Abstract
Background
Uridine 5′‐diphospho‐glucuronosyltransferase 1A1 (UGT1A1*27) is known to impair the effect of UGT in basic research; however, little clinical investigation has been conducted. To evaluate the effect of the UGT1A1*27 polymorphism in irinotecan therapy, we conducted a prospective study.
Methods
Eligibility criteria included: lung cancer patients; scheduled irinotecan therapy doses of single ≥ 80, combination ≥ 50, radiation with single ≥ 50, or radiation with combination ≥ 40 mg/m2; age ≥ 20; and Eastern Cooperative Oncology Group performance score (PS) 0–2. Patients were examined for UGT1A1*28 and *6 polymorphisms and received irinotecan. When the UGT1A1*28 polymorphism was detected, a search for UGT1A1*27 was conducted. Fifty patients were enrolled, with 48 patients determined eligible.
Results
UGT1A1 polymorphisms *28/*28, *6/*6, *28/*6, *28/−, *6/−, −/− observed 0 (0%), 1 (2%), 1 (2%), 7 (15%), 17 (35%) and 22 (46%), respectively. UGT1A1*27 were examined in nine patients including one ineligible patient; however, no polymorphisms were found. The study ceased after interim analysis. In an evaluation of the side effects of irinotecan, patients with UGT1A1*28 and UGT1A1*6 polymorphisms had a higher tendency to experience febrile neutropenia than wild type (25% and 32% vs. 14%). Incidences of grade 3/4 leukopenia and neutropenia were significantly higher in patients with UGT1A1*28 polymorphisms compared with wild type (75% vs. 32%, P = 0.049; 75% vs. 36%, P = 0.039, respectively).
Conclusion
Our prospective study did not locate the UGT1A1*27 polymorphism, suggesting that UGT1A1*27 does not significantly predict severe irinotecan toxicity in cancer patients.
Keywords: Gene polymorphism, irinotecan, lung cancer, prospective, UGT1A1
Introduction
Irinotecan, a camptothecin analog with anticancer activity through the inhibition of topoisomerase I, is widely used against solid tumors including lung, colorectal, gastric, gynecologic, and other types of cancer.1, 2, 3, 4 However, it causes adverse events, such as severe diarrhea and neutropenia in 13–25% of patients.5, 6 Irinotecan is a prodrug metabolized by carboxylesterase to form an active metabolite SN‐38, which is subsequently metabolized in the liver by uridine 5′‐diphospho‐glucuronosyltransferase 1A1 (UGT1A1) to an inactive metabolite SN‐38 glucuronide (SN‐38G).7 Polymorphism of the UGT1A1 gene is known to play an important role in irinotecan pharmacokinetics and severe toxicity.8 Decreased UGT1A1 activity resulting from genetic polymorphisms, such as UGT1A1*28 and UGT1A1*6, are associated with increased SN‐38 and the occurrence of adverse events in irinotecan chemotherapy.9, 10, 11, 12
The UGT1A1*27 gene is in linkage disequilibrium with UGT1A1*28. Basic research has suggested that these polymorphisms exist together (haplotype), to reduce UGT enzyme activity.13, 14 However, the impact of UGT1A1*27 on the toxicity of irinotecan is not well understood because its frequency is rare as 0.5–1%. Two previous clinical studies reported only seven patients with UGT1A1*27 heterozygous gene polymorphisms who received irinotecan, with severe adverse events associated in six patients (86%).11, 12, 13, 14, 15
Based on these results, we conducted a prospective study of the UGT1A1*27 gene polymorphism. The main objective of the study was to determine the clinical impact of the UGT1A1*27 gene polymorphism in patients who receive irinotecan‐based chemotherapy. A secondary objective was to evaluate the effects of gene polymorphisms UGT1A1*28 and UGT1A1*6 on irinotecan toxicity.
