Abstract
Background
The efficacy of pemetrexed‐based first‐line chemotherapy in anaplastic lymphoma kinase (ALK)‐positive non‐small cell lung cancer (NSCLC) has been demonstrated in several studies; however, there is a lack of data from Chinese populations.
Methods
The clinicopathological characteristics and treatment outcomes of 52 patients with ALK‐positive advanced NSCLC who received pemetrexed as first‐line chemotherapy at the Department of Medical Oncology, Cancer Hospital, Chinese Academy of Medical Sciences were retrospectively reviewed. The primary end points were response rate and progression‐free survival (PFS).
Results
The gender proportion was balanced and the median age was 51 years (range 26–76). Of the 52 patients, 46 (88.5%) had stage IV disease, predominantly adenocarcinoma (98.1%). Sixteen patients were current/former smokers and 36 were never/light smokers. The most common sites of metastasis were the pleura (36.5%), bone (30.8%), lung (26.9%), and brain (17.3%). The median PFS was 9.5 months (95% confidence interval 7.454–11.536). At the time of analysis, partial remission was achieved in 18 (34.6%) patients, stable disease in 26 (50.0%), and progressive disease in eight (15.4%); none of the patients achieved complete remission. The objective response rate was 34.6% and the disease control rate was 84.6%. Common adverse events with pemetrexed were neutropenia (53.8%), nausea and vomiting (51.9%), leukopenia (32.7%), and fatigue (25.0%), mainly at grades 1 or 2.
Conclusions
Pemetrexed is efficient and tolerated as first‐line treatment for ALK‐positive NSCLC in a cohort of Chinese patients and may prove to be an alternative option for the treatment of ALK‐positive NSCLC.
Keywords: Anaplastic lymphoma kinase, first‐line treatment, non‐small cell lung cancer, pemetrexed
Introduction
The recent discovery of anaplastic lymphoma kinase (ALK) gene rearrangements has defined one of the newest molecular subsets of non‐small‐cell lung cancer (NSCLC). ALK gene rearrangements, most often consisting of a chromosome two inversion that leads to fusion with the echinoderm microtubule‐associated protein‐like four (EML4) gene, result in abnormal expression and activation of tyrosine kinase in the cytoplasm of cancer cells.1 ALK gene rearrangements, which exist in about 3–5% of NSCLC patients, are associated with distinct clinicopathologic features, including younger age, never or light smoking, and adenocarcinoma histology.1, 2, 3, 4 This fusion gene rarely overlaps with epidermal growth factor receptor (EGFR) or Kirsten rat sarcoma oncogene homolog (KRAS) mutations.5 Crizotinib, an oral small‐molecule tyrosine kinase inhibitor of ALK, MET, and ROS proto‐oncogene 1 kinases, has demonstrated dramatic efficacy and tolerability in patients with advanced ALK‐positive NSCLC.6, 7, 8, 9, 10 In a randomized phase 3 trial (PROFILE 1007) involving patients with advanced ALK‐positive NSCLC who had received previous platinum‐based chemotherapy, crizotinib showed efficacy superior to that of single‐agent second‐line chemotherapy with either pemetrexed or docetaxel.9 PROFILE 1014 showed that Crizotinib was superior to standard first‐line pemetrexed‐plus‐platinum chemotherapy in patients with previously untreated advanced ALK‐positive NSCLC in terms of a longer progression‐free survival (PFS; median, 10.9 vs. 7.0 months; 95% confidence interval [CI], 0.35–0.60; P < 0.001) and a better objective response rate (ORR; 74% vs. 45%, respectively, P < 0.001).10 However, many questions remain regarding the clinical characteristics and treatment outcomes of different standard cytotoxic chemotherapies as first‐line treatment in ALK‐positive NSCLC patients, and more data are needed, in particular, of Chinese populations.
