16: 54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Baud V, Jacque E. [The alternative NF‐κB activation pathway and cancer: Friend or foe?] Med Sci 2008; 24: 1083–8. (In French.) [DOI] [PubMed] [Google Scholar]
- 23. Xu Y, Josson S, Fang F et al. RelB enhances prostate cancer growth: Implications for the role of the nuclear factor‐kappaB alternative pathway in tumorigenicity. Cancer Res 2009; 69: 3267–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Zhu L, Zhu B, Yang L, Zhao X, Jiang H, Ma F. RelB regulates Bcl‐xl expression and the irradiation‐induced apoptosis of murine prostate cancer cells. Biomed Rep 2014; 2: 354–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Mineva ND, Wang X, Yang S et al. Inhibition of RelB by 1, 25‐dihydroxyvitamin D3 promotes sensitivity of breast cancer cells to radiation. J Cell Physiol 2009; 220: 593–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Xu J, Zhou P, Sun A Guo F. [Effect of nuclear transcription factor RelB on the proteasome inhibitor‐sensitivity of chronic lymphocytic leukemia cells.] Chin J Hematol 2014; 35: 524–7. (In Chinese.) [DOI] [PubMed] [Google Scholar]
- 27. Tan HY, Wang N, Man K, Tsao SW, Chen CM, Feng Y. Autophagy‐induced RelB/p52 activation mediates tumour‐associated macrophage repolarisation and suppression of hepatocellular carcinoma by natural compound baicalin. Cell Death Dis 2015; 6: e1942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Dimitrakopoulos FI, Antonacopoulou AG, Kottorou A et al. NSCLC and the alternative pathway of NF‐κB: Uncovering an unknown relation. Virchows Arch 2012; 460: 515–23. [DOI] [PubMed] [Google Scholar]


