Abstract
Background
We compared video‐assisted thoracoscopic surgery (VATS) lobectomy and stereotactic body radiation therapy (SABR) to explore clinical outcomes in the treatment of patients with early stage NSCLC.
Methods
Major medical databases were systematically searched to identify studies on VATS and SBRT published between January 2010 and October 2015. English publications of stage I and II NSCLC with adequate patients and SBRT doses were included. A multivariate random effects model was used to perform meta‐analysis to compare overall survival (OS) and disease‐free survival (DFS) between VATS and SBRT, adjusting for median age and operable patient numbers.
Results
Thirteen VATS (3436 patients) and 24 SBRT (4433) studies were eligible. The median age and follow‐up duration was 68 years and 42 months for VATS and 74 years and 29.4 months for SBRT patients. After adjusting for the proportion of operable patients and median age, the estimated OS rates at one, two, three, and five years with VATS were 94%, 89%, 84%, and 69% compared with 96%, 94%, 89%, and 82% for SBRT. The estimated DFS rates at one, two, three, and five years with VATS were 97%, 93%, 87%, and 77% compared with 86%, 80%, 73%, and 58% for SBRT.
Conclusion
Before adjustment, patients treated with SBRT had poorer clinical outcomes compared to those treated with VATS. A substantial difference between median age and operability exists between patients treated with SBRT and VATS. After adjusting for these differences, OS and DFS did not differ significantly between the two techniques.
Keywords: Lung cancer, SBRT, VATS
Introduction
Lung cancer is the most common cause of cancer‐related death worldwide, a finding partly resulting from the small proportion of patients presenting with early‐stage disease.1, 2, 3 The recommended treatment for early‐stage non‐small‐cell lung cancer (NSCLC) is a lobectomy, but many patients with stage I NSCLC do not undergo surgery because of comorbidities or patient preference.4
The adoption of minimally invasive techniques for lobar resection has been one of the important advances in thoracic surgery. As a minimally invasive alternative to open thoracotomy, video‐assisted thoracic surgery (VATS) is the preferred modality in the latest American College of Chest Physicians Evidence‐based Guidelines for early‐stage NSCLC.5 VATS lobectomy is an accepted oncologic approach for early‐stage NSCLC.6, 7, 8
Stereotactic body radiotherapy (SBRT) is a treatment option for stage I patients who are medically inoperable or refuse surgery. SBRT has achieved local control (LC) and overall survival (OS) rates comparable with lobectomy in non‐randomized studies in medically inoperable or elderly patients.9, 10, 11 SBRT can also achieve high LC and low toxicity in patients with peripheral lung metastases and limited oligometastatic disease.12, 13, 14
Until now, no randomized trials comparing VATS lobectomy with SBRT have been conducted and non‐randomized comparisons may be hampered by imbalances in baseline characteristics between both groups. Propensity score analysis allows for matching across a broad range of baseline factors, creating two similar groups for comparison. As both VATS and SABR are routinely available to patients worldwide, we carried out a meta‐analysis using a mixed effects model to compare OS and disease‐free survival (DFS) after both treatments for patients with clinical stage I–II NSCLC.
Methods
Literature search strategy
We conducted a bibliographic search for original research articles, using multiple electronic databases, including PubMed, MEDLINE, Embase, and ISI Web of Science. For comparison, both VATS and SBRT studies were retrieved from the same databases within the same publication period. To effectively identify relevant articles, a protocol for structured literature retrieval was followed. The retrieval results for each step are detailed in Figure 1.
Figure 1.
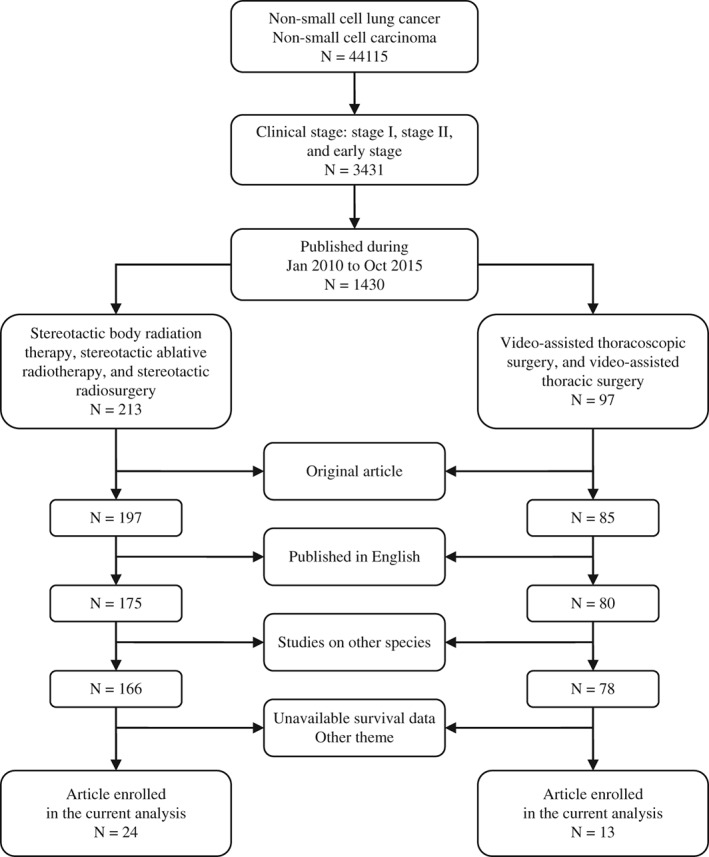
Selection strategy of studies enrolled in the current meta‐analysis.
Selection criteria
The following eligibility criteria were applied: (i) original English articles published between January 2010 and October 2015; (ii) early‐stage NSCLC strictly limited to stage I and II disease with reference to the 7th edition of Cancer Staging by the American Joint Committee on Cancer (AJCC); (iii) VATS was the abbreviation for video‐assisted thoracoscopic surgery, equivalent to video‐assisted thoracic surgery; and (iv) SBRT was the abbreviation for stereotactic body radiation therapy, equivalent to stereotactic ablative radiotherapy and stereotactic radiosurgery.