Pemetrexed is a multitarget antifolate with activity against several key enzymes involved in nucleotide synthesis, including thymidylate synthase (TS), dihydrofolate reductase (DHFR), glycinamide ribonucleotide formyltransferase (GARFT) and 5‐aminoimidazole‐4‐carboxamide ribonucleotide formyltransferase/inosine monophosphate cyclohydrolase (ATIC).11, 12 The drug was approved for the treatment of patients with nonsquamous NSCLC in combination with cisplatin or carboplatin as a first‐line treatment, and as a single agent in second‐line or maintenance therapy.13, 14, 15, 16 Pemetrexed has a better tolerability and toxicity profile compared with other cytotoxic agents.16 To date, several retrospective studies have demonstrated that ALK‐positive patients who received pemetrexed‐based chemotherapy showed a prolonged PFS and a greater response rate (RR) than ALK wild type (WT) patients.17, 18, 19 In the PROFILE 1007 study, RR and PFS in the pemetrexed group was 29% and 4.2 months versus 7% and 2.6 months in the group using docetaxel as a second‐line regimen.9 These findings raise the possibility of using ALK rearrangement as a predictive biomarker of enhanced pemetrexed sensitivity.
Knowing that ALK rearrangements have largely been reported among lung cancers of adenocarcinoma histology, we chose pemetrexed as the first line treatment in advanced ALK‐positive NSCLC patients to determine whether pemetrexed‐based chemotherapy was associated with efficiency and tolerability. In addition, we conducted a retrospective analysis of clinical outcomes in ALK‐positive NSCLC patients who were treated with pemetrexed‐based chemotherapy during the pre‐ALK inhibitor period.
Methods
Patient eligibility
Patients who were histologically or cytologically diagnosed as having local advanced recurrent or metastatic nonsquamous NSCLC positive for ALK rearrangement, and who received treatment at the Department of Medical Oncology, Cancer Hospital, Chinese Academy of Medical Sciences (Beijing, China) between May 2011 and June 2015, were included in the study. None of the included patients had received systemic treatment for advanced disease. Other eligibility criteria included: patients older than 18 years; the presence of measurable lesions according to Response Evaluation Criteria in Solid Tumors (RECIST), version 1.1; an Eastern Cooperative Oncology Group (ECOG) performance status of 0–2; and adequate hepatic, renal, and bone marrow function.20, 21 Patients with treated brain metastases were eligible if the metastases were neurologically stable for at least two weeks before enrollment and the patients had no ongoing requirement for glucocorticoids. The exclusion criterion was incomplete clinical information.
Patient demographics and clinical characteristics were obtained from medical records, including age, gender, tumor stage, histological type, ECOG PS score, smoking history, EGFR mutation, and whether pemetrexed was administered via monotherapy or combination therapy. All patients signed informed consent before recruitment. The characteristics of the study population are outlined in Table 1.
Table 1.
Demographic and clinical data of the study population (n = 52)
| Variable 0020 | n | % |
|---|---|---|
| Gender | ||
| Male | 26 | 50.0 |
| Female | 26 | 50.0 |
| Age | ||
| < 65 years | 46 | 88.5 |
| ≥ 65 years | 6 | 11.5 |
| Tumor stage | ||
| IIIB | 6 | 11.5 |
| IV | 46 | 88.5 |
| Histological type | ||
| Adenocarcinoma | 51 | 98.1 |
| Large‐cell carcinoma | 1 | 1.9 |
| ECOG PS | ||
| 0–1 | 42 | 80.8 |
| 2 | 10 | 19.2 |
| Smoking history | ||
| Yes | 16 | 30.8 |
| No | 36 | 69.2 |
| EGFR mutation | ||
| Yes | 3 | 5.8 |
| No | 43 | 82.7 |
| Unknown | 6 | 11.5 |
| Brain metastasis | ||
| Yes | 9 | 17.3 |
| No | 43 | 82.7 |
| Regimen | ||
| Pemetrexed monotherapy | 2 | 3.8 |
| Pemetrexed combination | 50 | 96.2 |
| PEM + DDP | 35 | 67.3 |
| PEM + CBP | 15 | 28.8 |
| Maintenance treatment after first‐line | ||
| None | 26 | 52.0 |
| Pemetrexed | 16 | 32.0 |
| Crizotinib | 5 | 10.0 |
| TKIs | 3 | 6.0 |
CBP, carboplatin; ECOG, Eastern Cooperative Oncology Group; EGFR, epidermal growth factor receptor; DDP, cisplatin; PEM, pemetrexed; PS, performance score; TKI, tyrosine kinase inhibitors.
