Abstract
Background
The aim of this study was to evaluate the diagnostic accuracy of integrated 18 F‐fluorodeoxyglucose positron emission tomography/computed tomography (FDG‐PET/CT) in hilar and mediastinal lymph node (HMLN) staging of non‐small cell lung cancer (NSCLC), and to investigate potential risk factors for false‐negative and false‐positive HMLN metastases.
Methods
We examined the data of 388 surgically resected NSCLC patients preoperatively staged by integrated FDG‐PET/CT. Risk factors for false‐negative and false‐positive HMLN metastases were analyzed using univariate and multivariate analyses of clinicopathological factors.
Results
The sensitivity, specificity, positive predictive value, negative predictive value, and accuracy of integrated FDG‐PET/CT in detecting HMLN metastases were 47.4%, 91.0%, 56.3%, 87.7%, and 82.5%, respectively. On multivariate analysis, the maximum standardized uptake value (SUVmax) of the tumor (P = 0.042), adenocarcinoma (P = 0.003), and tumor size (P = 0.017) were risk factors for false‐negative HMLN metastases, and history of lung disease (P = 0.006) and tumor location (central; P = 0.025) were risk factors for false‐positive HMLN metastases.
Conclusions
The present study identified risk factors for false‐negative and false‐positive HMLN metastases in NSCLC patients staged by preoperative integrated FDG‐PET/CT. These findings would be helpful in selecting appropriate candidates for mediastinoscopy or endobronchial ultrasound‐guided transbronchial needle aspiration.
Keywords: Lymph node staging, non‐small cell lung cancer, positron emission tomography
Introduction
Lung cancer remains the leading cause of death among all cancers. Studies have demonstrated a relationship between tumor node metastasis (TNM) stage and survival rate.1 Thus, accurate staging of non‐small cell lung cancer (NSCLC) provides important prognostic information and determines the best treatment approach.2 The determination of hilar and mediastinal lymph node (HMLN) metastases is an important part of staging in patients with NSCLC. Metastasis to mediastinal lymph nodes (LNs) is an inoperable condition, while patients without LN metastases or only intrapulmonary or hilar lymph node involvement can undergo surgery.3 Neoadjuvant chemotherapy with surgery or concurrent or sequential chemoradiotherapy (CRT) are legitimate choices for patients with positive N2 LNs.4
Chest computed tomography (CT) has been the gold‐standard modality for LN staging in NSCLC patients. Contrast‐enhanced CT is the most common imaging modality for TNM staging, but it has limitations in evaluating LN status because the prediction of positive LNs on CT is based on size criteria alone.5
In NSCLC patients, 18F‐fluorodeoxyglucose positron emission tomography (FDG‐PET) has increasingly been used for LN staging. FDG‐PET is a functional imaging modality that is based on the observation that malignant cells typically show increased glucose metabolism.6 To overcome the inherent disadvantages of FDG‐PET, such as poor quality of anatomical information, new imaging systems using integrated FDG‐PET/CT have recently been developed.7 This integrated approach provides higher sensitivity than CT alone in LN staging for NSCLC. Although some previous studies have indicated that integrated FDG‐PET/CT is more effective for detecting HMLN metastases, results regarding the extent of its benefits have been inconsistent.8 Furthermore, the rate of occult LN metastases in NSCLC patients showing negative uptake by FDG‐PET/CT is 7–16%, and false‐positive findings from inflammatory or granulomatous lesions are still problematic on FDG‐PET/CT.9, 10, 11
The aim of the present study was to evaluate the diagnostic accuracy of integrated FDG‐PET/CT in HMLN staging of NSCLC, and to investigate potential risk factors for false‐negative and false‐positive HMLN metastases with integrated FDG‐PET/CT, because accurate prediction of occult LN involvement based on associated risk factors would be helpful in selecting appropriate candidates for mediastinoscopy or endobronchial ultrasound‐guided transbronchial needle aspiration (EBUS‐TBNA).
Methods
Patient eligibility
Between April 2007 and July 2015, 453 consecutive patients underwent pulmonary resection for lung cancer at Sagamihara Kyodo Hospital (Sagamihara, Kanagawa, Japan). Of these, 388 patients who were diagnosed with clinical stage I–IIIA NSCLC based on integrated FDG‐PET/CT according to the 7th edition TNM classification and who underwent complete surgical resection with systematic LN dissection were reviewed. Complete resection was defined as an absence of residual cancer both macroscopically and microscopically (R0). Patients who underwent incomplete resection or neoadjuvant chemotherapy/radiotherapy were excluded. No patient underwent preoperative mediastinoscopy or EBUS‐TBNA in this period.