Surgical procedures in early‐stage NSCLC could be either full anatomical resections including lobectomy, bilobectomy, and pneumonectomy, or limited lung resection including sublobar resection, segmentectomy, and wedge resection. Studies with treatment using hypofractionated radiation therapy with fraction dose > 8 Gy and fraction number ≤ 8, were categorized as SBRT according to the SBRT definition.6, 15, 16 OS and/or DFS data were reported or could be extrapolated based on published results. Some authors had more than one report meeting that met the inclusion criteria. To minimize data overlap, each report was analyzed to ensure that only the report with the latest results and largest patient number was enrolled. If the patient population was from a different time period or different outcomes were reported, both reports were included in the analysis.
Data extraction
Two reviewers independently extracted the data from all studies. Additional data from SBRT studies obtained included: first author; publication year; research type; research year range; total patients; operable patient percent; male percent; median age; clinical stage; path; tumor size; dose range; biological equivalent dose (BED)10; and follow‐up period. Additional data from VATS studies included: first author; publication year; research type; research year range; total patients; male percent; median age; surgery process; clinical stage; and follow‐up period. Disagreements were resolved by consensus between the two reviewers. The majority of studies were retrospective (all 13 VATS studies were retrospective and 2 out of 24 SBRT studies were prospective). Survival data, not available within the context, were extracted from the survival curve using Engauge Digitizer V4.1 (Slashdot Media, La Jolla, CA, USA). At the same time, the extracted survival data were confirmed with data available in context in the same year. The acceptable error was ± 0.05.
Data analysis
The primary objective of the meta‐analysis was to compare OS rates of SBRT patients with those of surgery patients with early‐stage NSCLC. As a first step, overall raw summary statistics for each outcome measure were calculated by treatment. These statistics were not calculated to compare outcomes between treatments (because of the substantial differences in patient populations) but rather to summarize typical outcomes following treatment of a particular patient population. A multivariate random effects model was used to provide a meta‐analysis of the summary survival curve data while adjusting for potential confounders.17, 18 Survival estimates were ln‐minus‐ln transformed, whereas ln(time) was included as a covariate in addition to fixed covariates for treatment (SBRT or VATS), age, and portion of operable patients. The resulting estimated survival curves were in the Weibull family. Study‐specific random effects for the intercept and the slope of time were included to account for correlation within the study over time. An unstructured between‐study covariance matrix was used. Parameters were estimated in an iterative manner, updating the within‐study correlations, using repeated calls of process mixed in SAS V9.4 (SAS Institute Inc., Cary, NC, USA). Using this mixed random effects model, we calculated the hazard ratio (HR) for treatment adjusting for age and portion of operable patients, as well as the estimated survival probabilities at fixed times when the median age was 70 years and percentage of operable patients was 100%. Because standard errors or confidence intervals (CIs) of the survival values were not often provided, we estimated the within‐study variance–covariance matrix using the reported survival proportions and the number of subjects in follow‐up at a given time point.18, 19 The number of subjects in follow‐up was estimated from reported median follow‐up times and an assumed exponential loss to follow‐up time distribution. The median of the reported median follow‐up times was used for the studies that did not report follow‐up times. Confidence intervals were calculated based on variability between study level outcomes, representing both between‐study heterogeneity and sampling variation. Various sensitivity analyses were performed to assess the robustness of the results to modeling choices, such as which confounding variables to include.
Results
Literature search and characteristics
A total of 54 VATS and 87 SBRT articles meeting the initial screen criteria were collected for full‐text review. Among these, 19 were prospective and 122 were retrospective studies, with a total of 9821 patients. After excluding duplicate studies, studies that did not report survival data, and focused on other species, 13 retrospective VATS articles with a total of 3436 patients (Table 1), and 24 SBRT articles of two prospective studies and 22 retrospective studies with a total of 4433 patients (Table 2) were included in this study. Overall, the patients who received VATS treatment were significantly younger than those who received SBRT (67.1 ± 4.9 vs. 74.5 ± 6.5 years, respectively, P < 0.0001). There were no significant differences in gender between the two treatment modalities (51.0% vs. 61.1% for men in the VATS and SBRT groups, respectively; P = 0.0406). The mean follow‐up was longer in VATS than in SBRT studies (27.8 vs. 41.3 months, respectively).
Table 1.
Data of VATS studies (January 2010 to October 2015) included in the meta‐analysis
| Author | Publication year | Reseach type | Research year range | Total patients | Male | Med ian age | Surgical Procedure (no. of patients) | Clinical stage | Follow‐up | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (%) | IA | IB | IIA | IIB | (m) | |||||||
| Kim et al. 20 | 2010 | R | 2003–2008 | 436 | / | / | LR (436) | 248 | 188 | 13 | 44 | >20 |
| Puri et al. 21 | 2010 | R | 2000–2006 | 841 | / | 65 | / | 621 | 220 | >24 | ||
| Sugi et al. 22 | 2010 | R | 2001–2004 | 139 | 36.2 | 64 | LLR (43), LR (95) | 128 | 11 | >60 | ||
| Yamashita et al. 23 | 2011 | R | 2003–2008 | 109 | 60.6 | 70 | LLR (38), LR (71) | 83 | 26 | / | / | >27.5 |
| Marty‐Ané et al. 24 | 2013 | R | 1996–2011 | 312 | 65.1 | 62 | LR (364) | 183 | 90 | 10 | 29 | >60 |
| Verstegen et al. 9 | 2013 | R | 2007–2013 | 64 | 56.3 | 68 | LR (64) | 39 | 24 | >48 | ||
| Battoo et al. 25 | 2013 | R | 2002–2012 | 67 | 43 | 65 | LR (67) | 23 | 25 | / | ||
| Gonzalez‐Rivas et al. 26 | 2014 | R | 2010–2012 | 87 | 70.1 | 65 | LR (87) | 59 | 15 | 6 | 1 | / |
| Nakano et al. 27 | 2014 | R | 2010–2012 | 464 | 55.7 | 68 | LR (464) | / | / | / | / | / |
| Ghaly et al. 28 | 2015 | R | 2000–2013 | 91 | 37 | 72 | LLR (91) | 85 | 6 | ‐ | ‐ | >21.5 |
| Murakawa et al. 29 | 2015 | R | 2001–2010 | 101 | 51.5 | 69 | LR (101) | 51 | 30 | 18 | 2 | 60 |
| Nwogu et al. 30 | 2015 | R | 2004–2010 | 175 | 48 | 69 | LR (175) | / | / | / | / | 60 |
| Zhou et al. 31 | 2015 | R | 2006–2012 | 550 | 38 | 68 | LR (493), LLR (57) | / | / | / | / | >32.4 |
LLR, limited lung resection; LR, lobar resection; M, months; R, retrospective; VATS, video‐assisted thoracic surgery; /, not reported or obscure.