Methods
The study protocol was reviewed and approved by the protocol committee of the Lung Oncology Group in Kyusyu (LOGiK) and the ethics committee of each institution. Written informed consent was obtained from all study participants. This study was an independent collaborative (unsponsored) group study, and is registered at University Hospital Medical Information Network (UMIN) in Japan (UMIN000006164).
Patients and evaluation
Eligibility criteria for patients in this study included: histologically and/or cytologically confirmed diagnosis of lung cancer; scheduled doses of irinotecan therapy of single ≥ 80 mg/m2, combination ≥ 50 mg/m2, radiation with single ≥ 50 mg/m2, or radiation with combination ≥ 40 mg/m2; age ≥ 20 years old; and Eastern Cooperative Oncology Group (ECOG) performance status (PS) ≤ 2. Exclusion criteria were: interstitial pneumonia determined by chest X‐ray; active infection; other active malignancies; pregnancy or possible pregnancy; mental disease which made it difficult to participate in the study; high fever ≥ 38 degree; severe complication of myocardial infarction within three months, uncontrolled angina pectoris, heart failure, diabetes mellitus and hypertension; diarrhea; and paralysis of the intestine or ileus.
Treatment
Treatment commenced within one week of enrollment. Irinotecan was administered as a single agent or in combination chemotherapy. Irinotecan dissolved in 250 mL of 5% dextrose was infused intravenously over 60 minutes. Irinotecan was postponed if the leukocyte count < 3000/μL, platelet count < 10 × 104/μL, or any diarrhea occurred on the day or previous day, and was abandoned if the treatment was not administered within a week.
Genotyping assay and toxicity evaluation
After blood sampling, genomic DNA were isolated from the 50 patients enrolled in the trial. UGT1A1*28 and UGT1A1*6 gene polymorphisms were analyzed using Invader technology (BML, Inc., Tokyo, Japan). In patients with confirmed UGT1A1*28 heterozygous or homozygous gene polymorphisms, the existence of UGT1A1*27 gene polymorphisms was investigated (Fig 1).
Figure 1.
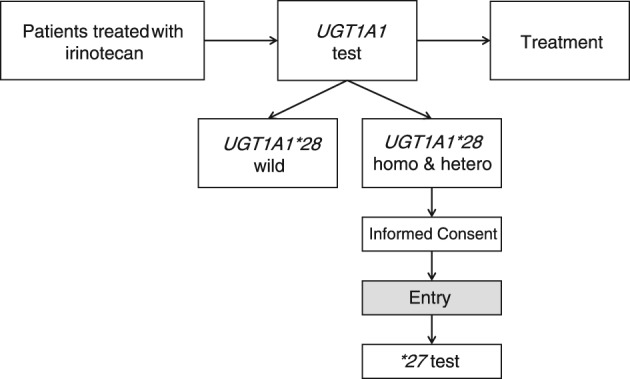
Study scheme. Hetero, heterozygous; homo, homozygous; UGT1A1, uridine 5'‐diphospho‐glucuronosyltransferase 1A1.
Drug toxicity was graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events v 3.0.16 Before the first cycle, blood cell count, urinalysis, and biochemistry tests were performed to assess renal and hepatic function and electrolytes. These tests were repeated during treatment, while other investigations were repeated, as necessary, to evaluate marker lesions.
Statistical analysis
The primary endpoint of this study was to determine the effect of the UGT1A1*27 gene polymorphism on irinotecan therapy. Binominal distribution was used. The control consisted of all wild type UGT1A1*28, UGT1A1*6, and UGT1A1*27. Assuming a frequency control of 35%, UGT1A1*27 gene polymorphism of 9%, grade 3–4 neutropenia of the control of 12.5%, and grade 3–4 neutropenia of the UGT1A1*27 gene polymorphism of 50%, were established. These data are recorded in another of our studies, currently unpublished. Alpha = 0.05, beta = 0.08, and the estimated required number of UGT1A1*27 gene polymorphism patients was 10. Considering a UGT1A1*27 gene polymorphism frequency of 9%, the required sample size of this study was determined to be 111. Toxicities and efficacies were compared by the chi‐square test between patient groups divided according to UGT1A1 gene polymorphisms. Progression‐free survival (PFS) was defined as the period from the start of irinotecan therapy to determination of treatment failure (death, documentation of disease progression) or the date of censoring at the last follow‐up examination. Overall survival (OS) was defined as the period from the start of irinotecan therapy until death from any cause or the date of censoring at the last follow‐up examination. Survival was evaluated by the Kaplan–Meier method, and differences in survival were analyzed by log‐rank test. The statistical significance level was set at P < 0.05. All statistical analyses were performed using the SPSS version 23.0 (SPSS Inc., Chicago, IL, USA).