The medical records of each patient were reviewed for the following clinicopathological information: age; gender; smoking habit (never or ever‐smoker); history of lung disease; concurrent diabetes; tumor laterality (right or left side); lobar distribution of tumor; tumor location; maximum standardized uptake value (SUVmax) of primary tumor; histological type; tumor size (cm); grade; pleural invasion (negative or positive); and lymph node metastasis (N0, N1, or N2). All clinical, intraoperative radiological, and pathological findings were reviewed from two hospitals in Kanagawa, Japan (Sagamihara Kyodo Hospital and Yuai Clinic). Table 1 shows the details of the patients’ characteristics and preoperative tumor evaluations. Histological classification of NSCLC was based on the World Health Organization (WHO) classification.12 Preoperative and postoperative staging were based on the TNM staging system.13 Data collection and analyses were approved and the need to obtain written informed consent from each patient was waived by the institutional review board in July 2015.
Table 1.
Tumor and patient characteristics (n = 388)
| Variable | Distribution (%) | |
|---|---|---|
| Age (years) | Mean ± SD | 69 ± 9.0 |
| Range | 35–86 | |
| Gender | Female | 130 (33.5) |
| Male | 258 (66.5) | |
| Smoking habits | Non‐smoker | 119 (30.7) |
| Ever‐smoker | 269 (69.3) | |
| History of lung disease† | Absent | 259 (66.8) |
| Present | 129 (33.2) | |
| Concurrent diabetes | Absent | 333 (85.8) |
| Present | 55 (14.2) | |
| Tumor laterality | Right | 238 (61.3) |
| Left | 150 (38.7) | |
| Lobar distribution of tumor | Right upper lobe | 110 (28.5) |
| Right middle lobe | 32 (8.2) | |
| Right lower lobe | 96 (24.6) | |
| Left upper lobe | 95 (24.5) | |
| Left lower lobe | 55 (14.2) | |
| Tumor location | Central | 60 (15.5) |
| Non‐central | 328 (84.5) | |
| SUVmax of tumor | Median | 3.9 |
| Mean ± SD | 5.4 ± 4.7 | |
| Range | 0.5–35.6 | |
| Histological type | Adenocarcinoma | 268 (69.0) |
| Squamous cell carcinoma | 90 (23.2) | |
| Other types | 30 (7.8) | |
| Tumor size (cm) | Median | 3.0 |
| Mean ± SD | 3.4 ± 1.9 | |
| Range | 0.5–18.0 | |
| Grade | Well/Moderate | 347 (89.5) |
| Poor | 41 (10.5) | |
| Pleural invasion (PL) | Absent | 328 (84.5) |
| Present | 60 (15.5) | |
| Lymph node metastasis | N0 | 312 (80.4) |
| N1 | 31 (8.0) | |
| N2 | 45 (11.6) |
History of lung disease includes interstitial lung disease, chronic obstructive pulmonary disorder, bronchial asthma, and tuberculosis.
Moderate/Poor, moderately or poorly‐differentiated carcinoma; SD, standard deviation; SUVmax, maximum standardized uptake value; Well, well‐differentiated carcinoma.
Computed tomography
Diagnostic‐quality contrast‐enhanced CT of the chest with 5 mm slice thickness was performed for all patients. A tumor was deemed central if its center was located in the inner one‐third of the lung parenchyma (adjacent to the mediastinum) on transverse CT. Peripherally located tumors were identified as those centered in the outer two‐thirds of the lung parenchyma on transverse CT. The maximal diameter of the lung nodules was measured on contrast‐enhanced CT of the chest. All CT was performed within four weeks of surgery.
Integrated 18 F‐fluorodeoxyglucose positron emission tomography (FDG‐PET) imaging
Each patient underwent integrated FDG‐PET/CT before surgical resection. All integrated FDG‐PET/CT was performed within four weeks of surgery. After fasting for six hours, FDG (3.5 MBq/kg body weight) was intravenously injected if the patient's blood sugar level was lower than 200 mg/dL. Image acquisition commenced an hour after the injection using a single PET/CT combined scanner (Eminence‐SOPHIA, Shimadzu Corporation, Kyoto, Japan).14 Image emission data from the eyes to the mid‐thigh area were continuously acquired over a period of approximately 20 minutes. After attenuation corrections were made for the resulting image data, reconstruction was performed using a dynamic row‐action expectation maximization algorithm.15 The reconstructed sectional images were then evaluated visually and quantitatively using the SUVmax inside a volume of interest (VOI) placed on the lesions. SUVmax was calculated as:
[(maximum activity in VOI)/(volume of VOI)]/[(injected FDG dose)/(patient weight)]
The quality of radiation measurements of the PET/CT scanner was assured by calibration in accordance with the National Electrical Manufacturers Association NU‐2 2001 standard.16
Nodal uptake with SUVmax > 2.5 was considered positive. For determination of SUV, a cylindrical region of interest (ROI) was manually placed over the tumor site on the hottest transaxial slice. The activity concentration within the ROI was determined and expressed as the SUV, where SUV is the ratio of the activity in the tissue to the decay‐corrected activity injected into the patient. All SUV measurements were normalized for patient body weight. SUVmax within a ROI was used as the reference measurement.17
Three experienced radiologists analyzed integrated FDG‐PET/CT images individually. Final assessment was made by consensus if the initial assessments differed.