Table 2.
Data of SBRT studies (January 2010 to October 2015) included in the meta‐analysis
| Author | Publication year | Research type | Research year range | Total patients | Operability | Male | Median age | Clinical stage | Pathological confirmation | Tumor size | Dose range | BED10 | Follow‐up (m) | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (%) | (%) | IA | IB | IIA | IIB | (%) | (mm) | |||||||||
| Baba et al. 32 | 2010 | R | 2004–2008 | 124 | 32.4 | 67.7 | 77 | 87 | 37 | / | / | 91.9 | m27 | 44,48,52 | 92.4105.6119,6 | 26 (7–66) |
| Ricardi et al. 33 | 2010 | P | 2003–2007 | 62 | 9.7 | 83.9 | 74 | 43 | 19 | / | / | 64.5 | <50 | 45 | 124 | 28 (9–60.7) |
| Timmerman et al. 34 | 2010 | P | 2004–2006 | 55 | 0 | 38 | 72 | 44 | 11 | / | / | 100 | <50 | 54 | 151.2 | 34.3 (4.8–49.9) |
| Haasbeek et al. 35 | 2011 | R | 2003–2009 | 63 | 0 | 67 | 74 | 46 | 17 | / | / | 38.1 | Major < 70, 2 > 70 | 60 | 105 | 35 |
| Matsuo et al. 36 | 2011 | R | 1998–2007 | 101 | 36.6 | 73.3 | 77 | 33 | 40 | 28 | / | 100 | <= 40 | 48 | 105.6 | 31.4 |
| Nath et al. 37 | 2011 | R | 2007–2009 | 48 | 0 | 62% | 79 | / | / | / | / | / | / | 48 | 105.6 | 21 (10–41) |
| Chang et al. 38 | 2012 | R | 2005–2009 | 130 | 26.2 | 51.5 | 74 | 112 | 18 | / | / | / | <50 | 50 | 112.5 | 26 |
| Grills et al. 14 | 2012 | R | 1998–2010 | 483 | 13 | 52 | 74 | 304 | 159 | 10 | 5* | 64 | / | 20–64 | 132 | 19.6 (1.2–87.6) |
| Nuyttens et al. 39 | 2012 | R | 2006–2009 | 56 | 5.4 | / | 73 | / | / | / | / | / | m41 | 48 | >86.4 | 23 |
| Senthi et al. 40 | 2012 | R | 2003–2011 | 676 | 31 | 61 | 73 | / | / | / | / | / | / | 54–60 | 105–180 | 32.9 (14.9–50.9) |
| Shibamoto et al. 41 | 2012 | R | 2004–2008 | 180 | 33.3 | 68.3 | 77 | 128 | 52 | / | / | 100 | 12–50 | 44,48,52 | 92.4105.6119.6 | 36 |
| Takeda et al. 42 | 2012 | R | 2005–2011 | 115 | 27 | 78 | 78 | / | / | / | / | 100 | / | 40–50 | 72–100 | 21.2 (6–63.7) |
| Badiyan et al. 43 | 2013 | R | 2004–2009 | 120 | 0 | 52 | 74 | / | / | / | / | 80.8 | / | 54 | 151.2 | 29 |
| Griffioen et al. 44 | 2013 | R | 2003–2012 | 62 | / | 66 | 72 | / | / | / | / | / | 10–69,6–42 | 54–60 | >100 | 44 |
| Yoon et al. 45 | 2013 | R | 2007–2009 | 93 | / | 80.6 | 61 | / | / | / | / | / | 20 (10–60) | 30–60 | / | 25.6 |
| Haidar et al. 46 | 2014 | R | 2002–2012 | 55 | 21.3 | 63.5 | 78 | 37 | 18 | / | / | 58.2 | 25 (10–5) | 48–56 | 106.5–168 | 24.2 (1.9–64.6) |
| Lucas et al. 47 | 2014 | R | 2003–2011 | 81 | / | 48.1 | 81 | 67 | 14 | 3 | 1 | / | 23 (16–29) | 54 | 151.2 | 29.4 |
| Ricardi et al. 48 | 2014 | R | 2003–2011 | 196 | 7.7 | 74.5 | 75 | 155 | 41 | / | / | / | 24.8 (9‐50) | 45–60 | 100–151.2 | 30 |
| Davis et al. 49 | 2015 | R | 2004–2014 | 111 | 15.3 | 53.1 | 69 | / | / | / | / | / | 22.2 | 37.5,48 | 93.6105.6 | 17 (1–72) |
| Davis et al. 50 | 2015 | R | 2004–2013 | 723 | 48 | 48% | 76 | / | / | / | / | / | / | 54 (10–80) | 151.2 (20–240) | 12 (1–87) |
| Kelley et al. 51 | 2015 | R | 2010–2012 | 67 | 0 | 36 | 79 | 52 | IB‐III:15 | / | Volume < 20cc | 48 | 105.6 | 24.5 (2.4–50.3) | ||
| Kohutek et al. 52 | 2015 | R | 2006–2012 | 211 | / | 43.6 | 77 | / | / | / | / | 100 | / | >45 | < 100 (19.2%), > = 100 (80.8) | 25.2 (4.3–75.2) |
| Zehentmayr et al. 53 | 2015 | R | 2002–2010 | 54 | / | 66.7 | 71 | 40 | 14 | / | 37.5 (5–103) | 73.8–90 | / | 28.5 (2–108) | ||
| Schanne et al. 54 | 2015 | R | 2003–2011 | 567 | / | 70.7 | 72 | 297$ | 223 | 30 | / | / | 37.5(12–64) | 72 (43–180) | 18.8 | |
| I (not specified):16 | ||||||||||||||||
BED10: biological equivalent dose with α/β = 10, calculated based on the linear‐quadratic equation BED10 = nd [1 + d/(α/β)], where n and d represent the number of fractions and the dose per fraction, respectively. m, median; M, months; P, prospective; R, retrospective; SBRT, stereotactic body radiation therapy; /, not reported or obscure.