Histology and molecular testing
Tumor histology was classified using standard World Health Organization (WHO) criteria. ALK status was detected by one of the following methods: fluorescence in situ hybridization (FISH) assay using the Vysis ALK Break Apart FISH probe kit (Abbott Molecular, Abbot Park, IL, USA), Ventana IHC anti‐ALK (D5F3) (Ventana Medical Systems, Tucson, Arizona, USA), and quantitative real‐time reverse transcription‐polymerase chain reaction (qRT‐PCR) technology. EGFR mutations were analyzed using a direct DNA sequencing method.
Therapeutic regimens
Tumor histology and subtype were classified according to WHO criteria. Pemetrexed was administered intravenously at a dose of 500 mg/m2 body surface area at 21 day intervals alone or in combination with either cisplatin at a dose of 75 mg/m2, or carboplatin at target area under the curve of 4–5 mg/mL per minute at three week intervals. Patients who received maintenance pemetrexed or targeted therapy after four or six cycles of pemetrexed‐cisplatin were classified into the combination group. All patients received folic acid, vitamin B12 supplementation, and dexamethasone to avoid adverse drug reactions. Treatment was continued until RECIST‐defined disease progression occurred, unacceptable toxicity was documented, the patient or physician decided to discontinue therapy, or the patient died.
Evaluation criteria
All patients were evaluated by thoracoabdominal computed tomography, electrocardiography (ECG), and contrast‐enhanced brain magnetic resonance imaging before the initiation of therapy. In addition, routine hematologic tests, biochemistry analysis, coagulation test, and urinalysis were performed. Treatment response was evaluated every two cycles during treatment until RECIST‐defined progression. Objective tumor response was assessed according to RECIST version 1.1.20 Adverse events were classified and graded according to the Common Terminology Criteria for Adverse Events, version 4.0.
Statistical analysis
Progression‐free survival was defined as the time from the commencement of pemetrexed treatment to the first documented radiologic/clinical disease progression or death from any cause. Overall survival (OS) was defined from the date of initiation of pemetrexed therapy until death from any cause or until the last follow‐up date. Patients who were alive but had not progressed at the time of the analysis (31 July 2015) were censored within the analyses. The objective tumor RR and disease control rate (DCR) were calculated from the data of each patient. PFS and survival curves were generated using the Kaplan–Meier method, and a log‐rank test was used for comparison. Hazard ratios (HRs) for univariate and multivariate survival analyses were calculated using the Cox proportional hazard model. The primary end point was PFS and ORR. Statistical analysis was performed at the last study follow‐up date (31 July 2015) using SPSS version 19.0 (SPSS, Chicago, IL, USA). A P value of less than 0.05 was considered to indicate statistical significance.
Results
Clinical characteristics
A total of 52 patients with advanced ALK‐positive NSCLC received pemetrexed‐based chemotherapy as first‐line treatment. The baseline demographic and clinical characteristics of the patients are presented in Table 1. Among these patients, the gender proportion was balanced and the median age was 51 years (range 26–76). Forty‐six (88.5%) patients had stage IV disease, predominantly adenocarcinoma (98.1%). Forty‐two of the 52 patients had an ECOG PS score of 0–1. Sixteen patients were current/former smokers (≥10 pack years in their lifetime) and 36 patients were never/light smokers (<10 pack years). The most common sites of metastasis were the pleura (36.5%, 19/52), bone (30.8%, 16/52), lung (26.9%, 14/52), brain (17.3%, 9/52), and adrenal gland (11.5%, 6/52). Fifty patients had been treated with platinum/pemetrexed combination chemotherapy and only two patients had received single‐agent pemetrexed treatment. Among the 50 patients treated with platinum/pemetrexed combinations, 35 patients received the platinum agent cisplatin and 15 patients were treated with carboplatin throughout their treatment periods. Twenty‐four of 50 (48%) patients received maintenance treatment after first‐line therapy, including pemetrexed, crizotinib, and EGFR‐tyrosine kinase inhibitors (TKIs).