Surgical resection
All patients underwent anatomical lung resection and radical lymphadenectomy in our hospital. Thoracic surgeons at Sagamihara Kyodo Hospital performed all surgical resections and nodal dissections. Techniques of nodal dissection at the time of surgical resection were also standardized. Systematic LN dissection according to American Thoracic Society criteria was performed in all patients, removing at least three hilar stations and three mediastinal stations.
Pathological examination
Experienced pulmonary pathologists examined all resected tumor specimens. Histological classification of NSCLC was based on WHO classification. Dissected LNs were examined histologically following hematoxylin and eosin staining.
Statistical analysis
Statistical analysis was performed using SPSS version 22.0 (SPSS, Chicago, IL, USA). Receiver operating characteristic (ROC) curve analysis was used to assess the best discriminative cut‐off value for SUVmax of HMLNs. Univariate analysis was conducted using Fisher's exact or Pearson's chi‐square tests. Multivariate analysis was conducted using the logistic regression (backwards stepwise) method. Values of P < 0.05 were considered significant.
Results
Patient characteristics
The characteristics and pathological features of nodal involvement for all 388 patients (130 women, 258 men; mean age 69 years, range 35–86) are listed in Table 1. Median tumor size was 3.0 cm, and the median SUVmax of the primary tumor was 3.9.
Evaluation of hilar and mediastinal lymph node (HMLN) status by pathological examination and integrated FDG‐PET‐computed tomography
Positive HMLNs were found in 19.6% (76/388) of the patients. All evaluating indicators of integrated FDG‐PET/CT examination are listed in Table 2. The sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), and accuracy of integrated FDG‐PET/CT in detecting HMLN metastases were 47.4% (36/76), 91% (284/312), 56.3% (36/64), 87.7% (284/324), and 82.5% (320/388), respectively. The ROC curve based on the SUVmax of HMLN status is shown in Figure 1. The SUVmax of nodes had an area under the curve of 0.797 (95% CI 0.740–0.853) with a cut‐off SUVmax of 1.7. If nodal uptake with SUVmax > 1.7 was interpreted as positive, the sensitivity, specificity, PPV, NPV, and accuracy of integrated FDG‐PET/CT were 80.3% (61/76), 59.9% (187/312), 32.8% (61/186), 92.6% (187/202), and 63.9% (248/388), respectively.
Table 2.
Histopathological findings and integrated FDG‐PET/CT results of HMLNs
| Metastasis | SUVmax ≤ 2.5 | SUVmax > 2.5 |
|---|---|---|
| Pathologically negative | 284 (TN) | 28 (FP) |
| Pathologically positive | 40 (FN) | 36 (TP) |
FDG‐PET/CT, 18F‐fluorodeoxyglucose positron emission tomography/computed tomography; FN, false negatives; FP, false positives; HMLN, hilar and mediastinal lymph node; SUVmax, maximum standardized uptake value; TN, true negatives; TP, true positives.
Figure 1.
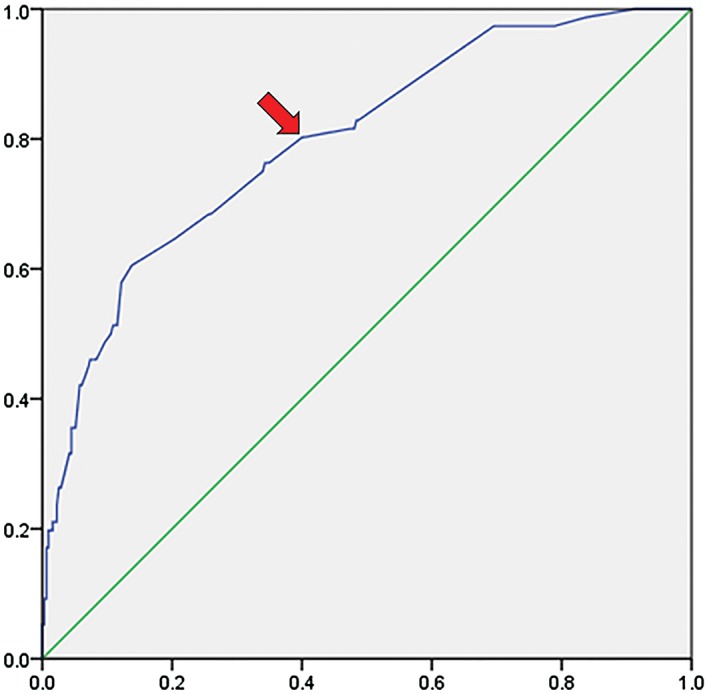
Area under the receiver operating characteristic curve for maximum standardized uptake value (SUVmax) of the hilar and mediastinal lymph nodes for predicting lymph node metastasis. Area under the curve, 0.797 (95% confidence interval, 0.740–0.853). An SUVmax of 1.7 (arrow) as the hypothetical threshold yields 80.3% sensitivity and 59.9% specificity.
