Abstract
Background
Biomarkers may help to improve non‐small cell lung cancer (NSCLC) prognosis. However, the prognostic effect of topoisomerase I (Topo I) on NSCLC is unknown. We evaluated the clinicopathologic and prognostic significance of tumor Topo I and thymidylate synthase (TS) protein expression in postoperative NSCLC patients.
Methods
One hundred and fifteen patients with postoperative NSCLC were enrolled. Topo I and TS protein were detected in removed tumors by immunohistochemistry. The correlations between Topo I/TS protein expression and clinicopathologic characters and outcomes of patients were analyzed.
Results
Increased expression of Topo I was found in 57 (49.6%) tumors. The largest diameter of the tumor was significantly different between patients with high and low Topo I expression (P = 0.035). TS staining showed that 35 (30.4%) carcinomas were TS positive. The level of TS expression was correlated with tumor differentiation (P = 0.037). Patients with low Topo I expression had significantly longer overall survival (OS) than those with high expression (P = 0.004). The correlation between Topo I expression and OS was demonstrated among patients with squamous cell carcinoma (P = 0.030) and patients in pathological tumor node metastasis stage I (P = 0.027). Topo I expression was positively correlated with TS expression in tumor tissue (R = 0.251, P = 0.007).
Conclusions
Low Topo I expression is an independent favorable prognostic factor for longer OS in postoperative NSCLC patients, especially in squamous cell carcinoma. There is a correlation between the expression of TS and Topo I in removed tumor tissue.
Keywords: Non‐small cell lung cancer, prognosis, topoisomerase I
Introduction
Lung cancer is the most frequently diagnosed cancer and the leading cause of cancer‐related death globally.1 Non‐small cell lung cancer (NSCLC), which accounts for 80% of lung cancers, is further distinguished as adenocarcinoma, squamous cell carcinoma (SCC), and other subtypes.2 Complete resection of the primary tumor is a major therapeutic method for stage I–IIIa patients with NSCLC.3 However, even after resection, some NSCLC patients still experience a poor outcome, primarily as a result of the high recurrence rate and postoperative metastasis.4
The international lung cancer tumor node metastasis (TNM) staging system is the most powerful tool for predicting the survival rate in NSCLC patients.5 However, NSCLC patients who are in the same TNM stage may have different prognoses, as NSCLC cell types have marked heterogeneity. Therefore, oncologists need to perform a systematic classification of the molecular counterparts of lung carcinomas, as well as their histological tumor type. In this respect, biomarkers can be used to identify NSCLC patients at a high risk of relapse and poor prognosis. With technological developments in molecular biology, the detection of genetic mutations and biomarkers plays a fundamental role in the individual management of patients with NSCLC.
DNA topoisomerases are ubiquitous nuclear enzymes found in all cell types. These enzymes act to regulate DNA supercoiling by catalyzing the winding and unwinding of DNA strands. Topoisomerase I (Topo I) changes the supercoiling degree of DNA by causing single‐strand breaks and re‐ligation.6 Topo I is expressed in various kinds of human tumors and is an important target for the treatment of malignant disease.7, 8 Although it is debated whether Topo I expression can be used to predict the response to anti‐Topo I chemotherapy, many studies show it is valuable in the prognosis of patients with colorectal cancer.9, 10 For patients with small cell lung cancer, Topo I messenger ribonucleic acid (mRNA) analysis can predict the cisplatin outcome and prognosis.11However, the prognostic effect of Topo I on NSCLC is unknown.
Thymidylate synthase (TS) catalyzes the methylation of deoxyuridylate monophosphate (dUMP) to deoxythymidylate monophosphate (dTMP) using 5,10‐methylenetetrahydrofolate (methylene‐THF) as a cofactor. This function maintains the dTMP pool critical for DNA replication and repair.12, 13 TS inhibitors, such as pemetrexed and 5‐fluorouracil (5‐FU), show suitable pharmacological activity in various tumors. Consequently, determining the inhibitor of TS is an important direction of anticancer research. Previous studies have supported an association between TS expression and many carcinomas, such as gastric and colorectal cancers; thus, TS expression can be of predictive value.14, 15 As for patients with NSCLC, several previous studies have identified the significance of TS expression as a prognostic or predictive biomarker.16, 17, 18 TS expression in tumor tissue also has predictive significance in postoperative NSCLC patients.19 In a meta‐analysis, Liu et al. reported that TS expression had prognostic value in NSCLC patients who received TS inhibitor treatment.20
Xu et al. found a correlation between TS and Topo I expression in colorectal cancer patients, revealing their prognostic value.10 However, there is little information about the expression of Topo I in NSCLC. Considering the correlation between TS and Topo I expression in tumors, we hypothesized that Topo I could be a surrogate biomarker in patients with NSCLC, which was similar to TS. To the best of our knowledge, little research had been conducted in this respect. The aim of this study was to investigate the association between Topo I and TS in tumor tissue via immunostaining and to correlate it with clinicopathological variables and patient outcomes in resectable NSCLC.
Methods
Patients
This study retrospectively evaluated lung cancer patients who underwent primary tumor resection at the Thoracic Surgery Department of Beijing Chest Hospital, Capital Medical University, from May 2008 to August 2009. All enrolled patients were diagnosed with NSCLC by postoperative pathology. Pathological TNM (pTNM) staging was made according to the 7th edition of the International Union Against Cancer (UICC) TNM system. A total of 115 patients were enrolled in this study. All patients received radical resection of primary lung cancer and were naïve to any treatments, such as chemotherapy, radiation therapy, targeted therapy, and biological immune therapy, before the surgery. Patients who had a history of other tumors were excluded. The ethics committee of Beijing Chest Hospital, Capital Medical University, approved this study and informed consent was obtained from all patients.