Results
Fifty patients from two institutions were enrolled between October 2011 and December 2013. Two patients received lower starting doses of irinotecan, which was considered a protocol violation. Thus, the data of 48 patients were finally evaluated for the existence of UGT1A1 gene polymorphisms, toxicity, response, and survival. The baseline patient characteristics are shown in Table 1. UGT1A1 gene polymorphisms *28/*28, *6/*6, *28/*6, *28/−, *6/−, −/− observed 0, 1, 1, 7, 17 and 22, respectively (Table 2). The existence of UGT1A1*27 was investigated in nine patients, including one ineligible patient with *28/*28; however, no UGT1A1*27 gene polymorphisms were detected. The statistical commission recommended the study be concluded because it was unlikely that a target of 10 patients with UGT1A1*27 polymorphisms could be achieved.
Table 1.
Patient characteristics
| Characteristics | Number of patients |
|---|---|
| Gender | |
| Male | 41 |
| Female | 7 |
| Age (years) | |
| Median | 72 |
| Range | 51–87 |
| Performance status (ECOG) | |
| 0 | 14 |
| 1 | 33 |
| 2 | 1 |
| Histology | |
| Adenocarcinoma | 16 |
| Squamous cell carcinoma | 15 |
| Small cell carcinoma | 12 |
| Large cell carcinoma | 1 |
| Others | 4 |
ECOG, Eastern Cooperative Oncology Group.
Table 2.
UGT1A1 gene polymorphism
| Total | *28/*28 | *6/*6 | *28/*6 | *28/− | *6/− | −/− | |
|---|---|---|---|---|---|---|---|
| n | 48 | 0 | 1 | 1 | 7 | 17 | 22 |
| (%) | (100) | (0) | (2) | (2) | (15) | (35) | (46) |
UGT1A1, uridine 5′‐diphospho‐glucuronosyltransferase 1A1.
Treatment administration
Irinotecan therapy was administered 153 times, at a median of three times per patient: once in seven patients (15%); twice in nine (19%); three in 19 (40%); four in three (6%); five in one (2%); and six in nine (19%). Irinotecan was used as combination chemotherapy in 32 (67%) patients, with cisplatin in 12 (25%); carboplatin in 10 (21%); gemcitabine in nine (19%); and paclitaxel in one (2%). In the remaining 16 patients (33%), only single irinotecan therapy was administered. Radiotherapies were administered concurrently in 23 (48%) patients, with a median of 60 Gy (range 40–61.4).
Toxicity
The grade 3–4 hematological and non‐hematological toxicities experienced by each patient and separated by UGT1A1 gene polymorphism are listed in Table 3. Febrile neutropenia was observed at a higher tendency in patients with UGT1A1*6 (32%) and UGT1A1*28 (25%) gene polymorphisms, compared with wild type (14%), but had no significant difference. Grade 3–4 leukopenia and neutropenia were observed in six out of eight patients with UGT1A1*28 gene polymorphisms, a significantly higher rate compared with wild type (75% vs. 32%, P = 0.049; 75% vs. 36%, P = 0.039, respectively). There was no difference between UGT1A1 gene polymorphisms and wild type in regard to other toxicities. No pneumonitis or treatment‐related death occurred.
Table 3.