Assessment of efficacy
During a median follow‐up duration of 17.6 months (range 1.5–77.9), 10 (19.2%) patients died, and six (11.5%) were being treated with a pemetrexed‐based regimen at the time of data cut‐off. The median PFS of ALK‐positive patients was 9.50 months (95% CI 7.454–11.536, Fig 1) and the median OS was 20.73 months (95% CI, 14.133–27.329, Figs 1, 2). According to the Cox proportional hazards model, no clinical features had a statistical impact on PFS or OS. At the time of analysis, partial response (PR) occurred in 18 (34.6%) cases, stable disease (SD) in 26 (50.0%), and progressive disease (PD) in eight (15.4%); none of the patients achieved complete response (CR), resulting in an ORR of 34.6% (18/52), and a DCR of 84.6% (44/52). In subgroup patients who received pemetrexed combination therapy without maintenance treatment, 28 patients obtained a PFS of 9.1 months (95% CI, 5.133–13.068), and an ORR of 39.3%.
Figure 1.
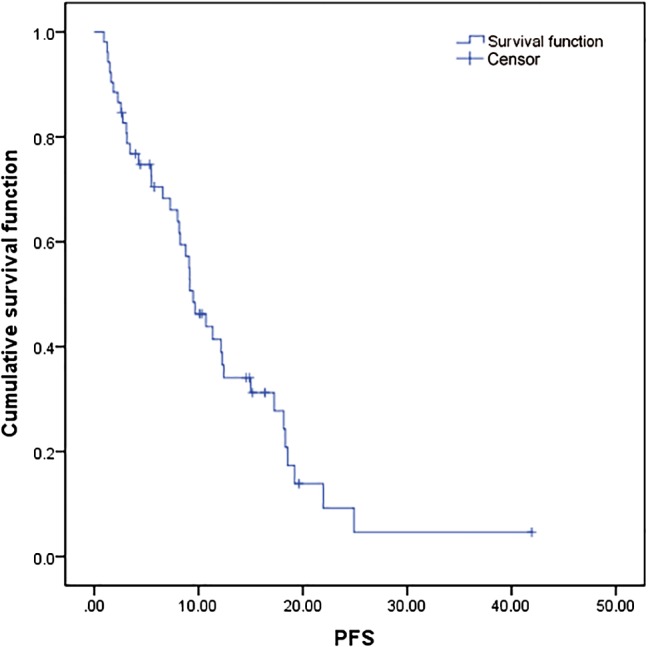
Progression‐free survival (PFS) curves of the 52 patients in the study.
Figure 2.
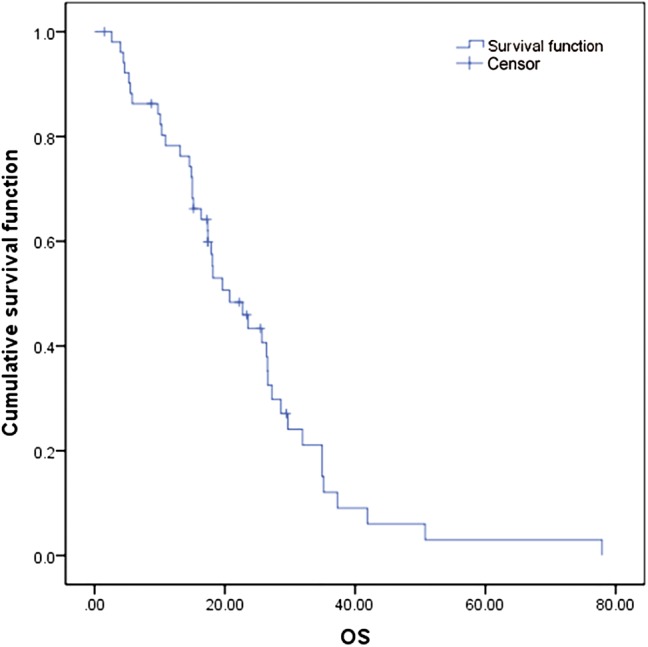
Overall survival (OS) curves of the 52 patients in the study.