Immunohistochemistry
Topo I and TS expression were detected in the tissues of the 115 enrolled NSCLC patients via immunohistochemistry (IHC). The primary antibodies used in this study were mouse anti‐human Topo I monoclonal antibody (MAB2290, Abnova Corporation, Taipei, Taiwan) and mouse anti‐human TS monoclonal antibody (Quanhui Trade Co., Ltd. Beijing, China). Slices 4 μm thick were cut from formalin‐fixed paraffin‐embedded representative tissues for IHC. The sections were then dewaxed and rehydrated through graded alcohol. For antigen retrieval, sections were treated with the working solution of citrate buffer (pH 6.0; CB07103011, Quanhui Enterprise Co., Ltd. Zhuhai, China) in an autoclave for four minutes. Primary antibody with a dilution of 1:400 covered a whole section. The slides were incubated overnight at 4°C, rinsed with phosphate buffered saline (PBS; pH 7.4–7.6), and incubated with ready‐to‐use MaxVisionTM reagent (KIT‐5030, Maixin Biotech Co., Ltd., Fuzhou, China) at room temperature for 30 minutes. DAB solution (DAB‐1031, Maixin Biotech. Co., Ltd.) was freshly prepared and mounted on slides for IHC staining. Finally, sections were counterstained with hematoxylin.
Interpretation of results
Immunohistochemistry (IHC) evaluation
In order to eliminate errors, one pathologist, blind to the clinical information, independently scored each slide.
IHC scoring method for Topo I protein
Immunohistochemistry staining was evaluated at a magnification of 200×. Two hundred tumor cells from five representative areas were counted. Tumor cells with nuclear staining were calculated positive and the rate of positive staining was obtained. The mean score (range 0–100%) was used for statistical analysis. A rate of positive staining less than 10% was interpreted as negative or low Topo I expression, while 11–100% was positive or high Topo I expression.
IHC scoring method for thymidylate synthase (TS) protein
A semi‐quantitative IHC test was performed. Four hundred cells were counted under a microscope at a magnification of 200×. IHC results were interpreted with semi‐quantitative scoring. The average score was used for statistical comparison. Cells with nuclear or cytoplasm yellow, gold or yellow‐brown stain were considered positive. Both positive cell proportion and staining intensity were considered in the scoring system. Positive cell proportion scoring was as follows: 0–10%, 0; 11–25%, 1; 26–50%, 2; and > 51%, 3. The scoring criteria for staining intensity were as follows: no staining, 0; light yellow, 1; yellow, 2; and brown, 3. Multiplication of the positive ratio and staining intensity determined the final total score (range 0–9) for statistical analysis. An IHC score of 0–3 was considered a negative or low level of TS expression, while 3.1–9 was a positive or high level of expression.
Follow‐up
Overall survival (OS) was defined as the interval between the date of surgery and the date of death or last follow‐up (31 October 2015). By the end of this study, all 115 patients had completed five‐year follow‐up.
Statistical analysis
SPSS version 22.0 was used for data analysis (IBM Corp., Armonk, NY, USA). The chi‐square test was used to correlate TS and Topo I expression with the following categorical variables: age group, gender, histology type, smoking status, tumor stage, tumor differentiation, and nodal status. The correlation between TS and Topo I expression was assessed by Spearman correlation analysis. The correlation between TS and Topo I expression at the largest diameter of the tumor was assessed by Mann–Whitney U test. In univariate analysis, OS was calculated using Kaplan–Meier analysis. The log‐rank test was used to test the differences between survival curves. A univariate Cox proportional hazard model was used to analyze the relative risks between OS with Topo I/TS expression and other clinicopathologic characters. Survival analysis of all parameters was calculated using multivariate Cox proportional hazard model. All tests were two‐tailed, and a P value < 0.05 was taken to have a significant difference.
Results
Patient characteristics
The 115 patients comprised of 93 men and 22 women with an age range of 27–84 years (median 59). The characteristics of all patients are listed in Table 1. Fifty‐seven (49.6%) patients received adjuvant chemotherapy, while 58 (50.4%) did not. The proportion of patients with stage I tumor treated with adjuvant chemotherapy was 22 (44%), stage II 18 (52.9%), and stage III 17 (54.8%).
Table 1.
Base characteristics of the patients
| Characteristics | Number (%) |
|---|---|
| Age (years) | |
| Median | 59 |
| Range | 27–84 |
| Gender | |
| Male | 93 (80.9) |
| Female | 22 (19.1) |
| Histology types | |
| ADC | 48 (41.7) |
| SCC | 57 (49.6) |
| Other types | 10 (8.7) |
| Tumor differentiation | |
| Poorly differentiated | 49 (42.6) |
| Moderately and well differentiated | 66 (57.4) |
| TNM stage | |
| Stage I | 50 (43.4) |
| Stage II | 34 (29.6) |
| Stage III | 31 (27.0) |
| Nodal status | |
| N0 | 74 (64.3) |
| N1 and N2 | 41 (35.7) |
| Smoking status | |
| Smoker | 73 (63.5) |
| Non‐smoker | 42 (36.5) |
| Surgical style | |
| Lobectomy | 92 (80.0) |
| Pneumonectomy | 23 (20.0) |
| Adjuvant chemotherapy | |
| Yes | 57(49.6) |
| No | 58 (50.4) |
ADC, adenocarcinoma; SCC, squamous cell carcinoma; TNM, tumor node metastasis.