Unadjusted outcomes of overall survival (OS) and disease‐free survival (DFS)
Overall survival rates at one, two, and three years were one of the primary endpoints in all but one of the studies.26 The unadjusted OS rates at one, two, three, and five years for SBRT were 85.4%, 68.1.6%, 54.6%, and 29.7%, respectively. The corresponding OS rates for VATS were higher at 94.6%, 86.9%, 82.8%, and 74.0%,, respectively. The mean unadjusted DFS rates at one, two, three, and five years for VATS were 94.2%, 89.1%, 84.8%, and 74.0% compared with 83.9%, 70.9%, 65.2%, and 58.1% for SBRT (Figure 2). Without considering the differences in patient characteristics, such as age between VATS and SBRT groups, OS and DFS rates were numerically higher in the VATS patients (Figure 3).
Figure 2.
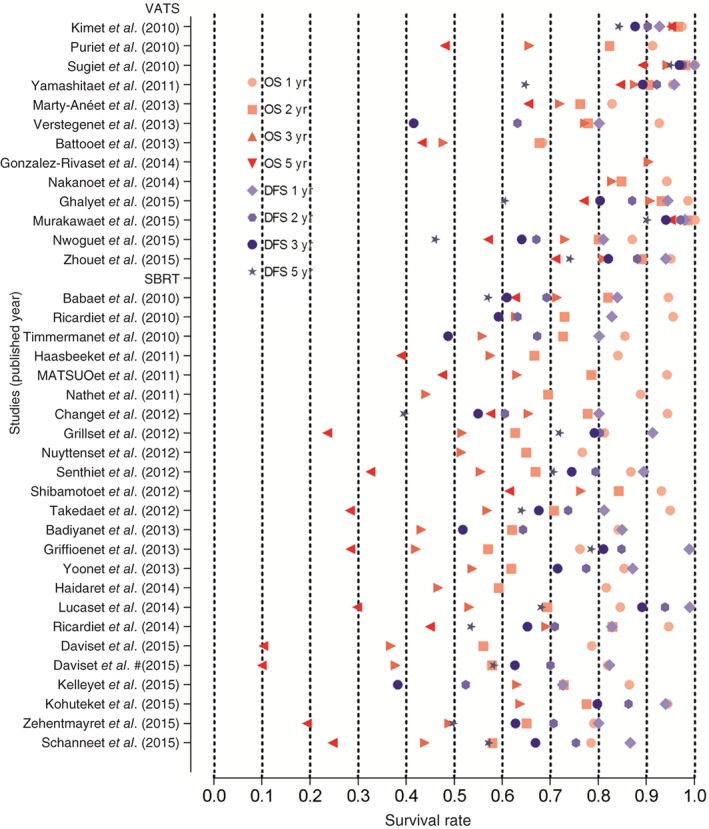
Overall survival (OS) and disease‐free survival (DFS) comparison between video‐assisted thoracic surgery (VATS) and stereotactic body radiation therapy (SBRT) for early‐stage non‐small cell lung cancer. The figure shows a pooled presentation of one, two, three and five year OS and DFS from all studies enrolled in the meta‐analysis with corresponding data available.
Figure 3.
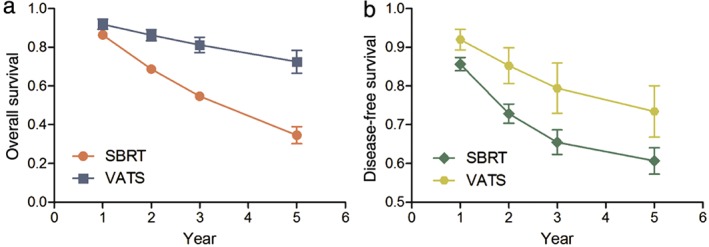
(a) Overall survival and (b) disease‐free survival comparison between video‐assisted thoracic surgery (VATS) and stereotactic body radiation therapy (SBRT) in early‐stage (stage Ia, Ib, IIa, and IIb) non‐small cell lung cancer.
Adjusted outcomes of OS and DFS by age and portion of operable patients
All but one of the studies reported the median age of patients, which was 68 years for VATS and 74 years for SBRT patients, respectively (67.1 ± 4.9 vs. 74.5 ± 6.5 years, respectively, P < 0.0001).20 This result suggested that age might be a confounder affecting clinical outcomes. Analyses regarding the effect of age showed that reported OS was significantly related to the reported median age for patients in a trial (P < 0.05; Fig 4a,b). DFS was similarly negatively correlated with median age (P < 0.05 at 3 and 5 years; Fig 4c,d).
Figure 4.