Safety and adverse events
The most common hematologic toxicity with pemetrexed was neutropenia (53.8%), with grade 3–4 neutropenia occurring in 13.5% of the patients. Other common non‐hematologic adverse events were nausea and vomiting (51.9%), transaminase elevation (23.1%), and fatigue (25.0%), mainly at grades 1 or 2. No grade 5 toxicity was observed (Table 2).
Table 2.
Adverse reactions observed among the 52 patients in the study
| Grade | 0 | 1 | 2 | 3 | 4 | Incidence rate (%) | Incidence rate of grade 3, 4 (%) |
|---|---|---|---|---|---|---|---|
| Leukopenia | 35 | 3 | 13 | 1 | 0 | 32.7 | 1.9 |
| Neutropenia | 24 | 6 | 15 | 7 | 0 | 53.8 | 13.5 |
| Thrombocytopenia | 51 | 0 | 0 | 1 | 0 | 1.9 | 1.9 |
| Nausea and vomiting | 25 | 12 | 11 | 4 | 0 | 51.9 | 7.7 |
| Elevated transaminase | 40 | 6 | 5 | 1 | 0 | 23.1 | 1.9 |
| Rash | 49 | 2 | 1 | 0 | 0 | 5.7 | 0 |
| Fatigue | 51 | 10 | 3 | 0 | 0 | 25.0 | 0 |
Discussion
According to National Comprehensive Cancer Network guidelines, crizotinib is recommended as first‐line treatment of patients with ALK‐positive lung cancer. Since the Food and Drug Administration approved crizotinib in 2011, it has been clinically available; however, crizotinib is not yet covered by medical insurance in China. Many ALK‐positive patients cannot afford such treatment; therefore, chemotherapeutic agents must be used as first‐line therapy in many cases. Indeed, nine patients in our series refused crizotinib treatment when definitively diagnosed with ALK‐positive NSCLC because of financial difficulty.
Pemetrexed is a multi‐targeted antifolate agent that exerts its potent antitumor activity by disrupting the nucleotide synthesis of pyrimidines and purines. Cisplatin‐pemetrexed has been shown to be superior to cisplatin‐gemcitabine for tumors with nonsquamous histologic characteristics.13 In addition, this drug has a better safety profile than other cytotoxic agents. The benefits of pemetrexed‐based first‐line chemotherapy in advanced ALK positive patients have been documented in two studies, in terms of a prolonged PFS and a greater RR.17, 22 Hence, pemetrexed could be preferentially selected for patients who are intolerant to crizotinib.