Topoisomerase (Topo) I and TS expression in primary tumors
Positive immunoexpression of Topo I was observed in 86 tumors, including 29 (25.2%) with less than 10% positive stained cells, 49 (42.6%) with 10–50% positive stained cells and 8 (7%) with 50–90% positive stained cells (Figs 1, 2). We used positive staining in more than 10% of cells, the median value, as a cut‐off point. Therefore, there were 57 (49.6%) tumors with positive or high Topo I protein expression. TS staining showed that 35 (30.4%) carcinomas were TS positive via semi‐quantitative IHC (Figs 3, 4).
Figure 1.
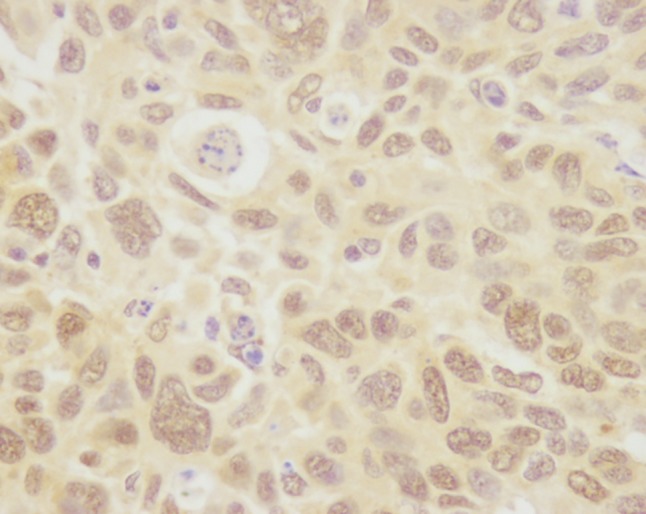
Positive immunoexpression of topoisomerase I.
Figure 2.
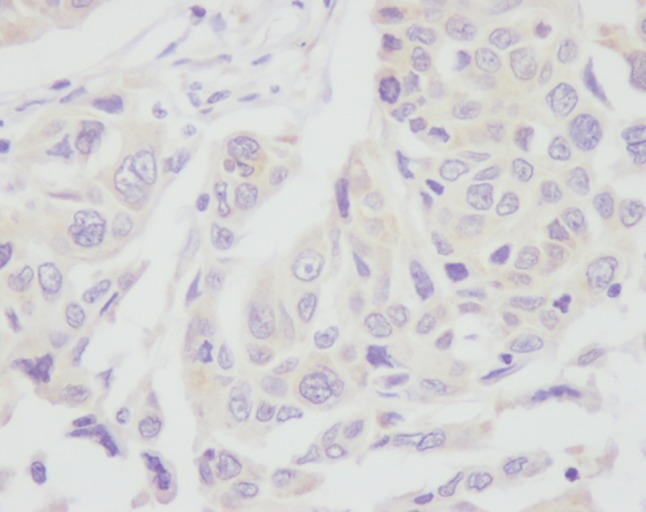
Negative immunoexpression of topoisomerase I.
Figure 3.
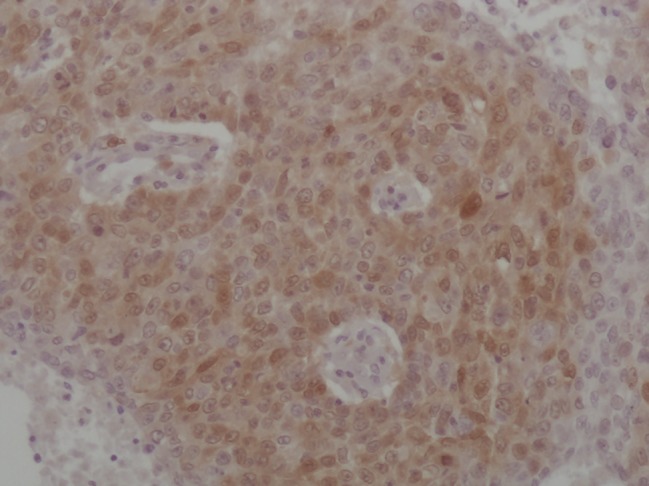
Positive immunoexpression of thymidylate synthase.
Figure 4.
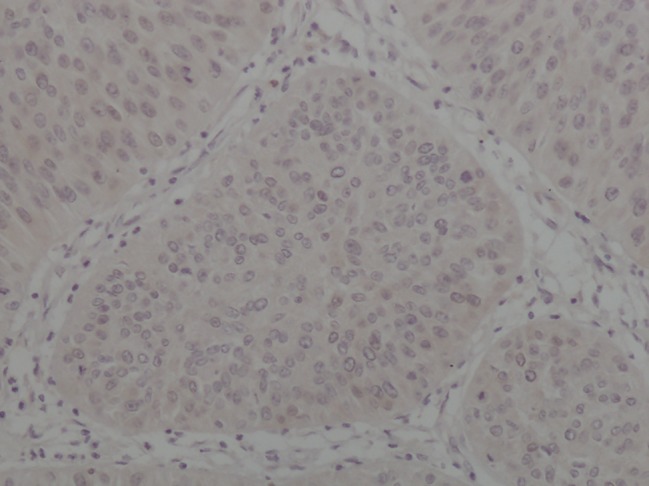
Negative immunoexpression of thymidylate synthase.