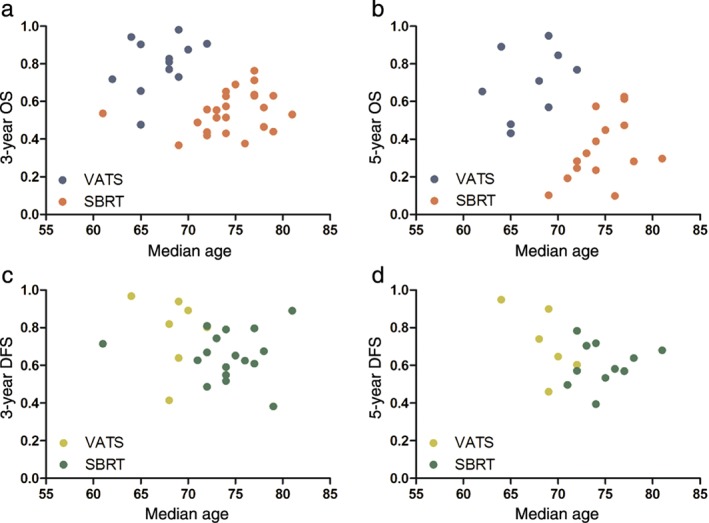
Age‐dependent overall survival (OS) and disease‐free survival (DFS) comparison between video‐assisted thoracic surgery (VATS) and stereotactic body radiation therapy (SBRT). Estimated (a) three‐year OS, (b) five‐year OS, (c) three‐year DFS, and (d) five‐year DFS for VATS versus SBRT by median trial age.
In 18 SBRT studies (3365 patients) reporting the proportion of SBRT patients who were operable, mean operability was 17.5% (range 0–48%; median 14.2%). Not surprisingly, mean OS improved significantly with increasing operability (P < 0.05 at every time point), as shown in Figure 5a,b. The corresponding Spearman correlation coefficients between operability and three and five‐year OS were 0.63 and 0.52, respectively. DFS was not correlated with operability (P > 0.05 at 3 and 5 years; Fig. 5c,d).
Figure 5.
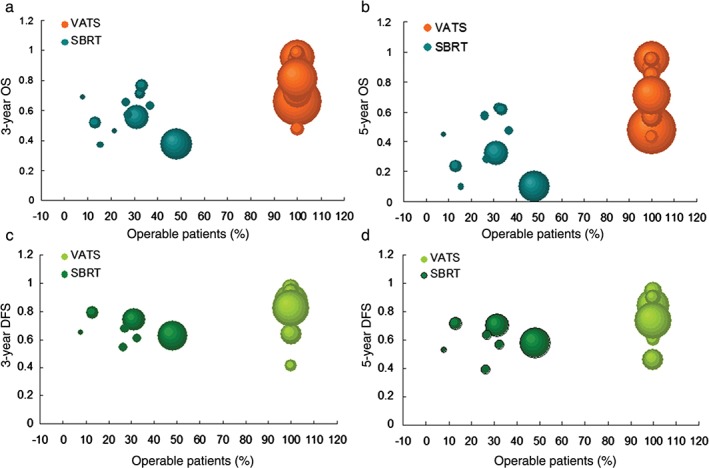
Operability‐dependent overall survival (OS) and disease‐free survival (DFS) comparison between video‐assisted thoracic surgery (VATS) and stereotactic body radiation therapy (SBRT). Estimated (a) three‐year OS, (b) five‐year OS, (c) three‐year DFS, and (d) five‐year DFS for VATS versus SBRT by median trial age by proportion. Dot sizes are proportional to the number of patients in specific studies.
Given the nonrandomized nature of the data, it is crucial to control or adjust for potential confounders when making comparisons between treatments. To do so, we could only use variables that were measured and reported. One such overall confounder, which encompasses many other factors, such as comorbidities, is patient eligibility for surgery. We also used age, which, in addition, to being frequently reported, differed significantly between SBRT and surgery series and was related to OS. After we controlled for these two confounders in a regression model, there were no longer any significant differences in OS between treatments (P = 0.36). Specifically, after adjusting for the proportion of operable patients and median age,, there were no significant differences in OS between VATS and SBRT (HR 2.02, 95% CI 1.45–3.07; P = 0.47; Table 3 and Fig 6a). Similarly, after adjustment, there were no significant differences in DFS between treatments (P = 0.49), or between VATS and SBRT (HR 0.42, 95% CI 0.21–1.12; P = 0.52; Table 3 and Fig 6b). From the fitted regression model, we calculated the expected OS at one, two, three, and five years at fixed values of the covariate age (70 years of age) and operability (100% operable; Table 4). Although SBRT had higher expected OS rates but lower expected DFS rates, these differences were not statistically significant.
Table 3.
Model‐based overall and disease‐free survival estimates in trials with a median age of 70 and 100% operable patients
| Model | Year | SBRT | VATS | ||
|---|---|---|---|---|---|
| Estimate | 95% CI | Estimate | 95% CI | ||
| Overall survival | 1 | 0.96 | 0.92–0.99 | 0.94 | 0.88–0.96 |
| 2 | 0.94 | 0.81–0.96 | 0.89 | 0.79–0.94 | |
| 3 | 0.89 | 0.79–0.93 | 0.84 | 0.73–0.88 | |
| 5 | 0.82 | 0.72–0.91 | 0.69 | 0.61–0.73 | |
| Disease‐free survival | 1 | 0.86 | 081–0.92 | 0.97 | 0.95–0.99 |
| 2 | 0.8 | 0.77–087 | 0.93 | 0.86–0.96 | |
| 3 | 0.73 | 0.65–0.78 | 0.87 | 0.78–0.95 | |
| 5 | 0.58 | 0.51–0.67 | 0.77 | 0.63–0.89 | |
CI, confidence interval; VATS, video‐assisted thoracic surgery; SBRT, stereotactic body radiation therapy.
Figure 6.