Risk factors associated with false‐negative detection of HMLN metastasis of maximum standardized uptake value (SUVmax) ≤ 2.5
The incidence of occult HMLN metastasis in this study was 12.3% (40/324). Table 3 summarizes the results of univariate analysis for factors associated with HMLN metastasis in 324 integrated FDG‐PET/CT‐negative patients. Factors significantly associated with HMLN metastasis were: tumor location (central; P = 0.037), SUVmax of tumor (>3.9; P = 0.007), histological type (adenocarcinoma; P = 0.001), and tumor size (>3 cm; P = 0.002). Multivariate risk‐factor analysis identified the SUVmax of the tumor (>3.9), histological type (adenocarcinoma), and tumor size (>3 cm) as risk factors for false‐negative HMLN metastasis (Table 4).
Table 3.
Univariate analysis for factors associated with false‐negatives in integrated FDG‐PET/CT‐negative patients
| Variable | Status | Pathological N0 | Pathological N1–N2 | P |
|---|---|---|---|---|
| Age (years) | ≤65 | 91 | 10 | N/S |
| >65 | 193 | 30 | ||
| Gender | Female | 102 | 16 | N/S |
| Male | 182 | 24 | ||
| Smoking habits | Non‐smoker | 95 | 14 | N/S |
| Ever‐smoker | 189 | 26 | ||
| History of lung disease | Absent | 202 | 25 | N/S |
| Present | 82 | 15 | ||
| Concurrent diabetes | Absent | 243 | 34 | N/S |
| Present | 41 | 6 | ||
| Tumor laterality | Right | 169 | 25 | N/S |
| Left | 115 | 15 | ||
| Lobar distribution of tumor | Upper or middle lobe† | 184 | 23 | N/S |
| Lower lobe‡ | 100 | 17 | ||
| Tumor location | Central | 31 | 9 | 0.037* |
| Non‐central | 253 | 31 | ||
| SUVmax of tumor | ≤3.9 | 171 | 15 | 0.007* |
| >3.9 | 113 | 25 | ||
| Histological type | Adenocarcinoma | 204 | 34 | 0.001* |
| Other types | 80 | 6 | ||
| Tumor size (cm) | ≤3 | 165 | 13 | 0.002* |
| >3 | 119 | 27 | ||
| Grade | Well/Moderate | 255 | 38 | N/S |
| Poor | 29 | 2 | ||
| Pleural invasion (PL) | Absent | 244 | 30 | N/S |
| Present | 40 | 10 |
Significant.
Upper or middle lobe includes right upper lobe, right middle lobe, and left upper lobe.
Lower lobe includes right lower lobe, left lower lobe.
18F‐FDG‐PET/CT, fluorodeoxyglucose positron emission tomography/computed tomography; N/S, not significant; Poor, poorly‐differentiated carcinoma SUVmax, maximum standardized uptake value; Well/Moderate, well or moderately‐differentiated carcinoma;
Table 4.
Multivariate analysis for factors associated with false negatives in integrated FDG‐PET/CT‐negative patients
| Variable | Odds ratio | Confidence interval | P |
|---|---|---|---|
| SUVmax of tumor (>3.9) | 2.316 | 1.029–5.212 | 0.042* |
| Histological type (adenocarcinoma) | 4.951 | 1.715–14.294 | 0.003* |
| Tumor size (>3 cm) | 2.724 | 1.199–6.190 | 0.017* |
Significant.
18F‐FDG‐PET/CT, fluorodeoxyglucose positron emission tomography/computed tomography; SUVmax, maximum standardized uptake value.