Correlation of Topo I and TS expression with clinicopathological features
No statistically significant difference of Topo I staining in clinicopathological features was found (Table 2). However, the median largest diameter of a tumor in the Topo I low expression group was 3.1 cm (range 0.7–14) compared with 4 cm (range 1.5–13) in the Topo I high expression group; a statistically significant difference (P = 0.035). The level of TS expression was not correlated with gender, histology type, smoking status, tumor stage, or nodal status. Increased TS expression occurred more frequently in tumors with poorer differentiation (χ2 = 4.346; P = 0.037; Table 3). The level of TS staining was higher in the age group under 45 years (50%) compared with the age group over 70 years (15%), but this result was not statistically significant (P = 0.078). Topo I expression was positively correlated with TS expression in tumor tissue using Spearman correlation analysis (R = 0.251, P = 0.007).
Table 2.
Topoisomerase I expression in different clinicopathological features
| Variables | Topo I positive (%) | χ2 | P |
|---|---|---|---|
| Total | |||
| Age group | |||
| ≤ 45 years | 40.0 | 0.403 | 0.817 |
| 46–69 years | 50.6 | — | — |
| ≥ 70 years | 50.0 | — | — |
| Gender | |||
| Male | 52.7 | 1.897 | 0.168 |
| Female | 36.4 | — | — |
| Histology types | |||
| ADC | 47.9 | 0.633 | 0.729 |
| SCC | 52.6 | — | — |
| Other types | 40.0 | — | — |
| Tumor differentiation | |||
| Poorly differentiated | 55.1 | 1.047 | 0.306 |
| Moderately and well differentiated | 45.5 | — | — |
| TNM stage | |||
| Stage I | 40.0 | 3.341 | 0.188 |
| Stage II | 58.8 | — | — |
| Stage III | 54.8 | — | — |
| Nodal status | |||
| N0 | 45.9 | 1.088 | 0.297 |
| N1 and N2 | 56.1 | — | — |
| Smoking status | |||
| Smoker | 49.3 | 0.005 | 0.944 |
| Non‐smoker | 50.0 | — | — |
ADC, Adenocarcinoma; SCC, Squamous cell carcinoma; TNM, tumor‐node‐metastasis; Topo I, Topoisomerase I.
Table 3.
Thymidylate synthase expression in different clinicopathological features
| Variables | TS positive | χ2 | P |
|---|---|---|---|
| Total | |||
| Age group | |||
| ≤ 45 years | 50.0 | 4.129 | 0.127 |
| 46–69 years | 31.8 | — | — |
| ≥ 70 years | 15.0 | — | — |
| Gender | |||
| Male | 30.1 | 0.025 | 0.875 |
| Female | 31.8 | — | — |
| Histology types | |||
| ADC | 25.0 | 1.328 | 0.515 |
| SCC | 33.3 | — | — |
| Other types | 40.0 | — | — |
| Tumor differentiation | |||
| Poorly differentiated | 40.8 | 4.346 | 0.037 |
| Moderately and well differentiated | 22.7 | — | — |
| TNM stage | |||
| Stage I | 32.0 | 0.431 | 0.806 |
| Stage II | 32.4 | — | — |
| Stage III | 25.8 | — | — |
| Nodal status | |||
| No | 29.7 | 0.049 | 0.825 |
| N1 and N2 | 31.7 | — | — |
| Smoking status | |||
| Smoker | 30.1 | 0.008 | 0.927 |
| Nonsmoker | 31.0 | — | — |
ADC, adenocarcinoma, SCC, squamous cell carcinoma; TNM, tumor‐node‐metastasis; TS, thymidylate synthase.
Topo I/TS expression and survival rates
After the last follow‐up on 31 October 2015, the median OS was 79 months (range 3–90). In the whole cohort, the one, three, and five‐year survival rates were 90.4%, 69.6%, and 61.7%, respectively. In univariate analysis, Topo I expression was significantly associated with decreased OS (P = 0.004). The pTNM stage of cancer, age group (with a cut‐off point of 70 years), nodal status (N1and N2 vs. N0), and adjuvant chemotherapy were also associated with OS in univariate analysis (P < 0.001, P = 0.024, P = 0.006, P = 0.025, respectively). No significant correlation between OS and TS expression in tumor tissue was observed (P = 0.542).
Multivariate analysis indicated that positive Topo I expression (P = 0.045), advanced TNM stage (P = 0.001), and no adjuvant chemotherapy (P = 0.019) were independent factors of poor prognosis for OS (Table 4).
Table 4.
Univariate and multivariate analysis of overall survival
| Factors | Univariate analysis | Multivariate analysis | ||||
|---|---|---|---|---|---|---|
| P | HR | 95% CI | P | HR | 95% CI | |
| Age (< 70 vs. ≥ 70 years) | 0.028 | 2.046 | 1.081–3.870 | 0.077 | 1.765 | 0.930–3.352 |
| Gender (male vs. female) | 0.490 | 1.279 | 0.637–2.568 | — | — | — |
| Histology types (ADC vs. SCC vs. other types) | 0.349 | 1.248 | 0.785–1.987 | — | — | — |
| Tumor differentiation (poorly vs. moderately and well‐differentiated) | 0.521 | 0.830 | 0.471–1.465 | — | — | — |
| TNM stage (stage I vs. stage II vs. stage III) | 0.001 | 1.836 | 1.292–2.610 | 0.001 | 1.811 | 1.265–2.592 |
| Nodal status (N0 vs. N1 and N2) | 0.007 | 2.182 | 1.236–3.852 | 0.781 | 1.100 | 0.465–2.605 |
| Smoking status (smoker vs. non‐smoker) | 0.163 | 1.501 | 0.848–2.656 | — | — | — |
| Adjuvant chemotherapy (yes vs. no) | 0.028 | 0.520 | 0.289–0.933 | 0.019 | 0.492 | 0.272–0.889 |
| Topo I expression (positive vs. negative) | 0.006 | 2.282 | 1.271–4.099 | 0.045 | 1.844 | 1.013–3.354 |
| TS expression (positive vs. negative) | 0.545 | 1.208 | 0.656–2.224 | — | — | — |
ADC, adenocarcinoma; CI, confidence interval; HR, hazard ratio; SCC, squamous cell carcinoma; TNM, tumor‐node‐metastasis; Topo I, topoisomerase I; TS, thymidylate synthase.