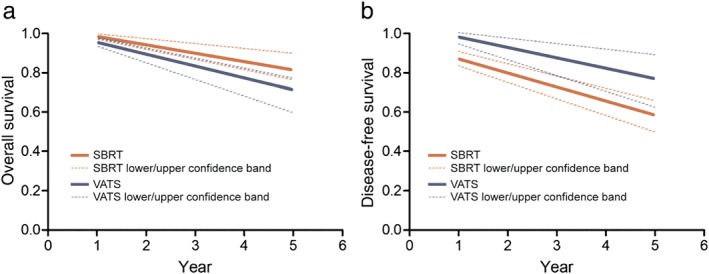
Estimated (a) overall survival and (b) disease‐free survival by video‐assisted thoracic surgery (VATS) versus stereotactic body radiation therapy (SBRT) in patients with a median age of 70 and 100% operability.
Table 4.
Hazard ratio (VATS to SBRT) of overall and disease‐free survival estimates from the multivariate mixed effects model
| Model | Hazard ratio | 95% CI | P value |
|---|---|---|---|
| Overall survival | |||
| Treatment | 0.38 | ||
| VATS to SBRT | 2.02 | 1.45–3.07 | 0.47 |
| Median age | 1.11 | 0.54–1.78 | 0.0002 |
| Percentage of operability | 0.96 | 0.88–1.04 | 0.04 |
| Disease‐free survival | |||
| Treatment | 0.49 | ||
| VATS to SBRT | 0.42 | 0.21–1.12 | 0.52 |
| Median age | 0.99 | 0.96–1.04 | 0.01 |
| Percentage of operability | 1.09 | 1.04–1.14 | 0.23 |
CI, confidence interval; VATS, video‐assisted thoracic surgery; SBRT, stereotactic body radiation therapy.
Discussion
Patients treated with SBRT had poorer clinical outcomes (OS and DFS) compared with those treated with VATS. However, patient characteristics of median age and portion of operability differed substantially between VATS and SBRT. After adjustment for these two confounders, the OS rate for SBRT patients was higher than for VATS, while the DFS rate for VATS was higher than for SBRT. However, there was no statistically significant difference between VATS and SBRT in patients with early stage NSCLC.
The confounders of treatment with VATS and SBRT are abundant, including clinical stage, tumor size, radiotherapy dose, bronchioloalveolar carcinoma (BAC), pathological confirmation, presumed lung cancer, peripheral or central NSCLC location, operability, and age of patients. According to Baba et al. and Ricardi et al., no difference in LC between stage IA and IB tumors exists, despite the difference in tumor size.32, 33 However, the benefit of increasing the SBRT dose for larger tumors should be investigated further. Compared with other NSCLC subtypes, BAC appears to have similar patterns of failure and survival after treatment with SBRT; however there may be an increased risk of distant metastases in BAC.43 There was no significant difference in OS between patients with pathologically confirmed NSCLC and those with presumed lung cancer (which was deemed most likely NSCLC).46 OS was favorable for patients with central lung tumors treated with SBRT.49 Centrally located NSCLC was compared with cases of peripheral tumor location, with OS and local progression‐free survival rates of central location NSCLC being better than peripheral.54
In the current meta‐analysis, we found that age and operability affect the clinical outcomes of VATS and SBRT in the treatment of early stage NSCLC.
In conclusion, considering the confounders of age and the portion of operable patients, VATS and SBRT showed inconspicuousness differences. The two treatments for early stage NSCLC are feasible and effective. However, a randomized prospective trial is needed to compare the clinical outcomes of VATS and SBRT.
Disclosure
No authors report any conflict of interest.
References
- 1. Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. (Published erratum appears in CA Cancer J Clin 2011; 61: 134.) CA Cancer J Clin 2011; 61: 69–90. [DOI] [PubMed] [Google Scholar]
- 2. Zheng R, Zeng H, Zhang S et al. Lung cancer incidence and mortality in China, 2010. Thorac Cancer 2014; 5: 330–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Chen W, Zheng R, Zhang S, Zou X, Zhao P, He J. Lung cancer incidence and mortality in China, 2009. Thorac Cancer 2013; 4: 102–8. [DOI] [PubMed] [Google Scholar]
- 4. Palma D, Visser O, Lagerwaard FJ, Belderbos J, Slotman BJ, Senan S. Impact of introducing stereotactic lung radiotherapy for elderly patients with stage I non‐small‐cell lung cancer: A population‐based time‐trend analysis. J Clin Oncol 2010; 28: 5153–9. [DOI] [PubMed] [Google Scholar]
- 5. Detterbeck FC, Postmus PE, Tanoue LT. The stage classification of lung cancer: Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence‐based clinical practice guidelines. Chest 2013; 143 (Suppl 5): e191S–210S. [DOI] [PubMed] [Google Scholar]
- 6. McKenna RJ Jr, Houck W, Fuller CB. Video‐assisted thoracic surgery lobectomy: Experience with 1,100 cases. Ann Thorac Surg 2006; 81: 421–5. [DOI] [PubMed] [Google Scholar]
- 7. Takagi H, Matsui M, Umemoto T. Long‐term survival of VATS versus open lobectomy. Ann Thorac Surg 2011; 92: 408–9. [DOI] [PubMed] [Google Scholar]
- 8. Wright GM, Thursfield VJ, Ball DL et al. Surgical resection and long‐term survival outcome for non‐small cell lung cancer: A comparison of Victorian population‐based studies spanning a decade. Asia‐Pac J Clin Oncol 2014; 10: 75–9. [DOI] [PubMed] [Google Scholar]
- 9. Verstegen NE, Oosterhuis JW, Palma DA et al. Stage I‐II non‐small‐cell lung cancer treated using either stereotactic ablative radiotherapy (SABR) or lobectomy by video‐assisted thoracoscopic surgery (VATS): Outcomes of a propensity score‐matched analysis. Ann Oncol 2013; 24: 1543–8. [DOI] [PubMed] [Google Scholar]
- 10. Onishi H, Shirato H, Nagata Y et al. Stereotactic body radiotherapy (SBRT) for operable stage I non‐small‐cell lung cancer: Can SBRT be comparable to surgery? Int J Radiat Oncol Biol Phys 2011; 81: 1352–8. [DOI] [PubMed] [Google Scholar]