In this study, we evaluated the efficacy and safety of pemetrexed‐based chemotherapy as first‐line treatment for advanced ALK‐positive NSCLC patients. Of the 52 patients enrolled, the median age of patients was 51 years, with 69.2% non‐smokers with predominantly adenocarcinoma (98.1%), which is consistent with previous data.3, 4 Although the ALK fusion gene rarely overlaps with EGFR or KRAS mutations, we found three patients harboring concomitant EGFR mutation and EML4‐ALK fusion in our study.5 One of these patients received TKIs and crizotinib during the treatment period, and both regimens showed a disease control response. Although EGFR mutations and ALK translocations are generally considered mutually exclusive, sporadic cases with concomitant EGFR and ALK alteration have been reported.23, 24, 25, 26 The prevalence of dual positive cases differed and their responses to EGFR and/or ALK inhibitors were conflicting. Although ALK inhibitors and TKIs were effective for the treatment of dual‐positive patients in our study, treatment strategies should be explored for patients with concomitant EGFR and ALK alterations in the future.27
We also confirmed that PFS and ORR of crizotinib as first‐line treatment for patients with advanced ALK‐positive adenocarcinoma were superior to those who received systemic chemotherapy (PFS 10.9 vs. 9.50 months; ORR: 74% vs. 36.4%). However, the PFS in our study was close to that of crizotinib and was longer than the PFS of chemotherapy regimens in the PROFILE 1014 (9.50 vs. 7.0 months) and other previous studies (9.50 vs. 8.5 months).22 This was probably because pemetrexed was not continued beyond the planned six cycles of pemetrexed‐plus‐platinum chemotherapy in PROFILE 1014. As shown in Table 1, 16 patients received pemetrexed maintenance therapy, five continued with crizotinib, and three were treated with TKIs after four to six cycles of cisplatin‐pemetrexed without disease progression. A previous study reported that pemetrexed maintenance therapy increased median PFS over the placebo by 1.3 months from the start of maintenance therapy, indicating that pemetrexed maintenance therapy could improve the survival outcome.14 The SATURN study showed that maintenance therapy with erlotinib for patients with NSCLC could significantly prolong PFS, compared with the placebo. Therefore, we analyzed a subgroup of 28 patients who received pemetrexed combination therapy without maintenance treatment and obtained a PFS of 9.1 months (95% CI, 5.133–13.068), which is still longer than the 7.0 months of the PROFILE 1014 study.10 The ORR of the subgroup was slightly lower than in PROFILE 1014 (39.3% vs. 45%), which could possibly be attributed to the scattered therapeutic regimens and small patient sample size. However, it was similar to that of pemetrexed as first‐line therapy in Lee et al.’s study (39.3% vs. 33.3%) and other historical controls, and was higher than previous second‐line chemotherapy studies, such as PROFILE 1007 (39.3% vs. 29% of pemetrexed and 7% of docetaxel), showing that ALK‐positive patients clinically benefit from pemetrexed.28, 29, 30
Previous studies have shown that never‐smoking status is associated with a greater sensitivity to pemetrexed in an ALK‐positive group, suggesting that smoking status should be considered an important factor in choosing the treatment regimen.13, 19, 22 However, the Cox proportional hazard model in our study showed no statistically significant correlation of any clinical feature with prolonged PFS and OS in patients using pemetrexed‐based chemotherapy as the first‐line regimen. This may be because our study was a retrospective non‐randomized study in which an imbalance between the cohort and several variables may have affected the PFS outcome. The overall size of the analyzed population was relatively small and some bias in sample selection could not be avoided. Thus, further research involving larger cohorts of patients is required to investigate the factors associated with the clinical significance of pemetrexed.
In summary, we can conclude that crizotinib is still the drug of choice as a first‐line treatment for patients with advanced ALK‐positive lung cancer. Meanwhile, pemetrexed is efficient and well tolerated as first‐line treatment for ALK‐positive NSCLC in a cohort of Chinese patients, and should be preferentially considered for the treatment of ALK‐positive lung adenocarcinoma when the use of crizotinib is not feasible.
Disclosure
No authors report any conflict of interest.