In univariate analysis, when a cut‐off point of 0% was used for Topo I expression, the negative NSCLC patients still had a higher OS rate than the Topo I positive patients (P = 0.022). However, we failed to demonstrate this association in multivariate analysis (P = 0.063).
In subgroup analysis, the correlation between Topo I expression and OS was statistically significant among patients with SCC (Table 5) and patients with pTNM stage I tumor (Table 6).
Table 5.
Univariate analysis of overall survival in lung cancer patients with different histology types
| Factors | SCC | Non‐SCC | ||||
|---|---|---|---|---|---|---|
| P | HR | 95% CI | P | HR | 95% CI | |
| Topo I expression (positive vs. negative) | 0.037 | 2.604 | 1.059–6.400 | 0.074 | 2.038 | 0.934–4.448 |
| TS expression (positive vs. negative) | 0.567 | 1.289 | 0.540–3.074 | 0.719 | 1.172 | 0.492–2.791 |
CI, confidence interval; HR, hazard ratio; SCC, squamous cell carcinoma, non‐SCC, not squamous cell carcinoma, including adenocarcinoma and other types; Topo I, topoisomerase I; TS, thymidylate synthase.
Table 6.
Univariate analysis of overall survival in patients with different TNM stage tumors
| Factors | Stage I | Stage II | Stage III | ||||||
|---|---|---|---|---|---|---|---|---|---|
| P | HR | 95% CI | P | HR | 95% CI | P | HR | 95% CI | |
| Topo I expression (positive vs. negative) | 0.036 | 3.037 | 1.077–8.562 | 0.225 | 2.277 | 0.602–8.610 | 0.364 | 1.483 | 0.633–3.472 |
| TS expression (positive vs. negative) | 0.727 | 1.211 | 0.414–‐3.544 | 0.788 | 0.833 | 0.221–3.144 | 0.098 | 2.169 | 0.868–5.424 |
CI, confidence interval; HR, hazard ratio; TNM, tumor node metastasis; Topo I, topoisomerase I; TS, thymidylate synthase.
Discussion
The present study provides an evaluation of Topo I expression in NSCLC. The cut‐off value was set at 10%, the median value, to separate low from high expression. The high Topo I protein expression rate in NSCLC was 49.6%. Relevant reports have shown Topo I positive expression rates of 49% in colorectal cancer (cut‐off value 1.5%) and 55% in NSCLC (cut‐off value 40%).21, 22 The median values were also used as cut‐off points in those studies. The level of Topo I expression was not correlated with age group, gender, histology type, tumor differentiation, or nodal status. Increased Topo I expression occurred in tumors of larger size. Guo et al. reported that in SCLC patients, Topo I expression was positively correlated with tumor stage.23 Topo I plays an essential role in various cellular processes, particularly regulating DNA topology, which is critical in cancer cell proliferation.24 Our study demonstrated that Topo I expression could reflect the tumor load to a certain extent. However, no significant correlation has been found between Topo I protein expression and tumor stage in colorectal cancer or penile carcinoma.25, 26 As little is known about Topo I protein expression in NSCLC, the reason for this diversity requires further study.
In the whole cohort, the one, three, and five‐year survival rates were 90.4%, 69.6%, and 61.7% respectively, which is consistent with a previous study.27 In univariate analysis, Topo I negative NSCLC patients had a higher OS rate than Topo I positive patients. Statistical analysis also revealed a correlation between OS rate with pTNM stage, age, adjuvant chemotherapy, and nodal status. In multiple Cox regression analysis, Topo I expression, adjuvant chemotherapy, and pTNM stage were independent prognostic factors for OS. pTNM stage was confirmed as a paramount prognostic factor for patients with NSCLC.28
There is controversy regarding the prognostic value of Topo I in many types of tumors. A previous study reported that Topo I mRNA expression influenced progression‐free survival in small‐cell lung cancer patients.11 However, another study did not identify an association between Topo I and prognosis in gastric cancer patients.29 In the present study, we found that Topo I expression was an independent prognostic factor in NSCLC patients. Topo I is a crucial enzyme in DNA replication. High levels of Topo I expression show active DNA replication; therefore, cancers with high Topo I protein expression are associated with a poor clinical outcome. Mukai et al. assessed Topo I expression using IHC and polymerase chain reaction in primary breast, gastric, and NSCLC tumors, and revealed that high levels of Topo I expression were related to a high recurrence rate.30 In this respect, Topo I expression is related to tumor proliferation and recurrence and could be a prognostic factor.
Further analysis showed that the correlation between Topo I expression and OS only applied to patients with stage I tumors. In patients with stage II and III tumors, Topo I expression was not of prognostic value, nor was it related to tumor pTNM stage. Patients with higher Topo I expression had a relatively poor prognosis, even if they were at an early tumor stage. In patients of advanced tumor stage, the influence of Topo I expression on survival was not significant, likely because of their shorter survival duration. Fifty‐seven patients received regimens of platinum‐doublet in adjuvant chemotherapy, including navelbine, paclitaxel, docetaxel, and gemcitabine; 21 patients received cisplatin‐based adjuvant chemotherapy; 31 received carboplatin‐based adjuvant chemotherapy; and five received both. We did not observe a significant correlation between OS and different regimens. Interestingly, our analysis showed that Topo I expression was associated with prognosis in surgically resected NSCLC patients who did not receive adjuvant chemotherapy after the surgery. Multivariate analysis also confirmed this result. This may be attributed to the fact that the chemotherapy regimens in this study consisted of paclitaxel, gemcitabine, and docetaxel that were not Topo I inhibitors.