- 11. Grills IS, Mangona VS, Welsh R et al. Outcomes after stereotactic lung radiotherapy or wedge resection for stage I non‐small‐cell lung cancer. J Clin Oncol 2010; 28: 928–35. [DOI] [PubMed] [Google Scholar]
- 12. Norihisa Y, Nagata Y, Takayama K et al. Stereotactic body radiotherapy for oligometastatic lung tumors. Int J Radiat Oncol Biol Phys 2008; 72: 398–403. [DOI] [PubMed] [Google Scholar]
- 13. Rusthoven KE, Kavanagh BD, Burri SH et al. Multi‐institutional phase I/II trial of stereotactic body radiation therapy for lung metastases. J Clin Oncol 2009; 27: 1579–84. [DOI] [PubMed] [Google Scholar]
- 14. Grills IS, Hope AJ, Guckenberger M et al. A collaborative analysis of stereotactic lung radiotherapy outcomes for early‐stage non‐small‐cell lung cancer using daily online cone‐beam computed tomography image‐guided radiotherapy. J Thorac Oncol 2012; 7: 1382–93. [DOI] [PubMed] [Google Scholar]
- 15. Chang BK, Timmerman RD. Stereotactic body radiation therapy: A comprehensive review. Am J Clin Oncol 2007; 30: 637–44. [DOI] [PubMed] [Google Scholar]
- 16. Potters L, Kavanagh B, Galvin JM et al. American Society for Therapeutic Radiology and Oncology (ASTRO) and American College of Radiology (ACR) practice guideline for the performance of stereotactic body radiation therapy. Int J Radiat Oncol Biol Phys 2010; 76: 326–32. [DOI] [PubMed] [Google Scholar]
- 17. Arends LR, Hunink MG, Stijnen T. Meta‐analysis of summary survival curve data. Stat Med 2008; 27: 4381–96. [DOI] [PubMed] [Google Scholar]
- 18. Zheng X, Schipper M, Kidwell K et al. Survival outcome after stereotactic body radiation therapy and surgery for stage I non‐small cell lung cancer: A meta‐analysis. Int J Radiat Oncol Biol Phys 2014; 90: 603–11. [DOI] [PubMed] [Google Scholar]
- 19. Dear KB. Iterative generalized least squares for meta‐analysis of survival data at multiple times. Biometrics 1994; 50: 989–1002. [PubMed] [Google Scholar]
- 20. Kim K, Kim HK, Park JS et al. Video‐assisted thoracic surgery lobectomy: Single institutional experience with 704 cases. Ann Thorac Surg 2010; 89: S2118–22. [DOI] [PubMed] [Google Scholar]
- 21. Puri V, Garg N, Engelhardt EE et al. Tumor location is not an independent prognostic factor in early stage non‐small cell lung cancer. Ann Thorac Surg 2010; 89: 1053–9. [DOI] [PubMed] [Google Scholar]
- 22. Sugi K, Kobayashi S, Sudou M, Sakano H, Matsuda E, Okabe K. Long‐term prognosis of video‐assisted limited surgery for early lung cancer. Eur J Cardiothorac Surg 2010; 37: 456–60. [DOI] [PubMed] [Google Scholar]
- 23. Yamashita S, Chujo M, Kawano Y et al. Clinical impact of segmentectomy compared with lobectomy under complete video‐assisted thoracic surgery in the treatment of stage I non‐small cell lung cancer. J Surg Res 2011; 166: 46–51. [DOI] [PubMed] [Google Scholar]
- 24. Marty‐Ané CH, Canaud L, Solovei L, Alric P, Berthet JP. Video‐assisted thoracoscopic lobectomy: An unavoidable trend? A retrospective single‐institution series of 410 cases. Interact Cardiovasc Thorac Surg 2013; 17: 36–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Battoo A, Jahan A, Yang Z et al. Thoracoscopic pneumonectomy: An 11‐year experience. Chest 2014; 146: 1300–9. [DOI] [PubMed] [Google Scholar]
- 26. Gonzalez‐Rivas D, Fieira E, Delgado M, Mendez L, Fernandez R, de la Torre M. Is uniportal thoracoscopic surgery a feasible approach for advanced stages of non‐small cell lung cancer? J Thorac Dis 2014; 6: 641–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Nakano T, Tetsuka K, Endo T et al. Extraction bag lavage cytology during video‐assisted thoracoscopic surgery for primary lung cancer. Interact Cardiovasc Thorac Surg 2014; 18: 770–4. [DOI] [PubMed] [Google Scholar]
- 28. Ghaly G, Kamel M, Nasar A et al. Video‐assisted thoracoscopic surgery is a safe and effective alternative to thoracotomy for anatomical segmentectomy in patients with clinical stage I non‐small cell lung cancer. Ann Thorac Surg 2015; 101: 465–72. [DOI] [PubMed] [Google Scholar]
- 29. Murakawa T, Ichinose J, Hino H, Kitano K, Konoeda C, Nakajima J. Long‐term outcomes of open and video‐assisted thoracoscopic lung lobectomy for the treatment of early stage non‐small cell lung cancer are similar: A propensity‐matched study. World J Surg 2015; 39: 1084–91. [DOI] [PubMed] [Google Scholar]
- 30. Nwogu CE, D'Cunha J, Pang H et al. VATS lobectomy has better perioperative outcomes than open lobectomy: CALGB 31001, an ancillary analysis of CALGB 140202 (Alliance). Ann Thorac Surg 2015; 99: 399–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Zhou H, Tapias LF, Gaissert HA et al. Lymph node assessment and impact on survival in video‐assisted thoracoscopic lobectomy or segmentectomy. Ann Thorac Surg 2015; 100: 910–6. [DOI] [PubMed] [Google Scholar]
- 32. Baba F, Shibamoto Y, Ogino H et al. Clinical outcomes of stereotactic body radiotherapy for stage I non‐small cell lung cancer using different doses depending on tumor size. Radiat Oncol 2010; 5: 81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ricardi U, Filippi AR, Guarneri A et al. Stereotactic body radiation therapy for early stage non‐small cell lung cancer: Results of a prospective trial. Lung Cancer 2010; 68: 72–7. [DOI] [PubMed] [Google Scholar]