UGT1A1 gene polymorphism and toxicities (≥ grade 3)
| Side effect | *6/*6 | *28/*6 | *28/− | *6/− | −/− | P | |
|---|---|---|---|---|---|---|---|
| *28 | *6 | ||||||
| Febrile neutropenia | 0 | 0 | 2 | 6 | 3 | 0.589 | 0.230 |
| (%) | (0) | (0) | (29) | (35) | (14) | — | — |
| Leukopenia | 0 | 0 | 6 | 9 | 7 | 0.049* | 0.486 |
| (%) | (0) | (0) | (86) | (53) | (32) | — | — |
| Neutropenia | 1 | 0 | 6 | 9 | 8 | 0.039* | 0.465 |
| (%) | (100) | (0) | (86) | (53) | (36) | — | — |
| Anemia | 0 | 0 | 0 | 4 | 2 | 1 | 0.390 |
| (%) | (0) | (0) | (0) | (24) | (9) | — | — |
| Thrombocytopenia | 0 | 0 | 0 | 2 | 3 | 0.545 | 1 |
| (%) | (0) | (0) | (0) | (12) | (14) | — | — |
| Diarrhea | 1 | 0 | 1 | 2 | 1 | 0.469 | 0.321 |
| (%) | (100) | (0) | (14) | (12) | (5) | — | — |
| Pneumonitis | 0 | 0 | 0 | 0 | 0 | 1 | 1 |
| (%) | (0) | (0) | (0) | (0) | (0) | — | — |
*Indicates significant difference. Statistic analyses were performed between “*28/−, *28/*6” versus “−/−”, and “*6/*6, *28/*6, *6/−” versus “−/−.” UGT1A1, uridine 5'‐diphospho‐glucuronosyltransferase 1A1.
Efficacy
Of the 48 patients, 43 were assessable for tumor response. Objective tumor response was observed in 19 patients with an overall response rate of 44%. Tumor responses separated by UGT1A1 gene polymorphisms are shown in Table 4. The tumor response of 57% in UGT1A1*28 or UGT1A1*6 gene polymorphisms was higher compared with 30% in wild type, but was not statistically different (P = 0.150). At survival assessment in June 2014, 27 patients were still alive, while 21 patients had died. The median PFS in the 48 patients was 6.8 months and the one‐year survival rate was 20.8%. Median PFS separated by UGT1A1 gene polymorphisms were 10.1 months in UGT1A1*28 heterozygous, 8.5 in UGT1A1*6 heterozygous, and 6.8 in both wild types, respectively. The median OS of 48 patients was 15.7 months and one and two‐year survival rates were 57.7% and 40.3%, respectively. Median OS separated by UGT1A1 gene polymorphisms was not reached in UGT1A1*28 heterozygous, was 16.4 months in UGT1A1*6 heterozygous, and 12.3 months in wild type, respectively. Kaplan–Meier plots and log‐rank tests showed no difference in PFS or OS between the three groups.
Table 4.
UGT1A1 gene polymorphism and tumor response (n = 43)
| UGT1A1 | n | CR | PR | SD | PD | Response (%) | P |
|---|---|---|---|---|---|---|---|
| *6/*6 | 1 | 0 | 0 | 1 | 0 | 0 | — |
| *28/*6 | 1 | 0 | 1 | 0 | 0 | 100 | — |
| *28/− | 5 | 1 | 2 | 2 | 0 | 60 | 0.175 |
| *6/− | 16 | 0 | 9 | 7 | 0 | 56 | 0.312 |
| −/− | 20 | 1 | 5 | 12 | 2 | 30 | — |
P: compared with “−/−” by 2 × 2 Chi square & Fisher's test. CR, complete response; PD, progressive disease; PR, partial response; SD, stable disease; UGT1A1, uridine 5'‐diphospho‐glucuronosyltransferase 1A1.