Other researchers have shown that Topo I expression is correlated with sensitivity to irinotecan‐containing adjuvant chemotherapy in resected colorectal cancer.9 It remains controversial whether Topo I expression can be of predictive value for anti‐Topo I chemotherapy. In subgroup analysis, this survival benefit appeared to apply only to patients with SCC. SCC of the lung, which is the second most frequent histologic subtype of NSCLC, is still a significant public health problem, despite its gradually decreasing incidence.31, 32 The lack of encouraging new therapeutic methods is a major threat to the successful treatment of SCC. As Topo I expression could be an indicator of sensitivity for Topo I inhibitors in esophageal and oral SCC, lung SCC patients may benefit from Topo I inhibitor therapy when their tumor exhibits increased Topo I expression; however, this requires further investigation.33, 34 The detection of Topo I expression in removed tumors may help oncologists to distinguish patients at a higher risk of relapse.
The correlation between the expressions of the two biomarkers was also evaluated. We found that the frequency of TS positive tumors was significantly higher in patients with high Topo I expression. This finding is consistent with results previously reported by Paradiso et al., Ichikawa et al., and Xu et al. in colorectal cancer, and may be attributed to the fact that both Topo I and TS protein are biomarkers associated with more aggressive behavior.10, 35, 36 Albor et al. reported interaction between Topo I and p53 in vitro, specifically, wild‐type human p53 and certain point mutations interacted with Topo I and enhanced its catalytic activity.37 Catalytic activity of Topo I is correlated with enzyme expression levels in many tumor types in vivo.38 Therefore, there is a link between p53 and Topo I expression. p53 expression is regulated by TS protein at a translational level.39 In this respect, Topo I expression is regulated by TS in an indirect way. In addition, there is a likely direct relationship between Topo I and TS. With the exception of its function in enzyme catalysis, TS is also an RNA binding protein and could form ribonucleoprotein complexes with several cellular RNA species.40, 41 In this way, TS functions as a regulator of cellular gene expression and probably regulates Topo I expression.
Thymidylate synthase expression was also explored in this study. The percentage and intensity of cells stained positive for TS were characterized. A high expression of TS was found in 35 (30.4%) of 115 postoperative NSCLC patients, less than the 57.4% positive expression reported in a recent study.19 This is probably a result of differences in the source of TS antibodies. The level of TS expression was correlated with tumor differentiation; TS expression was increased in patients with poorer tumor differentiation. The correlation between TS expression and tumor differentiation is consistent with Tanaka et al.’s results, although they used a different technique to evaluate TS status.42 TS is an essential enzyme in DNA replication/repair and plays a critical role in cancer cell proliferation. Considering its biological role, TS gene expression is significantly increased along with tumor cells with higher proliferative activity, which perhaps accounts for the correlation between TS and tumor differentiation.43 Zhao et al. reported that TS expression was significantly higher in young NSCLC patients, using 60 years of age as the cut‐off.19 Our study also revealed a correlation between TS expression and age group, although statistical differences were not reached. Further research is needed to assess this issue. Unlike Topo I, no association between TS expression and OS in NSCLC patients was observed. This may be a result of the small sample size, which caused diverse levels of TS expression. Some studies have shown that low TS expression is positively correlated with prognosis, while others have taken an opposite view.19, 44, 45, 46 The prognostic and predictive role of TS expression in NSCLC patients is still under debate.
In conclusion, our analysis reveals that high Topo I expression in tumor tissue is inversely correlated with OS rate in patients with surgically resected NSCLC, especially in SCC patients. There is a correlation between TS and Topo I expression in removed tumor tissue. Further studies are needed to evaluate the prognostic value of Topo I and TS expression in patients with NSCLC.
Disclosure
No authors report any conflict of interest.
Acknowledgments
This study was funded by the Beijing Tuberculosis and Thoracic Tumor Research Institute (2‐92).