References
- 1. Soda M, Choi YL, Enomoto M et al. Identification of the transforming EML4‐ALK fusion gene in non‐small‐cell lung cancer. Nature 2007; 448: 561–6. [DOI] [PubMed] [Google Scholar]
- 2. Rikova K, Guo A, Zeng Q et al. Global survey of phosphotyrosine signaling identifies oncogenic kinases in lung cancer. Cell 2007; 131: 1190–203. [DOI] [PubMed] [Google Scholar]
- 3. Shaw AT, Yeap BY, Mino‐Kenudson M et al. Clinical features and outcome of patients with non‐small‐cell lung cancer who harbor EML4‐ALK. J Clin Oncol 2009; 27: 4247–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Camidge DR, Doebele RC. Treating ALK‐positive lung cancer–early successes and future challenges. Nat Rev Clin Oncol 2012; 9: 268–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wong DW, Leung EL, So KK et al. The EML4‐ALK fusion gene is involved in various histologic types of lung cancers from nonsmokers with wild‐type EGFR and KRAS. Cancer 2009; 115: 1723–33. [DOI] [PubMed] [Google Scholar]
- 6. Christensen JG, Zou HY, Arango ME et al. Cytoreductive antitumor activity of PF‐2341066, a novel inhibitor of anaplastic lymphoma kinase and c‐Met, in experimental models of anaplastic large‐cell lymphoma. Mol Cancer Ther 2007; 6: 3314–22. [DOI] [PubMed] [Google Scholar]
- 7. Kwak EL, Bang YJ, Camidge DR et al. Anaplastic lymphoma kinase inhibition in non‐small‐cell lung cancer. (Published erratum appears in N Engl J Med 2011; 364: 588.) N Engl J Med 2010; 363: 1693–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Camidge DR, Bang YJ, Kwak EL et al. Activity and safety of crizotinib in patients with ALK‐positive non‐small‐cell lung cancer: Updated results from a phase 1 study. Lancet Oncol 2012; 13: 1011–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Shaw AT, Kim DW, Nakagawa K et al. Crizotinib versus chemotherapy in advanced ALK‐positive lung cancer. (Published erratum appears in N Engl J Med 2015; 373: 1582.) N Engl J Med 2013; 368: 2385–94. [DOI] [PubMed] [Google Scholar]
- 10. Solomon BJ, Mok T, Kim DW et al. First‐line crizotinib versus chemotherapy in ALK‐positive lung cancer. (Published erratum appears in N Engl J Med 2015; 373: 1582.) N Engl J Med 2014; 371: 2167–77. [DOI] [PubMed] [Google Scholar]
- 11. Shih C, Chen VJ, Gossett LS et al. LY231514, a pyrrolo[2, 3‐d]pyrimidine‐based antifolate that inhibits multiple folate‐requiring enzymes. Cancer Res 1997; 57: 1116–23. [PubMed] [Google Scholar]
- 12. Mendelsohn LG, Shih C, Chen VJ, Habeck LL, Gates SB, Shackelford KA. Enzyme inhibition, polyglutamation, and the effect of LY231514 (MTA) on purine biosynthesis. Semin Oncol 1999; 26: 42–7. [PubMed] [Google Scholar]
- 13. Scagliotti GV, Parikh P, von Pawel J et al. Phase III study comparing cisplatin plus gemcitabine with cisplatin plus pemetrexed in chemotherapy‐naive patients with advanced‐stage non‐small‐cell lung cancer. J Clin Oncol 2008; 26: 3543–51. [DOI] [PubMed] [Google Scholar]
- 14. Paz‐Ares L, de Marinis F, Dediu M et al. Maintenance therapy with pemetrexed plus best supportive care versus placebo plus best supportive care after induction therapy with pemetrexed plus cisplatin for advanced non‐squamous non‐small‐cell lung cancer (PARAMOUNT): A double‐blind, phase 3, randomised controlled trial. Lancet Oncol 2012; 13: 247–55. [DOI] [PubMed] [Google Scholar]
- 15. Paz‐Ares LG, de Marinis F, Dediu M et al. PARAMOUNT: Final overall survival results of the phase III study of maintenance pemetrexed versus placebo immediately after induction treatment with pemetrexed plus cisplatin for advanced nonsquamous non‐small‐cell lung cancer. J Clin Oncol 2013; 31: 2895–902. [DOI] [PubMed] [Google Scholar]