Risk factors associated with false‐positive detection of HMLN metastasis of SUVmax > 2.5
A total of 64 patients who had an HMLN SUVmax of > 2.5 were diagnosed as positive cases by integrated FDG‐PET/CT. Postoperatively, 28 patients were confirmed as false positive. Univariate analysis indicated history of lung disease (P < 0.001) and tumor location (central; P = 0.021) as risk factors for false‐positive uptake. Risk factors that were significantly associated with false‐positive uptake on multivariate analysis were history of lung disease (P = 0.006) and tumor location (central; P = 0.025). Some of these results are shown in Tables 5 and 6.
Table 5.
Univariate analysis for factors associated with false‐positives in integrated FDG‐PET/CT‐positive patients
| Variable | Status | Pathological N0 | Pathological N1–N2 | P |
|---|---|---|---|---|
| Age (years) | ≤65 | 6 | 9 | N/S |
| >65 | 22 | 27 | ||
| Gender | Female | 5 | 7 | N/S |
| Male | 23 | 29 | ||
| Smoking habits | Non‐smoker | 3 | 7 | N/S |
| Ever‐smoker | 25 | 29 | ||
| History of lung disease | Absent | 6 | 26 | <0.001* |
| Present | 22 | 10 | ||
| Concurrent diabetes | Absent | 25 | 31 | N/S |
| Present | 3 | 5 | ||
| Tumor laterality | Right | 22 | 22 | N/S |
| Left | 6 | 14 | ||
| Lobar distribution of tumor | Upper or middle lobe† | 15 | 15 | N/S |
| Lower lobe‡ | 13 | 21 | ||
| Tumor location | Central | 13 | 7 | 0.021* |
| Non‐central | 15 | 29 | ||
| SUVmax of tumor | ≤3.9 | 5 | 4 | N/S |
| >3.9 | 23 | 32 | ||
| Histological type | Adenocarcinoma | 10 | 20 | N/S |
| Other types | 18 | 16 | ||
| Tumor size (cm) | ≤3 | 7 | 14 | N/S |
| >3 | 21 | 22 | ||
| Grade | Well/Moderate | 25 | 29 | N/S |
| Poor | 3 | 7 | ||
| Pleural invasion (PL) | Absent | 23 | 31 | N/S |
| Present | 5 | 5 |
Significant.
Upper or middle lobe includes right upper lobe, right middle lobe, and left upper lobe.
Lower lobe includes right lower lobe, left lower lobe.
18F‐FDG‐PET/CT, fluorodeoxyglucose positron emission tomography/computed tomography; N/S, not significant; SUVmax, maximum standardized uptake value; Well/Mod,well or moderately differentiated carcinoma; Poor, poorly differentiated carcinoma.
Table 6.
Multivariate analysis for factors associated with false‐positives in integrated FDG‐PET/CT‐positive patients
| Variable | Odds ratio | Confidence interval | P |
|---|---|---|---|
| History of lung disease† (present) | 7.621 | 1.768–32.846 | 0.006* |
| Tumor location (central) | 7.599 | 1.288–44.832 | 0.025* |
Significant.
18F‐FDG‐PET/CT, fluorodeoxyglucose positron emission tomography/computed tomography.
History of lung disease includes interstitial lung disease, chronic obstructive pulmonary disorder, bronchial asthma, and tuberculosis.
Discussion
18F‐Fluorodeoxyglucose positron emission tomography is widely used for the staging of NSCLC and provides a promising optimization tool for LN staging.18 Originally, integrated FDG‐PET/CT provided useful information about HMLN metastases in NSCLC; however, microscopic LN metastases are barely detectable.
In this study, as in a previous study, a traditional value of 2.5 for SUVmax was chosen.19 HMLNs with SUVmax > 2.5 were, thus, considered positive.
In a meta‐analysis, the pooled sensitivity and specificity on a per‐patient analysis were 71.9% and 89.8%, respectively.8 This suggested PET/CT had more specificity but less sensitivity for LN staging.
We performed the present study to evaluate the sensitivity and specificity of integrated FDG‐PET/CT. The false‐negatives and false‐positives accounted for 12.3% (40/324) and 43.8% (28/64) of patients, respectively. Integrated FDG‐PET/CT provides somewhat unsatisfactory sensitivity (47.4%), specificity (91%), and accuracy (82.5%) for nodal staging of NSCLC.
In order to maximize the accuracy of integrated FDG‐PET/CT, ROC curve analysis was performed. The curve identified an optimal cut‐off SUVmax value of 1.7, which maximized the sensitivity and specificity and, consequently, the accuracy. When a SUVmax of 1.7 or greater is used to label any LN as positive and less than 1.7 as negative on integrated FDG‐PET/CT, the sensitivity of all patients is 80.3% at the expense of specificity. On ROC curve analysis, it is obvious that increasing the cut‐off value of SUVmax increases specificity at the expense of sensitivity.