- 34. Timmerman R, Paulus R, Galvin J et al. Stereotactic body radiation therapy for inoperable early stage lung cancer. JAMA 2010; 303: 1070–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Haasbeek CJ, Lagerwaard FJ, Slotman BJ, Senan S. Outcomes of stereotactic ablative radiotherapy for centrally located early‐stage lung cancer. J Thorac Oncol 2011; 6: 2036–43. [DOI] [PubMed] [Google Scholar]
- 36. Matsuo Y, Shibuya K, Nagata Y et al. Prognostic factors in stereotactic body radiotherapy for non‐small‐cell lung cancer. Int J Radiat Oncol Biol Phys 2011; 79: 1104–11. [DOI] [PubMed] [Google Scholar]
- 37. Nath SK, Sandhu AP, Kim D et al. Locoregional and distant failure following image‐guided stereotactic body radiation for early‐stage primary lung cancer. Radiother Oncol 2011; 99: 12–7. [DOI] [PubMed] [Google Scholar]
- 38. Chang JY, Liu H, Balter P et al. Clinical outcome and predictors of survival and pneumonitis after stereotactic ablative radiotherapy for stage I non‐small cell lung cancer. Radiat Oncol 2012; 7: 152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Nuyttens JJ, van der Voort van Zyp NC, Praag J et al. Outcome of four‐dimensional stereotactic radiotherapy for centrally located lung tumors. Radiother Oncol 2012; 102: 383–7. [DOI] [PubMed] [Google Scholar]
- 40. Senthi S, Lagerwaard FJ, Haasbeek CJ, Slotman BJ, Senan S. Patterns of disease recurrence after stereotactic ablative radiotherapy for early stage non‐small‐cell lung cancer: A retrospective analysis. Lancet Oncol 2012; 13: 802–9. [DOI] [PubMed] [Google Scholar]
- 41. Shibamoto Y, Hashizume C, Baba F et al. Stereotactic body radiotherapy using a radiobiology‐based regimen for stage I nonsmall cell lung cancer: A multicenter study. Cancer 2012; 118: 2078–84. [DOI] [PubMed] [Google Scholar]
- 42. Takeda A, Kunieda E, Sanuki N, Aoki Y, Oku Y, Handa H. Stereotactic body radiotherapy (SBRT) for solitary pulmonary nodules clinically diagnosed as lung cancer with no pathological confirmation: Comparison with non‐small‐cell lung cancer. Lung Cancer 2012; 77: 77–82. [DOI] [PubMed] [Google Scholar]
- 43. Badiyan SN, Bierhals AJ, Olsen JR et al. Stereotactic body radiation therapy for the treatment of early‐stage minimally invasive adenocarcinoma or adenocarcnioma in situ (formerly bronchioloalveolar carcinoma): A patterns of failure analysis. Radiat Oncol 2013; 8: 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Griffioen GH, Lagerwaard FJ, Haasbeek CJ, Smit EF, Slotman BJ, Senan S. Treatment of multiple primary lung cancers using stereotactic radiotherapy, either with or without surgery. Radiother Oncol 2013; 107: 403–8. [DOI] [PubMed] [Google Scholar]
- 45. Yoon SM, Lim YS, Park MJ et al. Stereotactic body radiation therapy as an alternative treatment for small hepatocellular carcinoma. PLoS One 2013; 8 (11): e79854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Haidar YM, Rahn DA III, Nath S et al. Comparison of outcomes following stereotactic body radiotherapy for non‐small cell lung cancer in patients with and without pathological confirmation. Ther Adv Respir Dis 2014; 8: 3–12. [DOI] [PubMed] [Google Scholar]
- 47. Lucas JT Jr, Kuremsky JG, Soike M et al. Comparison of accelerated hypofractionation and stereotactic body radiotherapy for stage 1 and node negative stage 2 non‐small cell lung cancer (NSCLC). Lung Cancer 2014; 85: 59–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Ricardi U, Frezza G, Filippi AR et al. Stereotactic ablative radiotherapy for stage I histologically proven non‐small cell lung cancer: An Italian multicenter observational study. Lung Cancer 2014; 84: 248–53. [DOI] [PubMed] [Google Scholar]
- 49. Davis JN, Medbery C, Sharma S et al. Stereotactic body radiotherapy for centrally located early‐stage non‐small cell lung cancer or lung metastases from the RSSearch(®) patient registry. Radiat Oncol 2015; 10: 113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Davis JN, Medbery C III, Sharma S et al. Stereotactic body radiotherapy for early‐stage non‐small cell lung cancer: Clinical outcomes from a National Patient Registry. J Radiat Oncol 2015; 4: 55–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Kelley KD, Benninghoff DL, Stein JS et al. Medically inoperable peripheral lung cancer treated with stereotactic body radiation therapy. Radiat Oncol 2015; 10: 120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Kohutek ZA, Wu AJ, Zhang Z et al. FDG‐PET maximum standardized uptake value is prognostic for recurrence and survival after stereotactic body radiotherapy for non‐small cell lung cancer. Lung Cancer 2015; 89: 115–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Zehentmayr F, Wurstbauer K, Deutschmann H et al. DART‐bid: Dose‐differentiated accelerated radiation therapy, 1.8 Gy twice daily: High local control in early stage (I/II) non‐small‐cell lung cancer. Strahlenther Onkol. 2015; 191: 256–63. [DOI] [PubMed] [Google Scholar]
- 54. Schanne DH, Nestle U, Allgäuer M et al. Stereotactic body radiotherapy for centrally located stage I NSCLC: A multicenter analysis. Strahlenther Onkol 2015; 191: 125–32. [DOI] [PubMed] [Google Scholar]