References
- 1. Torre LA, Bray F, Siegel RL, Ferlay J, Lortet‐Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin 2015; 65: 87–108. [DOI] [PubMed] [Google Scholar]
- 2. Spira A, Ettinger DS. Multidisciplinary management of lung cancer. (Published erratum appears in N Engl J Med 2009; 360:1917.) N Engl J Med 2004; 350: 379–92. [DOI] [PubMed] [Google Scholar]
- 3. Non‐small Cell Lung Cancer Collaborative Group . Chemotherapy in non‐small cell lung cancer: A meta‐analysis using updated data on individual patients from 52 randomised clinical trials. BMJ 1995; 311: 899–909. [PMC free article] [PubMed] [Google Scholar]
- 4. Williams BA, Sugimura H, Endo C et al. Predicting postrecurrence survival among completely resected nonsmall‐cell lung cancer patients. Ann Thorac Surg 2006; 81: 1021–7. [DOI] [PubMed] [Google Scholar]
- 5. Brundage MD, Davies D, Mackillop WJ. Prognostic factors in non‐small cell lung cancer: A decade of progress. Chest 2002; 122: 1037–57. [DOI] [PubMed] [Google Scholar]
- 6. Gilbert DC, Chalmers AJ, El‐Khamisy SF. Topoisomerase I inhibition in colorectal cancer: Biomarkers and therapeutic targets. Br J Cancer 2012; 106: 18–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bronstein IB, Vorobyev S, Timofeev A, Jolles CJ, Alder SL, Holden JA. Elevations of DNA topoisomerase I catalytic activity and immunoprotein in human malignancies. Oncol Res 1996; 8 (1): 17–25. [PubMed] [Google Scholar]
- 8. Yu J, Miller R, Zhang W et al. Copy‐number analysis of topoisomerase and thymidylate synthase genes in frozen and FFPE DNAs of colorectal cancers. Pharmacogenomics 2008; 9: 1459–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kostopoulos I, Karavasilis V, Karina M et al. Topoisomerase I but not thymidylate synthase is associated with improved outcome in patients with resected colorectal cancer treated with irinotecan containing adjuvant chemotherapy. BMC Cancer 2009; 9: 339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Xu JM, Zhu BD, Mangia A et al. [Prognostic value of thymidylate synthase, topoisomerase‐1 and Ki‐67 in advanced colorectal cancer patients on irinotecan and fluorouracil treatment.] Chin J Oncol 2005; 27: 312–5. (In Chinese.) [PubMed] [Google Scholar]
- 11. Sereno M, Cejas P, Moreno V et al. ERCC1 and topoisomerase I expression in small cell lung cancer: Prognostic and predictive implications. Int J Oncol 2012; 40: 2104–10. [DOI] [PubMed] [Google Scholar]
- 12. Hong B, Maley F, Kohen A. Role of Y94 in proton and hydride transfers catalyzed by thymidylate synthase. Biochemistry 2007; 46: 14188–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Doan LT, Martucci WE, Vargo MA, Atreya CE, Anderson KS. Nonconserved residues Ala287 and Ser290 of the Cryptosporidium hominis thymidylate synthase domain facilitate its rapid rate of catalysis. Biochemistry 2007; 46: 8379–91. [DOI] [PubMed] [Google Scholar]
- 14. Lenz HJ, Leichman CG, Danenberg KD et al. Thymidylate synthase mRNA level in adenocarcinoma of the stomach: A predictor for primary tumor response and overall survival. J Clin Oncol 1996; 14: 176–82. [DOI] [PubMed] [Google Scholar]
- 15. Qiu LX, Tang QY, Bai JL et al. Predictive value of thymidylate synthase expression in advanced colorectal cancer patients receiving fluoropyrimidine‐based chemotherapy: Evidence from 24 studies. Int J Cancer 2008; 123: 2384–9. [DOI] [PubMed] [Google Scholar]
- 16. Nicolson MC, Fennell DA, Ferry D et al. Thymidylate synthase expression and outcome of patients receiving pemetrexed for advanced nonsquamous non‐small‐cell lung cancer in a prospective blinded assessment phase II clinical trial. J Thorac Oncol 2013; 8: 930–9. [DOI] [PubMed] [Google Scholar]
- 17. Lee SH, Noh KB, Lee JS et al. Thymidylate synthase and ERCC1 as predictive markers in patients with pulmonary adenocarcinoma treated with pemetrexed and cisplatin. Lung Cancer 2013; 81: 102–8. [DOI] [PubMed] [Google Scholar]
- 18. Kasai D, Ozasa H, Oguri T et al. Thymidylate synthase gene copy number as a predictive marker for response to pemetrexed treatment of lung adenocarcinoma. Anticancer Res 2013; 33: 1935–40. [PubMed] [Google Scholar]
- 19. Zhao HY, Ma GW, Zou BY et al. Prognostic significance of thymidylate synthase in postoperative non‐small cell lung cancer patients. Onco Targets Ther 2014; 7: 1301–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Liu Q, Yu Z, Xiang Y et al. Prognostic and predictive significance of thymidylate synthase protein expression in non‐small cell lung cancer: A systematic review and meta‐analysis. Cancer Biomark 2015; 15 (1): 65–78. [DOI] [PubMed] [Google Scholar]
- 21. Silvestris N, Simone G, Partipilo G et al. CES2, ABCG2, TS and topo‐I primary and synchronous metastasis expression and clinical outcome in metastatic colorectal cancer patients treated with first‐line FOLFIRI regimen. Int J Mol Sci 2014; 15: 15767–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Giaccone G, van Ark‐Otte J, Scagliotti G et al. Differential expression of DNA topoisomerases in non‐small cell lung cancer and normal lung. Biochim Biophys Acta 1995; 1264: 337–46. [DOI] [PubMed] [Google Scholar]
- 23. Guo QS, Liu YX, Yu JM, Wang JL, Zhong WX, Liu XJ. [Expression and significance of DNA topoisomerase I (topo I) in small cell lung cancer.] Chin J Oncologia 2007; 29: 124–6. (In Chinese.) [PubMed] [Google Scholar]