Occult lymph node metastasis was detected in 40 of the 324 patients (12.3%) in the present study; thus, NPV was 87.7%. This finding is consistent with comparable studies. In some reported studies, not all patients underwent systematic LN dissection, which may have resulted in an underestimation of the true prevalence of occult nodal disease.20, 21 A study by Gomez‐Caro et al. reported a 32% prevalence of occult nodal disease.22 In a study by Park et al., the frequency of nodal upstaging in patients with cT1N0M0 NSCLC by PET/CT was 14.3%.20 The frequencies of N1 and N2 involvement were 9.5% and 4.8%, respectively. Veeramachaneni et al. reported a 10% prevalence of occult nodal disease (N1 disease, n = 6; N2 disease, n = 5) for clinical N0 by PET/CT.21 Casiraghi et al. reported a 13% rate of occult nodal metastasis.23 Li et al. reported a 9% rate of occult nodal metastasis in Chinese NSCLC N0 patients.24
In the present study, the SUVmax of the primary tumor, adenocarcinoma, and tumor size were independent predictors of occult LN metastasis for patients with NSCLC by integrated FDG‐PET/CT on multivariate analysis.
The incidence of LN metastasis is known to increase as tumor size increases.25, 26 Asamura et al. found that, among patients with resected peripheral NSCLC, the prevalence of LN metastases increased from 19.5% in tumors ≤2 cm in diameter to 32.5% in tumors 2–3 cm in diameter.26 In the current study, large clinical tumor size was significantly associated with increased prevalence of occult LN metastases.
Bryant et al. demonstrated the clinical importance and relevance of SUVmax for primary NSCLC.27 SUVmax provides information on the biological aggressiveness, key pathological features, and the potential of tumor spread. Several previous studies have also reported that a higher SUVmax for primary lung cancer was significantly associated with nodal involvement, although great heterogeneity is seen in the definition of an SUV threshold among studies. Casiraghi et al. reported higher SUVmax of the primary tumor in patients with pathological nodal involvement compared with patients without nodal involvement, while Cerfolio et al. showed that the SUVmax of lung tumors increased as the cancer progressed from N0 to N3.7, 23 Nambu et al. demonstrated that the likelihood of LN metastasis rose with an increase in the SUVmax of the primary tumor.28 No occult nodal metastases were observed in lung cancers with SUVmax of the primary tumor <2.5, and lung cancers with SUVmax > 12 showed a 70% frequency of occult nodal metastasis. In a study by Park et al., SUVmax > 7.3 in primary tumors offered an independent predictor of occult nodal metastasis in patients with cT1N0M0 NSCLC, according to PET/CT.20
Adenocarcinoma was also identified as a risk factor for occult LN metastasis in the present patient cohort. Kanzaki et al. posited that occult LN metastasis was more likely to occur in patients with lung adenocarcinoma.29 Conversely, the series by Al‐Sarraf et al. found that primary tumor cell type did not affect the incidence of occult MLN metastasis.9
The accuracy of integrated FDG‐PET/CT, as an imaging modality for NSCLC staging, is challenged by the increased glycolytic activity of benign tumors and inflammatory tissue, in addition to that of malignant tumors. As expected, up to 43.8% (28/64) of patients were confirmed as false positive with upstaging from N0 to N1 or N2 by integrated FDG‐PET/CT. These false‐positive results may have been caused by reactive hyperplasia or active inflammation as a result of granulomatous disease, such as tuberculosis.30 Risk factors for false‐positives in the present study were history of lung disease and tumor location (central). It is speculated that centrally located tumors tend to cause obstructive pneumonia and atelectasis, which results in the accumulation of FDG in macrophages and lymphocytes in LNs. Shiraishi et al. demonstrated that false‐positive FDG‐PET results in mediastinal and pulmonary LNs were closely related to the proportion of macrophages and lymphocytes.31 Therefore, we still recommend biopsies of all suspicious LNs with a high SUVmax that should not be equated with malignancy until tissue confirmation is obtained.
The main limitation of this study was the retrospective nature of the work. To clarify the true incidence of false‐negative and false‐positive diagnoses of LN metastases of NSCLC patients staged by integrated FDG‐PET/CT, prospective or randomized trials are warranted. In the present study, patients treated with neoadjuvant chemotherapy and radiotherapy were excluded, as these cases can lead to considerable inaccuracy. Although this criterion would have caused some degree of selection bias, a relatively large number of patients were examined overall; therefore, this bias should not have been significant.
The present study demonstrated that SUVmax of the primary tumor, adenocarcinoma, and tumor size were risk factors for false‐negatives, and history of lung disease and tumor location were risk factors for false‐positives in patients with NSCLC diagnosed by preoperative integrated FDG‐PET/CT. False‐negative and false‐positive results are inevitable, but may be somewhat predictable using some risk factors. The results of this study may assist pre‐therapy evaluation and decision‐making.