- 16. Hanna N, Shepherd FA, Fossella FV et al. Randomized phase III trial of pemetrexed versus docetaxel in patients with non‐small‐cell lung cancer previously treated with chemotherapy. J Clin Oncol 2004; 22: 1589–97. [DOI] [PubMed] [Google Scholar]
- 17. Camidge DR, Kono SA, Lu X et al. Anaplastic lymphoma kinase gene rearrangements in non‐small cell lung cancer are associated with prolonged progression‐free survival on pemetrexed. J Thoracic Oncol 2011; 6: 774–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lee JO, Kim TM, Lee SH et al. Anaplastic lymphoma kinase translocation: A predictive biomarker of pemetrexed in patients with non‐small cell lung cancer. J Thoracic Oncol 2011; 6: 1474–80. [DOI] [PubMed] [Google Scholar]
- 19. Park S, Park TS, Choi CM et al. Survival benefit of pemetrexed in lung adenocarcinoma patients with anaplastic lymphoma kinase gene rearrangements. Clin Lung Cancer 2015; 16: e83–9. [DOI] [PubMed] [Google Scholar]
- 20. Eisenhauer EA, Therasse P, Bogaerts J et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1). Eur J Cancer 2009; 45: 228–47. [DOI] [PubMed] [Google Scholar]
- 21. Oken MM, Creech RH, Tormey DC et al. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol 1982; 5: 649–55. [PubMed] [Google Scholar]
- 22. Shaw AT, Varghese AM, Solomon BJ et al. Pemetrexed‐based chemotherapy in patients with advanced, ALK‐positive non‐small cell lung cancer. Ann Oncol 2013; 24: 59–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Gainor JF, Varghese AM, Ou SH et al. ALK rearrangements are mutually exclusive with mutations in EGFR or KRAS: An analysis of 1, 683 patients with non‐small cell lung cancer. Clin Cancer Res 2013; 19: 4273–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Chen X, Zhang J, Hu Q, Li X, Zhou C. A case of lung adenocarcinoma harboring exon 19 EGFR deletion and EML4‐ALK fusion gene. Lung Cancer 2013; 81: 308–10. [DOI] [PubMed] [Google Scholar]
- 25. Santelmo C, Ravaioli A, Barzotti E et al. Coexistence of EGFR mutation and ALK translocation in NSCLC: Literature review and case report of response to gefitinib. Lung Cancer 2013; 81: 294–6. [DOI] [PubMed] [Google Scholar]
- 26. Yang JJ, Zhang XC, Su J et al. Lung cancers with concomitant EGFR mutations and ALK rearrangements: Diverse responses to EGFR‐TKI and crizotinib in relation to diverse receptors phosphorylation. Clin Cancer Res 2014; 20: 1383–92. [DOI] [PubMed] [Google Scholar]
- 27. Won JK, Keam B, Koh J et al. Concomitant ALK translocation and EGFR mutation in lung cancer: A comparison of direct sequencing and sensitive assays and the impact on responsiveness to tyrosine kinase inhibitor. Ann Oncol 2015; 26: 348–54. [DOI] [PubMed] [Google Scholar]
- 28. Lee HY, Ahn HK, Jeong JY et al. Favorable clinical outcomes of pemetrexed treatment in anaplastic lymphoma kinase positive non‐small‐cell lung cancer. Lung Cancer 2013; 79: 40–5. [DOI] [PubMed] [Google Scholar]
- 29. Ardizzoni A, Boni L, Tiseo M et al. Cisplatin‐versus carboplatin‐based chemotherapy in first‐line treatment of advanced non‐small‐cell lung cancer: An individual patient data meta‐analysis. J Natl Cancer Inst 2007; 99: 847–57. [DOI] [PubMed] [Google Scholar]
- 30. Koh Y, Kim DW, Kim TM et al. Clinicopathologic characteristics and outcomes of patients with anaplastic lymphoma kinase‐positive advanced pulmonary adenocarcinoma: Suggestion for an effective screening strategy for these tumors. J Thorac Oncol 2011; 6: 905–12. [DOI] [PubMed] [Google Scholar]