Discussion
The objective of the present study, to determine the clinical impact of the UGT1A1*27 gene polymorphism in patients receiving irinotecan‐based chemotherapy was not met, as we did not locate any patients with UGT1A1*27 gene polymorphisms. We only searched for UGT1A1*27 gene polymorphisms in patients with confirmed UGT1A1*28 haplotype because the UGT1A1*27 gene is in linkage disequilibrium with UGT1A1*28 and they were thought to exist at all times together (haplotype); however, this hypothesis might be inaccurate.13
Irinotecan is a semi‐synthetic water‐soluble prodrug metabolized by carboxylesterase to an active metabolite SN‐38, which has a 100–1000‐fold higher cytotoxicity than irinotecan. UGT further metabolizes SN‐38 to an inactive metabolite, SN‐38G.17 UGT1A1 is mainly expressed in the liver and is thought to be responsible for SN‐38 inactivation. UGT1A1 genetic polymorphisms result in reduced enzyme activity and increased irinotecan toxicities. The most extensively studied gene polymorphism, UGT1A1*28, a variation of the TATA box, has been determined as a significant risk factor for severe irinotecan‐related toxicity in Caucasians and Asians.10, 11 In 2005, the United States Food and Drug Administration approved the diagnostic UGT1A1 test, the Invader UGT1A1 Molecular Assay (Third Wave Technologies Inc., Madison, WI, USA) and advised that patients with UGT1A1*28 homozygous polymorphisms are at increased risk of irinotecan‐related neutropenia.18 UGT1A1*6 single nucleotide polymorphisms (SNPs) in exon one of the UGT1A1 gene (211G > A [G71R]), which also represents an increased risk of irinotecan‐related toxicities, are mainly found in Asians.11, 12, 19 UGT1A1*27, the variant sequence in exon one (686C > A [Pro229Gln]), reduces SN‐38 glucuronidation in vitro.14, 20 Sai et al. sequenced the UGT1A1 gene in 195 Japanese subjects, including the enhancer/promoter regions, all exons, and their flanking regions, and assigned haplotypes using the detected SNPs.13 The UGT1A1 haplotypes were classified into four groups (*1, *60, *6, *28); as *28 and *6 are present on a mutually exclusive chromosome, they exert an additive effect on irinotecan toxicity. By contrast, the *27 allele had links to *28 and was classified to the *28 haplotype group. For this reason, the UGT1A1*27 gene polymorphism was only investigated in patients who had UGT1A1*28 gene polymorphisms.
To determine whether the *27 haplotype exists with the *28, Ando et al. retrospectively investigated the impact of the UGT1A1 genetic polymorphism in irinotecan‐related severe toxicity in 118 patients. In 18 patients (15.3%) heterozygous for the UGT1A1*28 haplotype, one patient (0.8%) was heterozygous for UGT1A1*27. In seven patients (5.9%) homozygous for the UGT1A1*28 haplotype, two patients (1.7%) were heterozygous for UGT1A1*27. Although three patients out of 118 (2.5%), is low, all three patients heterozygous for UGT1A1*27 haplotypes existing with UGT1A1*28 experienced severe toxicity after irinotecan chemotherapy. Thus, UGT1A1*27 might considerably affect irinotecan susceptibility when it coexists with a variant sequence in the promoter UGT1A1*28. Minami et al. also found four additional *27 haplotypes, including three heterozygous *28 and one homozygous *28, in addition to *27 in their patients.21 Why, then, was the UGT1A1*27 haplotype not found in our study? The first possible reason, although our method was correct, is that the UGT1A1*27 haplotype is too rare; thus, UGT1A1*27 does not have significant meaning for clinical practice. Second, UGT1A1*27 may have medical significance but we did not find the haplotype in this population because we only searched for UGT1A1*27 in patients with the UGT1A1*28 polymorphisms. We did not investigate UGT1A1*27 status in patients with wild type UGT1A1*28.