Disclosure
No authors report any conflict of interest.
Acknowledgments
The authors acknowledge the assistance of Mr. Tomoyuki Kanno, Yuai Clinic, for data acquisition.
References
- 1. Sheel AR, McShane J, Poullis MP. Survival of patients with or without symptoms undergoing potentially curative resections for primary lung cancer. Ann Thorac Surg 2013; 95: 276–84. [DOI] [PubMed] [Google Scholar]
- 2. Bilfinger TV. Surgical viewpoints for the definitive treatment of lung cancer. Respir Care Clin N Am 2003; 9: 141–62. [DOI] [PubMed] [Google Scholar]
- 3. Bayman NA, Blackhall F, Jain P, Lee L, Thatcher N, Faivre‐Finn C. Management of unresectable stage III non‐small‐cell lung cancer with combined‐modality therapy: A review of the current literature and recommendations for treatment. Clin Lung Cancer 2008; 9: 92–101. [DOI] [PubMed] [Google Scholar]
- 4. Tieu BH, Sanborn RE, Thomas CR Jr. Neoadjuvant therapy for resectable non‐small cell lung cancer with mediastinal lymph node involvement. Thorac Surg Clin 2008; 18: 403–15. [DOI] [PubMed] [Google Scholar]
- 5. Birim O, Kappetein AP, Stijnen T, Bogers AJ. Meta‐analysis of positron emission tomographic and computed tomographic imaging in detecting mediastinal lymph node metastases in nonsmall cell lung cancer. Ann Thorac Surg 2005; 79: 375–82. [DOI] [PubMed] [Google Scholar]
- 6. Rohren EM, Turkington TG, Coleman RE. Clinical applications of PET in oncology. Radiology 2004; 231: 305–32. [DOI] [PubMed] [Google Scholar]
- 7. Cerfolio RJ, Ojha B, Bryant AS, Raghuveer V, Mountz JM, Bartolucci AA. The accuracy of integrated PET‐CT compared with dedicated PET alone for the staging of patients with nonsmall cell lung cancer. Ann Thorac Surg 2004; 78: 1017–23. [DOI] [PubMed] [Google Scholar]
- 8. Zhao L, He ZY, Zhong XN, Cui ML. (18)FDG‐PET/CT for detection of mediastinal nodal metastasis in non‐small cell lung cancer: A meta‐analysis. Surg Oncol 2012; 21: 230–6. [DOI] [PubMed] [Google Scholar]
- 9. Al‐Sarraf N, Aziz R, Gately K et al. Pattern and predictors of occult mediastinal lymph node involvement in non small cell lung cancer patients with negative mediastinal uptake on positron emission tomography. Eur J Cardiothorac Surg 2008; 33: 104–9. [DOI] [PubMed] [Google Scholar]
- 10. Lee PC, Port JL, Korst RJ, Liss Y, Meherally DN, Altorki NK. Risk factors for occult mediastinal metastases in clinical stage I non‐small cell lung cancer. Ann Thorac Surg 2007; 84: 177–81. [DOI] [PubMed] [Google Scholar]
- 11. Cerfolio RJ, Bryant AS, Ohja B, Bartolucci AA. The maximum standardized uptake values on positron emission tomography of a non‐small cell lung cancer predict stage, recurrence, and survival. J Thorac Cardiovasc Surg 2005; 130: 151–9. [DOI] [PubMed] [Google Scholar]
- 12. Travis WD, Brambilla E, Müller‐Hermelink HK. et al., eds. Histological Typing of Lung and Pleural Tumours, 4th edn. IARC Press, Lyon: 2004. [Google Scholar]
- 13. Goldstraw P, Crowley J, Chansky K et al. The IASLC lung cancer staging project: Proposals for the revision of the TNM stage groupings in the forthcoming (seventh) edition of the TNM classification of malignant tumours. J Thorac Oncol 2007; 2: 706–14. [DOI] [PubMed] [Google Scholar]
- 14. Matsumoto K, Kitamura K, Mizuta T et al. Performance characteristics of a new 3‐dimensional continuous‐emission and spiral‐transmission high sensitivity and high‐resolution PET camera evaluated with the NEMA NU 2‐2001 standard. J Nucl Med 2006; 47: 83–90. [PubMed] [Google Scholar]
- 15. Kitamura K, Takahashi S, Tanaka A et al. 3D continuous emission and spiral transmission scanning for high‐throughput whole‐body PET. IEEE Nucl. Sci. Symp. Conf. Rec. 2004; 5: 2801–5. [Google Scholar]