- 24. Gupta M, Fujimori A, Pommier Y. Eukaryotic DNA topoisomerases I. Biochim Biophys Acta 1995; 1262 (1): 1–14. [DOI] [PubMed] [Google Scholar]
- 25. Boonsong A, Curran S, McKay JA, Cassidy J, Murray GI, McLeod HL. Topoisomerase I protein expression in primary colorectal cancer and lymph node metastases. Hum Pathol 2002; 33: 1114–9. [DOI] [PubMed] [Google Scholar]
- 26. Berney DM, Stankiewicz E, Adlan AM et al. DNA topoisomerase I and IIalpha expression in penile carcinomas: Assessing potential tumour chemosensitivity. BJU Int 2008; 102: 1040–4. [DOI] [PubMed] [Google Scholar]
- 27. Dela Cruz CS, Tanoue LT, Matthay RA. Lung cancer: Epidemiology, etiology, and prevention. Clin Chest Med 2011; 32: 605–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Yoshida Y, Murayama T, Sato Y, Suzuki Y, Saito H, Tanaka N. Validation of 7th TNM staging system for lung cancer, based on surgical outcomes. Asian Cardiovasc Thorac Ann 2013; 21: 693–9. [DOI] [PubMed] [Google Scholar]
- 29. Skarlos DV, Bai M, Goussia A et al. Expression of a molecular marker panel as a prognostic tool in gastric cancer patients treated postoperatively with docetaxel and irinotecan. A study of the Hellenic Cooperative Oncology Group. Anticancer Res 2007; 27: 2973–83. [PubMed] [Google Scholar]
- 30. Mukai M, Sato S, Ninomiya H et al. Sensitivity to CPT‐11 and platinum derivatives of stage I/II node‐negative breast, lung, and gastric cancer with occult neoplastic cells in lymph node sinuses. Oncol Rep 2007; 18 (1): 33–9. [PubMed] [Google Scholar]
- 31. Ginsberg MS, Grewal RK, Heelan RT. Lung cancer. Radiol Clin North Am 2007; 45: 21–43. [DOI] [PubMed] [Google Scholar]
- 32. Janssen‐Heijnen ML, Coebergh JW. The changing epidemiology of lung cancer in Europe. Lung Cancer 2003; 41: 245–58. [DOI] [PubMed] [Google Scholar]
- 33. Nakajima Y, Miyake S, Nagai K, Kawano T, Iwai T. CPT‐11 may provide therapeutic efficacy for esophageal squamous cell cancer and the effects correlate with the level of DNA topoisomerase I protein. Jpn J Cancer Res 2001; 92: 1335–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Hafian H, Venteo L, Sukhanova A, Nabiev I, Lefevre B, Pluot M. Immunohistochemical study of DNA topoisomerase I, DNA topoisomerase II alpha, p53, and Ki‐67 in oral preneoplastic lesions and oral squamous cell carcinomas. Hum Pathol 2004; 35: 745–51. [DOI] [PubMed] [Google Scholar]
- 35. Paradiso A, Xu J, Mangia A et al. Topoisomerase‐I, thymidylate synthase primary tumour expression and clinical efficacy of 5‐FU/CPT‐11 chemotherapy in advanced colorectal cancer patients. Int J Cancer 2004; 111: 252–8. [DOI] [PubMed] [Google Scholar]
- 36. Ichikawa W, Uetake H, Nihei Z, Mastuo K, Fujita H, Yamada Y. Topoisomerase I (Topo‐1) expression correlates to thymidylate synthase (TS) expression in colorectal cancer (CRC). Proc Am Soc Clin Oncol 1999; 18: 946 (abstract). [Google Scholar]
- 37. Albor A, Kaku S, Kulesz‐Martin M. Wild‐type and mutant forms of p53 activate human topoisomerase I: A possible mechanism for gain of function in mutants. Cancer Res 1998; 58: 2091–4. [PubMed] [Google Scholar]
- 38. Husain I, Mohler JL, Seigler HF, Besterman JM. Elevation of topoisomerase I messenger RNA, protein, and catalytic activity in human tumors: Demonstration of tumor‐type specificity and implications for cancer chemotherapy. Cancer Res 1994; 54: 539–46. [PubMed] [Google Scholar]
- 39. Chu E, Copur SM, Ju J et al. Thymidylate synthase protein and p53 mRNA form an in vivo ribonucleoprotein complex. Mol Cell Biol 1999; 19: 1582–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Chu E, Voeller DM, Morrison PF et al. The effect of reducing reagents on binding of thymidylate synthase protein to thymidylate synthase messenger RNA. J Biol Chem 1994; 269: 20289–93. [PubMed] [Google Scholar]
- 41. Chu E, Cogliati T, Copur SM et al. Identification of in vivo target RNA sequences bound by thymidylate synthase. Nucleic Acids Res 1996; 24: 3222–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Tanaka F, Wada H, Fukui Y, Fukushima M. Thymidylate synthase (TS) gene expression in primary lung cancer patients: A large‐scale study in Japanese population. Ann Oncol 2011; 22: 1791–7. [DOI] [PubMed] [Google Scholar]
- 43. Nakagawa T, Otake Y, Yanagihara K et al. Expression of thymidylate synthase is correlated with proliferative activity in non‐small cell lung cancer (NSCLC). Lung Cancer 2004; 43: 145–9. [DOI] [PubMed] [Google Scholar]
- 44. Grimminger PP, Schneider PM, Metzger R et al. Low thymidylate synthase, thymidine phosphorylase, and dihydropyrimidine dehydrogenase mRNA expression correlate with prolonged survival in resected non‐small‐cell lung cancer. Clin Lung Cancer 2010; 11: 328–34. [DOI] [PubMed] [Google Scholar]
- 45. Kaira K, Ohde Y, Nakagawa K et al. Thymidylate synthase expression is closely associated with outcome in patients with pulmonary adenocarcinoma. Med Oncol 2012; 29: 1663–72. [DOI] [PubMed] [Google Scholar]
- 46. Zheng Z, Li X, Schell MJ et al. Thymidylate synthase in situ protein expression and survival in stage I nonsmall‐cell lung cancer. Cancer 2008; 112: 2765–73. [DOI] [PMC free article] [PubMed] [Google Scholar]