UGT1A1*28 and UGT1A1*6 polymorphisms were detected approximately half as often as both wild types (46%), followed by *6 heterozygous (35%), and *28 heterozygous (15%). Homozygous and both heterozygotes were rare (4%). The UGT1A1*6 gene polymorphism is associated with severe irinotecan toxicity in Asians, which was confirmed by our study; however, our results did not reach statistical significance.12, 22 In patients with the UGT1A1*28 gene polymorphism, including both heterozygous and homozygous types, grade 3–4 leukopenia and neutropenia were observed at a significantly higher frequency compared with wild type. Our results were consistent with previous studies in that UGT1A1*28 gene polymorphisms are a significant risk factor for irinotecan‐related toxicities.10, 11 The United States Food and Drug Administration declared that patients with homozygous UGT1A1*28 polymorphisms have an increased risk of neutropenia with irinotecan treatment.
In conclusion, our prospective study did not find the UGT1A1*27 gene polymorphism, which may suggest that the UGT1A1*27 gene polymorphism does not significantly predict severe irinotecan toxicity in cancer patients. By contrast, the UGT1A1*28 gene polymorphism is associated with irinotecan‐related leukopenia and neutropenia.
Acknowledgments
Analysis of UGT1A1*27 was supported by Sekisui Medical Co., Ltd. We thank the Clinical Research Support Center, Kyushu for their support of data management.
Disclosure
Takashi Seto received an honoraria from Daiichi Sankyo, and research funding from Daiichi Sankyo and Yakult Honsha. No other authors report any conflict of interest.
References
- 1. Boku N, Yamamoto S, Fukada H et al. Fluorouracil versus combination of irinotecan plus cisplatin versus S‐1 in metastatic gastric cancer: A randomized phase 3 study. Lancet Oncol 2009; 10: 1063–9. [DOI] [PubMed] [Google Scholar]
- 2. Bodurka DC, Levenback C, Wolf JK et al. Phase II trial of irinotecan in patients with metastatic epithelial ovarian cancer or peritoneal cancer. J Clin Oncol 2003; 21: 291–7. [DOI] [PubMed] [Google Scholar]
- 3. Noda K, Nishiwaki Y, Kawahara M et al. Irinotecan plus cisplatin compared with etoposide plus cisplatin for extensive small‐cell lung cancer. N Engl J Med 2002; 346: 85–91. [DOI] [PubMed] [Google Scholar]
- 4. Meyerhardt JA, Mayer RJ. Systemic therapy for colorectal cancer. N Engl J Med 2005; 352: 476–87. [DOI] [PubMed] [Google Scholar]
- 5. Fukuoka M, Niitani H, Suzuki A et al. A phase II study of CPT‐11, a new derivative of camptothecin, for previously untreated non‐small‐cell lung cancer. J Clin Oncol 1992; 10: 16–20. [DOI] [PubMed] [Google Scholar]
- 6. Shimada Y, Yoshino M, Wakui A et al. Phase II study of CPT‐11, a new camptothecin derivative, in metastatic colorectal cancer. CPT‐11 Gastrointestinal Cancer Study Group. J Clin Oncol 1993; 11: 909–13. [DOI] [PubMed] [Google Scholar]
- 7. Kawato Y, Aonuma M, Hirota Y, Kuga H, Sato K. Intracellular roles of SN‐38, a metabolite of the camptothecin derivative CPT‐11, in the antitumor effect of CPT‐11. Cancer Res 1991; 51: 4187–91. [PubMed] [Google Scholar]
- 8. de Forni M, Bugat R, Chabot GG et al. Phase I and pharmacokinetic study of the camptothecin derivative irinotecan, administered on a weekly schedule in cancer patients. Cancer Res 1994; 54: 4347–54. [PubMed] [Google Scholar]