- 16. National Electrical Manufacturers Association . Performance Measurement of Positron Emission Tomographs. NEMA Standards Publication NU 2–2001. NEMA, Rosslyn, VA: 2001. [Google Scholar]
- 17. Nabi HA, Zubeldia JM. Clinical applications of (18)F‐FDG in oncology. J Nucl Med Technol 2002; 30: 3–9. [PubMed] [Google Scholar]
- 18. Fischer B, Lassen U, Mortensen J et al. Preoperative staging of lung cancer with combined PET‐CT. (Published erratum appears in N Engl J Med 2011; 364: 982.) N Engl J Med 2009; 361: 32–9. [DOI] [PubMed] [Google Scholar]
- 19. Hu M, Han A, Xing L et al. Value of dual‐time point FDG PET/CT for mediastinal nodal staging in non‐small‐cell lung cancer patients with lung comorbidity. Clin Nucl Med 2011; 36: 429–33. [DOI] [PubMed] [Google Scholar]
- 20. Park HK, Jeon K, Koh WJ et al. Occult nodal metastasis in patients with non‐small cell lung cancer at clinical stage IA by PET/CT. Respirology 2010; 15: 1179–84. [DOI] [PubMed] [Google Scholar]
- 21. Veeramachaneni NK, Battafarano RJ, Meyers BF, Zoole JB, Patterson GA. Risk factors for occult nodal metastasis in clinical T1N0 lung cancer: A negative impact on survival. Eur J Cardiothorac Surg 2008; 33: 466–9. [DOI] [PubMed] [Google Scholar]
- 22. Gómez‐Caro A, Garcia S, Reguart N et al. Incidence of occult mediastinal node involvement in cN0 non‐small cell lung cancer patients after negative uptake of positron emission tomography/computed tomography scan. Eur J Cardiothorac Surg 2010; 37: 1168–74. [DOI] [PubMed] [Google Scholar]
- 23. Casiraghi M, Travaini LL, Maisonneuve P et al. Lymph node involvement in T1 non‐small cell lung cancer: Could glucose uptake and maximal diameter be predictive criteria? Eur J Cardiothorac Surg 2011; 39: e38–43. [DOI] [PubMed] [Google Scholar]
- 24. Li X, Zhang H, Xing L et al. Mediastinal lymph nodes staging by 18F‐FDG PET/CT for early stage non‐small cell lung cancer: A multicenter study. Radiother Oncol 2012; 102: 246–50. [DOI] [PubMed] [Google Scholar]
- 25. Shields TW. Pathology of carcinoma of the lung In: Shields TW, LoCicero J, Reed CE, Feins RH. (eds). General Thoracic Surgery, 7th edn, Vol. II. Lippincott Williams &Wilkins, New York: 2009; 1311–40. [Google Scholar]
- 26. Asamura H, Nakayama H, Kondo H, Tsuchiya R, Shimosato Y, Naruke T. Lymph node involvement, recurrence, and prognosis in resected small, peripheral, non–small cell lung carcinomas: Are these carcinomas candidates for video‐assisted lobectomy? J Thorac Cardiovasc Surg 1996; 111: 1125–34. [DOI] [PubMed] [Google Scholar]
- 27. Bryant AS, Cerfolio RJ, Klemm KM, Ojha B. Maximum standard uptake value of mediastinal lymph nodes on integrated FDG‐PET‐CT predicts pathology in patients with non‐small cell lung cancer. Ann Thorac Surg 2006; 82: 413–22. [DOI] [PubMed] [Google Scholar]
- 28. Nambu A, Kato S, Sato Y et al. Relationship between maximum standardized uptake value (SUVmax) of lung cancer and lymph node metastasis on FDG‐PET. Ann Nucl Med 2009; 23: 269–75. [DOI] [PubMed] [Google Scholar]
- 29. Kanzaki R, Higashiyama M, Fujiwara A et al. Occult mediastinal lymph node metastasis in NSCLC patients diagnosed as clinical N0‐1 by preoperative integrated FDG‐PET/CT and CT: Risk factors, pattern, and histopathological study. Lung Cancer 2011; 71: 333–7. [DOI] [PubMed] [Google Scholar]
- 30. Shim SS, Lee KS, Kim BT et al. Non‐small cell lung cancer: Prospective comparison of integrated FDG PET/CT and CT alone for preoperative staging. Radiology 2005; 236: 1011–9. [DOI] [PubMed] [Google Scholar]
- 31. Shiraki N, Hara M, Ogino H et al. False‐positive and true‐negative hilar and mediastinal lymph nodes on FDG‐PET – radiological‐pathological correlation. Ann Nucl Med 2004; 18: 23–8. [DOI] [PubMed] [Google Scholar]