- 9. Hahn KK, Wolff JJ, Kolesar JM. Pharmacogenetics and irinotecan therapy. Am J Health Syst Pharm 2006; 63: 2211–7. [DOI] [PubMed] [Google Scholar]
- 10. Innocenti F, Undevia SD, Iyer L et al. Genetic variants in the UGP‐glucuronosyltransferase 1A1 gene predict the risk of severe neutropenia of irinotecan. J Clin Oncol 2004; 22: 1382–8. [DOI] [PubMed] [Google Scholar]
- 11. Ando Y, Saka H, Ando M et al. Polymorphisms of UDP‐glucuronosyltransferase gene and irinotecan toxicity: A pharmacogenetic analysis. Cancer Res 2000; 60: 6921–6. [PubMed] [Google Scholar]
- 12. Han JY, Lim HS, Shin ES et al. Comprehensive analysis of UGT1A1 polymorphisms predictive for pharmacokinetics and treatment outcome in patients with non‐small‐cell lung cancer treated with irinotecan and cisplatin. J Clin Oncol 2006; 24: 2237–44. [DOI] [PubMed] [Google Scholar]
- 13. Sai K, Saeki M, Saito Y et al. UGT1A1 haplotypes associated with reduced glucuronidation and increased serum bilirubin in irinotecan‐administered Japanese patients with cancer. Clin Pharmacol Ther 2004; 75: 501–15. [DOI] [PubMed] [Google Scholar]
- 14. Jinno H, Tanaka‐Kagawa T, Hanioka N et al. Glucuronidation of 7‐ethyl‐10‐hydroxycamptothecin (SN‐38), an active metabolite of irinotecan (CPT‐11), by human UGT1A1 variants, G71R, P229Q, and Y486D. Drug Metab Dispos 2003; 31: 108–13. [DOI] [PubMed] [Google Scholar]
- 15. Nakamura Y, Soda H, Oka M et al. Randomized phase II trial of irinotecan with paclitaxel or gemcitabine for non‐small cell lung cancer: Association of UGT1A1*6 and UGT1A1*27 with severe neutropenia. J Thorac Oncol 2011; 6: 121–7. [DOI] [PubMed] [Google Scholar]
- 16. National Cancer Institute . Common Terminology Criteria for Adverse Events v 3.0. National Cancer Institute, Bethesda, MD 2006. [Cited 14 Jan 2014] Available from URL: http://ctep.cancer.gov/protocolDevelopment/electronic_applications/docs/ctcaev3.pdf
- 17. Iyer L, King CD, Whitington PF et al. Genetic predisposition to the metabolism of irinotecan (CPT‐11). Role of uridine diphosphate glucuronosyltransferase isoform 1A1 in the glucuronidation of its active metabolite (SN‐38) in human liver microsomes. J Clin Invest 1998; 101: 847–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kim TW, Innocenti F. Insights, challenges, and future directions in irinogenetics. Ther Drug Monit 2007; 29: 265–70. [DOI] [PubMed] [Google Scholar]
- 19. Akaba K, Kimura T, Sasaki A et al. Neonatal hyperbilirubinemia and mutation of the bilirubin uridine diphosphate‐glucuronosyltransferase gene: A common missense mutation among Japanese, Koreans and Chinese. Biochem Mol Biol Int 1998; 46: 21–6. [DOI] [PubMed] [Google Scholar]
- 20. Gagné JF, Montminy V, Belanger P, Journault K, Gaucher G, Guillemette C. Common human UGT1A polymorphisms and the altered metabolism of irinotecan active metabolite 7‐ethyl‐10‐hydroxycamptothecin (SN‐38). Mol Pharmacol 2002; 62: 608–17. [DOI] [PubMed] [Google Scholar]
- 21. Minami H, Sai K, Saeki M et al. Irinotecan pharmacokinetics/pharmacodynamics and UGT1A genetic polymorphisms in Japanese: Roles of UGT1A1*6 and *28 . Pharmacogenet Genomics 2007; 17: 497–504. [DOI] [PubMed] [Google Scholar]
- 22. Onoue M, Terada T, Kobayashi M et al. UGT1A1*6 polymorphism is most predictive of severe neutropenia induced by irinotecan in Japanese cancer patients. Int J Clin Oncol 2009; 14: 136–42. [DOI] [PubMed] [Google Scholar]


